Abstract
Alterations in extracellular fluid volume regulation and sodium balance may result in the development and maintenance of salt-dependent hypertension, a major risk factor for cardiovascular disease. Numerous pathways contribute to the regulation of sodium excretion and blood pressure, including endothelin and purinergic signaling. Increasing evidence suggests a link between purinergic receptor activation and endothelin production within the renal collecting duct as a means of promoting natriuresis. A better understanding of the relationship between these two systems, especially in regard to sodium homeostasis, will fill a significant knowledge gap and may provide novel antihypertensive treatment options. Therefore, this review focuses on the cross talk between endothelin and purinergic signaling as it relates to the renal regulation of sodium and blood pressure homeostasis.
Keywords: Sodium, blood pressure, natriuresis, kidney
since its discovery in 1988, the potent vasoactive peptide endothelin-1 (ET-1) has emerged as a fundamental regulator of sodium homeostasis and blood pressure (17). It accomplishes these physiological actions through two distinct receptors, ETA and ETB. Functional distribution of these receptors throughout the body determines the ultimate hemodynamic and renal tubular effects of ET-1 (25). ETA receptors are primarily located on vascular smooth muscle cells and are responsible for the vasoconstrictor properties of ET-1. Activation of ETA receptors is associated with renal hypertrophy, fibrosis, inflammation, and proteinuria (7, 9, 28, 29). ETB receptors are located primarily on endothelial cells and collecting duct epithelial cells (17). Activation of ETB receptors induces endothelium-dependent vasodilation in the vasculature and inhibits sodium and water reabsorption in the distal nephron (17). Increased renal tubular flow in response to high dietary sodium promotes inner medullary release of ET-1. This, in turn, facilitates sodium excretion mainly through ETB receptor-mediated inhibition of epithelial sodium channel activity (ENaC) (4, 17, 30).
In recent decades, it has become clear that purinergic signaling is also important in regulating sodium balance and blood pressure (19, 32). ATP-binding purinoceptors (P2 receptors) are widely distributed in epithelial cells and vascular elements of the kidney (20). P2 receptors are classified as ionotropic P2X (P2X1–7) or G protein-coupled P2Y (P2Y1,2,4,6,11–14) based on their structure and signaling systems (5, 19). Autocrine or paracrine transduction of extracellular nucleotide signaling via P2 receptors is associated with a number of renal processes, which include tubular transport function. Specifically, increased sodium intake enhances luminal release of ATP, which activates P2 receptors and lowers the open probability of ENaC resulting in increased sodium excretion (33).
Therefore, renal P2 and ET receptors are important regulators of tubular sodium transport and extracellular fluid volume, key components in the long-term control of blood pressure. Genetic loss of either P2Y2 or ETB receptors has been implicated in the loss of blood pressure regulation in association with alterations in sodium and water handling (13, 24, 26, 31). Despite the similarities in the physiological effects of these two classes of receptors, the exact relation between these two systems in the kidney is not established yet. In this brief review, we discuss the potential link between renal purinergic and ET-1 signaling.
Purinergic Regulation of Renal Endothelin System
Recent evidence highlights a role for renal P2 receptors as regulators of ET-1 production. Pandit et al. (23) demonstrated that activation of P2 receptors, specifically P2Y2 and P2X7, increased ET-1 expression in inner medullary collecting duct cells. Our group has also shown that intramedullary infusion of the purinergic antagonist suramin attenuated ET-1-induced natriuresis, and combined blockade of ETA and ETB receptors inhibited the natriuretic response to medullary infusion of the P2Y2/4 agonist UTP (14). This implies a possible role for P2Y2 and P2Y4 in regulating the production or release of ET-1 within the renal medulla (14). Cha et al. (6) observed that pretreatment of outer medullary collecting tubules with ATP inhibited ET-1-induced increase in intracellular calcium concentration (6), suggesting that ATP might prevent calcium mobilization by ET-1 signaling. Additionally, a recent study examining the development of renal fibrosis in response to overexpression of CD39, a key purinergic enzyme in the hydrolysis of ATP, revealed that increased CD39 activity enhanced the expression of ET-1 after unilateral ureteric obstruction (27). These findings support the hypothesis that P2 receptors are up stream of the ET-1 signaling cascade. Thus, ATP exerts significant effects on ET-1, indicating that activation of P2 receptors via ATP may regulate ET-1 release/production, which is particularly relevant in hypertension (Fig. 1).
Fig. 1.
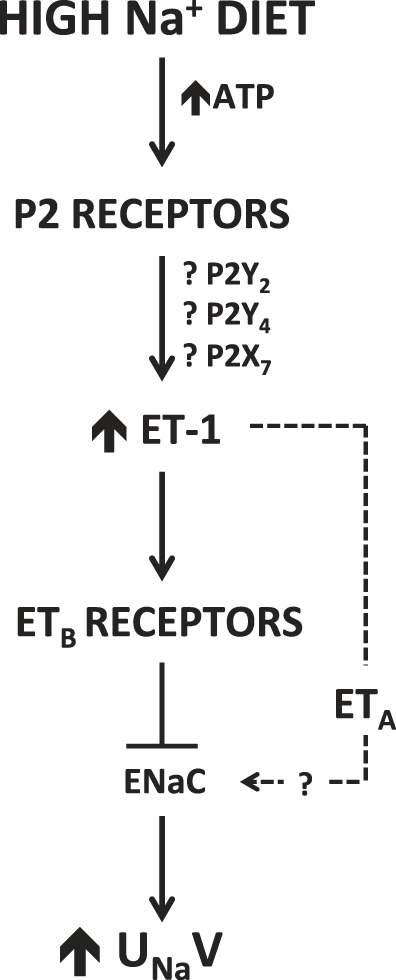
Proposed mechanistic relationship between P2 receptors and ET-1 signaling on renal sodium handling in response to high-salt intake. Dashed line reflects our previously published data demonstrating a role for the ETA receptor to increase urinary sodium excretion (UNaV) in female rats (22), but the relation to P2 activation has yet to be resolved.
Extrarenal Purinergic and Endothelin System Interactions
The potential interaction between P2 and ET-1 signaling in the kidney is supported by previous evidence demonstrating a common dependency between these two systems in extrarenal tissues including cardiovascular (1, 2, 10, 12, 15, 16, 34, 36), nervous (3, 8), gastrointestinal (11), reproductive (6, 18, 21), and visual systems (34). Specifically, some studies have demonstrated that P2 signaling regulates ET-1 (1, 6, 15, 16, 34). Others suggest that P2 signaling was enhanced by ET-1 (3, 10–12, 18). Opposing to these findings, one study has shown that activation of P2 receptors blocked the effects of ET-1 (36).
Cardiovascular system.
Horckmans et al. (15) observed that loss of P2Y2 receptors downregulated ET-1 expression and protected against myocardial infarction. Similarly, blockade of P2X2/3 receptors inhibited the effects of ET-1 in vascular endothelial cells (16). In pancreatic arteries, activation of P2Y14 induced vasoconstriction via elevation of intracellular calcium levels along with an increase in ET-1 production (1). Furthermore, P2 receptors may also be involved in the potentiating effects of ET-1 on the sympathetic response of cutaneous arteries (12). The purinergic antagonist PPADS prevented ET-1-induced intracellular calcium mobilization and partially attenuated ET-1-induced accumulation of total inositol trisphosphates in endothelial cells (34). These studies suggest that P2 and ET-1 pathways work cooperatively within the cardiovascular system. Nevertheless, ATP inhibited the cardiac hypertrophy induced by ET-1 in cardiac myocytes (36), suggesting that the interaction between purinergic and ET-1 signaling still remains inadequately defined.
Nervous system.
Barr et al. (3) showed that ET-1 increased neuronal sensitivity to ATP in sensory neurons, possibly due to sensitization of P2X4 receptors. This mechanism was independent of the ET-1-driven rise in intracellular calcium (3). On the other hand, ATP completely blocked subsequent ET-1-evoked calcium responses in astrocytes (8), whereas ET-1 failed to desensitize the increase in intracellular calcium elicited by ATP, suggesting a dominant role of P2 receptors in modulating calcium signal of astrocytes.
Gastrointestinal system.
In duodenal epithelial cells, ATP release was increased in subepithelial fibroblasts treated with ET-1 (11). Additionally, ET-1 recovered the suppression in ATP release in response to raising cAMP levels (11).
Male reproductive system.
ET-1 potentiated the contractility of vas deferens via postjunctional mechanisms, which are mainly due to purinergic contractile responses (21). Moreover, ET-1 potentiated vas deferens contraction evoked by ATP and α, β-methylene ATP (18). These studies highlight that ET-1-induced facilitation of sympathetic neurotransmission in the vas deferens is due to potentiation of the postjunctional effects of the cotransmitter ATP acting at P2X receptors. Data also show that this potentiating effect is observed with ET-1 but not ET-3, suggesting an ETA receptor-dependent mechanism (18).
Visual system.
Similar findings were reported in the visual system, where Vogler et al. (35) found that ET-1 inhibits osmotic swelling of rat retinal glial and bipolar cells by activation of growth factors and that this effect was associated with release of ATP and activation of P2Y1 receptor. Collectively, these findings suggest a potential cooperative interaction between P2 and ET-1 signaling in different physiological functions.
Perspectives
Extracellular ATP and ET-1 are autocrine/paracrine agents that play important roles in renal tubular reabsorption of sodium and water. Recent evidence suggests these two signaling pathways are interdependent in the tubular elements of the kidney. Better understanding of the complexity of the interplay between P2 receptors and ET-1 signaling will help to understand the mechanism of blood pressure control and explore the therapeutic potential of new drugs for hypertension.
GRANTS
This work was supported by a postdoctoral grant from the American Heart Association (15POST25090329 to E. Y. Gohar), American Society of Nephrology (Ben J. Lipps Research Fellowship Program, Joseph A. Carlucci Research Fellowship to M. Kasztan), and by a grant from the National Heart Lung and Blood Institute (P01 HL095499 to D. M. Pollock).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). E. Y. Gohar is also affiliated with Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Egypt.
AUTHOR CONTRIBUTIONS
E.Y.G. and M.K. prepared figures; E.Y.G. and M.K. drafted manuscript; E.Y.G., M.K., and D.M.P. edited and revised manuscript; E.Y.G., M.K., and D.M.P. approved final version of manuscript.
REFERENCES
- 1.Alsaqati M, Latif ML, Chan SL, Ralevic V. Novel vasocontractile role of the P2Y14 receptor: characterization of its signalling in porcine isolated pancreatic arteries. Br J Pharmacol 171: 701–713, 2014. doi: 10.1111/bph.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballew JR, Watts SW, Fink GD. Effects of salt intake and angiotensin II on vascular reactivity to endothelin-1. J Pharmacol Exp Ther 296: 345–350, 2001. [PubMed] [Google Scholar]
- 3.Barr TP, Hrnjic A, Khodorova A, Sprague JM, Strichartz GR. Sensitization of cutaneous neuronal purinergic receptors contributes to endothelin-1-induced mechanical hypersensitivity. Pain 155: 1091–1101, 2014. doi: 10.1016/j.pain.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36: 1127–1139, 1997. doi: 10.1016/S0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 6.Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Chen DD, Dong YG, Yuan H, Chen AF. Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension 59: 1037–1043, 2012. doi: 10.1161/HYPERTENSIONAHA.111.183368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho YJ, Lee SY, Kim YS, Lee EJ, Seo MS, Yeon G, Lee KH, Lee KJ, Jo YK, Rha HK. Adenosine triphosphate-induced heterologous desensitization of endothelin-1- and glutamate-evoked calcium increases in cultured rat cortical astrocytes. Neurosci Lett 286: 33–36, 2000. doi: 10.1016/S0304-3940(00)01073-9. [DOI] [PubMed] [Google Scholar]
- 9.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension 45: 283–287, 2005. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- 10.Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol 31: 1–8, 1998. doi: 10.1016/S0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 11.Furuya K, Sokabe M, Furuya S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: cell-shape-dependent ATP release and P2Y1 signaling. J Cell Sci 118: 3289–3304, 2005. doi: 10.1242/jcs.02453. [DOI] [PubMed] [Google Scholar]
- 12.García-Villalón AL, Padilla J, Monge L, Fernández N, Gómez B, Diéguez G. Role of the purinergic and noradrenergic components in the potentiation by endothelin-1 of the sympathetic contraction of the rabbit central ear artery during cooling. Br J Pharmacol 122: 172–178, 1997. doi: 10.1038/sj.bjp.0701359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gohar EY, Speed JS, Kasztan M, Jin C, Pollock DM. Activation of purinergic receptors (P2) in the renal medulla promotes endothelin-dependent natriuresis in male rats. Am J Physiol Renal Physiol 311: F260–F267, 2016. doi: 10.1152/ajprenal.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horckmans M, Esfahani H, Beauloye C, Clouet S, di Pietrantonio L, Robaye B, Balligand JL, Boeynaems JM, Dessy C, Communi D. Loss of mouse P2Y4 nucleotide receptor protects against myocardial infarction through endothelin-1 downregulation. J Immunol 194: 1874–1881, 2015. doi: 10.4049/jimmunol.1401364. [DOI] [PubMed] [Google Scholar]
- 16.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci 33: 2849–2859, 2013. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau WA, Ventura S, Jiang Q, Pennefather JN. Endothelin-induced facilitation of sympathetic neurotransmission to the rat vas deferens: effects of suramin. Eur J Pharmacol 272: 31–38, 1995. doi: 10.1016/0014-2999(94)00624-G. [DOI] [PubMed] [Google Scholar]
- 19.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiol (Oxf) 213: 232–241, 2015. doi: 10.1111/apha.12412. [DOI] [PubMed] [Google Scholar]
- 20.Mironova E, Boiko N, Bugaj V, Kucher V, Stockand JD. Regulation of Na+ excretion and arterial blood pressure by purinergic signalling intrinsic to the distal nephron: consequences and mechanisms. Acta Physiol (Oxf) 213: 213–221, 2015. doi: 10.1111/apha.12372. [DOI] [PubMed] [Google Scholar]
- 21.Mutafova-Yambolieva V, Radomirov R. Effects of endothelin-1 on postjunctionally-mediated purinergic and adrenergic components of rat vas deferens contractile responses. Neuropeptides 24: 35–42, 1993. doi: 10.1016/0143-4179(93)90038-C. [DOI] [PubMed] [Google Scholar]
- 22.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F541–F552, 2015. doi: 10.1152/ajprenal.00456.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertens 17: 70–75, 2008. doi: 10.1097/MNH.0b013e3282f34b02. [DOI] [PubMed] [Google Scholar]
- 26.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 27.Roberts V, Lu B, Chia J, Cowan PJ, Dwyer KM. CD39 overexpression does not attenuate renal fibrosis in the unilateral ureteric obstructive model of chronic kidney disease. Purinergic Signal 12: 653–660, 2016. doi: 10.1007/s11302-016-9528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54: 979–988, 2011. doi: 10.1007/s00125-010-2021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56: 942–949, 2010. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorokin A, Staruschenko A. Inhibition of ENaC by endothelin-1. Vitam Horm 98: 155–187, 2015. doi: 10.1016/bs.vh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension 41: 657–662, 2003. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 32.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallon V, Stockand J, Rieg T. P2Y receptors and kidney function. Wiley Interdiscip Rev Membr Transp Signal 1: 731–742, 2012. doi: 10.1002/wmts.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigne P, Pacaud P, Urbach V, Feolde E, Breittmayer JP, Frelin C. The effect of PPADS as an antagonist of inositol (1,4,5)trisphosphate induced intracellular calcium mobilization. Br J Pharmacol 119: 360–364, 1996. doi: 10.1111/j.1476-5381.1996.tb15994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogler S, Grosche A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Endothelins inhibit osmotic swelling of rat retinal glial and bipolar cells by activation of growth factor signaling. Neurochem Res 41: 2598–2606, 2016. doi: 10.1007/s11064-016-1971-4. [DOI] [PubMed] [Google Scholar]
- 36.Zheng JS, Boluyt MO, Long X, O’Neill L, Lakatta EG, Crow MT. Extracellular ATP inhibits adrenergic agonist-induced hypertrophy of neonatal cardiac myocytes. Circ Res 78: 525–535, 1996. doi: 10.1161/01.RES.78.4.525. [DOI] [PubMed] [Google Scholar]


