Abstract
Both the incidence and prevalence of chronic kidney disease are increasing in the elderly population. Although aging is known to induce kidney injury, the underlying molecular mechanisms remain unclear. Sirtuin 1 (Sirt1), a longevity gene, is known to protect kidney cell injury from various cellular stresses. In previous studies, we showed that the podocyte-specific loss of Sirt1 aggravates diabetic kidney injury. However, the role of Sirt1 in aging-induced podocyte injury is not known. Therefore, in this study we sought to determine the effects of podocyte-specific reduction of Sirt1 in age-induced kidney injury. We employed the inducible podocyte-specific Sirt1 knockdown mice that express shRNA against Sirt1 (Pod-Sirt1RNAi) and control mice that express shRNA for luciferase (Pod-LuciRNAi). We found that reduction of podocyte Sirt1 led to aggravated aging-induced glomerulosclerosis and albuminuria. In addition, urinary level of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative stress, was markedly increased in aged Pod-Sirt1RNAi mice compared with aged Pod-LuciRNAi mice. Although podocyte-specific markers decreased in aged mice compared with the young controls, the decrease was further exacerbated in aged Pod-Sirt1RNAi compared with Pod-LuciRNAi mice. Interestingly, expression of cellular senescence markers was significantly higher in the glomeruli of Pod-Sirt1RNAi mice than Pod-LuciRNAi mice, suggesting that cellular senescence may contribute to podocyte loss in aging kidneys. Finally, we confirmed that Pod-Sirt1RNAi glomeruli were associated with reduced activation of the transcription factors peroxisome proliferator-activated receptor (PPAR)-α coactivador-1 (PGC1α)/PPARγ, forkhead box O (FOXO)3, FOXO4, and p65 NF-κB, through SIRT1-mediated deacetylation. Together, our data suggest that SIRT1 may be a potential therapeutic target to treat patients with aging-related kidney disease.
Keywords: SIRT1, aging, chronic kidney disease, senescence, podocytes
chronic kidney disease is a major concern in the elderly, with both an increasing incidence of end-stage renal failure and a high prevalence of earlier stages of chronic kidney disease (30). the aging process leads to progressive loss of tissue and organ functions over time, including those of the kidney. Several potential underlying mechanisms have been postulated as to how aging affects renal function (3,5), but the exact molecular mechanisms remain incompletely understood.
The sirtuin family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases, a homolog of yeast Sir2 (silent mating type information regulation 2), has been shown to play an important role in aging. In yeast, the Sir2 gene has been shown to be a prolongevity factor and mediates the beneficial effects of caloric restriction on life span extension (32, 46). Similarly, the mammalian Sir2 ortholog Sirtuin 1 (Sirt1) is upregulated by caloric restriction and mediates the longevity effect of calorie restriction by regulation of glucose and lipid metabolism (36, 40). These findings are further corroborated by the observation that activation of Sirt1 by resveratrol extends the life span in mice fed high-caloric diet (2). On the cellular level, Sirt1 is involved in variety of processes that include autophagy (41), energetic homeostasis (40), mitochondrial biogenesis (42), and apoptosis (34). These biological effects of Sirt1 are thought to be mediated through the transcription repression by deacetylation of histones and multiple transcription factors, including p53, forkhead box O (FOXO), RelA, signal transducer and activator of transcription 3 (STAT3), peroxisome proliferator-activated receptor (PPAR)-α coactivador-1 (PGC1α), and PPARγ (37). In kidneys, several studies have shown a critical role of Sirt1 in protecting tubular cells from cellular stresses (24, 25, 28). In addition, increased renal tubular Sirt1 expression was also reported to attenuate albuminuria in the setting of diabetic kidney disease (23). However, the studies of Sirt1 in glomerular cells are relatively limited.
Several studies have also implicated the role of Sirt1 with renal aging and age-associated kidney disease. Sirt1 level decreases with aging kidneys, and recent report by Guan et al. (20) showed that nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to acute kidney injury in a Sirt1-dependent manner, which is thought to be mediated in part by the modulation of JNK signaling pathway through the deacetylation of JNK phosphatase, DUSP16. Kume et al. (28) also reported a protective role of Sirt1 against hypoxia in aging kidneys under calorie restriction that is mediated by Sirt1-dependent regulation of mitochondrial function and mitophagy. However, the role of Sirt1 in aging-induced podocyte injury has not been previously examined.
Our previous work showed that Sirt1 is expressed in the kidney podocyte and that it is reduced in diabetic kidney (9). Inhibition of advanced glycation end product (AGE) formation in db/db mice by pyridoxamine treatment attenuated proteinuria and podocyte injury, restored Sirt1 expression, and reduced p65 NF-κB and STAT3 acetylation. Conditional podocyte deletion of Sirt1 in diabetic db/db mice led to aggravated kidney injury and proteinuria in comparison with wild-type diabetic db/db mice, and was associated with increased acetylation of p65 NF-κB and STAT3. Our findings strongly support a critical role for Sirt1 in diabetic kidney injury (33). Recently, we engineered genetically modified mice with inducible, reversible, and tissue-specific RNAi (RNA interference)-mediated Sirt1 reduction (10). We found that mice with ~80% knockdown of overall renal Sirt1 expression have normal glomerular function under the basal condition. However, in the setting of adriamycin-induced glomerular injury, Sirt1 knockdown mice developed marked albuminuria, glomerulosclerosis, mitochondrial injury, and impaired autophagy of damaged mitochondria, suggesting an important homeostatic role of Sirt1 in podocytes. In this study, using a podocyte-specific knockdown of Sirt1, we have sought to interrogate the role of Sirt1 in age-related glomerular function and injury.
MATERIALS AND METHODS
Inducible podocyte-specific Sirt1 knockdown mouse model.
We employed a doxycycline (Dox)-inducible podocyte-specific RNAi model for Sirt1 (Pod-Sirt1RNAi) and its control (Pod-LuciRNAi) that were previously described (10). To induce the expression of shRNA against Sirt1 or luciferase, Pod-Sirt1RNAi or Pod-LuciRNAi mice were fed with 625 mg/kg Dox-supplemented chow (Bio-Serv, Frenchtown, NJ) starting at 5 mo of age until 26–28 mo of age, at which time the kidneys were harvested for analysis. Our previous work showed that under basal conditions podocyte Sirt1 was dispensable in young mice and that Pod-Sirt1RNAi or Pod-LuciRNAi mice were identical in phenotype when fed with Dox at 6 wk and examined up to 40–50 wk of age (10). Thus 3-mo-old mice of either Pod-Sirt1RNAi or Pod-LuciRNAi genotypes that were not induced with Dox were used for a single young control group. There were at least six mice in each group analyzed. All animal studies were performed in accordance with the approved protocol and guidelines of Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai (New York, NY). Mice were housed in a specific pathogen-free facility with free access to chow and water and a 12-h day-night cycle.
Measurement of renal function.
Blood urea nitrogen was measured from mouse sera using a commercially available kit (BioAssay Systems), according to manufacturer’s protocol. Urine albumin was quantified by ELISA using a kit from Bethyl Laboratories (Houston, TX). Urine creatinine levels were measured in the same samples using QuantiChrom creatinine assay kit (DICT-500; BioAssay Systems) according to the manufacturer's instruction. The urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
Kidney histology.
Kidneys were removed and fixed with 4% paraformaldehyde for 16 h at 4°C. The 4-μm sections were cut from paraffin-embedded kidney tissues. Sections were stained with periodic acid-Schiff for histology analysis. To quantify glomerulosclerosis, a score of 0 to 4 was used as described before (22). A score of 0 indicated normal glomerulus, a score of 1 indicated mesangial expansion or sclerosis involving up to 25% of the glomerular tuft, a score of 2 indicated sclerosis 25 to 50%, a score of 3 indicated sclerosis 50 to 75% and/or segmental extracapillary fibrosis or proliferation, and a score of 4 indicated global sclerosis (>75%) or global extracapillary fibrosis or proliferation, or complete collapse of the glomerular tuft. At least 50 glomeruli were counted per mouse.
Isolation of glomeruli from mice for RNA and protein extraction.
Mouse glomeruli were isolated by perfusion of ferric oxide, as described previously (1). Briefly, animals were perfused with Hanks’ buffered salt solution containing 2.5 mg/ml iron oxide and 1% bovine serum albumin. At the end of perfusion, kidneys were removed, decapsulated, minced into 1-mm3 pieces, and digested in Hanks’ buffered salt solution containing 1 mg/ml collagenase A and 100 units/ml deoxyribonuclease I. Digested tissue was then passed through a 100-μm cell strainer and collected by centrifugation. The pellet was resuspended in 2 ml of Hanks' buffered salt solution, and glomeruli were collected using a magnet. The purity of glomerular was verified under microscopy. Total RNA was isolated using TRIzol (Invitrogen), or protein was extracted using NP40-containing lysis buffer from isolated glomeruli, as previously described (14).
Immunohistochemical and immunofluoresence staining.
Immunohistochemical and immunofluoresence staining was conducted on paraffin-embedded kidney sections using standard procedures. Briefly, deparaffinized sections were incubated with primary antibody against Sirt1 (Abcam), p57 (Santa Cruz Biotechnology), or H2A.X (phospho-S139) (Abcam) at 4°C overnight. After being washed, sections were incubated with biotinylated secondary antibodies, followed by incubation with either avidin-biotin-peroxidase complex for DAB substrate development using the ABC kit (Vector Laboratories) or with cy3-labeled avidin at room temperature, counterstained with DAPI, and mounted using Aqua PolyMount (Polysciences). Images were acquired using AxioVision IIe microscope with a digital camera.
Quantitative real-time PCR.
Primers for quantitative (q)RT-PCR were designed by using the National Center for Biotechnology Information Primer-BLAST tool, and the sequences for primer pairs were as follows: Sirt1 (5′- CACTGTAACTGGGGGCAACT-3′ and 5′-CACTTCTTGTCAGCGTCGAA-3′); Nphs1 (5′-GTGCCCTGAAGGACCCTACT-3′ and 5′-CCTGTGGATCCCTTTGACAT-3′); Nphs2 (5′-CTTGGCACATCGATCCCTCA-3′ and 5′-CGCACTTTGGCCTGTCTTTG-3′); synaptopodin (5′-CTTTGGGGAAGAGGCCGATTG-3′ and 5′-GTTTTCGGTGAAGCTTGTGC-3′); WT1 (5′-GAGAGCCAGCCTACCATCC-3′ and 5′-GGGTCCTCGTGTTTGAAGGAA-3′); and GAPDH (5′-GCCATCAACGACCCCTTCAT-3′ and 5′-ATGATGACCCGTTTGGCTCC-3′). PCR was performed using SYBR Green Master Mix (Applied Biosystems), and the Applied Biosystems 7500 Real-time PCR system. Gene expression was normalized to housekeeping gene GAPDH, and fold change in expression relative to the control group was calculated using the 2−ΔΔCt method.
Immunoprecipitation and Western blot analysis.
Tissues were lysed with buffer containing 1% NP40 with a protease and phosphatase inhibitor cocktail, as previously described (15). Immunoprecipitation was carried out using ~0.5 mg of precleared glomerular lysates with standard protocol using the following antibodies: FOXO3 (NB100-163; Novus Biologic), FOXO4 (9472; Cell Signaling), PPARγ (sc-7196; Santa Cruz Biotechnology), PGC1α (ab54481; Abcam), p65 NF-κB (sc-372; Santa Cruz Biotechnology). The following specific antibodies were used for immunoblot analysis: SIRT1 (ab28170; Abcam), PPARγ (sc-7196; Santa Cruz Biotechnology), PGC1α (ab54481; Abcam), and β-actin (A5316; Sigma-Aldrich), acetylated-lysine (9681; Cell Signaling Technology), acetyl-p65 NF-κB (K310) (3045; Cell Signaling). Western blots were imaged and band intensities were quantified using ImageJ.
Statistical analysis.
Data are expressed as means ± SEM. The two-sided unpaired t-test was used to analyze data between two groups after determination of data distributions and variance. The ANOVA with Bonferroni correction was used when more than two groups were present. GraphPad Prism software was used for statistical analysis. P < 0.05 was considered to be statistically significant.
RESULTS
Knockdown of Sirt1 in podocytes aggravates glomerular injury in aging mice.
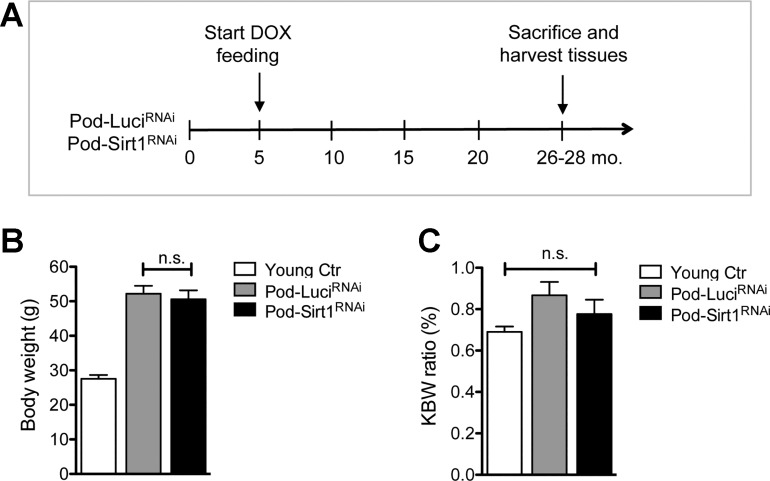
We have generated inducible podocyte-specific Sirt1 knockdown (Pod-Sirt1RNAi) mice and their control mice that carry an inducible shRNA against firefly luciferase gene (Pod-LuciRNAi), as described previously (10). Both Pod-Sirt1RNAi and Pod-LuciRNAi were fed with doxycycline-containing chow starting at 5 mo of age, and mice were kept in the same condition until the mice were killed at 26–28 mo of age (Fig. 1A). There were six or more mice in each group. Our previous work showed that under basal conditions, young mice with global knockdown of Sirt1 (CAGs-Sirt1RNAi mice) or young Pod-Sirt1RNAi displayed no defects but were identical to their LuciRNAi control counterparts in phenotype when examined up to 40–50 wk of age. Therefore, the young control group consisted of 3-mo-old mice of either Pod-Sirt1RNAi or Pod-LuciRNAi genotypes without Dox treatment. We found that at the end of 26–28 mo, both the body weight and the kidney-to-body weight ratio did not differ between old Pod-Sirt1RNAi and Pod-LuciRNAi mice (Fig. 1, B and C).
Fig. 1.
Podocyte-specific induction of Sirtuin 1 (Sirt1) knockdown in aging mice. A: schematics of study design. Pod-Sirt1RNAi or Pod-LuciRNAi mice were fed with doxycycline (Dox)-containing chow starting at 5 mo of age to induce the expression of specific shRNA against Sirt1 or luciferase, respectively. Mice between ages of 26–28 mo were killed for analysis. Young mice of 3 mo of age were used as controls (Ctr). B: body weights of Pod-Sirt1RNAi or Pod-LuciRNAi mice did not differ at 26–28 mo of age. C: kidney-to-body weight (KBW) ratio did not differ significantly among all 3 groups of mice (n = 6 or more in each group).
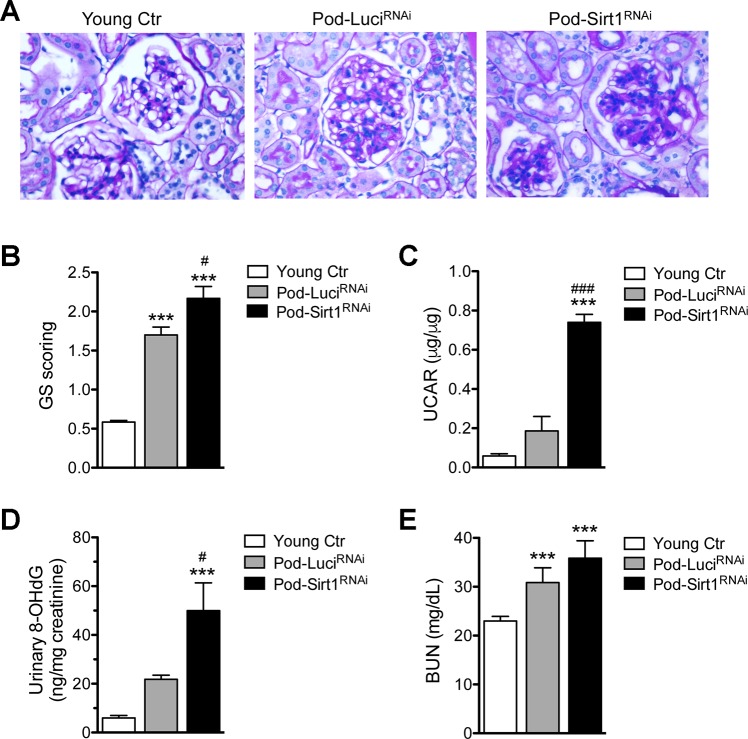
Histologically, in comparison with the young mice both old Pod-LuciRNAi and Pod-Sirt1RNAi mice displayed sclerotic glomeruli (Fig. 2A), consistent with observations reported previously (6, 50). However, there was increased glomerulosclerosis observed in the old Pod-Sirt1RNAi in comparison with old Pod-LuciRNAi. In addition, although older mice showed increased proteinuria in comparison with the young mice, old Pod-Sirt1RNAi mice had markedly higher albuminuria than old Pod-LuciRNAi mice (Fig. 2B). These data suggest that reduced SIRT1 expression in podocytes aggravated aging-associated glomerular injury. Since oxidative stress is known to contribute to aging kidney disease (5), we measured the levels of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidized nucleoside of DNA that is used as a biomarker of cellular oxidative stress (13). Level of 8-OHdG normalized to creatinine was indeed markedly higher in old Pod-Sirt1RNAi mice in comparison with both young and old Pod-LuciRNAi mice (Fig. 2C). The renal function as assessed by blood urea nitrogen levels did not differ significantly between old Pod-Sirt1RNAi and Pod-LuciRNAi mice (Fig. 2D), consistent with the histological findings of minimal tubulo-interstitial injury in both old Pod-Sirt1RNAi and Pod-LuciRNAi.
Fig. 2.
Podocyte-specific knockdown of Sirt1 exacerbates aging-related glomerulosclerosis and albuminuria. A: representative images of periodic acid-Schiff-stained kidney sections show increased glomerulosclerosis in aged Pod-Sirt1RNAi in comparison with Pod-LuciRNAi mice. B: semiquantitative scoring of severity of glomerulosclerosis (GS) in kidneys of mice in all groups. 50 glomeruli were counted per mouse. C: urinary albumin-to-creatinine ratio (UACR) is significantly increased in aged Pod-Sirt1RNAi mice compared with other groups. D: urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) normalized to creatinine is also significantly increased in aged Pod-Sirt1RNAi mice compared with other groups. E: renal function assessed by blood-urea nitrogen (BUN) is significantly increased in aged mice compared with young controls. ***P < 0.001, compared with young controls; #P < 0.05 and ###P < 0.001, compared with Pod-LuciRNAi mice (n = 6 mice in each group).
Knockdown of Sirt1 in podocytes aggravates podocyte injury in aging mice.
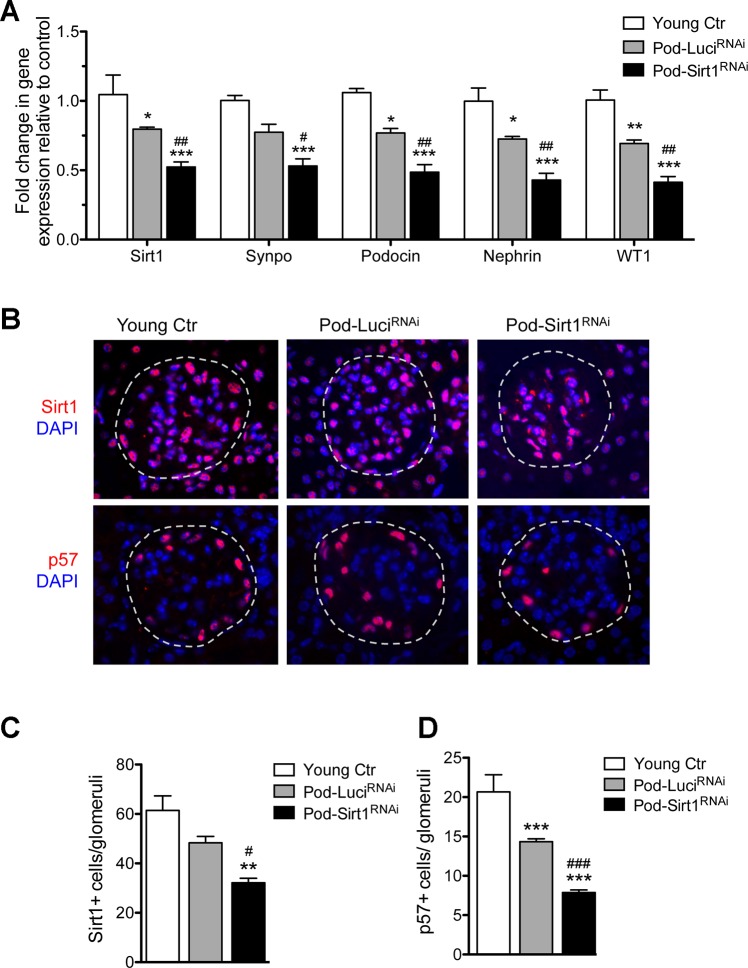
Next, we examined the effects of podocyte-specific Sirt1 knockdown by comparing the gene expression levels of Sirt1 and several podocyte markers in the isolated glomeruli from the mice by qPCR. Compared with the young controls, there was a significant reduction in expression of Sirt1 and of podocyte markers synaptopodin, podocin, nephrin, and WT1 from isolated glomeruli of aged Pod-Sirt1RNAi and Pod-LuciRNAi mice (Fig. 3A). However, a much further reduction of all these markers was observed in Pod-Sirt1RNAi mice as compared with Pod-LuciRNAi mice (Fig. 3A). By immunostaining we confirmed a reduction in number of SIRT1+ cells in old mice as compared with young mice and a further reduction of SIRT1 in Pod-Sirt1RNAi mice as compared with Pod-LuciRNAi mice (Fig. 3B). Concomitant with the reduction in SIRT1+ cells, we also observed a reduction of podocyte number in old mice as compared with young mice, as detected by immunostaining for p57, which is constitutively expressed in mature podocytes (43). Consistent with the above mRNA analysis, further reduction of p57+ cells was observed in Pod-Sirt1RNAi mice as compared with Pod-LuciRNAi mice (Fig. 3B). Quantification of changes in SIRT1 and p57 is shown in Fig. 3, C and D.
Fig. 3.
Podocyte-specific knockdown of Sirt1 accelerates aging-related podocyte expression markers. A: expression of Sirt1 and podocyte-specific markers were assessed from mRNAs of isolated glomeruli in young control and in aged mice. B: glomerular Sirt1 (top) and podocyte-specific p57 expressions (bottom) are detected by immunostaining. Glomeruli are outlined with dotted lines. C and D: quantification of Sirt1 (C) and p57 (D) per glomeruli is shown. **P < 0.01 and ***P < 0.001, compared with young controls; #P < 0.05 and ###P < 0.001, compared with Pod-LuciRNAi mice (n = 6 mice in each group).
Knockdown of Sirt1 in podocytes increases expression of senescence markers in glomeruli of aging mice.
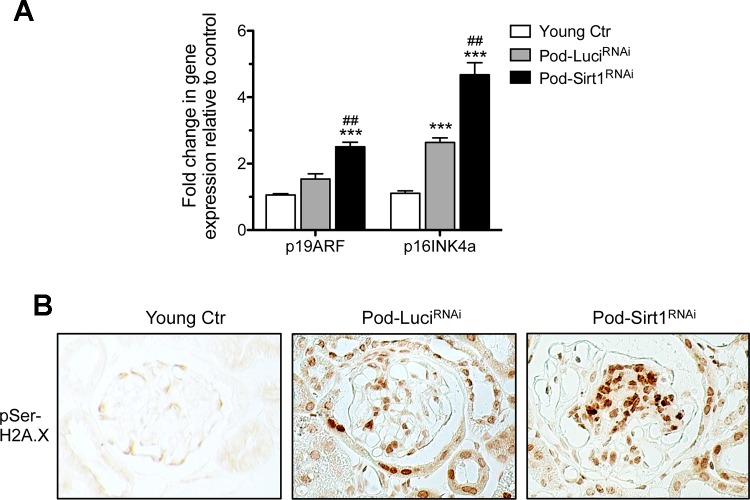
Cellular senescence increases with aging, and SIRT1 is known to antagonize cellular senescence (49). Therefore, we examined whether the podocyte-specific reduction of Sirt1 would increase cellular senescence in aging glomeruli. By real-time PCR, we found that expression of senescence markers p19ARF and p16INK4a wsd significantly higher in the glomeruli of old Pod-Sirt1RNAi mice as compared with old Pod-LuciRNAi mice (Fig. 4A). Immunostaining for phosphorylated histone H2A.X (pSer139) also confirmed that senescence was increased in glomerular cells in Pod-Sirt1RNAi as compared with those of Pod-LuciRNAi mice (Fig. 4B). Together with the reduced number of podocytes from above data, these results suggest that the increased senescence may contribute to the loss of podocytes in aging kidneys.
Fig. 4.
Podocyte-specific knockdown of Sirt1 increases markers of senescence in glomeruli. A: Expression of senescence markers p19ARF and p16INK4a, assessed mRNAs of isolated glomeruli, were significantly increased aged mice compared with the young controls, and their increases were further heightened in Pod-Sirt1RNAi mice compared with Pod-LuciRNAi mice. B: expression of pSer139-H2A.X was significantly increased in glomeruli of aged Pod-Sirt1RNAi mice by immunohistochemical analysis. ***P < 0.001, compared with young controls; ##P < 0.01, compared with Pod-LuciRNAi mice (n = 6 mice in each group).
Knockdown of Sirt1 in podocytes decreases the expression of PGC1α and the acetylation of transcription factors FOXO3, FOXO4, and NF-κB in aging mice.
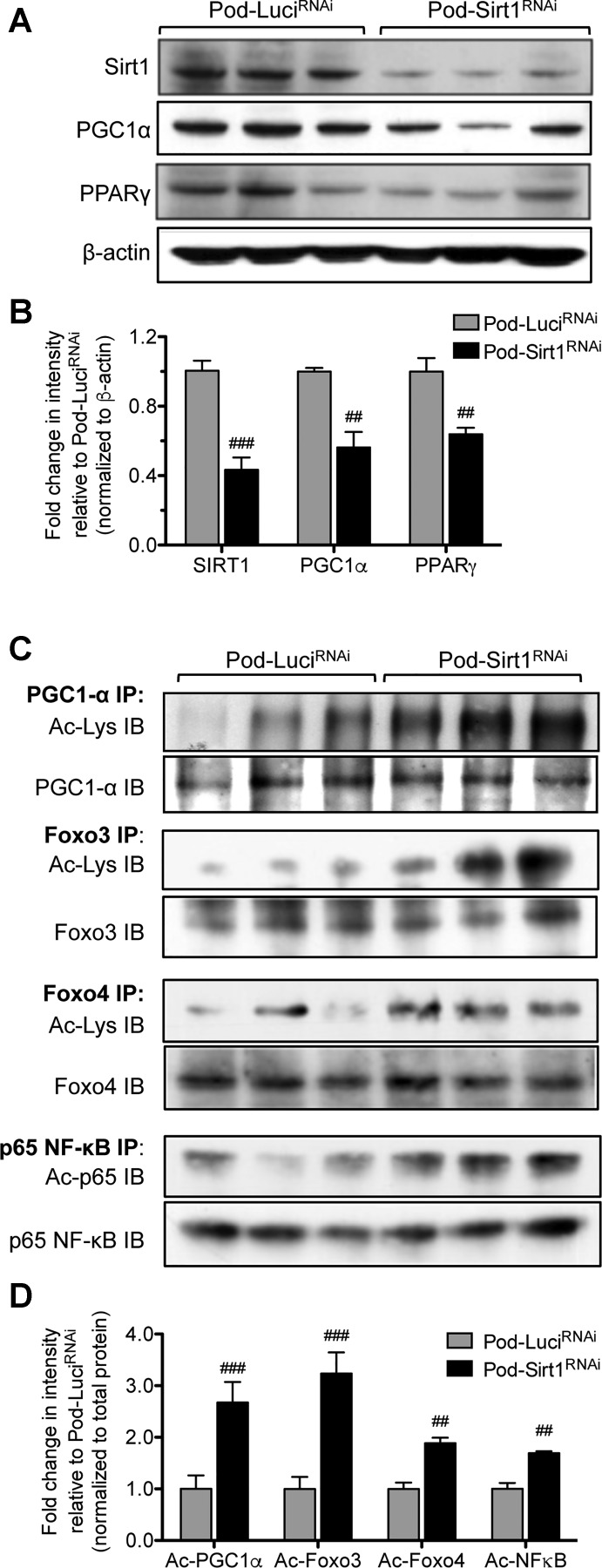
SIRT1 is known to protect cells from aging-induced stress through the activation of PGC1α/PPARγ pathway and attenuation of FOXO3/FOXO4 pathways (17, 39), and PGC1α expression is reduced with aging (8, 31). We have also previously shown that SIRT1 reduces inflammation in diabetic kidneys through deacetylation of p65 NF-κB (33). Therefore, we tested these pathways in the isolated glomeruli from Pod-LuciRNAi and Pod-Sirt1RNAi mice by Western blot analysis. We found that the glomeruli of old Pod-Sirt1RNAi mice had both decreased expression levels of PGC1α and PPARγ compared with old Pod-LuciRNAi mice (Fig. 5, A and B). Furthermore, the acetylation of PGC1α was markedly higher in old Pod-Sirt1RNAi mice compared with old Pod-LuciRNAi mice (Fig. 5, C and D). In addition, old Pod-Sirt1RNAi glomeruli also had increased acetylation of the transcription factors FOXO3, FOXO4, and p65 NF-κB acetylation as compared with those from old Pod-LuciRNAi mice (Fig. 5, C and D). Together, these data suggest that the podocyte Sirt1-knockdown results in aggravated cellular senescence and inflammation, resulting in heightened glomerulosclerosis and reduced overall glomerular function in aging kidneys.
Fig. 5.
Podocyte-specific knockdown of Sirt1 results in increased acetylation of peroxisome proliferator-activated receptor (PPAR)-α coactivador-1 (PGC1α), PPARγ, forkhead box O (FOXO)3, FOXO4, and p65 NF-κB. A: Western blot analysis of Sirt1, PGC1α, and PPARγ in glomerular lysates of aged Pod-Sirt1RNAi and Pod-LuciRNAi mice. B: densitometric analysis of Sirt1, PGC1α, and PPARγ immunoblotting is shown. Band intensities were normalized to corresponding β-actin and are shown as a relative fold change to old Pod-LuciRNAi. C: immunoprecipitation (IP) and immunoblotting (IB) of glomerular lysates show increased acetylation of PGC1α, FOXO3, FOXO4 and p65 NF-κB in Pod-Sirt1RNAi compared with Pod-LuciRNAi mice. D densitometric analysis of acetylated FOXO3, FOXO4, p65 NF-κB, and PGC1α is shown. Band intensities were normalized to total protein and are shown as a relative fold change to old Pod-LuciRNAi. #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with Pod-LuciRNAi mice (n = 3 samples in each group).
DISCUSSION
In this study, we used an inducible podocyte-specific knockdown mouse model of Sirt1 to confirm its role in aging-associated glomerular and podocyte injury. Our data suggest that the podocyte loss in aging kidneys is likely due to increased senescence as a consequence of reduced SIRT1 expression. Although several studies suggest an important role of SIRT1 in aging kidney disease, most have investigated its role in aging-induced tubular cell injury (20, 28). To our knowledge, this is the first study to determine the specific role of podocyte SIRT1 in aging-induced glomerular and podocyte injury. Our findings clearly indicate that reduced SIRT1 expression in podocytes aggravates age-induced podocyte loss, albuminuria, and glomerulosclerosis.
Loss of podocytes has been described in aging rodents and has been considered as a major contributor for development of glomerulosclerosis (4, 26). However, the mechanism of podocyte loss in aging kidneys is not entirely clear. The mechanism of podocyte loss may occur through detachment from the glomerular basement membrane, cell death, and/or dedifferentiation (27, 52). Our data suggest that senescence may be one of the contributing factors of podocyte loss in aging kidneys.
SIRT1’s role is well established in aging (21), and it may contribute to aging kidney disease through increased oxidative stress and mitochondrial injury (19, 28). In kidney cells, reduction in SIRT1 is associated with high glucose-induced mesangial cell senescence (51) and aldosterone-induced senescence in proximal tubular cells (16). Short-term caloric restriction reduced renal senescence through activation of SIRT1 and AMPK pathway, thereby increasing autophagy and reducing oxidative damage (38). Sirt1 depletion in vascular endothelial cells mediates endothelial dysfunction and leads to development of nephrosclerosis in mice through downregulation of matrix metalloproteinase-14 (47). Recent studies suggest that autophagy plays a major role in aging kidneys (29). Since SIRT1 is a known regulator of autophagy (41), reduced SIRT1 may contribute to renal aging through dysregulation of autophagy or mitophagy (10, 28). SIRT1 also regulates apoptosis (12), and we demonstrated previously that FOXO4 is a major transcription factor that mediates AGE-induced podocyte apoptosis and that SIRT1 inhibits podocyte apoptosis by deacetylating FOXO4 (9, 11). Future studies are required to further dissect the interaction among these cellular mechanisms by which SIRT1 protect podocytes from injury.
We have shown here that Sirt1 reduction in podocytes increased the acetylation status of PGC1α, FOXO3, FOXO4, and p65 NF-κB in aging mouse glomeruli. SIRT1 is known to deacetylate PGC1α, resulting in the activation of PPARγ pathway and maintainenance of normal mitochondrial biogenesis (18, 45). SIRT1 also regulates the activity of many transcriptional factors, such as the FOXO family of transcription factors (7, 35). It has been shown that SIRT1 has a dual effect on FOXO3 function: SIRT1 increases FOXO3’s ability to induce cell cycle arrest and resistance to oxidative stress but inhibits its ability to induce cell death. Thus SIRT1 may increase organismal longevity by tipping the FOXO3-dependent responses away from apoptosis and toward stress resistance (44, 48). We have previously shown that SIRT1 deacetylates FOXO4 to attenuate podocyte apoptosis in the setting of diabetic nephropathy (9) and that SIRT1 exerts anti-inflammatory effects in diabetic mice through deacetylation of p65 NF-κB (33). Consistent with these reports, our results here also show that reduced Sirt1 led to increased FOXO3, FOXO4, and NF-κB acetylation, suggesting that dysregulation of SIRT1 is associated with increased the downstream pathways of cell death, oxidant stress, and inflammation in podocytes.
In summary, our current study demonstrates a podocyte-specific role of Sirt1 in aging-induced kidney injury. Reduced podocyte SIRT1 expression exacerbated age-induced glomerulosclerosis and albuminuria and increased podocyte loss in aging mouse kidneys, in part through dysregulation of PGC1α-, FOXO3-, FOXO4-, and NF-κB-mediated pathways. Out study suggests SIRT1 as a potential drug target to treat aging-induced chronic kidney disease.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-098126 (to P. Y. Chuang) and P01-DK-056492 (to J. C. He) and National Nature Science Foundation of China Grant 81500511 and Shanghai Pujiang Talent Foundation of China Grant 15PJ1407100 (both to X. Li).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.H. conceived and designed research; P.Y.C., W.C., X.L., L.F., J.X., and R.Y. performed experiments; P.Y.C., W.C., X.L., L.F., J.X., R.Y., J.C.H., and K.L. analyzed data; P.Y.C., J.C.H., and K.L. interpreted results of experiments; PYC, WC, and K.L. prepared figures. J.C.H. and K.L. drafted manuscript; J.C.H. and K.L. edited and revised manuscript; P.Y.C., J.C.H., and K.L. approved final version of manuscript.
REFERENCES
- 1.Baelde JJ, Bergijk EC, Hoedemaeker PJ, de Heer E, Bruijn JA. Optimal method for RNA extraction from mouse glomeruli. Nephrol Dial Transplant 9: 304–308, 1994. [PubMed] [Google Scholar]
- 2.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol 9: 699–709, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bitzer M, Wiggins J. Aging biology in the kidney. Adv Chronic Kidney Dis 23: 12–18, 2016. doi: 10.1053/j.ackd.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev 14: 65–80, 2014. doi: 10.1016/j.arr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Bolton WK, Benton FR, Maclay JG, Sturgill BC. Spontaneous glomerular sclerosis in aging Sprague-Dawley rats. I. Lesions associated with mesangial IgM deposits. Am J Pathol 85: 277–302, 1976. [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 8.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang PY, Dai Y, Liu R, He H, Kretzler M, Jim B, Cohen CD, He JC. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 6: e23566, 2011. doi: 10.1371/journal.pone.0023566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang PY, Xu J, Dai Y, Jia F, Mallipattu SK, Yacoub R, Gu L, Premsrirut PK, He JC. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol 184: 1940–1956, 2014. doi: 10.1016/j.ajpath.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 13.Cooke MS, Evans MD, Herbert KE, Lunec J. Urinary 8-oxo-2′-deoxyguanosine–source, significance and supplements. Free Radic Res 32: 381–397, 2000. doi: 10.1080/10715760000300391. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Li X, Xiao W, Fu J, Harris RC, Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, Lee K, He JC, Schlondorff D, Chuang PY. BAMBI elimination enhances alternative TGF-β signaling and glomerular dysfunction in diabetic mice. Diabetes 64: 2220–2233, 2015. doi: 10.2337/db14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Y, Xiao W, Li Z, Li X, Chuang PY, Jim B, Zhang W, Wei C, Wang N, Jia W, Xiong H, Lee K, He JC. RTN1 mediates progression of kidney disease by inducing ER stress. Nat Commun 6: 7841, 2015. doi: 10.1038/ncomms8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, Yatabe M, Felder RA, Ohsaki H, Rafiq K, Sherajee SJ, Noma T, Nishiyama A, Nakano D. Aldosterone/mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology 152: 680–688, 2011. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- 17.Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol 14: 408–412, 2004. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes P, Outeiro TF, Cavadas C. Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci 36: 756–768, 2015. doi: 10.1016/j.tips.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide mononucleotide, an NAD(+) precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J Am Soc Nephrol 28: 2337–2352, 2017. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 22.Hartner A, Cordasic N, Klanke B, Müller U, Sterzel RB, Hilgers KF. The alpha8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am J Pathol 160: 861–867, 2002. doi: 10.1016/S0002-9440(10)64909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19: 1496–1504, 2013. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285: 13045–13056, 2010. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015. doi: 10.1681/ASN.2014080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenoir O, Jasiek M, Hénique C, Guyonnet L, Hartleben B, Bork T, Chipont A, Flosseau K, Bensaada I, Schmitt A, Massé JM, Souyri M, Huber TB, Tharaux PL. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11: 1130–1145, 2015. doi: 10.1080/15548627.2015.1049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W; Centers for Disease Control and Prevention Expert Panel . Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis 53: 522–535, 2009. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Lim JH, Kim EN, Kim MY, Chung S, Shin SJ, Kim HW, Yang CW, Kim YS, Chang YS, Park CW, Choi BS. Age-associated molecular changes in the kidney in aged mice. Oxid Med Cell Longev 2012: 171383, 2012. doi: 10.1155/2012/171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128, 2000. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM, Chuang PY, He JC. Role of transcription factor acetylation in diabetic kidney disease. Diabetes 63: 2440–2453, 2014. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 35.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563, 2004. doi: 10.1016/S0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 36.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2: 105–117, 2005. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa T, Guarente L. Sirtuins at a glance. J Cell Sci 124: 833–838, 2011. doi: 10.1242/jcs.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning YC, Cai GY, Zhuo L, Gao JJ, Dong D, Cui S, Feng Z, Shi SZ, Bai XY, Sun XF, Chen XM. Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage. Mech Ageing Dev 134: 570–579, 2013. doi: 10.1016/j.mad.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett 582: 46–53, 2008. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 41.Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal 21: 1356–1360, 2009. doi: 10.1016/j.cellsig.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813: 1269–1278, 2011. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int 58: 674–683, 2000. doi: 10.1046/j.1523-1755.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 44.Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, Mori R. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell 14: 707–709, 2015. doi: 10.1111/acel.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang BL. Sirt1 and the mitochondria. Mol Cells 39: 87–95, 2016. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410: 227–230, 2001. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 47.Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol 25: 276–291, 2014. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6: 505–514, 2007. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett 585: 986–994, 2011. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 50.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F, Chen X. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev 133: 387–400, 2012. doi: 10.1016/j.mad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11: 535–545, 2015. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]