SLC26A3 [down regulated in adenoma (DRA)] mediates intestinal luminal NaCl absorption and is downregulated in irritable bowel disease-associated diarrhea. Since both DRA and caudal-type homeobox protein-2 (CDX2) are reduced in intestinal inflammation and the DRA promoter harbors CDX2 binding sites, we examined whether the DRA gene is regulated by CDX2. Our studies, for the first time, demonstrate transcriptional regulation of DRA expression by CDX2 via direct binding to the DRA promoter, suggesting that reduced expression of DRA in irritable bowel disease-associated diarrhea could, in part, be attributed to downregulation of CDX2.
Keywords: CDX2, SLC26A3, transcription
Abstract
SLC26A3 [downregulated in adenoma (DRA)] plays a key role in mammalian intestinal NaCl absorption, in that it mediates apical membrane Cl−/ exchange. DRA function and expression are significantly decreased in diarrhea associated with inflammatory bowel disease. DRA is also considered to be a marker of cellular differentiation and is predominantly expressed in differentiated epithelial cells. Caudal-type homeobox protein-2 (CDX2) is known to regulate genes involved in intestinal epithelial differentiation and proliferation. Reduced expression of both DRA and CDX2 in intestinal inflammation prompted us to study whether the DRA gene is directly regulated by CDX2. Our initial studies utilizing CDX2 knockout (CDX2fV/fV;Cre+) mice showed a marked reduction in DRA mRNA and protein levels in proximal and distal colon. In silico analysis of the DRA promoter showed two consensus sites for CDX2 binding. Therefore, we utilized Caco-2 cells as an in vitro model to examine if DRA is a direct target of CDX2 regulation. siRNA-mediated silencing of CDX2 in Caco-2 cells resulted in a marked (~50%) decrease in DRA mRNA and protein levels, whereas ectopic overexpression of CDX2 upregulated DRA expression and also stimulated DRA promoter activity, suggesting transcriptional regulation. Electrophoretic mobility shift and chromatin immunoprecipitation assays demonstrated direct binding of CDX2 to one of the two putative CDX2 binding sites in the DRA promoter (+645/+663). In summary, our studies, for the first time, demonstrate transcriptional regulation of DRA expression by CDX2, implying that reduced expression of DRA in inflammatory bowel disease-associated diarrhea may, in part, be due to downregulation of CDX2 in the inflamed mucosa.
NEW & NOTEWORTHY SLC26A3 [downregulated in adenoma (DRA)] mediates intestinal luminal NaCl absorption and is downregulated in inflammatory bowel disease-associated diarrhea. Since both DRA and caudal-type homeobox protein-2 (CDX2) are reduced in intestinal inflammation and the DRA promoter harbors CDX2 binding sites, we examined whether the DRA gene is regulated by CDX2. Our studies, for the first time, demonstrate transcriptional regulation of DRA expression by CDX2 via direct binding to the DRA promoter, suggesting that reduced expression of DRA in inflammatory bowel disease-associated diarrhea could, in part, be attributed to downregulation of CDX2.
the mammalian intestinal apical membrane Cl−/ exchanger SLC26A3 [downregulated in adenoma (DRA)] mediates NaCl absorption via its direct coupling to the Na+/H+ exchanger NHE3. Earlier studies have shown that mutations in the DRA gene are linked to congenital Cl− diarrhea (CLD) (17). Also, mice deficient in the DRA gene exhibit a phenotype similar to CLD (21). Expression of DRA is also reported to be significantly reduced in animal models of colitis (8). For example, in IL-10 knockout (KO) mice and in mice with dextran sulfate sodium (DSS)-induced colitis, DRA expression in colonic epithelium is significantly reduced (4, 27, 29). Similarly, patients with active ulcerative colitis (UC) exhibit reduced DRA expression (3). Since DRA has emerged as a novel therapeutic target for diarrheal diseases, it is critical to understand the mechanisms of regulation of DRA function and expression in health and disease.
Previous studies from our group showed that DRA gene transcription is regulated at the promoter level by different transcription factors. For example, its basal transcriptional activity requires hepatocyte nuclear factor (HNF)-4α (1) and interaction of transcription factor Ying Yang 1 (YY1) (2) and an unidentified GATA factor (1). Another transcription factor, STAT1, was shown to mediate IFN-γ inhibition of DRA gene expression (20). Furthermore, transcription of the DRA gene is known to be stimulated by butyrate, a metabolic by-product of endogenous colonic flora (1), as well as by the probiotic bacteria Lactobacillus acidophilus (LA) (19) and Bifidobacterium (13) species-secreted soluble factors.
Earlier studies showed that ectopic or transgenic overexpression of CDX2, an intestine-specific transcription factor (26), in an esophageal keratinocyte cell line or in vivo model of murine esophagus, respectively, induced DRA expression only when the cells were treated with 5′-azacytidine, a DNA methyltransferase inhibitor (11). This suggests that CDX2 regulation of DRA could involve epigenetic mechanisms. Furthermore, a marked decrease in CDX2 expression was found in patients with active UC, as well as in animal models of DSS-induced colitis (5, 6). CDX2 is the “master regulator” of terminal differentiation of the intestine (26). This nuclear transcription factor is also known to directly regulate expression of several genes related to epithelial function, e.g., mucin 2 (MUC2) (28), and several transport proteins, such as cystic fibrosis transmembrane conductor regulator (10), apical Na+-dependent bile acid transporter (16), Na+-coupled monocarboxylate transporter 1 (9), and peptide transporter 1 (23). Interestingly, in silico analysis of the DRA promoter showed two putative binding sites for CDX2; however, the role of CDX2 in regulating DRA function and expression has not been investigated. Since expression of both CDX2 and DRA has been reported to be downregulated in models of intestinal inflammation and also in human inflammatory bowel disease (IBD), it was of interest to investigate whether CDX2 directly regulates expression of DRA. Our current studies demonstrate that CDX2 transcriptionally activates basal expression of DRA via its direct binding to the promoter region of DRA.
MATERIALS AND METHODS
Antibodies and reagents.
For Western blot and immunocytochemical analysis, anti-CDX2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For chromatin immunoprecipitation (ChIP) assays, anti-CDX2 antibody (catalog no. BL3194) was obtained from Bethyl Laboratories (Montgomery, TX). Anti-DRA antibody was raised in rabbit against its 745–764 amino acid sequence, INTNGGLRNRVYEPVETKF, at the COOH terminal, as described previously (19). Total RNA was extracted utilizing the RNeasy kit (Qiagen, Valencia, CA). Human CDX2 siRNA was obtained from Thermo Fisher Scientific-Dharmacon (Waltham, MA). FLAG-tagged CDX2 overexpression plasmid was a kind gift from Dr. Makoto Taketo (Kyoto University, Kyoto, Japan). All other chemicals were of at least reagent grade and were obtained from Sigma Chemical (St. Louis, MO) or Fisher Scientific (San Jose, CA).
Mice.
All control and CDX2fV/fV;Cre+ mice were generated and maintained at the animal care facility at Rutgers University according to the guidelines of the Animal Care and Use Committee at Rutgers University, which approved the experimental protocol, as described previously (24, 25).
Cell culture.
Caco-2 cells, a human colon carcinoma cell line (American Type Culture Collection, Manassas, VA), were grown in Eagle’s minimum essential medium containing 20% heat-inactivated FBS (GIBCO/Thermo Fisher Scientific, Waltham, MA) and 1% antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; GIBCO/Thermo Fisher Scientific) and maintained at 37 °C in a humidified 5% CO2 incubator.
siRNA silencing and ectopic overexpression of CDX2 in Caco-2 cells.
For CDX2 knockdown in Caco-2 cells, cells seeded in six-well plates and grown to ~60% confluency were transfected with a CDX2-specific double-stranded siRNA (siCDX2) or scrambled siRNA (both obtained from Thermo Fisher Scientific-Dharmacon) (Table 1) in a total concentration of 50 nM. The siRNA duplexes were used in a ratio of 1:5 relative to Lipofectamine 2000 reagent in OptiMEM. siRNA sequences are shown in Table 1. This procedure was repeated every 3rd day. Finally, cells were harvested on day 10 and processed for mRNA and protein analysis.
Table 1.
List of primers used in nonradioactive gel-shift and ChIP assays
| Sequence (5′–3′) | |
|---|---|
| CDX2 probe for site 1 (645–663) | |
| Sense | AAC ATA GGG TTT ATT GCT CTT AGG AAGA |
| Antisense | TCT TCC TAA GAG CAA TAA ACC CTA TGTT |
| CDX2 probe for site 2 (360–378) | |
| Sense | AAG TGT GTT AAA AAT TTT TTT TTA |
| Antisense | TAA AAA AAA ATT TTT AAC ACA CTT |
| CDX2 mutated probe for site 1 (645–663) | |
| Sense | AAC ATA GGG TTT CGC ACC AGT AGG AAG A |
| Antisense | TCT TCC TAC TGG TGC GAA ACC CTA TGT T |
| DRA ChIP binding primer | |
| Forward | CTC TAA TCC CAG CTA CTC AGG TTA AA |
| Reverse | AAA GCA TTT GGC ATT GCT GAC TAC ACTT |
ChIP, chromatin immunoprecipitation; DRA, downregulated in adenoma.
For CDX2 overexpression, Caco-2 cells were transfected with 1 μg of CDX2 expression vector containing two FLAG tags at the NH2 terminus (kind gift from Dr. Taketo) or the corresponding empty vector (Qiagen, Germantown, MD) using Amaxa Nucleofector transfection reagents (Lonza, Walkersville, MD) according to the manufacturer’s instructions. Cell lysates were prepared 48 h after transfection, and overexpression was measured by quantitative RT-PCR and immunoblotting with respective antibodies.
RNA extraction and quantitative real-time PCR.
Total RNA was extracted using an RNA extraction minikit (Qiagen) according to the manufacturer’s instructions. Specific transcripts were amplified using SYBR Green (Agilent Technologies, Santa Clara, CA) in a fluorescence reader (Stratagene Biosystems). The sequences of the gene-specific primers are presented in Table 1. Levels of each transcript were normalized to internal control GAPDH to calculate the relative mRNA levels of a specific gene.
Preparation of lysates and Western blotting.
Cells and tissues were lysed using 1× cell lysis buffer (Cell Signaling Technology, Danvers, MA) in the presence of 1× diluted proteinase inhibitor cocktail, as described previously (19). Protein concentrations in the lysates were measured using the Bradford method. Control and experimental samples were run on SDS-polyacrylamide gels and blotted onto nitrocellulose membrane. After blocking, the membrane was probed for different primary antibodies and their respective secondary antibodies (see results). Bands were visualized using enhanced chemiluminescence detection reagents (Bio-Rad, Hercules, CA) and a Bio-Rad image analyzer according to the manufacturer’s instructions.
Immunofluorescence staining in mouse colonic tissues.
Paraffin sections (5-μm) of mouse proximal and distal colon were cut using a microtome. Sections were deparaffinized in xylene and passed through graded alcohol. For antigen retrieval, the slides were boiled in citrate buffer for 15 min, cooled to room temperature, blocked with 5% normal goat serum for 1 h, and then incubated overnight with DRA antibody (1:100 dilution) in PBS with 1% normal goat serum at 4°C. After they were washed with PBS, the sections were incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG (Invitrogen) for 60 min, washed, and then mounted using SlowFade Gold antifade with 4′,6-diaminido-2-phenylindole reagent (Invitrogen). Sections were imaged using a fluorescence microscope (model B51X170, Olympus, Tokyo, Japan) equipped with a ×20 objective.
DRA promoter activity.
Caco-2 cells were cotransfected with CDX2 expression vector and DRA promoter (p-1183/+114) fragment cloned upstream of the luciferase reporter gene in pGL2-basic along with β-galactosidase (β-Gal) expression vector by electroporation using the Amaxa Nucleofector transfection kit (Lonza), as described previously (13). Luciferase reporter activity was measured using a luciferase reporter assay system kit (Promega, Madison, WI) according to the manufacturer’s instructions, and β-Gal luciferase reporter vector was used to normalize for transfection efficiency.
Preparation of nuclear extracts and electrophoretic mobility shift assays.
The binding of CDX2 to its putative binding sites in the DRA promoter was detected utilizing the nonradioactive digoxigenin (DIG) gel shift kit (Roche, Indianapolis, IN). Nuclear extracts from control or CDX2-overexpressed Caco-2 cells were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher) according to the manufacturer’s instructions. Protein concentrations in nuclear extracts were determined by the Bradford method. Recombinant terminal transferase and DIG-11-ddUTP were used to label the 3′ end of the oligonucleotide probes representing CDX2 binding sites in the DRA promoter (Table 1). The labeled probes were incubated with the nuclear extracts, with or without competition by unlabeled oligonucleotides, with or without specific CDX2 antibodies, for analysis of competition, as described previously. Nuclear extracts and probes were run on a 4% polyacrylamide gel. An alkaline phosphatase-conjugated anti-DIG antibody was bound to the DIG-labeled oligonucleotide-protein complex, and the immobilized alkaline phosphatase removed a phosphate group from the chemiluminescent substrate CSPD, thereby emitting a signal that was detected and proportionate to the amount of bound probe. Specificity of binding was ascertained by competition with excess cold consensus oligonucleotides as well as by measurement of binding to the mutated probe (Table 1).
Chromatin immunoprecipitation assay.
ChIP was performed utilizing the EpiTect ChIP OneDay Kit (Qiagen) according to the manufacturer’s protocol. Briefly, control and CDX2-overexpressed Caco-2 cells were fixed with 1% formaldehyde for 10 min at room temperature, and chromatin was sheared by sonication. Sheared DNA was incubated with the CDX2 antibody for 1 h. Protein-DNA complexes were precipitated by protein A-coupled agarose beads. After purification of the DNA from the immunoprecipitated complexes by reversal of cross-linking followed by proteinase K treatment, real-time RT-PCR was performed using Brilliant SYBR Green quantitative PCR master mix (Stratagene, La Jolla, CA) and Mx3000 (Stratagene) with the primer pair (Table 1) that encompassed the putative CDX2 binding element in the DRA promoter. Normal rabbit IgG was used as the control (negative) group.
Statistical analysis.
Values are means ± SE of at least three independent experiments. The difference between control and experimental groups was analyzed by unpaired t-test. P < 0.05 was considered statistically significant.
RESULTS
DRA expression is reduced in intestine-specific CDX2fV/fV;Cre+mice.
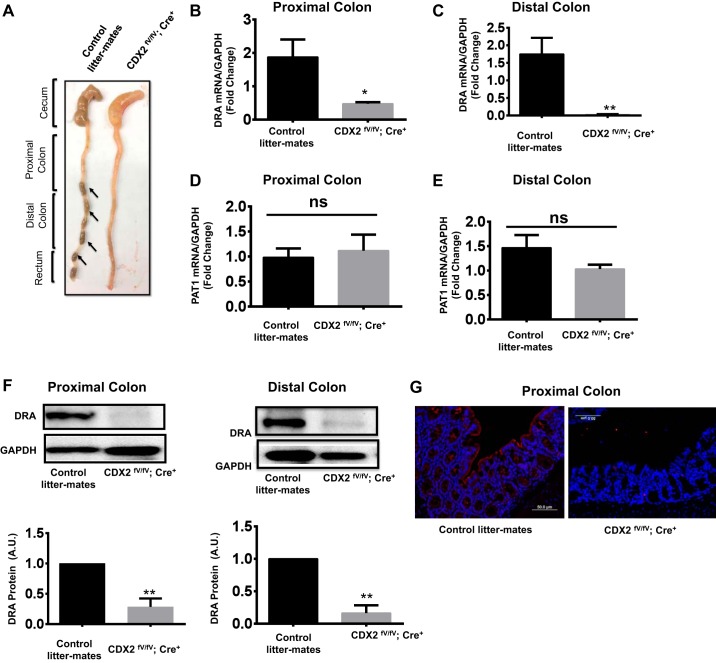
Previous studies in IBD patients showed decreased DRA, as well as CDX2, protein levels in the colonic mucosa compared with the control group (3, 6). However, no link between CDX2 and DRA expression has been reported in intestinal epithelial cells. In our current studies, CDX2fV/fV;Cre+ mice exhibited the diarrheal phenotype with no fecal pellets in the colon compared with their wild-type littermates (Fig. 1A). Since DRA function and expression are known to be downregulated in diarrheal diseases, we examined whether altered expression of CDX2 has an effect on DRA expression. We used CDX2fV/fV;Cre+ mice, where CDX2 was knocked out in the intestine using villin CreERT2 mice in a tamoxifen-inducible manner (24, 25). DRA mRNA levels were significantly reduced in proximal and distal colon of CDX2fV/fV;Cre+ mice: 1.87 ± 0.53 (control) and 0.51 ± 0.08 (CDX2fV/fV;Cre+) in proximal colon (P < 0.05) and 1.75 ± 0.46 (control) and 0.02 ± 0.02 (CDX2fV/fV;Cre+) in distal colon (P < 0.05) (Fig. 1, B and C). However, the absence of CDX2 does not alter the levels of PAT1 (SLC26A6) (Fig. 1, D and E), another intestinal transporter involved in Cl−/ exchange, or mRNA levels of NHE3 in the ileum (data not shown), suggesting that the effects were specific to DRA. Similarly, DRA protein levels in proximal and distal colon, as determined by immunoblotting, were significantly lower in CDX2fV/fV;Cre+ mice than in the specific control mice (Fig. 1F), which was further confirmed by immunofluorescence staining in proximal colon (Fig. 1G).
Fig. 1.
Diarrheal phenotype and reduced expression of downregulated in adenoma (DRA, SLC26A3) in proximal and distal colon of CDX2fV/fV;Cre+ mice. A: colon of a CDX2fV/fV;Cre+ mouse showing excess fluid with no intact pellets compared with a control littermate exhibiting intact fecal pellets (arrows). B and C: quantitative PCR analysis of relative mRNA levels of DRA in proximal and distal colon of CDX2fV/fV;Cre+ mice compared with control littermates. D and E: quantitative PCR analysis of relative mRNA levels of PAT1 mRNA in proximal and distal colon of CDX2fV/fV;Cre+ mice compared with control littermates. F: immunoblots showing DRA protein levels in proximal and distal colon and densitometric analysis of the respective band intensities. In all cases, GAPDH was used as an internal control. AU, arbitrary units. Values are means ± SE; n = 5. *P < 0.05; **P < 0.01; ns, not significant. G: paraffin-embedded sections from proximal colon from wild-type (control littermate) and CDX2fV/fV;Cre+ mice stained with DRA antibodies for immunofluorescence analysis. Representative images show DRA expression as detected by red fluorescence in CDX2fV/fV;Cre+ mice compared with control littermates.
siRNA-mediated attenuation of CDX2 decreased DRA expression in Caco-2 cells.
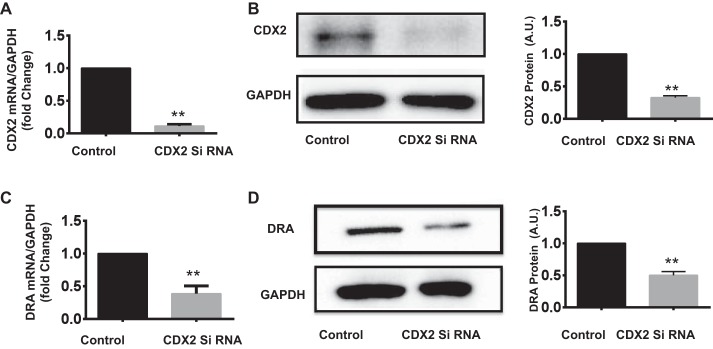
Since DRA expression was reduced in CDX2fV/fV;Cre+ mice, it was of interest to use an in vitro model to investigate the mechanisms of regulation of DRA expression by CDX2. Caco-2 cells were first transfected with CDX2-specific siRNA to knock down its expression and allow examination of the effects on DRA mRNA and protein levels. Our results show that attenuation of CDX2 expression by siRNA (Fig. 2, A and B) results in a marked (~50%) decrease in DRA mRNA and protein levels (Fig. 2, C and D).
Fig. 2.
CDX2 silencing decreased DRA expression in Caco-2 cells. Caco-2 cells were transfected with CDX2 siRNA or control siRNA, and mRNA and protein levels of CDX2 and DRA were analyzed by quantitative PCR and Western blotting, respectively. A: CDX2 mRNA levels normalized to GAPDH. B: representative immunoblot of CDX2 protein and densitometric analysis of band intensities normalized to GAPDH. C and D: relative levels of DRA mRNA and protein and densitometric analysis of protein bands. Values are means ± SE of 3 independent experiments. **P < 0.01.
Ectopic overexpression of CDX2 in Caco-2 cells increased DRA mRNA and protein levels.
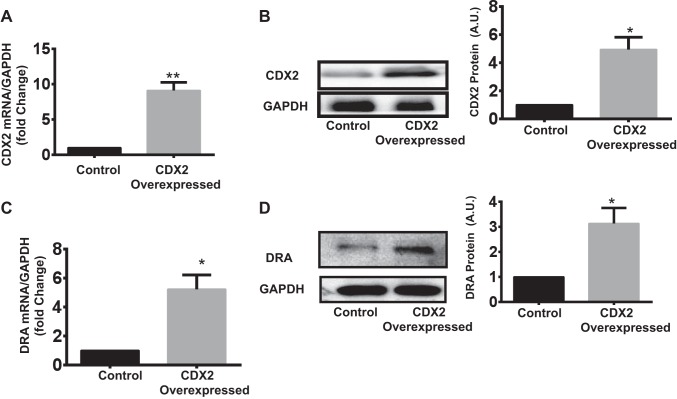
To determine whether DRA expression is induced by CDX2, we overexpressed CDX2 ectopically in Caco-2 cells by transfecting the CMV vector encoding CDX2 (see materials and methods). Overexpression of CDX2 (~9-fold in mRNA and 4.94-fold in protein) (Fig. 3. A and B) resulted in significant upregulation of DRA mRNA and protein levels (~5- and ~3-fold, respectively) in Caco-2 cells (Fig. 3, C and D).
Fig. 3.
CDX2 overexpression increased DRA mRNA and protein levels in Caco-2 cells. A and B: AMAXA was used to transfect Caco-2 cells with the mammalian p-CMV expression vector encoding CDX2. At 48 h posttransfection, CDX2 overexpression was confirmed by measurement of its mRNA level (with GAPDH as internal control) and by densitometric analysis of band intensities to determine protein level. C and D: CDX2 overexpression significantly increased DRA mRNA and protein levels. Values are means ± SE of 3 independent experiments. *P < 0.05; **P < 0.01.
CDX2 is a direct transcriptional activator of the DRA promoter.
In silico analysis of the DRA promoter region (p-1183/+114) utilizing MatInspector software (Genomatix) showed two putative binding sites for CDX2, −360/−378 with the sequence aaaaattTTTAacacactt and +645/+663 with the sequence catagggtTTATtgctctt (Fig. 4A). Therefore, we sought to examine if CDX2 regulation of DRA expression involves a transcriptional mechanism via direct binding of CDX2 to their putative sites. We used Caco-2 cells to overexpress CDX2, and after 24 h, we used luciferase assay to measure promoter activity. Overexpression of CDX2 stimulated DRA promoter activity by ~10-fold (Fig. 4B), indicating its role in transcriptional regulation.
Fig. 4.
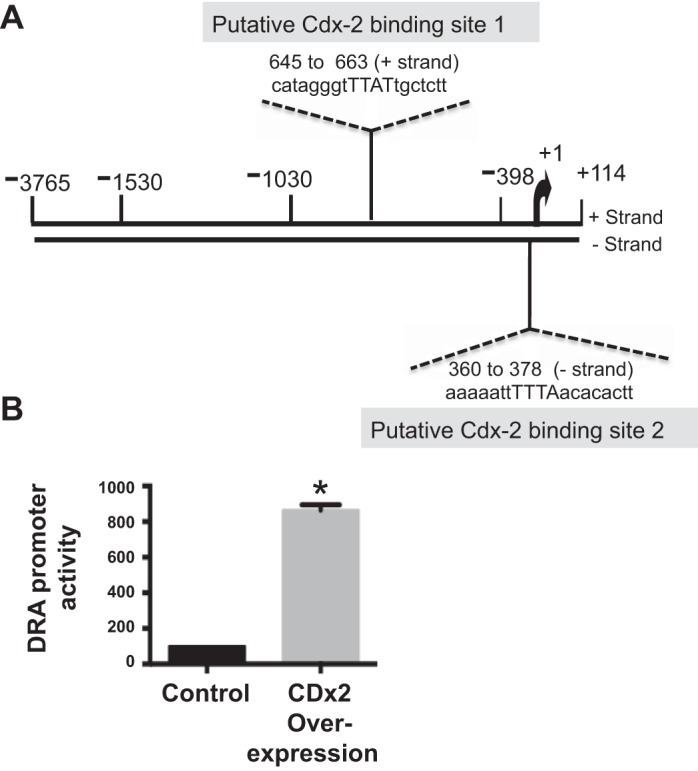
CDX2 overexpression stimulates DRA promoter activity. A: DRA promoter region showing 2 CDX2 binding sites as predicted by in silico analysis by MatInspector software (Genomatix). B: luciferase activity measured in response to overexpression of CDX2 in Caco-2 cells cotransfected with or without the DRA promoter and β-galactosidase (β-Gal) plasmid. Relative luciferase activities were normalized to β-Gal activities in different groups. Values are means ± SE of 3 independent experiments. *P < 0.05.
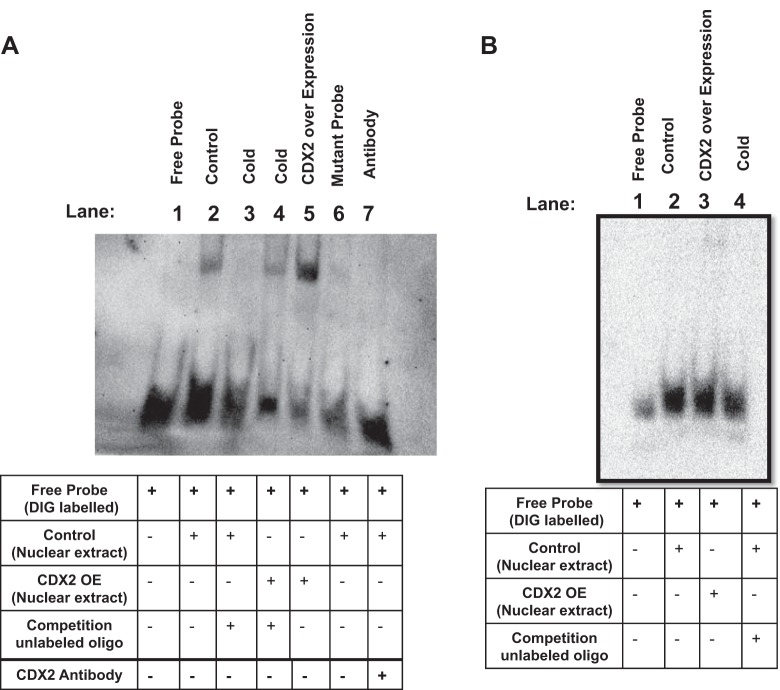
We next utilized nonradioactive electrophoretic mobility shift assay to determine whether CDX2 directly binds to the DRA promoter. Nuclear extracts prepared from control and CDX2-overexpressed Caco-2 cells were incubated with the 3′-DIG-labeled oligonucleotide probes AAG TGT GTT AAA AAT TTT TTT TTA for CDX2 binding site 1 (+645/+663) and AAC ATA GGG TTT AAT GCT CTT AGG AAGA for CDX2 binding site 2 (−360/−378), along with their mutated probes, and run on 4% polyacrylamide gels. As shown in Fig. 5A, the oligonucleotide probe corresponding to site 1 showed increased DNA/protein binding in response to CDX2 overexpression (lane 5) compared with the control group (lane 2). The specificity of CDX2 protein interaction with this site was shown by competition assay, in which the band was competed out with a 200-fold excess of cold oligonucleotides in lanes corresponding to nuclear extracts from control, as well as CDX2-overexpressed, cells (lanes 3 and 4, respectively). The specificity was further confirmed by the finding that there was no band with the mutated probe (lane 6). CDX2 antibody did not show supershift but competed out the binding, presumably by masking the motif in CDX2 protein required for its binding to site 1 in the DRA promoter (lane 7). These results indicate that CDX2 interacts physically with the DRA promoter via site 1. Interestingly, the oligonucleotide probe corresponding to site 2 (Fig. 5B) did not show binding to CDX2.
Fig. 5.
Digoxigenin (DIG) nonradioactive gel shift assay showed direct binding of CDX2 to the DRA promoter. Synthetic oligonucleotides containing the 2 putative CDX2 binding sites (Fig. 4A) were used. A: +645/+663 site showed direct binding of DIG-labeled probe with nuclear extract (lane 2). This interaction was increased with ectopic overexpression of CDX2 (lane 5). In both cases, CDX2 binding was attenuated when competed with a 200-fold excess of cold oligonucleotide (lanes 3 and 4) or when mutated probe was used (lane 6). B: probes containing −360/−378 (−) binding site did not show an interaction between CDX2 and DRA. Blot is representative of 3 independent experiments.
CDX2 shows direct binding to the DRA promoter in ChIP.
To further demonstrate direct in vivo interaction of CDX2 with the DRA gene, ChIP was performed in Caco-2 cells. Interaction of CDX2 with the DRA promoter region corresponding to site 1 (+645/+663) was increased in the CDX2 antibody-immunoprecipitated group compared with the control IgG-precipitated group (Fig. 6). The real-time PCR results showed 33.59-fold enrichment in the CDX2 antibody-precipitated group. The specificity of the ChIP assay was verified using control chromatin, and no PCR amplification was visible. However, the primers corresponding to site 2 (−360/−378) did not show binding. These data confirm the direct binding of CDX2 to the specific cis element in the DRA promoter in vivo.
Fig. 6.
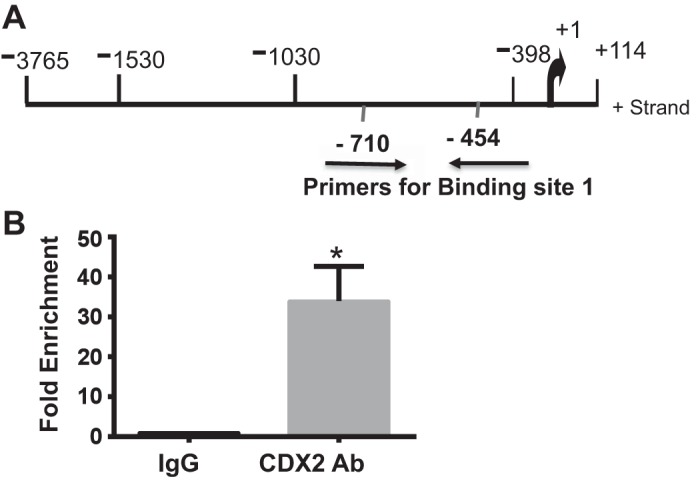
Chromatin immunoprecipitation (ChIP) showed enhanced CDX2 binding to DRA promoter following its overexpression. A: schematic diagram of DRA promoter region. Arrows show primers used for ChIP assay. B: ChIP assay confirmed binding of CDX2 to DRA promoter in Caco-2 cells. Results are shown as enrichment of DRA promoter relative to empty vector. Normal IgG was used as negative control. Values are means ± SE of 3 independent experiments. *P < 0.05.
DISCUSSION
The predominant route of Na+ and Cl− absorption in mammalian ileum and colon involves coupled operation of Na+/H+ and Cl−/ exchangers, where DRA is the key molecular species mediating Cl−/ exchange. Reduced expression and function of DRA have been implicated in the pathophysiology of diarrheal disorders associated with inflammation or enteric pathogen infection. Mutations in the DRA gene lead to CLD associated with high fecal Cl− content (17), and DRA KO mice exhibited the diarrheal phenotype (21) and were more susceptible to experimental colitis (8). Similarly, reduction in the colonic crypt Cl−/ exchange activity with a parallel decrease in DRA mRNA expression has been reported in patients with UC and in animal models of experimental colitis, such as IL-10 KO (29) and DSS-treated (4) mouse models. A genome-wide association study identified DRA polymorphism, which is associated with lower DRA expression, as a risk factor for the development of UC (3). These studies suggest that strategies aimed at upregulation of DRA expression and function will be of therapeutic importance for diarrhea and IBD. Therefore, it is critical to understand the mechanisms involved in DRA regulation in normal physiology as well as in diarrheal diseases. Previous studies from our laboratory showed that basal DRA expression is regulated by the transcription factor HNF4α, whereas butyrate-induced transcriptional activation is mediated via YY1 and GATA transcription factors (1). We have also shown that DRA expression is reduced in response to the proinflammatory cytokine IFN-γ by binding of STAT1 to the GAS element on the DRA promoter (20). We also showed that various agents, such as the soluble factor(s) secreted by the probiotic bacteria LA and Bifidobacterium species (13, 19) and dietary components, e.g., lysophosphatidic acid (27) and all-trans-retinoic acid (18), upregulate DRA expression via transcriptional activation. LA also counteracted inflammation-induced downregulation of DRA expression in vitro and in vivo (22). Another recent study showed that DRA is completely abolished in intestine-specific HNF1α/HNF1β double-KO mice and that these mice exhibited the diarrheal phenotype (7). We and others have also shown severe reduction in DRA expression in Citrobacter rodentium-induced colitis in susceptible mice, and LA was able to counteract C. rodentium inhibition of DRA expression (4, 12). In the current study we have demonstrated, for the first time, that the SLC26A3 gene is a novel target for the transcription factor CDX2.
CDX2 plays an important role in development of the intestinal mucosa (26). It is also critical for development of the crypt-villus axis pattern and cellular lineages in the intestine (24, 26). In addition to these critical cellular processes, there is increasing evidence linking CDX2 to intestinal inflammation (6). The expression of CDX2 was decreased in the epithelium of patients with UC and was inversely correlated with the level of TNF-α (6). High levels of TNF-α affected the expression of CDX2 target genes and reduced the binding of CDX2 to gene regulatory regions of these targets in Caco-2 cells (6). CDX2 is a downstream target of inflammatory cytokines, its expression is reduced in inflamed tissues, and CDX2 heterozygous mice exhibit increased sensitivity to DSS-induced colitis (5). CDX2 also plays a critical role in the regulation of genes (e.g., Muc2 and Claudin-2) involved in mucosal permeability (15, 28). Together, these studies indicate that CDX2 could be a key player in intestinal inflammation. A number of CDX2 target genes, including marker genes for intestinal differentiation, such as sucrase-isomaltase (14), a gene encoding MUC2 (28), and genes encoding intestinal transporters, have been identified. Therefore, it is expected that altered CDX2 expression and activity in IBD may change several functions and properties of the epithelium, including the expression of ion and nutrient transporters.
Our previous studies showed extensive downregulation of DRA in DSS-induced colitis and other models of intestinal inflammation. To examine if CDX2 is linked to reduced DRA expression in inflammation, we utilized CDX2fV/fV;Cre+ mice (Fig. 1A) (26) to measure colonic mucosal DRA mRNA and protein levels. In CDX2fV/fV;Cre+ mice, DRA mRNA and protein levels were significantly decreased in proximal and distal colon compared with their wild-type littermates. These effects were specific to DRA, as there was no change in the mRNA levels of PAT1 (another apical membrane transporter suggested to be involved in Cl−/ exchange) and NHE3 (the key apical membrane Na+/H+ exchanger) in CDX2fV/fV;Cre+ mice. Earlier studies in CDX2fV/fV;Cre+ mice showed reduced alkaline phosphatase in the small intestine compared with the control group, indicating the presence of immature enterocytes in the intestinal mucosa (24). Since DRA expression is known to be highest in mature enterocytes, reduced DRA expression in CDX2 KO mice could, in part, be due to the presence of immature enterocytes. However, since CDX2 has also been shown to transcriptionally regulate multiple genes in differentiated enterocytes (24), we examined if DRA could be a target gene for CDX2 regulation. In Caco-2 cells, siRNA-mediated silencing of CDX2 reduced DRA mRNA and protein expression, whereas overexpression of CDX2 augmented DRA mRNA and protein levels, suggesting direct regulation of DRA expression by CDX2. Furthermore, DRA promoter activity in Caco-2 cells overexpressing CDX2 increased ~10-fold, indicating a direct transactivation of the DRA gene by CDX2. Indeed, transcription factor binding analysis of the DRA promoter by MatInspector software (Genomatix) revealed two putative CDX2 binding sites, and, utilizing electrophoretic mobility shift and ChIP assays, we experimentally showed direct binding of CDX2 to one of these sites. These findings may suggest that decreased DRA expression in intestinal inflammation could, at least in part, be secondary to downregulation of CDX2.
Earlier studies showed dynamic interactions of CDX2 with other transcription factors, such as GATA6 and HNF4α, to regulate distinct gene expression in intestinal epithelial cells (7). We previously demonstrated the role of HNF4α in basal expression of DRA (1). However, in the current study we did not observe a significant change in HNF4α mRNA levels in CDX2 KO mice (data not shown), suggesting that effects of CDX2 on DRA transcription are not mediated via HNF4α. Furthermore, the level of CDX1, another caudal-type homeobox protein, remained unaltered in CDX2fV/fV;Cre+ mice (data not shown). In view of the downregulation of both DRA and CDX2 in inflammation and results of our current studies showing direct binding of CDX2 to DRA, it appears that DRA suppression in gut inflammation may be, in part, due to CDX2 downregulation.
Despite reduced expression of DRA in inflammation secondary to downregulation of CDX2, the diarrheal phenotype observed in CDX2fV/fV;Cre+ mice may not be solely attributable to reduced expression of DRA by CDX2, as diarrhea is multifactorial in nature. We did not observe alterations in the expression of NHE3, the apical membrane Na+/H+ exchanger that normally acts in concert with DRA in mediating luminal NaCl absorption. Furthermore, although the NHE3 promoter region has been shown to harbor putative binding sites for CDX family proteins, there have been no reports showing CDX2 regulation of NHE3 gene transcription. CDX2 has also been shown to be critical for the expression of cystic fibrosis transmembrane conductor regulator, which plays a pivotal role in the pathophysiology of secretory diarrhea (10). Altered expression of epithelial tight junction proteins governing paracellular permeability could also contribute to diarrhea. Therefore, extensive mechanistic studies are needed to ascertain the physiological and molecular basis of the diarrheal phenotype associated with CDX2 deficiency.
In summary, our studies, for the first time, demonstrate a direct role of CDX2 in regulating transcription of the DRA gene, thereby modulating basal expression of this key transporter mediating intestinal NaCl absorption. This study also raises the possibility that downregulation of DRA in inflamed mucosa could be attributable, at least in part, to reduced expression of CDX2. Further studies examining altered interactions of CDX2 with DRA or other ion transporter genes under inflammatory conditions could uncover new avenues to counteract reduced DRA expression in inflammation-associated diarrhea.
GRANTS
This work was supported by Department of Veterans Affairs Merit Award BX002011 (P. K. Dudeja), Senior Research Career Scientist Award (P. K. Dudeja), and Career Scientist Award BX000152 (W. A. Alrefai); National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016, DK-81858, and DK-92441 (P. K. Dudeja), DK-98170 (R. K. Gill), DK-71596 and DK-109709 (W. A. Alrefai), and K01 DK-088868 (M. Verzi), and a grant from the Human Genetics Institute of New Jersey (M. Verzi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.C., A.K., R.M.C.M., and O.P.-C. performed experiments; I.C., A.K., and O.P.-C. analyzed data; I.C., R.K.G., W.A.A., and A.B. interpreted results of experiments; I.C. and A.K. prepared figures; I.C. and A.B. drafted manuscript; R.K.G., W.A.A., A.B., M.V., and P.K.D. edited and revised manuscript; P.K.D. conceived and designed research; P.K.D. approved final version of manuscript.
REFERENCES
- 1.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007. doi: 10.1152/ajpgi.00029.2007. [DOI] [PubMed] [Google Scholar]
- 2.Anbazhagan AN, Priyamvada S, Alakkam A, Kumar A, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. Transcriptional modulation of SLC26A3 (DRA) by sphingosine-1-phosphate. Am J Physiol Gastrointest Liver Physiol 310: G1028–G1035, 2016. doi: 10.1152/ajpgi.00308.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, Matsumoto T, Matsui T, Kakuta Y, Kinouchi Y, Shimosegawa T, Hosokawa M, Arimura Y, Shinomura Y, Kiyohara Y, Tsunoda T, Kamatani N, Iida M, Nakamura Y, Kubo M. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet 41: 1325–1329, 2009. doi: 10.1038/ng.482. [DOI] [PubMed] [Google Scholar]
- 4.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calon A, Gross I, Lhermitte B, Martin E, Beck F, Duclos B, Kedinger M, Duluc I, Domon-Dell C, Freund JN. Different effects of the Cdx1 and Cdx2 homeobox genes in a murine model of intestinal inflammation. Gut 56: 1688–1695, 2007. doi: 10.1136/gut.2007.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun M. The role of CDX2 in inflammatory bowel disease. Dan Med J 61: B4820, 2014. [PubMed] [Google Scholar]
- 7.D’Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1α and β control terminal differentiation and cell fate commitment in the gut epithelium. Development 137: 1573–1582, 2010. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton MJ, Sinnamon MJ, Lyng GD, Glickman JN, Wang X, Xing W, Krilis SA, Blumberg RS, Adachi R, Lee DM, Stevens RL. Essential role for mast cell tryptase in acute experimental colitis. Proc Natl Acad Sci USA 108: 290–295, 2011. doi: 10.1073/pnas.1005758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakizaki F, Aoki K, Miyoshi H, Carrasco N, Aoki M, Taketo MM. CDX transcription factors positively regulate expression of solute carrier family 5, member 8 in the colonic epithelium. Gastroenterology 138: 627–635, 2010. doi: 10.1053/j.gastro.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Kerschner JL, Harris A. Transcriptional networks driving enhancer function in the CFTR gene. Biochem J 446: 203–212, 2012. doi: 10.1042/BJ20120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong J, Nakagawa H, Isariyawongse BK, Funakoshi S, Silberg DG, Rustgi AK, Lynch JP. Induction of intestinalization in human esophageal keratinocytes is a multistep process. Carcinogenesis 30: 122–130, 2009. doi: 10.1093/carcin/bgn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Anbazhagan AN, Coffing H, Chatterjee I, Priyamvada S, Gujral T, Saksena S, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol 311: G817–G826, 2016. doi: 10.1152/ajpgi.00173.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307: C1084–C1092, 2014. doi: 10.1152/ajpcell.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorentz O, Duluc I, Arcangelis AD, Simon-Assmann P, Kedinger M, Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol 139: 1553–1565, 1997. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3: e977176, 2015. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Jüttner M, Kullak-Ublick GA, Eloranta JJ. Regulation of the gene encoding the intestinal bile acid transporter ASBT by the caudal-type homeobox proteins CDX1 and CDX2. Am J Physiol Gastrointest Liver Physiol 302: G123–G133, 2012. doi: 10.1152/ajpgi.00102.2011. [DOI] [PubMed] [Google Scholar]
- 17.Mäkelä S, Kere J, Holmberg C, Höglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002. doi: 10.1002/humu.10139. [DOI] [PubMed] [Google Scholar]
- 18.Priyamvada S, Anbazhagan AN, Gujral T, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. All-trans-retinoic acid increases SLC26A3 DRA (down-regulated in adenoma) expression in intestinal epithelial cells via HNF-1β. J Biol Chem 290: 15066–15077, 2015. doi: 10.1074/jbc.M114.566356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. doi: 10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010. doi: 10.1152/ajpgi.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 22.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. doi: 10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spanier B. Transcriptional and functional regulation of the intestinal peptide transporter PEPT1. J Physiol 592: 871–879, 2014. doi: 10.1113/jphysiol.2013.258889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verzi MP, Shin H, He HH, Sulahian R, Meyer CA, Montgomery RK, Fleet JC, Brown M, Liu XS, Shivdasani RA. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell 19: 713–726, 2010. doi: 10.1016/j.devcel.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verzi MP, Shin H, Ho LL, Liu XS, Shivdasani RA. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol 31: 2026–2039, 2011. doi: 10.1128/MCB.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verzi MP, Shin H, San Roman AK, Liu XS, Shivdasani RA. Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding. Mol Cell Biol 33: 281–292, 2013. doi: 10.1128/MCB.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Xiao F, He J, Lan X, Ding Q, Li J, Seidler U, Zheng Y, Tian D. Lysophosphatidic acid increases SLC26A3 expression in inflamed intestine and reduces diarrheal severity in C57BL/6 mice with dextran-sodium-sulfate-induced colitis. Chin Med J (Engl) 127: 1737–1743, 2014. [PubMed] [Google Scholar]
- 28.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun 300: 813–818, 2003. doi: 10.1016/S0006-291X(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 275: G1445–G1453, 1998. [DOI] [PubMed] [Google Scholar]