Abstract
Alzheimer’s disease (AD) is characterized by an extensive accumulation of amyloid-β (Aβ) peptide, which triggers a set of deleterious processes, including synaptic dysfunction, inflammation, and neuronal injury, leading to neuronal loss and cognitive impairment. A large body of evidence supports that nuclear receptor (NR) activation could be a promising therapeutic approach for AD. NRs are ligand-activated transcription factors that regulate gene expression and have cell type-specific effects. In this review, we discuss the mechanisms that underlie the beneficial effects of NRs in AD. Moreover, we summarize studies reported in the last 10–15 years and their major outcomes arising from the pharmacological targeting of NRs in AD animal models. The dissection of the pathways regulated by NRs in the context of AD is of importance in identifying novel and effective therapeutic strategies.
Keywords: inflammation, nuclear receptors/liver X receptor, nuclear receptors/peroxisome proliferator-activated receptor, nuclear receptors/retinoic acid receptor, nuclear receptors/retinoid X receptor, neuroprotection, neurodegeneration
ALZHEIMER’S DISEASE
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the leading cause of dementia in the elderly. There is an urgent need for the development of novel and effective therapeutic strategies for the prevention and/or treatment of AD, given the personal and financial burdens associated with this disease (1). Although several hypotheses have been proposed, the dominant model of AD pathogenesis was put forward more than 20 years ago, in which the amyloid-β (Aβ) peptide plays a central role and subsequently drives the dysregulation of the cytoskeletal protein, tau. The amyloid hypothesis is supported by a substantial body of scientific and clinical evidence (2). AD is typified by the progressive Aβ deposition in the brain parenchyma as both diffuse and dense-core plaques, starting in isocortical areas, followed by limbic and allocortical structures, and finally subcortical structures (3). Aβ peptide is generated by sequential cleavage of the amyloid precursor protein (APP) by the β and γ secretases, yielding a heterogeneous pool of Aβ species with different lengths, most prominently those of 40 or 42 amino acids in length. The Aβ(1-42) species is more hydrophobic and highly self-aggregating, and is the principal species that is initially deposited in the brain of AD patients. APP can also be processed in a nonamyloidogenic pathway mediated by α-secretase activity, generating a soluble APPα fragment (sAPPα), which is considered to have beneficial effects on neurons (4, 5). Genetically inherited familial forms of AD are caused by mutations in APP or in γ-secretase that favor Aβ(1-42) generation, followed by aggregation and deposition. On the other hand, patients with “sporadic” late-onset AD, exhibit an impairment of Aβ clearance from the brain, likely underlying the pathological Aβ accumulation observed in this type of patient (6, 7). Thus, perturbation of Aβ homeostasis caused by either increased production (familial AD) or impaired clearance (sporadic late-onset AD) of Aβ peptides leads to the pathological accumulation of this peptide in the form of toxic Aβ oligomers, disrupting synaptic function and plasticity, which is postulated to underlie the cognitive deficits observed in this disease. The deposition of Aβ in the form of plaques results in neuritic dystrophy (8) and triggers glial activation leading to the induction of a robust inflammatory response. This cascade of events further leads to neuronal injury and hyperphosphorylation and aggregation of tau protein, culminating in widespread synaptic dysfunction and neuronal loss (2). Thus, therapeutic strategies aiming to decrease Aβ burden, by increasing clearance or reducing Aβ generation, were expected to have a beneficial effect in AD patients, although this has yet to be demonstrated in clinical trials. New therapeutic options that are not limited to modulation of Aβ levels are now under development.
Because the exact etiology of sporadic late onset AD is unknown, most of the animal models that have been developed to study this disease rely on the utilization of mutations related to familial forms of AD. The majority of animal models mentioned in this review are based on transgenic mice carrying mutated human APP and presenilin-1 (PSEN1) transgenes with a neuron-specific expression, resulting in increased Aβ production and accumulation, ultimately leading to the onset and progression of AD-like pathology. Some models only carry one transgene with a particular familial AD mutation, such as the case of the APP23 and Tg2576 mouse models, which express human APP harboring the Swedish mutation (KM670/671NL) (APPswe) (9, 10), and the APPV717I model, which expresses the human APP with the London mutation (V717I) (11). Other models were developed to exhibit two familial AD mutations in a particular transgene, such as the APPswe,ind mice that express the human APP not only bearing the Swedish mutation, but also the Indiana mutation (V717F) (12, 13). Furthermore, the APPswe/PSENd1E9 mouse model carries APPswe together with the human PSEN1 containing the AD-related deletion of exon 9 (14, 15). Another popular model is 5XFAD mice, which express the human APP harboring three familial AD mutations, the Swedish, Florida (I716V), and London mutations, together with PSEN1 containing two familial AD mutations, M146L and L286V (16). Some mouse models were also generated to reflect the tau-related pathology of AD, namely, the P301S mouse model, which expresses tau protein bearing the P301S familial AD-related mutation (17), and other models that incorporate both features of tau and Aβ pathologies, such as the case of the 3xTg-AD mouse model, which carries three familial AD mutations, as it expresses the APPswe, the human P301L tau mutation, and PSEN1 harboring the M146V mutation (18, 19). The disease onset and progression is specific for each model, and it is a crucial factor to take into consideration when comparing studies and testing new therapeutic strategies.
NUCLEAR RECEPTORS
Nuclear receptors (NRs) belong to a superfamily of ligand-activated transcription factors that regulate the expression of a large number of genes involved in a wide variety of biological processes, regulating energy and lipid metabolism in response to environmental and dietary changes (20). For the last 10–15 years, numerous studies demonstrated that the pharmacological targeting of particular NRs is beneficial in AD animal models (Table 1), namely, the liver X receptor (LXR), PPARs, the retinoid X receptor (RXR), and, to a lesser extent, the retinoic acid receptor (RAR). LXR, PPARs, and RAR belong to the type II family of NRs, a group that encompasses nonsteroid NRs that form obligate heterodimers with RXR. The heterodimeric receptors bind to sequence-specific DNA elements positioned in the enhancers and promoters of their target genes, and act to directly regulate gene transcription. The heterodimeric receptors are retained in the nucleus regardless of their ligand binding status (21). Importantly, LXR and PPAR heterodimers with RXR are considered to be “permissive,” meaning that the heterodimer is activated by ligation of either member of the receptor pair and, when simultaneously ligated, it can respond in an additive or synergistic fashion. In contrast, heterodimers of RAR with RXR are “conditionally permissive;” they are not activated by RXR ligands alone; it is the binding of a RAR ligand that activates the dimer and subsequently allows the binding of RXR ligands, increasing the transcriptional potential of RAR. There are also “non-permissive” RXR heterodimers, such as the thyroid hormone receptor, which only respond to ligands of the nonpermissive binding partner and not RXR ligands (22). Importantly, in the absence of ligand binding, the RXR heterodimers are bound to DNA and function as transcriptional repressors to silence gene expression. This repression mechanism is mediated by an interaction with corepressor complexes that contain the NR corepressor (NCoR) or the silencing mediator of retinoic acid thyroid hormone receptors (SMRT), together with HDAC3. Upon the binding of a ligand, the heterodimer changes its conformation resulting in the dismissal of the corepressor complex and the association with transcriptional coactivators, such as p300 and members of the steroid receptor coactivator (SRC) subfamily, which catalyzes the assembly of large protein complexes mediating the induction of gene transcription (23). Additionally, other repression mechanisms mediated by these NRs that do not include direct binding do DNA (transrepression) have been reported in macrophages. Upon binding to their ligands, monomers of PPARγ and LXRs can be sumoylated, which leads them to interact and prevent the clearance of NCoR corepressor complexes from chromatin. This mechanism is responsible for maintaining the repression of a particular subset of genes that are activated by the nuclear factor-κB (NF-κB) in response to inflammatory signals, most prominently in immune cells (23).
TABLE 1.
Effects of NR agonists in AD animal models
| NR | Ligand | Dosage | Length of Treatment | Administration | Animal Model | Pathology Effects | Inflammation Markers | Behavioral Outcomes | Ref. |
| PPARγ | Pioglitazone | 20 mg/kg/day | 16 weeks | Oral: food | Tg2576 | ↓sol Aβ | — | — | 93 |
| Pioglitazone | 18 mg/kg/day | 2 months | Oral: food | TGFbeta OE | ↓sol Aβ(1-42) | ↓ | — | 94 | |
| Pioglitazone | 40 mg/kg/day | 7 days | Oral: food | APPV717I | ↓Plaques; ↓sol Aβ | ↓ | — | 95 | |
| Pioglitazone | 40 mg/kg/day | 7 days | Oral: food | APPV717I | ↓Intracellular Aβ | — | — | 62 | |
| Pioglitazone | 20 mg/kg/day | 6–8 weeks | Oral: food | APPswe,ind | n.c. | ↓ | n.c. MWM | 96 | |
| Pioglitazone | 80 mg/kg/day | 9 days | Oral: gavage | APPswe/PSEN1dE9 | ↓Plaques; ↓sol Aβ | ↓ | ↑CFC | 52 | |
| Pioglitazone | 18 mg/kg/day | 14 weeks | Oral: food | 3xTg-AD | ↓Intracellular Aβ ↓p-tau | — | ↑Active avoidance learning | 97 | |
| Pioglitazone | 20 mg/kg/day | 9 months | Oral: food | PS1-KIm146v | — | — | ↑MWM; ↑NOR in females | 98 | |
| Pioglitazone | 20 mg/kg/day | 9 months | Oral: food | 3xTg-AD | — | n.c. | 98 | ||
| Pioglitazone | 20 mg/kg/day | 6 months | Oral: food | APPswe,ind/TGF-b1 | n.c. | ↓ | n.c. | 99 | |
| Pioglitazone | 20 mg/kg/day | 3 months | Oral: food | APPswe,ind/TGF-b1 | n.c. | ↓ | n.c. | 99 | |
| Pioglitazone | 15 mg/kg | 21 days | Oral: gavage | Aβ(1-42) brain injection in Wistar rats | — | ↓ | ↑MWM | 100 | |
| Pioglitazone | 30 mg/kg | 21 days | Oral: gavage | Aβ(1-42) brain injection in Wistar rats | — | ↓ | ↑MWM | 100 | |
| Pioglitazone | 80 mg/kg/day | 9 days | Oral: gavage | APPswe/PS1dE9 | ↓sol Aβ(1-40); ↓amyloid burden; n.c. sol Aβ(1-42); n.c. insol Aβ | ↓ | ↑CFC | 101 | |
| Pioglitazone | 80 mg/kg/day | 9 days | Oral: gavage | APPswe/PS1dE9 | ↓sol Aβ(1-42) | — | ↑Rotarod test | 102 | |
| Rosiglitazone | 4 mg/kg/day | 15 weeks | Oral: food | Tg2576 | ↓sol Aβ(1-42) | — | ↑Radial arm maze | 103 | |
| Rosiglitazone | 5 mg/kg/day | 10 weeks | Oral: food | APPswe,ind | — | — | ↑NOR | 104 | |
| Rosiglitazone | 5 mg/kg/day | 4 weeks | Oral: food | APPswe,ind | — | — | ↑NOR | 104 | |
| Rosiglitazone | 3 mg/kg/day | 12 weeks | Oral: gavage | APPswe/PSEN1dE9 | ↓Plaques | ↓ | ↑MWM | 105 | |
| Rosiglitazone | 5 mg/kg/day | 4–16 weeks | Oral: gavage | APPswe,ind | ↓Plaques; ↓sol Aβ ↓p-tau | ↓ | ↑NOR, MWM | 106 | |
| Rosiglitazone | 0.18 mg/day | 1 month | Oral: food | Tg2576 | n.c. | — | ↑CFC (9 months only) | 78 | |
| Rosiglitazone | 6 mg/kg/day | 4 weeks | Oral | APPswe/PSEN1dE9 | ↓Plaques; ↓insol Aβ(1-42) | ↓ | ↑MWM | 107 | |
| Rosiglitazone | 0.18 mg/day | 1 month | Oral: food | Tg2576 | n.c. | — | ↑CFC | 79 | |
| DSP-8658 | 150 mg/kg/day | 3 months | Oral: food | APPswe/PSEN1dE9 | ↓Plaques and sol Aβ | ↑Phagocytosis | ↑MWM | 58 | |
| β-Caryophyllene | 48 mg/kg/day | 10 weeks | Oral: gavage | APPswe/PSEN1dE9 | ↓Aβ burden | ↓ | ↑MWM | 108 | |
| Curcumin | 150 mg/kg/day | 4 weeks | IP | APPswe/PS1dE9 | — | ↓ | ↑MWM | 109 | |
| 3-O-β-glucopyranoside | 5 mg/kg/day | 2 months | Oral: gavage | APPswe/PS1dE9 | — | — | ↑MWM | 110 | |
| PPARγ | Genistein | 0.022 mg/kg/day | 3–6 days | APPswe//PSEN1dE9 | ↓Plaques; ↓sol Aβ ↓Aβ burden | — | ↑NOR; ↑PAT; ↑OHT; ↑HWM | 111 | |
| PPARα | WY-14643 | 0.2 g/l | 60 days | Oral: water | APPswe/PSEN1dE9 | ↓Plaques; ↓p-tau | ↓ | ↑MWM | 112 |
| 4-PB | 10 mg/l | 60 days | Oral: water | APPswe/PSEN1dE9 | ↓Plaques; ↓p-tau | ↓ | ↑MWM | 112 | |
| Simvastatin | 1 mg/kg/day | 2 weeks | Oral: gavage | 5XFAD | — | ↑Barnes maze | 34 | ||
| PPARβ/δ | GW742 | 30 mg/kg/day | 1 month | Oral: food | 5XFAD | ↓Plaques | ↓Glial activation | — | 55 |
| GW0742 | 30 mg/kg/day | 2 weeks | Oral: gavage | 5XFAD | ↓Brain APP/Aβ | ↓ | 87 | ||
| PPARγ and PPARβ/δ | T3D-959 | 0.3–3.0 mg/kg/day | 28 days | Oral: gavage | STZ-induced AD | ↓Aβ(1-42); ↓p-tau | — | ↑MWM | 113 |
| pan-PPAR | Bezafibrate | 0.5% food | 9 months | Oral: food | P301S | ↓tau pathology; ↓p-tau | ↓ | ↓Locomotor deficits and anxiety | 114 |
| pan-PPAR | GFT1803 | 1 mg/kg/day | 2 months | Oral: food | APPswe/PSEN1dE9 | n.c. plaques; n.c. sol Aβ ↓insol Aβ | — | ↑MWM | 115 |
| GFT1803 | 10 mg/kg/day | 2 months | Oral: food | APPswe/PSEN1dE9 | ↓Plaques; ↓sol Aβ(1-38, 40); n.c. Aβ(1-42); ↓sol Aβ | — | ↑MWM | 115 | |
| Pioglitazone | 50 mg/kg/day | 2 months | Oral: food | APPswe/PSEN1dE9 | n.c. plaques; n.c. sol Aβ ↓insol Aβ(1-38, 40); n.c. insol Aβ(1-42) | — | n.c. MWM | 115 | |
| LXR | GW3965 | 33 mg/kg/day | 4 months | Oral: food | Tg2576 | ↓Plaques; sol Aβ | — | ↑CFC | 116 |
| GW3965 | 2.5 mg/kg/day | 8 or 24 weeks | Oral: food | APPswe/PSEN1dE9 | ↑sol Aβ | — | ↑NOR; ↑MWM | 117 | |
| GW3965 | 33 mg/kg/day | 8 weeks | Oral: food | APPswe/PSEN1dE9 | ↓Plaques; ↑sol Aβ | — | ↑NOR; ↑MWM | 117 | |
| GW3965 | 33 mg/kg/day | 2 weeks | Oral: gavage | Tg2576 | ↓Plaques and sol Aβ | — | ↑OHB | 118 | |
| GW3965 | 50 mg/kg/day | 9 days | Oral: gavage | APPswe/PSEN1dE9 | n.c. sol and insol Aβ ↓amyloid burden | ↓ | ↑CFC | 101 | |
| GW3965 | 33 mg/kg | 12 weeks | Oral | 3xTg-AD | — | ↓ | ↑MWM | 77 | |
| GW3965 | 50 mg/kg/day | 6 days | Oral | 3xTg-AD | n.c. | — | ↑MWM | 76 | |
| TO901317 | 50 mg/kg/day | 6 days | Oral: gavage | APP23 | ↓sol Aβ | — | 67 | ||
| TO901317 | 10 mg/kg/day | 7 days | Oral: gavage | Tg2576 | n.c. | — | 119 | ||
| TO901317 | 30 mg/kg/day | 7 days | Oral: gavage | Tg2576 | ↓sol Aβ(1-42) | — | |||
| TO901317 | 50 mg/kg/day | 7 days | Oral: gavage | Tg2576 | ↓sol Aβ(1-42) | — | ↑CFC | 119 | |
| TO901317 | 50 mg/kg/day | 1 day | Oral: gavage | APP23 | — | n.c. | 120 | ||
| TO901317 | 20 mg/kg/day | 4 weeks | Oral: gavage | APP23 | ↓insol Aβ | ↓ | 120 | ||
| TO901317 | 25 mg/kg/day | 4 months | Oral: food | APP23 | ↓Plaques; and sol Aβ | — | ↑MWM | 121 | |
| TO901317 | 30 mg/kg/day | 6–9 weeks | Oral: food | APPswe/PSEN1dE9 | n.c. plaques | — | ↑NOR and object location | 122 | |
| TO901317 | 50 mg/kg/day | 7 weeks | Oral: gavage | APP23 | ↓Plaques and sol Aβ | — | ↑MWM (consolidation) | 59 | |
| TO901317 | 50 mg/kg/day | 6 days | Oral: gavage | APP23 | — | ↑Glia/plaques | 59 | ||
| LXR | TO901317 | 30 mg/kg/day | 30 days | Oral: gavage | APPswe/PSEN1dE9 | ↓Plaques | ↓ | ↑MWM | 123 |
| TO901317 | 25 mg/kg/day | 15 days | Oral: food | APP23 | ↓ISF Aβ(1-42) | — | 124 | ||
| TO901317 | 25 mg/kg/day | 50 days | Oral: food | APP23 | n.c. plaques; n.c. sol Aβ | — | ↑CFC; ↑RWM | 124 | |
| Compound 19 | 10 mg/kg; 3×/week | 6 weeks | IP | APPswe/PSEN1dE9 | ↓Plaques and sol Aβ(1-42) | — | 125 | ||
| Compound 9 | 50 mg/kg | 3 weeks | SC | Tg2576 | n.c. sol Aβ (↓trend) | — | ↑Locomotor phenotype | 126 | |
| Compound 9 | 20 mg/kg/day | 2 weeks | Oral | Rehsus monkey | ↑CSF Aβ | — | 126 | ||
| RXR | Bexarotene | 100 mg/kg/day | 3, 7, or 14 days | Oral: gavage | APPswe/PSEN1dE9 | ↓Plaques and sol Aβ | — | ↑CFC; ↑MWM | 51 |
| Bexarotene | 100 mg/kg/day | 90 days | Oral: gavage | APPswe/PSEN1dE9 | ↓sol Aβ n.c. plaques | — | ↑CFC; ↑MWM | 51 | |
| Bexarotene | 100 mg/kg/day | 20 days | Oral: gavage | APPPS1-21 | ↓Plaques, ↓sol Aβ | — | ↑CFC; ↑MWM | ||
| Bexarotene | 100 mg/kg/day | 3 or 9 days | Oral: gavage | Tg2576 | — | — | ↑OHB; ↑nesting | 51 | |
| Bexarotene | 100 mg/kg/day | 3 or 7 days | Oral: gavage | APPswe/PSEN1dE9 | n.c. plaques; n.c. sol Aβ | — | 127 | ||
| Bexarotene | 100 mg/kg/day | 15 days | Oral: gavage | APPswe/PSEN1dE9 | ↓ISF Aβ n.c. plaques | — | ↑RWM | 128 | |
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | APPswe/PSEN1dE9 | n.c. plaques; n.c. sol and insol Aβ | — | 129 | ||
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | 5XFAD | n.c. plaques; ↓sol Aβ(1-40); n.c. sol Aβ(1-42); n.c. insol Aβ | — | 129 | ||
| Bexarotene | 100 mg/kg/day | 26 days | Oral: gavage | APPPS1-21 | n.c. plaques; n.c. sol and insol Aβ | — | 129 | ||
| Bexarotene | 100 mg/kg/day | 19 days | Oral: gavage | APPPS1-21 | n.c. plaques; n.c. sol Aβ(1-40) | — | Unclear | 130 | |
| Bexarotene | 100 mg/kg/day | 0–36 h | Oral: gavage | APPswe/PSEN1dE9 | ↓ISF Aβ(1-40) | — | 131 | ||
| Bexarotene | 100 mg/kg/day | 3, 7, or 14 days | Oral: gavage | APPswe/PSEN1dE9 | n.c. plaques | n.c. | n.c. CFC | 132 | |
| Bexarotene | 2.5 mg/day | 10 days | Oral: gavage | ApoE4-TR | ↓Neuronal Aβ(1-42); ↓p-tau | — | ↑MWM; ↑NOR | 75 | |
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | E4FAD | Hippocampus: ↓sol. Aβ(1-42); ↓sol. oAβ n.c. total Aβ(1-42). Cortex: ↑sol Aβ(1-42); ↑sol. oAβ n.c. total Aβ(1-42) | — | — | 133 | |
| Bexarotene | 100 mg/kg/day | 7 days | Hydrogel | E4FAD | Hippocampus: n.c. except ↓sol. oAβ | — | — | 133 | |
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | E3FAD | Hippocampus: n.c except ↑sol. Aβ(1-42). Cortex: n.c. except ↑sol. oAβ | — | — | 133 | |
| Bexarotene | 100 mg/kg/day | 30 days | Hydrogel | E4FAD | n.c. Aβ | — | — | 133 | |
| Bexarotene | 100 mg/kg/day | 30 days | Hydrogel | E3FAD | n.c. Aβ | — | — | 133 | |
| Bexarotene | 25 mg/kg/day | 7 days | Oral: gavage | 5XFAD | n.c. Aβ | Mixed | n.c. Y maze | 134 | |
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | APPswe/PSEN1dE9 | ↓Plaque burden; ↓insol Aβ(1-42); n.c. insol Aβ(1-40) | ↑Phagocytosis | — | 57 | |
| Bexarotene | 100 mg/kg/day | 7 days | Oral: gavage | APPswe/PSEN1dE9 | Hippocampus: ↓sol. Aβ n.c. insol Aβ n.c. Aβ burden; n.c. plaques Cortex: n.c. | ↑ | ↑NOR; n.c. CFC | 135 | |
| RXR | LG100268 | 104.3 mg/kg/day | 7 days | Oral: gavage | E4FAD | Hippocampus: ↓sol. Aβ(1-42); ↓sol. oAβ n.c. total Aβ(1-42) Cortex: ↑sol Aβ(1-42); ↑sol. oAβ n.c. total Aβ(1-42) | — | — | 133 |
| LG100268 | 104.3 mg/kg/day | 7 days | Hydrogel | E4FAD | Hippocampus: n.c. except ↓sol. oAβ | — | — | 133 | |
| LG100268 | 104.3 mg/kg/day | 7 days | Oral: gavage | E3FAD | Hippocampus: n.c except ↑total Aβ(1-42). Cortex: n.c except ↑sol. Aβ(1-42) and sol. oAβ | — | — | 133 | |
| LG100268 | 104.3 mg/kg/day | 30 days | Hydrogel | E4FAD | n.c. Aβ | — | — | 133 | |
| LG100268 | 104.3 mg/kg/day | 30 days | Hydrogel | E3FAD | n.c. Aβ | — | — | 133 | |
| RARα | Am580 | 1 mg/kg; 3×/week | 12 weeks | IP | Tg2576 | ↓Plaques | — | ↑T maze; ↑nesting | 56 |
| Am580 | 1 mg/kg; 3×/week | 12 weeks | IP | APPswe/tau (P301L) | ↓p-tau | — | |||
| Am80 | 0.5 mg/kg/day | 14 weeks | Oral: food | APP23 | ↓insol Aβ(1-42); n.c. sol Aβ(1-40, 42) and insol Aβ(1-40) | — | n.c. MWM | 136 | |
| Am80 | 1 mg/kg/day | 4 weeks | Oral: food | SAMP8 | n.c. Aβ | — | 8-Arm radial maze test (reduction of age-related learning deterioration) | 137 | |
| RARβ | CD2019 | 1 mg/kg; 3×/week | 12 weeks | IP | Tg2576 | n.c. | — | n.c. T maze; n.c. nesting | 56 |
| RARγ | CD437 | 1 mg/kg; 3×/week | 12 weeks | IP | Tg2576 | n.c. | — | n.c. T maze; n.c. nesting | 56 |
| pan-RAR | Acitretin | 1 μl of 100 mM solution | Stereotactic injection | APPswe/PSEN1dE9 | ↓sol Aβ | — | — | 138 | |
| RAR and RXR | All-trans-retinoic acid | 20 mg/kg; 3×/week | 8 weeks | IP | APPswe/PSEN1dE9 | ↓Plaques and p-tau | ↓Glial activation | ↑MWM | 139 |
Ref., reference; IP, intraperitoneal; SC, subcutaneous; sol Aβ, soluble Aβ insol Aβ, insoluble Aβ oAβ, Aβ oligomers; n.c., no change; ISF, interstitial fluid; MWM, Morris water maze; CFC, contextual fear conditioning; NOR, novel object recognition; RWM, radial arm water maze; HWB, Hebb-Williams maze; PAT, passive avoidance test; OHT, odor habituation test; CSF, cerebrospinal fluid.
The role of NRs in the brain is not as well understood as in other periphery organs. However, it is clear that these receptors regulate a wide array of processes, such as lipid homeostasis, anti-inflammatory response, and synaptic function.
LXRs
LXRs are essential regulators of cholesterol homeostasis, lipogenesis, and inflammation. These receptors are activated by endogenous oxysterols. There are two LXR isoforms, LXRα and LXRβ, and both are expressed in the brain, although LXRβ exhibits the highest and most widespread expression pattern in this organ (24). LXR double knockout mice suffer from several brain abnormalities, including excessive lipid deposition, proliferation of astrocytes, and extensive neuronal loss (25). LXRβ-specific knockout mice also exhibit lipid accumulation, motor neuron degeneration, and astrogliosis (26). Both genetic models highlight the critical role of LXRs in the brain.
PPARs
In general, PPARs act as lipid sensors and regulate whole body metabolism and energy homeostasis. Fatty acids and other lipids are endogenous ligands of the PPARs (27). A recent study analyzed the PPAR expression pattern in the adult mouse and human brain, and it revealed that all PPAR isoforms are more highly expressed in neurons than other cell types. The order of abundance in the brain was found to be PPARβ/δ > PPARα ≥ PPARγ (28), which is in line with previous findings (29). It is noteworthy, however, that the authors did not report any expression analysis in oligodendrocytes, but solely in neurons, astrocytes, and microglia, although it has been reported that PPARs have relevant biological actions in oligodendrocytes (30). PPARs have a variety of roles in the brain. PPARγ has been widely associated with anti-inflammatory response, neuroprotection, neuronal differentiation, and neuronal function (31, 32). PPARα has been related to the regulation of energy homeostasis (33), synaptic function (34), neuroprotection, and anti-inflammatory response (35). PPARβ/δ is involved in neuroprotection, astroglial differentiation, oligodendrocyte differentiation, and myelination (36).
RARs
The functions of RARs in the brain have not been studied as extensively as the other NRs. Nonetheless, RARs seem to play a role related to cognitive function, neuronal differentiation, and locomotion in coordination with RXRs (37). It has also been suggested that RARs are important regulators of sleep and the circadian cycle (38). There are three isoforms, RARα, RARβ, and RARγ. These receptors are activated by vitamin A (all-trans retinol) and other retinoids (37). RARs are expressed throughout several regions in the adult brain, and the expression profile seems to be sex-specific, with each isoform exhibiting its own unique expression pattern (39, 40). Using genetic models for these receptors, it was demonstrated that mice lacking RARβ exhibit cognitive deficits associated with impaired long-term depression and long-term potentiation (41). Knockout of RARα revealed that this receptor is required for the homeostatic synaptic plasticity mediated by all-trans retinoic acid, highlighting the importance of these receptors for proper brain function (42).
RXRs
The roles of RXRs are very diverse owing to their ability to dimerize with other type II NRs, activating many different genes and pathways. Additionally, RXRs are able to switch between a homotetramer and homodimer structure, modulating DNA architecture (22). Although RXR homodimers have been shown to possess the ability to regulate PPARα metabolic pathways in vivo (43), only a small number of targets have been reported to be modulated by these homodimers, namely the chemokine (C-C motif) ligands 6 and 9 (44), and p21 (45). There are three isoforms of these receptors, RXRα, RXRβ, and RXRγ. RXRs are expressed in several regions of the adult brain, with specific patterns depending on the isoform and sex (39, 40). It is not clear what is the primary endogenous ligand(s) for these receptors. In spite of 9-cis-retinoic acid being initially proposed to be the endogenous ligand for RXR, several inconsistencies raised many doubts about this assumption. Moreover, phytanic acid and n-3 polyunsaturated fatty acids, such as the docosahexaenoic acid, have also been identified as RXR ligands (46).
NRs AND AD
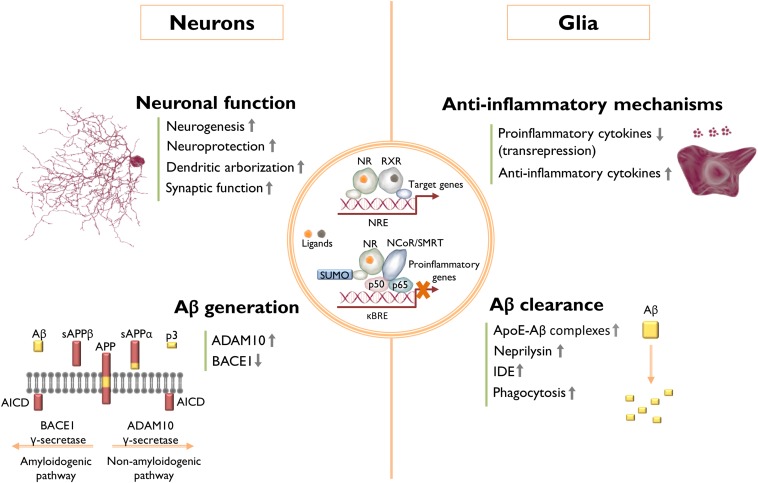
NRs have been extensively investigated in animal models of CNS disorders, and agonists of these receptors have a broad range of salutary effects in murine models of AD, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, stroke, and aging. A list of studies reported in the last 10–15 years and a summary of their major results arising from the pharmacological targeting of LXRs, PPARs, RXRs, and RARs in AD animal models are listed in Table 1. Overall, although there are inconsistencies among some reports, these NRs seem to be promising therapeutic targets for AD. It is likely that their beneficial effects do not rely on one particular mechanism, but rather on an array of different pathways, in some cases cell type-specific, which is in line with the fact that AD is a multifactorial disease. The mechanisms that have been described, so far, as underlying the beneficial effects of these NRs can be systematized into four categories: Aβ clearance; Aβ generation; anti-inflammatory mechanisms; and neuronal function (Fig. 1). Although some of these pathways are surely intertwined, many reports were able to pin-point, very specifically, the mechanisms through which NRs elicited their effects in the brain.
Fig. 1.
Effects of NR activation in AD. The underlying mechanisms by which the activation of NRs exerts beneficial effects in AD are not completely understood. Nonetheless, a number of studies have been able to dissect some of these mechanisms, which seem to be cell type-specific, and can be grouped in four different classes: 1) Aβ clearance: mainly through modulation of Aβ phagocytosis and enzymatic degradation; 2) anti-inflammatory mechanisms: repression and induction of pro-inflammatory and anti-inflammatory genes, respectively; 3) Aβ generation: modulation of APP processing by inhibiting BACE1 production or by shifting to the nonamyloidogenic pathway by induction of ADAM10, reducing Aβ production in both cases; and 4) neuronal function: increased synaptic function, neurogenesis, dendritic development, and protection against neuronal insults. The overall effect of a particular NR agonist likely arises from the modulation of several different pathways, rather than only one particular mechanism, and it is also likely that many of the different mechanisms reported for a specific NR might be, to some extent, related to each other. AICD, APP intracellular domain; NCoR/SMRT, NCoR/silencing mediator of retinoic acid thyroid hormone receptors; κBRE, NF-κB response element; NRE, NR response element; sAPPβ, soluble APPβ fragment.
Aβ clearance
The stimulation of Aβ clearance is one of the most popular strategies to ameliorate AD pathology. NRs are able to induce Aβ clearance primarily by stimulating its enzymatic degradation, either extracellularly or through microglial phagocytosis. The clearance of soluble forms of Aβ from the brain is regulated by apoE (47). Interestingly, the major source of apoE in the brain is astrocytes (48), and its expression and secretion are under the regulation of LXR:RXR heterodimers (49). Jiang et al. (50) were the first to demonstrate that ABCA1-mediated lipidation of apoE stimulates proteolytic degradation of Aβ through microglial neprilysin and extracellular insulin-degrading enzyme (IDE). Because apoE and ABCA1 are canonical targets of LXR, the authors treated the AD mouse model, Tg2576, with the LXR agonist, GW3965, to induce apoE lipidation. This treatment led to a marked reduction in both Aβ plaque burden and soluble Aβ, together with a dramatic improvement in contextual memory. Importantly, the ability of microglial cells to degrade Aβ was apoE isoform dependent, with isoform E2 being the most effective, followed by E3, and the less effective E4, which is also the more poorly lipidated isoform (50). This work provided a putative mechanistic explanation for the fact that the APOE4 allele is the major genetic risk factor for late-onset AD (2). Importantly, this report also paved the way for the following development of novel apoE-directed therapeutics for AD using NR agonists, such as for the case of the RXR agonist, bexarotene (51). The advantage of using RXR agonists relies on the activation of both LXRs and PPARs. PPARγ is also able to increase apoE and ABCA1 in the brains of AD animal models through the targeted increase in LXR expression (52). Additionally, PPARγ has been shown to induce IDE expression in neurons (53), and the Aβ degrading activity of an IDE-like metalloproteinase (54). PPARβ/δ activation in the AD mouse model, 5XFAD, drives the expression of neprilysin and IDE (55), which can also contribute to an increased clearance of Aβ. Similarly, RARα was shown to increase the expression of neprilysin and IDE, and stimulate microglia-mediated Aβ clearance (56). Interestingly, these authors also observed an increase in microglial apoE expression upon activation of RARα. Compelling evidence indicates that NR activation stimulates the phagocytic clearance of deposited forms of amyloid, resulting in the rapid reduction of plaque burden in murine models of AD (51, 52, 57–60). NR induction of Aβ phagocytosis by brain myeloid cells seems to rely on the expression of the phagocytic receptors, Axl and MerTK, both of which are induced by the RXR agonist, bexarotene. Furthermore, inhibiting the MerTK receptor abrogated the induction of phagocytosis by bexarotene in brain slices of the AD mouse model, APPswe/PSEN1dE9 (57). Thus, it has been proposed that the induction of MerTK and Axl expression by bexarotene licenses phagocytic activity of plaque-associated myeloid cells, promoting plaque clearance in AD. Additionally, PPARγ activation has been shown to stimulate microglial Aβ phagocytosis by increasing the expression of the scavenger receptor, CD36, and, interestingly, the combined treatment with PPARγ and RXR agonists was shown to have an additive effect on Aβ uptake by myeloid cells (58). Importantly, it has also been reported that LXR activation with TO901317 leads to an increase in microglial Aβ phagocytosis through the induction of ABCA1 and apoE specifically in astrocytes, which positively regulates phagocytosis in microglia (59). Thus, the increase in the efficiency of Aβ phagocytosis by brain myeloid cells mediated by NR agonists seems to be underlined by different cellular mechanisms in both myeloid cells, themselves, and also in surrounding nonmyeloid cells, depending on which NR is being targeted.
Aβ generation
Another mechanism by which NRs are able to reduce Aβ burden, is to suppress the generation of this peptide by modulation of APP processing. Recently, PPARα activation was shown drive the α-secretase ADAM metallopeptidase domain 10 (ADAM10) expression, shifting APP processing toward the non-amyloidogenic pathway, decreasing Aβ levels and increasing sAPPα (61). Knocking out PPARα from 5XFAD mice exacerbated Aβ deposition and, interestingly, the same effect was observed in Ppara−/− mice, which exhibited increased levels of endogenous Aβ. Furthermore, it has been observed that PPARγ represses β-secretase 1 (BACE1) promoter activity and expression (62), which might be explained, in part, by the induction of miR-188-3p, which targets BACE1 (63). Additionally, the reduction of BACE1 expression and Aβ levels, mediated by the PPARγ coactivator-1α (PGC-1α) (64) and by the NR interacting protein-1 (RIP140) (65), are both PPARγ-dependent, further supporting the role of this receptor in the modulation of Aβ production. Moreover, RARα and RARβ are also able to lower Aβ generation by driving ADAM10 expression, which leads to an increase in sAPPα as well (65). Interestingly, in vitro and in vivo data also support a mechanism by which LXR leads to a decrease in Aβ production. This mechanism is dependent on the upregulation of ABCA1, but possibly not dependent on cholesterol efflux (66, 67).
Anti-inflammatory mechanisms
The NRs act broadly to suppress pro-inflammatory gene expression, and many of the salutary actions of NR agonists arise from their anti-inflammatory actions. AD-associated neuroinflammation is primarily driven by microglia, the resident myeloid cells in the brain, and escalates with disease progression. Microglia are responsible for the production of a diverse range of pro-inflammatory cytokines and chemokines and other inflammatory mediators (68). Apart from resident microglia, it has been proposed that there is also infiltration of peripherally derived monocytes or macrophages into the AD brain, also contributing to the pathophysiology of the disease (69). Both the brain resident or peripherally derived myeloid cells respond to NR agonists by switching from an “activated” pro-inflammatory state to an “alternative activation” phenotype characterized by inhibition of pro-inflammatory gene expression and induction of anti-inflammatory genes. Importantly, alternative activation phenotypes include the induction of genes associated with the resolution of inflammation, tissue repair, and increased phagocytosis (69). Interestingly, it seems that there is still no consensus about the dominance of each of these states in AD and, in fact, analysis of brain samples from AD patients revealed the presence of markers from both activation states (70). These results suggest the presence of a heterogeneous population of immune effector cells, or even hybrid activation states, in the AD brain. Furthermore, astrocytes are also active players in AD-associated neuroinflammation. Similarly to microglia, Aβ stimulates the activation of astrocytes and triggers inflammatory signaling cascades (68). Several studies have shown that LXRs and PPARs exhibit an anti-inflammatory effect in AD animal models (Table 1). Although the transrepression of NF-κB target genes mediated by the sumoylation of LXR and PPARγ was studied in peripheral macrophages (23), one would expect that a similar mechanism of transrepression of inflammation is likely to occur in microglia and astrocytes. Interestingly, it has been reported that the activation and sumoylation of both LXRα and LXRβ in astrocytes leads them to interact with the signal transducer and activator of transcription 1 (STAT1), inhibiting the STAT1-mediated inflammatory gene expression (71), which may also account for the anti-inflammatory effects of LXR ligands. Additionally, the anti-inflammatory mechanisms of LXR in the context of AD pathology seem to be coupled to an increase in microglia ability to phagocyte Aβ (72).
Neuronal function
Several lines of evidence indicate that the activation of NRs is beneficial for neuronal function and development, as well as being neuroprotective. The identification of the mechanisms behind the modulation of neuronal function by NRs could be relevant in AD, allowing the identification of novel therapeutic targets. ChIP-seq and RNA-seq analysis of mice with targeted replacement of the endogenous murine apoE gene with the human APOE3 or APOE4 alleles (apoE3-TR and apoE4-TR mice) treated with the RXR agonist, bexarotene, revealed that this compound stimulates genetic programs associated with neuronal differentiation and development in an APOE isoform-independent fashion. This induction possibly occurs through epigenetic changes in these genes, namely, an enrichment in the histone marker associated with active promoter, H3K4me3, and a decrease in the marker related to promoter repression, H3K27me3 (73, 74). Bexarotene has been shown to increase the number of neuronal progenitors in the dentate gyrus of apoE3-TR and apoE4-TR mice and, more importantly, it rescued compromised dendritic structures in the hippocampus of apoE4-TR mice (73), cognitive impairment, and overall APOE4-driven brain pathology (75). Interestingly, the comparison of the RNA-seq data obtained from the brains of bexarotene-treated apoE3-TR and APP/PS1dE9/apoE3-TR mice revealed that Aβ accumulation affects the bexarotene-elicited changes in the transcriptome (74). Although bexarotene was shown to induce an upregulation of genes related to neurogenesis, neuronal development, and neuroprotection in both genotypes, the genes that are downregulated by this compound cluster in very distinct categories between genotypes. This is the case of genes related to the immune system and inflammatory response, which are predominantly downregulated in APP/PS1dE9/apoE3-TR mice, when compared with apoE3-TR mice, in response to bexarotene treatment (74). Importantly, this work highlights how the genetic program modulated by NR agonists might change in the context of AD, in comparison to a nonpathological state.
Recently, it was reported that activation of LXRs in a triple transgenic mouse model of AD (3xTg-AD) leads to a change in the DNA methylation status of genes related to synaptic function (Syp, Syn1, and Dlg3) and neurogenesis (Hmgb3 and Rbbp7), suggesting an increase in their expression, which was, in fact, confirmed for Syn1 (76). These results are in line with previous work published by the same authors, demonstrating that the beneficial effect of LXR on synaptic function in 3xTg-AD mice is dependent on synaptic-related protein synthesis, which is disrupted by Aβ (77). Another recent study reported that the activation of PPARα by simvastatin is able to improve memory function in 5XFAD mice, which is likely underlined by an increase in cAMP-response element binding protein (CREB) and brain-derived neurotrophic factor (34). Moreover, it has been shown that PPARγ activation by rosiglitazone rescues cognitive deficits in a Tg2576 AD mouse model, but it does not affect WT mouse performance, which was proposed to be underlined by a normalization of a dysregulated mitogen-activated protein kinase (ERK) signaling pathway in AD brains (78, 79). Further study of this mechanism led to the suggestion that PPARγ activation is able to restore memory consolidation in AD animals through an interaction with phosphorylated ERK in a multiprotein complex, including mitogen-activated protein kinase kinase (MEK) and ribosomal S6 kinase α-1 (p90RSK) (80). The authors propose a model in which PPARγ restores an AD-associated dysfunction of ERK signaling involved in memory consolidation through the recruitment of a multi-protein complex, for which a potential partner is suggested to be CREB-binding protein (CBP), a cofactor for both CREB and PPARγ, restoring proper ERK-dependent regulation of transcription of target genes involved in memory formation. This work is in agreement with the observation that rosiglitazone normalizes presynaptic function, ameliorates aberrant firing, and restores the output function of Tg2576 mouse dentate gyrus granule cells (81, 82). The authors propose that the regulation of different presynaptic vesicular proteins and potassium and calcium channels by rosiglitazone might be an underlying mechanism responsible for the beneficial effects of PPARγ activation. Furthermore, it has also been shown that the activation of PPARγ with rosiglitazone attenuated the decrease in dendritic filopodia and synapse density elicited by Aβ(1-42) in cultured rat hippocampal neurons, and it protected hippocampal slices from Aβ(1-42)-induced long-term potentiation deficits as well (83). These authors observed that Aβ(1-42) induces a reduction in the number of mitochondria in neuronal dendrites and spines, which is prevented by rosiglitazone; thus, it was proposed that this mitochondrial effect might be an underlying mechanism by which PPARγ activation by rosiglitazone prevents Aβ(1-42)-induced deficits in synapse formation and plasticity. Moreover, it has been found that activation of PPARα and PPARγ are also neuroprotective against Aβ-induced toxicity. Based on in vitro work, the underlying mechanism is proposed to be related to modulation of Wnt signaling reflected by an increase in β-catenin and reduction in glycogen synthase kinase-3β activity in neurons (84, 85). Moreover, NRs have recently been demonstrated to have direct neuroprotective effects in 5XFAD mice, as administration of the RXR agonist, bexarotene, resulted in prevention of neuronal loss observed in this model, which was accompanied by elevation of both pre- and postsynaptic markers and behavioral improvement (86). Similarly, the PPARβ/δ agonist, GW0742, has also been shown to have a neuroprotective effect in the 5XFAD model, particularly preventing neuronal loss in the subiculum (87). RARs have also been shown to be neuroprotective, driving the expression of brain-derived neurotrophic factor when activated, although not in an AD setting (88).
CLINICAL TRIALS
Although the animal studies are very promising, the clinical efficacy of NR activation in AD has yet to be demonstrated. A phase III trial to test the PPARγ agonist, rosiglitazone, as monotherapy in mild to moderate AD failed to show clinical efficacy (89), which may be related to the poor brain penetrance of this drug. Recently, Takeda and Zinfandel Pharmaceuticals initiated a phase III trial to test the efficacy of the PPARγ agonist, pioglitazone, in AD, and this trial is currently underway. Additionally, Heneka, Fink, and Doblhammer (90) found that chronic treatment of diabetic patients with pioglitazone is associated with a reduction of dementia risk by 47%, through analysis of data obtained from the German health insurance registry over a period of 6 years, suggesting a neuroprotective effect. Cummings et al. (91) have recently reported the outcome of a small phase II trial of the RXR ligand, bexarotene, in AD patients. The trial, involving a 30 day treatment of mild to moderate AD patients, reported a significant decrease in brain amyloid burden in APOE4 noncarriers accompanied by a concomitant increase in the levels of serum Aβ(1-42), suggesting an increased clearance of Aβ from the brain to the periphery. There was no change in amyloid burden in APOE4 carriers. The study was not powered to assess cognition. Importantly, bexarotene treatment caused a significant elevation of serum triglycerides, which may represent a cardiovascular risk for the patients. A phase Ib trial was also performed in healthy subjects, homozygous for the APOE3 allele, to determine whether bexarotene could modulate apoE and Aβ levels in these subjects (92). This drug modestly increased apoE levels in cerebrospinal fluid and failed to reduce Aβ levels, which was attributed to the poor penetration of bexarotene into the CNS, which might hamper the utility of this drug in clinical practice. It is noteworthy that bexarotene effects depend on the APOE genotype, which might warrant further investigation.
CONCLUSIONS
The activation of NRs seems to be a promising therapeutic strategy for AD; however, the mechanisms underlying the salutary effects of NRs are still not fully understood. The identification of the precise mechanisms that are regulated by these NRs could open the door for novel and more targeted pharmacological interventions directed at new therapeutic targets. Furthermore, it is also important to keep in mind the systemic effects of NR agonists, their pharmacodynamics, and brain penetrance, as these types of drugs are considered for use in CNS disorders. A more detailed characterization of the pathophysiological mechanisms in AD is also warranted, especially as new players are being identified, such as the case of infiltrating monocytes. Moreover, targeting different classes of NRs, such as estrogen receptors, might also be of therapeutic interest for AD.
Footnotes
Abbreviations:
- Aβ
- amyloid-β AD, Alzheimer’s disease
- ADAM10
- ADAM metallopeptidase domain 10
- APP
- amyloid precursor protein
- APPswe
- human amyloid precursor protein harboring the Swedish mutation
- BACE1
- β-secretase 1
- CREB
- cAMP-response element binding protein
- IDE
- insulin-degrading enzyme
- LXR
- liver X receptor
- NCoR
- nuclear receptor corepressor
- NF-κB
- nuclear factor-κB
- NR
- nuclear receptor
- PSEN1
- presenilin-1
- RAR
- retinoic acid receptor
- RXR
- retinoid X receptor
- sAPPα
- soluble amyloid precursor protein α fragment
This work was supported by a Weston Brain Institute Postdoctoral Fellowship Award from the W. Garfield Weston Foundation.
REFERENCES
- 1.Brookmeyer R., Johnson E., Ziegler-Graham K., and Arrighi H. M.. 2007. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 3: 186–191. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D. J., and Hardy J.. 2016. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano-Pozo A., Frosch M. P., Masliah E., and Hyman B. T.. 2011. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1: a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kummer M. P., and Heneka M. T.. 2014. Truncated and modified amyloid-beta species. Alzheimers Res. Ther. 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow V. W., Mattson M. P., Wong P. C., and Gleichmann M.. 2010. An overview of APP processing enzymes and products. Neuromolecular Med. 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., Yarasheski K. E., and Bateman R. J.. 2010. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 330: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., et al. 2011. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 3: 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan P., and Grutzendler J.. 2016. Attenuation of beta-amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J. Neurosci. 36: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K. H., Mistl C., Rothacher S., Ledermann B., Burki K., Frey P., Paganetti P. A., et al. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA. 94: 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., and Cole G.. 1996. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 274: 99–102. [DOI] [PubMed] [Google Scholar]
- 11.Moechars D., Dewachter I., Lorent K., Reverse D., Baekelandt V., Naidu A., Tesseur I., Spittaels K., Haute C. V., Checler F., et al. 1999. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274: 6483–6492. [DOI] [PubMed] [Google Scholar]
- 12.Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., and McConlogue L.. 2000. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20: 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chishti M. A., Yang D. S., Janus C., Phinney A. L., Horne P., Pearson J., Strome R., Zuker N., Loukides J., French J., et al. 2001. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276: 21562–21570. [DOI] [PubMed] [Google Scholar]
- 14.Borchelt D. R., Ratovitski T., van Lare J., Lee M. K., Gonzales V., Jenkins N. A., Copeland N. G., Price D. L., and Sisodia S. S.. 1997. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 19: 939–945. [DOI] [PubMed] [Google Scholar]
- 15.Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., et al. 2004. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 13: 159–170. [DOI] [PubMed] [Google Scholar]
- 16.Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., et al. 2006. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26: 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshiyama Y., Higuchi M., Zhang B., Huang S. M., Iwata N., Saido T. C., Maeda J., Suhara T., Trojanowski J. Q., and Lee V. M.. 2007. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 53: 337–351. [DOI] [PubMed] [Google Scholar]
- 18.Oddo S., Caccamo A., Shepherd J. D., Murphy M. P., Golde T. E., Kayed R., Metherate R., Mattson M. P., Akbari Y., and LaFerla F. M.. 2003. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 39: 409–421. [DOI] [PubMed] [Google Scholar]
- 19.Billings L. M., Oddo S., Green K. N., McGaugh J. L., and LaFerla F. M.. 2005. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 45: 675–688. [DOI] [PubMed] [Google Scholar]
- 20.Evans R. M., and Mangelsdorf D. J.. 2014. Nuclear receptors, RXR, and the big bang. Cell. 157: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sever R., and Glass C. K.. 2013. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 5: a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson M. I., and Xia Z.. 2012. The retinoid X receptors and their ligands. Biochim. Biophys. Acta. 1821: 21–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass C. K., and Saijo K.. 2010. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10: 365–376. [DOI] [PubMed] [Google Scholar]
- 24.Whitney K. D., Watson M. A., Collins J. L., Benson W. G., Stone T. M., Numerick M. J., Tippin T. K., Wilson J. G., Winegar D. A., and Kliewer S. A.. 2002. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol. Endocrinol. 16: 1378–1385. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Schuster G. U., Hultenby K., Zhang Q., Andersson S., and Gustafsson J. A.. 2002. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. USA. 99: 13878–13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson S., Gustafsson N., Warner M., and Gustafsson J. A.. 2005. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc. Natl. Acad. Sci. USA. 102: 3857–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y. X. 2010. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 20: 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warden A., Truitt J., Merriman M., Ponomareva O., Jameson K., Ferguson L. B., Mayfield R. D., and Harris R. A.. 2016. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 6: 27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno S., Farioli-Vecchioli S., and Ceru M. P.. 2004. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 123: 131–145. [DOI] [PubMed] [Google Scholar]
- 30.Heneka M. T., and Landreth G. E.. 2007. PPARs in the brain. Biochim. Biophys. Acta. 1771: 1031–1045. [DOI] [PubMed] [Google Scholar]
- 31.Kapadia R., Yi J. H., and Vemuganti R.. 2008. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 13: 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintanilla R. A., Utreras E., and Cabezas-Opazo F. A.. 2014. Role of PPAR gamma in the differentiation and function of neurons. PPAR Res. 2014: 768594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakravarthy M. V., Zhu Y., Lopez M., Yin L., Wozniak D. F., Coleman T., Hu Z., Wolfgang M., Vidal-Puig A., Lane M. D., et al. 2007. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J. Clin. Invest. 117: 2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy A., Jana M., Kundu M., Corbett G. T., Rangaswamy S. B., Mishra R. K., Luan C. H., Gonzalez F. J., and Pahan K.. 2015. HMG-CoA reductase inhibitors bind to PPARalpha to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. 22: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidaleo M., Fanelli F., Ceru M. P., and Moreno S.. 2014. Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARalpha) and its lipid ligands. Curr. Med. Chem. 21: 2803–2821. [DOI] [PubMed] [Google Scholar]
- 36.Hall M. G., Quignodon L., and Desvergne B.. 2008. Peroxisome proliferator-activated receptor beta/delta in the brain: facts and hypothesis. PPAR Res. 2008: 780452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane M. A., and Bailey S. J.. 2005. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 75: 275–293. [DOI] [PubMed] [Google Scholar]
- 38.Ransom J., Morgan P. J., McCaffery P. J., and Stoney P. N.. 2014. The rhythm of retinoids in the brain. J. Neurochem. 129: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krezel W., Kastner P., and Chambon P.. 1999. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 89: 1291–1300. [DOI] [PubMed] [Google Scholar]
- 40.Arfaoui A., Lobo M. V., Boulbaroud S., Ouichou A., Mesfioui A., and Arenas M. I.. 2013. Expression of retinoic acid receptors and retinoid X receptors in normal and vitamin A deficient adult rat brain. Ann. Anat. 195: 111–121. [DOI] [PubMed] [Google Scholar]
- 41.Chiang M. Y., Misner D., Kempermann G., Schikorski T., Giguere V., Sucov H. M., Gage F. H., Stevens C. F., and Evans R. M.. 1998. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 21: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 42.Sarti F., Schroeder J., Aoto J., and Chen L.. 2012. Conditional RARalpha knockout mice reveal acute requirement for retinoic acid and RARalpha in homeostatic plasticity. Front. Mol. Neurosci. 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IJpenberg A., Tan N. S., Gelman L., Kersten S., Seydoux J., Xu J., Metzger D., Canaple L., Chambon P., Wahli W., et al. 2004. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 23: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Núñez V., Alameda D., Rico D., Mota R., Gonzalo P., Cedenilla M., Fischer T., Bosca L., Glass C. K., Arroyo A. G., et al. 2010. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc. Natl. Acad. Sci. USA. 107: 10626–10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka T., Suh K. S., Lo A. M., and De Luca L. M.. 2007. p21WAF1/CIP1 is a common transcriptional target of retinoid receptors: pleiotropic regulatory mechanism through retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer and RXR/RXR homodimer. J. Biol. Chem. 282: 29987–29997. [DOI] [PubMed] [Google Scholar]
- 46.Desvergne B. 2007. RXR: from partnership to leadership in metabolic regulations. Vitam. Horm. 75: 1–32. [DOI] [PubMed] [Google Scholar]
- 47.Holtzman D. M. 2001. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer’s disease and cerebral amyloid angiopathy. J. Mol. Neurosci. 17: 147–155. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., and Liu Q.. 2015. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 6: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang Y., Lin S., Beyer T. P., Zhang Y., Wu X., Bales K. R., DeMattos R. B., May P. C., Li S. D., Jiang X. C., et al. 2004. A liver X receptor and retinoid X receptor heterodimer mediates apolipoprotein E expression, secretion and cholesterol homeostasis in astrocytes. J. Neurochem. 88: 623–634. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., et al. 2008. ApoE promotes the proteolytic degradation of Abeta. Neuron. 58: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., Casali B. T., Restivo J. L., Goebel W. D., James M. J., et al. 2012. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 335: 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandrekar-Colucci S., Karlo J. C., and Landreth G. E.. 2012. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 32: 10117–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du J., Zhang L., Liu S., Zhang C., Huang X., Li J., Zhao N., and Wang Z.. 2009. PPARgamma transcriptionally regulates the expression of insulin-degrading enzyme in primary neurons. Biochem. Biophys. Res. Commun. 383: 485–490. [DOI] [PubMed] [Google Scholar]
- 54.Espuny-Camacho I., Dominguez D., Merchiers P., Van Rompaey L., Selkoe D., and De Strooper B.. 2010. Peroxisome proliferator-activated receptor gamma enhances the activity of an insulin degrading enzyme-like metalloprotease for amyloid-beta clearance. J. Alzheimers Dis. 20: 1119–1132. [DOI] [PubMed] [Google Scholar]
- 55.Kalinin S., Richardson J. C., and Feinstein D. L.. 2009. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 6: 431–437. [DOI] [PubMed] [Google Scholar]
- 56.Goncalves M. B., Clarke E., Hobbs C., Malmqvist T., Deacon R., Jack J., and Corcoran J. P.. 2013. Amyloid beta inhibits retinoic acid synthesis exacerbating Alzheimer disease pathology which can be attenuated by an retinoic acid receptor alpha agonist. Eur. J. Neurosci. 37: 1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage J. C., Jay T., Goduni E., Quigley C., Mariani M. M., Malm T., Ransohoff R. M., Lamb B. T., and Landreth G. E.. 2015. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer’s disease. J. Neurosci. 35: 6532–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M. P., and Heneka M. T.. 2012. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32: 17321–17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terwel D., Steffensen K. R., Verghese P. B., Kummer M. P., Gustafsson J. A., Holtzman D. M., and Heneka M. T.. 2011. Critical role of astroglial apolipoprotein E and liver X receptor-alpha expression for microglial Abeta phagocytosis. J. Neurosci. 31: 7049–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hjorth E., Zhu M., Toro V. C., Vedin I., Palmblad J., Cederholm T., Freund-Levi Y., Faxen-Irving G., Wahlund L. O., Basun H., et al. 2013. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 35: 697–713. [DOI] [PubMed] [Google Scholar]
- 61.Corbett G. T., Gonzalez F. J., and Pahan K.. 2015. Activation of peroxisome proliferator-activated receptor alpha stimulates ADAM10-mediated proteolysis of APP. Proc. Natl. Acad. Sci. USA. 112: 8445–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sastre M., Dewachter I., Rossner S., Bogdanovic N., Rosen E., Borghgraef P., Evert B. O., Dumitrescu-Ozimek L., Thal D. R., Landreth G., et al. 2006. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 103: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., Hu M., Teng Z., Tang Y. P., and Chen C.. 2014. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J. Neurosci. 34: 14919–14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katsouri L., Parr C., Bogdanovic N., Willem M., and Sastre M.. 2011. PPARgamma co-activator-1alpha (PGC-1alpha) reduces amyloid-beta generation through a PPARgamma-dependent mechanism. J. Alzheimers Dis. 25: 151–162. [DOI] [PubMed] [Google Scholar]
- 65.Blondrath K., Steel J. H., Katsouri L., Ries M., Parker M. G., Christian M., and Sastre M.. 2016. The nuclear cofactor receptor interacting protein-140 (RIP140) regulates the expression of genes involved in Abeta generation. Neurobiol. Aging. 47: 180–191. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y., Yao J., Kim T. W., and Tall A. R.. 2003. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J. Biol. Chem. 278: 27688–27694. [DOI] [PubMed] [Google Scholar]
- 67.Koldamova R. P., Lefterov I. M., Staufenbiel M., Wolfe D., Huang S., Glorioso J. C., Walter M., Roth M. G., and Lazo J. S.. 2005. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer’s disease. J. Biol. Chem. 280: 4079–4088. [DOI] [PubMed] [Google Scholar]
- 68.Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., Jacobs A. H., Wyss-Coray T., Vitorica J., Ransohoff R. M., et al. 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cameron B., and Landreth G. E.. 2010. Inflammation, microglia, and Alzheimer’s disease. Neurobiol. Dis. 37: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colton C. A., Mott R. T., Sharpe H., Xu Q., Van Nostrand W. E., and Vitek M. P.. 2006. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflammation. 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J. H., Park S. M., Kim O. S., Lee C. S., Woo J. H., Park S. J., Joe E. H., and Jou I.. 2009. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol. Cell. 35: 806–817. [DOI] [PubMed] [Google Scholar]
- 72.Zelcer N., Khanlou N., Clare R., Jiang Q., Reed-Geaghan E. G., Landreth G. E., Vinters H. V., and Tontonoz P.. 2007. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver X receptors. Proc. Natl. Acad. Sci. USA. 104: 10601–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mounier A., Georgiev D., Nam K. N., Fitz N. F., Castranio E. L., Wolfe C. M., Cronican A. A., Schug J., Lefterov I., and Koldamova R.. 2015. Bexarotene-activated retinoid X receptors regulate neuronal differentiation and dendritic complexity. J. Neurosci. 35: 11862–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nam K. N., Mounier A., Fitz N. F., Wolfe C., Schug J., Lefterov I., and Koldamova R.. 2016. RXR controlled regulatory networks identified in mouse brain counteract deleterious effects of Abeta oligomers. Sci. Rep. 6: 24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boehm-Cagan A., and Michaelson D. M.. 2014. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J. Neurosci. 34: 7293–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandoval-Hernández A. G., Hernandez H. G., Restrepo A., Munoz J. I., Bayon G. F., Fernandez A. F., Fraga M. F., Cardona-Gomez G. P., Arboleda H., and Arboleda G. H.. 2016. Liver X receptor agonist modifies the DNA methylation profile of synapse and neurogenesis-related genes in the triple transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 58: 243–253. [DOI] [PubMed] [Google Scholar]
- 77.Sandoval-Hernández A. G., Buitrago L., Moreno H., Cardona-Gomez G. P., and Arboleda G.. 2015. Role of liver X receptor in AD pathophysiology. PLoS One. 10: e0145467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Rivera J., Denner L., and Dineley K. T.. 2011. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav. Brain Res. 216: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denner L. A., Rodriguez-Rivera J., Haidacher S. J., Jahrling J. B., Carmical J. R., Hernandez C. M., Zhao Y., Sadygov R. G., Starkey J. M., Spratt H., et al. 2012. Cognitive enhancement with rosiglitazone links the hippocampal PPARgamma and ERK MAPK signaling pathways. J. Neurosci. 32: 16725–16735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jahrling J. B., Hernandez C. M., Denner L., and Dineley K. T.. 2014. PPARgamma recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 34: 4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nenov M. N., Laezza F., Haidacher S. J., Zhao Y., Sadygov R. G., Starkey J. M., Spratt H., Luxon B. A., Dineley K. T., and Denner L.. 2014. Cognitive enhancing treatment with a PPARgamma agonist normalizes dentate granule cell presynaptic function in Tg2576 APP mice. J. Neurosci. 34: 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nenov M. N., Tempia F., Denner L., Dineley K. T., and Laezza F.. 2015. Impaired firing properties of dentate granule neurons in an Alzheimer’s disease animal model are rescued by PPARgamma agonism. J. Neurophysiol. 113: 1712–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu S., Liu G., Bao X., Wu J., Li S., Zheng B., Anwyl R., and Wang Q.. 2014. Rosiglitazone prevents amyloid-beta oligomer-induced impairment of synapse formation and plasticity via increasing dendrite and spine mitochondrial number. J. Alzheimers Dis. 39: 239–251. [DOI] [PubMed] [Google Scholar]
- 84.Santos M. J., Quintanilla R. A., Toro A., Grandy R., Dinamarca M. C., Godoy J. A., and Inestrosa N. C.. 2005. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J. Biol. Chem. 280: 41057–41068. [DOI] [PubMed] [Google Scholar]
- 85.Inestrosa N. C., Godoy J. A., Quintanilla R. A., Koenig C. S., and Bronfman M.. 2005. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp. Cell Res. 304: 91–104. [DOI] [PubMed] [Google Scholar]
- 86.Mariani M. M., Malm T., Lamb R., Jay T. R., Neilson L., Casali B., Medarametla L., and Landreth G. E.. 2017. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep. 7: 42270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malm T., Mariani M., Donovan L. J., Neilson L., and Landreth G. E.. 2015. Activation of the nuclear receptor PPARdelta is neuroprotective in a transgenic mouse model of Alzheimer’s disease through inhibition of inflammation. J. Neuroinflammation. 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsuki H., Kurimoto E., Takemori S., Kurauchi Y., Hisatsune A., Isohama Y., Izumi Y., Kume T., Shudo K., and Akaike A.. 2009. Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J. Neurochem. 110: 707–718. [DOI] [PubMed] [Google Scholar]
- 89.Gold M., Alderton C., Zvartau-Hind M., Egginton S., Saunders A. M., Irizarry M., Craft S., Landreth G., Linnamagi U., and Sawchak S.. 2010. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 30: 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heneka M. T., Fink A., and Doblhammer G.. 2015. Effect of pioglitazone medication on the incidence of dementia. Ann. Neurol. 78: 284–294. [DOI] [PubMed] [Google Scholar]
- 91.Cummings J. L., Zhong K., Kinney J. W., Heaney C., Moll-Tudla J., Joshi A., Pontecorvo M., Devous M., Tang A., and Bena J.. 2016. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers Res. Ther. 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosal K., Haag M., Verghese P. B., West T., Veenstra T., Braunstein J. B., Bateman R. J., Holtzman D. M., and Landreth G. E.. 2016. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement. 2: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan Q., Zhang J., Liu H., Babu-Khan S., Vassar R., Biere A. L., Citron M., and Landreth G.. 2003. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 23: 7504–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lacombe P., Mathews P. M., Schmidt S. D., Breidert T., Heneka M. T., Landreth G. E., Feinstein D. L., and Galea E.. 2004. Effect of anti-inflammatory agents on transforming growth factor beta over-expressing mouse brains: a model revised. J. Neuroinflammation. 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heneka M. T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O’Banion K., Klockgether T., Van Leuven F., and Landreth G. E.. 2005. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 128: 1442–1453. [DOI] [PubMed] [Google Scholar]
- 96.Nicolakakis N., Aboulkassim T., Ongali B., Lecrux C., Fernandes P., Rosa-Neto P., Tong X. K., and Hamel E.. 2008. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J. Neurosci. 28: 9287–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Searcy J. L., Phelps J. T., Pancani T., Kadish I., Popovic J., Anderson K. L., Beckett T. L., Murphy M. P., Chen K. C., Blalock E. M., et al. 2012. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 30: 943–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Masciopinto F., Di Pietro N., Corona C., Bomba M., Pipino C., Curcio M., Di Castelnuovo A., Ciavardelli D., Silvestri E., Canzoniero L. M., et al. 2012. Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death Dis. 3: e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Papadopoulos P., Rosa-Neto P., Rochford J., and Hamel E.. 2013. Pioglitazone improves reversal learning and exerts mixed cerebrovascular effects in a mouse model of Alzheimer’s disease with combined amyloid-beta and cerebrovascular pathology. PLoS One. 8: e68612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prakash A., and Kumar A.. 2014. Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of beta-amyloid animal model of Alzheimer’s disease. Neurotox. Res. 25: 335–347. [DOI] [PubMed] [Google Scholar]
- 101.Skerrett R., Pellegrino M. P., Casali B. T., Taraboanta L., and Landreth G. E.. 2015. Combined liver X receptor/peroxisome proliferator-activated receptor gamma agonist treatment reduces amyloid beta levels and improves behavior in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 290: 21591–21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toba J., Nikkuni M., Ishizeki M., Yoshii A., Watamura N., Inoue T., and Ohshima T.. 2016. PPARgamma agonist pioglitazone improves cerebellar dysfunction at pre-Abeta deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem. Biophys. Res. Commun. 473: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 103.Pedersen W. A., McMillan P. J., Kulstad J. J., Leverenz J. B., Craft S., and Haynatzki G. R.. 2006. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 199: 265–273. [DOI] [PubMed] [Google Scholar]
- 104.Escribano L., Simon A. M., Perez-Mediavilla A., Salazar-Colocho P., Del Rio J., and Frechilla D.. 2009. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochem. Biophys. Res. Commun. 379: 406–410. [DOI] [PubMed] [Google Scholar]
- 105.Toledo E. M., and Inestrosa N. C.. 2010. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry. 15: 272–285. [DOI] [PubMed] [Google Scholar]
- 106.Escribano L., Simon A. M., Gimeno E., Cuadrado-Tejedor M., Lopez de Maturana R., Garcia-Osta A., Ricobaraza A., Perez-Mediavilla A., Del Rio J., and Frechilla D.. 2010. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 35: 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Reilly J. A., and Lynch M.. 2012. Rosiglitazone improves spatial memory and decreases insoluble Abeta(1-42) in APP/PS1 mice. J. Neuroimmune Pharmacol. 7: 140–144. [DOI] [PubMed] [Google Scholar]
- 108.Cheng Y., Dong Z., and Liu S.. 2014. beta-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 mice through CB2 receptor activation and the PPARgamma pathway. Pharmacology. 94: 1–12. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z. J., Li Z. H., Liu L., Tang W. X., Wang Y., Dong M. R., and Xiao C.. 2016. Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 7: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song N., Zhang L., Chen W., Zhu H., Deng W., Han Y., Guo J., and Qin C.. 2016. Cyanidin 3-O-beta-glucopyranoside activates peroxisome proliferator-activated receptor-gamma and alleviates cognitive impairment in the APP(swe)/PS1(DeltaE9) mouse model. Biochim. Biophys. Acta. 1862: 1786–1800. [DOI] [PubMed] [Google Scholar]
- 111.Bonet-Costa V., Herranz-Perez V., Blanco-Gandia M., Mas-Bargues C., Ingles M., Garcia-Tarraga P., Rodriguez-Arias M., Minarro J., Borras C., Garcia-Verdugo J. M., et al. 2016. Clearing amyloid-beta through PPARgamma/ApoE activation by genistein is a treatment of experimental Alzheimer’s disease. J. Alzheimers Dis. 51: 701–711. [DOI] [PubMed] [Google Scholar]
- 112.Inestrosa N. C., Carvajal F. J., Zolezzi J. M., Tapia-Rojas C., Serrano F., Karmelic D., Toledo E. M., Toro A., Toro J., and Santos M. J.. 2013. Peroxisome proliferators reduce spatial memory impairment, synaptic failure, and neurodegeneration in brains of a double transgenic mice model of Alzheimer’s disease. J. Alzheimers Dis. 33: 941–959. [DOI] [PubMed] [Google Scholar]
- 113.Tong M., Deochand C., Didsbury J., and de la Monte S. M.. 2016. T3D-959: a multi-faceted disease remedial drug candidate for the treatment of Alzheimer’s disease. J. Alzheimers Dis. 51: 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dumont M., Stack C., Elipenahli C., Jainuddin S., Gerges M., Starkova N., Calingasan N. Y., Yang L., Tampellini D., Starkov A. A., et al. 2012. Bezafibrate administration improves behavioral deficits and tau pathology in P301S mice. Hum. Mol. Genet. 21: 5091–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kummer M. P., Schwarzenberger R., Sayah-Jeanne S., Dubernet M., Walczak R., Hum D. W., Schwartz S., Axt D., and Heneka M. T.. 2015. Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol. Neurobiol. 51: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang Q., Heneka M., and Landreth G. E.. 2008. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS Drugs. 22: 1–14. [DOI] [PubMed] [Google Scholar]
- 117.Donkin J. J., Stukas S., Hirsch-Reinshagen V., Namjoshi D., Wilkinson A., May S., Chan J., Fan J., Collins J., and Wellington C. L.. 2010. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 285: 34144–34154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wesson D. W., Borkowski A. H., Landreth G. E., Nixon R. A., Levy E., and Wilson D. A.. 2011. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J. Neurosci. 31: 15962–15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riddell D. R., Zhou H., Comery T. A., Kouranova E., Lo C. F., Warwick H. K., Ring R. H., Kirksey Y., Aschmies S., Xu J., et al. 2007. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Mol. Cell. Neurosci. 34: 621–628. [DOI] [PubMed] [Google Scholar]
- 120.Lefterov I., Bookout A., Wang Z., Staufenbiel M., Mangelsdorf D., and Koldamova R.. 2007. Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol. Neurodegener. 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fitz N. F., Cronican A., Pham T., Fogg A., Fauq A. H., Chapman R., Lefterov I., and Koldamova R.. 2010. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J. Neurosci. 30: 6862–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vanmierlo T., Rutten K., Dederen J., Bloks V. W., van Vark-van der Zee L. C., Kuipers F., Kiliaan A., Blokland A., Sijbrands E. J., Steinbusch H., et al. 2011. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging. 32: 1262–1272. [DOI] [PubMed] [Google Scholar]
- 123.Cui W., Sun Y., Wang Z., Xu C., Peng Y., and Li R.. 2012. Liver X receptor activation attenuates inflammatory response and protects cholinergic neurons in APP/PS1 transgenic mice. Neuroscience. 210: 200–210. [DOI] [PubMed] [Google Scholar]
- 124.Fitz N. F., Castranio E. L., Carter A. Y., Kodali R., Lefterov I., and Koldamova R.. 2014. Improvement of memory deficits and amyloid-beta clearance in aged APP23 mice treated with a combination of anti-amyloid-beta antibody and LXR agonist. J. Alzheimers Dis. 41: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu Y., Yang Y., Yu Y., Wen G., Shang N., Zhuang W., Lu D., Zhou B., Liang B., Yue X., et al. 2013. Synthesis and identification of new flavonoids targeting liver X receptor beta involved pathway as potential facilitators of Abeta clearance with reduced lipid accumulation. J. Med. Chem. 56: 6033–6053. [DOI] [PubMed] [Google Scholar]
- 126.Stachel S. J., Zerbinatti C., Rudd M. T., Cosden M., Suon S., Nanda K. K., Wessner K., DiMuzio J., Maxwell J., Wu Z., et al. 2016. Identification and in vivo evaluation of liver X receptor beta-selective agonists for the potential treatment of Alzheimer’s disease. J. Med. Chem. 59: 3489–3498. [DOI] [PubMed] [Google Scholar]
- 127.Price A. R., Xu G., Siemienski Z. B., Smithson L. A., Borchelt D. R., Golde T. E., and Felsenstein K. M.. 2013. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 340: 924-d. [DOI] [PubMed] [Google Scholar]
- 128.Fitz N. F., Cronican A. A., Lefterov I., and Koldamova R.. 2013. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 340: 924-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Veeraraghavalu K., Zhang C., Miller S., Hefendehl J. K., Rajapaksha T. W., Ulrich J., Jucker M., Holtzman D. M., Tanzi R. E., Vassar R., et al. 2013. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 340: 924-f. [DOI] [PubMed] [Google Scholar]
- 130.Tesseur I., Lo A. C., Roberfroid A., Dietvorst S., Van Broeck B., Borgers M., Gijsen H., Moechars D., Mercken M., Kemp J., et al. 2013. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 340: 924-e. [DOI] [PubMed] [Google Scholar]
- 131.Ulrich J. D., Burchett J. M., Restivo J. L., Schuler D. R., Verghese P. B., Mahan T. E., Landreth G. E., Castellano J. M., Jiang H., Cirrito J. R., et al. 2013. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol. Neurodegener. 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.LaClair K. D., Manaye K. F., Lee D. L., Allard J. S., Savonenko A. V., Troncoso J. C., and Wong P. C.. 2013. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol. Neurodegener. 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tai L. M., Koster K. P., Luo J., Lee S. H., Wang Y. T., Collins N. C., Ben Aissa M., Thatcher G. R., and LaDu M. J.. 2014. Amyloid-beta pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. J. Biol. Chem. 289: 30538–30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Casali B. T., Corona A. W., Mariani M. M., Karlo J. C., Ghosal K., and Landreth G. E.. 2015. Omega-3 fatty acids augment the actions of nuclear receptor agonists in a mouse model of Alzheimer’s disease. J. Neurosci. 35: 9173–9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corona A. W., Kodoma N., Casali B. T., and Landreth G. E.. 2016. ABCA1 is necessary for bexarotene-mediated clearance of soluble amyloid beta from the hippocampus of APP/PS1 mice. J. Neuroimmune Pharmacol. 11: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawahara K., Suenobu M., Ohtsuka H., Kuniyasu A., Sugimoto Y., Nakagomi M., Fukasawa H., Shudo K., and Nakayama H.. 2014. Cooperative therapeutic action of retinoic acid receptor and retinoid X receptor agonists in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 42: 587–605. [DOI] [PubMed] [Google Scholar]
- 137.Kitaoka K., Shimizu N., Ono K., Chikahisa S., Nakagomi M., Shudo K., Ishimura K., Sei H., and Yoshizaki K.. 2013. The retinoic acid receptor agonist Am80 increases hippocampal ADAM10 in aged SAMP8 mice. Neuropharmacology. 72: 58–65. [DOI] [PubMed] [Google Scholar]
- 138.Tippmann F., Hundt J., Schneider A., Endres K., and Fahrenholz F.. 2009. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 23: 1643–1654. [DOI] [PubMed] [Google Scholar]
- 139.Ding Y., Qiao A., Wang Z., Goodwin J. S., Lee E. S., Block M. L., Allsbrook M., McDonald M. P., and Fan G. H.. 2008. Retinoic acid attenuates beta-amyloid deposition and rescues memory deficits in an Alzheimer’s disease transgenic mouse model. J. Neurosci. 28: 11622–11634. [DOI] [PMC free article] [PubMed] [Google Scholar]