Abstract
Connexin-based therapeutics have shown the potential for therapeutic efficacy in improving wound healing. Our previous work demonstrated that the connexin43 (Cx43) mimetic peptide juxtamembrane 2 (JM2) reduced the acute inflammatory response to a submuscular implant model by inhibiting purinergic signaling. Given the prospective application in improving tissue-engineered construct tolerance that these results indicated, we sought to determine the mechanism of action for JM2 in the present study. Using confocal microscopy, a gap-FRAP cell communication assay, and an ethidium bromide uptake assay of hemichannel function we found that the peptide reduced cell surface Cx43 levels, Cx43 gap junction (GJ) size, GJ communication, and hemichannel activity. JM2 is based on the sequence of the Cx43 microtubule binding domain, and microtubules have a confirmed role in intracellular trafficking of Cx43 vesicles. Therefore, we tested the effect of JM2 on Cx43-microtubule interaction and microtubule polymerization. We found that JM2 enhanced Cx43-microtubule interaction and that microtubule polymerization was significantly enhanced. Taken together, these data suggest that JM2 inhibits trafficking of Cx43 to the cell surface by promoting irrelevant microtubule polymerization and thereby reduces the number of hemichannels in the plasma membrane available to participate in proinflammatory purinergic signaling. Importantly, this work indicates that JM2 may have therapeutic value in the treatment of proliferative diseases such as cancer. We conclude that the targeted action of JM2 on Cx43 channels may improve the tolerance of implanted tissue-engineered constructs against the innate inflammatory response.
Keywords: cancer, connexin43, gap junction, hemichannel, inflammation
poor cell survival in implanted tissue-engineered constructs remains a roadblock to the successful integration of these laboratory-grown exogenous tissues into host organ systems. Although few studies have quantitatively measured cell survival in implanted tissue-engineered constructs, the available data suggest that most exogenous cells in implanted constructs die within a week, if not a few days, of implantation (42, 60). Recent evidence demonstrates that even in autologous models implanted cells are largely eliminated within a few days post-introduction (3). Importantly, these effects were coincident with a robust innate inflammatory response. Similarly, Suzuki et al. (59) have also correlated implanted cell death with acute inflammation. Thus, techniques to inhibit acute innate inflammation may be key to improving implanted cell survival in tissue-engineered constructs.
The innate inflammatory response is an unavoidable consequence of any tissue damage, and any implanted device, material, or cellularized construct is no exception (50). Over the past decade or more, connexin-based therapeutics have been shown to be effective at inhibiting inflammation and improving the wound healing/foreign body response (31). Connexin proteins are the subunits of membrane channels that allow for the passive diffusion of molecules less than ~1 kDa in size (39). These channels exist in the plasma membrane in two modes: intercellular channels within gap junctions (GJs), and half-intercellular channels called hemichannels that primarily reside within a domain adjacent to GJs called the perinexus (51, 52).
Of particular interest, the connexin43 (Cx43) isoform of connexin proteins has been demonstrated to play a major role in the inflammatory response to injury and implanted medical devices (26, 57). Most recently, we have demonstrated that purinergic (extracellular ATP) signals transmitted through Cx43 hemichannels are a key pathway in initiation of the acute inflammatory response to an implanted device (5). Specifically, we found that application of the Cx43 peptidomimetic juxtamembrane 2 (JM2) to the submuscular implant pocket in which a strip of silicone was placed significantly reduced the amount of inflammatory infiltrate surrounding the implant, and in particular attenuated the neutrophil response. Importantly, this effect was found to be dependent on extracellular ATP, and in cultured endothelial cells it was shown that JM2 inhibited ATP release in a connexin-dependent manner (5). Here, we explore the mechanism of action of JM2.
JM2 is a mimetic of the Cx43 microtubule binding domain (5). Interaction between Cx43 and β-tubulin (a protein subunit of microtubules) was first described in 2001 (27). In that study, the authors found that Cx43 possesses a microtubule binding domain in the juxtamembrane (hence the name JM2) region of the Cx43 COOH terminus, and that domain is unique to the Cx43 isoform.
A number of potential functions for Cx43-β-tubulin interaction have been elucidated to date. Noncanonical roles for Cx43-β-tubulin interaction include modulation of transforming growth factor-β (TGF-β) signaling through competition with SMAD-β-tubulin binding (11), and regulation of cell polarity through stabilization and membrane targeting of microtubules (23, 49). In addition to these nonchannel roles for Cx43-β-tubulin interaction, a number of studies support a role for microtubules in Cx43 trafficking to the plasma membrane. The primary evidence for this comes from studies showing that application of microtubule polymerization inhibitors decrease Cx43 GJ labeling (32, 38) and reduce the recovery of Cx43-GFP GJs subjected to FRAP (40, 61). Importantly, two of these studies also show that movement of Cx43-containing vesicles is impeded in cells treated with microtubule polymerization inhibitors, further reinforcing a microtubule trafficking model (40, 61).
Other studies with Cx43 peptidomimetics suggest that these peptides act through inhibition of Cx43 interaction with target protein of the mimicked domain. For example, we have shown that the Cx43 mimetic peptide ACT1, which is based on the Cx43-ZO-1 interaction domain, competitively inhibits the interaction of Cx43 and ZO-1 (35, 51). We therefore tested the hypothesis that JM2 competitively inhibits Cx43-β-tubulin interaction and that disruption of the interaction reduces cell-surface trafficking of Cx43 thereby limiting the number of hemichannels available to release ATP.
MATERIALS AND METHODS
Peptide synthesis.
The JM2 peptide and control peptide were generated at American Peptide Company (Sunnyvale, CA) or Petron (Daejeon, Korea). The JM2 peptide was composed, from NH2 to COOH terminus, of an 8-mer poly-d-arginine internalization vector (66), and the Cx43 COOH-terminal amino acids 231–245 (VFFKGVKDRVKGRSD) that encompass the microtubule binding sequence [amino acids 234–243 (27)]; biotin-rrrrrrrr-VFFKGVKDRVKGRSD. An alanine-substituted control peptide (CP) was generated by replacing valines, lysines, and prolines (amino acids that are common to tubulin binding domains; see “The Microtubule Interaction Site” (http://www.neurobiologie.uni-osnabrueck.de/index.php?cat=Research&page=MT-Interaction-Site) with alanines: biotin-rrrrrrrr-AFFAGAADRAAGRSD. For both peptides the NH2 terminus was capped with a biotin tag, and the COOH-terminal end was left as free acetic acid.
Synthesis was carried out using Trt-Cl resins, and Fmoc amino acids were purchased from GL Biochem (Shanghai, China). Coupling regents and cleavage cocktail reagents were purchased from Sigma Aldrich, and other solvents were purchased form Daejung Chemical (Korea). Peptides were synthesized by Fmoc SPPS (solid-phase peptide synthesis) using ASP48S (Peptron, Korea) and biotin conjugation to the NH2 terminus was synthesized using biotin (8 eq), HOBT (8 eq), HBTU (8 eq), and DIPEA (16 eq) in DMF for 2 h.
When the desired sequence was complete, crude peptide was cleaved from the resin using a mixture of TFA/EDT/thioanisole/TIS/DW (90/2.5/2.5/2.5/2.5 volume) for 2 h. The peptide was then purified by reverse-phase HPLC (Shimadzu Prominence HPLC, Japan) using a Vydac Everest C18 column (250 mm × 22 mm, 10 μm) to >95% purity. Elution was carried out with a water-acetonitrile linear gradient (10~75% (vol/vol) of acetonitrile) containing 0.1% (vol/vol) trifluoroacetic acid. Molecular weights of the purified peptide were confirmed using LC/MS (Agilent HP1100 series). Peptides were lyophilized using a FDT-12012 freeze-dryer (Operon, Korea).
Cell culture.
Parental wild-type (WT) HeLa cells (ATCC, Manassas, VA), and HeLa cells stably expressing Cx43 (Cx43-HeLa; a generous gift from Klaus Willecke) were cultured as previously described (51). Human microvascular endothelial cells (HMVECs; Lonza, Allendale, NJ) were cultured in EGM-2MV medium (Lonza) according to manufacturer instructions and used for experiments at passage 5 or below.
Cytotoxicity.
HMVECs were grown to confluence on 96-well plates and treated with either vehicle (H2O, “No Peptide”), or JM2 at 12.5, 25, 50, 100, and 200 μM concentrations for 2 h at 37°C, 5% CO2. Following the incubation period, the medium was sampled and analyzed for lactate dehydrogenase (LDH) using an LDH cytotoxicity assay (Thermo Fisher Scientific, Rockford, IL) according to manufacturer instructions.
Western blots.
Standard cell lysates were resolved by “any kD” SDS-PAGE (Bio-Rad, Hercules, CA). Western blot detection was performed with Cx43 antibodies (C6219; Sigma-Aldrich, St. Louis, MO), α-tubulin antibodies (2144; Cell Signaling Technology, Danvers, MA), and actin antibodies (A5441; Sigma-Aldrich). Results were confirmed by repetition in at least three separate experiments. Nonlinear level adjustments were applied to Western blot images to enhance visibility.
Pull-down.
Pull-downs were performed according to the method of Hunter et al. (35). Briefly, 2 μg of glutathione S-transferase (GST)-tagged β-tubulin (GST-β-tubulin; Sigma-Aldrich) was coupled to 50 μl of glutathione-Sepharose 4B beads (GE Healthcare Bio-Sciences, Pittsburg, PA) according to manufacturer instructions. Five hundred microliters 500 μl of PBS (vehicle) or 50 μM JM2 in PBS was then incubated with the GST-β-tubulin-coupled beads for 1 h at room temperature.
During the peptide incubation, Cx43-HeLa cells were lysed in 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2 mM EGTA, 1% NP-40, 0.25% Na-deoxycholate, and Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Lysates were incubated for 30 min at 4°C with vortexing every 5 min, and then centrifuged at 16,000 g for 10 min at 4°C. H2O (vehicle) or 25 μM JM2 was added to clarified lysates, which were then combined with the peptide-incubated (or vehicle), GST-β-tubulin-coupled beads for 1 h at room temperature. Pelleted material and standard cell lysates were resolved by “any kD” SDS-PAGE (Bio-Rad). Western blot detection was performed with Cx43 antibodies (C6219; Sigma-Aldrich) and β-tubulin antibodies (ab52901; Abcam, Cambridge, MA). Results were confirmed by repetition in at least three separate experiments. Nonlinear level adjustments were applied to Western blot images to enhance visibility.
Immunohistochemistry and in situ protein interaction by proximity ligation assay.
Cx43/microtubule labeling: HMVECs plated on glass-bottom dishes (MatTek, Ashland, MA) were treated for 2 h with either vehicle (H2O) or 50 μM JM2. Cells were then washed and fixed in −20°C methanol. Fixed cells were blocked (1% bovine serum albumin, 0.1% Triton X-100, PBS) and labeled with Cx43 antibodies (C6219; Sigma-Aldrich) and α-tubulin antibodies ([no. 8203; Sigma-Aldrich (55)]. Labeled cells were imaged on a TCS SP5 laser scanning confocal microscope (LSCM) equipped with a ×63/1.4 numerical aperture (NA) oil objective (Leica, Buffalo Grove, IL). Images were analyzed for Cx43 GJ size, GJ and vesicle number, and microtubule fluorescence using ImageJ software (version 1.42q; National Institutes of Health, Bethesda, MD) as previously described (51).
For Cx43/TGN38 (trans-Golgi network protein, 38-kDa) colocalization, HMVECs were plated on glass-bottom dishes (MatTek) and treated for 2 h with either vehicle (H2O) or 50 μM JM2. Cells were then washed and fixed in 2% paraformaldehyde. Fixed cells were blocked (1% bovine serum albumin, 0.1% Triton X-100, PBS) and labeled with Cx43 antibodies (C6219; Sigma-Aldrich) and TGN38 antibodies (MA3-063; ThermoFisher Scientific, Waltham, MA). Labeled cells were imaged on a TCS SP5 LSCM equipped with a ×63 /1.4 NA oil objective (Leica). Images were analyzed for colocalization by measurement of the Pearson’s coefficient using the Intensity Correlation Analysis macro in the McMaster Biophotonics Facility ImageJ software package (version 1.42q), and as previously described by calculating the percentage of colocalized pixels (51).
Proximity ligation assay.
HMVECs plated on Laboratory-Tek II 4-chamber slides (Thermo Fisher Scientific) were treated for 2 h with either vehicle (H2O) or 50 μM JM2. Cells were then washed and fixed in 2% paraformaldehyde, then labeled with antibodies against Cx43 (MAB3067; Millipore, Billerica, MA) and β-tubulin (ab52901; Abcam). Following primary antibody labeling, Duolink proximity ligation assay (Sigma-Aldrich) was performed according to manufacturer instructions. Labeled cells were imaged on a BX63 motorized upright microscope (Olympus, Center Valley, PA). Images were analyzed for the number of Duolink signals. Controls included leaving out primary and Duolink antibodies. Signal was not observed when antibodies were left out.
Gap-FRAP.
Gap junction intercellular communication (GJIC) was measured by gap fluorescence recovery after photobleaching (gap-FRAP) as previously described (51). Briefly, HMVECs were grown to confluence on 60-mm culture dishes and treated for 2 h with either vehicle (H2O), 50 μM flufenamic acid (FFA; Abcam), or 50 μM JM2. Cells were then loaded with 1 μM calcein green, AM (BD Biosciences, San Jose, CA), washed with PBS, and imaged on a TCS SP5 LSCM equipped with a ×20 /0.75 NA water immersion objective using the Leica FRAP LAS-AF application wizard. Images were acquired once every 10 s for 5 min post-bleaching. Fluorescence recovery of the bleached cell was measured using ImageJ. The maximal recovery (F∞) and rate constant (k) were determined using nonlinear regression performed on GraphPad Prism software (version 5.0d for Mac OS X; GraphPad, San Diego, CA), using the following equation:
where t is time after photobleaching, F(t) is normalized fluorescence intensity, F0 is theoretical fluorescence intensity immediately after bleaching (estimated from the first post-bleach image), F∞ is the asymptotic theoretical maximal recovery value to which post-bleach fluorescence intensity tends, and k is the rate constant. This exponential decay function is a well-established model of GJ-mediated fluorescence recovery (1, 53).
Ethidium bromide uptake.
Cells were analyzed for hemichannel function by ethidium bromide (EtBr) uptake assay as previously described (51). Briefly, subconfluent Cx43-HeLa cells, WT HeLa cells, or HMVECs were treated for 2 h with either vehicle (H2O), 50 μM FFA, 5 μM mefloquine (MFQ; Sigma-Aldrich), or 50 μM JM2 or control peptide. We note that while we found the 5 μM MFQ concentration effective in this study, others have found that higher concentrations are necessary to block Cx43 (10), and this may be due to the stereoisomer contained in a given preparation of MFQ (36). Cells were then exposed to Opti-MEM without phenol red (Life Technologies) containing 500 nM EtBr for 15 min, followed by fixation in 2% paraformaldehyde. Fixed monolayers were nuclear labeled with Hoechst 33258 (Sigma-Aldrich), and imaged on an Olympus BX63 motorized upright microscope. EtBr uptake was determined in these images by measuring the mean gray values of fluorescent images using ImageJ and normalizing to the number of cells in the field.
Microtubule polymerization.
Microtubule polymerization was assessed using a tubulin polymerization HTS assay kit (BK004P; Cytoskeleton, Denver, CO) according to manufacturer instructions. Briefly, GTP, purified tubulin, and one of the following compounds were combined in a 96-well plate, and absorbance at 340 nm was monitored on a BioTek Synergy HT microplate reader (BioTek Instruments, Winooski, VT). Substances tested included control (H2O), 10 μM paclitaxel, 10 μM nocodazole, and 5, 10, and 20 μM JM2 or control peptide. Several parameters were measured from the resulting growth curves: The maximum OD340 reading; the time at which the curve reached 50% of the maximum OD340 reading (tOD(50)); and the lag phase, which was defined as the time at which a line drawn tangent to the steepest slope of the microtubule polymerization growth phase intersected with the initial absorbance value.
Statistics.
Statistical analysis was performed using Prism 5 software (version 5.0f; GraphPad Software, La Jolla, CA). An unpaired t-test was used for comparing two groups. For multiple comparisons, a Dunnett’s post hoc analysis was performed, except in the case of the HeLa cell EtBr uptake assay in which a Bonferroni post hoc analysis was used. A value of P < 0.05 was considered statistically significant.
RESULTS
JM2 is not cytotoxic to microvascular endothelial cells.
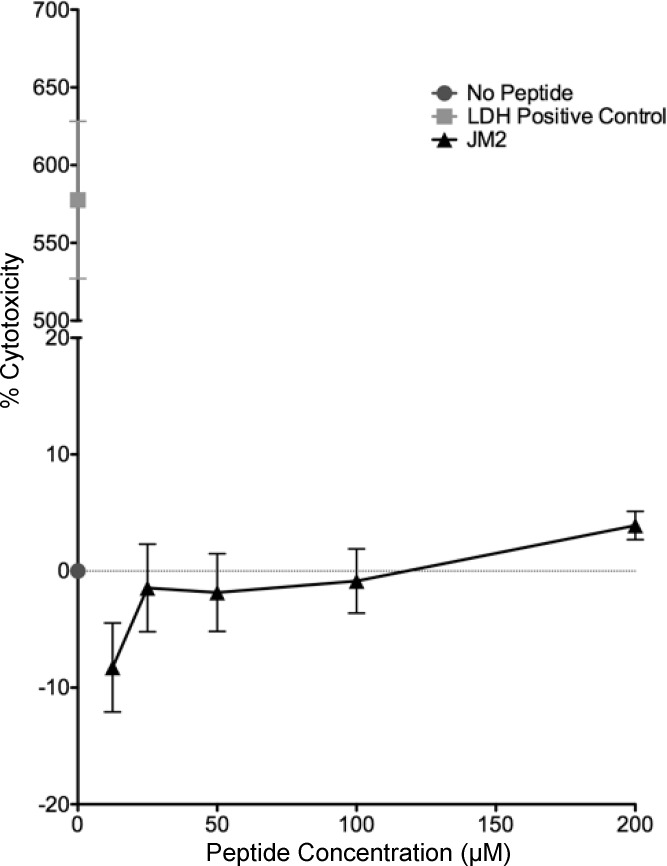
To focus on the dynamic effects of modulating Cx43-β-tubulin interaction, a 2 h time course of JM2 treatment was used for all cell culture experiments, as in previous studies (51). To determine the optimal concentration of peptide, human microvascular endothelial cells (HMVECs), which were chosen as a model cell type for their role in purinergic signaling during the inflammatory phase of injury, and the part that HMVEC Cx43 expression plays in these events (5, 41, 50), were exposed to JM2 concentrations ranging from 12.5 to 200 μM in line with prior reports on Cx43-based peptides (35, 46, 51, 65). None of the concentrations tested displayed significant cytotoxicity; however, a trend toward lower and higher cytotoxicity was noted for 12.5 and 200 μM JM2, respectively (Fig. 1). As such, 50 μM JM2 was used in all cell culture studies to minimize cytotoxicity, while maximizing effects on Cx43-β-tubulin interaction.
Fig. 1.
Juxtamembrane 2 (JM2) is not cytotoxic. Human microvascular endothelial cells (HMVECs) were treated with JM2 at 12.5, 25, 50, 100, and 200 μM concentrations for 2 h at 37°C, 5% CO2. The media were then sampled for lactate dehydrogenase (LDH) as a marker for cell death. No significant differences were observed between control (No Peptide) and any concentration of JM2. n = 3, with each n representing the average of 3 replicates; error bars represent SE.
JM2 enhances Cx43-β-tubulin interaction.
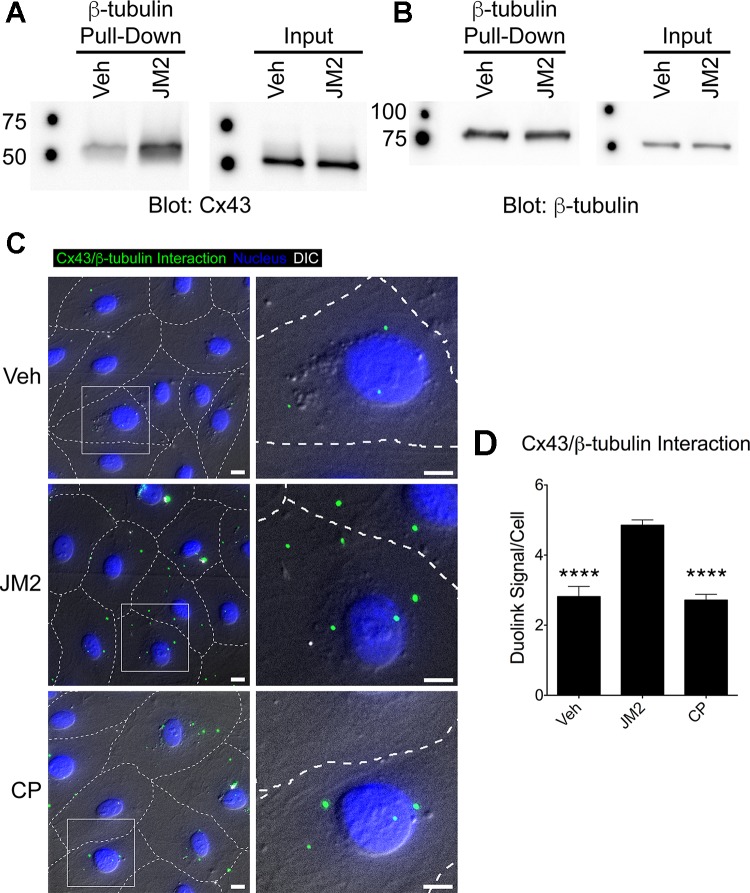
In our previous work, a peptide targeting Cx43-ZO-1 interaction (ACT1) was shown to competitively inhibit Cx43-ZO-1 interaction (35). Therefore, we anticipated that JM2 would act similarly and competitively inhibit Cx43-β-tubulin interaction. Counter to our expectations, we found that JM2 dissolved in cell lysates increased the amount of Cx43 recovered by pull-down with β-tubulin (Fig. 2A).
Fig. 2.
JM2 specifically enhances connexin43 (Cx43)-β-tubulin interaction. A and B: Cx43-HeLa cell lysates were incubated with β-tubulin-glutathione S-transferase (GST) bound to glutathione-Sepharose beads plus vehicle (H2O; Veh) or 25 μM JM2. Eluted proteins were analyzed by immunoblotting for Cx43 (A) or β-tubulin (B). β-Tubulin pulled down Cx43 in control conditions (vehicle), and this was increased in the presence of JM2 (A, “β-tubulin Pull-Down”), indicating that JM2 specifically enhances the interaction between Cx43 and β-tubulin. This was supported by the observation that the input amounts of Cx43 and β-tubulin subjected to pull-down were equivalent between Veh and JM2 treatments (A and B “Input” blots, respectively) and that JM2 did not affect the amount of β-tubulin pulled down by a β-tubulin antibody (B, “β-tubulin Pull-Down”). C: HMVECs were treated for 2 h with either vehicle (H2O; “Veh”), 50 μM JM2, or 50 μM control peptide (CP). Cells were fixed and labeled for Cx43-β-tubulin interaction (green), and the nucleus (blue). Differential interference contrast (DIC; gray scale) was used to approximate cell-cell borders (dashed lines). Boxed regions indicate the location of the enlarged insets. Scale bar = 10 μm. D: Cx43-β-tubulin interaction was quantified as the number of Duolink signals detected per cell. The number of signals detected in JM2-treated cells was significantly greater than in vehicle (Veh) or control peptide (CP). n = 3, with each n representing the average of 5 replicates; error bars represent SD. Nonlinear level adjustments were applied to Western blot images to enhance visibility. ****P < 0.001.
The unforeseen nature of the results led us to use a second experimental system to confirm the outcome of the pull-down assay. Specifically, we used a Duolink proximity ligation assay (PLA) to label Cx43-β-tubulin interaction in situ in intact, fixed HMVEC monolayers. In vehicle and control peptide (CP) treated cells we observed ~3 interaction labels per cell (Fig. 2, B and C). JM2 treated cells displayed a significant increase of nearly double the number of interaction signals (Fig. 2, B and C). These results demonstrate that application of JM2 to cultured cells specifically increases Cx43-β-tubulin interaction.
JM2 Reduces GJ size and increases intracellular Cx43 and microtubule labeling.
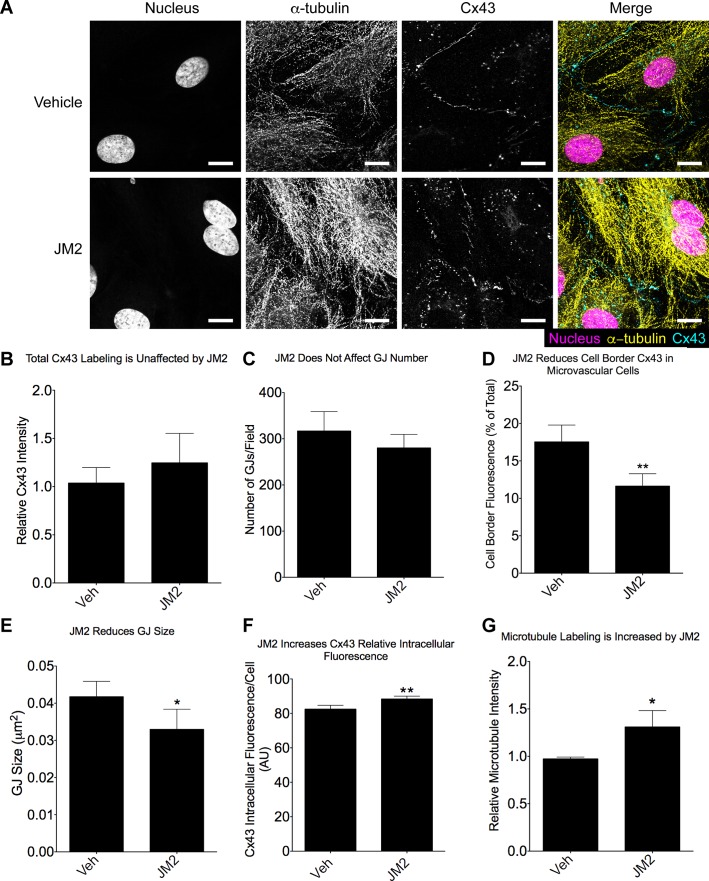
The effects of JM2 on GJ structure, Cx43 subcellular localization, and the microtubule cytoskeleton were studied by treating HMVECs with JM2, followed by labeling the fixed cells for Cx43 and microtubules. We note that microtubule labeling was carried out using an α-tubulin antibody instead of a β-tubulin antibody to obviate the potential of JM2 interaction with β-tubulin to alter microtubule fluorescence intensity by epitope masking or unmasking. The JM2-treated cells displayed less Cx43 membrane fluorescence, more intracellular Cx43 labeling, and greater microtubule labeling (Fig. 3A). Quantitative analysis was carried out by measuring the total Cx43 and microtubule fluorescence, the size, intensity, and number of membrane-associated GJ structures, and the level of intracellular fluorescence. No significant difference in total Cx43 level was observed between vehicle and JM2 treatment (Fig. 3B). Furthermore, the average number of GJ structures was very similar between the two treatment groups (Fig. 3C). In contrast, the level of cell border-associated Cx43 labeling and size of GJs was significantly reduced by JM2 (Fig. 3, D and E), and this change corresponded to a relative increase in intracellular Cx43 labeling (Fig. 3F). Intriguingly, these changes in Cx43 labeling were associated with increased microtubule labeling in cells treated with JM2 (Fig. 3G). Importantly, we did not observe any changes in cell number or morphology in JM2-treated cells, indicating that the differences in microtubule labeling were not an artifact of cell rounding or overlapping.
Fig. 3.
JM2 decreases gap junction (GJ) size while increasing labeling for microtubules. HMVECs were treated for 2 h with either vehicle (H2O; “Veh”) or 50 μM JM2. A: cells were fixed and labeled for Cx43 (cyan), α-tubulin (yellow), and the nucleus (magenta); Scale bar = 10 μm. B: total Cx43 fluorescence was unaffected by JM2. C and D: similarly, GJ number was measured and found to be unaffected by JM2 (C), while there was a significant decrease in the level of cell border-associated Cx43 fluorescence (D). E: consistent with the reduction in cell border Cx43 fluorescence, GJ size was reduced. F: concomitantly, the level of intracellular Cx43 fluorescence was significantly increased in JM2-treated cells. G: microtubule fluorescence was also significantly increased by JM2 treatment. For all graphs, n = 3, with each n representing the average of 5 replicates; error bars represent SD. *P < 0.05, **P < 0.01.
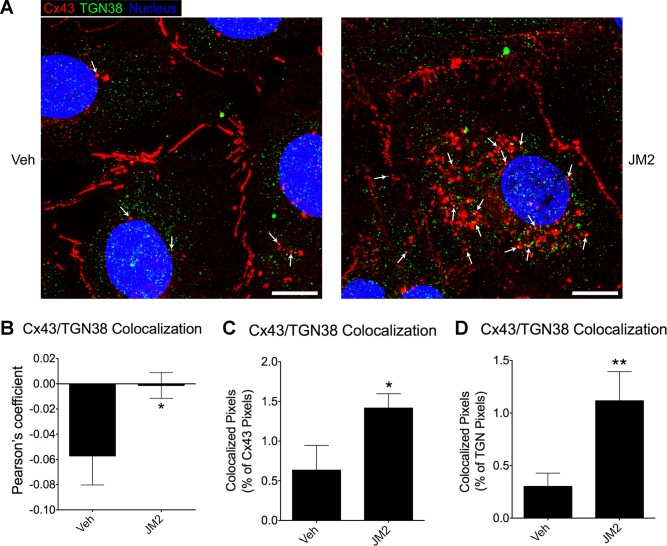
The identity of the Cx43-containing intracellular vesicles was examined by colabeling HMVECs treated with JM2 for Cx43 and the trans-Golgi network (TGN) protein TGN38. TGN38 has been shown to localize to the TGN, secretory vesicles, and the cell surface and is important for transport of proteins to the cell surface (64). We found that JM2-treated cells displayed increased colocalization of Cx43 and TGN38, suggesting that JM2 increases the accumulation of secretory vesicles containing Cx43 (Fig. 4).
Fig. 4.
JM2 increases the accumulation of Cx43-containing secretory vesicles. HMVECs were treated for 2 h with either vehicle (H2O; “Veh”) or 50 μM JM2. A: cells were fixed and labeled for Cx43 (red), the trans-Golgi network (TGN) protein TGN38 (green), and the nucleus (blue). Arrows indicate a number of puncta with Cx43 TGN38 colocalization that are likely secretory vesicles; Scale bar = 10 μm. B–D: colocalization of Cx43 and TGN38 was significantly increased by JM2 as assessed by Pearson’s coefficient (B), the amount of colocalized pixels as a % of total Cx43 pixels (C), and the amount of colocalized pixels as a % of total TGN38 pixels (D); n = 3, with each n representing the average of 3 replicates; error bars represent SD. *P < 0.05, **P < 0.01.
JM2 does not affect Cx43 or tubulin protein levels.
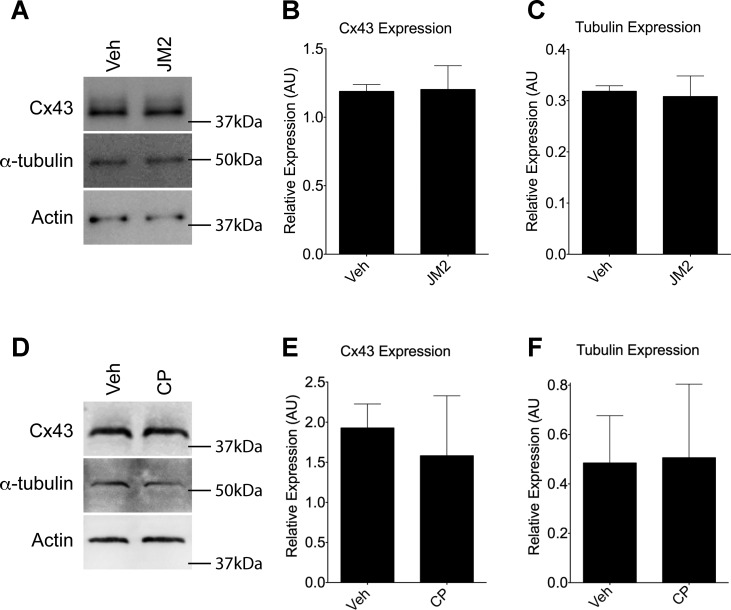
To determine whether any of the changes detailed above were due to changes in protein expression, Western blotting was performed for Cx43, tubulin (using the same α-tubulin antibody as in the imaging experiment described above), and actin on lysates of HMVECs exposed to JM2 (Fig. 5A). We found that there were no statistical differences in actin-normalized Cx43 and tubulin levels between vehicle-treated and JM2-treated cells (Fig. 5, B and C). Similar results were found in control peptide-treated cells (Fig. 5, D–F). Taken together, these results indicated that JM2 treatment resulted in limited trafficking of Cx43 to the cell surface.
Fig. 5.
Cx43 and tubulin expression are unaffected by JM2. A and D: Cx43, α-tubulin, and actin immunoblots of HMVEC lysates from cultures treated with vehicle (H2O; “Veh”), 50 μM JM2 (A), or 50 μM control peptide (D). B, C, E, and F: densitometry was performed, and Cx43 (B and E) and α-tubulin (C and F) levels were normalized to actin for JM2 (B and C) and control peptide (E and F) treated cells. For all graphs, n = 3; error bars represent SD. No significant changes were observed. Nonlinear level adjustments were applied to Western blot images to enhance visibility. AU, arbitrary units.
JM2 reduces both GJIC and hemichannel activity.
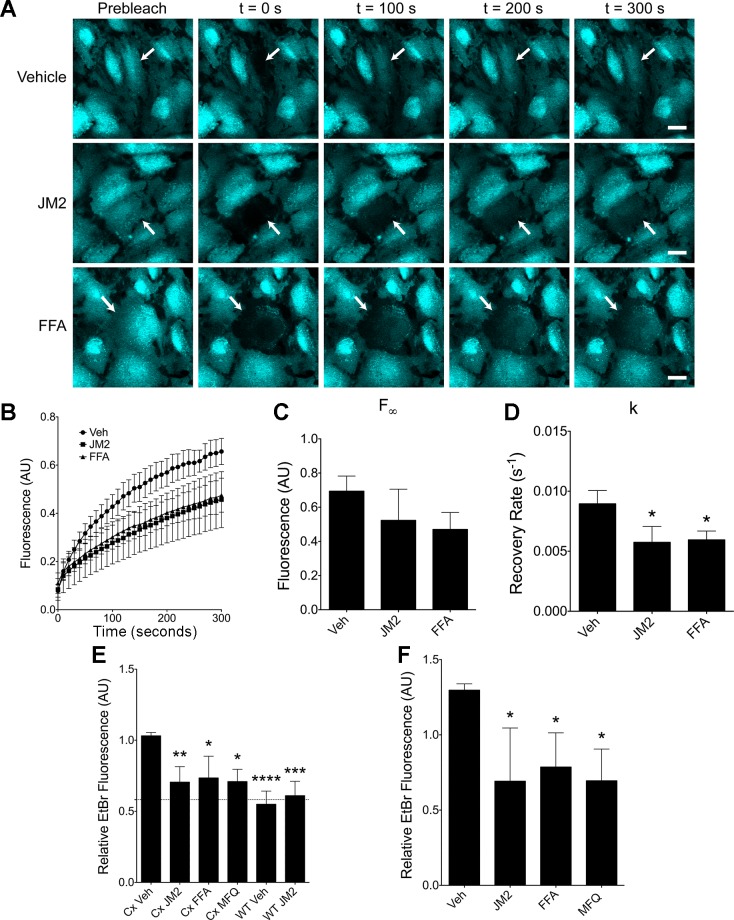
We next sought to determine whether the structural changes we observed in JM2-treated cells were associated with changes in Cx43 channel function. Therefore, gap junction fluorescence recovery after photobleaching (gap-FRAP) was carried out to measure GJIC. Briefly, HMVECs were loaded with calcein ester, yielding a membrane impermeable, but GJ-permeable fluorescent tracer, a selected cell was photobleached, and recovery of fluorescence in that cell was monitored over time. In control conditions, HMVECs displayed robust fluorescence recovery, achieving ~70% of initial fluorescence levels over a 5 min period (Fig. 6, A and B), confirming the presence of functional GJs. As a positive control for inhibition of GJIC, cells were treated with 50 μM flufenamic acid (FFA), a commonly used GJ and hemichannel blocker (19, 28, 48, 56). Furthermore, at the concentration used in this study, FFA specifically blocks connexin hemichannels and not pannexin channels (2). As expected, FFA clearly reduced cell-cell coupling (Fig. 6, A and B). Similarly, and consonant with the observation that the size of GJs was decreased with dysregulation Cx43-β-tubulin interaction, HMVECs treated with JM2 showed similar reduced levels of GJIC as those treated with FFA (Fig. 6 A and B).
Fig. 6.
JM2 treatment inhibits both gap junction intercellular communication and hemichannel function. A: HMVECs were treated with either vehicle (H2O and EtOH), 50 μM JM2, or 50 μM flufenamic acid (FFA) for 2 h, and calcein-AM was loaded during the final 30 min. Selected cells (arrows) were then photobleached by high-intensity laser light and recovery was monitored. Scale bar = 20 μm. B: average fluorescence readings were plotted over time. n = 3, with each n representing the average of 3 replicates; error bars represent SE. C and D: nonlinear regression to an exponential decay function was used to determine the maximum predicted recovery (F∞; C) and the recovery rate constant (k; D). Error bars represent SD. E: connexin43 (Cx43) and and wild-type (WT) HeLa cells were treated with either vehicle (H2O and EtOH; “Veh”), 50 μM JM2, 50 μM FFA, or 5 μM mefloquine (MFQ) for 2 h, then exposed to ethidium bromide (EtBr) for 15 min. Cells were fixed and imaged, and the quantified relative fluorescence is displayed. Dashed line indicates the level of autofluorescence in Cx43-HeLa cells. F: HMVECs were treated, imaged, and quantified as in E. For graphs in E and F, n = 4, with each n representing the average of 5 replicates; error bars represent SD. *P < 0.05, **P < 0.01, ***P< 0.001, ****P < 0.001.
To quantify fluorescence recovery, nonlinear regression to an exponential decay function was used to determine a maximal predicted recovery value (F∞) and rate constant (k). F∞ was not significantly affected by either JM2 or FFA, suggesting that given enough time all treatment groups would recover to the same degree (Fig. 6C). Even so, the rate of recovery was significantly reduced in both JM2- and FFA-treated cells (Fig. 6D), verifying that GJIC is reduced in JM2-treated cells in correlation with reduced GJ size (Fig. 3E).
We also assessed hemichannel function in cells treated with JM2. First, we used Cx43-expressing HeLa (Cx43-HeLa) cells and wild-type (WT) HeLa cells subjected to an ethidium bromide (EtBr) uptake assay to measure hemichannel activity. In control conditions, fluorescence measurements showed that vehicle-treated Cx43 HeLa cells took up EtBr over a 15 min period significantly greater than autofluorescence levels (P < 0.001; Fig. 6E). Conversely, WT HeLa cells [which do not express connexins with the exception of “marginal” levels of Cx45 (35, 63)] did not display EtBr fluorescence levels significantly different from autofluorescence, indicating that the assay is specific for connexin expression (Fig. 6E). Mefloquine (MFQ) and FFA (5 and 50 μM, respectively) were used as positive controls for hemichannel blockade (48, 51, 62), and Cx43-HeLa cells treated with MFQ and FFA exhibited significant reductions in EtBr uptake compared with vehicle control (Fig. 6E). Strikingly, hemichannel activity in JM2-treated Cx43-HeLa cells was also significantly inhibited, while EtBr uptake was unaffected by JM2 in WT HeLa cells, demonstrating that JM2 decreases membrane permeability in a Cx43-dependent manner (Fig. 6E).
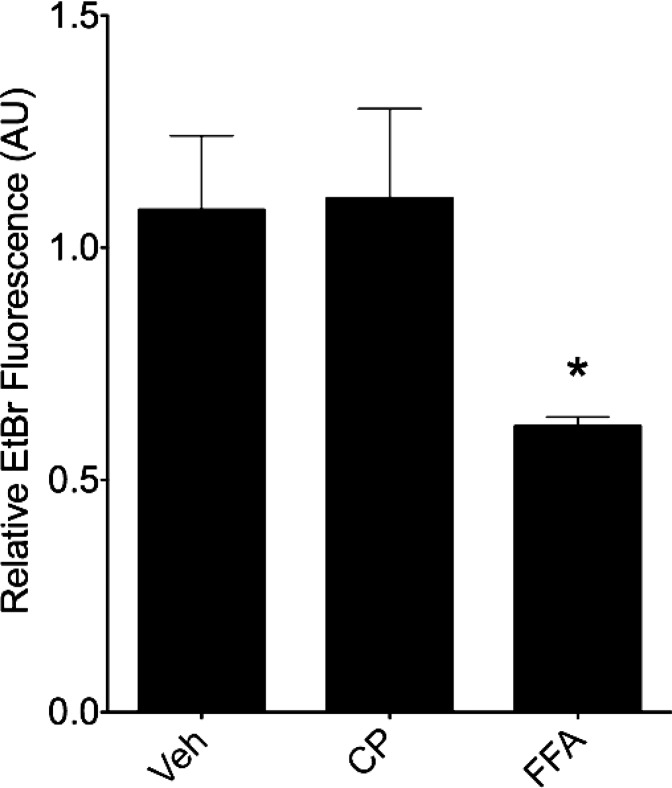
Hemichannel function was also studied in HMVECs. As with the HeLa cells above, HMVECs were treated with JM2, MFQ, or FFA followed by exposure to EtBr. Similar to the HeLa cells, HMVECs treated with either JM2, MFQ, or FFA displayed significant reductions in EtBr uptake (Fig. 6F), consistent with a model in which hemichannel function was blocked by dysregulation of Cx43-β-tubulin interaction in microvascular endothelial cells. Conversely, a control peptide showed no effect on EtBr uptake (Fig. 7).
Fig. 7.
A control peptide does not affect hemichannel function. HMVECs were treated with either vehicle (H2O and EtOH; “Veh”), 50 μM control peptide (CP), or 50 μM FFA for 2 h, then exposed to EtBr for 15 min. Cells were fixed and imaged, and the quantified relative fluorescence is displayed. No significant difference was observed between vehicle control and control peptide treatment conditions; n = 3, with each n representing the average of 5 replicates; error bars represent SD. *P < 0.05.
JM2 enhances microtubule polymerization.
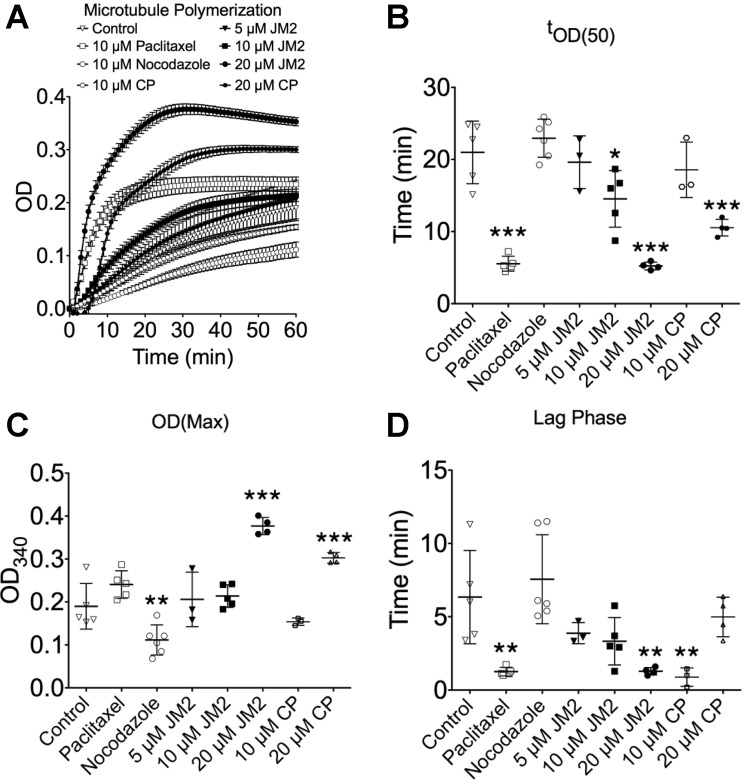
The results showing that JM2 enhanced Cx43-β-tubulin interaction in vitro, and microtubule quantity in cultured cells were unexpected (Figs. 2 and 3, respectively). We therefore explored the possibility that the Cx43 microtubule binding domain affects microtubule polymerization. An in vitro microtubule polymerization assay in which purified tubulin is combined with GTP in an optimized buffer, and absorbance at 340 nm (OD340) is monitored, was employed. Paclitaxel and nocodazole were used as controls for enhanced and inhibited polymerization, respectively. Figure 8A shows the averaged curves for control, 5, 10, and 20 μM JM2, 10, and 20 μM control peptide, 10 μM paclitaxel, and 10 μM nocodazole treatment conditions. Importantly, JM2 appeared to enhance microtubule polymerization in a dose-dependent manner. Therefore, time to 50% maximum OD340 (tOD(50); Fig. 8B), the maximum OD340 obtained (OD(Max); Fig. 8C), and duration of the lag phase (Fig. 8D) were quantified as measures of polymerization rate, peak conversion, and nucleation, respectively.
Fig. 8.
The Cx43 tubulin binding domain induces microtubule polymerization. A: microtubule polymerization was spectrophotometrically monitored by reading absorbance at 340 nm over a period of 1 h in control, 5, 10, and 20 μM JM2, 10 and 20 μM control peptide (CP), 10 μM paclitaxel, and 10 μM nocodazole treatment conditions. Error bars represent SE. B: time (t) to 50% maximum OD340 (tOD(50)) was determined as a proxy for reaction rate. C: maximum OD was measured to quantify the peak conversion of tubulin to microtubules. D: the duration of the lag phase was used as a measure of the nucleation phase. For B, C, D, and E, n ≥ 3; error bars represent SD. *P < 0.05, **P < 0.01, ***P< 0.001.
We found that JM2 significantly reduced tOD(50) at both 10 and 20 μM concentrations, with 20 μM JM2 most closely resembling paclitaxel in its effects, suggesting that JM2 enhances the rate of microtubule polymerization (Fig. 8B). However, unlike paclitaxel, 20 μM JM2 significantly enhanced OD(Max), indicating that JM2 also stabilized the newly polymerized microtubules (Fig. 8C). Finally, 20 μM JM2 also significantly decreased the duration of the lag phase, again in a comparable manner to paclitaxel (Fig. 8D), signifying that JM2 may also enhance the nucleation phase of microtubule polymerization. We note that the 20 μM control peptide did display some intermediate effects on microtubule polymerization, significantly affecting tOD(50) and OD(Max), but not lag phase, suggesting that there are some nonspecific effects occurring at the 20 μM peptide concentrations in the in vitro environment of the polymerization assay.
DISCUSSION
Herein, we have resolved the mechanism of action for the anti-inflammatory peptide JM2. Specifically, the results of this study show that JM2 inhibits Cx43 trafficking to the cell surface and thereby reduces the number of hemichannels available to participate in signaling between the inside and outside of the cells. We draw this conclusion from the observations that JM2 promoted Cx43-β-tubulin interaction (Fig. 2), reduced cell surface Cx43 levels, GJ size, GJ activity, and hemichannel function (Figs. 3 and 6), and promoted microtubule polymerization both in cultured cells and using isolated tubulin (Figs. 3 and 8). Taken together, these data support a model in which the Cx43 microtubule binding domain promotes forward trafficking of Cx43 to the cell surface in control conditions, but that JM2 dysregulates this process by activating irrelevant microtubule polymerization.
We note that alternative models need to be taken into account. For example, a number of other proteins contain a similar sequence to JM2 (>60% identity as discovered in a BLAST search), which leaves open the possibility that JM2 may affect GJIC and hemichannel activity indirectly, and/or have some pleiotropic effects. The data in this study also cannot rule out the possibility that JM2 does not have a direct effect on channel gating. Future work utilizing techniques such as patch clamp will be necessary to determine whether JM2 affects channel conductance. We also note that not all of the accumulated intracellular Cx43 and TGN38 puncta in Fig. 4 are small enough to be secretory vesicles, but are more in the size range of annular junctions [0.5–2 μm in diameter (21)]. This opens the possibility that JM2 is also disrupting GJ stability.
Finally, future work will be necessary to determine the exact molecular mechanism of the action of JM2 on microtubule polymerization. This is of particular interest considering that the effect of JM2 on microtubule polymerization was somewhat nonspecific in the in vitro assay, while the effects observed in cell culture appeared more specific. This suggests that there is a factor in live cells that is lacking in the in vitro assay that might confer specificity to JM2. It is a possibility that full-length, endogenous Cx43 is that very factor, but this remains to be seen.
It is interesting to compare our results with others that have examined the microtubule binding domain of Cx43. Francis et al. (23) showed that expression of Cx43dT (presumably obviating the possibility of Cx43-β-tubulin interaction) also inhibited GJIC. It seems contradictory that either enhancing or inhibiting Cx43-β-tubulin interaction would both result in reduced trafficking of Cx43 to the plasma membrane. One possibility is that the primary effect of JM2 is its action on microtubule polymerization, and not Cx43-β-tubulin interaction. Specifically, the excess of microtubules in JM2-treated cells may create trafficking routes that fail to reach the cell surface.
Another possibility is that by enhancing Cx43-β-tubulin interaction, JM2 causes Cx43 to “adhere” to microtubules. This mechanism is more consistent with the published literature than one in which Cx43-β-tubulin interaction directly facilitates transport of Cx43 along the microtubule cytoskeleton to the cell surface, as could be surmised from Francis et al. (23). Microtubules are likely important for transport of Cx43 to the plasma membrane, as application of microtubule polymerization inhibitors retards the movement of Cx43-containing vesicles (38, 61). Additionally, Fort et al. (22) has shown that Cx32 is trafficked along microtubules by Kinesin-1, and recent work by Delmar and colleagues has shown that Cx43 colocalizes with the Kinesin-1 isoform Kif5b (8), indicating that Cx43 is trafficked by a similar mechanism. However, trafficking of Cx43-containing vesicles along microtubules by kinesin motors is likely to be incompatible with a direct interaction between Cx43 and microtubules during the trafficking process, as kinesin motors are large—Kinesin-1 is on the order of 70 nm long (34).
The molecular mechanism by which JM2 enhances Cx43-β-tubulin interaction is also a unique question raised by this study. To date, a number of other Cx43 mimetic peptides have been generated, and they seem to invariably inhibit intermolecular interactions between Cx43 and other Cx43 molecules or a binding partner [e.g., ACT1, Gap26, Gap27, PEP-1 (20, 25, 35)], or inhibit intramolecular interactions [e.g., TAT-L2, Gap19 (46, 65)]. In this light, JM2 is novel among other connexin mimetic peptides. One possibility is that by generating more microtubules, JM2 creates more sites for endogenous Cx43 molecules to bind. Additionally, to our knowledge, the site(s) on β-tubulin that interacts with Cx43 has yet to be elucidated. This leads to a second intriguing possibility that binding of β-tubulin to the Cx43 microtubule domain is cooperative. In other words, binding of JM2 to β-tubulin could facilitate binding of endogenous Cx43 to a second binding site on β-tubulin or to another β-tubulin molecule nearby in the same microtubule.
The findings of this study have the potential to impact a broad range of fields. In particular, we demonstrated that targeting Cx43-β-tubulin interaction potently inhibits GJIC and hemichannel function (Fig. 6). GJIC has been implicated in injury spread through the bystander effect in the heart (6), brain (67), retina (45), spinal cord (9), and dermis (58). Similarly, opening of hemichannels due to tissue damage is also suggested to play an important role in exacerbating the extent of injury in the heart (37, 65), central nervous system (14, 44), retina (12), skin (26, 47), and during the foreign body response to both implanted medical devices and tissue-engineered constructs (5, 50, 57).
The mechanism behind the deleterious effects of hemichannel opening are due to the release of purinergic signals [e.g., ATP (4, 50) and other cytoplasmic molecules small enough to diffuse through hemichannels such as IP3, glutamate, glucose, and prostaglandins (9, 44)]. ATP released through Cx43 hemichannels in damaged or stressed cells may contribute directly to cell death (15). Additionally, it is becoming increasingly recognized that extracellular ATP plays a major role in the innate inflammatory response to injury (18, 43). In particular, ATP has been shown to be a potent neutrophil chemoattractant (7).
Tissue-engineered constructs have the potential to replace and/or regenerate damaged tissues and organs. However, the most basic challenge facing tissue engineers is the limited survival of implanted cells and living tissue-engineered constructs observed in vivo (16, 42). A number of factors contribute to poor cell survival in implanted engineered constructs. In particular, many biomaterials succumb to the foreign body response (50). However, whether or not encapsulation occurs, any implanted device or tissue faces the same initial fate as any injury, namely, neutrophil infiltration of the damaged tissue within minutes of the initial injury (24). Neutrophils are well documented to cause collateral tissue damage during the wound healing response (17, 26, 47) and to correlate with increased capsule size and contractility in response to a silicone implant (57). A neutrophil response to implanted biomaterials has also been documented (54), and recent data show that “naked” myogenic cells injected submuscularly are attacked and eliminated by neutrophils and macrophages (3). We propose that strategies to reduce connexin-mediated purinergic signals will limit cell death in implanted tissue-engineered constructs.
Finally, we note that the effect of JM2 on microtubule polymerization indicate the possibility of chemotherapeutic properties. We demonstrated that JM2 enhances microtubule polymerization both in vitro (Fig. 8), and in live cells (Fig. 3). In vitro, JM2 was an effective microtubule polymerization agent in the same concentration range as paclitaxel (Fig. 8). Paclitaxel is an effective chemotherapeutic agent that is unfortunately associated with a number of adverse side effects, which are exacerbated by its vehicle Cremophor EL (33). Although water-soluble paclitaxel formulations are currently being tested as a way to avoid the issues with Cremophor EL (33), JM2 is water soluble and was associated with no cytotoxic effects in the 10–20 μM range for which it was an effectual microtubule polymerization agent (Figs. 1 and 8). Other connexin-based therapeutics display chemotherapeutic properties (30), including ACT1 (29). Whether or not JM2 is as effective as paclitaxel at inducing cell cycle arrest and apoptosis in cancer cells will require further study (13), but its targeted effects indicate therapeutic potential.
Concluding remarks.
A basic regulatory mechanism in Cx43 biology has been elucidated by the results of the present study. Cx43-induced microtubule polymerization is indicated as a mechanism by which Cx43 is trafficked to the plasma membrane. Although the possibility of arrhythmogenic effects or other negative consequences of GJ blockade must be taken into account, targeting Cx43-β-tubulin interaction with JM2 is suggested as potentially beneficial in treating multiple pathologies from myocardial infarction to cancer and may significantly improve cell survival in implanted tissue-engineered constructs, making replacement of damaged tissue with engineered constructs a reality.
GRANTS
This work was supported by a Clinical and Translational Science Award from National Institutes of Health (UL1 TR000062 to J. M. Rhett) and by National Institute of Dental and Craniofacial Research Grant R01-DE019355 (to M. J. Yost). This study used the services of the Morphology, Imaging and Instrumentation Core, which is supported by National Institute of General Medical Sciences Grant P30 GM103342 to the South Carolina Centers of Biomedical Research Excellence (COBRE) for Developmentally Based Cardiovascular Diseases.
DISCLOSURES
R. G. Gourdie is a coinventor of the ACT1 peptide and cofounded FirstString Research. R. G. Gourdie is a member of the Scientific Advisory Board of FirstString Research and has stock options issued by the company.
AUTHOR CONTRIBUTIONS
J.M.R., S.A.F., R.G.G., and M.J.Y. conceived and designed research; J.M.R. and H.B. performed experiments; J.M.R. and M.J.Y. analyzed data; J.M.R., B.W.C., R.G.G., and M.J.Y. interpreted results of experiments; J.M.R. prepared figures; J.M.R. drafted manuscript; J.M.R., R.G.G., and M.J.Y. edited and revised manuscript; J.M.R., B.W.C., S.A.F., R.G.G., and M.J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the laboratory for crucial discussion and helpful advice on the manuscript.
REFERENCES
- 1.Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques 45: 33–62, 2008. doi: 10.2144/000112810. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92: 1033–1043, 2005. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 3.Burdzinska A, Gala K, Kowalewski C, Zagozdzon R, Gajewski Z, Pa̦czek L. Dynamics of acute local inflammatory response after autologous transplantation of muscle-derived cells into the skeletal muscle. Mediators Inflamm 2014: 482352, 2014. doi: 10.1155/2014/482352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Introductory overview of purinergic signalling. Front Biosci (Elite Ed) E3: 896–900, 2011. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 5.Calder BW, Rhett MJ, Bainbridge H, Fann SA, Gourdie RG, Yost MJ. Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response. Tissue Eng Part A 21: 1752–1762, 2015. doi: 10.1089/ten.tea.2014.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal 3: ra45, 2010. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm 9: 1133–1140.e6, 2012. doi: 10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci 39: 152–160, 2008. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA 101: 12364–12369, 2004. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell 18: 2264–2273, 2007. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O’Carroll SJ, Nicholson LF, Johnson CS, Green CR. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain 135: 506–520, 2012. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- 13.Das GC, Holiday D, Gallardo R, Haas C. Taxol-induced cell cycle arrest and apoptosis: dose-response relationship in lung cancer cells of different wild-type p53 status and under isogenic condition. Cancer Lett 165: 147–153, 2001. doi: 10.1016/S0304-3835(01)00404-9. [DOI] [PubMed] [Google Scholar]
- 14.Davidson JO, Green CR, Nicholson LF, Bennet L, Gunn AJ. Connexin hemichannel blockade is neuroprotective after, but not during, global cerebral ischemia in near-term fetal sheep. Exp Neurol 248: 301–308, 2013. doi: 10.1016/j.expneurol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D’Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ 16: 151–163, 2009. doi: 10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 16.Don CW, Murry CE. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med 17: 1355–1362, 2013. doi: 10.1111/jcmm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol 73: 448–455, 2003. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185: 93–102, 2002. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- 20.Evans WH, Bultynck G, Leybaert L. Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol 245: 437–449, 2012. doi: 10.1007/s00232-012-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong JT, Kells RM, Gumpert AM, Marzillier JY, Davidson MW, Falk MM. Internalized gap junctions are degraded by autophagy. Autophagy 8: 794–811, 2012. doi: 10.4161/auto.19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fort AG, Murray JW, Dandachi N, Davidson MW, Dermietzel R, Wolkoff AW, Spray DC. In vitro motility of liver connexin vesicles along microtubules utilizes kinesin motors. J Biol Chem 286: 22875–22885, 2011. doi: 10.1074/jbc.M111.219709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One 6: e26379, 2011. doi: 10.1371/journal.pone.0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants – a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32: 6692–6709, 2011. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 25.Gangoso E, Thirant C, Chneiweiss H, Medina JM, Tabernero A. A cell-penetrating peptide based on the interaction between c-Src and connexin43 reverses glioma stem cell phenotype. Cell Death Dis 5: e1023, 2014. doi: 10.1038/cddis.2013.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghatnekar GS, O’Quinn MP, Jourdan LJ, Gurjarpadhye AA, Draughn RL, Gourdie RG. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen Med 4: 205–223, 2009. doi: 10.2217/17460751.4.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11: 1364–1368, 2001. doi: 10.1016/S0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 28.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 29.Grek CL, Rhett JM, Bruce JS, Abt MA, Ghatnekar GS, Yeh ES. Targeting connexin 43 with α-connexin carboxyl-terminal (ACT1) peptide enhances the activity of the targeted inhibitors, tamoxifen and lapatinib, in breast cancer: clinical implication for ACT1. BMC Cancer 15: 296, 2015. doi: 10.1186/s12885-015-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grek CL, Rhett JM, Bruce JS, Ghatnekar GS, Yeh ES. Connexin 43, breast cancer tumor suppressor: missed connections? Cancer Lett 374: 117–126, 2016. doi: 10.1016/j.canlet.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Grek CL, Rhett JM, Ghatnekar GS. Cardiac to cancer: connecting connexins to clinical opportunity. FEBS Lett 588: 1349–1364, 2014. doi: 10.1016/j.febslet.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Y, Martinez-Williams C, Rannels DE. Gap junction-microtubule associations in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1213–L1221, 2003. doi: 10.1152/ajplung.00066.2003. [DOI] [PubMed] [Google Scholar]
- 33.Gupta N, Hatoum H, Dy GK. First line treatment of advanced non-small-cell lung cancer - specific focus on albumin bound paclitaxel. Int J Nanomedicine 9: 209–221, 2014. doi: 10.2147/IJN.S41770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279: 519–526, 1998. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 35.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16: 5686–5698, 2005. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iglesias R, Spray DC, Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes 16: 131–137, 2010. doi: 10.3109/15419061003642618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyyathurai J, D’hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology 75: 491–505, 2013. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RG, Meyer RA, Li XR, Preus DM, Tan L, Grunenwald H, Paulson AF, Laird DW, Sheridan JD. Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp Cell Res 275: 67–80, 2002. doi: 10.1006/excr.2002.5480. [DOI] [PubMed] [Google Scholar]
- 39.Laird DW. Life cycle of connexins in health and disease. Biochem J 394: 527–543, 2006. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99: 10446–10451, 2002. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95: 269–280, 2012. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam BK, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ, Davis DR. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 35: 133–142, 2014. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330: 362–366, 2010. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 44.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118: 826–840, 2011. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paschon V, Higa GS, Resende RR, Britto LR, Kihara AH. Blocking of connexin-mediated communication promotes neuroprotection during acute degeneration induced by mechanical trauma. PLoS One 7: e45449, 2012. doi: 10.1371/journal.pone.0045449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponsaerts R, De Vuyst E, Retamal M, D’hondt C, Vermeire D, Wang N, De Smedt H, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J 24: 4378–4395, 2010. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 47.Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 13: 1697–1703, 2003. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA 104: 8322–8327, 2007. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee DY, Zhao XQ, Francis RJ, Huang GY, Mably JD, Lo CW. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 136: 3185–3193, 2009. doi: 10.1242/dev.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhett JM, Fann SA, Yost MJ. Purinergic signaling in early inflammatory events of the foreign body response: modulating extracellular ATP as an enabling technology for engineered implants and tissues. Tissue Eng Part B Rev 20: 392–402, 2014. doi: 10.1089/ten.teb.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22: 1516–1528, 2011. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245: 411–422, 2012. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salmon ED, Leslie RJ, Saxton WM, Karow ML, McIntosh JR. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol 99: 2165–2174, 1984. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials 26: 7457–7470, 2005. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 55.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128: 547–560, 2007. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skeberdis VA, Rimkute L, Skeberdyte A, Paulauskas N, Bukauskas FF. pH-dependent modulation of connexin-based gap junctional uncouplers. J Physiol 589: 3495–3506, 2011. doi: 10.1113/jphysiol.2011.209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soder BL, Propst JT, Brooks TM, Goodwin RL, Friedman HI, Yost MJ, Gourdie RG. The connexin43 carboxyl-terminal peptide ACT1 modulates the biological response to silicone implants. Plast Reconstr Surg 123: 1440–1451, 2009. doi: 10.1097/PRS.0b013e3181a0741d. [DOI] [PubMed] [Google Scholar]
- 58.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419: 261–272, 2009. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki K, Murtuza B, Beauchamp JR, Brand NJ, Barton PJ, Varela-Carver A, Fukushima S, Coppen SR, Partridge TA, Yacoub MH. Role of interleukin-1beta in acute inflammation and graft death after cell transplantation to the heart. Circulation 110, Suppl 1: II219–II224, 2004. doi: 10.1161/01.CIR.0000138388.55416.06. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki K, Murtuza B, Beauchamp JR, Smolenski RT, Varela-Carver A, Fukushima S, Coppen SR, Partridge TA, Yacoub MH. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J 18: 1153–1155, 2004. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 61.Thomas T, Jordan K, Simek J, Shao Q, Jedeszko C, Walton P, Laird DW. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci 118: 4451–4462, 2005. doi: 10.1242/jcs.02569. [DOI] [PubMed] [Google Scholar]
- 62.Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci 120: 4016–4024, 2007. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- 63.Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch 440: 366–379, 2000. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Howell KE. The luminal domain of TGN38 interacts with integrin beta 1 and is involved in its trafficking. Traffic 1: 713–723, 2000. doi: 10.1034/j.1600-0854.2000.010904.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol 108: 309, 2013. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA 97: 13003–13008, 2000. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon JJ, Green CR, O’Carroll SJ, Nicholson LF. Dose-dependent protective effect of connexin43 mimetic peptide against neurodegeneration in an ex vivo model of epileptiform lesion. Epilepsy Res 92: 153–162, 2010. doi: 10.1016/j.eplepsyres.2010.08.014. [DOI] [PubMed] [Google Scholar]