Abstract
The early events that signal renal dysfunction in presymptomatic heart failure are unclear. We tested the hypothesis that functional and mechanistic changes occur in the kidney that precede the development of symptomatic heart failure. We employed a transgenic mouse model with cardiomyocyte-specific overexpression of mutant α-B-crystallin that develops slowly progressive cardiomyopathy. Presymptomatic transgenic mice displayed an increase in serum creatinine (1.17 ± 0.34 vs. wild type 0.65 ± 0.16 mg/dl, P < 0.05) and in urinary neutrophil gelatinase-associated lipocalin (NGAL; 278.92 ± 176.24 vs. wild type 49.11 ± 22.79 ng/ml, P < 0.05) but no renal fibrosis. Presymptomatic transgenic mouse kidneys exhibited a twofold upregulation of the Ren1 gene, marked overexpression of renin protein in the tubules, and a worsened response to ischemia-reperfusion injury based on serum creatinine (2.77 ± 0.66 in transgenic mice vs. 2.01 ± 0.58 mg/dl in wild type, P < 0.05), urine NGAL (9,198.79 ± 3,799.52 in transgenic mice vs. 3,252.94 ± 2,420.36 ng/ml in wild type, P < 0.05), tubule dilation score (3.4 ± 0.5 in transgenic mice vs. 2.6 ± 0.5 in wild type, P < 0.05), tubule cast score (3.2 ± 0.4 in transgenic mice vs. 2.5 ± 0.5 in wild type, P < 0.05), and TdT-mediated dUTP nick-end labeling (TUNEL)-positive nuclei (10.1 ± 2.1 in the transgenic group vs. 5.7 ± 1.6 per 100 cells counted in wild type, P < 0.01). Our findings indicate functional renal impairment, urinary biomarker elevations, and induction of renin gene and protein expression in the kidney that occur in early presymptomatic heart failure, which increase the susceptibility to subsequent acute kidney injury.
Keywords: cardiorenal syndrome, acute kidney injury, ischemia-reperfusion injury, neutrophil gelatinase-associated lipocalin, renin
cardiorenal syndrome (CRS) is currently defined as a complex heterogeneous group of disorders of the heart and kidney, in which acute or chronic dysfunction in one organ initiates or aggravates acute or chronic dysfunction in the other (2, 5, 7, 10). Acute forms of CRS are characterized by the development of acute kidney injury (AKI) in the clinical settings of acute heart failure or cardiogenic shock. Experimental and human studies have elucidated the roles of both arterial underfilling as well as venous congestion, with subsequent activation of the renin-angiotensin-aldosterone system (RAAS) and inflammatory pathways in the pathogenesis of AKI in acute CRS (10). In contrast, chronic CRS is defined as chronic abnormalities in heart function leading to kidney dysfunction or injury. The most common clinical manifestation of chronic CRS is in patients with congenital or acquired chronic congestive heart failure, more than 50% of whom display evidence for chronic kidney disease (CKD) (3). A myriad of systemic and renal events, mediated by both hemodynamic and nonhemodynamic factors, have been implicated in the inexorable progression of CKD in chronic CRS (2, 5, 7, 10). However, no hierarchy has been identified in the multitude of factors and mechanisms (2), in part due to the lack of reliable animal models that recapitulate typical clinical settings in a temporally relevant manner (12). In a recent rat model of myocardial infarction due to coronary artery ligation, a reduction in glomerular filtration rate (GFR) and increased renal interstitial fibrosis were documented at 16 wk (13). Importantly, an increase in kidney nuclear phospho-Smad2 immunostaining was evident within 1 wk after coronary ligation, preceding the appearance of renal fibrosis and implicating Smad2-dependent signaling pathways in early CRS (13). In both clinical and experimental settings, there remains a major unmet need for a better understanding of early mechanisms and biomarkers that signal renal dysfunction in presymptomatic heart failure, which may be amenable to early diagnosis and therapeutic intervention before the development of renal interstitial fibrosis.
In this study, we employed a previously described transgenic mouse model of slowly progressive cardiomyopathy that develops symptomatic congestive heart failure after 24 wk of life (14, 25, 26). We tested the hypothesis that structural, functional, biomarker, and mechanistic changes occur in the kidney that precede the development of symptomatic heart failure and overt renal fibrosis. Using a serial and systematic approach, we show evidence for functional renal impairment and novel biomarker, gene expression, and protein expression changes that precede symptoms of heart failure but dramatically increase the susceptibility to subsequent kidney ischemia-reperfusion injury.
MATERIALS AND METHODS
Transgenic mouse model of cardiomyopathy.
The derivation and cardiac characterization of a transgenic mouse model with cardiac-specific overexpression of mutant α-B-crystallin (CryAB) have been previously published (14, 25, 26). Briefly, FVB/N mice that overexpress CryAB containing the R120G missense mutation (driven by the cardiomyocyte-specific α-myosin heavy chain promoter) develop cardiomyocyte mitochondrial dysfunction by 8 wk of life, disruption of the desmin network and increased myocyte size by 12 wk, grossly enlarged and dilated hearts by 24 wk, and symptomatic congestive heart failure after 24 wk. For this study, transgenic mice were identified by PCR analysis of DNA isolated from tail clips as previously described (14, 25, 26) and compared with wild-type nontransgenic FVB/N mice (Taconic Farms, Germantown, NY). All mice were housed with 12:12-h light-dark cycles with free access to food and water. Animals were studied serially at 10, 15, and 24 wk of life, before the appearance of any symptoms of cardiac failure. The study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
Renal function measurements.
At 10, 15, and 24 wk of life, mice (n = 20 at each time point) were anesthetized with pentobarbital (50 mg/kg ip), and blood was collected into microhematocrit capillary tubes by retro-orbital bleeding. All mice showed full recovery after this procedure, and no complications were encountered. The serum creatinine in these samples was measured using a quantitative colorimetric assay (Sigma-Aldrich, St. Louis, MO).
Urinary biomarker measurements.
Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early, sensitive, and specific biomarker of kidney injury in mice (15, 16) and humans (17). At 10, 15, and 24 wk of life, before retro-orbital bleeding, mice (n = 20 at each time point) were placed in metabolic cages for urine collection. Urine samples were centrifuged at 1,500 g to remove debris, and the supernatant was analyzed. Urine NGAL was measured using a commercially available mouse NGAL ELISA kit (NGAL ELISA Kit 042; Bioporto, Grusbakken, Denmark).
Gene expression studies.
At 24 wk of life, wild-type and transgenic mice (n = 3 from each group) were anesthetized with triple sedative (ketamine, xylazine, and acepromazine), euthanized, and both kidneys were harvested. Gross examination confirmed the presence of enlarged and dilated hearts in the mice identified as transgenic by tail clip PCR analysis. Whole kidney RNA was isolated using the RNeasy kit (Qiagen) after the tissue was homogenized with the Tissue Lyser II (Qiagen). RNA-Seq using TruSeq technology (Illumina, San Diego, CA) was performed in triplicate, with a minimum of 20 million reads per sample. Data were submitted to the GEO database (Accession No. GSE99703).
Western blotting.
Proteins were extracted from frozen kidneys after homogenization with protease inhibitors in lysis buffer and quantified by the Bradford method. For detection of renin protein, 10 µg of kidney total protein samples were electrophoretically separated and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). Blots were incubated with a goat anti-renin primary antibody (R&D Systems) and with horseradish-peroxidase-conjugated secondary antibody (donkey anti-goat, Santa Cruz Biotechnology). Detection was accomplished with enhanced chemiluminescence (Pierce ECL system; Thermo Scientific). Blots were incubated with β-actin as a loading control.
Immunohistochemistry.
Paraffin-embedded kidney sections were subjected to descending grades of ethanol and stained with a goat anti-renin primary antibody (R&D Systems) followed by incubations with anti-goat Ig peroxidase and peroxidase substrate solution and counterstaining with Harris modified hematoxylin.
Mouse model of renal ischemia-reperfusion injury.
At 24 wk of life, wild-type and transgenic mice (n = 10 from each group) were anesthetized with triple sedative (ketamine, xylazine, and acepromazine) and subjected to ischemia-reperfusion injury. We employed an established model in which the structural and functional consequences of ischemia-reperfusion have been well documented (15, 16). Briefly, animals were placed on a warming table to maintain a rectal temperature of 37°C. Both renal pedicles were occluded with a nontraumatic vascular clamp for 30 min, during which time the kidney was kept warm and moist. The clamps were then removed, the kidney was observed for return of blood flow, and the incision was sutured. The mice were allowed to recover in a warmed cage. After 24 h of reperfusion, the animals were placed in metabolic cages to obtain urine for NGAL determination and then reanesthetized, the abdominal cavity was opened, and blood was obtained via puncture of the inferior vena cava for measurement of serum creatinine. The mice were euthanized, the kidneys were perfused fixed in situ with 4% paraformaldehyde in PBS, and both kidneys were harvested. Kidneys were then fixed in formalin, paraffin-embedded, and subjected to microscopy.
Microscopy.
Paraffin-embedded kidneys obtained from wild-type and transgenic mice at 24 wk of life both before and after ischemia-reperfusion injury (n = 10 from each group) were sectioned at 4 μm and stained with hematoxylin and eosin. Slides were scored for histopathologic evidence for acute damage by one investigator (P. Devarajan) in a blinded fashion (15, 16). For each slide, we assessed three parameters (tubule dilation, tubule cast formation, and tubule cell necrosis) in five high-power fields (×40), and an average of the five fields were determined for each section. Each parameter was scored on a scale of 0 to 4, ranging from none (0), mild (1), moderate (2), severe (3), to extensive/very severe (4). Kidney sections were also stained for detection of renal fibrosis using trichrome stain and picrosirius red (8).
Kidney sections were also subjected to the TdT-mediated dUTP nick-end labeling (TUNEL) assay using the ApoAlert DNA Fragmentation Assay Kit (Clontech, La Jolla, CA) as previously described (15, 16). Sections were counterstained with DAPI to identify all nuclei and visualized with a fluorescence microscope in a blinded manner. TUNEL-positive nuclei per 100 DAPI-positive nuclei counted in an average of five high-power fields (×40) in each section were reported.
Statistical analysis.
Data are presented as means ± SD. SPSS software was used to generate means, SD, distributions, range, and skewness. All data were normally distributed. For continuous variables, unpaired t-test and one-way ANOVA were used as appropriate to compare means ± SD among different groups. Paired t-test was used for comparisons of serial measurements obtained within the same group. P < 0.05 was considered statistically significant.
For the gene expression studies, data were analyzed using Strand NGS software. Bam files were generated using mouse build mm10. Data were filtered on read quality metrics, including removal of reads aligning to more than one position in the genome, as well as expression level, requiring at least five normalized reads per kilobase of transcript per million in two of six samples. Replicate analyses were carried out with one-way ANOVA, requiring P < 0.05 without correction, and data were filtered on a fold-change of 1.5.
RESULTS
Mice with presymptomatic cardiomyopathy develop functional renal injury.
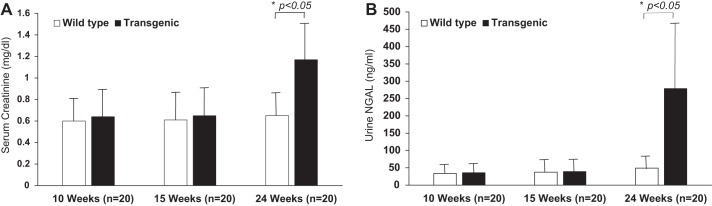
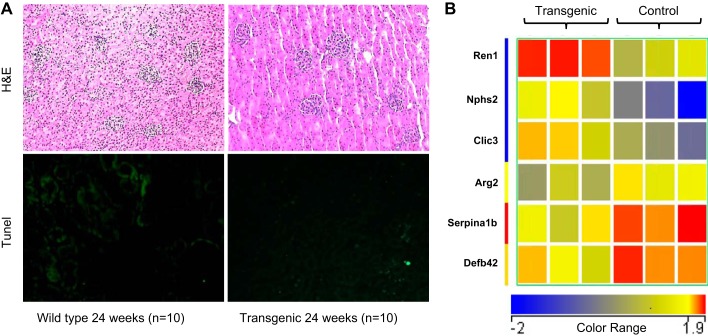
We serially examined the renal consequences in a transgenic mouse model of isolated slowly progressive cardiomyopathy that develops symptomatic congestive heart failure after 24 wk of life. Compared with wild-type controls, mice with cardiomyopathy displayed no differences in either serum creatinine or urine NGAL at 10 or 15 wk of life. However, by 24 wk, before any symptoms of cardiac failure, the transgenic mice displayed a small but significant increase in serum creatinine (1.17 ± 0.34 vs. wild type 0.65 ± 0.16 mg/dl, P < 0.05), as shown in Fig. 1A. At the same time point, the transgenic mice also displayed a small but significant increase in urine NGAL (278.92 ± 176.24 vs. wild type 49.11 ± 22.79 ng/ml, P < 0.05), as shown in Fig. 1B. However, histologic examination of the kidneys showed only mild and subtle differences. Hematoxylin and eosin staining showed mild tubule dilation and glomerular congestion of varying degrees in the transgenic mice, but no other evidence for acute or chronic structural injury, as shown in Fig. 2A, top. This was confirmed by TUNEL assay, which showed only isolated evidence for TUNEL-positive nuclei in both the wild-type and transgenic mice (<2% in both groups), as shown in Fig. 2A, bottom. Furthermore, staining with trichrome stain and picrosirius red did not reveal significant areas of fibrosis in either the wild-type or the transgenic mice (not shown).
Fig. 1.
Renal functional and urinary biomarker changes in presymptomatic cardiomyopathy (transgenic). Serum creatinine (A) and urine neutrophil gelatinase-associated lipocalin (NGAL; B) at 10, 15, and 24 wk of life; n = 20 for each of the 2 groups (transgenic and wild type) at each time point. Bars represent means, and the error bars represent SD. *P < 0.05.
Fig. 2.
Renal structural and gene expression changes in presymptomatic cardiomyopathy (transgenic). A, top: staining with hematoxylin and eosin (H&E), showing mild tubule dilation and glomerular congestion in transgenic kidneys. A, bottom: TdT-mediated dUTP nick-end labeling (TUNEL)-positive nuclei. Each picture is representative of n = 10. B: heatmap comparing gene expression profiles of transgenic (presymptomatic cardiomyopathy) kidneys (n = 3), compared with wild-type kidneys (n = 3), showing 6 genes with fold-change >1.5. Red color indicates upregulated genes, and blue color illustrates downregulated genes. Transgenic kidneys displayed upregulation of Ren1, Nphs2, and Clic3 and downregulation of the Arg2, Serpin, and Defb42.
Mice with presymptomatic cardiomyopathy develop renal gene expression changes.
RNA seq analysis of transgenic mice at 24 wk of life, before any cardiac symptoms, showed very few genes differently expressed when filtered on a fold-change of 1.5 compared with age-matched gender-matched nontransgenic controls. Transgenic kidneys displayed a 2-fold or greater upregulation of the genes encoding Ren1, Nphs2, and Clic3, and a 1.5- to 2-fold downregulation of the Arg2, Serpina1b, and Defb42 genes, as illustrated in the heatmap in Fig. 2B and quantified in Table 1. Based on the average reads per kilobase of transcript per million, the most dramatic upregulation in the transgenic kidneys was noted for the Ren1 gene.
Table 1.
Differently expressed genes in age- and sex-matched transgenic kidneys, compared with wild-type kidneys
| Gene | Control, RPKM | Transgenic, RPKM | Fold-Change |
|---|---|---|---|
| Nphs2 | 6.5 (2.2) | 15.73 (2.9) | 2.48 |
| Ren1 | 180.9 (30.3) | 429.7 (26.1) | 2.40 |
| Clic3 | 3.0 (0.5) | 6.1 (1.4) | 2.00 |
| Serpina1b | 11.7 (2.5) | 6.3 (1.3) | −1.86 |
| Defb42 | 7.6 (1.1) | 4.8 (1.1) | −1.58 |
| Arg2 | 5.3 (0.6) | 3.5 (0.5) | −1.53 |
Values are average reads per kilobase of transcript per million (RPKM) and SD is in parenthesis. Differently expressed genes in age- and sex-matched transgenic (presymptomatic cardiomyopathy) kidneys (n = 3), compared with wild-type kidneys (n = 3), are shown with fold-change >1.5.
Mice with presymptomatic cardiomyopathy overexpress renin protein.
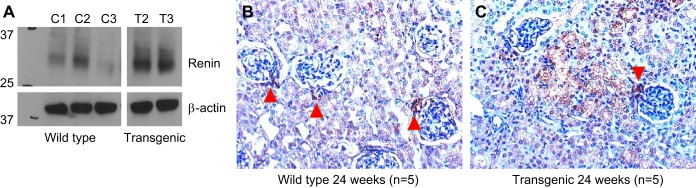
Overexpression of the protein product of the Ren1 gene in transgenic kidneys was documented using two complementary techniques. Western blots of whole kidney lysates revealed a three- to fourfold increase in renin protein in transgenic kidneys (Fig. 3A). Immunohistochemistry showed renin protein staining to be largely localized to the juxta-glomerular apparatus in wild-type kidneys (Fig. 3B). In contrast, kidneys from transgenic mice displayed renin protein expression in the juxta-glomerular apparatus as well as in a wide distribution in kidney tubule epithelial cells (Fig. 3C).
Fig. 3.
Renal renin protein expression changes in presymptomatic cardiomyopathy (transgenic). A: Western blot showing expression of renin protein in whole kidney lysates from wild-type (n = 3) and transgenic (n = 2) animals. B: immunohistochemistry showing renin expression largely restricted to the juxta-glomerular apparatus in wild-type kidneys (red arrowhead, n = 5). C: immunohistochemistry showing renin expression in the juxta-glomerular apparatus (red arrowhead) as well as diffusely in tubule epithelial cells in transgenic animals (n = 5).
Mice with presymptomatic cardiomyopathy display increased susceptibility to acute ischemia-reperfusion injury.
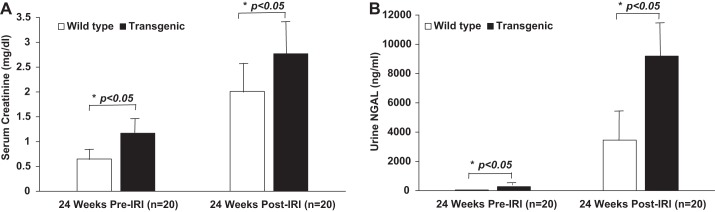
We examined the consequences of acute renal ischemia-reperfusion injury in a transgenic mouse model of isolated slowly progressive cardiomyopathy that develops symptomatic congestive heart failure after 24 wk of life. Wild-type age-matched mice displayed the expected rise in serum creatinine and urine NGAL, as previously described (15, 16). However, these increases in serum creatinine and urine NGAL were dramatically exaggerated in the presymptomatic transgenic mice, as shown in Fig. 4, A and B, respectively (serum creatinine 2.77 ± 0.66 in transgenic mice vs. 2.01 ± 0.58 mg/dl in wild type, P < 0.05, and urine NGAL 9,198.79 ± 3,799.52 in transgenic mice vs. 3,252.94 ± 2,420.36 ng/ml in wild type, P < 0.05).
Fig. 4.
Increased susceptibility to acute ischemia-reperfusion injury (IRI) based on renal functional measurements and urinary biomarker changes in presymptomatic cardiomyopathy (transgenic). Serum creatinine (A) and urine NGAL (B) at 24 wk of life pre-IRI and 24 h post-IRI. N = 20 for each of the 2 groups (transgenic and wild type) at each time point. Bars represent means, and the error bars represent SD. *P < 0.05.
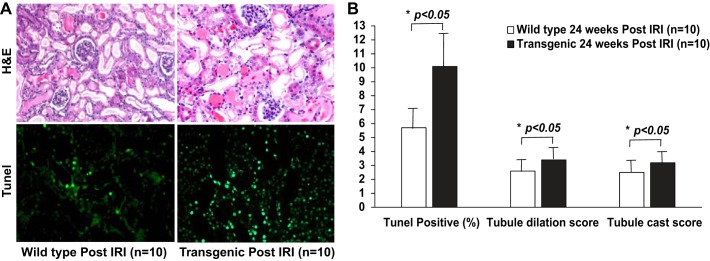
These findings were corroborated by our observations of acute structural damage. While both animal groups displayed the characteristic histologic consequences of renal ischemia-reperfusion injury, these changes were much worsened in the presymptomatic transgenic mice, as shown in Fig. 5A, top. In transgenic mice, hematoxylin-eosin staining showed a marked increase in tubule dilation score (3.4 ± 0.5 in transgenic mice vs. 2.6 ± 0.5 in wild type, P < 0.05) and tubule cast score (3.2 ± 0.4 in transgenic mice vs. 2.5 ± 0.5 in wild type, P < 0.05), as shown by quantitation in Fig. 5B. Furthermore, TUNEL assay showed moderate evidence for TUNEL-positive nuclei in the wild-type mice and more extensive TUNEL-positive nuclei in transgenic mice, as shown in Fig. 5A, bottom. By quantitation, there were 10.1 ± 2.1 TUNEL-positive nuclei per 100 nuclei counted in the transgenic group vs. 5.7 ± 1.6 TUNEL-positive nuclei per 100 nuclei counted in wild-type mice (P < 0.01), as shown in Fig. 5B.
Fig. 5.
Increased susceptibility to acute IRI based on structural changes in presymptomatic cardiomyopathy (transgenic). A, top: H&E staining 24 h post-IRI. A, bottom: staining for apoptotic nuclei with TUNEL assay 24 h post-IRI. Each picture is representative of n = 10. B: TUNEL-positive nuclei per 100 nuclei examined in an average of five high-power fields are reported. Tubule dilation and tubule cast score were assessed in hematoxylin-eosin stained sections and scored on a scale of 0 to 4. For each of the 2 groups (transgenic and wild type), n = 10. Bars represent means, and the error bars represent SD. *P < 0.05.
DISCUSSION
Several systemic and renal events, mediated by both hemodynamic and nonhemodynamic factors, have been implicated in the late progression of kidney disease in patients with chronic heart failure (2, 5, 7, 10). However, in both clinical and experimental settings, there remains an unmet need for a better understanding of early mechanisms and biomarkers that signal subtle renal dysfunction in presymptomatic heart failure, before the development of overt CKD and renal interstitial fibrosis. In this study, we have demonstrated the time course of early functional, biomarker, structural, and molecular changes in a mouse model of slowly progressive cardiomyopathy. Our findings indicate functional renal impairment, urinary biomarker elevation, and induction of renin gene and protein expression in the kidney that occur in early presymptomatic heart failure in the absence of chronic renal fibrotic changes. These changes dramatically increase the susceptibility to subsequent acute renal ischemia-reperfusion injury.
Transgenic mice with subclinical chronic heart failure displayed a small but significant increase in serum creatinine and urine NGAL. Histology revealed predominantly mild tubule dilation and glomerular congestion. Taken together, these findings are consistent with a functional form of “prerenal” AKI, likely resulting from arterial underfilling and renal hypoperfusion dictated by subclinical chronic heart failure (10). The glomerular congestion is likely the result of renal congestion or renal venous hypertension that have also been documented in cardiorenal syndrome (5, 10). Functional forms of AKI have traditionally been considered physiologic and largely reversible. However, accumulating clinical evidence indicates that even functional AKI is not necessarily benign, especially since it can be associated with short-term adverse outcomes, including increase in damage biomarkers such as NGAL, and dialysis requirement (19, 22, 24). In clinical practice, “prerenal” AKI due to chronic heart failure, as exemplified in this study, is unlikely to respond to increased fluid administration and will require improvement in cardiac function for reversal. Our findings indicate that failure to improve cardiac function before symptoms increases the susceptibility of the functionally injured kidney to subsequent insults, with ensuing worsening of structural kidney injury.
Our gene expression analysis revealed changes in a surprisingly small group of genes that could account for this increased susceptibility to structural AKI. Transgenic kidneys displayed a twofold or greater overexpression of the genes encoding Ren1, Nphs2, and Clic3, and a 1.5- to 2-fold downregulation of the Arg2, Serpina1b, and Defb42 genes. Some of these functional genomic changes are biologically plausible and may provide insights into the mechanisms underlying the observed kidney injury. Previous experimental and human studies of chronic heart failure have provided evidence for the eventual development of glomerular, tubular, and interstitial injury, evidenced by reduction in glomerular filtration rate, appearance of proteinuria, and increased concentrations of novel urinary biomarkers of kidney injury (6, 9, 21). Major implicated pathophysiological mechanisms include chronic activation of the renal and systemic RAAS, as well as the sympathetic nervous system, resulting from chronic arterial underfilling and venous congestion (5, 10). The present study’s findings of induction of Ren1, and in particular the dramatic overexpression of the encoded renin protein in the tubules of transgenic mice, provide evidence for the early activation of the intrarenal RAAS in asymptomatic cardiac failure, and also offers a pathophysiologic explanation for the observed functional “prerenal” form of AKI. Recent publications also raise the intriguing possibility that the induced Ren1 represents an early repair response to kidney injury, since 1) renin-expressing cells of the juxta-glomerular apparatus can differentiate into glomerular podocytes and tubule-interstitial pericytes during acute and chronic kidney disease (20), and 2) extraglomerular renin-positive cells give rise to intraglomerular mesangial cells after acute kidney injury (23). Similarly, induction of Nphs2 (which encodes for the podocyte slit diaphragm-associated protein podocin) may represent an early response to the podocyte injury that occurs in animal models of chronic congestive heart failure (21). Finally, Clic2 encodes a poorly studied intracellular channel protein that was previously shown to possess chloride channel activity in a lipid bilayer experiment. As such, it may regulate fundamental cellular processes including transepithelial transport, maintenance of intracellular pH, and regulation of cell volume (27), all potentially important processes to regenerating kidney tubule cells. In addition, Clic2 protein binds directly to the ryanodine receptor and inhibits intracellular Ca2+ release in heart muscle. Upregulation of Clic2 could therefore represent an additional protective mechanism by limiting calcium-induced cell death in conditions such as ischemia.
The biological implications of the kidney genes downregulated in the present study of early cardiac failure are less apparent. Arg2 encodes arginase type 2, the predominant arginase isoform expressed in kidney tubules. Arginases catalyze hydrolysis of l-arginine and compete with nitric oxide synthases for the common substrate l-arginine. Genetic and pharmacologic inhibition of arginase 2 conferred kidney protection by attenuating functional kidney injury, albuminuria, and histopathological changes in a diabetic mouse model (18). Thus, the downregulation of Arg2 observed in our model of presymptomatic cardiomyopathy likely represents a protective response. Serpina 1b encodes α-1-antitrypsin 1–2 (AAT), a serine protease inhibitor and acute phase reactant that protects tissues from enzymes of inflammatory cells, especially neutrophil elastase. In addition to protease inhibition, AAT expresses anti-inflammatory, immunomodulatory, and antimicrobial properties. The role of AAT in acute and chronic kidney diseases remains poorly elucidated. Similarly, kidney-specific information on the product of the Defb42 gene is currently limited. Collectively, the induction of Ren1, Nphs2, and Clic2, combined with the downregulation of Arg2, might represent early compensatory protective mechanisms in our model of presymptomatic cardiomyopathy that minimize structural and functional kidney damage in the steady state. However, these mechanisms are insufficient to protect against a subsequent renal ischemia-reperfusion insult.
The role of novel biomarkers such as NGAL as an indicator of kidney injury in chronic cardiorenal syndrome has attracted increasing attention. In several cohorts of patients with established congestive heart failure, the concentrations of NGAL in either urine or blood have been elevated in comparison with matched control populations and correlated with decreased kidney function, increased mortality, and increased hospitalizations (1, 4, 6). In a recent report, asymptomatic children with cardiomyopathy but with normal kidney function and no proteinuria displayed an increased concentration of several novel urinary biomarkers including NGAL (11). Our current findings of elevated urinary NGAL levels in mice with presymptomatic cardiomyopathy lend further support to the notion of employing urinary biomarkers to detect subclinical kidney injury in asymptomatic patients.
In summary, our findings indicate functional renal impairment, urinary biomarker elevations, novel gene expression changes, and new tubular overexpression of renin protein that occur in early presymptomatic heart failure in the absence of chronic renal fibrotic changes, which dramatically increase the susceptibility to subsequent acute renal injury. Our results have direct translational implications for the timely detection and therapeutic intervention of early, subclinical kidney injury in cardiorenal syndrome, before the development of irreversible renal interstitial fibrosis.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P50-DK-096418 (to P. Devarajan).
DISCLOSURES
P. Devarajan is a coinventor on patents submitted for the use of NGAL as a biomarker of kidney disease.
AUTHOR CONTRIBUTIONS
L.P., K.D., J.R., and P.D. conceived and designed research; L.P., Q.M., P.P., K.D., B.J.S., and K.S.-W. performed experiments; L.P., Q.M., M.D., P.P., K.D., and P.D. analyzed data; L.P., M.D., P.P., K.D., B.J.S., K.S.-W., J.R., and P.D. interpreted results of experiments; M.D., K.D., and P.D. prepared figures; P.D. drafted manuscript. L.P., Q.M., M.D., P.P., K.D., B.J.S., J.R., and P.D. edited and revised manuscript; L.P., Q.M., M.D., P.P., K.D., B.J.S., K.S.-W., J.R., and P.D. approved final version of manuscript.
REFERENCES
- 1.Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res 12: 7–14, 2009. doi: 10.1089/rej.2008.0803. [DOI] [PubMed] [Google Scholar]
- 2.Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome–current understanding and future perspectives. Nat Rev Nephrol 10: 48–55, 2014. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 3.Cruz DN, Bagshaw SM. Heart-kidney interaction: epidemiology of cardiorenal syndromes. Int J Nephrol: 2010: 351291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med 50: 1533–1545, 2012. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182: 117–136, 2013. doi: 10.1159/000349968. [DOI] [PubMed] [Google Scholar]
- 6.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail 10: 997–1000, 2008. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 36: 1437–1444, 2015. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farris AB, Alpers CE. What is the best way to measure renal fibrosis?: a pathologist’s perspective. Kidney Int Suppl (2011) 4: 9–15, 2014. doi: 10.1038/kisup.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haapio M, House AA, de Cal M, Cruz DN, Lentini P, Giavarina D, Fortunato A, Menghetti L, Salgarello M, Lupi A, Soffiati G, Fontanelli A, Zanco P, Ronco C. Heart-kidney biomarkers in patients undergoing cardiac stress testing. Int J Nephrol 425923: 2010, 2011. doi: 10.4061/2011/425923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.House AA. Cardiorenal syndrome: new developments in the understanding and pharmacologic management. Clin J Am Soc Nephrol 8: 1808–1815, 2013. doi: 10.2215/CJN.02920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaddourah A, Goldstein SL, Basu R, Nehus EJ, Terrell TC, Brunner L, Bennett MR, Haffner C, Jefferies JL. Novel urinary tubular injury markers reveal an evidence of underlying kidney injury in children with reduced left ventricular systolic function: a pilot study. Pediatr Nephrol 31: 1637–1645, 2016. doi: 10.1007/s00467-016-3360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G protein-coupled receptor-g-protein βγ-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J Am Soc Nephrol 28: 197–208, 2017. doi: 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekawanvijit S, Kompa AR, Zhang Y, Wang BH, Kelly DJ, Krum H. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: implications for cardiorenal syndrome. Am J Physiol Heart Circ Physiol 302: H1884–H1893, 2012. doi: 10.1152/ajpheart.00967.2011. [DOI] [PubMed] [Google Scholar]
- 14.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 112: 3451–3461, 2005. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15: 3073–3082, 2004. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 18.Morris SM Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes 60: 3015–3022, 2011. doi: 10.2337/db11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81: 1254–1262, 2012. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309: F341–F358, 2015. doi: 10.1152/ajprenal.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 125: 1402–1413, 2012. doi: 10.1161/CIRCULATIONAHA.111.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto K, Campos P, Pinto I, Rodrigues B, Frade F, Papoila AL, Devarajan P. The risk of chronic kidney disease and mortality are increased after community-acquired acute kidney injury. Kidney Int 90: 1090–1099, 2016. doi: 10.1016/j.kint.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol 26: 48–54, 2015. doi: 10.1681/ASN.2014030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 25: 1833–1839, 2010. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res 89: 84–91, 2001. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Klevitsky R, Huang W, Glasford J, Li F, Robbins J. AlphaB-crystallin modulates protein aggregation of abnormal desmin. Circ Res 93: 998–1005, 2003. doi: 10.1161/01.RES.0000102401.77712.ED. [DOI] [PubMed] [Google Scholar]
- 27.Witham S, Takano K, Schwartz C, Alexov E. A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins 79: 2444–2454, 2011. doi: 10.1002/prot.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]