Abstract
(Pro)renin receptor (PRR) is highly expressed in the distal nephron, but it has an unclear functional implication. The present study was conducted to explore a potential role of renal PRR during high K+ (HK) loading. In normal Sprague-Dawley rats, a 1-wk HK intake increased renal expression of full-length PRR and urinary excretion of soluble PRR (sPRR). Administration of PRO20, a decoy peptide antagonist of PRR, in K+-loaded animals elevated plasma K+ level and decreased urinary K+ excretion, accompanied with suppressed urinary aldosterone excretion and intrarenal aldosterone levels. HK downregulated Na+-Cl− cotransporter (NCC) expression but upregulated CYP11B2 (cytochrome P-450, family 11, subfamily B, polypeptide 2), renal outer medullary K+ channel (ROMK), calcium-activated potassium channel subunit α1 (α-BK), α-Na+-K+-ATPase (α-NKA), and epithelial Na+ channel subunit β (β-ENaC), all of which were blunted by PRO20. After HK loading was completed, urinary, but not plasma renin, was upregulated, which was blunted by PRO20. The same experiments that were performed using adrenalectomized (ADX) rats yielded similar results. Interestingly, spironolactone treatment in HK-loaded ADX rats attenuated kaliuresis but promoted natriuresis, which was associated with the suppressed responses of β-ENaC, α-NKA, ROMK, and α-BK protein expression. Taken together, we discovered a novel role of renal PRR in regulation of K+ homeostasis through a local mechanism involving intrarenal renin-angiotensin-aldosterone system and coordinated regulation of membrane Na+- and K+-transporting proteins.
Keywords: (Pro)renin receptor, potassium, kidney, CYP11B2, Na+-Cl− cotransporter, aldosterone
potassium (K+), the main intracellular cation together with sodium (Na+), maintains total body Na+ and K+ balance and is essential to the survival of most species. Total body K+ balance not only depends on a continuous balance between dietary K+ intake and K+ excretion, but also on the distribution between extracellular fluid and intracellular fluid compartments. The effective excretion of K+ must be tightly regulated to maintain blood K+ concentrations in the range of 3.8 to 5 mM (21). Aldosterone is thought to be critical for K+ homeostasis. The aldosterone-sensitive distal nephron of the kidney is responsible for >90% of whole body K+ excretion and has a remarkable capacity to regulate K+ excretion to match K+ intake, while the aldosterone-sensitive distal colon contributes to <10% of whole body K+ excretion (20, 21). The regulation of K+ homeostasis is thought to follow a classic feedback model: high K+ (HK) intake increases plasma [K+], which directly stimulates aldosterone secretion by the adrenal glands, and then stimulates renal K+ secretion to restore plasma K+ to normal levels (59). In this model, the role of adrenal gland-derived aldosterone is supported by the observation that hypoaldosteronism, as occurs in Addison’s disease, causes hyperkalemia, and the opposite is true that hyperaldosteronism, as occurs in Conn syndrome, induces hypokalemia. However, this model cannot explain the rapid kaliuretic response to dietary K+ load before the elevation of circulating aldosterone (63). Moreover, the responses of renal K+ excretion (29, 79), along with Na+ and K+ conductance in the collecting duct (CD) (74) to dietary K+ load, remain intact in adrenalectomized animals.
(Pro)renin receptor (PRR), a newly discovered component of the renin-angiotensin system (RAS), is a single-transmembrane receptor for renin and prorenin, with almost equal binding affinity, and this binding leads to increased catalytic activity (46, 48). So far, three molecular forms of PRR have been identified: the full-length form (fPRR) (48), a truncated transmembrane form with a COOH-terminal region (8.9 kDa called M8.9 was reported to complex with V-ATPase) (40); and a truncated soluble form with a NH2-terminal region (sPRR) generated intracellularly by furin (11) or ADAM19-mediated cleavage (77). sPRR can be secreted into the extracellular space, such as plasma (11) and urine (24), and into a variety of cell types (77), but can also be retained inside the cell through a stable and direct or indirect membrane association (56). Both fPRR and sPRR bind renin and prorenin (11) to increase renin activity and induce nonproteolytic activation of prorenin, respectively (24). In addition to its role in enhancing prorenin enzymatic activity toward the generation of ANG I, PRR binding triggers intracellular signaling cascades, such as the phosphorylation of ERK-1/2; PRR is also required as a subunit of V-ATPase, which actively transports protons into vesicles, and as an adaptor protein between V-ATPase and low-density lipoprotein receptor-related protein 6, which are members of the Wnt receptor complex (51).
Although a series of studies have showed that the handle region decoy peptide (HRP, 10–19 amino acid residues of the prorenin prosegment, R10IPLKKMPSV19) inhibits the conformational change and nonproteolytic activation of prorenin (71) and prevents the development of rat diabetic nephropathy (27) and cardiac fibrosis in spontaneously hypertensive rats (28), other studies showed controversial results (4, 5, 14, 45, 62). PRO20 is a newly developed PRR decoy inhibitor, the first 20 amino acid residues of the prorenin prosegment (L1PTRTATFERIPLKKMPSVR20), which binds to PRR and effectively suppresses the activation of ERK1/2 induced by prorenin and attenuates hypertension induced by ANG II, prorenin, or DOCA-salt via inhibiting the activation of the local renin-angiotensin system (35, 66).
Within the kidney, immunofluorescence demonstrated that PRR is detected in the renal vasculature, distal convoluted tubules (DCT), proximal tubules, connecting tubules, and CDs (1, 12, 39, 66). Renal expression of PRR is altered by changing salt balance, implicating a potential role of PRR in the regulation of electrolyte metabolism (15, 26, 42). This notion is also supported by the observation that overexpression of human PRR in transgenic rats elevates aldosterone production (7). Previously, we showed PRR functions as a regulator of local renin-angiotensin-aldosterone system (RAAS) to control aldosterone synthesis during HK treatment in primary rat inner medullary CD cells. These results implicate a potential role of PRR in regulation of K+ homeostasis. In the present study, we aimed to validate the role of PRR in the regulation of renal derived aldosterone release and K+ homeostasis in animal models.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley (SD) rats (250–300 g, Charles River Laboratories, Wilmington, MA) were all given free access to tap water and were fed the standard diet (Na+: 0.5% and K+: 0.8%). Rats were housed in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle. The animal protocols were approved by the Animal Care and Use Committee at Sun Yat-sen University, China.
Animal Treatment
Protocol I.
SD rats were fed a normal K+ (NK) diet (Na+: 0.5% and K+: 0.8%), a high K+ (HK) diet (Na+: 0.5% and K+: 5%), or a HK diet in combination with PRO20 treatment (HK + PRO20) (700 μg·kg−1·day−1 sc, 3 times a day for every 8 h) for 7 days. The NK and HK groups received 0.9% NaCl (3 times a day for every 8 h, subcutaneously). K+ was added to the standard diet as 8% KCl to obtain a HK diet (5%), as previously described (59). Rats were housed in metabolic cages in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle for 24-h urine collection and to measure body weight and 24-h food and water intake. At day 7, rats were euthanized, and blood and kidneys were harvested. Urine and plasma electrolytes were determined with an automatic analyzer (9180 Electrolyte analyzer, Roche, Berlin, Germany).
Protocol II.
Under anesthesia, all rats were subjected to a right uninephrectomy and then to bilateral adrenalectomy (ADX) with 1-wk interval, as previously described (54). In the second surgery, an infusion catheter was chronically implanted in the kidney (42). Briefly, the left kidney was exposed, and the tip of the polyethylene-10 catheter was inserted into the left kidney cortex, ~2 mm in depth, and glued with Vetbond (3M Animal Care Products, Saint Paul, MN) to prevent dislodging. The other end of the catheter was connected to an osmotic minipump (model no. 1007D, Alzet, Cupertino, CA) that was placed under the skin. After the surgery, all rats were given 0.9% NaCl containing 0.2 mg/l dexamethasone as the drinking fluid. They received a NK diet, a HK diet, or a HK diet + PRO20. PRO20 was administered at 700 μg·kg−1·day−1 in 0.9% NaCl via the intrarenal infusion catheter. The other two groups received intrarenal infusion of 0.9% NaCl. The treatment was for 1 wk. The sample collections were the same as described in Protocol I.
Protocol III.
While under anesthesia, all rats were subjected to ADX. After the surgery, all rats were given 0.9% NaCl containing 0.2 mg/l dexamethasone as the drinking fluid. Rats were then randomly assigned to one of the following groups: 1) NK + vehicle group, in which rats were given a NK diet and treated with 0.5% ethanol in the drinking water; 2) NK + spironolactone group, in which rats were given NK diet and simultaneously treated with the mineralocorticoid receptor blocker spironolactone [spironolactone dissolved in ethanol at 25 mg/ml and added to drinking water (40 mg·kg−1·day−1)], as previously described (9, 44); 3) HK + vehicle group, in which rats were given HK diet and treated with 0.5% ethanol in drinking water; and 4) HK + spironolactone group, in which rats were given HK diet and simultaneously treated with spironolactone. The treatment was for 1 wk. The sample collections were the same as described in protocol I.
Renin Activity Assay
Renin activity assay was performed as previously described (8). Briefly, renin activity in plasma and urine was determined by the delta value of the ANG I generation using an ELISA kit from the sample incubating at 4°C and 37°C for 1 h, respectively. Total renin content was measured with excessive angiotensinogen plus trypsinization, and active renin content with excessive angiotensinogen. Urine and plasma samples were spiked with 1 μM synthetic renin substrate tetradecapeptide (RST, R8129; Sigma-Aldrich, St. Louis, MO). After incubation at 37°C for 18 h, ANG I generation was assayed by using an ANG I EIA kit (S-1188; Peninsula Laboratories International, San Carlos, CA), according to the manufacturer’s instructions. The values were expressed as nanograms per milliliter per hour of generated ANG I. For measurement of total renin content, trypsinization was performed to activate prorenin to renin (57). The samples were incubated with trypsin derived from bovine pancreas (100 g/l, T1426, Sigma-Aldrich) in 37°C for 18 h. The reaction was then terminated with soybean trypsin inhibitor (100 g/l, T6522; Sigma-Aldrich) at 37°C for 1 h.
qRT-PCR
Snap-frozen renal samples (n = 5 per group) were homogenized in TRIzol reagent (cat. no. 15596018, Life Technologies, Carlsbad, CA). Total RNA isolation and RT were performed as previously described (52). Total RNA concentrations were determined using NANODROP 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. We used 1 μg of total RNA as a template for RT by using the Transcriptor first-strand cDNA synthesis kit (cat. no. 04379012001; Roche, Berlin, Germany), according to the manufacturer’s instructions. qPCR was performed using the ABI Prism StepOnePlus System (Applied Biosystems, Life Technologies, Waltham, MA) and the SYBR Premix Ex Taq kit (Tli RNaseH Plus) (cat. no. DRR420A, Takara, Dalian, China), according to the manufacturer’s instructions. Oligonucleotides were designed using Primer3 software (available at http://bioinfo.ut.ee/primer3-0.4.0/); their sequences are shown in Table 1. All reactions were run in duplicate. Relative mRNA expression levels were calculated from threshold cycle numbers (CT), i.e., 2−ΔΔCT, according to the manufacturer's suggestion. The data were shown as a relative value normalized by GAPDH.
Table 1.
Sequences of oligonucleotides used for qRT-PCR
| Gene | PubMed Number | Sequence (5′ → 3′) | Product Size, bp |
|---|---|---|---|
| PRR | NM_001007091.1 | (F) TGGGAAGCGTTATGGAGAAG | 215 |
| (R) GGTTGTAGGGACTTTGGGTGT | |||
| NCC | NM_019345.3 | (F) TCTACTGGCTGTTTGACGATGG | 135 |
| (R) CCTTTCTCTCTTCATCCATCCTGT | |||
| CYP11B2 | NM_012538.2 | (F) TGAGACGTGGTGTGTTCTTGC | 140 |
| (R) GGCCTCCAAGAAGTCCCTTGC | |||
| GAPDH | NM_017008.4 | (F) GTCTTCACTACCATGGAGAAGG | 197 |
| (R) TCATGGATGACCTTGGCCAG |
qPRT-PCR, quantitative RT-PCR; NCC, sodium chloride cotransporter; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Immunoblot Analysis
Tissue samples from the renal cortex, outer medulla, and inner medulla were lysed and subsequently sonicated in RIPA buffer (Biocolors, Shanghai, China) with protease inhibitor cocktail (Roche, Berlin, Germany). Protein concentrations were determined with the Pierce BCA protein assay kit (cat. no. NCI3225CH; Thermo Scientific, Rockford, IL), according to the manufacturer’s instructions. As previously described (43), to verify linearity of the detection system, one-half of the protein amount was loaded to verify doubling of signal intensity; to verify uniform loading, loading gels were run, and random bands were quantified. Samples were resolved by SDS-PAGE and transferred onto polyvinylidene fluoride membrane (Immobilion-P, Millipore, Bedford, MA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST) for 1 h at room temperature, followed by incubation with primary antibodies [PRR: 1:1,000 dilution, ab40790; Abcam, Cambridge, MA; NCC: 1:1000 dilution, SPC-402D, StressMarq, Victoria, BC, Canada; α-epithelial sodium channel (α-ENaC): 1:1,000 dilution, SPC-403D, StressMarq; β-ENaC: 1:1,000 dilution, SPC-404D, StressMarq; γ-ENaC: 1:1,000 dilution, SPC-405D, StressMarq; CYP11B2, 1:1,000 dilution, MAB6020, Merck Millipore; renal outer medullary K+ channel (ROMK), 1:1,000 dilution, 20953-1-AP, Proteintech; α Na+-K+-ATPase (α-NKA), 1:1,000 dilution, ab76020, Abcam; calcium-activated potassium channel subunit α1 (α-BK): 1:10 dilution, clone L6/60, 73-022, NeuroMab; β-actin: 1:10,000 dilution, A-2066, Sigma-Aldrich] diluted in antibody dilution buffer (1.5 g BSA, 0.1 g NaN3, 50 ml TBST) overnight at 4°C. After being washed with TBST, membranes were incubated with secondary antibodies [goat anti-rabbit/mouse horseradish peroxidase (HRP)-conjugated secondary antibody] (Thermo Scientific) for 1 h at room temperature and visualized with enhanced chemiluminescence (Thermo Scientific). Signals on immunoblots were detected using Tanon 5200 Luminescent Imaging Workstation (Tanon, Shanghai, China) and quantitated using Image-Pro Plus version 6.0 software. Values were normalized to the mean intensity measured in the NK groups defined as 1.0. The expression of protein was calculated in relation to β-actin.
ELISA Assays for sPRR and Aldosterone
sPRR and aldosterone levels, released into the plasma and urine, were determined by using a soluble (Pro)renin receptor assay kit (cat. no. 27782; Immuno-Biological Laboratories, Gunma, Japan) and aldosterone EIA kit (cat. no. 10004377; Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions. Renal aldosterone assay was performed as previously described (55, 75). Briefly, homogenized kidney tissue was extracted by methylene chloride (1:2 vol/vol). After evaporation of the methylene chloride, the extract was dissolved into enzyme immunoassay buffer and added to the assay wells (50 μl per well) for ELISA assays.
Liquid Chromatography Tandem Mass Spectrometry Assays for Free and Total Aldosterone
Before the experiment, acetic acid glacial was added to urine samples as a preservative. For the measurement of free aldosterone, 20 μl of 5-sulfosalicylic acid dehydrate were added to 80-μl urine samples. For the measurement of total aldosterone, urine samples were hydrolyzed by adding 20 μl of 3.2 M HCl into 200-μl urine samples, followed by incubating at 37°C for 4 h in dark. A six-point calibration curve was prepared with water-based standards of aldosterone; the final calibrator concentrations were 0, 5, 25, 50, 100, and 200 ng/ml. Aldosterone analysis was performed using an Agilent 1200L, and an API Q-trap 4000 LC-MS/MS spectrometer was equipped with a TurboIonSpray interface (AB Sciex, Concord, ON, Canada). Data acquisition and analyses were performed using Analyst software version 1.5 (AB Sciex).
Immunofluorescence
Kidneys were fixed overnight at 4°C in 3% paraformaldehyde/0.1 M phosphate salt buffer and were processed for immunohistochemistry analysis, as previously described (37). Briefly, paraffin-embedded tissue samples were cut into 3-μm-thick sections, deparaffinized, and rehydrated. After microwave treatment and consumption of endogenous peroxidase with 3% H2O2 in methanol, slides were preincubated with 3% BSA to block nonspecific reactions. Cryosections were incubated with primary antibodies [NCC: 1:75 dilution, SPC-402D, StressMarq; Calbindin D28K (C-20): 1:500 dilution, sc-28285, Santa Cruz Biotechnology, Santa Cruz, CA; AQP2: 1:200 dilution, sc-9882, Santa Cruz Biotechnology] at 4°C overnight and with fluorescent dye-conjugated secondary antibodies at room temperature for 1 h. Calbindin D28K is an unequivocal identification of distal tubule segments and CDs (36); aquaporin 2 (AQP2) is exclusively localized in the apical and subapical regions of CDs (19). Images were captured using a Leica DMI4000B fluorescence microscope (Wetzlar, Germany).
Statistical Analysis
Data are summarized as means ± SE. Statistical analysis was performed by using one-way ANOVA with the Bonferroni test for multiple comparisons or by unpaired Student's t-test for two comparisons using IBM SPSS 19 software. P < 0.05 was considered statistically significant.
RESULTS
In Vivo Experiments Using Normal Rats
Normal SD rats were treated for 7 days with NK diet, a HK diet alone, or HK combined with PRO20 treatment. As shown in Table 2, animals that received the HK diet gained less weight and exhibited polydipsia, polyuria, accompanied with hypoosmotic urine, compared with the NK group, but none of these were affected by PRO20 treatment.
Table 2.
General physiological data in rats
| NK | HK | HK + PRO20 | |
|---|---|---|---|
| ΔBody weight, g | 46.9 ± 2.6 | 16.3 ± 1.5*** | 16.8 ± 1.2*** |
| Food intake, g/24 h | 25.1 ± 2.1 | 21.5 ± 0.9* | 21.9 ± 0.7* |
| Water intake, ml/24 h | 50.9 ± 5.1 | 122.5 ± 5.9*** | 118.1 ± 5.8*** |
| Urine volume, ml/24 h | 27.8 ± 2.7 | 101.3 ± 5.2*** | 98.4 ± 4.0*** |
| Urine osmolality, mosmol/kg⋅H2O | 1445.3 ± 111.3 | 1082.8 ± 11.5** | 1156.2 ± 55.8* |
| Plasma osmolality, mosmol/kg⋅H2O | 312.8 ± 0.4 | 312.8 ± 0.8 | 314.0 ± 4.8 |
Values are expressed as means ± SE. ΔBody weight, change in body weight.
P < 0.05 vs. NK.
P < 0.01 vs. NK.
P < 0.001 vs. NK.
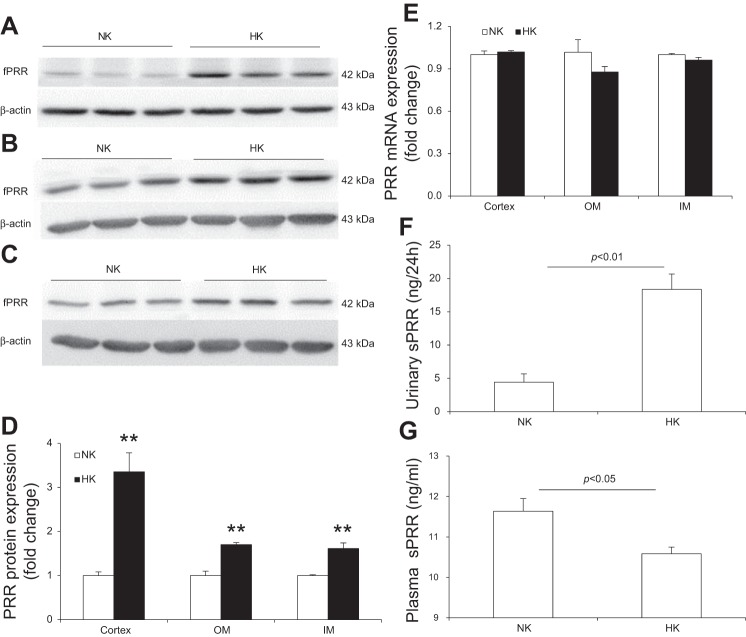
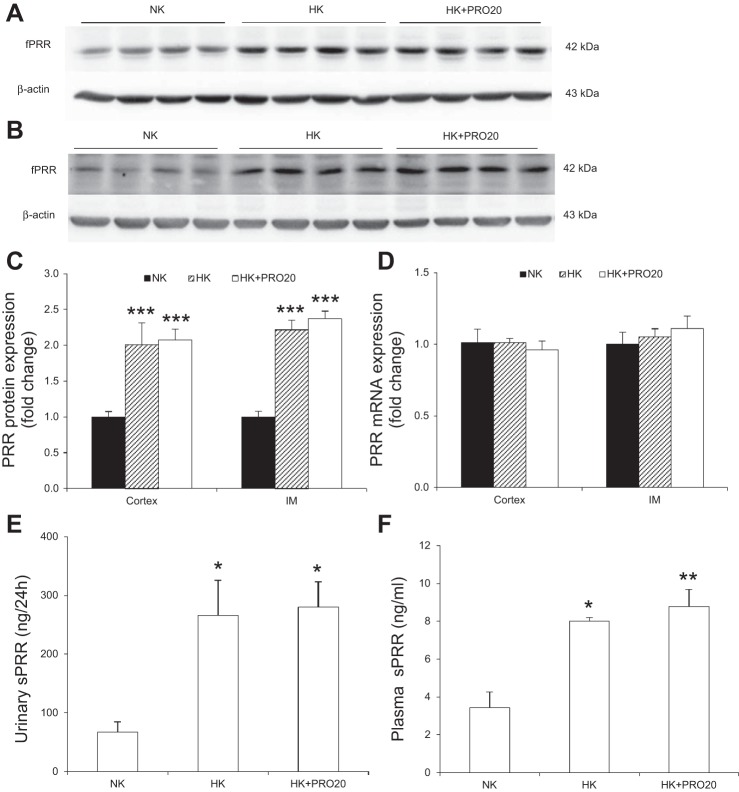
To examine renal PRR regulation after a HK diet, normal SD rats were treated with a NK or HK diet for 7 days and renal PRR expression, plasma sPRR, and urinary sPRR were analyzed. As shown in Fig. 1, HK intake increased renal fPRR protein expression in both the cortex (Fig. 1A), outer medulla (Fig. 1B), and inner medulla (Fig. 1C). The densitometry data were shown in Fig. 1D. In contrast, renal PRR mRNA expression remained unchanged in these regions (Fig. 1E). By ELISA, urinary sPRR was remarkably increased after HK intake (Fig. 1F), contrasting to decreased plasma sPRR concentration (Fig. 1G).
Fig. 1.
Effect of HK intake on renal PRR and sPRR levels in normal rats. A, B, and C: representative immunoblotting of PRR in the renal cortex (A), outer medulla (B), and inner medulla (C). D: densitometric analysis of PRR protein; expression was normalized by β-actin. E: quantitative RT-PCR (qRT-PCR) analysis of renal PRR mRNA expression; expression was normalized by GAPDH. F: ELISA analysis of urinary sPRR. G: ELISA analysis of plasma sPRR concentration. n = 5 per group. Data are expressed as means ± SE. **P < 0.01 vs. NK.
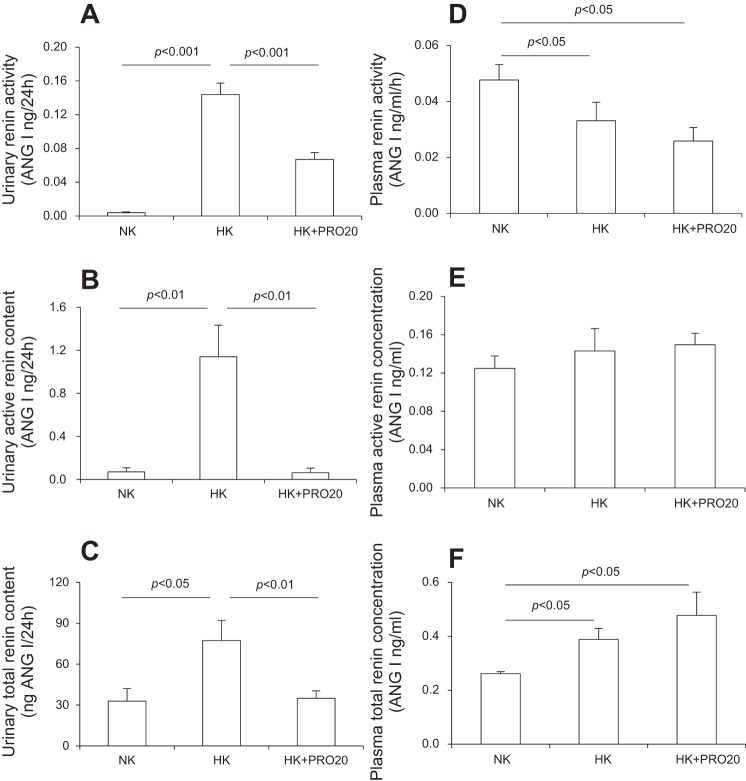
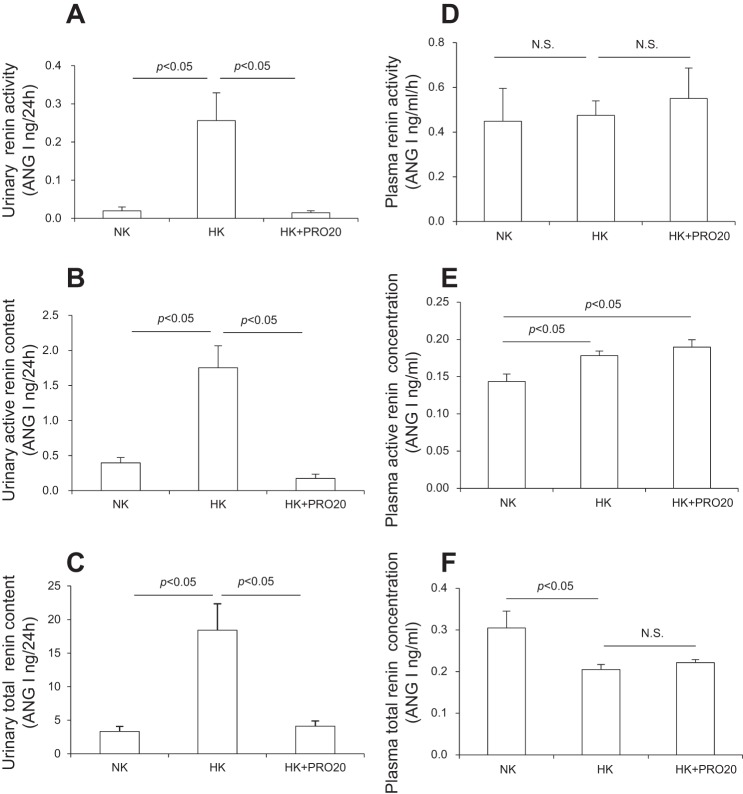
We examined renin levels in plasma and urine in NK, HK, and HK + PRO20 rats. PRO20 was given by subcutaneous injections. HK intake significantly elevated urinary renin activity (Fig. 2A), active renin concentration (Fig. 2B), and total renin concentration (Fig. 2C), which were all attenuated by PRO20 treatment. In contrast, plasma renin exhibited a different response to HK intake with decreased renin activity (Fig. 2D) and increased total renin concentration (Fig. 2F) but no change in active renin concentration (Fig. 2E). Importantly, none of the plasma renin parameters were affected by PRO20 treatment.
Fig. 2.
Effect of PRO20 on renin levels in K+-loaded normal rats. SD rats were randomly divided into the following three groups: NK, HK, or HK + PRO20. A: urinary renin activity. B: urinary active renin content. C: urinary total renin content. D: plasma renin activity. E: plasma active renin concentration. F: plasma total renin concentration. n = 5 per group. Data are expressed as means ± SE.
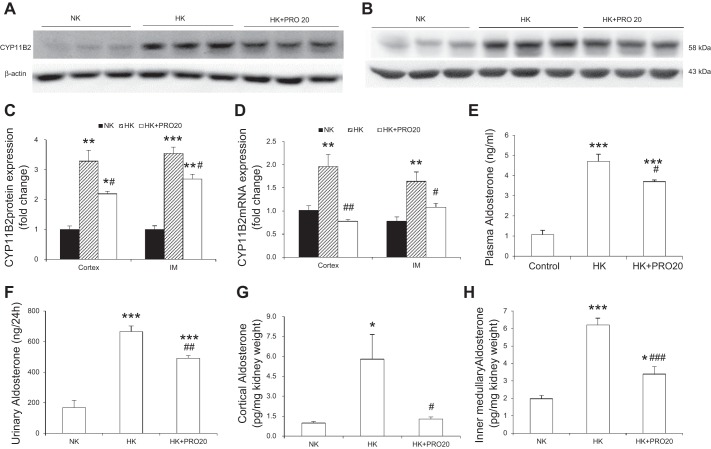
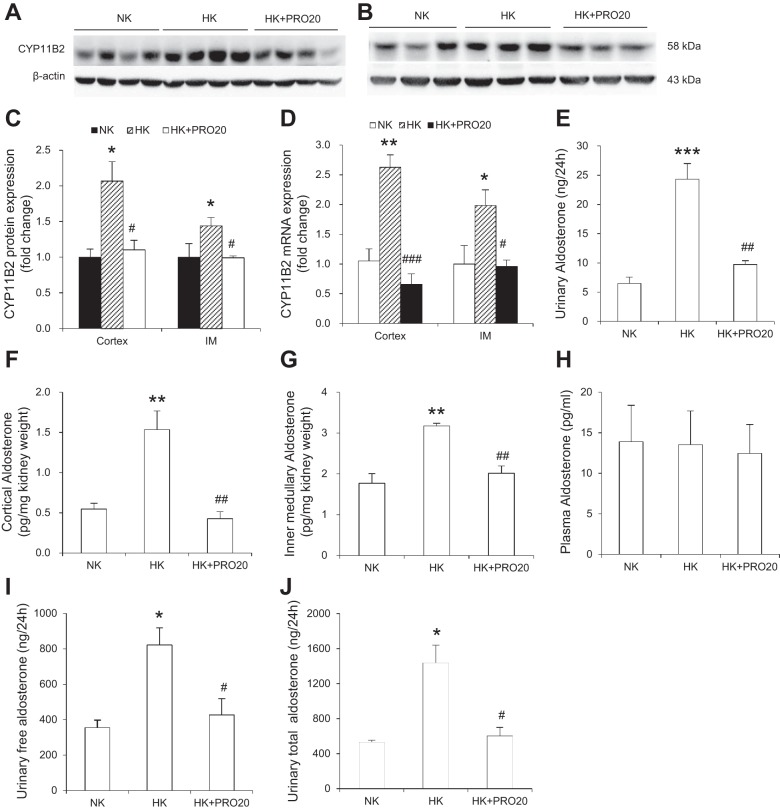
In light of the evidence arguing against the role of adrenal derived aldosterone in K+ homeostasis, we attempted to assess the contribution of local generation of aldosterone in the kidney. CYP11B2, a cytochrome P-450 oxidase, contributes to the conversion of deoxycorticosterone to aldosterone (53). We examined the effect of HK on renal CYP11B2 expression and aldosterone production in vivo. In normal SD rats, HK intake significantly increased CYP11B2 protein in renal cortex (Fig. 3, A and C) and inner medulla (Fig. 3, B and C), and CYP11B2 mRNA expression in these regions (Fig. 3D), and this increase was attenuated by PRO20 treatment. In parallel, PRO20 treatment significantly attenuated HK-induced rises in aldosterone levels in plasma (Fig. 3E), urine (Fig. 3F), the renal cortex (Fig. 3G), and the inner medulla (Fig. 3H), as assessed by ELISA.
Fig. 3.
Effect of PRO20 on renal CYP11B2 expression in K+-loaded normal rats. A–C: representative immunoblotting of CYP11B2 in the renal cortex (A) and inner medulla (B). C: densitometric analysis of CYP11B2 protein (IM, inner medulla); expression was normalized by β-actin. D: qRT-PCR analysis of CYP11B2 mRNA; expression was normalized by GAPDH. E: measurement of plasma aldosterone by ELISA. F: measurement of urinary aldosterone by ELISA. G: measurement of renal cortex aldosterone by ELISA. H: measurement of renal inner medullary aldosterone by ELISA. n = 5 per group. Data are expressed as means ± SE. *P < 0.05 vs. NK, **P < 0.01 vs. NK, ***P < 0.001 vs. NK, #P < 0.05 vs. HK, ##P < 0.01 vs. HK, and ###P < 0.001 vs. HK.
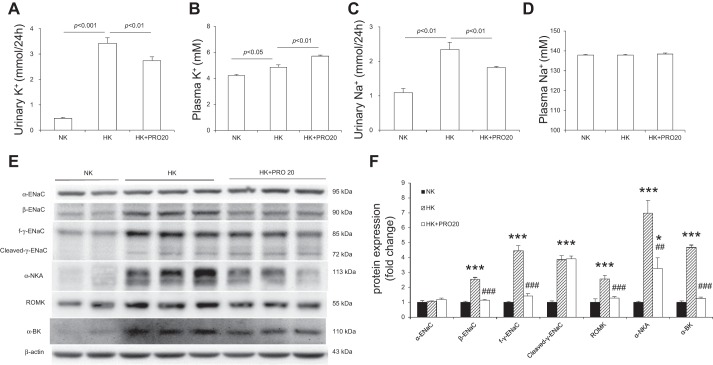
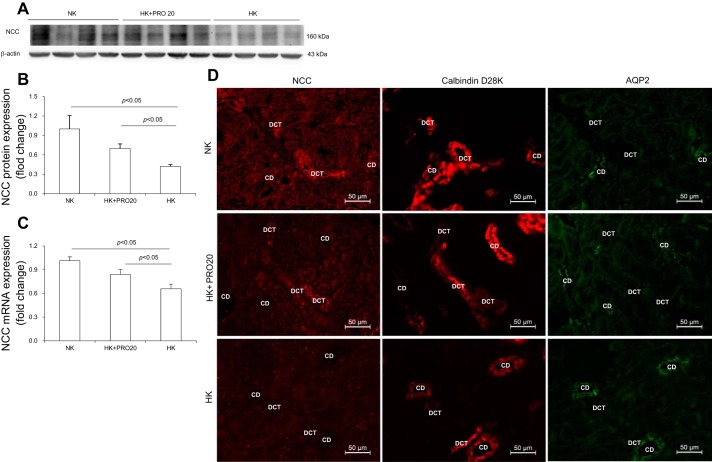
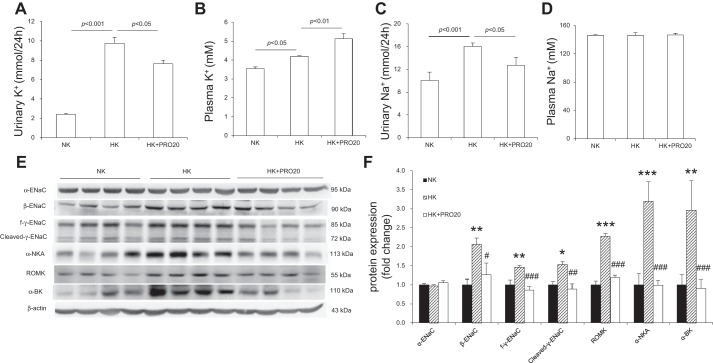
As compared with the NK control group, HK rats exhibited increased 24-h urinary K+ excretion (UKV) (Fig. 4A), plasma K+ concentration (Fig. 4B), and 24-h urinary Na+ excretion (UNaV) (Fig. 4C). HK + PRO20 rats had a greater increase in plasma K+, accompanied with attenuated UKV and UNaV, but plasma Na+ concentration was similar among the three groups (Fig. 4D). It is known that HK intake upregulates ENaC but downregulates NCC, which allows Na+-dependent K+ secretion in the CD. This K+ secretion process depends on activation of ROMK and α-BK on the apical membrane and NKA on the basolateral membrane. We found that HK intake enhanced the protein expression of β-ENaC, full-length γ-ENaC (f-γ-ENaC), cleaved-γ-ENaC, ROMK, and α-NKA, but not α-ENaC. PRO20 treatment attenuated the upregulation of β-ENaC, f-γ-ENaC, ROMK, α-BK, and α-NKA, without affecting cleaved-γ-ENaC (Fig. 4, E and F). As shown in Fig. 5, PRO20 significantly inhibited HK-induced downregulation of NCC protein (Fig. 5, A and B) and mRNA expression (Fig. 5C). Immunofluorescence for NCC also showed that PRO20 increased the immunofluorescence staining of NCC in the renal DCT compared with that in the HK group (Fig. 5D).
Fig. 4.
Urinary K+ (A), plasma K+ (B), urinary Na+ (C), plasma Na+ (D), immunoblotting (E), and densitometric analysis (F) of α-ENaC, β-ENaC, γ-ENaC, ROMK, α-BK, and α-NKA protein expression in the renal cortex of normal rats treated with NK, HK, or HK + PRO20. Expression was normalized by β-actin. n = 5 per group. Data are expressed as means ± SE. *P < 0.05 vs. NK, ***P < 0.001 vs. NK, ##P < 0.01 vs. HK, and ###P < 0.001 vs. HK.
Fig. 5.
NCC expression in the renal cortex of normal rats treated with vehicle, HK, or HK + PRO20. Representative immunoblotting (A) and densitometric analysis (B) of NCC protein; expression was normalized by β-actin. C: qRT-PCR analysis of NCC mRNA; mRNA expression was normalized by GAPDH. D: representative immunofluorescence images of NCC in DCT of normal rats treated with vehicle, HK, or HK + PRO20, tubules are identified by costaining for calbindin D28K and AQP2. DCT, distal convoluted tubules; CD, collecting duct. Data are expressed as means ± SE.
In Vivo Experiments Using Adrenalectomized Rats With Intrarenal PRO20 Infusion
To assess the direct role of PRR in K+ homeostasis, in the absence of adrenal derived aldosterone, we examined the effect of PRO20 in adrenalectomized rats. PRO20 was given via a catheter chronically placed in the left kidney. To prevent the compensatory response, the right kidney was removed. As shown in Table 3, ADX rats fed the HK diet lost more weight and also exhibited polydipsia, polyuria, but hypertonic urine, as compared with the NK group.
Table 3.
General physiological data in adrenalectomized rats treated by intrarenal PRO20 infusion
| NK | HK | HK + PRO20 | |
|---|---|---|---|
| ΔBody weight, g | −47.0 ± 6.0 | −68.4 ± 9.1* | −71.1 ± 6.4* |
| Food intake, g/24 h | 16.0 ± 1.8 | 12.2 ± 1.9* | 12.4 ± 1.9* |
| Water intake, ml/24 h | 52.6 ± 3.4 | 78.8 ± 13.2* | 94.3 ± 9.8** |
| Urine volume, ml/24 h | 49.7 ± 7.8 | 67.4 ± 8.4* | 73.7 ± 7.1* |
| Urine osmolality, mosmol/kg⋅H2O | 723.8 ± 77.3 | 1035.5 ± 94.4* | 939.6 ± 20.1* |
| Plasma osmolality, mosmol/kg⋅H2O | 309.0 ± 5.8 | 310.3 ± 3.8 | 309.4 ± 2.1 |
Values are expressed as means ± SE.
P < 0.05 vs. NK.
P < 0.01 vs. NK.
In ADX rats, HK intake increased renal fPRR protein expression (Fig. 6, A–C) but not PRR mRNA expression (Fig. 6D) in the renal cortex and inner medulla. This treatment induced a remarkable increase in urinary sPRR secretion (Fig. 6E) and plasma sPRR concentration (Fig. 6F), all of which were unaffected by intrarenal PRO20 infusion. HK intake also increased urinary renin activity (Fig. 7A), active renin concentration (Fig. 7B), and total renin concentration (Fig. 7C), all of which were completely blocked by intrarenal PRO20 infusion. In contrast, HK treatment unchanged plasma renin activity (Fig. 7D) and decreased plasma total renin concentration (Fig. 7F), with a modest elevation of plasma active renin concentration (Fig. 7E). Importantly, none of the plasma renin parameters was affected by intrarenal PRO20 infusion.
Fig. 6.
Upregulation of renal PRR expression in K+-loaded ADX rats. ADX rats were fed a NK or HK diet. Representative immunoblotting of PRR in the renal cortex (A) and inner medulla (B). C: densitometric analysis of PRR. Expression was normalized by β-actin; n = 4 per group. D: qRT-PCR analysis of PRR mRNA in the renal cortex and inner medulla; expression was normalized by GAPDH. E: urinary sPRR excretion. F: plasma sPRR concentration. NK: n = 4; HK: n = 4; HK+PRO20: n = 5. Data are expressed as means ± SE. *P < 0.05 vs. NK, **P < 0.01 vs. NK, and ***P < 0.001 vs. NK.
Fig. 7.
Effect of PRO20 on urinary and plasma renin levels in K+-loaded ADX rats. ADX rats were randomly divided into the following three groups: NK, HK, or HK + PRO20. A: urinary renin activity. B: urinary active renin content. C: urinary total renin content. D: plasma renin activity. E: plasma active renin concentration. F: plasma total renin concentration. NK: n = 4; HK: n = 4; HK+PRO20: n = 5. Data are expressed as means ± SE.
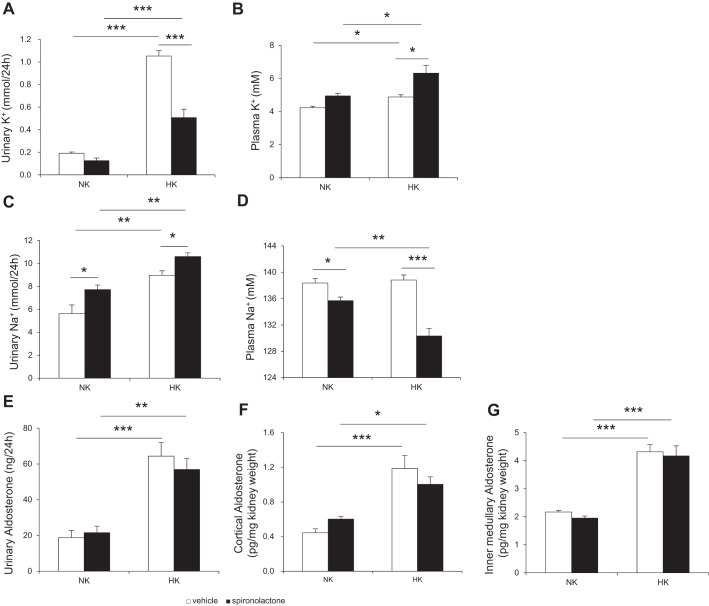
To test the possibility that PRR may promote K+ excretion via release of aldosterone, we detected renal CYP11B2 expression by immunoblotting and performed both ELISA and LC-MS/MS assays to measure urinary aldosterone in these animals. We found intrarenal PRO20 infusion attenuated the upregulation of CYP11B2 protein (Fig. 8, A–C) and mRNA (Fig. 8D). By ELISA, HK intake significantly promoted urinary aldosterone excretion (Fig. 8E) and intrarenal aldosterone level in renal cortex (Fig. 8F) and the inner medulla (Fig. 8G), which were suppressed by intrarenal PRO20 infusion, but plasma aldosterone concentration (Fig. 8H) was extremely low in all ADX rats, as previously reported (22, 75), and remained unchanged by either HK loading or PRO20. Similarly, urinary free (Fig. 8I) and total (Fig. 8J) aldosterone, as detected by LC-MS/MS method, were increased by HK intake, and this increase was abolished by intrarenal PRO20 infusion. Intrarenal PRO20 infusion in ADX rats further increased plasma K+ following HK intake, accompanied with attenuated UKV and UNaV, contrasting to unchanged plasma Na+ concentration (Fig. 9). HK-induced upregulation of β-ENaC, f-γ-ENaC, cleaved-γ-ENaC, ROMK, α-BK, and α-NKA protein expression was also attenuated (Fig. 9, E and F).
Fig. 8.
Responses in renal CYP11B2 expression and aldosterone excretion to HK intake in ADX rats treated with or without PRO20. Representative immunoblotting of CYP11B2 in the renal cortex (A) and inner medulla (B). C: densitometric analysis of CYP11B2 protein (IM, inner medulla); expression was normalized by β-actin. D: qRT-PCR analysis of CYP11B2 mRNA in the renal cortex and inner medulla; expression was normalized by GAPDH. E: measurement of urinary aldosterone by ELISA. F: measurement of renal cortex aldosterone by ELISA. G: measurement of renal inner medullary aldosterone by ELISA. H: measurement of plasma aldosterone by ELISA; n = 13 per group. I: measurement of urinary free aldosterone by LC-MS/MS. J: measurement of urinary total aldosterone by LC-MS/MS. NK: n = 4; HK: n = 4; HK+PRO20: n = 5. Data are expressed as means ± SE. *P < 0.05 vs. NK, **P < 0.01 vs. NK, ***P < 0.001 vs. NK, #P < 0.05 vs. HK, ##P < 0.01 vs. HK, and ###P < 0.001 vs. HK.
Fig. 9.
Urinary K+ (A), plasma K+ (B), urinary Na+ (C), plasma Na+ (D), and immunoblotting (E), and densitometric analysis (F) of α-ENaC, β-ENaC, γ-ENaC, ROMK, α-BK, and α-NKA in the renal cortex in K+-loaded ADX rats treated with or without PRO20. Expression was normalized by β-actin; n = 4 per group. Data are expressed as means ± SE *P < 0.05 vs. NK, **P < 0.01 vs. NK, ***P < 0.001 vs. NK, #P < 0.05 vs. HK, ##P < 0.01 vs. HK, and ###P < 0.001 vs. HK.
In Vivo Experiments Using Adrenalectomized Rats With Spironolactone Treatment
To further confirm the role of intrarenal aldosterone in K+ homeostasis, we examined the effect of mineralocorticoid receptor antagonist spironolactone in ADX rats. Spironolactone was given in the drinking water. As shown in Table 4, in HK-fed ADX rats treated with spironolactone, 24-h urine volume was slightly increased with lower urine osmolality, while NK-fed ADX rats treated with spironolactone gained more body weight and had decreased 24-h urine volume and increased urine osmolality, as compared with the vehicle group.
Table 4.
General physiological data in ADX rats treated by spironolactone
| Vehicle |
Spironolactone |
|||
|---|---|---|---|---|
| NK | HK | NK | HK | |
| ΔBody weight, g | −15.6 ± 2.5 | −38.4 ± 3.0** | 8.2 ± 3.0### | −43.2 ± 5.5*** |
| Food intake, g/24 h | 16.2 ± 1.2 | 12.5 ± 0.8* | 17.1 ± 1.0 | 12.2 ± 1.2* |
| Water intake, ml/24 h | 42.4 ± 7.5 | 61.3 ± 1.4* | 39.4 ± 5.4 | 65.6 ± 3.8* |
| Urine volume, ml/24 h | 34.0 ± 1.7 | 46.2 ± 2.2* | 27.5 ± 1.7# | 53.8 ± 2.1* |
| Urine osmolality, mosmol/kg⋅H2O | 1027.6 ± 59.1 | 1298.4 ± 76.0* | 1485.2 ± 148.3# | 1097.8 ± 55.6* |
| Plasma osmolality, mosmol/kg⋅H2O | 307.2 ± 2.9 | 311.0 ± 2.3 | 309.6 ± 2.9 | 312.0 ± 1.1 |
Values are expressed as means ± SE.
P < 0.05 vs. NK.
P < 0.01 vs. NK.
P < 0.001 vs. NK.
P < 0.05 vs. Vehicle.
P < 0.001 vs. Vehicle.
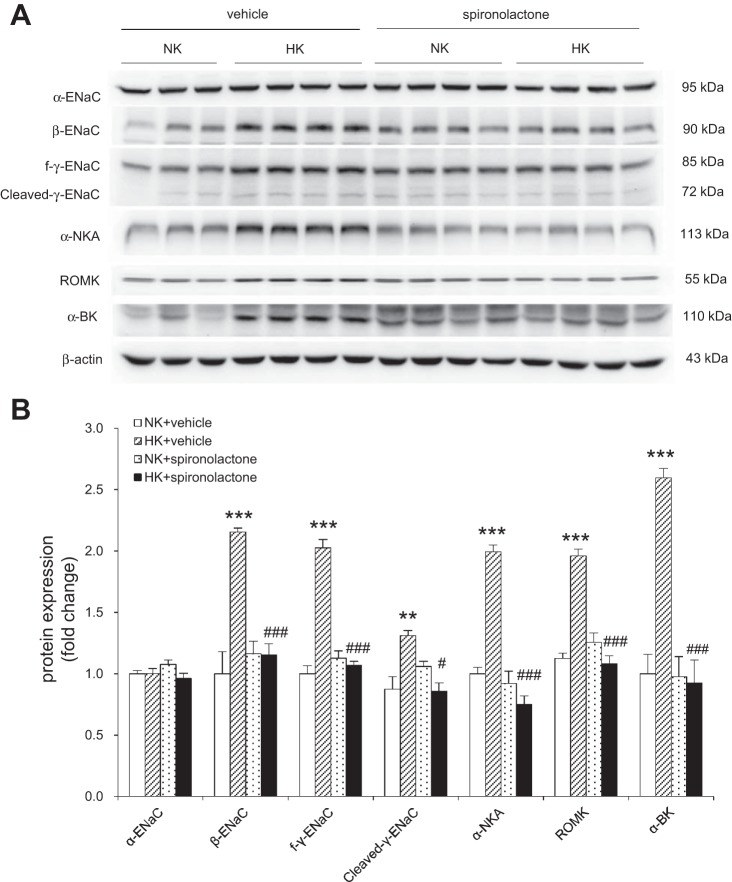
In ADX rats, HK intake increased plasma K+, UKV, and UNaV, and spironolactone treatment further increased plasma K+ and UNaV, but decreased plasma Na+ and UKV, in both NK and HK group rats (Fig. 10, A–D). In the ELISA, plasma aldosterone was barely detectable in all ADX rats, as described above, whereas urinary aldosterone was significantly increased by HK intake which was unaffected by spironolactone (Fig. 10E), as well as intrarenal aldosterone level in renal cortex (Fig. 10F) and inner medulla (Fig. 10G). HK-induced upregulation of β-ENaC, f-γ-ENaC, cleaved-γ-ENaC, α-NKA, ROMK, and α-BK protein expression were also attenuated by spironolactone treatment (Fig. 11).
Fig. 10.
Urinary K+ (A), plasma K+ (B), urinary Na+ (C), plasma Na+ (D), urinary aldosterone (E), renal cortex aldosterone (F), and renal inner medullary aldosterone (G) in K+-loaded ADX rats treated with or without spironolactone. n = 5 per group. Data are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 11.
Immunoblotting (A) and densitometric analysis (B) of α-ENaC, β-ENaC, γ-ENaC, ROMK, α-BK, and α-NKA in the renal cortex in K+-loaded ADX rats treated with or without spironolactone. Expression was normalized by β-actin. n = 5 per group. Data are expressed as means ± SE. **P < 0.01 vs. NK + vehicle, ***P < 0.001 vs. NK + vehicle, #P < 0.05 vs. HK + spironolactone, and ###P < 0.001 vs. HK + spironolactone.
DISCUSSION
In the present study, we investigated the role of renal PRR in K+ homeostasis and further explored the underlying mechanism with emphasis on analysis of intrarenal RAAS and distal nephron Na+ and K+ transporters/channels. In normal rat experiments, HK intake elevated renal PRR protein expression and urinary sPRR excretion, in parallel with increased urinary aldosterone and renal CYP11B2 expression, as well as increased intrarenal renin and aldosterone levels. PRR antagonism with PRO20 exacerbated hyperkalemia following HK intake associated with a restoration of NCC expression and a reduction of ENaC, ROMK, BK, and NKA expression. Most of these effects of PRO20 persisted after adrenalectomy. These results suggest a novel role of PRR in promoting K+ excretion during HK intake, likely through activation of a local RAAS and subsequent stimulation of K+ -secreting machinery in the distal nephron.
The present study provides in vivo evidence that HK upregulates renal PRR expression. To rule out the influence of adrenal derived steroids, the in vivo studies were conducted in both normal and ADX rats. In line with our previous results in cultured renal cells, immunoblotting consistently demonstrated increased renal fPRR expression following HK intake, irrespective of adrenal glands. Renal fPRR expression correlated well with urinary sPRR and renin levels but not plasma sPRR. PRR is cleaved by furin or ADAM19 to generate sPRR (human, residues 17–274; rat and mouse, residues 18–275) that is secreted into the extracellular space and is ultimately found in blood (11, 77). Although plasma sPRR concentration is elevated in heart failure (18), diabetes (10, 30, 70), chronic kidney disease (25), gestation (70), hypertension (24, 51, 69), primary aldosteronism (49), and Gitelman syndrome (49), it is independent of plasma renin, prorenin, and aldosterone concentrations (34, 49). We suspect that urinary sPRR may be of renal origin, and its increase predicts overactivation of intrarenal RAS, whereas plasma sPRR may derive from other sources in addition to the kidney.
Aldosterone is thought to derive primarily from the zona glomerulosa of the adrenal cortex and to play an important role in regulating electrolyte homeostasis, particularly promoting Na+ reabsorption and K+ secretion (3, 6). Studies have found that local renal aldosterone system was regulated by salt, diabetes, and ANG II type 1 receptor (75). Diabetes increased renal aldosterone release and CYP11B2 expression, which contributes to the development of renal inflammation (55, 75). However, the renal source of aldosterone and its role in K+ homeostasis have not well been established. In the present study, we showed that both plasma and urinary aldosterone was significantly increased in 5% K+-fed normal rats, which was inhibited by PRO20. Moreover, PRO20 decreased HK-induced urinary and intrarenal aldosterone levels in ADX rats. Therefore, the use of ADX rats has ruled out adrenal glands as a source of aldosterone in the current experimental model.
Further evidence for the involvement of renal-derived aldosterone came from the study of renal CYP11B2 regulation. CYP11B2 is a rate-limiting enzyme for aldosterone synthesis (31) and has been found in tissues other than the adrenal gland, such as blood vessels (76), heart (23, 32), brain (22, 41), and kidney (55, 75). Here, our data confirmed the expression, functional, and regulatory role of CYP11B2 in the kidney. We found that HK intake remarkably induced renal CYP11B2 expression in both normal and ADX rats. The upregulation of CYP11B2 by HK was consistently blocked by PRO20. These findings suggest CYP11B2 as an enzymatic source for PRR-dependent intrarenal generation of aldosterone.
Na+-dependent K+ secretion in the CD appears to require coordinated action of NCC and ENaC, which determines the CD Na+ reabsorption rate, the driving force of K+ secretion (9, 33). In response to HK intake, NCC expression and activity were remarkably downregulated, allowing increased Na+ delivery to the CD for K+ secretion, which explains the observed K+-induced natriuresis (9, 17, 60, 61). In the present study, we showed that the PRR antagonism attenuated HK-induced downregulation of NCC protein and mRNA expression and the excretion of Na+. Indeed, PRR is abundantly expressed in the DCT (1). However, the mechanism of how PRR regulates NCC expression during HK intake is unclear. Because aldosterone exerts a stimulatory effect on NCC expression (50) and activity (34), it appears likely that PRR-mediated NCC downregulation is through an aldosterone-independent mechanism. This notion is supported by the observation that HK-induced natriuresis is blunted by PRO20 but enhanced by spironolactone, despite the similar attenuation of HK-induced kaliuresis.
Renal K+ excretion is dependent on K+ secretion in the distal nephron because the filtered K+ is almost completely reabsorbed in the proximal convoluted tubule (2). In the CD, Na+ reabsorption through ENaC generates a lumen-negative potential that serves as the driving force for K+ secretion (78). In CD, ENaC is directly stimulated by aldosterone (10, 47). As previously described (61), we found increased expression of β-ENaC, f-γ-ENaC, and cleaved-γ-ENaC in HK rats, which was blunted by PRO20 treatment, in parallel with the changes in aldosterone level. Spironolactone treatment also inhibited HK-induced β-ENaC, f-γ-ENaC, and cleaved-γ-ENaC protein expression. These results suggest PRR mediation of aldosterone-dependent stimulation of ENaC expression. However, a possibility exists that PRR may regulate ENaC independently of aldosterone. In fact, we recently found that activation of PRR by prorenin elevates ENaC activity in cultured mpkCCD cells within minutes (38), an acute model where aldosterone is not expected to play a role (16). This notion is in line with the observation arguing against a major role of aldosterone in regulation of K+ hemostasis (59). Additionally, HK intake stimulates renin activity that leads to the release of ANG II that increases ENaC activity independently and additively to aldosterone.
The ROMK and BK channels mediate the downhill movement of K+ across the apical membrane that is driven by high intracellular K+ concentration generated by the Na+ pump on the basolateral membrane. Therefore, we studied the effect of HK intake on the expression of the ROMK, BK, and NKA. Aldosterone and K+ regulate the abundance of ROMK, BK, and NKA (61, 63, 64, 73), but low Na+ or high Na+-induced changes of circulating aldosterone levels does not contribute to the regulation of BK channel expression/activity in mammalian cortical CDs (13). Renal NKA activity is suppressed by adrenalectomy and restored by administration of mineralocorti-coids (64). These results are in line with our present observations in both normal and ADX rats, in which the expression of renal ROMK, BK, and NKA was remarkably increased in HK intake and blunted by PRO20 treatment, in parallel with the changes in urinary aldosterone level. Moreover, spironolactone inhibited HK-induced ROMK, BK, and NKA protein expression in ADX rats, supporting a role of intrarenal generation of aldosterone. Of note, Wei et al. (71) reported ANG II significantly increased ROMK channel activity in CD in response to high dietary K+ intake, while in normal dietary K+ diet, ANG II is shown to inhibit or stimulate ROMK activity, an effect that is dependent on the dose (29, 72).
The central role of intrarenal aldosterone in regulation of K+ response, as discussed above, disagree with the previous reports showing independence of NCC and ROMK regulation on aldosterone (58, 59). Terker et al. (58) report that NCC activity is reduced in nephron-specific MR KO mice under basal condition, but NCC expression is still stimulated by a low-K+ diet. The assumption is that the inhibitory effect of a HK diet on NCC expression will be unaffected in the null mice, but this needs to be experimentally tested. On the other hand, Todkar et al. (59) observed a largely negative phenotype in aldosterone synthase (AS) KO mice during HK loading. This is in contrast to severe salt wasting, hypotension, and hyperkalemia in nephron MR KO mice. The quite remarkable difference in the severity of the phenotype between the two null mice may suggest that AS may not be the only enzymatic source of aldosterone. Another consideration is that since adrenalectomy is not performed in either one of these studies, they are unable to discriminate the roles of adrenal vs. extra-adrenal generation of aldosterone. Our study design is unique in that we used both normal and ADX animals. Overall, despite the lack of consensus on the role of aldosterone in regulation of K+ homeostasis, the present study helps set up a new stage for reevaluation of this issue by focusing on PRR-mediated intrarenal aldosterone production.
During the past decade, there has been a paradigm shift in our understanding about the role of local vs. systemic RAS in blood pressure control. In particular, abundant evidence has established an essential role of intrarenal RAS in pathogenesis of ANG II-induced hypertension (79). Following ANG II infusion, urinary and renal medullary renin is upregulated, whereas plasma and renal cortical renin are suppressed, clearly dissociating the local and systemic RAS (67, 68). In normal or ADX rats, HK intake induced robust and consistent increases in renin activity and active and total renin content in the urine, which were all blunted by PRO20 treatment. In contrast, the changes in the renin parameters in the plasma were relatively inconsistent. More importantly, none of the plasma renin parameters were affected by PRO20 treatment. These results suggest activation of intrarenal RAS during HK intake. Of note, PRO20 treatment not only suppressed renin activity but also reduced renin content, indicating a possibility that PRR may regulate prorenin/renin stability or expression beyond their activity. Similarly, HK intake induced a significant increase in urinary aldosterone level, and this increase was abolished by PRO20 treatment, contrasting to unchanged plasma aldosterone concentration. Interestingly, this phenomenon persisted after adrenalectomy, supporting renal generation of aldosterone. Aldosterone was initially measured by ELISA and subsequently validated by LC-MS/MS. Although the absolute aldosterone values were variable with the two assays, the pattern of changes in aldosterone levels was quite consistent. We provided further evidence for CYP11B2 as a potential source of PRR-dependent generation of aldosterone in renal cells. Overall, these results represent compelling evidence for PRR-dependent activation of intrarenal RAAS during HK intake.
PRO20, a specific antagonist of PRR with 10 amino acids longer than HRP, contains all PRR binding sites in prorenin, and its NH2 terminus is in close proximity to PRR binding domain (S149QGVLKEDVF158) in prorenin (35). Li et al. (35) reported PRO20 can specifically bind PRR, completely block prorenin-induced phosphorylation of ERK1/2, and attenuate prorenin- or deoxycorticosterone acetate-salt-induced hypertension. In addition, our group, employing PRO20, has demonstrated a positive association between PRR and the local RAS in the kidney, as well as a positive role of renal PRR in ANG II-induced hypertension (66) and in the regulation of urine-concentrating capability (65). In the present study, we employed PRO20 to demonstrate an important role of PRR in regulation of K+ homeostasis during HK loading. Although these results favor PRO20 as an effective and specific inhibitor of PRR, a full evaluation of this peptide inhibitor needs to be conducted in the future studies. As a decoy inhibitor disrupting the binding between the prosegment of prorenin and PRR, PRO20 is expected to bind the extracellular domain of PRR, e.g., the full-length or soluble form, but this has to be experimentally validated. Moreover, future studies, particularly those using PRR knockout mice, will be necessary to fully address the pharmacological actions of PRO20.
In summary, the present study provides evidence that PRR functions as a kaliuretic factor during HK intake. The mechanism of the kaliuretic action of PRR involves coordinated regulation of NCC, ENaC, ROMK, BK, and NKA in a way to promote K+ secretion. This process is, at least in part, dependent on activation of the local RAAS. Overall, these results contribute to the identification of a novel PRR-dependent local mechanism that appears important for K+ homeostasis.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 91439205 and 31330037, National Basic Research Program of China (973 Program) 2012CB517600 (no. 2012CB517602), Veterans Affairs Merit Review, and National Institutes of Health Grant DK-094956.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.X., A.L., H.W., H.F., L.Z., and P.S. performed experiments; C.X., A.L., H.W., L.Z., and T.Y. analyzed data; C.X. and T.Y. interpreted results of experiments; C.X. prepared figures; C.X. and T.Y. drafted manuscript; T.Y. conception and design of research; T.Y. edited and revised manuscript; T.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
T. Yang is an Established Investigator from the American Heart Association and a Research Career Scientist in the Department of Veterans Affairs.
REFERENCES
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Alpern RJ, Cogan MG, Rector FC Jr. Flow dependence of proximal tubular bicarbonate absorption. Am J Physiol 245: F478–F484, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26: 115–123, 2011. doi: 10.1152/physiol.00049.2010. [DOI] [PubMed] [Google Scholar]
- 4.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burcklé CA, Müller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens 25: 2441–2453, 2007. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 5.Batenburg WW, van den Heuvel M, van Esch JH, van Veghel R, Garrelds IM, Leijten F, Danser AH. The (pro)renin receptor blocker handle region peptide upregulates endothelium-derived contractile factors in aliskiren-treated diabetic transgenic (mREN2)27 rats. J Hypertens 31: 292–302, 2013. doi: 10.1097/HJH.0b013e32835c1789. [DOI] [PubMed] [Google Scholar]
- 6.Berl T, Katz FH, Henrich WL, de Torrente A, Schrier RW. Role of aldosterone in the control of sodium excretion in patients with advanced chronic renal failure. Kidney Int 14: 228–235, 1978. doi: 10.1038/ki.1978.114. [DOI] [PubMed] [Google Scholar]
- 7.Burcklé CA, Jan Danser AH, Müller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Nussberger J, Stowasser M, Danser AH, Morganti A, Frandsen E, Ménard J. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem 55: 867–877, 2009. doi: 10.1373/clinchem.2008.118000. [DOI] [PubMed] [Google Scholar]
- 9.Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K+ intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol 306: F1507–F1519, 2014. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Zhang X, Zhang W. Regulation of αENaC transcription. Vitam Horm 98: 101–135, 2015. doi: 10.1016/bs.vh.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 12.Daryadel A, Bourgeois S, Figueiredo MF, Gomes Moreira A, Kampik NB, Oberli L, Mohebbi N, Lu X, Meima ME, Danser AH, Wagner CA. Colocalization of the (Pro)renin receptor/Atp6ap2 with H+-ATPases in mouse kidney but prorenin does not acutely regulate Intercalated cell H+-ATPase activity. PLoS One 11: e0147831, 2016. doi: 10.1371/journal.pone.0147831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. doi: 10.1152/ajprenal.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51: 682–688, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 15.Foster ES, Jones WJ, Hayslett JP, Binder HJ. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology 88: 41–46, 1985. doi: 10.1016/S0016-5085(85)80130-X. [DOI] [PubMed] [Google Scholar]
- 16.Frindt G, Palmer LG. Acute effects of aldosterone on the epithelial Na channel in rat kidney. Am J Physiol Renal Physiol 308: F572–F578, 2015. doi: 10.1152/ajprenal.00585.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010. doi: 10.1152/ajprenal.00323.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 168: 4313–4314, 2013. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 19.Fushimi K, Uchida S, Harat Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 20.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Giebisch G, Krapf R, Wagner C. Renal and extrarenal regulation of potassium. Kidney Int 72: 397–410, 2007. doi: 10.1038/sj.ki.5002288. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab 288: E342–E346, 2005. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of aldosterone in the rat heart. Endocrinology 145: 4796–4802, 2004. doi: 10.1210/en.2004-0295. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848–856, 2013. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Siragy HM. Sodium depletion enhances renal expression of (pro)renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension 59: 317–323, 2012. doi: 10.1161/HYPERTENSIONAHA.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 47: 894–900, 2006. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- 29.Javkhedkar AA, Lokhandwala MF, Banday AA. Defective nitric oxide production impairs angiotensin II-induced Na-K-ATPase regulation in spontaneously hypertensive rats. Am J Physiol Renal Physiol 302: F47–F51, 2012. doi: 10.1152/ajprenal.00270.2011. [DOI] [PubMed] [Google Scholar]
- 30.Kanda A, Noda K, Saito W, Ishida S. (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia 55: 3104–3113, 2012. doi: 10.1007/s00125-012-2702-2. [DOI] [PubMed] [Google Scholar]
- 31.Kater CE, Biglieri EG, Rost CR, Schambelan M, Hirai J, Chang BC, Brust N. The constant plasma 18-hydroxycorticosterone to aldosterone ratio: an expression of the efficacy of corticosterone methyloxidase type II activity in disorders with variable aldosterone production. J Clin Endocrinol Metab 60: 225–228, 1985. doi: 10.1210/jcem-60-2-225. [DOI] [PubMed] [Google Scholar]
- 32.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 85: 2519–2525, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko B, Mistry AC, Hanson L, Mallick R, Wynne BM, Thai TL, Bailey JL, Klein JD, Hoover RS. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol 305: F645–F652, 2013. doi: 10.1152/ajprenal.00053.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 37.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 41.MacKenzie SM, Clark CJ, Fraser R, Gómez-Sánchez CE, Connell JM, Davies E. Expression of 11β-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol 24: 321–328, 2000. doi: 10.1677/jme.0.0240321. [DOI] [PubMed] [Google Scholar]
- 42.Matavelli LC, Huang J, Siragy HM. In vivo regulation of renal expression of (pro)renin receptor by a low-sodium diet. Am J Physiol Renal Physiol 303: F1652–F1657, 2012. doi: 10.1152/ajprenal.00204.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonough AA, Veiras LC, Minas JN, Ralph DL. Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015. doi: 10.1152/ajpcell.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation 109: 1933–1937, 2004. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- 45.Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension 51: 676–681, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- 46.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006. doi: 10.3892/ijmm.18.3.483. [DOI] [PubMed] [Google Scholar]
- 47.Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol 303: F1289–F1299, 2012. doi: 10.1152/ajprenal.00247.2012. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen G, Blanchard A, Curis E, Bergerot D, Chambon Y, Hirose T, Caumont-Prim A, Tabard SB, Baron S, Frank M, Totsune K, Azizi M. Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension 63: 297–302, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02217. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol 283: F923–F933, 2002. doi: 10.1152/ajprenal.00015.2002. [DOI] [PubMed] [Google Scholar]
- 51.Oshima Y, Morimoto S, Ichihara A. Roles of the (pro)renin receptor in the kidney. World J Nephrol 3: 302–307, 2014. doi: 10.5527/wjn.v3.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E2. Am J Physiol Renal Physiol 287: F152–F159, 2004. doi: 10.1152/ajprenal.00287.2003. [DOI] [PubMed] [Google Scholar]
- 53.Raff H, Ball DL, Goodfriend TL. Low oxygen selectively inhibits aldosterone secretion from bovine adrenocortical cells in vitro. Am J Physiol Endocrinol Metab 256: E640–E644, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Rafiq K, Nakano D, Ihara G, Hitomi H, Fujisawa Y, Ohashi N, Kobori H, Nagai Y, Kiyomoto H, Kohno M, Nishiyama A. Effects of mineralocorticoid receptor blockade on glucocorticoid-induced renal injury in adrenalectomized rats. J Hypertens 29: 290–298, 2011. doi: 10.1097/HJH.0b013e32834103a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol 93: 817–824, 2008. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki-Nakagawa C, Nishimura M, Noda M, Iwata H, Hattori M, Ebihara A, Suzuki F, Nakagawa T. Intracellular retention of the extracellular domain of the (pro)renin receptor in mammalian cells. Biosci Biotechnol Biochem 78: 1187–1190, 2014. doi: 10.1080/09168451.2014.915732. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki F, Nakagawa T, Kakidachi H, Murakami K, Inagami T, Nakamura Y. The dominant role of the prosegment of prorenin in determining the rate of activation by acid or trypsin: studies with molecular chimeras. Biochem Biophys Res Commun 267: 577–580, 2000. doi: 10.1006/bbrc.1999.1997. [DOI] [PubMed] [Google Scholar]
- 58.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH. Direct and Indirect Mineralocorticoid Effects Determine Distal Salt Transport. J Am Soc Nephrol 27: 2436–2445, 2016. doi: 10.1681/ASN.2015070815. doi: 10.1681/ASN.2015070815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015. doi: 10.1681/ASN.2013111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 62.van Esch JH, van Veghel R, Garrelds IM, Leijten F, Bouhuizen AM, Danser AH. Handle region peptide counteracts the beneficial effects of the renin inhibitor aliskiren in spontaneously hypertensive rats. Hypertension 57: 852–858, 2011. doi: 10.1161/HYPERTENSIONAHA.110.169060. [DOI] [PubMed] [Google Scholar]
- 63.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Phsyiol 275: F239–F245, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Wald H, Scherzer P, Popovtzer MM. Parallel changes in red blood cell and renal Na-K-ATPase activity in adrenal and electrolyte disorders in the rat. Pflügers Arch 404: 56–60, 1985. doi: 10.1007/BF00581491. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic ation of collecting duct (pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhu SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol 307: F25–F32, 2014. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A. Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension 60: 1250–1256, 2012. doi: 10.1161/HYPERTENSIONAHA.112.197418. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Ando T, Kimura T, Sago H, Ichihara A. Association between soluble (Pro)renin receptor concentration in cord blood and small for gestational age birth: a cross-sectional study. PLoS One 8: e60036, 2013. doi: 10.1371/journal.pone.0060036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K+ channel activity in the renal cortical collecting duct during high dietary K+ adaptation. Am J Physiol Renal Physiol 307: F833–F843, 2014. doi: 10.1152/ajprenal.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013. doi: 10.1152/ajprenal.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest 70: 579–586, 1982. doi: 10.1172/JCI110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46: 584–590, 2005. doi: 10.1161/01.HYP.0000175814.18550.c0. [DOI] [PubMed] [Google Scholar]
- 76.Takeda Y. Vascular synthesis of aldosterone: role in hypertension. Mol Cell Endocrinol 217: 75–79, 2004. doi: 10.1016/j.mce.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 34: 599–605, 2011. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 78.Zaika O, Mamenko M, Boukelmoune N, Pochynyuk O. IGF-1 and insulin exert opposite actions on ClC-K2 activity in the cortical collecting ducts. Am J Physiol Renal Physiol 308: F39–F48, 2015. doi: 10.1152/ajprenal.00545.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal renin-angiotensin system: a critical review of classical and new paradigms. Front Endocrinol (Lausanne) 4: 166, 2013. doi: 10.3389/fendo.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]