Abstract
The P2Y12 receptor is a validated target for prevention of major adverse cardiovascular events in patients with acute coronary syndrome. The aim of this study was to compare two direct‐acting, reversible P2Y12 antagonists, ACT‐246475 and ticagrelor, in a rat thrombosis model by simultaneous quantification of their antithrombotic efficacy and surgery‐induced blood loss. Blood flow velocity was assessed in the carotid artery after FeCl3‐induced thrombus formation using a Doppler flow probe. At the same time, blood loss after surgical wounding of the spleen was quantified. Continuous infusions of ACT‐246475 and ticagrelor prevented the injury‐induced reduction of blood flow in a dose‐dependent manner. High doses of both antagonists normalized blood flow and completely abolished thrombus formation as confirmed by histology. Intermediate doses restored baseline blood flow to ≥65%. However, ACT‐246475 caused significantly less increase of blood loss than ticagrelor; the difference in blood loss was 2.6‐fold (P < 0.01) at high doses and 2.7‐fold (P < 0.05) at intermediate doses. Potential reasons for this unexpected difference were explored by measuring the effects of ACT‐246475 and ticagrelor on vascular tone. At concentrations needed to achieve maximal antithrombotic efficacy, ticagrelor compared with ACT‐246475 significantly increased carotid blood flow velocity in vivo (P = 0.003), induced vasorelaxation of precontracted rat femoral arteries, and inhibited contraction of femoral artery induced by electrical field stimulation or by phenylephrine. Overall, ACT‐246475 showed a significantly wider therapeutic window than ticagrelor. The absence of vasodilatory effects due to high selectivity of ACT‐246475 for P2Y12 provides potential arguments for the observed safety advantage of ACT‐246475 over ticagrelor.
Keywords: ACT‐246475, P2Y12, thrombosis, ticagrelor
Abbreviations
- 2‐MeS‐ADP
2‐methyl‐S‐adenosine di‐phosphate (stable ADP analog)
- ABC
area between curves
- ACS
acute coronary syndrome
- ADP
adenosine di‐phosphate
- FeCl3
ferric chloride
- GPCR
G protein‐coupled receptor
- U46619
thromboxane receptor agonist
Introduction
When vascular integrity is compromised, circulating platelets adhere to the damaged vessel wall and aggregate, forming a plug that seals off the site of injury to prevent blood loss. Autopsy studies demonstrated that rupture of atherosclerotic plaques can lead to uncontrolled platelet thrombus formation and vessel occlusion (Davies et al. 1986). Inhibition of platelet aggregation is recognized as an effective strategy for the prevention of atherothrombotic events in patients with atherosclerotic disease in the coronary, peripheral, and cerebrovascular circulation (Davi and Patrono 2007).
The ADP receptors P2Y1 and P2Y12 play a critical role in platelet activation and aggregation (Andre et al. 2003). Inhibition of the P2Y12 receptor is a validated concept for prevention of major adverse cardiovascular events in patients with acute coronary syndromes (ACS) as demonstrated by the thienopyridines ticlopidine, clopidogrel, and prasugrel (Franchi and Angiolillo 2015). Following metabolic activation, these drugs irreversibly block the P2Y12 receptor and platelet function (Antman et al. 2004; Anderson et al. 2011), resulting in increased efficacy and increased bleeding (Wiviott et al. 2007). In studies using P2Y12 knockout mice, it was shown that clopidogrel and prasugrel caused more blood loss following tail transection as compared with vehicle indicating that the increased blood loss may be due to off‐target effects of the thienopyridines (Andre et al. 2011).
Preclinical studies with the direct‐acting, reversibly binding P2Y12 antagonist ticagrelor demonstrated a wider therapeutic window in rat and dog thrombosis models as compared with clopidogrel (van Giezen et al. 2009; Becker and Gurbel 2010). It was argued that the reversibility of the binding of ticagrelor to P2Y12 might account for this difference. In patients, ticagrelor achieved a higher extent of inhibition of ADP‐induced platelet aggregation than clopidogrel (Husted et al. 2006; Storey et al., 2010), and in the pivotal phase III trial (PLATO), ticagrelor showed superior efficacy, and no significant difference in the risk of major bleeding events to clopidogrel. However, a significant increase in fatal intracranial bleedings and in major or minor bleedings according to the study criteria was reported for ticagrelor (Wallentin et al. 2009).
The need for novel P2Y12 antagonists with further reduced bleeding risk is illustrated by post hoc analyses of antiplatelet clinical trials (Eikelboom et al. 2006; Amlani et al. 2010). In more than 30,000 ACS patients (Eikelboom et al. 2006), an increasing risk of death or new ischemic events was observed with increasing severity of bleeding, emphasizing the need for new antiplatelet drugs that are efficacious and have a reduced propensity to cause bleedings.
ACT‐246475 is a potent P2Y12 antagonist synthesized in our laboratories (Caroff et al. 2015). We used the FeCl3 rat thrombosis model to search for compounds showing a wide therapeutic window. The aim of this study was to compare two direct‐acting, reversibly binding P2Y12 antagonists, ACT‐246475 and ticagrelor, by simultaneous measurements of their effects on thrombus formation and on surgery‐induced blood loss. In this setting, ACT‐246475 was shown to have a significantly wider therapeutic window than ticagrelor. Therefore, mechanistic studies were performed to better understand the observed difference between the two P2Y12 antagonists. Both molecules were profiled for potential interference with vascular tone.
Materials and Methods
Animals
This study was conducted in accordance with Swiss Animal Protection Laws. Male Wistar rats were purchased from Harlan Laboratories B.V. (Venray, NL). All animals were housed in accordance with local guidelines (Basel‐Landschaft Cantonal Veterinary Office).
Reagents
ACT‐246475 was synthesized in the Department of Medicinal Chemistry at Idorsia Pharmaceuticals Ltd, Allschwil, Switzerland. Acetylcholine, phenylephrine, and U46619 were purchased from Sigma (St Louis, MO, USA). Ticagrelor was synthesized according to the published protocols. The chemical structures of ACT‐246475 and ticagrelor are shown in Figure 1.
Figure 1.
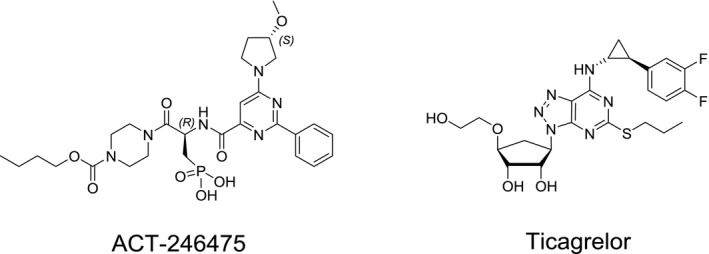
Chemical structures of ACT‐246475 and ticagrelor.
Preparation of rat platelet‐rich plasma and platelet aggregation
Wistar rats were anesthetized by an intraperitoneal injection of thiobutabarbital sodium salt hydrate 150 mg/kg (Inactin®; Sigma‐Aldrich GmbH, Buchs, Switzerland). After tracheotomy, an intravenous cannula (Introcan, 20G × 1¼, B Braun, REF 4252110 B, Sempach, Switzerland) was inserted into the right carotid artery and 7–10 mL blood was removed into a syringe containing 1 mL napsagatran (1 mmol/L in 0.9% NaCl, 1% DMSO), a direct thrombin inhibitor (Ro 46‐6240 from Hoffmann‐La Roche, Basel, Switzerland). The blood was centrifuged for 5 min at 650 g and platelet‐rich plasma (PRP) was stored at 37°C. Platelet aggregation was assessed by light transmission aggregometry (LTA) with a four‐channel Chronolog Lumi‐Aggregometer 490‐4D (Probe & Go Labordiagnostica GmbH, Osburg, Germany) with the AggroLink software package. Aggregation was started by addition of 10 μL of ADP solution into 240 μL PRP at 520 × 106 platelets/mL and monitored for up to 8 min. IC50 values were calculated using XLfit software (IDBS, Guildford, UK).
Rat FeCl3 thrombosis and blood loss model
FeCl3 model was performed based on previous reports (Kurz et al. 1990). Specifically, rats were anesthetized by an intraperitoneal injection of 150 mg/kg thiobutabarbital sodium salt hydrate (Inactin®). The body temperature was maintained at 36–38°C with a thermostatically controlled heating table. After tracheotomy, a catheter was inserted into the right femoral vein for infusion of test compounds during 50 min using a Precidor pump (Infors AG, Bottmingen, Switzerland) at the rate of 0.05 mL/kg/min. A second catheter was inserted in the right femoral artery to collect blood samples. The right carotid artery was gently dissected free of connective tissue, and a silastic‐tubing flow transducer (D‐20‐0.8 mm; Triton Technologies Inc., Arizona, USA) was placed on the artery for blood flow velocity measurement. After 20 min of infusion of vehicle, ACT‐246475, or ticagrelor, filter paper was soaked with ferric chloride 200 mg/mL (20%) (FeCl3, Fluka N°44943, Buchs, Switzerland) and placed on the carotid artery anterior to the flow transducer for 5 min to cause arterial injury, and carotid blood flow velocity was measured for 30 min. After FeCl3 deposition, the spleen was punctured with a 4‐mm biopsy punch (Stiefel, München, Germany) and blood was collected for 30 min. The amount of blood loss is reported in weight (g). All experiments were blinded to the treatment allocation. At the end of the experiment, rats were euthanized by an intravenous infusion of pentobarbital (100 mg/kg; Esconarkon, Streuli Pharma AG, Switzerland).
Carotid blood flow velocity
Carotid blood flow velocity was continuously recorded on a PowerLab acquisition system using IOX software (IOX Data acquisition, Emka Technologies, Paris, France) and analyzed using Datanalyst (version 2.10.17; Emka Technologies). Baseline blood flow was calculated for each rat by using the mean blood flow velocity during 100–110 consecutive heart beats at the end of FeCl3 deposition. The area between curves (ABC) was calculated using delta blood flow (change of baseline flow in %) versus time (30 min treatment). Calculation of ABC is an integrated analytical method to quantify the responses over the entire assessment period.
Vasoreactivity of rat femoral artery
Following euthanasia, rings of femoral artery were prepared from rats using a standard technique (Duckles et al. 1985). Briefly, the right and left femoral arteries were isolated. Two arterial rings (1.5 mm) were prepared from each artery, and vessels suspended between 40‐μm stainless wires in a Mulvany–Halpern myograph system (10 mL) containing modified Krebs–Henseleit buffer of the following composition (mmol/L): NaCl 115; KCl 4.7; MgSO4 1.2; KH2PO4 1.5; CaCl2 2.5; NaHCO3 25; glucose 10. Care was taken to avoid damage to the endothelium. Bathing solution was maintained at 37°C and aerated with 95%O2/5%CO2/(pH7.4). An initial resting force of 3.92 mN was applied to the vessel, and changes in force generation were measured using an isometric force recorder (Multi Wire Myograph System Model 610 M Version 2.2; DMT A/S, Aarhus, Denmark) coupled to a EMKA data acquisition system (EMKA Technologies Inc, Paris, France). Viability of the femoral artery was tested by measuring contraction to KCl (60 mmol/L) and the presence of a functional endothelium confirmed by measuring the ability of acetylcholine (10−5 mol/L) to relax arterial rings contracted with 9,11‐dideoxy‐9α,11α‐metha‐noepoxy prostaglandin F2α (U46619; 10−6 mol/L). Vasorelaxation to ACT‐246475 and ticagrelor was measured in rings of femoral artery which had been precontracted with U46619 (EC70 concentration relative to contraction to KCl 60 mM). When the developed force to U46619 had stabilized, cumulative concentration‐relaxation curves to ACT‐246475 and ticagrelor were obtained. The interval between additions of higher concentrations of compounds to the baths was determined by the time required for the response to reach plateau.
In experiments that measured contraction of femoral artery to electrical field stimulation (EFS), rings of artery were placed between platinum electrodes and stimulated every 5 min (17V, 0.5 msec pulse width, 10 sec, 4–24 Hz) using an electrical stimulator (EMKA Technologies Inc). Two frequency‐contraction curves were obtained in each vessel: an initial control response followed, after a period of recovery, by a second curve in the presence of vehicle or test compound (20 min). Contraction in the presence of test compound was expressed as a percentage of the maximal contraction in the first control response.
Compliance with design and statistical analysis requirements
Data are presented as mean ± SEM. Statistical analyses were performed by multiple Student's t‐test using Graph Pad Prism (V5). Results were considered significant at P < 0.05.
Results
ACT‐246475 and ticagrelor inhibit ADP‐induced aggregation of rat platelets
The inhibitory potencies of ACT‐246475 and ticagrelor were tested in aggregation experiments. In rat platelet‐rich plasma, ACT‐246475 inhibited platelet aggregation, induced by 1 μmol/L ADP, with an IC50 of 55 ng/mL (106 nmol/L) and ticagrelor with an IC50 of 165 ng/mL (316 nmol/L). The rat was, therefore, considered a suitable responder species to determine the dose‐dependent antithrombotic efficacy and to compare surgical blood loss at equi‐efficacious doses of these two direct‐acting, reversible P2Y12 antagonists.
ACT‐246475 and ticagrelor inhibit FeCl3‐induced thrombus formation in rat carotid artery
The FeCl3‐induced thrombosis model was used to determine the dose‐dependent antithrombotic effects of ACT‐246475 and ticagrelor. To ensure an unbiased, objective comparison of the two P2Y12 antagonists, we established continuous infusion protocols resulting in plasma exposures of either compound translating to low (30–50%), intermediate (55–75%), or high (80–100%) inhibition of platelet aggregation. In the presence of vehicle, deposition of FeCl3 induced a rapid decrease in blood flow velocity. Within 11 ± 1 min after vessel injury, the blood flow velocity was reduced to zero, indicative of complete thrombotic occlusion of the artery (Fig. 2A).
Figure 2.
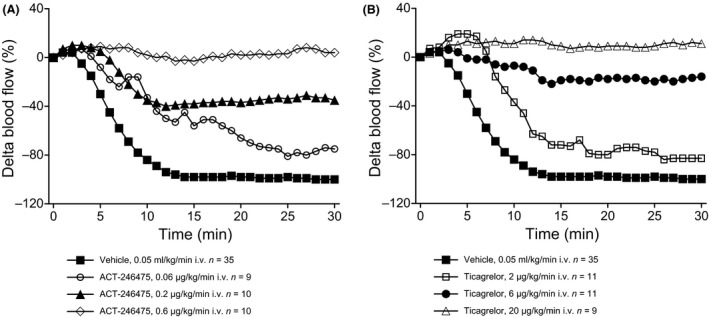
Blood flow velocity after FeCl3‐induced arterial injury. The maximal decrease of blood flow in the presence of vehicle was defined as 100% reduction of blood flow velocity. Effects of ACT‐246475 (Panel A) and ticagrelor (Panel B) are expressed as % change in carotid blood flow velocity relative to baseline flow. ACT‐246475 and ticagrelor were dissolved in PEG 400 (7.5% v/v), PG (7.5% v/v), Cremophor (5% v/v) (Chremophor EL, Fluka N°27963, Buchs, Switzerland), in phosphate buffer pH = 7.4 at a concentration of 1 mg/mL. Vehicle: PEG 400 (7.5%), PG (7.5%), Cremophor (5%) phosphate buffer, pH = 7.4. Data are presented as means, n = 9–35.
ACT‐246475 dose dependently inhibited the FeCl3‐induced reduction of carotid blood flow velocity. The extent of this inhibition was of 25 ± 9% with 0.06 μg/kg/min (low dose) and 65 ± 16% with 0.2 μg/kg/min (intermediate dose) at 30 min after vessel injury. At the high dose of 0.6 μg/kg/min ACT‐246475, the baseline blood flow remained unchanged, suggesting that no flow impairing thrombi were formed (Fig. 2A).
At the end of ACT‐246475 infusion at 0.06, 0.2, and 0.6 μg/kg/min, the number of injured arteries that remained open were 0 of 9, 6 of 10, and 10 of 10, respectively (Table 1). Plasma concentration of ACT‐246475 during continuous infusion was determined after 20 and 50 min of infusion, that is, at the beginning and at the end of the assessment period. The infusions of low, intermediate, and high dose resulted in mean plasma concentrations of 28–57, 72–141, and 243–425 ng/mL of ACT‐246475, respectively.
Table 1.
Antithrombotic effects of P2Y12 antagonists in FeCl3 model in rats
| ACT‐246475 infusion (μg/kg/min) | 0 | 0.06 | 0.2 | 0.6 |
| Mean area of vascular lumen occluded by thrombi (n = 4) | 100% | 52% | 29% | 20% |
|
Blood flow (means ± SEM) n = number of rats |
0% n = 35 |
25 ± 9% n = 9 |
65 ± 16% n = 10 |
104 ± 2% n = 10 |
| Patency of carotid artery | ||||
| Open | 0/35 | 0/9 | 6/10 | 10/10 |
| Partially occluded | 0/35 | 4/9 | 1/10 | 0/10 |
| Occluded | 35/35 | 5/9 | 3/10 | 0/10 |
| Ticagrelor infusion (μg/kg/min) | 0 | 2 | 6 | 20 |
| Mean area of vascular lumen occluded by thrombi (n = 4) | 100% | 94% | 37% | 19% |
|
Blood flow (mean ± SEM) n = number of rats |
0% n = 35 |
17 ± 5% n = 11 |
84 ± 12% n = 11 |
111 ± 4% n = 9 |
| Patency of carotid artery | ||||
| Open | 0/35 | 0/11 | 6/11 | 9/9 |
| Partially occluded | 0/35 | 2/11 | 4/11 | 0/9 |
| Occluded | 35/35 | 9/11 | 1/11 | 0/9 |
Quantification of platelet‐rich thrombi. Paraffin sections of the carotid artery thrombi were deparaffinized, rehydrated and stained with hematoxylin and eosin for histopathological and morphometric evaluation of platelet‐rich thrombi. The mean percentage area of occluded vascular lumen was calculated from one section per rat (n = 4). Quantification of blood flow at 30 min. The mean blood flow at the end of infusion (i.e., at 30 min) was quantified and expressed as percentage from baseline blood flow measured in each rat before vessel injury. Assessment of vessel patency. At the end of the infusion, the patency of the carotid artery was designated either as open (no impairment of blood flow), partially occluded (less than baseline blood flow) or occluded (no blood flow).
Ticagrelor also dose dependently inhibited the FeCl3‐induced decrease in blood flow velocity. The extent of this inhibition was 17 ± 5% with 2 μg/kg/min (low dose) and 84 ± 12% with 6 μg/kg/min (intermediate dose) at 30 min after vessel injury. At the high dose of ticagrelor (20 μg/kg/min), the blood flow was no longer reduced (Fig. 2B). In contrary, at the high dose of ticagrelor, the increase in carotid blood flow velocity versus baseline (ABC of 302 ± 50%*min) was significantly higher (P = 0.003) as compared with that observed at the high dose of ACT‐246475 (ABC of 97 ± 37%*min).
At the end of ticagrelor infusion of 2, 6, and 20 μg/kg/min, the number of injured carotid arteries that remained open were 0 of 11, 6 of 11, and 9 of 9, respectively (Table 1). Plasma exposures of ticagrelor were determined at the beginning and at the end of the period in which the antithrombotic effect was assessed. The infusions of low, intermediate, and high dose resulted in mean plasma concentrations of ticagrelor of 84–119, 185–337, and 1019–1679 ng/mL, respectively. The need for higher plasma concentrations of ticagrelor relative to ACT‐246475 to achieve comparable antithrombotic effects is in good agreement with the threefold lower potency of ticagrelor in rat platelet aggregation.
To confirm that the observed changes in blood flow are a consequence of thrombus formation, cross‐sections of FeCl3‐treated blood vessels were histologically examined. In the vehicle‐treated arteries, platelet‐rich thrombi were prominent and filled the entire vascular lumen. At the low and intermediate doses of ACT‐246475, thrombi mostly formed a ring‐like shape leading to partial occlusion of the vascular lumen (Fig. 3). At the high dose of ACT‐246475, only small mural thrombi were detectable. Morphometric analyses of the cross‐sections revealed that 100%, 52%, 29%, and 20% of the lumen were occluded by thrombi in the vehicle, low‐dose, intermediate‐dose, and high‐dose group, respectively (Table 1). For ticagrelor, morphometric analyses of the respective samples revealed 100%, 94%, 37%, and 19% of thrombus‐occluded vascular lumen in vehicle, low‐dose, intermediate‐dose, and high‐dose group, respectively (Table 1). Thus, improvements of blood flow velocity correlated well with inhibition of thrombus formation.
Figure 3.
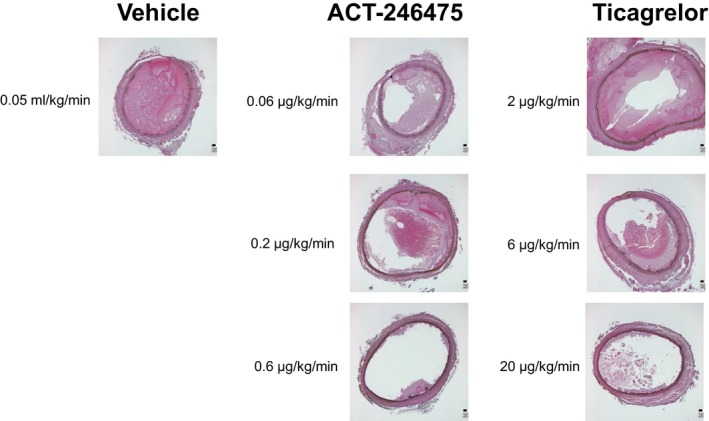
Representative histological images of platelet‐rich thrombi obtained from the FeCl3‐injured carotid arteries. At end of the experiment, carotid arteries of a subset of four rats per group were fixed in 4% paraformaldehyde solution and embedded in paraffin. Paraffin sections of the thrombi (2 μm thick) were deparaffinized, rehydrated, and stained with hematoxylin and eosin for histopathological and morphometric evaluation of platelet‐rich thrombi. Micrographs were analyzed with an Olympus BX45 microscope with 4× magnification and pictures taken with a Colorview IIIu camera. Representative examples are shown.
ACT‐246475 causes less surgery‐induced blood loss than ticagrelor
We then investigated how much blood is lost as result of an injury of the spleen of all treated rats. This method to assess the bleeding risk was used due to its high reproducibility as compared with bleeding time measurements. For this purpose, a standardized surgical wound of the spleen was placed immediately after deposition of FeCl3‐soaked filter paper onto the carotid artery. The amount of blood leaking from the punctured spleen was collected for 30 min, that is, during the time period of the assessment of thrombus formation, and the weight of lost blood was determined. In vehicle‐treated rats, the blood loss amounted to 0.13 ± 0.02 g (mean ± SEM, n = 35), indicating that this method is highly reproducible. Nevertheless, to exclude any experimental bias, the blood loss studies were performed by several investigators who were blinded to the treatment allocation.
Continuous infusion of the low dose of ACT‐246475 resulted in surgery‐induced blood loss of 0.28 ± 0.06 g. Blood loss was 0.58 ± 0.07 g and 0.96 ± 0.21 g at the intermediate and high doses, respectively (Fig. 4). Surgery‐induced blood loss in the ticagrelor‐treated rats was 0.48 ± 0.1 g at the low dose. At the intermediate dose blood loss was 1.54 ± 0.32 g and 2.53 ± 0.47 g at the high dose (Fig. 4).
Figure 4.
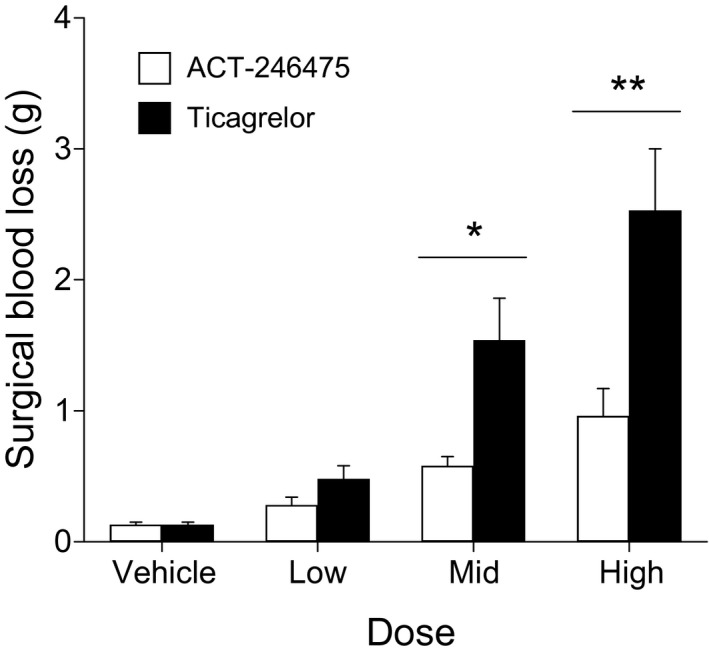
ACT‐246475 and ticagrelor dose dependently caused surgical blood loss after standardized punch biopsy of the spleen. Each study drug was administered by continuous infusion to rats to achieve low‐, intermediate‐, and high‐level inhibition of platelet aggregation. Rats were treated with vehicle, with low, intermediate (Mid), and high dose of ACT‐246475 (0.06, 0.2, 0.6 μL/kg/min) and with low, intermediate (Mid), and high dose of ticagrelor (2, 6, 20 μL/kg/min). After surgical wounding of the spleen, blood was collected for 30 min and the weight of lost blood was determined. Data are presented as means ± SEM, n = 9–35. *P < 0.05, **P < 0.01.
Comparison of blood loss at the low, intermediate, and high doses of the two P2Y12 antagonists showed that ACT‐246475 resulted in 1.7‐, 2.7‐ and 2.6‐fold less blood loss than ticagrelor, respectively. Therefore, at doses resulting in pronounced antithrombotic efficacy (≥65% inhibition), the surgery‐induced blood loss was significantly lower with ACT‐246475 as compared with ticagrelor.
ACT‐246475, in contrast to ticagrelor, does not induce relaxation nor prevent contraction of rat arteries ex vivo
Effects of the P2Y12 antagonists on three general mechanisms of vascular tone regulation were studied ex vivo in rat femoral arteries: (1) relaxation of vascular smooth muscle; (2) vasoconstriction to endogenously released norepinephrine (electrical field stimulation, EFS); and (3) vasoconstriction to exogenously added phenylephrine.
Relaxation of vascular smooth muscle was investigated using isolated rings of rat femoral artery. Rings were precontracted and the potential vasodilating effect of the two antagonists was measured (Fig. 5). ACT‐246475 did not relax arterial rings of femoral artery, whereas ticagrelor caused concentration‐dependent relaxation of precontracted femoral artery rings with an EC50 of 3.9 ± 0.4 μmol/L and an Emax of 84.2 ± 12.9%.
Figure 5.
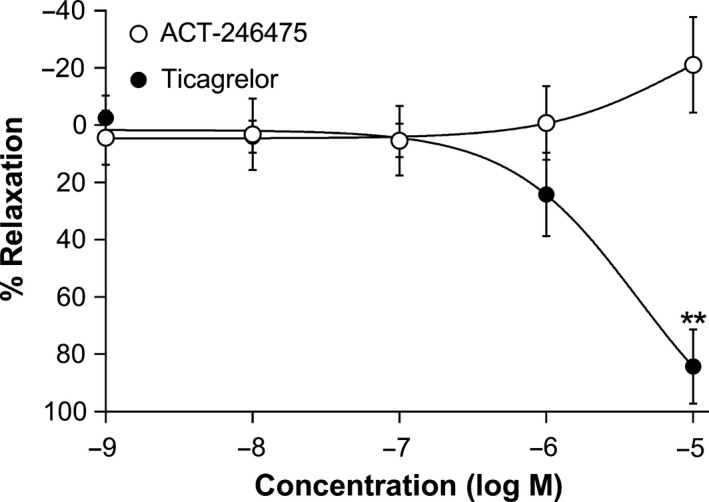
Relaxation of precontracted femoral artery. Relaxation of rat isolated femoral artery to ACT‐246475 (open circles) and ticagrelor (black circles). Rings of femoral artery were precontracted with 1 μmol/L U46619, and increasing concentrations of the two P2Y12 antagonists were added to the organ bath. Data are presented as means ± SEM, n = 4–6. **P < 0.01.
EFS caused frequency‐dependent (4–24 Hz) contraction of femoral artery that was abolished by tetrodotoxin (at 10−7 mol/L) and prazosin (at 10−7 mol/L), confirming that EFS‐induced vasoconstriction is neuronal in origin and mediated via activation of α1‐adrenoceptors. ACT‐246475 at 10−5 mol/L did not inhibit contraction of femoral artery by EFS (Fig. 6A). In contrast, ticagrelor at 3 × 10−6 and 10−5 mol/L significantly inhibited contraction of rat femoral artery to EFS at all frequencies tested (Fig. 6B). In addition, ACT‐246475 at 10−5 mol/L did not inhibit contraction to phenylephrine (Fig. 7A), whereas ticagrelor inhibited contraction of femoral artery in a concentration‐dependent manner. At 10−5 mol/L, ticagrelor almost completely abolished contraction to phenylephrine (Fig. 7B).
Figure 6.
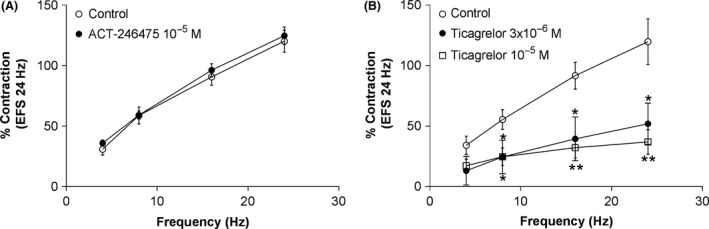
Prevention of contraction of rat femoral artery to electrical field stimulation. Effects of ACT‐246475 (A) and ticagrelor (B) on contraction of rat femoral artery to electrical field stimulation. Contraction observed in the first control response to 24 Hz was set as 100%. Control: open circles, study drugs: black circles and open squares. Data are presented as means ± SEM, n = 4–6. *P < 0.05, **P < 0.01.
Figure 7.
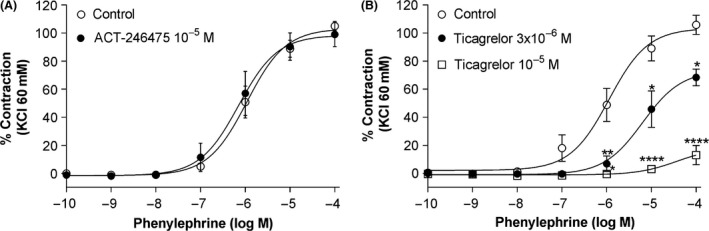
Prevention of contraction of femoral artery to phenylephrine. Effects of ACT‐246475 (A) and ticagrelor (B) on contraction of rat femoral artery to phenylephrine. Control: open circles, study drugs: black circles and open squares. Data are presented as means ± SEM, n = 4. *P < 0.05, **P < 0.01, ****P < 0.0001.
Discussion
In this study, we compared the extent of the therapeutic windows of two direct‐acting, reversibly binding P2Y12 antagonists, ACT‐246475 and ticagrelor, in a rat model of thrombus formation combined with simultaneous measurement of surgery‐induced blood loss. To ensure an objective, unbiased comparison of the two antagonists, a continuous infusion protocol was implemented. Doses were selected that resulted in plasma levels of each antagonist translating into low, intermediate, and high inhibition of platelet aggregation. The data indicated first that both P2Y12 antagonists fully prevented FeCl3‐induced thrombus formation in the carotid artery at the high doses. Second, at equivalent antithrombotic efficacy, ACT‐246475 caused 2.6‐times less blood loss than ticagrelor. Significant differences in blood loss were observed at the intermediate (P < 0.05) and the high doses (P < 0.01), which both resulted in ≥65% restoration of baseline blood flow. Third, ticagrelor significantly increased the carotid blood flow velocity in vivo as compared with ACT‐246475 (P = 0.003), caused relaxation of precontracted rat femoral arteries, and inhibited constriction of femoral arteries either induced by EFS or phenylephrine. In contrast, ACT‐246475 did not affect vascular tone. Thus, ACT‐246475 showed a clearly wider therapeutic window than ticagrelor in rats. Off‐target effects on vascular tone are a potential explanation for the increased bleeding propensity of ticagrelor as compared with ACT‐246475.
In the past decade, clinical and preclinical studies were conducted to determine which P2Y12 antagonist offers the best therapeutic window. In the TRITON‐TIMI, 38 trial patients with ACS were treated with prasugrel or clopidogrel (Wiviott et al. 2007). At the doses studied, prasugrel inhibited ADP‐induced platelet aggregation more consistently and to a greater extent than clopidogrel (Wiviott et al. 2007). Prasugrel significantly reduced the primary efficacy endpoint as compared with clopidogrel. However, prasugrel also significantly increased the risk of major or minor bleedings (Wiviott et al. 2007), supporting the notion that a higher extent of platelet aggregation inhibition translates into improved clinical efficacy albeit at the consequence of more bleedings. Next, clopidogrel was compared with the reversibly binding P2Y12 antagonist ticagrelor in ACS patients. Preclinical studies demonstrated a wider therapeutic window of ticagrelor in rat and dog thrombosis models compared with clopidogrel. It was argued that the reversibility of ticagrelor binding might account for this difference (van Giezen et al. 2009; Becker and Gurbel 2010). In patients, ticagrelor achieved a higher extent of inhibition of platelet aggregation than clopidogrel (Husted et al. 2006; Storey et al., 2010), and in the PLATO trial, ticagrelor showed superior efficacy and no significant difference in the risk of major bleedings to clopidogrel (Wallentin et al. 2009). Thus, despite the higher extent of platelet aggregation inhibition by ticagrelor, bleeding was not increased compared with clopidogrel. This is in contrast to findings from the TRITON data. Therefore, the PLATO data supported the conclusion that reversibly binding P2Y12 antagonists, such as ticagrelor, have a wider therapeutic window than the thienopyridines.
In preclinical studies, it was reported that thienopyridines induce P2Y12‐independent effects resulting in increased bleeding (Andre et al. 2011). When administered to P2Y12‐deficient mice, clopidogrel and prasugrel increased blood loss following tail transection compared to vehicle. In contrast, the reversible P2Y12 antagonist elinogrel did not increase blood loss in P2Y12 knockout mice. The authors showed that only the thienopyridines inhibited vasoconstriction of mesenteric veins induced by α,β‐metATP (Andre et al. 2011). Furthermore, we reported that the reversible P2Y12 antagonist ACT‐246475 has a wider therapeutic window in a rat thrombosis model than clopidogrel (Caroff et al. 2015), since clopidogrel caused clearly more blood loss than ACT‐246475 at maximal efficacy. Therefore, clinical and preclinical data show growing evidence that ticagrelor is the new standard of P2Y12 antagonism because it has three major advantages over clopidogrel. First, ticagrelor inhibits platelet aggregation in patients to a significantly higher extent than clopidogrel (Husted et al. 2006; Storey et al., 2010) and achieves superior efficacy (Wallentin et al. 2009). Second, ticagrelor is a direct‐acting, reversibly binding P2Y12 antagonist; and third, effects of clopidogrel on vascular tone resulting in increased bleeding (Andre et al. 2011) may have further decreased the therapeutic window of clopidogrel compared with ticagrelor.
We, therefore, decided to compare ACT‐246475 with ticagrelor in the FeCl3‐induced rat thrombosis model. Both compounds were continuously infused to achieve constant levels of platelet inhibition for a defined period of time during which antithrombotic efficacy and surgery‐induced blood loss could be assessed. In this model, both antagonists fully blocked thrombus formation at the respective highest dose. Bleeding time measurements are often used to evaluate the bleeding propensity in animals. However, rat tail bleeding time is reported to vary according to the antithrombotic principle and method applied (Dejana et al. 1982). A high variability of bleeding time is also reported for tongue‐bleeding time measurements in dog (van Giezen et al. 2009). By performing a standardized punch biopsy of the spleen, we established a new method to measure surgery‐induced blood loss. In vehicle‐treated rats, the blood loss amounted to 0.13 ± 0.02 g (mean ± SEM, n = 35), indicating good reproducibility. At doses resulting in comparable intermediate or high antithrombotic efficacy, ACT‐246475 caused 2.7‐fold and 2.6‐fold less surgical blood loss than ticagrelor (P < 0.05 and P < 0.01, respectively). Thus, although the compared antagonists are both direct‐acting and reversibly binding to P2Y12, ACT‐246475 revealed a significantly wider therapeutic window than ticagrelor in rats.
Several additional experiments explored the potential reasons for the improved safety profile of ACT‐246475, with emphasis placed on vascular tone regulation. The focus on vascular tone was triggered by reported data indicating that local vasodilation is associated with increased bleeding (Wangensteen et al. 1970; Dunlevy et al. 1996), and local vasoconstriction following arterial injury is considered as immediate physiological response to prevent massive hemorrhage (Lam et al. 1987; Hollenberg et al. 1988; Andre et al. 2011). Furthermore, a dose of 180 mg ticagrelor was shown to enhance adenosine‐induced coronary vasodilatory responses in human volunteers (Wittfeldt et al. 2013). In our study, the high dose of ticagrelor induced a significant increase of blood flow velocity compared with ACT‐246475. It is interesting to note that the vasodilatory effect in humans was observed at ticagrelor plasma concentrations of 1.0–3.0 μmol/L (Wittfeldt et al. 2013). In our rat study, the ticagrelor plasma concentration at the high dose was in a comparable range (1019–1679 ng/mL corresponding to 2.0–3.2 μmol/L).
It was previously reported that ticagrelor inhibits 2‐MeSADP‐ and ADP‐induced contraction of murine and human vessels in vitro (Hogberg et al. 2010) and prevents 2‐MeSADP‐induced contraction of rat tail arteries in vivo (Hogberg et al. 2010; Grzesk et al. 2012). Moreover, in a canine model, ticagrelor increased coronary blood flow in response to ischemia (van Giezen et al. 2012). The effect of ticagrelor on vascular tone was explained either by potential off‐target effects, for example, ticagrelor inhibits adenosine uptake in erythrocytes by interfering with nucleoside transporters (van Giezen et al. 2012; Cattaneo et al., 2014; Nylander and Schulz 2016) or by the presence of P2Y12 receptors in the vasculature. Indeed, it has been reported that brain capillary endothelial cells (Simon et al. 2002) and vascular smooth muscle cells (Wihlborg et al. 2004) express functional P2Y12 receptors, and in the latter case, P2Y12 mediates vasoconstriction. However, we found no evidence that ACT‐246475 increases carotid blood flow velocity in vivo nor induces relaxation of precontracted rat femoral artery. This differentiation of ACT‐246475 from ticagrelor triggered additional mechanistic experiments using rat femoral artery in which the antagonists were compared under identical conditions. The femoral artery was chosen as a model system due to its dense sympathetic innervation and high norepinephrine content (Todd 1980; Stassen et al. 1998). Contraction of rat femoral artery to EFS is neuronal in origin and mediated via activation of α1‐adrenoceptors (Zacharia et al. 2004). Ticagrelor, at 3 and 10 μmol/L, inhibited vasoconstriction of femoral artery to both EFS and phenylephrine, suggesting that ticagrelor acts postsynaptically to inhibit vasoconstriction mediated via activation of α1‐adrenoceptors. In contrast, ACT‐246475 did not affect vasoconstriction of femoral artery in response to EFS or to exogenously added phenylephrine. In this model system, the vasodilating activity of ticagrelor was seen at concentrations similar to those required for maximal antithrombotic efficacy, whereas ACT‐246475 was without any effect on vascular tone even at concentrations 10‐fold higher than those needed to achieve maximal antithrombotic efficacy. Since ACT‐246475 shows no effect on contraction of arteries, the effects of ticagrelor on vascular tone are probably not mediated via the P2Y12 receptor. Thus, our data indicate that ACT‐246475 is a P2Y12 antagonist without off‐target effects on vascular tone. Its high receptor selectivity was further supported by measurements of ACT‐246475 activity toward 89 de‐orphanized G protein‐coupled receptors (GPCRs) and 72 orphan GPCRs, where no relevant inhibitory or activating activity was identified (data not shown).
Based on our findings, we propose that a selective and potent P2Y12 antagonist such as ACT‐246475, by inhibiting platelet aggregation without causing vasodilation at the site of arterial injury, may also show in humans a clearly improved therapeutic window versus currently approved P2Y12 antagonists. Further development of promising P2Y12 antagonists such as ACT‐246475 in the clinical setting is warranted.
Authorship Contributions
Designed research and wrote manuscript: Steiner and Riederer. Conducted experiments and contributed to the writing of the manuscript: Rey and Morrison. Participated in research design and data analysis: Hess, Clozel, and Weber. Conducted experiments: Kramberg, Baumann, Ernst, and Haag. Provided new reagents and tools: Caroff and Hubler.
Disclosures
M. Rey, M. Kramberg, P. Hess, K. Morrison, R. Ernst, F. Haag, E. Weber, M. Clozel, M. Baumann, E. Caroff, F. Hubler, M. A. Riederer, and B. Steiner are all employees of Idorsia Pharmaceuticals Ltd., Switzerland.
Acknowledgements
The authors are indebted to Brigitte Butscha, Christophe Cattaneo, Isabelle Guillaumat, and Benoît Lack for technical assistance. All acknowledged persons are employees of Idorsia Pharmaceuticals Ltd., Switzerland.
Rey M., Kramberg M., Hess P., Morrison K., Ernst R., Haag F., Weber E., Clozel M., Baumann M., Caroff E., Hubler F., Riederer M. A., Steiner B.. The reversible P2Y12 antagonist ACT‐246475 causes significantly less blood loss than ticagrelor at equivalent antithrombotic efficacy in rat, Pharma Res Per, 5(5), 2017,e00338, https://doi.org/10.1002/prp2.338
References
- Amlani S, Nadarajah T, Afzal R, Pal‐Sayal R, Eikelboom JW, Natarajan MK (2010). Mortality and morbidity following a major bleed in a registry population with acute ST elevation myocardial infarction. J Thromb Thrombolysis 30: 434–440. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. (2011). 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123: e426–e579. [DOI] [PubMed] [Google Scholar]
- Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, et al. (2003). P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest 112: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, DeGuzman F, Haberstock‐Debic H, Mills S, Pak Y, Inagaki M, et al. (2011). Thienopyridines, but not elinogrel, result in off‐target effects at the vessel wall that contribute to bleeding. J Pharmacol Exp Ther 338: 22–30. [DOI] [PubMed] [Google Scholar]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. (2004). ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 110: 588–636. [DOI] [PubMed] [Google Scholar]
- Becker RC, Gurbel PA (2010). Platelet P2Y12 receptor antagonist pharmacokinetics and pharmacodynamics: a foundation for distinguishing mechanisms of bleeding and anticipated risk for platelet‐directed therapies. Thromb Haemost 103: 535–544. [DOI] [PubMed] [Google Scholar]
- Caroff E, Hubler F, Meyer E, Renneberg D, Gnerre C, Treiber A, et al. (2015). 4‐((R)‐2‐{[6‐((S)‐3‐Methoxypyrrolidin‐1‐yl)‐2‐phenylpyrimidine‐4‐carbonyl]amino}‐ 3‐phosphonopropionyl)piperazine‐1‐carboxylic Acid Butyl Ester (ACT‐246475) and Its Prodrug (ACT‐281959), a Novel P2Y Receptor Antagonist with a Wider Therapeutic Window in the Rat Than Clopidogrel. J Med Chem 58: 9133–9153. [DOI] [PubMed] [Google Scholar]
- Davi G, Patrono C (2007). Platelet activation and atherothrombosis. N Engl J Med 357: 2482–2494. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Schulz R, Nylander S (2014). Adenosine‐mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol 63: 2503–2509. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Thomas AC, Knapman PA, Hangartner JR (1986). Intramyocardial platelet aggregation in patients with unstable angina suffering sudden ischemic cardiac death. Circulation 73: 418–427. [DOI] [PubMed] [Google Scholar]
- Dejana E, Villa S, de Gaetano G (1982). Bleeding time in rats: a comparison of different experimental conditions. Thromb Haemost 48: 108–111. [PubMed] [Google Scholar]
- Duckles SP, Carter BJ, Williams CL (1985). Vascular adrenergic neuroeffector function does not decline in aged rats. Circ Res 56: 109–116. [DOI] [PubMed] [Google Scholar]
- Dunlevy TM, O'Malley TP, Postma GN (1996). Optimal concentration of epinephrine for vasoconstriction in neck surgery. Laryngoscope 106: 1412–1414. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S (2006). Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114: 774–782. [DOI] [PubMed] [Google Scholar]
- Franchi F, Angiolillo DJ (2015). Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 12: 30–47. [DOI] [PubMed] [Google Scholar]
- van Giezen JJ, Berntsson P, Zachrisson H, Bjorkman JA (2009). Comparison of ticagrelor and thienopyridine P2Y(12) binding characteristics and antithrombotic and bleeding effects in rat and dog models of thrombosis/hemostasis. Thromb Res 124: 565–571. [DOI] [PubMed] [Google Scholar]
- van Giezen JJ, Sidaway J, Glaves P, Kirk I, Bjorkman JA (2012). Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine‐mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther 17: 164–172. [DOI] [PubMed] [Google Scholar]
- Grzesk G, Kozinski M, Navarese EP, Krzyzanowski M, Grzesk E, Kubica A, et al. (2012). Ticagrelor, but not clopidogrel and prasugrel, prevents ADP‐induced vascular smooth muscle cell contraction: a placebo‐controlled study in rats. Thromb Res 130: 65–69. [DOI] [PubMed] [Google Scholar]
- Hogberg C, Svensson H, Gustafsson R, Eyjolfsson A, Erlinge D (2010). The reversible oral P2Y12 antagonist AZD6140 inhibits ADP‐induced contractions in murine and human vasculature. Int J Cardiol 142: 187–192. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Monteiro K, Sandor T (1988). Endothelial injury provokes collateral arterial vasoconstriction: response to a serotonin 2 antagonist, thromboxane antagonist or synthetase inhibition. J Pharmacol Exp Ther 244: 1164–1168. [PubMed] [Google Scholar]
- Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G (2006). Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double‐blind comparison to clopidogrel with aspirin. Eur Heart J 27: 1038–1047. [DOI] [PubMed] [Google Scholar]
- Kurz KD, Main BW, Sandusky GE (1990). Rat model of arterial thrombosis induced by ferric chloride. Thromb Res 60: 269–280. [DOI] [PubMed] [Google Scholar]
- Lam JY, Chesebro JH, Steele PM, Badimon L, Fuster V (1987). Is vasospasm related to platelet deposition? Relationship in a porcine preparation of arterial injury in vivo. Circulation 75: 243–248. [DOI] [PubMed] [Google Scholar]
- Nylander S, Schulz R (2016). Effects of P2Y12 receptor antagonists beyond platelet inhibition–comparison of ticagrelor with thienopyridines. Br J Pharmacol 173: 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Filippov AK, Goransson S, Wong YH, Frelin C, Michel AD, et al. (2002). Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem 277: 31390–31400. [DOI] [PubMed] [Google Scholar]
- Stassen FR, Maas RG, Schiffers PM, Janssen GM, De Mey JG (1998). A positive and reversible relationship between adrenergic nerves and alpha‐1A adrenoceptors in rat arteries. J Pharmacol Exp Ther 284: 399–405. [PubMed] [Google Scholar]
- Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon CP, Becker RC, Wallentin L (2010). Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 56: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Todd ME (1980). Development of adrenergic innervation in rat peripheral vessels: a fluorescence microscopic study. J Anat 131: 121–133. [PMC free article] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- Wangensteen SL, Kiechel SF, Ludewig RM, Madden JJ Jr (1970). The role of vasoconstriction in the suppression of hemorrhage from arteries. I. The completely severed artery. Surgery 67: 338–341. [PubMed] [Google Scholar]
- Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, et al. (2004). ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24: 1810–1815. [DOI] [PubMed] [Google Scholar]
- Wittfeldt A, Emanuelsson H, Brandrup‐Wognsen G, van Giezen JJJ, Jonasson J, Nylander S, et al. (2013). Ticagrelor enhances adenosine‐induced coronary vasodilatory responses in humans. J Am Coll Cardiol 61: 723–727. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, et al. (2007). Prasugrel compared with high loading‐ and maintenance‐dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation‐Thrombolysis in Myocardial Infarction 44 trial. Circulation 116: 2923–2932. [DOI] [PubMed] [Google Scholar]
- Zacharia J, Hillier C, Macdonald A (2004). Pharmacological characterization of alpha1‐adrenoceptors in mouse isolated femoral small arteries. Eur J Pharmacol 503: 155–163. [DOI] [PubMed] [Google Scholar]


