Abstract
White adipose tissue (WAT) has a critical role in lipid handling. Previous work demonstrated that SCD1 is an important regulator of WAT fatty acid (FA) composition; however, its influence on the various interconnected pathways influencing WAT lipid handling remains unclear. Our objective was to investigate the role of SCD1 on WAT lipid handling using Scd1 knockout (KO) mice and SCD1-inhibited 3T3-L1 adipocytes by measuring gene, protein, and metabolite markers related to FA reesterification, glyceroneogenesis, and lipolysis. Triacylglycerol (TAG) content was higher in inguinal WAT (iWAT) from KO mice compared with wild-type, but significantly lower in epididymal WAT (eWAT). The SCD1 desaturation index was decreased in both WAT depots in KO mice. FA reesterification, as measured with a NEFA:glycerol ratio, was reduced in both WAT depots in KO mice, as well as SCD1-inhibited 3T3-L1 adipocytes. Pck1, Atgl, and Hsl gene expression was reduced in both WAT depots of KO mice, while Pck2 and Pdk4 gene expression showed depot-specific regulation. Pck1, Atgl, and Hsl gene expression was reduced, and phosphoenolpyruvate carboxykinase protein content was ablated, in SCD1-inhibited adipocytes. Our data provide evidence that SCD1 has a broad impact on WAT lipid handling by altering TAG composition in a depot-specific manner, reducing FA reesterification, and regulating markers of lipolysis and glyceroneogenesis.
Keywords: SCD1−/− mice, inguinal adipose tissue, epididymal adipose tissue, SCD1 inhibitor, adipocytes
white adipose tissue (WAT) has an important role in whole body lipid handling. When energy intake outweighs energy expenditure (e.g., during the fed state or with obesity), adipocytes in WAT act as reservoirs and sequester this excess energy as triacylglycerol (TAG) in lipid droplets. In contrast, when the body requires energy (e.g., during fasting or exercise), TAGs are broken down and mobilized into nonesterified fatty acids (NEFA) (12). These NEFAs can then be oxidized directly within the adipocyte, secreted into circulation for use by other tissues (such as the liver and muscle), or reesterified back into TAG (~0.2%, 50.1%, and 49.7% of NEFAs, respectively) (6, 22, 25, 30). Perturbations in WAT lipid handling processes, such as lipogenesis and lipolysis, are characteristic of metabolic diseases such as obesity and type 2 diabetes (1, 22). Given the worldwide prevalence of these metabolic diseases, it is imperative to improve our understanding of the factors controlling WAT lipid handling [a term used hereon to encompass TAG synthesis, glyceroneogenesis, fatty acid (FA) reesterification and lipolysis].
TAG lipid stores in WAT are regulated by a number of highly interconnected and coordinated processes. TAG synthesis in adipocytes involves FA esterification to glycerol-3-phosphate (G3P) (6, 22). These FAs are either synthesized directly within the adipocyte via de novo lipogenesis or obtained through lipoprotein lipase (LPL)-mediated hydrolysis of TAG-enriched particles, such as chylomicrons or hepatic-derived lipoproteins (6, 12, 22, 25). In WAT, the formation of G3P from glycerol is negligible because of low glucokinase activity (25). Instead, G3P in WAT is primarily generated via glyceroneogenesis (6, 20, 25). During periods of energy demand, TAGs are broken down through the sequential action of several lipolytic enzymes—adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoglyceride lipase (MGL)—resulting in the release of NEFAs from the glycerol backbone (1, 12, 22). A significant proportion (up to 50%) of the NEFAs released during lipolysis is reesterified back into TAG within the adipocyte (6, 12, 25). Consequently, the highly interconnected processes of TAG synthesis, glyceroneogenesis, lipolysis, and FA reesterification regulate net NEFA release and, more broadly, WAT lipid handling. It is noteworthy that some of these processes have been shown to differ between visceral and subcutaneous WAT depots. For example, LPL expression is higher in subcutaneous compared with visceral WAT, the effects of catecholamines on lipolysis are greater in visceral WAT, and broad differences in the overall lipid composition of the depots have been reported (11). This implies that investigations into WAT lipid handling should consider different depots.
A key enzyme involved in TAG synthesis is stearoyl-CoA desaturase-1 (SCD1, encoded by the Scd1 gene), which catalyzes the conversion of saturated fatty acids palmitate (16:0) and stearate (18:0) into monounsaturated fatty acids (MUFAs) palmitoleate (16:1n7) and oleate (18:1n9), respectively (21, 26, 31). Moreover, it is these MUFAs that are preferentially esterified to G3P to form TAG (21, 31). Several lines of evidence now suggest that SCD1 may influence lipid handling more extensively than previously appreciated. First, a downregulation in hepatic Scd1 expression caused a marked decrease in phosphoenolpyruvate carboxykinase (PEPCK, encoded by the Pck1 gene) expression in rodents (9), which is the rate-limiting enzyme for glyceroneogenesis (6, 20, 25). Second, reduced SCD1 activity using a specific inhibitor decreased TAG levels in 3T3-L1 adipocytes, and concomitantly inhibited the expression of genes regulating TAG synthesis (23). Finally, SCD1 inhibition in cardiomyocytes was shown to decrease the levels of lipogenic proteins and increase lipolysis (2). Collectively, these independent studies suggest that SCD1 has a broad influence on lipid handling in various tissues.
The aim of the present study was to investigate the role of SCD1 on WAT lipid handling by examining key gene, protein, and metabolite markers of glyceroneogenesis (PEPCK), lipolysis (HSL and ATGL), and FA reesterification (glycerol and NEFA) in Scd1 knockout (KO) mice and 3T3-L1 adipocytes. Given the aforementioned observations that reduced SCD1 activity was associated with marked reductions in PEPCK expression and TAG levels in the liver and 3T3-L1 cells, respectively, we hypothesized that reduced SCD1 activity would broadly influence pathways regulating NEFA release. Moreover, since WAT lipid handling differs in a depot-specific manner, we speculated that reduced SCD1 activity may have different effects in visceral versus subcutaneous WAT depots. Collectively, this study provides new insights regarding the role of SCD1 as a critical regulator of WAT lipid handling.
MATERIALS AND METHODS
Chemicals and reagents.
Cell culture reagents including Dulbecco’s modified Eagle’s medium (DMEM), 0.25% trypsin-ethylenediaminetetraacetic acid, and penicillin-streptomycin were purchased from Hyclone (Logan, UT). Rosiglitazone, human insulin, dimethyl sulfoxide (DMSO; ≥99.9% purity), 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), fatty acid free bovine serum albumin (BSA; ≥98% purity), medium 199 (M199), and fetal bovine serum (FBS) were purchased from Sigma Aldrich (St. Louis, MO). Primary antibodies for SCD1 (no. 2438), ATGL (no. 2439), T-HSL (no. 4107), phospho Ser-660 HSL (no. 4126), phospho Ser-563 HSL (no. 4139), and phospho Ser-565 HSL (no. 4137) were purchased from Cell Signaling Technology (Danvers, MA), while the primary antibody for α-tubulin (no. 7291) was obtained from Abcam (Toronto, ON, Canada). The primary antibody for PEPCK (no. 10004943) and the SCD1 inhibitor (CAY10566) were purchased from Cayman Chemical (Ann Arbor, MI).
Animal husbandry and sample collection.
The Scd1 knockout line (C57BL/6 background) was a generous gift from Dr. James M. Ntambi (Scd1tm1Ntam, University of Wisconsin-Madison). Mice were generated at the University of Quebec in Montreal (UQAM) animal facilities. The UQAM Animal Care and Use Committee approved all animal experimental protocols. Only male mice were used in this study [Scd1+/+ (WT), Scd1+/− (HET), and Scd1−/− (KO) genotypes].
WT, HET, and KO mice were fed a standard chow diet (Charles River; rodent chow no. 5075) ad libitum from weaning until 9 wk of age. The mice were housed (maximum 4 per cage) at constant 23°C and 48% air humidity, with a standard 12:12-h light-dark cycle. After a 4-h fast, all mice were euthanized with isoflurane and CO2. Blood was collected by cardiac puncture and immediately used to measure circulating glucose with a glucometer, and then stored on ice for ~30 min. Serum was isolated after centrifugation at 4°C for 15 min at 1,500 g and stored at −80°C. Inguinal WAT (iWAT) and epididymal WAT (eWAT) were collected from all mice. Approximately 40 mg of each WAT tissue were immediately rinsed in 1 × PBS, flash frozen in liquid N2, and stored at −80°C for gas chromatography. The remainder of the tissue was used for adipose tissue organ culture (3), as described below.
Adipose tissue organ culture.
iWAT and eWAT from WT, HET, and KO mice (n = 6 per genotype per WAT depot) were excised, weighed, and immediately placed in 15 ml conical tubes containing oxygenated M199 media and 1% penicillin-streptomycin. Approximately 70 mg of tissue were minced and placed into a well of a 12-well plate containing 2 ml of oxygenated M199 media supplemented with 1% penicillin-streptomycin, 50 μU insulin, and 1.25 nM DEX. Samples were incubated at 37°C in 5% CO2 for 24 h. After 24 h, media were removed, tissue was washed with 1 × PBS, and 1 ml of fresh M199 (supplemented with 2% BSA and DMSO) was added. Aliquots of media were taken at 0 h, 2 h, 4 h, and 24 h and flash frozen in liquid N2. After 24 h, the minced tissue was collected, flash frozen in liquid N2, and stored at −80°C.
Gas chromatography.
All solvents were purchased from Fisher Scientific (Waltham, MA) unless otherwise specified. Excised iWAT and eWAT were homogenized in 3.0 ml of 0.1 M KCl. A chloroform:methanol solution (2:1 vol/vol) was added to homogenized samples, according to Folch et al. (5). Samples were vortexed, and then flushed with N2 before being stored at 4°C overnight. Samples were centrifuged at 420 g for 10 min to separate phases. The chloroform layer was dried under N2 and then reconstituted in 100 μl of chloroform. Silica G plates (VWR, Mississauga, ON, Canada) were activated by heating for 1 h at 100°C. Samples were run alongside standards for 30 min in a solvent mixture containing 80 ml petroleum ether, 20 ml ethyl ester, and 1 ml acetic acid. Fractions were visualized using 8-anilino-1-naphthalenesulfonic acid (Sigma Aldrich) under UV light. The band corresponding to TAG fraction was identified and transferred into acid-washed vials containing the internal standard 17:0 (10 mg/ml). Hexane (2 ml) and 14% boron trifluoride-methanol (2 ml; Sigma Aldrich) were added and samples were methylated for 1.5 h at 100°C. Samples were cooled and centrifuged at 420 g for 10 min. The lower hexane layer containing methylated FAs was transferred into GC vials, and dried down under a gentle stream of N2. TAG fractions were reconstituted in 1.5 ml of hexane in GC vials. FA methyl esters were quantified using an Agilent 6890N gas chromatograph with a flame ionized detector and separated on a Supelco SP-2560 fused silica capillary column (100m, 0.2 μm film thickness, 0.25 mm; Sigma Aldrich). Hydrogen was used as the carrier gas and set at a constant flow rate of 30 ml/min. Samples were injected in splitless mode, with injector and detector ports set at 250°C. FA methyl esters were eluted through the column using a temperature program as follows: 0.2 min at 60°C, then increasing 13°C/min until a temperature of 170°C was reached. 170°C was held for 4 min, then increased 6.5°C/min to 175°C, increased 2.6°C/min to 185°C, increased 1.3°C/min to 190°C, and finally increased 13°C/min to 240°C and held at this temperature for 13 min. The run time per sample was 37.77 min. FAs were identified by comparing peak retention times with those of the known FA standard peaks (Nu-Chek-Prep, Elysian, MN). Peak areas were determined using EZChrom Elite software (version 3.3.2) to quantify the individual FAs present.
Cell culture experiments.
Murine 3T3-L1 preadipocytes were obtained from ATCC (Rockville, MD). 3T3-L1 preadipocytes were cultured in 5% CO2 and 100% humidity at 37°C for all experiments. Prior to treatments, cells were maintained in basic media, which consisted of DMEM supplemented with 1% penicillin-streptomycin and 5% heat-inactivated FBS. Cells were seeded at a density of 6.0 × 104 cells per well in six-well plates or at 1.3 × 105 cells per T25 flask. The SCD1 inhibitor (SCD1inhib) and rosiglitazone (Rosi) were diluted in DMSO to stock concentrations of 10 μM and 1 mM, respectively. Final working concentrations of 10 nM SCD1inhib and 1 μM Rosi were made by further diluting the stock solutions in adipocyte culture media. These concentrations of SCD1inhib and Rosi were selected based on dose-response experiments to identify the dose that maximized effectiveness while avoiding cell toxicity. Toxicity was assessed with the Promega CytoTox 96 Non-Radioactive Cytotoxicity Assay (Madison, WI).
Adipocyte differentiation was induced 2 days postconfluence (i.e., day 0) using an established differentiation cocktail that consisted of IBMX (0.5 mM), DEX (1 μM) and human insulin (5 μg/ml) in basic media. On day 2 and day 4, medium was replaced with fresh maintenance media (MM) that consisted of basic media supplemented with only human insulin. On day 5, MM was replaced with serum-free MM (SF-MM) supplemented with 2% BSA for the remaining duration of the experiments. Throughout the entire experiment, adipocytes were also treated with either 10 nM SCD1inhib, 1 μM Rosi, or an equivalent volume of DMSO (control condition). RNA, protein, and media collections took place on day 7. All experiments were performed with technical replicates in at least three different passages to ensure that results were not passage specific.
Dual RNA and protein extraction.
Total RNA was extracted from 3T3-L1 adipocytes and adipose tissue organ culture (ATOC) tissue using the Qiagen RNeasy Mini Kit (Qiagen, Mississauga, ON, Canada). Total protein was extracted from 3T3-L1 adipocytes using a modified acetone precipitation protocol, as previously described (27). RNA and protein were stored at −80°C until further analysis.
Real-time RT-PCR.
Single-stranded cDNA was synthesized from 1 μg or 0.5 μg (for 3T3-L1 cells or ATOC tissue, respectively) total RNA using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Burlington, ON, Canada). Amplification of cDNA was conducted using a Bio-Rad CFX96 RT-PCR detection system and SSo FAST EvaGreen Supermix (Bio-Rad, Mississauga, ON, Canada) using the following protocol: 95°C for 30 s, 95°C for 4 s, and 55.9°C for 4 s, repeated for 40 cycles. Primers for Scd1, Scd2, Pdk4, Pck1, Pck2, Atgl, Hsl, Pparγ, and Nono were designed using the online Roche Universal Probe Library and Assay Design Center (Table 1). Data were normalized using Nono as the housekeeping gene and are expressed as fold changes relative to control samples using the comparative Δ-Ct (ΔΔCt) method.
Table 1.
DNA oligonucleotide sequences
| Oligo Sequence (5′–3′) |
||
|---|---|---|
| Gene | Forward | Reverse |
| Scd1 | AAGTGGCAACGAACACACTG | AACTGGTGATGTTCCAGAGGA |
| Scd2 | GGCCCACATACTGCAAGAG | TTCAAACTTCTCGCCTCCAT |
| Pdk4 | CGCTTAGTGAACACTCCTTCG | CTTCTGGGCTCTTCTCATGG |
| Pck1 | GGAGTACCCATTGAGGGTATCAT | GCTGAGGGCTTCATAGACAAG |
| Pck2 | CAGGGTCTTATCCGCAAACT | CACATCCTTGGGGTCTGTG |
| Atgl | TGACCATCTGCCTTCCAGA | TGTAGGTGGCGCAAGACA |
| Hsl | CACAAAGGCTGCTTCTACGG | GGAGAGAGTCTGCAGGAACG |
| Pparγ | TGCTGTTATGGGTGAAACTCTG | CTGTGTCAACCATGGTAATTTCTT |
| Nono | CCCCACCAATACCTGCAA | TTCAGGTCAATAGTCAAGCCTTC |
Scd1/2, stearoyl-CoA desaturase 1/2; Pdk4, pyruvate dehydrogenase kinase 4; Pck1/2, phosphoenolpyruvate carboxykinase 1/2; Atgl, adipose triglyceride lipase; Hsl, hormone-sensitive lipase; Ppar, peroxisome proliferator-activated receptor; Nono, non-POU-domain-containing, octamer binding protein.
Western blot analyses.
Equal amounts of protein (30 μg) were heat denatured for 10 min at 95°C and allowed to cool before separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% resolving gels. Proteins were transferred to nitrocellulose membranes at 200 mA and membranes were subsequently blocked in Tris-buffered saline-0.1% Tween 20 supplemented with 5% nonfat dry milk with gentle agitation for 1 h at room temperature. Primary antibodies were diluted in Tris-buffered saline-0.1% Tween 20-5% BSA. Membranes were incubated in primary antibody overnight at 4°C with gentle agitation. The membranes were then washed two times in Tris-buffered saline-0.1% Tween 20. Afterwards, membranes were incubated in Tris-buffered saline-0.1% Tween 20-1.0% nonfat dry milk supplemented with horseradish peroxidase-conjugated secondary antibodies (Jackson Immuno-Research, West Grove, PA) at a dilution of 1:2,000 for 1 h at room temperature. Protein bands were detected with ECL Plus and imaged using a FluorChem HD2 Imaging System (ProteinSimple, San Jose, CA). All primary antibody dilutions were used at 1:1,000, except for α-tubulin, which was 1:5,000. Relative band intensities were quantified using Alpha Innotech Software (San Leandro, CA), with α-tubulin used as the internal control.
NEFA and glycerol measurements.
The concentrations of NEFA and glycerol in culture media (both 3T3-L1 adipocytes and ATOC) and fasted serum were measured using commercially available kits (Wako Diagnostics, Richmond, VA, and Sigma Aldrich, respectively), following the manufacturer's instructions. Briefly, glycerol and NEFA were used to investigate FA reesterification, as previously reported (29). Using these measurements enables a comparison between actual FA release and theoretical FA release (i.e., 3 times glycerol release into the media). Importantly, this calculation assumes a negligible amount of FA oxidation and is a measure of absolute rates of reesterification. Samples were run in duplicate and the average coefficient of variance was <7%.
Statistical analyses.
A one-way ANOVA followed by Tukey's post hoc test was used for all analyses. Data are reported as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Blood glucose and NEFA:glycerol accumulation are reduced in Scd1 KO mice.
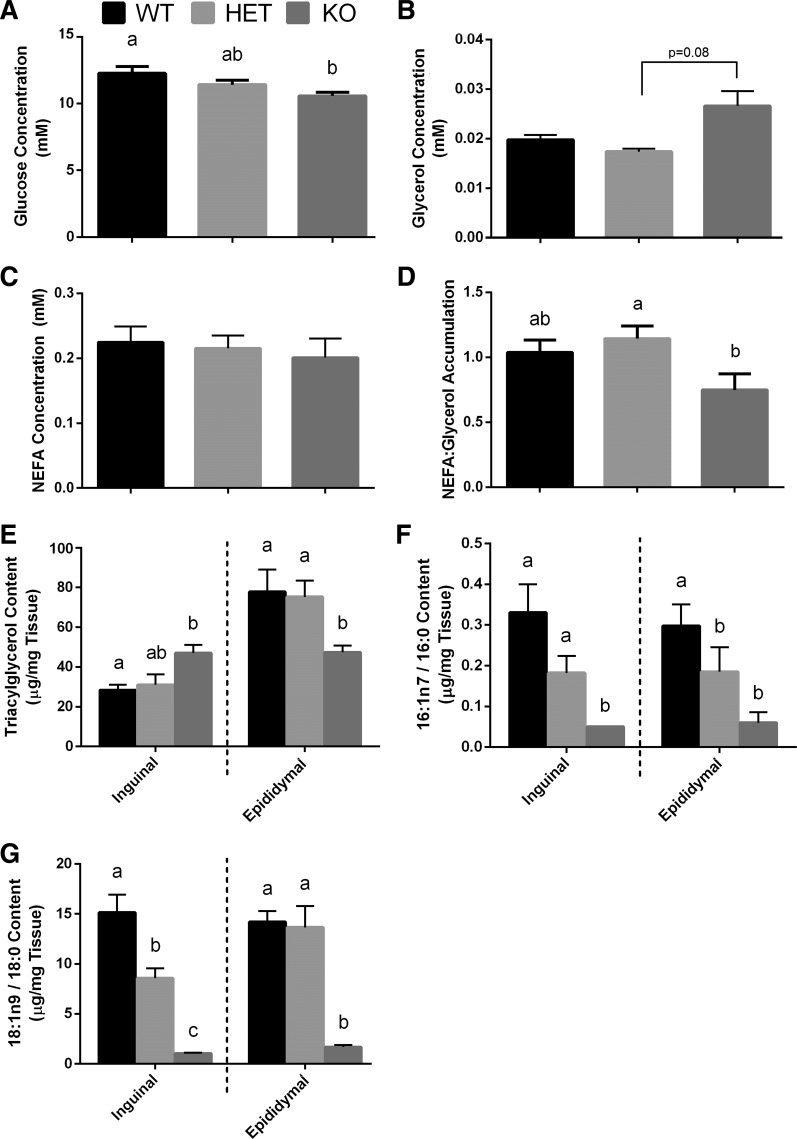
We first investigated general blood markers of metabolic health in mice. Blood glucose levels in KO mice were significantly lower than WT mice (Fig. 1A), with no differences seen between KO and HET or HET and WT groups. A trend towards an increase in serum glycerol levels in KO mice compared with HET (P = 0.08) was observed (Fig. 1B), but no difference was detected between KO and WT mice. Serum NEFA concentrations were comparable between the three genotypes (Fig. 1C); however, KO mice had a significantly lower NEFA:glycerol ratio compared with HET mice (Fig. 1D). There was no difference in the NEFA:glycerol ratio between HET and WT animals.
Fig. 1.
Blood metabolic markers and white adipose tissue (WAT) lipids in Scd1 knockout mice. A–D: blood glucose (A), serum glycerol (B), serum nonesterified fatty acid (NEFA; C), and serum NEFA:glycerol accumulation (D) are shown for the three genotypes [wild type (WT), heterozygote (HET), and knockout (KO)]. E: total triacylglycerol (TAG) content in inguinal WAT (iWAT) and epididymal WAT (eWAT). F and G: SCD desaturation indices were calculated accordingly: SCD-16 = 16:1n7/16:0 (F) and SCD-18 = 18:1n9 / 18:0 (G). ANOVA was used to determine differences between the three genotypes in a depot-specific manner. Bars with different letters are significantly different from one another (P < 0.05). All values are expressed as means ± SE (n = 6 per genotype).
WAT lipid composition is altered in Scd1 KO mice in a depot-specific manner.
We next investigated whether an SCD1 deficiency altered FA composition in a depot-specific manner. Depot differences were observed when considering WAT TAG content in the three genotypes. TAG content was significantly greater in iWAT from KO mice compared with WT mice, with HET mice showing intermediate levels (Fig. 1E). In contrast, TAG content was significantly lower in eWAT from KO mice compared with WT and HET animals. The abundance of key FAs related to SCD1 activity (i.e., 16:0, 16:1n7, 18:0, and 18:1n9) was measured in both iWAT and eWAT depots of all mice. Levels of 18:0 were significantly higher in both iWAT and eWAT from KO mice, with no change in 16:0 compared with WT and HET animals (Table 2). Levels of 16:1n7 were reduced in both depots from KO mice compared with WT and HET, while 18:1n9 was reduced only in eWAT (Table 2). Using these FA values, we also calculated SCD1 desaturation indices (i.e., SCD-16→16:1n7/16:0; SCD-18→18:1n9/18:0). As expected, SCD1 desaturation indices were reduced in iWAT and eWAT from KO mice compared with WT and HET mice (Fig. 1, F and G). FA data generally revealed that both indices in HET mice were intermediate to that observed for WT and KO animals. No difference was detected with the SCD-18 (i.e., 18:1n9/18:0 ratio) index in eWAT between WT and HET animals (Fig. 1G).
Table 2.
Triacylglycerol fatty acid composition in inguinal and epididymal white adipose tissue
| FA, μg/mg tissue | iWAT |
eWAT |
||||
|---|---|---|---|---|---|---|
| WT | HET | KO | WT | HET | KO | |
| 12:00 | 97.27 ± 15.58 | 81.01 ± 17.19 | 59.19 ± 7.36 | 134.90 ± 30.96ab | 118.22 ± 6.24a | 47.61 ± 9.28b |
| 14:00 | 574.95 ± 77.37 | 536.01 ± 130.69 | 827.44 ± 118.29 | 1239.57 ± 95.72a | 940.05 ± 94.76b | 859.16 ± 135.11b |
| 15:00 | 74.02 ± 5.92 | 130.62 ± 25.60 | 94.39 ± 9.84 | 183.42 ± 28.31 | 129.43 ± 14.77 | 112.05 ± 12.53 |
| 16:00 | 7,174.85 ± 811.27 | 11,007.89 ± 2,304.68 | 12,213.35 ± 1,337.55 | 22,575.92 ± 2,827.07 | 18,417.50 ± 2405.07 | 16,077.70 ± 2,878.11 |
| 18:00 | 683.63 ± 105.77a | 1,100.62 ± 312.06a | 8,809.89 ± 1,232.07b | 1,883.15 ± 283.19a | 1,786.34 ± 214.47a | 7,078.99 ± 1,143.10b |
| 20:00 | 35.60 ± 3.55a | 43.89 ± 9.83a | 120.10 ± 10.60b | 66.94 ± 5.45 | 60.67 ± 6.75 | 106.52 ± 20.70 |
| 24:00:00 | 38.54 ± 5.96a | 103.12 ± 14.56b | 36.04 ± 7.50a | 189.09 ± 48.86 | 106.96 ± 33.18 | 61.80 ± 13.88 |
| Total SFA | 86,78.86 ± 1,025.42a | 13,003.16 ± 2,814.61a | 22,160.4 ± 2,723.21b | 26,272.99 ± 3,319.56 | 21,559.16 ± 2,775.24 | 24,343.83 ± 4,212.71 |
| 14:1c9 | 122.32 ± 16.20 | 160.96 ± 24.96 | ND | 117.99 ± 25.30ab | 136.35 ± 6.71a | 60.49 ± 9.10b |
| 16:1n7 | 2,362.10 ± 285.23a | 2,143.09 ± 593.12a | 558.18 ± 61.03b | 6,752.46 ± 996.74a | 3,314.69 ± 424.35b | 794.00 ± 105.15c |
| 18:1n9 | 10,038.61 ± 1,202.41 | 8,391.72 ± 2148.11 | 9,086.70 ± 803.33 | 28,379.21 ± 5539.91a | 23,097.61 ± 3125.65a | 11,133.08 ± 1,384.33b |
| 18:1c11 | 711.60 ± 88.92 | 1,094.50 ± 240.43 | 748.44 ± 90.27 | 2,286.04 ± 368.14a | 1300.45 ± 224.29b | 778.86 ± 124.1b |
| 20:1c11 | 513.25 ± 48.24 | 385.29 ± 106.72 | 588.66 ± 73.51 | 1,062.98 ± 152.21a | 859.09 ± 135.43a | 618.31 ± 101.38b |
| 22:1n9 | 82.52 ± 11.49 | 97.42 ± 30.87 | 162.05 ± 27.18 | 58.04 ± 7.76a | 67.60 ± 15.02a | 33.42 ± 5.17b |
| Total MUFA | 13,830.4 ± 1,652.49 | 12,272.98 ± 3,144.21 | 11,144.03 ± 1,055.32 | 38,656.72 ± 7,090.06a | 28,775.79 ± 3,931.45a | 13,418.16 ± 1,729.23b |
| 18:2n6 | 7,422.26 ± 981.78 | 10,971.98 ± 2,513.73 | 10,132.61 ± 1,202.13 | 21,248.24 ± 3,916.41 | 15,978.86 ± 2,713.67 | 15,017.38 ± 2,120.03 |
| 18:3n6 | 52.73 ± 5.22 | 70.41 ± 13.21 | 53.15 ± 8.32 | 140.68 ± 27.87 | 97.24 ± 13.03 | 75.07 ± 11.51 |
| 20:2n6 | 67.42 ± 9.20 | 39.11 ± 8.42 | 49.60 ± 9.29 | 165.00 ± 20.42a | 62.72 ± 21.43b | 102.87 ± 10.57b |
| 20:3n6 | 113.27 ± 18.93 | 125.87 ± 25.70 | 80.33 ± 12.18 | 200.55 ± 32.18a | 157.32 ± 24.48a | 90.54 ± 11.09b |
| 20:4n6 | 83.51 ± 10.95 | 103.59 ± 28.01 | 81.03 ± 12.56 | 196.50 ± 57.54 | 175.37 ± 33.66 | 100.33 ± 15.41 |
| 22:4n6 | 69.05 ± 9.97 | 91.80 ± 16.90 | ND | 97.95 ± 20.85a | 93.75 ± 16.44a | 37.64 ± 8.17b |
| Total n-6 | 7,808.24 ± 1,036.05 | 11,402.76 ± 2,605.97 | 10,396.72 ± 1,244.48 | 22,048.92 ± 4,075.27 | 16,565.26 ± 2,822.71 | 15,423.83 ± 2,176.78 |
| 18:3n3 | 376.80 ± 44.95 | 548.99 ± 127.40 | 540.39 ± 56.59 | 1,091.10 ± 184.72 | 915.42 ± 114.14 | 784.29 ± 97.58 |
| 18:4n3 | 63.18 ± 11.96 | 82.33 ± 15.07 | 74.77 ± 10.75 | 162.52 ± 31.19a | 126.23 ± 19.51a | 80.17 ± 14.67b |
| 20:5n3 | 48.59 ± 5.63 | 126.44 ± 24.31 | 66.42 ± 10.29 | 172.49 ± 38.90 | 79.07 ± 6.42 | 96.31 ± 18.66 |
| 22:5n3 | 155.59 ± 70.59 | 98.86 ± 22.03 | 72.51 ± 7.34 | 164.05 ± 26.38a | 159.58 ± 21.13a | 66.73 ± 11.57b |
| 22:6n3 | 101.31 ± 12.00 | 140.74 ± 38.12 | 145.14 ± 16.13 | 271.34 ± 36.41 | 202.78 ± 40.46 | 131.67 ± 30.53 |
| Total n-3 | 745.47 ± 145.13 | 997.36 ± 226.93 | 899.23 ± 101.1 | 1,861.49 ± 317.60 | 1,483.09 ± 201.66 | 1,159.18 ± 173.01 |
Data correspond to mean μg fatty acid (FA)/mg tissue ± SE (n = 6 mice per genotype). FAs were analyzed between the 3 genotypes in a white adipose tissue (WAT) depot-specific manner using a 1-way ANOVA. FAs with different letters are significantly different from one another (P < 0.05). The absence of letters means there were no significant differences for a FA in a specific WAT depot between the 3 genotypes. iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue; WT, wild-type mice; HET, heterozygous mice; KO, Scd1 knockout mice; ND, not detected; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids.
FA reesterification is decreased in ex vivo WAT from Scd1 KO mice.
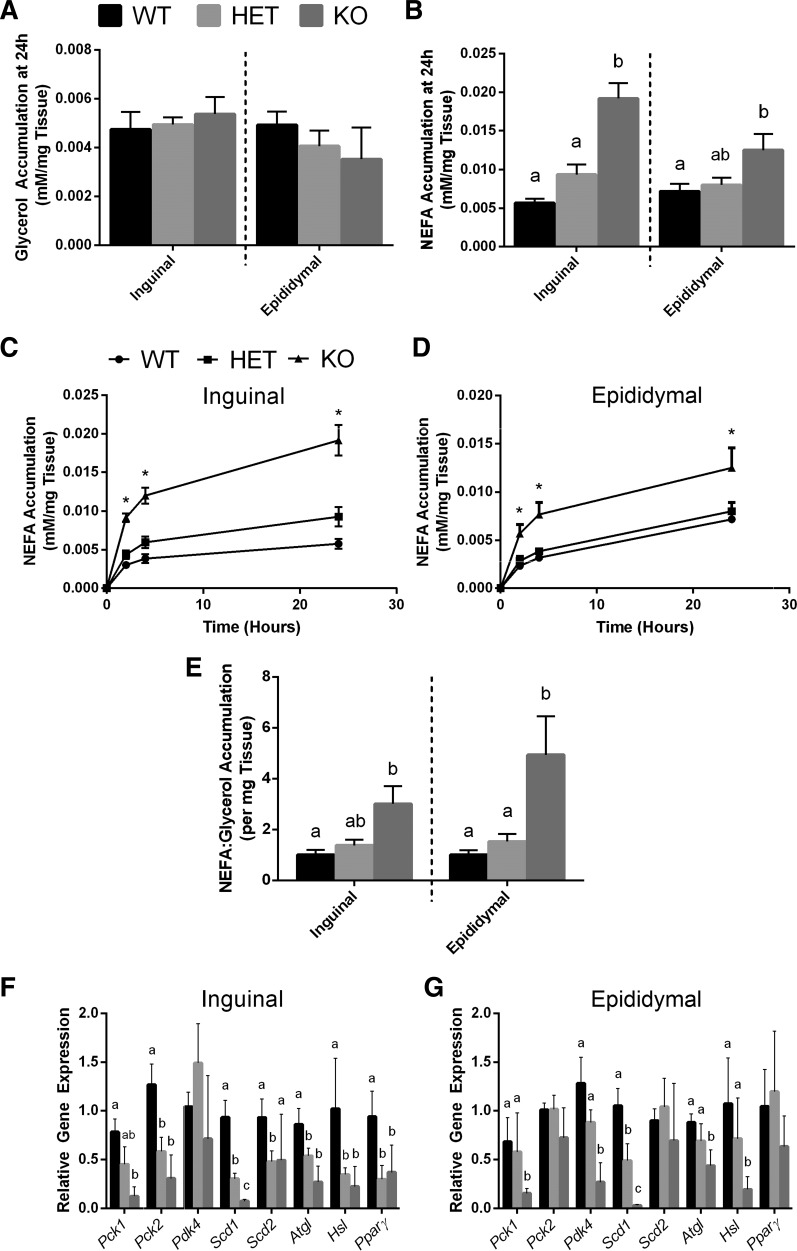
ATOC was then used to examine how SCD1 regulates lipid handling in isolated iWAT and eWAT depots. No significant difference in glycerol accumulation in ATOC media was observed between the mice (Fig. 2A); however, significant increases in NEFA accumulation in the media of cultured iWAT and eWAT from KO mice compared with WT mice were observed after 24 h (Fig. 2B). These differences were also seen more rapidly than 24 h in both depots, as evidenced by a higher NEFA accumulation in ATOC media in WAT from KO mice during a 24-h time course (Fig. 2, C and D). There was an increase in the NEFA:glycerol ratio in ATOC media collected from eWAT of KO mice compared with WT and HET animals, as well as an increase in ATOC media from iWAT of KO mice compared with WT mice (Fig. 2E).
Fig. 2.
Markers of lipid handling in ex vivo WAT are altered in Scd1 knockout mice. A and B: glycerol (A) and NEFA (B) measured after 24 h of WAT culture. C and D: NEFA accumulation during the 24 h time course in iWAT (C) and eWAT (D). *Significant difference (P < 0.05) between KO and WT. E: NEFA:glycerol accumulation in the media of cultured iWAT and eWAT. F and G: relative gene expression for key genes involved in WAT lipid handling iWAT (F) and eWAT (G). ANOVA was used to determine differences between the three genotypes in a depot-specific manner. Bars with different letters are significantly different from one another (P < 0.05). Bars with no letters indicate no difference between the three genotypes for that gene. All values expressed as means ± SE (n = 6 per genotype).
Expression of WAT lipid handling genes is reduced in Scd1 KO mice.
WAT used for ATOC experiments was also used to examine potential changes in the expression of key genes involved in lipid handling. Pck1, Scd1, Atgl, and Hsl were all significantly reduced in both WAT depots from KO mice compared with WT mice (Fig. 2, F and G). Pck2 and Pparγ gene expression was only significantly reduced in iWAT from KO and HET mice compared with WT mice, while Pdk4 was only lower in eWAT from KO mice compared with WT and HET mice. No differences in Scd2 expression were observed in either depot from KO mice. Data generally revealed that the expression of these genes in HET mice was intermediate to that observed for WT and KO animals.
Reduced SCD1 activity in differentiating 3T3-L1 preadipocytes decreases FA reesterification while altering the expression of markers of lipid handling.
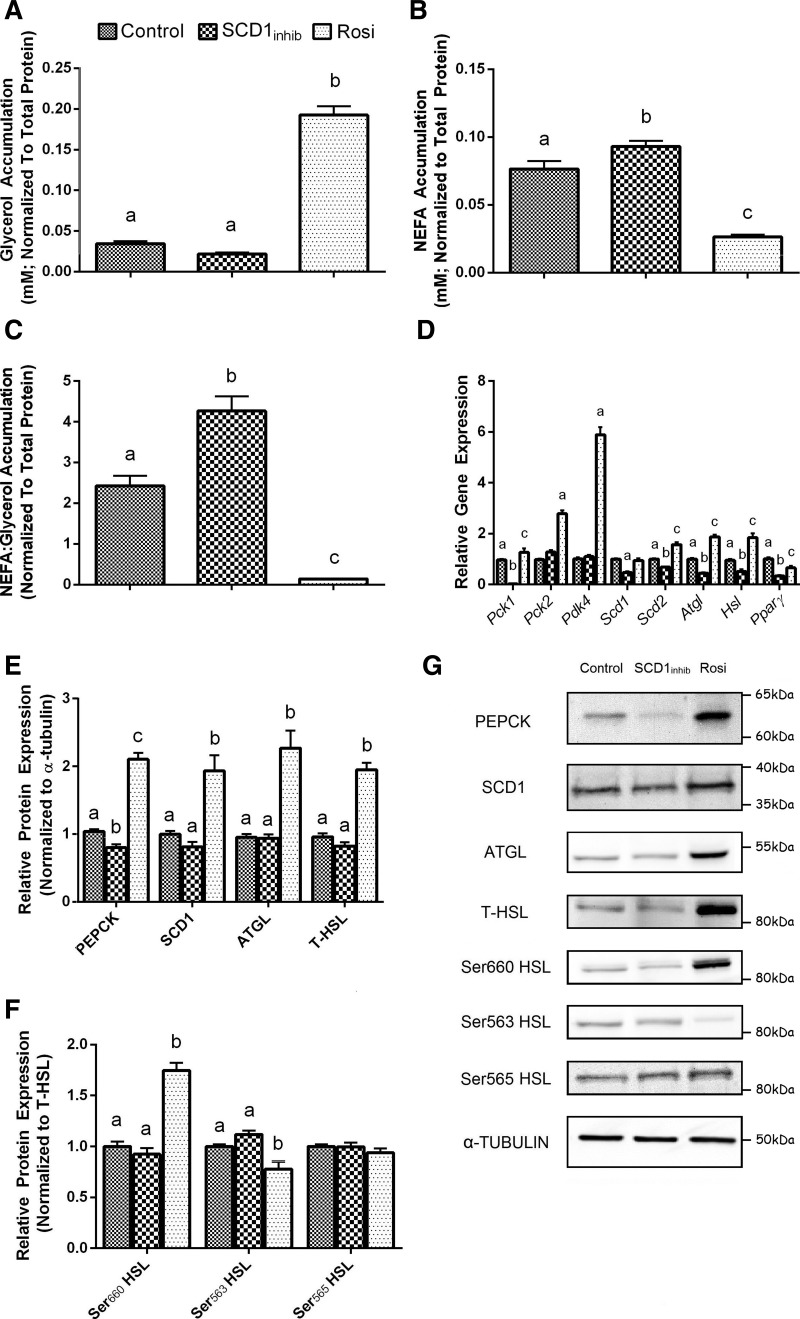
We next investigated whether the commonly used 3T3-L1 adipocyte cell culture model could be used to study the changes in WAT lipid handling observed in Scd1 KO mice. With SCD1inhib treatment, we observed no difference in glycerol accumulation (Fig. 3A), higher NEFA accumulation (Fig. 3B), and a significant increase in the NEFA:glycerol ratio in the cell media compared with the DMSO control (Fig. 3C). Rosiglitazone was used as a positive control and showed the opposite effects to SCD1inhib treatment, with an increase in glycerol accumulation (Fig. 3A), lower NEFA accumulation (Fig. 3B), and a decrease in the NEFA:glycerol ratio in the cell media compared with the DMSO control (Fig. 3C).
Fig. 3.
Stearoyl-CoA desaturase-1 (SCD1) regulation of lipid handling in differentiated 3T3-L1 preadipocytes. A–C: glycerol (A), NEFA (B), and NEFA:glycerol (C) accumulation in the media of preadipocytes treated with either DMSO, SCD1inhib, or rosiglitazone (Rosi). D and E: relative expression of key genes involved in WAT lipid handling (D) as well as corresponding total protein levels (E). PEPCK, phosphoenolpyruvate carboxykinase; HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase. F: different phosphorylated HSL sites (Ser660, Ser563, and Ser565). ANOVA was used to determine differences between the three treatment groups for each gene and protein. Bars with different letters are significantly different from one another (P < 0.05). G: representative protein blots. Data are expressed as means ± SE (n = 9 per treatment).
Differentiating preadipocytes treated with SCD1inhib showed a significant reduction in the expression of Pck1, Scd1, Scd2, Atgl, Hsl, and Pparγ genes (Fig. 3D). No changes in Pck2 or Pdk4 gene expression were observed with SCD1 inhibition. PEPCK protein content was lower with SCD1inhib treatment compared with the DMSO control; however, SCD1, ATGL, and T-HSL protein levels were unchanged (Fig. 3, E and G). There was no difference in p660-HSL, p563-HSL, or p565-HSL protein levels with SCD1inhib treatment (Fig. 3, F and G). As expected, rosiglitazone treatment generally increased gene and protein expression of the lipid handling markers (Fig. 3, D–G).
DISCUSSION
The present study examined the role of SCD1 on lipid handling in different WAT depots from Scd1 KO mice, as well as an adipocyte cell model treated with an SCD1 inhibitor. Collectively, our findings highlight the broad implications of reduced SCD1 activity in different WAT depots. Specifically, our results provide important new insights regarding the impact of SCD1 on markers of FA reesterification, glyceroneogenesis, and lipolysis in adipocytes. Together, we anticipate that these findings will help to better understand the role of SCD1 in WAT and its contribution to the perturbations in lipid handling observed with common metabolic diseases.
We observed a significant reduction in fasting blood glucose levels in Scd1 KO mice compared with WT mice, which suggests an improvement in overall metabolic health. This aligns with previous findings showing improved glucose tolerance and insulin action in Scd1 KO mice (18), and a downward trend in fasting glucose levels in Scd1 KO mice fed a chow diet (16). We found no difference in serum NEFA levels between KO and WT mice; however, NEFA:glycerol accumulation was reduced in KO mice, primarily because of an upward trend in serum glycerol content. In humans and rodents, it is estimated that ~80% of circulating glycerol stems from WAT lipolysis (10, 19). The remaining ~20% of glycerol comes from lipolysis in other tissues, such as liver and muscle, and/or release of glycerol via the hydrolysis of circulating very low density lipoproteins (10, 12, 22). Thus, the upward trend in systemic glycerol content may be indicative of elevated global lipolysis in Scd1 KO mice. A previous study by Lee et al. (14) suggested that the increased energy expenditure observed in Scd1 KO mice was, at least partly, related to increased thermogenesis and lipolysis in brown adipose tissue (BAT). Recently, Zhang et al. (33) showed that elevated lipolysis in both BAT and WAT was associated with reduced Scd1 expression in miR-378 transgenic mice. While the causal link between miR-378 and Scd1 was not definitive, it is interesting to note that miR-378 transgenic mice have a similar phenotype to that of Scd1 KO mice, including a lean body type and resistance to obesity (33). Thus, an elevation in whole body lipolytic activity aligns with the reduced adiposity phenotype observed in Scd1 KO mice (18).
We investigated the impact of reduced SCD1 levels on FA profiles in distinct WAT depots. Interestingly, we observed depot-specific changes in TAG levels in Scd1 KO mice compared with WT and HET animals. Specifically, Scd1 KO mice showed reduced TAG content in eWAT and increased TAG content in iWAT, compared with the other genotypes. The reduction in TAG content observed in eWAT aligns with previous data from our laboratory showing that reduced SCD1 activity in 3T3-L1 adipocytes led to a decrease in TAG levels (23). Reduced TAG content in BAT was also reported in Scd1 KO mice (14). The depot-specific changes in TAG content observed in the current study suggest an overall improvement in metabolic health of Scd1 KO mice. Indeed, eWAT lipid content is positively correlated with metabolic disease (13), whereas iWAT tends to be associated with metabolic benefits (13, 28). Thus, a decrease in TAG content in eWAT and an increase in iWAT suggests a repartitioning of FA storage that would improve whole body metabolic status. As such, the depot-specific changes in TAG content may provide a partial explanation for the improvements in metabolic function in Scd1 KO mice previously reported by Ntambi et al. (18).
We also observed the expected reduction in estimated SCD activity in Scd1 KO mice, as seen by decreases in 16:1n7 and 18:1n9 and increases in 18:0. Reduced SCD activity in Scd1 KO mice aligns with findings by Yew Tan et al. (32), who reported that adiposity in mice is positively correlated with estimated SCD activity. The lack of significant change in 16:0 levels and large increase in 18:0 in both WAT depots in Scd1 KO mice may be attributed to concomitant increases in ELOVL6 activity, which converts 16:0 into 18:0 (23, 32). We previously reported that 3T3-L1 adipocytes treated with a specific SCD1 inhibitor increased ELOVL6 activity (24). Moreover, Yew Tan et al. (32) showed that ELOVL6 has a high specificity for 16:0 (compared with 16:1n7) in both mice and human WAT. Thus, these results in mice align with those from previous in vitro work by our group (24).
The depot-specific differences in TAG content noted in Scd1 KO mice provided a strong rationale to more broadly examine lipid handling in these two WAT depots. We found that indices of FA reesterification in both iWAT and eWAT of Scd1 KO mice were significantly reduced compared with WT and HET animals. Relative rates of FA reesterification can be estimated by measuring the ratio of NEFA:glycerol accumulation in culture media, where a 3:1 NEFA:glycerol accumulation ratio would indicate absence of FA reesterification. In cultured WAT, NEFA accumulation was 3-fold and 4.8-fold higher for iWAT and eWAT, respectively, from Scd1 KO compared with WT mice. Since we did not detect significant differences in circulating blood NEFA levels, this suggests that the increased NEFAs released from WAT are most likely being taken up by peripheral tissues elsewhere in the body.
Reductions in FA reesterification seen in Scd1 KO mice were also observed in 3T3-L1 adipocytes treated with an SCD1 inhibitor (SCD1inhib). We observed lower glycerol alongside significantly higher NEFA and NEFA:glycerol accumulation (1.9:1) in the cell media of SCD1-inhibited adipocytes compared with the control. In contrast, 3T3-L1 adipocytes treated with rosiglitazone (our positive control) showed significantly higher glycerol and reduced NEFA accumulation and a subsequent decrease in NEFA:glycerol accumulation (0.06:1). This was expected, since rosiglitazone is known to reduce circulating NEFA levels (8), possibly in part by promoting FA reesterification and reducing lipolysis in WAT. Collectively, our data show that SCD1 plays an important role regulating FA reesterification in WAT.
We speculated that the reduction in FA reesterification may be due to a decreased production of G3P. Pck1 and Pck2 (both which encode the PEPCK protein), as well as Pdk4, are genes involved in glyceroneogenesis in WAT. We observed a reduced expression of Pck1 and Pck2 gene expression in iWAT, while Pck1 and Pdk4 gene expression was reduced in eWAT from Scd1 KO mice. We also saw a reduction in Pck1 gene expression, as well as PEPCK protein, in 3T3-L1 adipocytes treated with SCD1inhib, while rosiglitazone treatment upregulated these markers. In accordance, it was previously shown that reduced Scd1 activity was associated with a reduction in hepatic PEPCK expression and, correspondingly, hepatic gluconeogenesis (9). Thus, our data suggest that markers of glyceroneogenesis are decreased when SCD1 activity is reduced in WAT and adipocytes. However, it is not possible to reconcile the differential regulation of Pck2 and Pdk4 between the two WAT depots and 3T3-L1 cells when SCD1 activity was reduced. Global gene expression profiles have been shown to differ between various WAT depots and 3T3-L1 cells (17), and differences in the regulation of WAT lipid handling between visceral and subcutaneous adipose have been reported (11). Further confirmation of changes in Pck2 and Pdk4 gene expression are necessary before concluding that altered SCD1 activity influences these genes in a depot-specific manner. The culture of primary adipocytes isolated from each WAT depot would be particularly advantageous in this regard. In contrast, reduced SCD1 activity caused a marked reduction in Pck1 expression that was consistent in both WAT depots and 3T3-L1 cells; suggesting a reduction in glyceroneogenesis. However, to definitively show that SCD1 regulates the glyceroneogenic pathway in WAT will require future biochemical experiments using radiolabeled pyruvate.
ATGL and HSL (as well as MGL) are responsible for the sequential hydrolysis of TAG, resulting in NEFA and glycerol release. NEFA and glycerol accumulation, although not exact measures, are widely used end points of lipolytic flux, and an increase in these markers would indicate elevated lipolysis (7, 12, 15). As previously mentioned, we observed increased NEFA accumulation from cultured iWAT and eWAT. However, we also observed reduced Atgl and Hsl gene expression in iWAT and eWAT from Scd1 KO mice, suggesting a reduction in WAT lipolysis. Differentiating preadipocytes treated with SCD1inhib also showed a significant reduction in Atgl and Hsl gene expression and lower glycerol accumulation; however, ATGL and T-HSL protein levels were unchanged. Furthermore, there was no difference in the phosphorylation status of HSL. Similar to cultured WAT, NEFA accumulation was higher in SCD1-inhibited adipocytes. When considering both the WAT and 3T3-L1 data, we speculate that the impact of reduced SCD1 activity on WAT TAG lipolysis may be secondary to changes in FA re-esterification and glyceroneogenesis. We suggest that the increase in NEFA accumulation is due to reduced glyceroneogenesis and FA re-esterification in Scd1 KO mice, as previously discussed, and that the downregulation of Atgl and Hsl are possible compensatory mechanisms. In other words, since FA re-esterification is reduced, lipolysis may be downregulated to conserve TAG. However, it is important to note that the relationship between SCD1 activity and lipolysis may involve other tissues as well (e.g., liver). In comparison to the global Scd1 KO model used in the current study, Flowers et al. noticed no changes in lipolysis with a WAT-specific deletion of SCD1 in mice (4). Furthermore, previous studies reported increased lipolysis in the heart (2) and BAT (14) of Scd1 KO mice. The current study examined markers of lipolysis under non-stimulated conditions; therefore, future studies should consider examining the relationship between SCD1 and lipolysis in ATOC tissue and/or 3T3-L1 adipocytes under stimulated conditions.
In conclusion, the present study revealed that SCD1 has a broad role regulating WAT lipid handling, and that these effects vary in a depot-dependent manner. Our findings suggest that reduced SCD1 activity alters WAT lipid handling by regulating TAG synthesis, as well as key markers of FA reesterification, glyceroneogenesis (Pck1), and lipolysis (Atgl and Hsl). Moreover, the repartitioning of fat away from eWAT and towards iWAT in Scd1 KO mice is suggestive of a metabolically more favorable deposition of TAG. Importantly, we have shown that SCD1 has an important role in FA reesterification and, consequently, NEFA release from WAT. This is particularly relevant given that metabolically compromised states like obesity and type 2 diabetes are associated with increased SCD1 expression and activity (31). Together, our findings support the notion that therapeutic or lifestyle approaches that target a reduction in SCD1 activity in WAT may have strong benefits on whole body lipid handling and, consequently, metabolic health.
GRANTS
This work was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (D. M. Mutch; Grant No. 371546).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.D., K.F.B., D.C.W., C.M., and D.M.M. conceived and designed research; S.M.D., K.F.B., F.D., K.S., and D.M.M. performed experiments; S.M.D., K.S., and D.M.M. analyzed data; S.M.D., K.F.B., D.C.W., C.M., and D.M.M. interpreted results of experiments; S.M.D. and D.M.M. prepared figures; S.M.D. and D.M.M. drafted manuscript; S.M.D., K.F.B., F.D., K.S., D.C.W., C.M., and D.M.M. edited and revised manuscript; S.M.D., K.F.B., F.D., K.S., D.C.W., C.M., and D.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. James M. Ntambi (University of Wisconsin-Madison) for the generation of the Scd1 KO. We also extend our gratitude to Dr. Jessica Ralston for her technical expertise.
REFERENCES
- 1.Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, Skurk T, Rydén M, Frayn KN, Spalding KL. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478: 110–113, 2011. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bednarski T, Olichwier A, Opasinska A, Pyrkowska A, Gan AM, Ntambi JM, Dobrzyn P. Stearoyl-CoA desaturase 1 deficiency reduces lipid accumulation in the heart by activating lipolysis independently of peroxisome proliferator-activated receptor α. Biochim Biophys Acta 1861, 12 Pt A: 2029–2037, 2016. doi: 10.1016/j.bbalip.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Carswell KA, Lee MJ, Fried SK. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol Biol 806: 203–214, 2012. doi: 10.1007/978-1-61779-367-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flowers MT, Ade L, Strable MS, Ntambi JM. Combined deletion of SCD1 from adipose tissue and liver does not protect mice from obesity. J Lipid Res 53: 1646–1653, 2012. doi: 10.1194/jlr.M027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 6.Forest C, Tordjman J, Glorian M, Duplus E, Chauvet G, Quette J, Beale EG, Antoine B. Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase. Biochem Soc Trans 31: 1125–1129, 2003. doi: 10.1042/bst0311125. [DOI] [PubMed] [Google Scholar]
- 7.Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 298: C961–C971, 2010. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 8.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J 13: 17, 2014. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez-Juárez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest 116: 1686–1695, 2006. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MD, Chandramouli V, Schumann WC, Ekberg K, Previs SF, Gupta S, Landau BR. Sources of blood glycerol during fasting. Am J Physiol Endocrinol Metab 281: E998–E1004, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med 48: e215, 2016. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34: 1–11, 2013. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Dobrzyn A, Dobrzyn P, Rahman SM, Miyazaki M, Ntambi JM. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res 45: 1674–1682, 2004. doi: 10.1194/jlr.M400039-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.MacPherson RE, Dragos SM, Ramos S, Sutton C, Frendo-Cumbo S, Castellani L, Watt MJ, Perry CG, Mutch DM, Wright DC. Reduced ATGL-mediated lipolysis attenuates β-adrenergic-induced AMPK signaling, but not the induction of PKA-targeted genes, in adipocytes and adipose tissue. Am J Physiol Cell Physiol 311: C269–C276, 2016. doi: 10.1152/ajpcell.00126.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6: 484–496, 2007. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 4: 295–302, 2015. doi: 10.1080/21623945.2015.1040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99: 11482–11486, 2002. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 35: 1326–1331, 1986. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- 20.Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem 283: 27565–27574, 2008. doi: 10.1074/jbc.M804393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab 297: E28–E37, 2009. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proenca AR, Sertie RA, Oliveira AC, Campana AB, Caminhotto RO, Chimin P, Lima FB. New concepts in white adipose tissue physiology. Rev Bras Pesqui Med Biol 47: 192–205, 2014. doi: 10.1590/1414-431X20132911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralston JC, Badoud F, Cattrysse B, McNicholas PD, Mutch DM. Inhibition of stearoyl-CoA desaturase-1 in differentiating 3T3-L1 preadipocytes upregulates elongase 6 and downregulates genes affecting triacylglycerol synthesis. Int J Obes 38: 1449–1456, 2014. doi: 10.1038/ijo.2014.35. [DOI] [PubMed] [Google Scholar]
- 24.Ralston JC, Metherel AH, Stark KD, Mutch DM. SCD1 mediates the influence of exogenous saturated and monounsaturated fatty acids in adipocytes: effects on cellular stress, inflammatory markers and fatty acid elongation. J Nutr Biochem 27: 241–248, 2016. doi: 10.1016/j.jnutbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 278: 30413–30416, 2003. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 26.Sampath H, Ntambi JM. The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann NY Acad Sci 1243: 47–53, 2011. doi: 10.1111/j.1749-6632.2011.06303.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarr O, Dyck DJ, Mutch DM. [Letter to the Editor] Protein phosphorylation status is preserved following dual RNA and protein extraction using the Qiagen RNeasy Mini Kit. Biotechniques 61: 233–235, 2016. doi: 10.2144/000114471. [DOI] [PubMed] [Google Scholar]
- 28.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 6: 195–213, 2010. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem 274: 18243–18251, 1999. doi: 10.1074/jbc.274.26.18243. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Zang Y, Ling W, Corkey BE, Guo W. Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res 11: 880–887, 2003. doi: 10.1038/oby.2003.121. [DOI] [PubMed] [Google Scholar]
- 31.Warensjö E, Rosell M, Hellenius ML, Vessby B, De Faire U, Risérus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 8: 37, 2009. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yew Tan C, Virtue S, Murfitt S, Roberts LD, Phua YH, Dale M, Griffin JL, Tinahones F, Scherer PE, Vidal-Puig A. Adipose tissue fatty acid chain length and mono-unsaturation increases with obesity and insulin resistance. Sci Rep 5: 18366, 2015. doi: 10.1038/srep18366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Li C, Li H, Song Y, Zhao Y, Zhai L, Wang H, Zhong R, Tang H, Zhu D. miR-378 Activates the pyruvate-PEP futile cycle and enhances lipolysis to ameliorate obesity in mice. EBioMedicine 5: 93–104, 2016. doi: 10.1016/j.ebiom.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]