Abstract
Basement membranes (BMs), a specialized form of extracellular matrix, underlie nearly all cell layers and provide structural support for tissues and interact with cell surface receptors to determine cell behavior. Both macromolecular composition and stiffness of the BM influence cell-BM interactions. Collagen IV is a major constituent of the BM that forms an extensively cross-linked oligomeric network. Its deficiency leads to BM mechanical instability, as observed with glomerular BM in Alport syndrome. These findings have led to the hypothesis that collagen IV and its cross-links determine BM stiffness. A sulfilimine bond (S = N) between a methionine sulfur and a lysine nitrogen cross-links collagen IV and is formed by the matrix enzyme peroxidasin. In peroxidasin knockout mice with reduced collagen IV sulfilimine cross-links, we find a reduction in renal tubular BM stiffness. Thus this work provides the first direct experimental evidence that collagen IV sulfilimine cross-links contribute to BM mechanical properties and provides a foundation for future work on the relationship of BM mechanics to cell function in renal disease.
Keywords: collagen IV, sulfilimine cross-link, basement membrane, peroxidasin, elastic modulus
basement membranes (BMs), a sheetlike, specialized form of extracellular matrix (ECM), underlie nearly all epithelial, endothelial, and muscle cells. The tubular BM (TBM) provides structural support for the renal tubular epithelium. In addition to structural support, BMs also control growth factor signaling and interact with cell surface receptors, such as integrins, to control cell adhesion, migration, proliferation, and differentiation (16, 38). Much work on cell-BM interactions has focused on chemical cues provided by the BM to cells, with BM macromolecules acting as ligands for cell surface receptors or controlling growth factor availability (38). Recent work has demonstrated that the mechanical properties of the ECM alter cell behavior independent of chemical composition (7, 23). Much of this work has focused on organ fibrosis and cancer. Stiffer matrices promote fibroblast activation and fibrosis and enhance cancer cell metastatic potential (19, 24, 25, 33). While most work has found that increased stiffness correlates with disease progression, some work has also documented softer tissue or ECM in disease. For example, kidney glomeruli from mouse models of Alport syndrome and HIV nephropathy are softer early in disease but may eventually stiffen with disease progression (34). This suggests that matrix stiffness must remain within a homeostatic window to maintain tissue structure and function.
Although studies of ECM mechanics have focused primarily on the interstitial matrix, some work has addressed BM stiffness (23). In their classic work, Welling and Grantham used circumferential stress to measure elastic moduli (stiffness) of 7–10 MPa for renal TBMs (32). More recent work in retinal BM with atomic force microscopy demonstrated a similar modulus averaging 3–4 MPa (5). While these studies provide an estimate of BM stiffness, they do not address the molecular substrate that endows the BM with its mechanical properties.
Collagen IV is a core constituent of BMs, along with laminins, proteoglycans, and nidogens. Collagen IV is secreted as triple-helical protomers, which self-oligomerize into large networks. Compared with other BM constituents, the collagen IV network is uniquely reinforced with intermolecular, covalent cross-links (18). Cross-links in fibrillar collagens, primarily collagen I, enhance matrix stiffness. Lysyl oxidases form lysyl aldehyde-based cross-links in collagens and, as such, are considered potential targets for the treatment of fibrosis or cancer (2, 35). Because of its extensive cross-links, collagen IV is often assumed to define BM stiffness, particularly with tensile or circumferential stress. In support of this hypothesis, loss of function in collagen IV leads to mechanical disruption of BMs. For example, in Drosophila egg development, collagen IV deficiency prevents normal egg elongation, which involves tightening of a “molecular corset,” affecting collagen IV and its interactions with integrin receptors (13). Collagen IV knockout (KO) mice demonstrate normal formation of the BM but eventual embryonic lethality due to widespread BM rupture and mechanical instability (26). Finally, in human Alport syndrome, loss of the network of α3-, α4-, and α5-chains of collagen IV in the renal glomerular BM compromises its integrity, leading to hematuria, proteinuria, and progressive renal failure (15). While these data suggest a role for collagen IV in resilience to mechanical stress, they do not directly address whether collagen IV defines BM stiffness.
A sulfilimine bond (S = N) between a methionine sulfur and a lysine nitrogen is a cross-link of collagen IV that bridges the trimer-trimer interface formed when two protomers associate via their COOH-terminal noncollagenous (NC1) domains during oligomerization to yield a hexameric structure (31). Recent work has demonstrated that a BM-associated protein, peroxidasin, catalyzes the formation of sulfilimine cross-links in collagen IV. In Drosophila, loss of peroxidasin function and sulfilimine cross-links compromises gut muscular BM integrity, leading to perforation and larval lethality (3). Similarly, loss of collagen IV sulfilimine cross-links reduces egg elongation in Drosophila (22). These data demonstrate that collagen IV sulfilimine cross-links support BM stability but do not define the effect of sulfilimine cross-links on BM mechanics. Here, using peroxidasin KO mice and a newly developed method to characterize BM tensile stiffness in renal tubules, we find that reduced sulfilimine cross-link density in collagen IV lowers BM stiffness. This work provides experimental evidence that the sulfilimine cross-link of collagen IV directly contributes to BM stiffness and acts as a foundation for future studies to examine how perturbations in BM mechanical properties affect cell and tissue function.
MATERIALS AND METHODS
Animals.
The Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures, which were consistent with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Peroxidasin KO mice were generated using the “knock-out first” targeting vector created by the Trans-NIH Knockout Mouse Project (KOMP) and obtained from the KOMP Repository (Project ID CSD 80013; www.komp.org). The vector was introduced into B6/129 hybrid embryonic stem cells, yielding homologous recombination with eventual generation of mice heterozygous for the KO allele by Ingenious Targeting Laboratory (Ronkonkoma, NY). These mice were backcrossed for 10 generations with C57BL/6J mice to yield pure-background mice. Wild-type (WT) C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). WT and KO mice were used at 10–14 wk of age for all studies.
Renal histology.
Mouse kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin using standard techniques. Mouse kidneys were processed for transmission electron microscopy (TEM) and imaged in the Vanderbilt Cell Imaging Shared Resource-Research Electron Microscopy facility. Briefly, samples were fixed in 2.5% glutaraldehyde solution in 0.1 M sodium cacodylate buffer, pH 7.4, at room temperature (RT) for 1 h and then transferred to 4°C overnight. The samples were washed in 0.1 M cacodylate buffer, incubated for 1 h in 1% osmium tetroxide at RT, and then washed with 0.1 M cacodylate buffer. Subsequently, the samples were dehydrated through a graded ethanol series followed by three exchanges of 100% ethanol. Next, the samples were incubated for 5 min in 100% ethanol and propylene oxide (PO) followed by two exchanges of pure PO. Samples were then infiltrated with 25% Epon 812 resin and 75% PO for 30 min at RT. Next, they were infiltrated with Epon 812 resin-PO (1:1) for 1 h at RT and then overnight at RT. On the next day, the samples were subjected to a resin-PO (3:1) exchange for 3–4 h and then incubated with pure epoxy resin overnight. Samples were then incubated in two more changes of pure epoxy resin and allowed to polymerize at 60°C for 48 h. After 500- to 1,000-nm-thick sections were cut for ultrastructure identification, 70- to 80-nm ultrathin sections were cut from the region of interest, collected on 300-mesh copper grids, and poststained with 2% uranyl acetate and then with Reynolds’ lead citrate. Samples were subsequently imaged on the Philips/FEI Tecnai T12 electron microscope at various magnifications.
Purification of kidney collagen IV and analysis of sulfilimine cross-links.
Collagen IV NC1 hexamers were purified as previously described (10). Briefly, mouse kidneys were homogenized in PBS + protease inhibitors and then sequentially extracted with 1% deoxycholate, 1 M NaCl, and double-distilled water to isolate a crude ECM fraction. Crude ECM underwent collagenase digestion using chromatographically purified bacterial collagenase (CLSPA grade collagenase, Worthington Biochemical, Lakewood, NJ) overnight at 37°C. Solubilized NC1 hexamers were dialyzed against 50 mM Tris·Cl, pH 7.5, subjected to DEAE chromatography, and isolated as the flow-through fraction. Hexamers underwent SDS-PAGE and Coomassie blue staining to identify singly (D1) and doubly (D2) cross-linked dimeric subunits and un-cross-linked monomeric (M) subunits. Densitometric analysis of these subunit bands was used to calculate average sulfilimine cross-links per hexamer (9, 22): cross-links per hexamer = 3 * fraction D1 + 6 * fraction D2.
Renal tubule isolation.
Kidneys were removed from anesthetized mice through an abdominal incision, placed in cold PBS, cut into thin transverse sections, and immediately transferred to a dissecting microscope with a cooled stage at 4°C. Tubules were manually dissected in isolation buffer consisting of (in mM) 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 6 l-alanine, 0.1 l-arginine, 1.0 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 (4). For decellularized tubule experiments, cells were removed with detergent extraction using 1% sodium deoxycholate before stress-strain measurements.
Preparation and calibration of force measurement cantilevers.
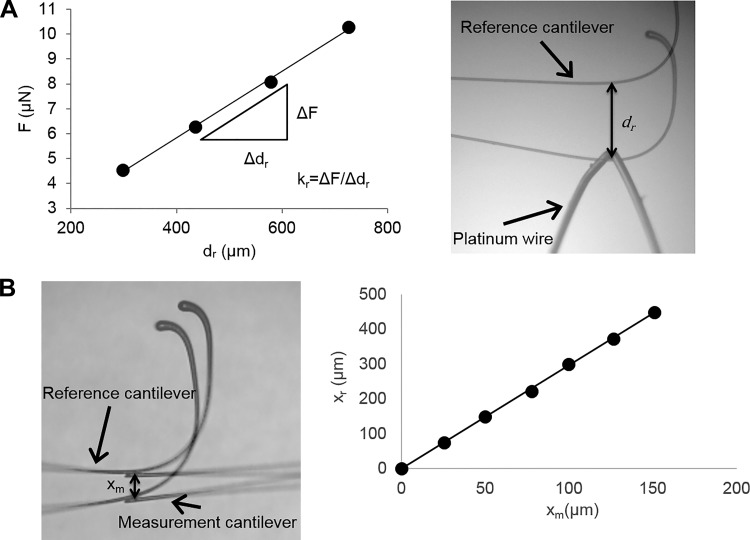
Force measurement cantilevers were fabricated from pulled glass capillary tubes (World Precision Instruments) using a vertical pipette puller (model 700C, David Kopf Instruments). The spring constant of the measurement cantilevers (km) was measured in a manner similar to that described by Shimamoto and Kapoor (29) (Fig. 1). Reference cantilevers were fabricated by pulling 1-mm solid glass rods. A microburner was used to fashion a hook on the end of the reference cantilevers. The spring constant of the reference cantilevers (kr) was determined by hanging lengths of platinum wire (0.05 mm diameter) from the end of the reference cantilevers and measuring the deflection with a ×4 objective. Force applied to the end of the reference cantilever by each length of platinum wire was calculated using the length and diameter of the wire and the density of platinum. The kr was calculated from the slope of the force vs. deflection plot (Fig. 1A). To determine km, the ends of the measurement and reference cantilevers were placed in contact, and the reference cantilever was displaced a fixed distance using a micromanipulator. Deflection of the measurement cantilever was measured under a microscope with a ×4 objective. The km was calculated as follows: km = kr(xr/xm), where xr is deflection of the reference cantilever and xm is deflection of the force measurement cantilever (Fig. 1B).
Fig. 1.
Cantilever calibration procedure. A: spring constant of reference cantilevers (kr) was determined by hanging lengths of platinum wire from the end of the reference cantilever (F = force applied to the cantilever) and measuring cantilever deflection (dr). Image is shown with an overlay of the original cantilever position. B: spring constant of the measurement cantilever was determined by displacing the reference cantilever relative to the measurement cantilever and measuring the displacement of the reference and measurement cantilevers (xr and xm, respectively). Measurement cantilever stiffness is calculated from the slope of the relative deflection plot and the known reference cantilever stiffness.
Measurement of TBM stiffness.
The measurement cantilever was attached to a manual micromanipulator, and a holding pipette (10-µm inner diameter) was attached to a motorized micromanipulator (World Precision Instruments). Experiments were performed on an inverted microscope (VWR) equipped with a ×4 objective and a digital camera for image acquisition. Isolated tubules were attached to the measurement cantilever by bringing the cantilever in proximity to the tubule and applying vacuum. The applied vacuum was sufficient to hold the tubule on the cantilever, and the lumen of the cantilever was sufficiently small to avoid aspiration of the tubule into the lumen. The tubule was moved away from the bottom of the dish, and vacuum was applied to the holding pipette to secure the free end of the tubule. The holding pipette was translated in 20-µm increments to stretch the tubule while simultaneously deflecting the measurement cantilever. Images acquired at each deflection increment were used to calculate the deflection of the measurement cantilever (dm) and the distance traversed by the holding pipette. The change in the length of the tubule (Δl) was calculated as the difference between the distance traveled by the holding pipette and the dm. The initial length of the tubule (lo) was measured from an image acquired before translation of the holding pipette. Stress (α) and strain (ε) were calculated as follows: α = (kmdm)/A and ε = Δl/lo, where A is cross-sectional area of the TBM. Area was calculated from the average diameter of the tubule measured at six different locations along the length of the tubule and the average thickness of the basement membrane as measured from TEM imaging. For linear elastic materials, elastic modulus is calculated from the slope of the linear stress-strain curve before yield. Given the nonlinear stress-strain behavior of the TBM, the elastic modulus was calculated from the slope of the linear regression of the stress-strain curve over 10% strain increments. While the overall stress-strain curve is nonlinear, there is reasonable linearity over smaller strain ranges.
Statistical analysis.
Statistical analysis is based on a minimum of three experimental replicates; n is the number of replicates for each experiment. Values are means ± SE. Statistical significance was determined using a Student’s t-test (unpaired, 2-tailed). P ≤ 0.05 was considered statistically significant.
RESULTS
Measurement of BM elastic modulus.
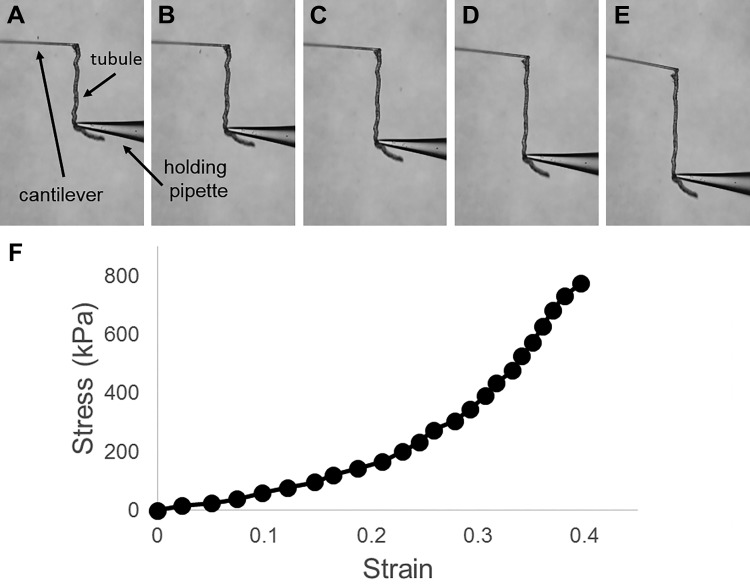
We developed a glass microcantilever system to measure the tensile stress-strain response of microdissected renal tubules. Tubules are composed of a single epithelial cell layer with an underlying BM. Prior work has suggested that the TBM primarily determines renal tubule stiffness, with minimal contribution from cells (32). Thus the system is a convenient way to specifically interrogate BM mechanics. The measurement system used in this work involves holding an isolated tubule between a hollow measurement cantilever and a holding pipette, each under vacuum (Fig. 2A). A fixed displacement is applied to the holding pipette, which stretches the tubule and simultaneously bends the measurement cantilever (Fig. 2, A–E; see Supplemental Video S1 in Supplemental Material for this article, available online at the Journal website). The force applied to the tubule is calculated from the bending of the precalibrated cantilever, and strain is determined from the dimensional change in tubule length. These measurements were used to generate stress-strain curves, which demonstrated that normal renal tubules exhibit nonlinear stress-strain behavior (Fig. 2F). Thus the tubule elastic modulus, defined as the slope of the stress-strain curve, rises with increasing strain.
Fig. 2.
Microcantilever method for measuring the stress-strain behavior of renal tubules. A–E: isolated tubule, measurement cantilever, and holding pipette at 0, 10, 21, 31, and 40% strain, respectively. F: stress-strain curve.
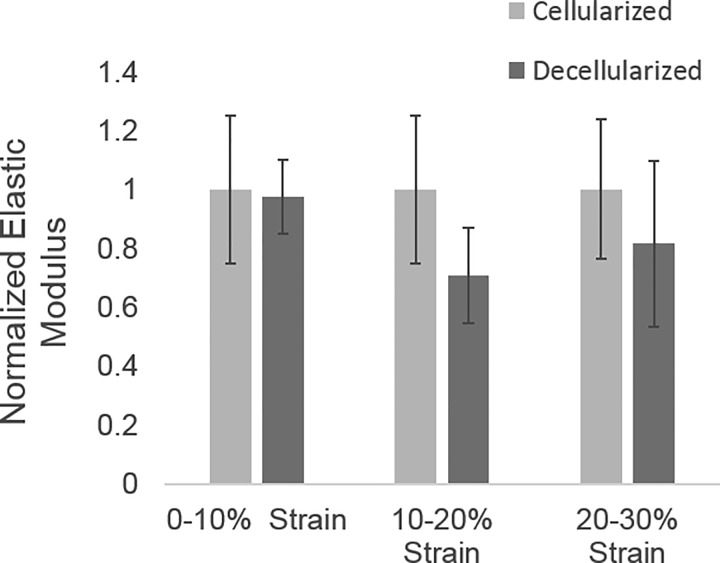
Since the goal of this work was to specifically examine BM stiffness, we wanted to determine whether the BM, rather than cells, primarily defines renal tubule elastic modulus in our system, which applies tensile stress, as opposed to circumferential stress, as in prior work (32). To do this, we compared stress-strain relationships from intact and decellularized tubules. These data showed no significant difference in elastic modulus between cellularized and decellularized tubules, suggesting that the TBM provides the primary resistance to tensile stress with a negligible cellular contribution (Fig. 3). Therefore, subsequent stress-strain relationships were determined using intact tubules.
Fig. 3.
Elastic modulus in cellularized and decellularized renal tubules. No significant difference in the elastic modulus was found between cellularized and decellularized tubules (n = 3). Elastic modulus was normalized to the cellularized elastic modulus at the corresponding strain range.
Peroxidasin KO mice exhibit normal BM structure with diminished collagen IV cross-links.
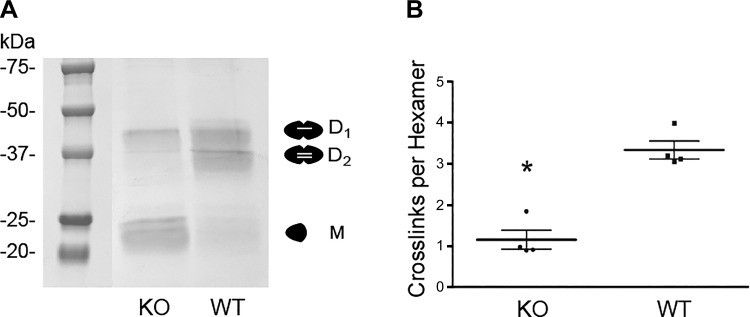
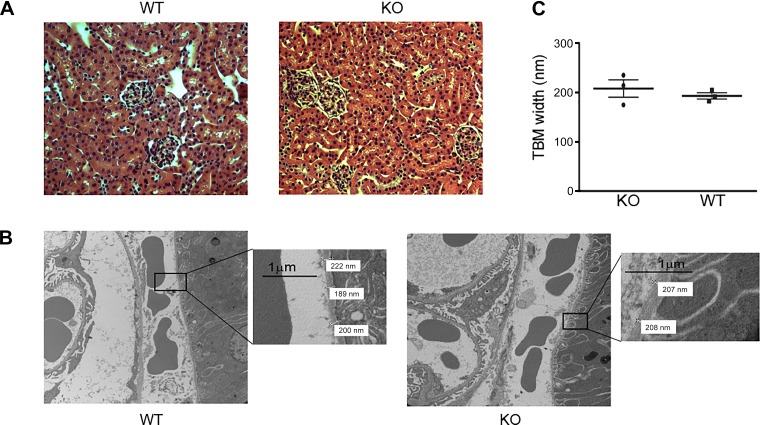
We hypothesized that collagen IV and its cross-links endow BM resistance to tensile stress. Since peroxidasin forms sulfilimine cross-links in collagen IV, peroxidasin KO mice provided a model system to directly test this hypothesis. These mice are born with the only overt phenotype of an anterior segment eye defect previously reported by others in mice and rare humans (6, 17, 36). To determine whether sulfilimine cross-links in collagen IV were altered in peroxidasin KO mice, we isolated collagen IV NC1 hexamers from WT and KO mouse kidneys. Since sulfilimine cross-links bridge NC1 hexamers, SDS dissociation and electrophoresis produce D1 or D2 subunits and M subunits (Fig. 4A). The distribution of these cross-linked and un-cross-linked forms can be used to quantify sulfilimine cross-links per hexamer, which ranges from zero to a maximum of six possible cross-links. As predicted, peroxidasin KO mice demonstrated a significant reduction in collagen IV sulfilimine cross-links from approximately three per NC1 hexamer in WT mice to approximately one per hexamer in KO mice (Fig. 4). Although peroxidasin KO mice possess fewer collagen IV sulfilimine cross-links, any structural BM alterations, including changes in BM thickness, would need to be accounted for in evaluating BM mechanical properties. Moreover, although the gross phenotype of these mice appeared normal, subtle structural differences could be present. To evaluate this possibility, we histologically examined renal tissue from WT and peroxidasin KO mice, including ultrastructural analysis using TEM. We could identify no light microscopic or ultrastructural morphological alterations in the renal glomeruli or tubules from KO vs. WT mouse kidneys (Fig. 5, A and B). Furthermore, TBM width was similar in peroxidasin KO and WT mice (Fig. 5C). Together, these findings show that peroxidasin KO mouse renal tubules are a reasonable model to test the specific effect of diminished sulfilimine cross-links in collagen IV on BM mechanical properties without significant confounding from altered BM structure.
Fig. 4.
Sulfilimine cross-link content in wild-type (WT) and peroxidasin knockout (KO) mice. A: Coomassie blue-stained gels after SDS-PAGE under nonreducing conditions of purified NC1 hexamers from WT and peroxidasin KO mice. Under denaturing conditions, hexamers dissociate into singly cross-linked dimeric (D1), doubly cross-linked dimeric (D2), and un-cross-linked monomeric (M) subunits. B: number of sulfilimine cross-links per hexamer was quantified using densitometry of D1, D2, and M subunits for WT and KO mice. Individual data points (n = 4) are displayed with mean and SE. *P < 0.05 (by unpaired t-test).
Fig. 5.
Renal tubular basement membrane structure in WT and peroxidasin KO mice. A: representative hematoxylin-eosin-stained images of WT and peroxidasin KO mouse kidneys. B: transmission electron microscopy images from WT and peroxidasin KO mouse kidney. Insets: magnified images demonstrating tubular basement membrane (TBM) width measurements. C: for measured TBM width, each data point represents mean of 40–100 replicate measurements of sections from 1 WT and 1 peroxidasin KO mouse kidney. Individual animal data points (n = 3 with 40–100 replicates) are displayed with mean and SE.
BM stiffness is reduced in peroxidasin KO mice.
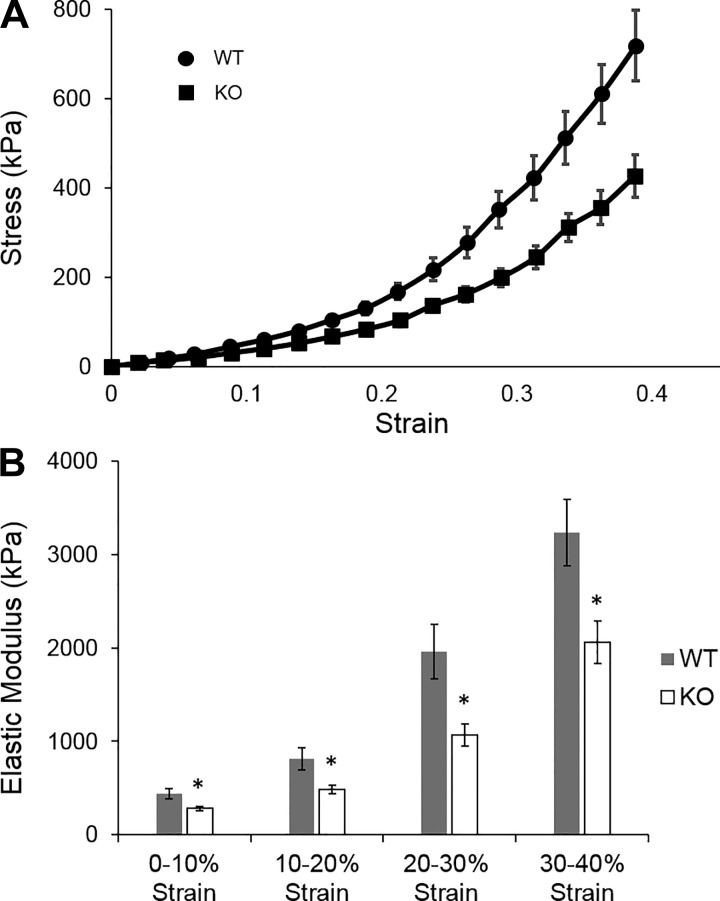
With a validated measurement of BM stiffness and a mouse model of deficient collagen IV sulfilimine cross-links, we tested the hypothesis that these cross-links contribute to BM resistance to tensile stress. Stress-strain curves for renal tubules from WT and peroxidasin KO mice are shown in Fig. 6A. The elastic modulus was decreased in renal tubules from peroxidasin KO compared with WT mice across all strain ranges (Fig. 6B). Low-strain (0–10%) elastic modulus was 438 ± 54 kPa in WT BM compared with 284 ± 90 kPa in KO BM. Elastic modulus at high strain (30–40%) was markedly higher in both WT (3,230 ± 356 kPa) and KO (2,056 ± 227 kPa) BM, illustrating strain stiffening in both WT and KO BM. However, elastic modulus was significantly lower in KO than WT BM over all strain ranges.
Fig. 6.
Mechanical properties of TBM from WT and peroxidasin KO mice. A: stress-strain curves for WT and KO TBM. Stress data were pooled in 2.5% increments of strain, and mean stress and strain were calculated from the pooled data. Values are means ± SE. Error bars for strain are obscured by individual data points. B: elastic modulus of TBMs as measured from the slope of the individual tubule stress-strain curves at 10% increments of strain. Values are means ± SE for 16 tubules from 4 different animals. *P < 0.05.
DISCUSSION
Complex interactions between macromolecules of the BM are thought to determine its mechanical properties. Because collagen IV is a major constituent of the BM and forms an extensively cross-linked oligomeric network and genetic loss of collagen IV leads to BM mechanical instability, collagen IV has been hypothesized to play a predominant role in the ability of the BM to resist deformation (13, 18, 26). Similarly, loss of sulfilimine cross-links in collagen IV increases BM sensitivity to mechanical stress, suggesting that these cross-links contribute to BM mechanical resilience (3, 22). However, none of these studies directly determined whether collagen IV and its cross-links govern the BM response to applied stress. We therefore endeavored to determine whether loss of sulfilimine cross-links in collagen IV led to reduced resistance to deformation in the BM. The primary conclusion of this work is that sulfilimine cross-links in collagen IV contribute to overall BM stiffness.
To define the role of sulfilimine cross-links in BM stiffness, we developed a system using renal tubules to measure tubular stress-strain relationships and elastic modulus. We focused on renal tubules, because renal tubule isolation, although cumbersome, has been extensively described in classic renal physiology, and prior work by others suggested that renal tubule stiffness was primarily determined by the TBM with little contribution from cells (32). In cell culture, the elastic modulus of an epithelial cell monolayer has been estimated at ~20 kPa (measured at 30–50% strain), which is ~2 orders of magnitude less than BM elastic modulus (14). Consistent with these data, we found that renal tubular elastic modulus was largely unaffected when tubules were decellularized, pointing to the BM as the primary determinant of stiffness.
Renal tubules exhibited nonlinear stress-strain relationships with higher elastic moduli with increasing strain ranging from ~0.5 MPa at low strain to 3 MPa at high strain. Notably, these measurements are qualitatively similar to previous estimates of BM elastic modulus ranging from 3 to 10 MPa using alternative measurement techniques or tissues (5, 32). The nonlinearity in the renal TBM stress-strain relationship, known as strain stiffening, is commonly observed in many filamentous network biomaterials, such as actin networks, fibrin gels, and fibrillar collagen gels (30). In general, the mechanical behavior of these materials is governed not simply by the intrinsic mechanical properties of the material that forms the filaments, but also by the complex three-dimensional interactions within and between the filaments in the network. Computational studies of biological networks, including collagen IV, also predict strain stiffening. Furthermore, these studies suggest that changes in the number of filamentous interactions or cross-links and, to a lesser degree, the strength of individual interactions significantly affect the stress-strain response of the material (12, 20). The data presented here qualitatively recapitulate previous experimentally observed and computationally predicted mechanical responses of normal BM.
Renal TBM stiffness was reduced in peroxidasin KO mice despite normal BM structure and collagen IV. These data suggest that collagen IV cross-links significantly determine BM resistance to tensile stress. In this context, the modest effect on TBM stiffness in peroxidasin KO mice is not surprising. First, sulfilimine cross-links are not completely abrogated in these mice (Fig. 4), which may also explain their relatively normal phenotype. Second, collagen IV has other covalent cross-links. Lysyl-derived cross-links catalyzed by lysyl oxidase 2 (LOXL2) and disulfide cross-links in the NH2-terminal “7S” domain of collagen IV and disulfide cross-links in the central collagenous portion of collagen IV also reinforce the network (1, 11, 27, 28).
This work directly demonstrates that sulfilimine cross-links in collagen IV contribute to BM stiffness. Future work can now evaluate the role of sulfilimine and other collagen IV cross-links and BM components in determining modulus and how cell behavior is modulated by BM stiffness. For example, the role of TBM mechanics and collagen IV cross-links in the response to renal injury can now be examined. In models of renal tubular injury and interstitial fibrosis, disruptions in the TBM have been observed (21, 37). We can now address whether TBM elastic modulus alters the frequency of TBM disruptions and possibly the role of these disruptions in subsequent renal repair and fibrosis. Whether collagen IV cross-links similarly determine glomerular BM stiffness is unknown. Given the role of glomerular hypertension and mechanical stress in the progression of chronic kidney disease (8), this will be a significant focus of future investigation.
GRANTS
TEM imaging experiments were performed through the use of the Vanderbilt Cell Imaging Shared Resource (supported by National Institutes of Health Grants CA-68485, DK-20593, DK-58404, DK-59637, and EY-08126). This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08 DK-097306 (to G. Bhave) and K01 DK-092357 (to N. Ferrell), Burroughs Wellcome Fund Career Award for Medical Scientists 13030995 (to G. Bhave), and developmental funds from the Vanderbilt University Medical Center Division of Nephrology (to G. Bhave).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.B. and N.F. conceived and designed research; G.B., S.C., and N.F. performed experiments; G.B., S.C., and N.F. analyzed data; G.B. and N.F. interpreted results of experiments; G.B., S.C., and N.F. prepared figures; G.B. and N.F. drafted manuscript; G.B., S.C., and N.F. edited and revised manuscript; G.B. and N.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Parvin Todd for technical assistance in the isolation of collagen IV NC1 hexamers from mouse kidney and Drs. Jeffrey Garvin and Pablo Cabral for training in tubule isolation.
REFERENCES
- 1.Añazco C, López-Jiménez AJ, Rafi M, Vega-Montoto L, Zhang MZ, Hudson BG, Vanacore RM. Lysyl oxidase-like-2 cross-links collagen IV of glomerular basement membrane. J Biol Chem 291: 25999–26012, 2016. doi: 10.1074/jbc.M116.738856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer 12: 540–552, 2012. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 3.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang JS, Pedchenko V, Fessler LI, Fessler JH, Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8: 784–790, 2012. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabral PD, Hong NJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol 299: F1185–F1192, 2010. doi: 10.1152/ajprenal.00112.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H. Biomechanical properties of native basement membranes. FEBS J 274: 2897–2908, 2007. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi A, Lao R, Ling-Fung Tang P, Wan E, Mayer W, Bardakjian T, Shaw GM, Kwok PY, Schneider A, Slavotinek A. Novel mutations in PXDN cause microphthalmia and anterior segment dysgenesis. Eur J Hum Genet 23: 337–341, 2015. doi: 10.1038/ejhg.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 8.Endlich N, Endlich K. The challenge and response of podocytes to glomerular hypertension. Semin Nephrol 32: 327–341, 2012. doi: 10.1016/j.semnephrol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Ero-Tolliver IA, Hudson BG, Bhave G. The ancient immunoglobulin domains of peroxidasin are required to form sulfilimine cross-links in collagen IV. J Biol Chem 290: 21741–21748, 2015. doi: 10.1074/jbc.M115.673996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA, Borza DB, Steele RE, Ivy MT, Hudson JK, Hudson BG; Aspirnauts . A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci USA 111: 331–336, 2014. doi: 10.1073/pnas.1318499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of α3, α4, and α5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem 273: 8767–8775, 1998. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 12.Gyoneva L, Segal Y, Dorfman KD, Barocas VH. Effect of supercoiling on the mechanical and permeability properties of model collagen IV networks. Ann Biomed Eng 43: 1695–1705, 2015. doi: 10.1007/s10439-014-1187-1. [DOI] [PubMed] [Google Scholar]
- 13.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331: 1071–1074, 2011. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris AR, Peter L, Bellis J, Baum B, Kabla AJ, Charras GT. Characterizing the mechanics of cultured cell monolayers. Proc Natl Acad Sci USA 109: 16449–16454, 2012. doi: 10.1073/pnas.1213301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan K, Rudkin A, Parry DA, Burdon KP, McKibbin M, Logan CV, Abdelhamed ZI, Muecke JS, Fernandez-Fuentes N, Laurie KJ, Shires M, Fogarty R, Carr IM, Poulter JA, Morgan JE, Mohamed MD, Jafri H, Raashid Y, Meng N, Piseth H, Toomes C, Casson RJ, Taylor GR, Hammerton M, Sheridan E, Johnson CA, Inglehearn CF, Craig JE, Ali M. Homozygous mutations in PXDN cause congenital cataract, corneal opacity, and developmental glaucoma. Am J Hum Genet 89: 464–473, 2011. doi: 10.1016/j.ajhg.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech 71: 357–370, 2008. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 28: 113–127, 2009. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B, Zhou X, Riching K, Eliceiri KW, Keely PJ, Guelcher SA, Weaver AM, Jiang Y. A three-dimensional computational model of collagen network mechanics. PLoS One 9: e111896, 2014. doi: 10.1371/journal.pone.0111896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157: 1380–1392, 2014. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RT. Mechanical properties of basement membrane in health and disease. Matrix Biol 57–58: 366-373, 2017. doi: 10.1016/j.matbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA 109: 10334–10339, 2012. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131: 1619–1628, 2004. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 27.Risteli J, Timpl R, Bächinger HP, Engel J, Furthmayr H. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem 108: 239–250, 1980. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- 28.Scott JE, Qian R, Henkel W, Glanville RW. An Ehrlich chromogen in collagen cross-links. Biochem J 209: 263–264, 1983. doi: 10.1042/bj2090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimamoto Y, Kapoor TM. Microneedle-based analysis of the micromechanics of the metaphase spindle assembled in Xenopus laevis egg extracts. Nat Protoc 7: 959–969, 2012. doi: 10.1038/nprot.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature 435: 191–194, 2005. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 31.Vanacore R, Ham A-JL, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond identified in collagen IV. Science 325: 1230–1234, 2009. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welling LW, Grantham JJ. Physical properties of isolated perfused renal tubules and tubular basement membranes. J Clin Invest 51: 1063–1075, 1972. doi: 10.1172/JCI106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta 1832: 884–890, 2013. doi: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyss HM, Henderson JM, Byfield FJ, Bruggeman LA, Ding Y, Huang C, Suh JH, Franke T, Mele E, Pollak MR, Miner JH, Janmey PA, Weitz DA, Miller RT. Biophysical properties of normal and diseased renal glomeruli. Am J Physiol Cell Physiol 300: C397–C405, 2011. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem 52: 113–133, 2012. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan X, Sabrautzki S, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Hrabě de Angelis M, Graw J. Peroxidasin is essential for eye development in the mouse. Hum Mol Genet 23: 5597–5614, 2014. doi: 10.1093/hmg/ddu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538, 2002. doi: 10.1172/JCI0216219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3: a004911, 2011. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]