Sulfate is an essential anion that is actively absorbed from the small intestine involving members of the Slc26 gene family. Here, we show that the main intestinal chloride transporter Slc26a3, known as downregulated in adenoma (DRA), also handles sulfate and contributes to its secretion into the lumen. In the absence of functional DRA (as in the disease congenital chloride diarrhea), net intestinal sulfate absorption was significantly enhanced resulting in substantial alterations to overall sulfate homeostasis.
Keywords: Ussing chamber, chloride secretion, Slc13a1, Slc26a1, downregulated in adenoma, putative anion transporter 1
Abstract
The ileum is considered the primary site of inorganic sulfate () absorption. In the present study, we explored the contributions of the apical chloride/bicarbonate (Cl−/) exchangers downregulated in adenoma (DRA; Slc26a3), and putative anion transporter 1 (PAT1; Slc26a6), to the underlying transport mechanism. Transepithelial 35 and 36Cl− fluxes were determined across isolated, short-circuited segments of the distal ileum from wild-type (WT), DRA-knockout (KO), and PAT1-KO mice, together with measurements of urine and plasma sulfate. The WT distal ileum supported net sulfate absorption [197.37 ± 13.61 (SE) nmol·cm−2·h−1], but neither DRA nor PAT1 directly contributed to the unidirectional mucosal-to-serosal flux (), which was sensitive to serosal (but not mucosal) DIDS, dependent on Cl−, and regulated by cAMP. However, the absence of DRA significantly enhanced net sulfate absorption by one-third via a simultaneous rise in and a 30% reduction to the secretory serosal-to-mucosal flux (). We propose that DRA, together with PAT1, contributes to by mediating sulfate efflux across the apical membrane. Associated with increased ileal sulfate absorption in vitro, plasma sulfate was 61% greater, and urinary sulfate excretion (USO4) 2.2-fold higher, in DRA-KO mice compared with WT controls, whereas USO4 was increased 1.8-fold in PAT1-KO mice. These alterations to sulfate homeostasis could not be accounted for by any changes to renal sulfate handling suggesting that the source of this additional sulfate was intestinal. In summary, we characterized transepithelial sulfate fluxes across the mouse distal ileum demonstrating that DRA (and to a lesser extent, PAT1) secretes sulfate with significant implications for intestinal sulfate absorption and overall homeostasis.
NEW & NOTEWORTHY Sulfate is an essential anion that is actively absorbed from the small intestine involving members of the Slc26 gene family. Here, we show that the main intestinal chloride transporter Slc26a3, known as downregulated in adenoma (DRA), also handles sulfate and contributes to its secretion into the lumen. In the absence of functional DRA (as in the disease congenital chloride diarrhea), net intestinal sulfate absorption was significantly enhanced resulting in substantial alterations to overall sulfate homeostasis.
inorganic sulfate () is an important anion, vital for normal growth and development, and serves as an essential factor in numerous physiological processes (11, 35). The majority of sulfate available to the body is sourced from the diet, either absorbed directly by the intestine in its inorganic form or released following the metabolism of sulfur-containing amino acids (32). The intestine therefore has a key role in sulfate acquisition, which, together with regulated reabsorption by the proximal renal tubule, contributes to overall sulfate homeostasis (32). The small bowel, in particular the ileum, has been identified as the primary site for active sulfate absorption by the intestine (2, 4, 52). The proposed transport mechanism consists of an apical sodium (Na+)-dependent entry step performed by the Na+- cotransporter, NaSi (Slc13a1), in series with the basolateral sulfate anion transporter SAT1 (Slc26a1; 33). In addition to NaSi, a number of studies using brush-border membrane vesicles (BBMVs) had also identified an accompanying Na+-independent sulfate transport pathway. This was characterized as a DIDS-sensitive /OH− exchanger and predicted to contribute to sulfate absorption (25, 43, 44, 61), but the identity of this apical transporter has not been determined.
The multifunctional Slc26 gene family of anion exchangers encode a number of sulfate transporters that are expressed in the small intestine, and potential candidates for this apical sulfate exchanger include the diastrophic dysplasia sulfate transporter (DTDST; Slc26a2), downregulated in adenoma (DRA; Slc26a3), and putative anion transporter 1 (PAT1; Slc26a6). DTDST is important for sulfate uptake by chondrocytes, osteoblasts, and fibroblasts (14, 20, 41), and mutations of the human DTDST gene are responsible for four inherited chondrodysplasias (24), but its role in the intestine has yet to be resolved. PAT1 is able to transport a variety of anions, including sulfate (23, 29, 62), and although early studies initially characterized recombinant DRA as a sulfate transporter (7, 8, 29, 37, 48), subsequent work cast doubt on this capability (10, 27, 50, 54). However, in human Caco-2 cells, apical DIDS-sensitive /OH− exchange was attributed to DRA (1), with only a very minor role for PAT1-mediated sulfate transport (16). More recently, we reported that DRA, and to a lesser extent PAT1, were responsible for active sulfate secretion by the mouse cecum (60). In the small intestine, nothing is known of the relative contributions made by DRA and PAT1 to sulfate transport, although apical in/out exchange has been demonstrated by the villous epithelium of the mouse duodenum, which was ascribed to PAT1 and not DRA (49, 50).
Studies with DRA- and PAT1-knockout (KO) mice have been critical to defining some of the roles of these two apical exchangers. In the distal ileum, PAT1 facilitates oxalate secretion via chloride (Cl−)/oxalate exchange, its absence contributing to the hyperoxaluric phenotype of the PAT1-KO model (15), although urinary sulfate excretion was unchanged (40), implying that PAT1 may not necessarily be important to sulfate homeostasis. DRA is considered the main Cl−/ exchanger in the lower intestine, and loss-of-function mutations of the human DRA gene cause congenital chloride diarrhea (CCD; 21). This phenotype has been reproduced with a DRA-KO mouse model (46), lacking net Cl− absorption by the distal ileum, and large intestine in vitro (17). This latter study also revealed a significant contribution of DRA to intestinal oxalate absorption, lower circulating oxalate levels, and reduced urinary oxalate excretion. Whether sulfate homeostasis is also altered in DRA-KO mice is not known, and no such indication has been reported for humans with CCD although the founder mutation of DRA in the Finnish population (p.V318del) is unable to transport sulfate, as well as Cl−, in vitro (37). Using DRA- and PAT1-KO mice, the aim of this work was to deduce the involvement of these apical anion exchangers in sulfate transport by the distal ileum, gaining some insight into the characteristics of the accompanying transport mechanism and their contribution to overall sulfate homeostasis.
MATERIALS AND METHODS
Experimental animals.
DRA-KO (Slc26a3−/−) and PAT1-KO (Slc26a6−/−) mice were obtained from respective colonies of breeding pairs at the University of Florida that had been bred onto a C57BL/6 background. Information on the targeting vector construction and subsequent generation of the PAT1- and DRA-KO mice have been described elsewhere (46, 59). Genotype analysis of the offspring was performed by PCR of DNA isolated from tail snips as detailed previously (46, 59). All mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility in the Biomedical Sciences Building at the University of Florida, where they were given free access to standard chow (diet 7912; Harlan Teklad, Indianapolis, IN) and sterile drinking water. Pedialyte (Walgreens, Deerfield, IL) was added to the drinking water of DRA-KO mice (50% vol/vol) to help offset the persistent electrolyte and volume losses associated with the chronic diarrhea experienced by these animals (57). A total of 120 mice of both sexes were used in the following experiments, ranging in age from 2 to 6 mo with a mean body mass of 26.1 ± 0.9 g, n = 47, for DRA-KO; 24.6 ± 1.1 g, n = 17, for PAT1-KO; and 29.3 ± 1.0 g, n = 56, for wild-type (WT) controls. Mice were euthanized by inhalation of 100% CO2 followed by exsanguination via cardiac puncture, after which the lower portion of the intestinal tract (midileum to distal colon) was removed to ice-cold buffer. All animal experimentation was approved by the University of Florida Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Transepithelial flux experiments.
In addition to the transport of sulfate we simultaneously measured Cl− fluxes since this is one of the primary substrates transported by DRA and PAT1. Having the accompanying fluxes of Cl− assisted our interpretation of the sulfate fluxes and accompanying changes to short-circuit current (Isc), as well as providing important information on DRA and PAT1 as Cl− transporters in this portion of the mouse small intestine. Unidirectional fluxes of and Cl− were measured across intact isolated segments of the distal ileum under short-circuit conditions as detailed for other previous studies (15, 17). In brief, following euthanasia and removal of the intestine, a pair of tissues was prepared from a 4-cm length of distal ileum taken adjacent to the ileocecal valve and mounted flat on a slider exposing a gross surface area of 0.3 cm2 (P2304; Physiologic Instruments, San Diego, CA), which was then secured between two halves of a modified Ussing chamber (P2300; Physiologic Instruments). Each tissue was bathed on both sides by 4 ml of buffered saline (pH 7.4), maintained at 37°C while simultaneously gassed and stirred with 95% O2-5% CO2. These preparations were continuously voltage-clamped (VCCMC6; Physiologic Instruments), and ~10 min after mounting, 0.27 μCi 35S (specific activity = 1,494 Ci/mmol) and 0.09 μCi 36Cl (specific activity = 571 μCi/mmol) were added to either the mucosal (M) or serosal (S) chamber, which was then designated as the “hot” side. At 10-min intervals for up to 70 min the appearance of these tracers was detected in 1-ml samples taken from the opposing “cold” side, where each 1-ml sample taken for counting was immediately replaced with 1 ml of warmed buffer. In addition, transepithelial potential difference (mV) and Isc (μA) were also recorded at each 10-min sampling interval. Flux experiments were often divided into two time periods, an initial “control” period (period I), representing the first three 10-min sampling intervals (0–30 min) and a second “treatment” period (period II) extending from 50 to 70 min. Experiments testing the acute effects of the anion transport inhibitor DIDS (500 μmol/l), loop diuretic bumetanide (100 μmol/l), and the cAMP agonist cocktail of forskolin/IBMX (10/100 μmol/l) were all performed as part of this paired, two-period design. These drugs were applied to the tissue at the end of period I, and the responses followed during period II. At the beginning and end of each flux experiment, 50-μl samples from the hot side were collected to calculate the specific activity of each isotope. The activity of 35S and 36Cl− in collected samples was determined by liquid scintillation spectrophotometry (Beckman LS6500; Beckman Coulter, Fullerton, CA) with quench correction. Using a series of external standards, the validity of counting dual-labeled samples was independently established, allowing the individual activities of 35S and 36Cl to be calculated on the basis of their relative counting efficiencies after minimizing and accounting for overlap in their energy spectra.
Urine and blood collections.
For the collection of urine, a random selection of mice with each genotype (WT, n = 15; DRA-KO, n = 15; PAT1-KO, n = 8) were placed in individual metabolic cages for a 24-h urine collection. During this time they all had access to sterile water, but no food; this was to avoid uneaten food falling from the cage and potentially contaminating the urine. The DRA-KO mice entering metabolic cages were temporarily switched from dilute Pedialyte to sterile deionized water for the duration of these urine collections. This was done to avoid the consumption of sulfate, present as ZnSO4 (0.64 mmol/l) in Pedialyte. Urine was collected under mineral oil (75 μl) with 20 μl of 2% (wt/vol) sodium azide as a preservative. Approximately 1 wk following urine collection, blood samples from each of these animals were obtained by cardiac puncture following CO2 narcosis. Blood was collected using a 25-gauge needle attached to a syringe that had been flushed with 5% (wt/vol) Na2-EDTA as an anticoagulant, and the blood was transferred into microcentrifuge tubes containing 20 μl Na2-EDTA and gently mixed by inversion. The amount of EDTA used equated to ~1.9 mg (5.1 μmol) per milliliter of blood collected. The blood was then immediately centrifuged at 3,000 rpm for 10 min, and the plasma was carefully aspirated before being frozen at −20°C until analysis.
Urine and blood plasma analysis.
Urine pH, chloride, and osmolality were determined immediately following retrieval of the collected urine from the metabolic cages and before the remainder of the urine was appropriately diluted and subsequently frozen at −20°C. The pH was measured using an Accumet combination micro-pH electrode (Fisher Scientific) connected to a Beckman 690 pH meter (Beckman Coulter). Osmolality was determined by freezing point depression (Fiske One-Ten osmometer; Fiske Associates, Norwood, MA). Chloride was measured by direct coulometric titration using a digital chloridometer (Labconco, Kansas City, MO). Creatinine was measured in urine and plasma with a modification of the Jaffe reaction as described previously (18). Sulfate was determined by turbidimetric assay based on the methods described by Berglund and Sorbo (5), and Lundquist et al. (30), using a barium chloride reagent [containing 40 mmol/l BaCl2, with 150 g/l polyethylene glycol (PEG) 6000 as a stabilizing agent, and seeded with 0.1 mmol/l Na2SO4]. Urinary sulfate was assayed following a 50-fold dilution and acidification with dilute HCl to achieve a final concentration of 0.1 N. Plasma sulfate was determined on aliquots that had been deproteinized with 8% (vol/vol) trichloroacetic acid (TCA) in a ratio of 1:1. To trace and subsequently correct for any losses of sulfate following deproteinization, each sample was spiked with Na235SO4 (0.12 μCi/μl plasma) beforehand. The mean ± SE recovery of 35S following deproteinization of the plasma was 92.9 ± 0.9% (n = 36). To perform the sulfate assay with diluted aliquots of acidified urine, the BaCl2-PEG reagent was added to each sample, Na2SO4 standard, and deionized water blank (which also contained 0.1 N HCl) in a ratio of 4:1 (sample-reagent) in a 96-well microplate, with the plate agitated immediately before reading at 600 nm. The deproteinized plasma samples were assayed in the same manner except that the accompanying standards and blank contained 4% TCA (rather than 0.1 N HCl).
Buffer solutions and reagents.
The standard bicarbonate buffer used in the flux experiments contained the following solutes (mmol/l): 139.4 Na+, 122.2 Cl−, 21 , 5.4 K+, 2.4 , 1.2 Ca2+, 1.2 Mg2+, 0.6 , and 0.5 , pH 7.4, with 10 glucose included in the serosal buffer and 10 mannitol added to the mucosal buffer. For ion substitution experiments, chloride-free buffer was prepared by replacing NaCl with an equimolar amount of isethionic acid Na+ salt, whereas CaCl2 and MgCl2 were replaced by their respective gluconate salts. To produce a /CO2-free buffer, NaHCO3 was replaced with equimolar 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) and gassed with 100% O2 (pH 7.4). The contribution of any endogenous CO2 production to the supply of was also limited by including the carbonic anhydrase inhibitor ethoxzolamide (Sigma-Aldrich, St. Louis, MO) in the /CO2-free buffer at a final concentration of 100 μmol/l. To inhibit spontaneous prostanoid production, all buffers contained 5 μmol/l indomethacin (Sigma-Aldrich). The radioisotope 35S was purchased as Na2SO4 from PerkinElmer (Billerica, MA), and 36Cl was purchased as HCl from Amersham Biosciences (Piscataway, NJ). Concentrated stock solutions of DIDS (Molecular Probes, Eugene, OR) and bumetanide (Sigma-Aldrich) were made fresh on the day of each experiment in DMSO. A cocktail of forskolin and 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) was also freshly prepared in DMSO. The total concentration of DMSO presented to the tissues in any experiment never exceeded 0.25%.
Calculations and statistical analyses.
The transepithelial fluxes of and Cl− in the absorptive direction, mucosal-to-serosal (), and secretory direction, serosal-to-mucosal (), were calculated from the change in activity of 35S and 36Cl detected on the cold side of the chamber at each 10-min sampling point, having corrected for dilution with replacement buffer between samples. These flux rates were expressed per square centimeter of tissue surface area per hour. The recordings of Isc (μA/cm2) and potential difference (mV) were used to calculate transepithelial conductance (GT, mS/cm2) following Ohm’s law. The net flux of each ion was calculated as = – from paired tissues matched on the basis of conductance (no greater than a ±15% difference in GT between pairs).
The following data are presented as means ± SE. Significant differences between WT, DRA-KO, and PAT1-KO mice were tested for by one-way ANOVA, and, as appropriate, multiple pairwise comparisons were made using Holm-Sidak post hoc tests. The effects of ion substitution/replacement on fluxes were directly compared with corresponding values obtained in standard bicarbonate buffer using an unpaired t-test. When evaluating the effect of DIDS (500 μmol/l), bumetanide (100 μmol/l), or forskolin/IBMX (10/100 μmol/l), during two-period flux experiments, a repeated-measures one-way ANOVA was used to test for significant changes at each subsequent 10-min time point (from 40 to 70 min) following its application and compared with the mean value for the prior control period (0–30 min). Multiple, pairwise comparisons with this control period were made using Holm-Sidak post hoc tests, as appropriate. For data failing to meet the assumptions of approximate normality and equality of variance the equivalent nonparametric tests were performed. The results of all tests were accepted as significant at P ≤ 0.05. Statistical analysis was performed using SigmaStat v3.5, and figures were drawn in SigmaPlot v11.0 (Systat Software, San Jose, CA).
RESULTS
Sulfate and chloride transport by the distal ileum.
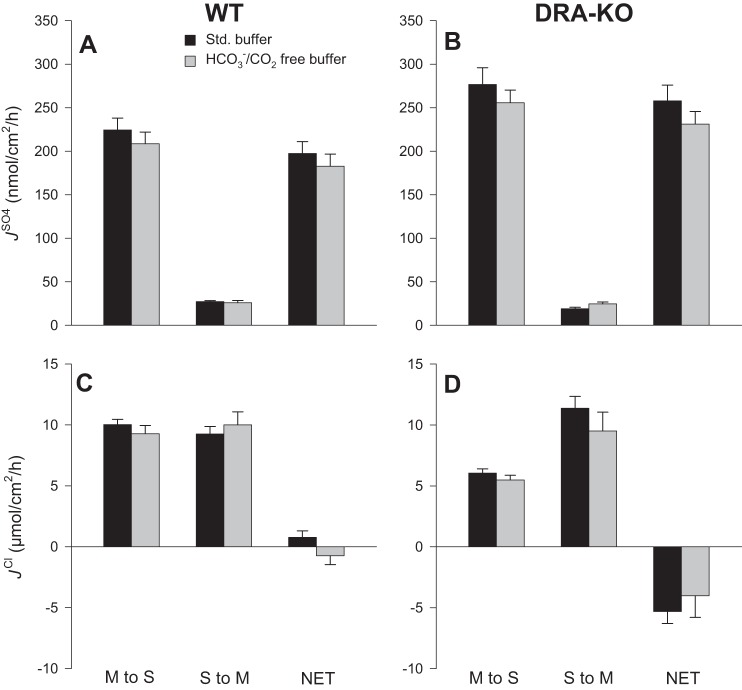
Table 1 reveals a clear, pronounced net absorption of sulfate by the distal ileum of WT mice under short-circuit conditions and in symmetrical standard bicarbonate buffer. By comparison, net sulfate absorption by the same segment from DRA-KO mice was significantly higher by almost one-third. This overall trend of enhanced net absorption was the combined result of an elevated, but nonsignificant, flux (F2,65 = 2.88, P = 0.064), together with a significant 30% reduction to the secretory flux. Sulfate fluxes across the PAT1-KO ileum displayed a very similar pattern, where was decreased 27%; however, unlike the DRA-KO model, was not statistically significant relative to the WT tissues following post hoc pairwise comparisons (P = 0.072). On a net basis, there was no substantial Cl− absorption by the ileum of WT mice (0.77 ± 0.53 μmol·cm−2·h−1), whereas the absence of DRA (but not PAT1) caused a 40% decrease in leading to robust net Cl− secretion that was comparable in magnitude and direction to the prevailing Isc. The ionic permeability of the ileum, indexed by GT, was not significantly different between genotypes.
Table 1.
Comparison of simultaneously measured fluxes of sulfate and chloride across pairs of isolated, short-circuited segments of distal ileum taken from wild-type, DRA-KO, and PAT1-KO mice in standard bicarbonate buffer, with the accompanying electrophysiological parameters, short-circuit current and transepithelial conductance
| , nmol·cm−2·h−1 | , μmol·cm−2·h−1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | M→S | S→M | Net | M→S | S→M | Net | Isc, μeq·cm−2·h−1 | GT, mS/cm2 |
| WT | 224.40 ± 13.64 (30) | 27.03 ± 1.25a (30) | 197.37 ± 13.61a (30) | 10.03 ± 0.43a (30) | 9.26 ± 0.61 (30) | 0.77 ± 0.53a (30) | −2.20 ± 0.16a (60) | 30.45 ± 1.11 (60) |
| DRA-KO | 276.74 ± 19.14 (31) | 18.94 ± 1.80b (31) | 257.80 ± 18.16b (31) | 6.05 ± 0.35b (31) | 11.37 ± 0.97 (31) | −5.31 ± 0.99b (31) | −4.85 ± 0.31b (62) | 29.78 ± 1.07 (62) |
| PAT1-KO | 285.44 ± 35.74 (7) | 19.70 ± 2.41b (7) | 265.74 ± 34.16a,b (7) | 10.09 ± 1.31a (7) | 7.96 ± 1.06 (7) | 2.13 ± 0.49a (7) | −1.97 ± 0.29a (14) | 31.33 ± 3.30 (14) |
Values are means ± SE with associated sample size in parentheses. Data are for the initial control period (0–30 min, period I) from each experiment performed with standard bicarbonate buffer. , sulfate flux; , chloride flux; Isc, short-circuit current; GT, transepithelial conductance; WT, wild type; KO, knockout. Statistically significant differences between genotypes for each measured parameter were determined by one-way ANOVA and, where appropriate, subsequent post hoc comparisons (P ≤ 0.05). Following Holm-Sidak multiple pairwise tests, values labeled with a different letter are significantly different, whereas values sharing the same letter are not.
Responses of sulfate and chloride transport to mucosal DIDS.
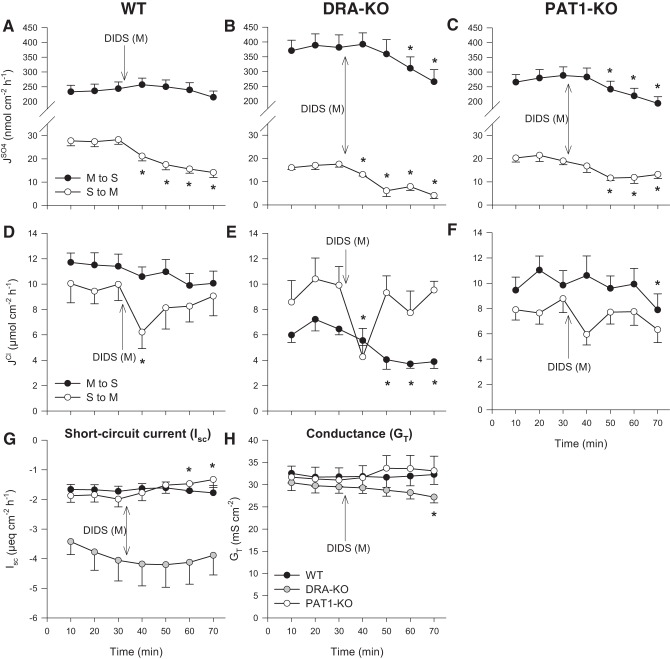
To investigate the possible role of an apical anion exchanger in sulfate fluxes across the distal ileum, we used the nonspecific anion transport inhibitor DIDS. Applying 500 μmol/l DIDS to the mucosal surface of the WT ileum did not diminish , although this flux displayed a small, but significant, degree of sensitivity in DRA- and PAT1-KO animals (Fig. 1, B and C). In terms of sulfate secretion, mucosal DIDS produced an immediate, sustained reduction to , resulting in an overall 44% decrease in this secretory flux during period II (Fig. 1A). In the absence of DRA, was more DIDS sensitive and could be reduced even further to just 6.0 ± 1.5 nmol·cm−2·h−1 (Fig. 1B). Compared with this flux across the WT ileum (28.1 ± 2.0 nmol·cm−2·h−1; Fig. 1A), and with no significant impact on GT (Fig. 1H), this would suggest that 79% of is transcellular and involves DRA and at least one other DIDS-sensitive apical transport pathway, most likely PAT1. Compared with the PAT1-KO ileum, was still inhibited by DIDS (Fig. 1C). In contrast to sulfate secretion, Cl− absorption was clearly resistant to mucosal DIDS (Fig. 1, D and F), except in the DRA-KO ileum (Fig. 1E). There was also a transient dip in the secretory flux by all three genotypes immediately following the addition of mucosal DIDS, appearing most prominent in the WT and DRA-KO tissues (Fig. 1, D and E). This might have reflected inhibition of electrogenic Cl− transport, although there were no concurrent changes to Isc (Fig. 1G).
Fig. 1.
Effect of 500 μmol/l mucosal DIDS on transepithelial fluxes of sulfate () and chloride () across the distal ileum from wild-type (WT), DRA-knockout (KO), and PAT1-KO mice. Unidirectional sulfate fluxes (A–C) and chloride fluxes (D–F) were measured simultaneously across isolated, short-circuited segments of the distal ileum in standard bicarbonate buffer. G and H compare the responses of short-circuit current (Isc) and transepithelial conductance (GT) between WT, DRA-KO, and PAT1-KO mice. Each data point represents mean ± SE of n = 12, 6, and 9 tissues taken from WT, DRA-KO, and PAT1-KO mice, respectively. *Statistically significant difference (P ≤ 0.05) as determined by one-way repeated-measures ANOVA, followed by Holm-Sidak pairwise comparisons with the preceding control period (0–30 min). M, mucosal; S, serosal.
Responses of sulfate and chloride transport to serosal DIDS.
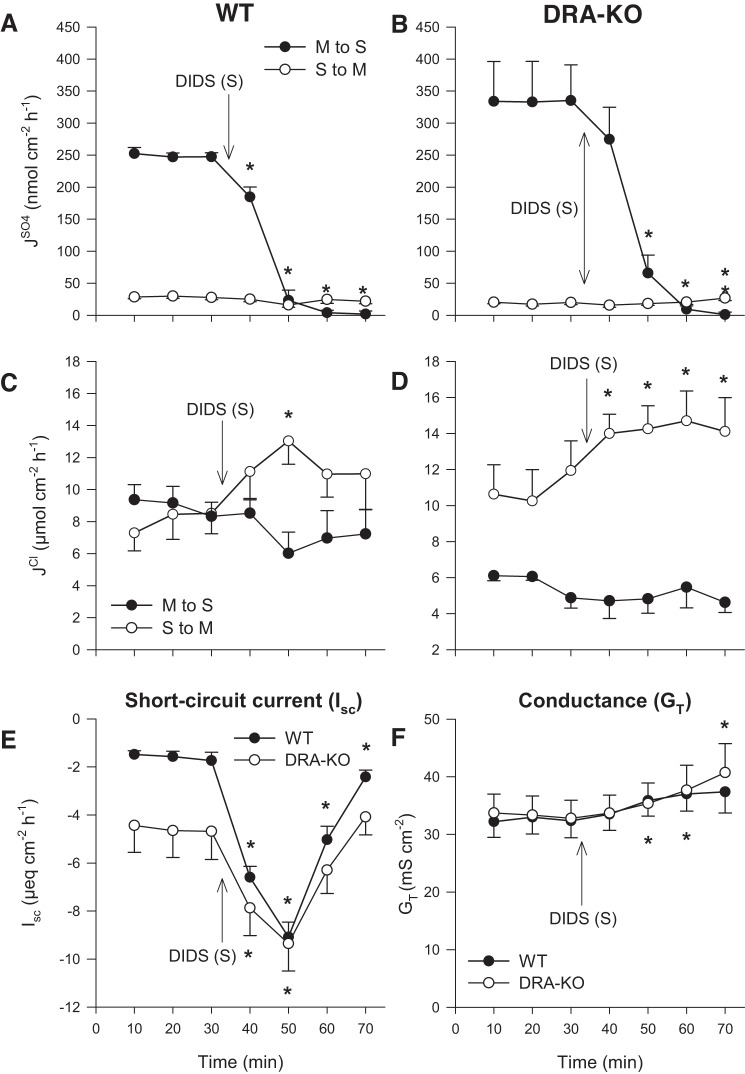
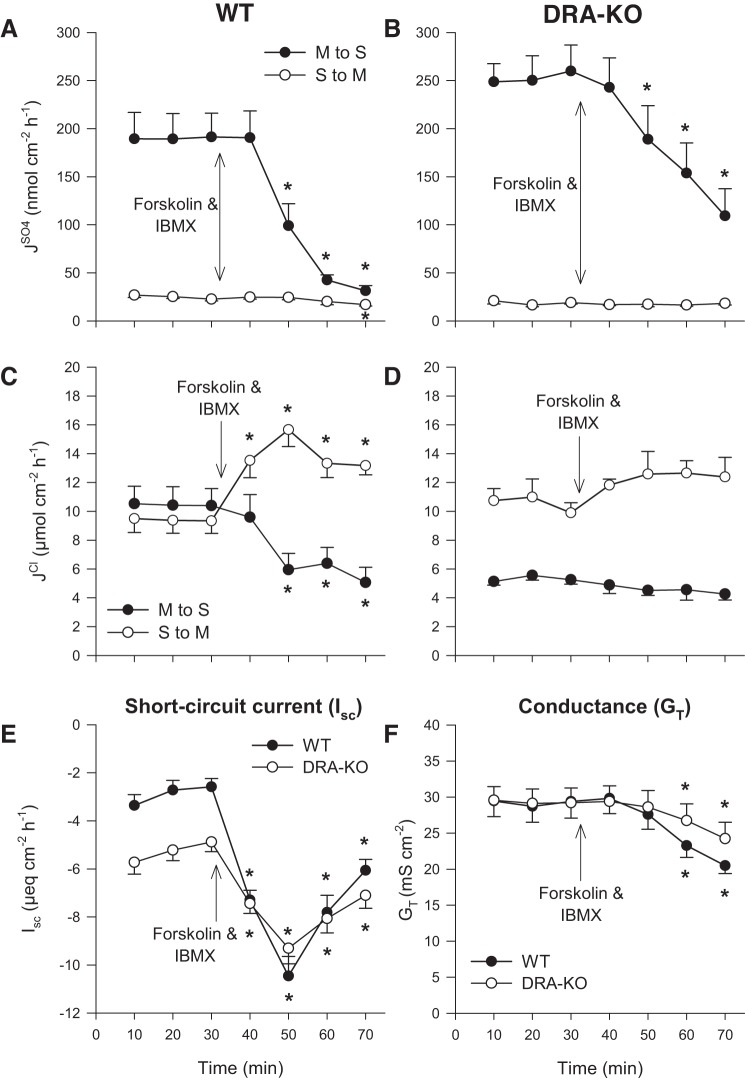
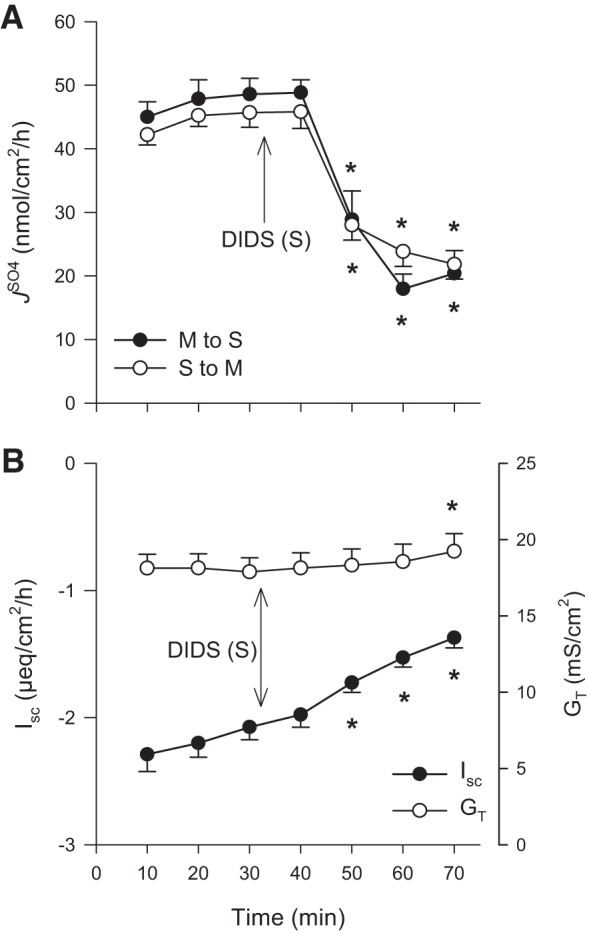
Application of 500 μmol/l DIDS to the serosal bath produced a far more dramatic impact on sulfate fluxes. The prominent absorptive flux, , was effectively driven to zero within 30 min of DIDS exposure for both WT (Fig. 2A) and DRA-KO (Fig. 2B) tissues. This not only implies involvement of a DIDS-sensitive pathway for sulfate absorption at the basolateral membrane but also suggests that there is very little paracellular contribution to , despite the trend toward increasing ionic permeability as indicated by GT (Fig. 2F). There was no significant inhibition of by serosal DIDS, which tended to increase slightly alongside GT. There were two main observations in terms of the effects on Cl− fluxes: 1) was not significantly responsive to serosal DIDS, which would appear to rule out a basolateral, DIDS-sensitive import pathway supporting apical Cl−/ exchange by DRA. 2) There was an unexpected and potent stimulation of net Cl− secretion in both WT and DRA-KO mice via a rapid increase in (Fig. 2, C and D). The concomitant increase to Isc in both cases indicates the induction of net electrogenic anion secretion. This surprising response was specific to the basolateral membrane since mucosal DIDS elicited no such effect (Fig. 1). In terms of Isc, the overall change was greatest for the WT ileum, although the peak current (−9.1 ± 0.6 μeq·cm−2·h−1) at the 50-min mark was well matched to that of the DRA-KO ileum (−9.8 ± 1.2 μeq·cm−2·h−1; Fig. 2E).
Fig. 2.
Effect of 500 μmol/l serosal DIDS on transepithelial fluxes of sulfate () and chloride () across the distal ileum from wild-type (WT) and DRA-knockout (KO) mice. Unidirectional sulfate fluxes (A and B) and chloride fluxes (C and D) were measured simultaneously across isolated, short-circuited segments of the distal ileum in standard bicarbonate buffer. E and F compare the responses of short-circuit current (Isc) and transepithelial conductance (GT) between WT and DRA-KO mice. Each data point represents mean ± SE of n = 5 and 6 tissues taken from WT and DRA-KO mice, respectively. *Statistically significant difference (P ≤ 0.05) as determined by one-way repeated-measures ANOVA, followed by Holm-Sidak pairwise comparisons with the preceding control period (0–30 min). M, mucosal; S, serosal.
Responses to serosal bumetanide.
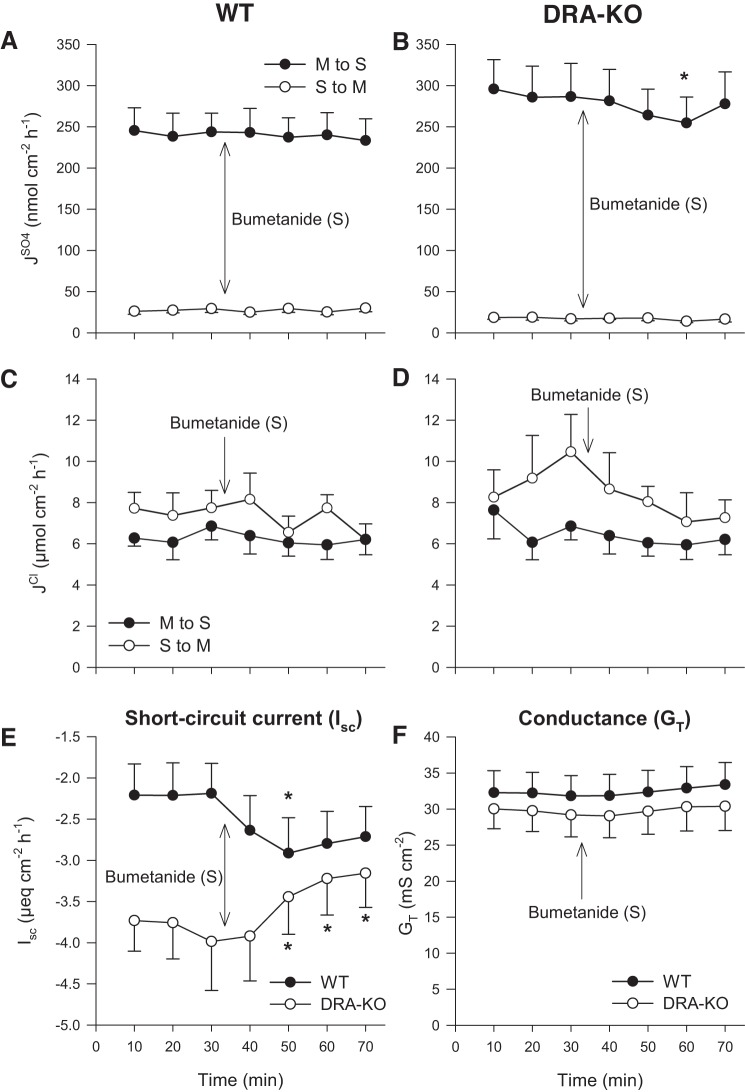
With no inhibition of following the application of 500 μmol/l serosal DIDS this would appear to discount a role for DIDS-sensitive basolateral anion exchange/cotransport as a primary contributor to transcellular sulfate secretion. To explore whether might be dependent on the Na+-K+-2Cl− cotransporter, NKCC1 (Slc12a2), another major basolateral anion transporter, we tested the effects of the loop diuretic, bumetanide. Figure 3 shows that 100 μmol/l bumetanide added to the serosal bath had no significant effect on unidirectional sulfate and Cl− fluxes in either the WT or DRA-KO ileum.
Fig. 3.
Effect of 100 μmol/l serosal bumetanide on transepithelial fluxes of sulfate () and chloride () across the distal ileum from wild-type (WT) and DRA-knockout (KO) mice. Unidirectional sulfate fluxes (A and B) and chloride fluxes (C and D) were measured simultaneously across isolated, short-circuited segments of the distal ileum in standard buffer. E and F compare the responses of short-circuit current (Isc) and transepithelial conductance (GT). Each data point represents mean ± SE of n = 6 and 5 tissues taken from WT and DRA-KO mice, respectively. *Statistically significant difference (P ≤ 0.05), as determined by one-way repeated-measures ANOVA, followed by Holm-Sidak pairwise comparisons with the preceding control period (0–30 min). M, mucosal; S, serosal.
Dependence of sulfate transport on extracellular chloride.
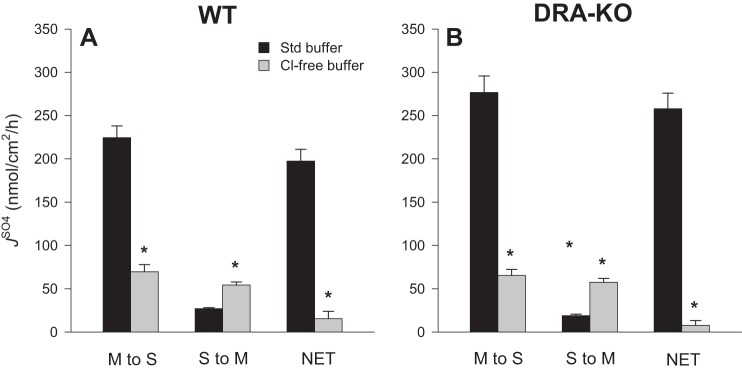
In symmetrical Cl−-free buffers there were striking reductions to net sulfate absorption by 92 and 97% for WT and DRA-KO mice, respectively, compared with corresponding fluxes in standard buffer (Fig. 4). This occurred through not only a substantial decrease in the absorptive flux but also a twofold increase in for WT ileum (Fig. 4A) and an even larger threefold increase in this secretory flux for DRA-KO ileum (Fig. 4B). To further probe this dramatic increase in , we repeated this experiment using Cl−-free buffer but added a second period to test the effects of serosal DIDS (Fig. 5A). Not only was the enhanced effectively inhibited, but also was reduced by ~50%. Notably, the large Isc characteristic of the DRA-KO epithelium (−4.85 ± 0.31 μeq·cm−2·h−1; Table 1) was significantly reduced in Cl−-free buffer to −2.99 ± 0.32 μeq·cm−2·h−1 (Table 2) and, compared with the Isc across the WT ileum (−2.20 ± 0.16 μeq·cm−2·h−1; Table 1), implies that a portion of this inflated current in the DRA-KO ileum may be carried by Cl−. Furthermore, the apparent induction of net electrogenic anion secretion observed previously with serosal DIDS in standard buffer (Fig. 2, E and F) was completely absent in Cl−-free buffer (Fig. 5B), suggesting that this DIDS-stimulated current was being carried by Cl−.
Fig. 4.
Comparison of unidirectional and net sulfate fluxes () across the distal ileum from wild-type (WT) and DRA-knockout (KO) mice in standard (Std) and chloride (Cl−)-free buffer. Sulfate fluxes were measured across isolated, short-circuited segments of the distal ileum after bilateral replacement of chloride. Bars represent means ± SE of n = 7 tissue pairs for each genotype. *Statistically significant difference (P ≤ 0.05) from the corresponding flux measured in standard buffer (reproduced from Table 1), as determined by independent t-test. The accompanying electrophysiological data are presented in Table 2. M, mucosal; S, serosal.
Fig. 5.
Effect of 500 μmol/l serosal DIDS on the transepithelial fluxes of sulfate () across the distal ileum from wild-type mice in chloride-free buffer. Unidirectional sulfate fluxes (A) were measured across isolated, short-circuited segments of the distal ileum after bilateral replacement of chloride. B: responses of short-circuit current (Isc) and transepithelial conductance (GT). Each data point represents mean ± SE from n = 8 tissues taken from WT mice. *Statistically significant difference (P ≤ 0.05) as determined by one-way repeated-measures ANOVA, followed by Holm-Sidak pairwise comparisons with the preceding control period (0–30 min). M, mucosal; S, serosal.
Table 2.
Short-circuit current and transepithelial conductance measured across isolated segments of distal ileum taken from wild-type and DRA-knockout mice following bilateral ion substitution of either Cl− or /CO2
|
Isc, μeq·cm−2·h−1 |
GT, mS/cm2 |
|||
|---|---|---|---|---|
| Genotype | Cl−-Free | /CO2-Free | Cl−-Free | /CO2-Free |
| WT | −1.87 ± 0.15 (14) | −2.16 ± 0.17 (12) | 19.39 ± 0.95 (14) | 29.82 ± 1.48 (12) |
| DRA-KO | −2.99 ± 0.32* (14) | −3.15 ± 0.55 (10) | 18.96 ± 1.39 (14) | 28.23 ± 1.56 (10) |
Values are means ± SE with associated sample size in parentheses. Isc, short-circuit current; GT, transepithelial conductance; WT, wild type; KO, knockout.
Statistically significant difference between genotypes as determined by independent t-test (P ≤ 0.05).
Dependence of sulfate and chloride transport on extracellular bicarbonate and carbonic anhydrase.
When and CO2 were removed (and carbonic anhydrase activity was simultaneously inhibited with 100 μmol/l ethoxzolamide), there were no significant changes to any of the sulfate fluxes relative to standard buffer (Fig. 6); hence net sulfate absorption was not dependent on a supply of , either endogenous or extracellular. Furthermore, Cl− transport was also not impacted to any significant degree (Fig. 6, C and D). The accompanying electrophysiology data in Table 2 show that Isc across the isolated ileum from DRA-KO mice was reduced in HEPES buffer (−3.15 ± 0.55 μeq·cm−2·h−1), to a similar degree as seen in Cl−-free buffer. The large Isc seen in the DRA-KO ileum therefore also includes a component as well as a contribution from Cl−.
Fig. 6.
Comparison of unidirectional and net fluxes of sulfate () and chloride () across the distal ileum from wild-type (WT) and DRA-knockout (KO) mice in standard and /CO2-free buffer. Sulfate fluxes (A and B) and chloride fluxes (C and D) were measured simultaneously across isolated, short-circuited segments of the distal ileum after bilateral replacement of and CO2, together with inhibition of carbonic anhydrase activity by the inclusion of 100 µmol/l ethoxzolamide. Bars represent means ± SE of n = 6 and 5 tissue pairs taken from WT and DRA-KO mice, respectively. For comparison, the corresponding flux data measured in standard buffer (black bars) have been reproduced from Table 1. The accompanying electrophysiological data are shown in Table 2. M, mucosal; S, serosal.
Effects of cAMP stimulation on sulfate and chloride transport.
Stimulating the production of cAMP with a cocktail of forskolin (10 μmol/l) and IBMX (100 μmol/l) resulted in an abrupt decline in for both WT and DRA-KO mice, without any significant changes to the secretory flux (Fig. 7, A and B). In the WT ileum, Cl− secretion was induced through a simultaneous reduction in alongside an increase to producing an overall net secretion of −8.3 ± 1.0 μmol·cm−2·h−1 (Fig. 7C). By contrast, forskolin/IBMX was without effect on either of the unidirectional Cl− fluxes across the DRA-KO ileum (Fig. 7D). Consistent with the stimulation of net electrogenic anion secretion, Isc was increased significantly and, as seen previously in Fig. 2E, both WT and DRA-KO tissues reached an identical zenith at ~50 min (Fig. 7E).
Fig. 7.
Effect of cAMP stimulation on transepithelial fluxes of sulfate () and chloride () across the distal ileum from wild-type (WT) and DRA-knockout (KO) mice. Unidirectional sulfate fluxes (A and B) and chloride fluxes (C and D) were measured simultaneously across isolated, short-circuited segments of the distal ileum in standard bicarbonate buffer in response to bilateral application of forskolin (10 μmol/l) and IBMX (100 μmol/l). E and F compare the responses of short-circuit current (Isc) and transepithelial conductance (GT). Each data point represents mean ± SE of n = 8 and 7 tissues taken from WT and DRA-KO mice, respectively. *Statistically significant difference (P ≤ 0.05) as determined by one-way repeated-measures ANOVA, followed by Holm-Sidak pairwise comparisons with the preceding control period (0–30 min). M, mucosal; S, serosal.
Analysis of urine and plasma from WT, DRA-KO, and PAT1-KO mice.
The WT mice were significantly heavier than either their DRA- or PAT1-KO counterparts, despite all animals being of similar age and an approximately even number of males and females. The only exception was the DRA-KO group, where the sex distribution was skewed toward females. Table 3 therefore shows mass-adjusted rates of fluid consumption and urine excretion. Urinary sulfate was significantly increased in DRA- and PAT1-KO mice (2.2- and 1.8-fold higher, respectively) relative to WT controls. Circulating levels of sulfate in the plasma were also higher in the absence of DRA and PAT1. The plasma sulfate of DRA-KO mice was 2.5 mmol/l and significantly greater than WT mice, whereas the mean plasma sulfate of PAT1-KO animals lay in between at 2.0 mmol/l and was not significantly different from either WT or DRA-KO mice. Notably, this accumulation of plasma sulfate in DRA-KO mice was associated with a 55% higher plasma creatinine, not observed in PAT1-KO mice. The DRA-KO mice not only consumed more deionized water but also produced a more acidic and less concentrated urine. Urinary Cl− excretion by DRA-KO mice was very low, and in 20% (3/15) of samples, Cl− was <1 mmol/l (the detection limit of the chloridometer). The elevated urinary creatinine excretion in DRA- and PAT1-KO mice contributed to the higher calculated creatinine clearances, although their sulfate clearances did not differ significantly from WT mice. The consequent clearance ratio of sulfate to creatinine was also consistent for each group, between 0.13 and 0.16, indicative of prominent net sulfate reabsorption along the nephron in all cases.
Table 3.
Comparison of the urine and plasma parameters from wild-type, DRA-KO, and PAT1-KO mice
| WT | DRA-KO | PAT1-KO | |
|---|---|---|---|
| Sex (male/female), n | 7/8 | 4/11 | 4/4 |
| Age, days | 128 ± 5a (15) | 125 ± 5a (15) | 148 ± 5b (8) |
| Body mass, g | 27.0 ± 1.5a (15) | 22.4 ± 0.9b (15) | 23.9 ± 1.6a,b (8) |
| Fluid intake, ml·g−1·24 h−1 | 0.132 ± 0.023a (15) | 0.230 ± 0.022b (15) | 0.157 ± 0.033a,b (8) |
| Urine volume, ml·g−1·24 h−1 | 0.043 ± 0.009a (15) | 0.082 ± 0.008b (15) | 0.054 ± 0.008a,b (8) |
| Urine pH | 6.218 ± 0.028a (14) | 5.275 ± 0.108b (15) | 6.058 ± 0.050a,b (7) |
| Urine osmolality, mosmol/kgH2O | 1,092 ± 116a (14) | 730 ± 62b (15) | 1,136 ± 142a (7) |
| Urine chloride, μmol·g−1·24 h−1 | 2.439 ± 0.303a (15) | 0.224 ± 0.048b (12) | 3.722 ± 0.464a (8) |
| Urine creatinine, μmol·g−1·24 h−1 | 0.120 ± 0.011a (15) | 0.280 ± 0.018b (15) | 0.184 ± 0.013c (8) |
| Urine sulfate, μmol·g−1·24 h−1 | 0.779 ± 0.070a (15) | 1.709 ± 0.134b (15) | 1.368 ± 0.181b (8) |
| Plasma creatinine, μmol/l | 32.8 ± 1.9a (14) | 50.7 ± 3.1b (14) | 36.3 ± 2.6a (8) |
| Plasma sulfate, mmol/l | 1.57 ± 0.14a (15) | 2.52 ± 0.29b (12) | 2.04 ± 0.14a,b (8) |
| Creatinine clearance, μl·min−1·g−1 | 2.57 ± 0.25a (14) | 4.02 ± 0.36b (14) | 3.65 ± 0.39a,b (8) |
| Sulfate clearance, μl·min−1·g−1 | 0.41 ± 0.08 (15) | 0.55 ± 0.08 (12) | 0.46 ± 0.04 (8) |
| Clearance ratio (sulfate-creatinine) | 0.16 ± 0.02 (14) | 0.15 ± 0.02 (12) | 0.13 ± 0.01 (8) |
Values are means ± SE with associated sample size in parentheses. All mice were housed individually in metabolic cages for a 24-h urine collection; during this time they each had access to sterile water but no food. Because of the differences in body mass between groups, fluid intake, urinary excretion and clearance rates were expressed per gram of body mass. WT, wild type; KO, knockout. Statistically significant differences between genotypes for each measured parameter were determined by one-way ANOVA and, where appropriate, subsequent post hoc comparisons (P ≤ 0.05). Following Holm-Sidak multiple pairwise tests, values labeled with a different letter are significantly different, whereas values sharing the same letter are not.
DISCUSSION
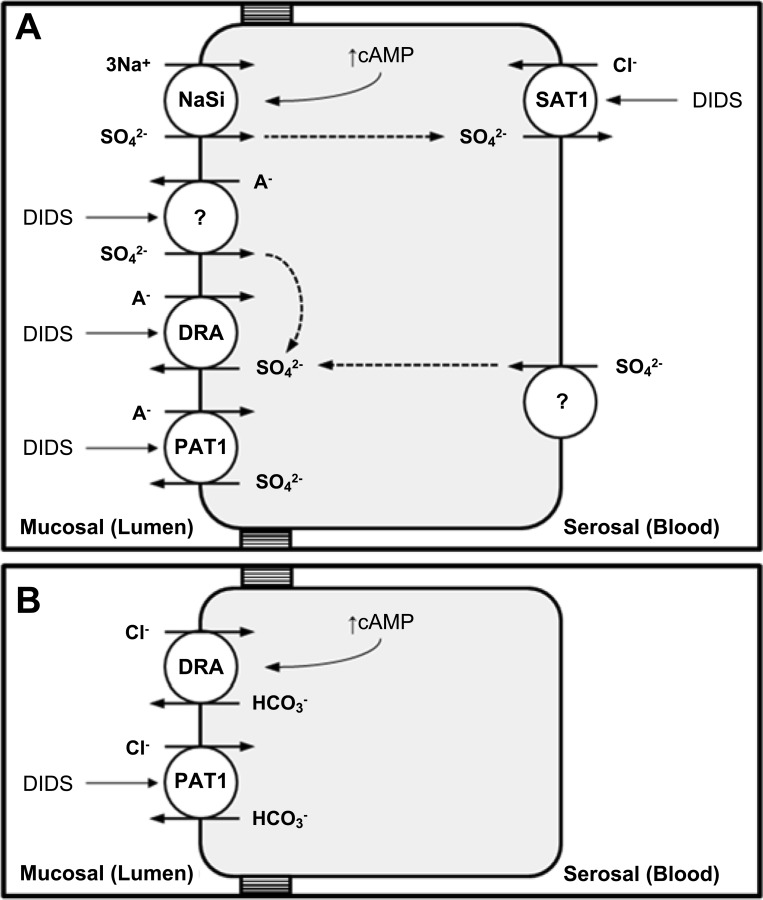
In the present study we have characterized the transepithelial fluxes of sulfate across the mouse distal ileum focusing on the apical anion exchangers DRA (Slc26a3) and PAT1 (Slc26a6). The isolated, short-circuited ileum supported a substantial net absorption of sulfate. Neither DRA nor PAT1 were direct contributors to this, but net sulfate absorption was significantly enhanced by the DRA-KO (and to a lesser extent, the PAT1-KO) ileum through a simultaneous rise in and reduction to . To explain this, we propose that DRA and PAT1 are mediating transcellular sulfate secretion in addition to a partial back flux of imported sulfate across the apical membrane (Fig. 8). Consistent with these elevated rates of sulfate absorption by the ileum in vitro, DRA-KO mice exhibited distinct hypersulfatemia and hypersulfaturia, whereas PAT1-KO mice excreted significantly more urinary sulfate. These alterations to sulfate homeostasis could not be attributed to any apparent changes in net renal sulfate transport. We therefore suggest that DRA and PAT1 contribute to intestinal sulfate secretion and their absence results in higher intestinal absorption of sulfate with significant implications for overall homeostasis.
Fig. 8.
Simple model illustrating the proposed roles of DRA (Slc26a3) and PAT1 (Slc26a6) in transcellular sulfate (A) and chloride (B) transport in the mouse distal ileum. A: the apical sodium-sulfate cotransporter, NaSi (Slc13a1), is primarily responsible for importing sulfate into the cell and the target for acute negative regulation by cAMP. In addition, an unidentified DIDS-sensitive transporter (apparently unmasked by the absence of either DRA or PAT1) may also be operating in parallel with NaSi contributing to . Sulfate absorption is completed by DIDS-sensitive /Cl− exchange at the basolateral membrane, most likely SAT1 (Slc26a1). In terms of DRA and PAT1, both contribute to DIDS-sensitive sulfate efflux across the apical membrane, which includes mediating a partial back flux of imported sulfate that would explain the enhanced net sulfate absorption observed by DRA-KO (and to a lesser extent, PAT1-KO) tissues. A− = Cl− or . B: DRA also functions as the main apical transporter responsible for Cl− absorption by the mouse distal ileum. As a Cl− transporter, DRA was characteristically resistant to DIDS and appeared to operate independently of , but it was acutely regulated by cAMP. PAT1 may partially compensate for the loss of DRA, albeit incompletely, by contributing to DIDS-sensitive Cl− absorption.
Apical DRA and PAT1 contribute to transcellular, DIDS-sensitive sulfate secretion.
The roles of DRA and PAT1 in sulfate transport by the small intestine have received little attention. Apical in/out exchange has been demonstrated in the mouse ileum (55, 56) and duodenum (49), implying a possible mechanism for sulfate absorption. However, we found that was unaffected by limiting cellular and insensitive to mucosal DIDS (at least in WT tissues), suggesting that apical / exchange was not a major absorptive pathway. In fact, the absence of DRA or PAT1 actually increased, rather than diminished, net sulfate absorption via . Curiously, this enhanced flux by DRA- and PAT1-KO tissues was modestly sensitive to DIDS. Whether the loss of DRA or PAT1 unmasked a DIDS-sensitive transporter contributing to absorption is an intriguing possibility (Fig. 8). A potential candidate would be DTDST; however, its role as an intestinal sulfate transporter has yet to be explored, and mRNA levels of DTDST were not upregulated in the PAT1-KO ileum (15).
The reduction to seen in the DRA-KO ileum suggests that DRA is directly mediating sulfate efflux across the apical membrane and contributing to secretion, similar to its purported role in the adjacent cecum, where we had proposed that it was a in/out exchanger (60). However, if DRA were functioning in this same mode in the ileum, then removal of extracellular /CO2 would have been expected to limit , but this was not the case. Alternatively, as a prominent Cl−/ exchanger, DRA may simply perform Cl−/ exchange, but was also independent of extracellular Cl−. These ion substitution experiments thus do not offer any clear insight into the possible identity of the extracellular anion(s) being exchanged for intracellular sulfate at the apical membrane. It is conceivable that Cl− and could interchangeably serve as counterions for sulfate, but we did not test this with a combined Cl−--free buffer.
In the PAT1-KO ileum, sulfate fluxes closely resembled those in DRA-KO tissues, but the change to net sulfate absorption was not statistically significant relative to WT controls. The contribution of PAT1, together with DRA, is supported by the ability of mucosal DIDS to consistently inhibit by their respective KO tissues. Indeed, the largest effect of DIDS was in the DRA-KO ileum, where was reduced to just 21% of its corresponding rate in the WT ileum. This indicates that not only is a large portion (79%) of this secretory flux transcellular but also there is at least one other apical DIDS-sensitive transporter participating alongside DRA. PAT1 is therefore the obvious candidate given its clear contribution to , together with its functional characterization as a DIDS-sensitive sulfate transporter (9, 23, 50, 62) and previously documented role (with DRA) in sulfate secretion by the cecum (60).
For DRA and PAT1 to contribute to they must have a supply of sulfate from the serosal bath, yet the identity of the basolateral import pathway was not obvious as neither DIDS nor bumetanide had any effect on precluding involvement of either a DIDS-sensitive anion transporter or NKCC1, respectively. The absence of extracellular Cl−, however, stimulated between twofold and threefold, an effect seen previously in the mouse cecum (60) and rabbit ileum (52), where the latter could be attenuated by SITS (28). In Cl−-free buffer, we found that this inflated secretory flux was sensitive to serosal DIDS suggesting that there may be a basolateral /Cl− exchange mechanism contributing to sulfate import in the mouse ileum. However, this was only revealed in the presence of a strong outwardly directed Cl− gradient and not exposed by cAMP-stimulated Cl− secretion (Fig. 7A).
With DRA responsible for ileal Cl− absorption (17), together with its characteristic resistance to DIDS (3, 10, 27, 54), it is difficult to explain why was sensitive to DIDS but was not, when our data imply that DRA was responsible for both. We previously attempted to reconcile these same perplexing observations for the cecum (60). Since DRA does not appear to function as a Cl−/ exchanger, one explanation could be that it mediates and by two distinct mechanisms bearing different sensitivities to DIDS. However, in Caco-2 cells, the divergent sensitivity of apical /OH− and Cl−/OH− exchange to DIDS was taken as indicative of two separate transporters, with DRA representing the former, DIDS-responsive, mechanism (1). The involvement of a distinct DIDS-sensitive sulfate transporter coupled to DRA in the mouse ileum therefore cannot be ruled out. We can, however, eliminate PAT1 as a possible candidate since the sensitivity of , and resistance of , to mucosal DIDS persisted in the PAT1-KO ileum.
Net sulfate absorption by the distal ileum is enhanced by the loss of DRA.
The apical transport pathway responsible for the dominant absorptive sulfate flux seen in Table 1 is most likely the Na+- cotransporter, NaSi, on the basis of Na+-dependent sulfate uptake by BBMVs from the ileum of NaSi-KO mice (12). Although we did not specifically probe for NaSi, the magnitude of and its lack of DIDS sensitivity are consistent with early work on the rabbit ileum where this large absorptive flux was Na+ dependent and resistant to the stilbene derivatives DIDS and SITS (43, 52). Indeed, subsequent functional characterizations have shown that NaSi is impervious to as much as 1 mmol/l DIDS (39, 42). To explain the significantly enhanced absorption of sulfate by DRA-KO tissues, we propose that DRA may be operating in parallel with NaSi and/or an accompanying apical DIDS-sensitive transporter, to mediate a portion of imported sulfate back out into the lumen (Fig. 8). The former scenario resembles the suggested coupling between PAT1 and NaSi in the renal proximal tubule whereby PAT1 functions as a /anion exchanger facilitating a back flux of sulfate into the tubular lumen (34).
Sulfate absorption at the basolateral membrane involves a DIDS-sensitive, Cl−-dependent pathway.
The abrupt inhibition of by serosal DIDS would be consistent with a role for SAT1 at the basolateral membrane (13), but its contribution to intestinal sulfate absorption has not been reported; thus other pathways cannot be excluded. Indeed, two basolateral DIDS-sensitive sulfate transport mechanisms have been described in the small intestine: Cl−/ exchange (19, 28, 45) and / exchange (26). In agreement with observations in rabbit ileum (28, 52), our data also support a major role for basolateral Cl−/ exchange in sulfate absorption (Fig. 8), since was reduced and net sulfate absorption virtually abolished in Cl−-free buffer whereas the removal of /CO2 had no impact.
DRA (but not PAT1) is required for chloride absorption by the ileum.
In addition to contributing to sulfate (60) and oxalate (17) transport, DRA is the major apical Cl−/ exchanger along the intestine. Similar to our previous report on Cl− fluxes across the DRA-KO ileum (17), we also showed a 40% reduction to resulting in a pronounced net secretion of Cl−. Hence the absence of DRA not only hampers Cl− uptake but also actually results in a net loss of Cl−. PAT1 has also been characterized as a Cl−/ exchanger in the proximal small intestine (47, 50, 58, 59), but we show that it is not a major Cl− transporter in the ileum. However, was sensitive to mucosal DIDS when DRA was absent. This may well represent some degree of compensatory Cl− absorption by PAT1, which (unlike DRA) appears consistently DIDS sensitive in a variety of in vitro settings (9, 15, 23, 54, 58).
Serosal DIDS stimulates net anion secretion but does not inhibit Cl− absorption.
Serosal DIDS failed to impact , ruling out DIDS-sensitive basolateral Cl−/ exchange or Na+- cotransport in Cl− absorption. However, an unexpected outcome was the induction of net anion secretion, based on the increase to in both WT and DRA-KO ileum, together with accompanying transient reductions in Isc. Previously, the cecum did not display this same reaction to serosal DIDS (60), but in the ileum this was specific to the basolateral aspect since mucosal DIDS had no such effect and it was therefore unlikely to be crossing the cell membrane and acting intracellularly. This was comparable to the concentration-dependent effects of serosal DIDS on electrogenic anion secretion seen in rabbit distal colon (53) and T84 cells (6), which bore the characteristics of intracellular Ca2+ (Ca2+i)-stimulated Cl− secretion. Indeed, when extracellular Cl− was removed, we found that the stimulation of Isc by serosal DIDS was eliminated, suggesting activation of Cl− secretion. Although we did not probe the signaling pathway concerned in the ileum, the response of to serosal DIDS was clearly different from how forskolin/IBMX affected Cl− fluxes, implying that DIDS was not triggering Cl− secretion via cAMP.
Sulfate and chloride absorption are simultaneously downregulated by cAMP.
The extent to which intestinal sulfate transport might be regulated and the pathways involved remain largely unknown. In rabbit ileum, compounds known to elicit intestinal Cl− secretion (via cAMP, Ca2+i, and cGMP) were all capable of reducing (52). Using forskolin/IBMX to stimulate cAMP, we also showed a dramatic inhibition of and net sulfate absorption. This may well reflect an acute decrease in NaSi function, since cAMP reportedly reduces the activity of this transporter in Xenopus oocytes (31). In terms of Cl−, the WT ileum displayed the classical response to cAMP stimulation whereby net Cl− secretion was induced and absorption abolished, manifesting as a rapid increase in followed by acute reduction of . Since is DRA mediated, this suggests that DRA is negatively regulated consistent with its endocytosis in Caco-2 cells and mouse jejunum following cAMP stimulation (38). We also note that in the WT ileum declined to a mean rate of 5.8 μmol·cm−2·h−1 during period II (Fig. 7C), comparable to DRA-KO tissues before treatment with forskolin/IBMX (5.3 μmol·cm−2·h−1; Fig. 7D), implying large-scale withdrawal and functional loss of DRA in response to cAMP. Despite this, was unaffected, which would appear to contrast with our proposal that DRA facilitates sulfate secretion (Fig. 8).
Altered sulfate homeostasis in DRA- and PAT1-KO mice is due to higher intestinal absorption.
At the glomerulus, sulfate is freely filtered and undergoes active, regulated, bidirectional transport along the proximal tubule. The end result is overwhelmingly sulfate conservation with 65–90% of the filtered load being reabsorbed (34, 36). In this study, net sulfate reabsorption was comparable, estimated to be ~85% across groups, based on the calculated sulfate-to-creatinine clearance ratios. Indeed, the consistency of sulfate clearance, together with the absence of DRA from the rodent kidney (3, 22, 48, 59), suggests that there were no net changes to renal sulfate handling that could readily explain the distinct hypersulfaturia and hypersulfatemia exhibited by DRA-KO mice. The additional sulfate burden in these animals may therefore be the result of greater intestinal absorption.
Higher sulfate absorption from the intestine will increase the amount filtered from the blood at the kidney and thus its availability for reabsorption along the proximal tubule. Once the capacity for tubular reabsorption is reached, increasing amounts of sulfate will appear in the urine (34, 36). With plasma sulfate elevated 61% and urinary excretion >2-fold higher in DRA-KO mice, these findings correspond to increased rates of net sulfate absorption by the distal ileum in vitro and, to a lesser extent, the cecum (60). In fact, with DRA functionally expressed in other segments (i.e., duodenum, jejunum, and distal colon; 17, 46, 51, 57), one might reasonably predict higher overall rates of intestinal sulfate absorption in vivo contributing to the hypersulfaturia and hypersulfatemia seen in DRA-KO mice.
In PAT1-KO mice, the rate of urinary sulfate excretion was also significantly higher than the WT group, contrasting with a previous urine electrolyte excretion profile for this model where sulfate was near-identical to their WT controls (40). The reason for this discrepancy is uncertain. In the proximal tubule, PAT1 is prominently expressed at the apical membrane, where it has been suggested to function alongside NaSi (34), similar to the role posited for DRA and PAT1 in the distal ileum (Fig. 8). The absence of PAT1 would therefore be anticipated to increase net reabsorption along the proximal tubule, raising plasma sulfate and reducing urinary sulfate excretion. However, the consistency of sulfate clearance and the sulfate-to-creatinine clearance ratio suggests that there were no alterations to net renal handling in PAT1-KO mice, yet urinary sulfate excretion was almost twofold higher (Table 3). This would also point to greater intestinal absorption by PAT1-KO mice too, even though this was not entirely borne out by the accompanying net sulfate fluxes and plasma sulfate concentration. It is, however, worth considering that we restricted our flux studies to the final 4 cm of the small intestine. Our in vitro observations therefore do not fully capture the enhanced sulfate absorption that may have been taking place in the more proximal ileum in vivo, as well as the other portions of the small intestine (i.e., the duodenum and jejunum), where PAT1 is also prominently functional (47, 50, 58, 59).
Summary.
This study represents the first characterization of transepithelial sulfate fluxes across the mouse distal ileum and investigation of the roles performed by DRA (Slc26a3) and PAT1 (Slc26a6). We report the following key findings: 1) Neither DRA nor PAT1 directly contributed to net sulfate absorption under symmetrical, short-circuit conditions in vitro. Additionally, did not display any characteristics of an apical anion exchange mechanism (i.e., inhibition by DIDS or dependence on Cl− or ), whereas the basolateral transport pathway was represented by a DIDS-sensitive, Cl−-dependent mechanism. 2) The absence of DRA (and to a lesser extent, PAT1) increased net sulfate absorption by the ileum through simultaneous enhancement of and reduction of . To explain this, we suggest that DRA, together with PAT1, contributes to sulfate secretion and mediates a partial back flux of imported sulfate across the apical membrane (Fig. 8). 3) The DRA-KO mouse exhibited distinct hypersulfatemia and hypersulfaturia, whereas urinary sulfate excretion was 1.8-fold higher in the PAT1-KO model. These impacts on sulfate homeostasis resulting from the absence of DRA or PAT1 could not be explained by changes to renal sulfate handling leading us to conclude that this additional sulfate was derived from elevated absorption by the intestine.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 56245, 81624, and 88892 to M. Hatch.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.W. and M.H. conceived and designed research; J.M.W. performed experiments; J.M.W. and M.H. analyzed data; J.M.W. and M.H. interpreted results of experiments; J.M.W. prepared figures; J.M.W. drafted manuscript; J.M.W. and M.H. edited and revised manuscript; J.M.W. and M.H. approved final version of the manuscript.
ACKNOWLEDGMENTS
We thank Carolyn Avila-Duran, Tara Braun, Kristina Fernandez, Heran Getachew, Morgan Parker, and Tisha Van Pelt for technical assistance and animal husbandry.
REFERENCES
- 1.Alrefai WA, Tyagi S, Mansour F, Saksena S, Syed I, Ramaswamy K, Dudeja PK. Sulfate and chloride transport in Caco-2 cells: differential regulation by thyroxine and the possible role of DRA gene. Am J Physiol Gastrointest Liver Physiol 280: G603–G613, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Anast C, Kennedy R, Volk G, Adamson L. In vitro studies of sulfate transport by small intestine of rat, rabbit, and hamster. J Lab Clin Med 65: 903–911, 1965. [PubMed] [Google Scholar]
- 3.Barmeyer C, Ye JHQ, Sidani S, Geibel J, Binder HJ, Rajendran VM. Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflügers Arch 454: 441–450, 2007. doi: 10.1007/s00424-007-0213-7. [DOI] [PubMed] [Google Scholar]
- 4.Batt ER. Sulfate accumulation by mouse intestine: influence of age and other factors. Am J Physiol 217: 1101–1104, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Berglund F, Sorbo B. Turbidimetric analysis of inorganic sulfate in serum, plasma and urine. Scand J Clin Lab Invest 12: 147–153, 1960. doi: 10.3109/00365516009062416. [DOI] [PubMed] [Google Scholar]
- 6.Brayden DJ, Krouse ME, Law T, Wine JJ. Stilbenes stimulate T84 Cl− secretion by elevating Ca2+. Am J Physiol Gastrointest Liver Physiol 264: G325–G333, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Byeon MK, Frankel A, Papas TS, Henderson KW, Schweinfest CW. Human DRA functions as a sulfate transporter in Sf9 insect cells. Protein Expr Purif 12: 67–74, 1998. doi: 10.1006/prep.1997.0809. [DOI] [PubMed] [Google Scholar]
- 8.Chapman JM, Knoepp SM, Byeon MK, Henderson KW, Schweinfest CW. The colon anion transporter, down-regulated in adenoma, induces growth suppression that is abrogated by E1A. Cancer Res 62: 5083–5088, 2002. [PubMed] [Google Scholar]
- 9.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005. doi: 10.1074/jbc.M411703200. [DOI] [PubMed] [Google Scholar]
- 10.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003. doi: 10.1113/jphysiol.2003.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson PA. Sulfate in fetal development. Semin Cell Dev Biol 22: 653–659, 2011. doi: 10.1016/j.semcdb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Dawson PA, Beck L, Markovich D. Hyposulfatemia, growth retardation, reduced fertility, and seizures in mice lacking a functional NaSi-1 gene. Proc Natl Acad Sci USA 100: 13704–13709, 2003. doi: 10.1073/pnas.2231298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Invest 120: 706–712, 2010. doi: 10.1172/JCI31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forlino A, Piazza R, Tiveron C, Della Torre S, Tatangelo L, Bonafè L, Gualeni B, Romano A, Pecora F, Superti-Furga A, Cetta G, Rossi A. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum Mol Genet 14: 859–871, 2005. doi: 10.1093/hmg/ddi079. [DOI] [PubMed] [Google Scholar]
- 15.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 16.Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297: G918–G929, 2009. doi: 10.1152/ajpgi.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freel RW, Whittamore JM, Hatch M. Transcellular oxalate and Cl− absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305: G520–G527, 2013. doi: 10.1152/ajpgi.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005. doi: 10.1152/ajprenal.00025.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hagenbuch B, Stange G, Murer H. Transport of sulphate in rat jejunal and rat proximal tubular basolateral membrane vesicles. Pflügers Arch 405: 202–208, 1985. doi: 10.1007/BF00582561. [DOI] [PubMed] [Google Scholar]
- 20.Hästbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell 78: 1073–1087, 1994. doi: 10.1016/0092-8674(94)90281-X. [DOI] [PubMed] [Google Scholar]
- 21.Höglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 22.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/ exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002. doi: 10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 24.Karniski LP. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: correlation between sulfate transport activity and chondrodysplasia phenotype. Hum Mol Genet 10: 1485–1490, 2001. doi: 10.1093/hmg/10.14.1485. [DOI] [PubMed] [Google Scholar]
- 25.Knickelbein RG, Aronson PS, Dobbins JW. Substrate and inhibitor specificity of anion exchangers on the brush border membrane of rabbit ileum. J Membr Biol 88: 199–204, 1985. doi: 10.1007/BF01868433. [DOI] [PubMed] [Google Scholar]
- 26.Knickelbein RG, Dobbins JW. Sulfate and oxalate exchange for bicarbonate across the basolateral membrane of rabbit ileum. Am J Physiol Gastrointest Liver Physiol 259: G807–G813, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Lamprecht G, Baisch S, Schoenleber E, Gregor M. Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pflügers Arch 449: 479–490, 2005. doi: 10.1007/s00424-004-1342-x. [DOI] [PubMed] [Google Scholar]
- 28.Langridge-Smith JE, Field M. Sulfate transport in rabbit ileum: characterization of the serosal border anion exchange process. J Membr Biol 63: 207–214, 1981. doi: 10.1007/BF01870982. [DOI] [PubMed] [Google Scholar]
- 29.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284: C769–C779, 2003. doi: 10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- 30.Lundquist P, Mårtensson J, Sörbo B, Ohman S. Turbidimetry of inorganic sulfate, ester sulfate, and total sulfur in urine. Clin Chem 26: 1178–1181, 1980. [PubMed] [Google Scholar]
- 31.Markovich D. Molecular regulation and membrane trafficking of mammalian renal phosphate and sulphate transporters. Eur J Cell Biol 79: 531–538, 2000. doi: 10.1078/0171-9335-00076. [DOI] [PubMed] [Google Scholar]
- 32.Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 81: 1499–1533, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Markovich D. Slc13a1 and Slc26a1 KO models reveal physiological roles of anion transporters. Physiology (Bethesda) 27: 7–14, 2012. doi: 10.1152/physiol.00041.2011. [DOI] [PubMed] [Google Scholar]
- 34.Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol 69: 361–375, 2007. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- 35.Morris ME, Murer H. Molecular mechanisms in renal and intestinal sulfate (re)absorption. J Membr Biol 181: 1–9, 2001. doi: 10.1007/s0023200100028. [DOI] [PubMed] [Google Scholar]
- 36.Morris ME, Sagawa K. Molecular mechanisms of renal sulfate regulation. Crit Rev Clin Lab Sci 37: 345–388, 2000. doi: 10.1080/10408360091174240. [DOI] [PubMed] [Google Scholar]
- 37.Moseley RH, Höglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 276: G185–G192, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol 296: G202–G210, 2009. doi: 10.1152/ajpgi.90350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norbis F, Perego C, Markovich D, Stange G, Verri T, Murer H. cDNA cloning of a rat small-intestinal Na+/SO4(2−) cotransporter. Pflügers Arch 428: 217–223, 1994. doi: 10.1007/BF00724500. [DOI] [PubMed] [Google Scholar]
- 40.Ohana E, Shcheynikov N, Moe OW, Muallem S. SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol 24: 1617–1626, 2013. doi: 10.1681/ASN.2013010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M, Ohana E, Choi SY, Lee M-S, Park JH, Muallem S. Multiple roles of the SO4(2−)/Cl−/OH− exchanger protein Slc26a2 in chondrocyte functions. J Biol Chem 289: 1993–2001, 2014. doi: 10.1074/jbc.M113.503466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perego C, Markovich D, Norbis F, Verri T, Sorribas V, Murer H. Expression of rat ileal Na (+)-sulphate cotransport in Xenopus laevis oocytes: functional characterization. Pflügers Arch 427: 252–256, 1994. doi: 10.1007/BF00374531. [DOI] [PubMed] [Google Scholar]
- 43.Schron CM, Knickelbein RG, Aronson PS, Della Puca J, Dobbins JW. Effects of cations on pH gradient-stimulated sulfate transport in rabbit ileal brush-border membrane vesicles. Am J Physiol Gastrointest Liver Physiol 249: G614–G621, 1985. [DOI] [PubMed] [Google Scholar]
- 44.Schron CM, Knickelbein RG, Aronson PS, Della Puca J, Dobbins JW. pH gradient-stimulated sulfate transport by rabbit ileal brush-border membrane vesicles: evidence for SO4-OH exchange. Am J Physiol Gastrointest Liver Physiol 249: G607–G613, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Schron CM, Knickelbein RG, Aronson PS, Dobbins JW. Evidence for carrier-mediated Cl-SO4 exchange in rabbit ileal basolateral membrane vesicles. Am J Physiol Gastrointest Liver Physiol 253: G404–G410, 1987. [DOI] [PubMed] [Google Scholar]
- 46.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 47.Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhöft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflügers Arch 455: 757–766, 2008. doi: 10.1007/s00424-007-0318-z. [DOI] [PubMed] [Google Scholar]
- 48.Silberg DG, Wang W, Moseley RH, Traber PG. The down regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem 270: 11897–11902, 1995. doi: 10.1074/jbc.270.20.11897. [DOI] [PubMed] [Google Scholar]
- 49.Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates basal Cl−/ exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005. doi: 10.1152/ajpgi.00493.2004. [DOI] [PubMed] [Google Scholar]
- 50.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007. doi: 10.1152/ajpgi.00354.2006. [DOI] [PubMed] [Google Scholar]
- 51.Singh AK, Liu Y, Riederer B, Engelhardt R, Thakur BK, Soleimani M, Seidler U. Molecular transport machinery involved in orchestrating luminal acid-induced duodenal bicarbonate secretion in vivo. J Physiol 591: 5377–5391, 2013. doi: 10.1113/jphysiol.2013.254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith PL, Orellana SA, Field M. Active sulfate absorption in rabbit ileum: dependence on sodium and chloride and effects of agents that alter chloride transport. J Membr Biol 63: 199–206, 1981. doi: 10.1007/BF01870981. [DOI] [PubMed] [Google Scholar]
- 53.Smith PL, Sullivan SK, McCabe RD. Concentration-dependent effects of disulfonic stilbenes on colonic chloride transport. Am J Physiol Gastrointest Liver Physiol 250: G44–G49, 1986. [DOI] [PubMed] [Google Scholar]
- 54.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011. doi: 10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchiyama H, Hayashi H, Suzuki Y. Functional characterization of Cl−/ exchange in villous cells of the mouse ileum. Biomed Res 27: 265–274, 2006. doi: 10.2220/biomedres.27.265. [DOI] [PubMed] [Google Scholar]
- 56.Uchiyama H, Hayashi H, Tanji K, Sugimoto O, Suzuki Y. pH stat studies on bicarbonate secretion in the isolated mouse ileum. Biomed Res 28: 239–246, 2007. doi: 10.2220/biomedres.28.239. [DOI] [PubMed] [Google Scholar]
- 57.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645–1653.e3, 2008. doi: 10.1053/j.gastro.2008.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/ exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 60.Whittamore JM, Freel RW, Hatch M. Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum. Am J Physiol Gastrointest Liver Physiol 305: G172–G184, 2013. doi: 10.1152/ajpgi.00084.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolffram S, Grenacher B, Scharrer E. Transport of selenate and sulphate across the intestinal brush-border membrane of pig jejunum by two common mechanism. Q J Exp Physiol 73: 103–111, 1988. doi: 10.1113/expphysiol.1988.sp003107. [DOI] [PubMed] [Google Scholar]
- 62.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]