Abstract
Tuberculosis therapy utilizes drugs that while effective cause treatment‐related toxicity. Modulation of antitubercular drugs‐induced toxicity by methionine and vitamin B‐complex in patients was evaluated. 285 treatment‐naïve tuberculosis patients at the Chest Clinics of Infectious Diseases Hospital, Yaba and General Hospital, Lagos in Lagos, Nigeria was prospectively recruited and allotted into test (antitubercular medicines, methionine and vitamin B‐complex) and control groups (antitubercular medicines). Data on adverse drug reactions and blood samples were collected at initiation, 2 months and 6 months, and then analyzed. Red blood cells and packed cell volume were significantly higher (P < 0.05) in the test group compared to control at 6 months of therapy. At the end of 2 months, results showed a significant decrease (P < 0.001) in aspartate aminotransferase, alkaline phosphatase, alanine aminotransferase, urea, creatinine and total bilirubin in the test group compared to control. Reduced glutathione and superoxide dismutase were significantly increased (P < 0.001) and malondialdehyde significantly decreased (P < 0.001) in the test versus control groups at the end of 2 and 6 months. Adverse drug reactions were significantly lower (P < 0.001) in the test group (32.4%) compared to control group (56.2%), with 1 death. Hepatotoxicity was significantly higher (P = 0.026) in control (6.9%), compared to test group (0%). Alcohol and cigarette smoking were significantly (P = 0.019 and P = 0.027) associated with the occurrence of adverse drug reactions. Methionine and vitamin B‐complex modulated hepatic, renal, hematological, antioxidant indices and adverse effects in patients administered antitubercular medicines. Such interventions can enhance compliance and better treatment outcomes in tuberculosis patients.
Keywords: Antioxidants, biomarkers, drug toxicity, methionine, modulation, tuberculosis
Abbreviations
- DILI
drug‐induced liver injury
- INH
isoniazid
- MDA
malondialdehyde
- PZA
pyrazinamide
- RIF
rifampin
- ROS
reactive oxygen species
- RUCAM
Roussel Uclaf Causality assessment method
- SAMe
S‐adenosyl‐L‐methionine
- TB
tuberculosis
Introduction
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, is a single leading cause of death from any single infectious agent and a major global public health problem worldwide (Mohajan 2015). Isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) are first‐line drugs used for tuberculosis therapy in adults for an initial 2 months (intensive phase), then a continuation phase of 4 months involving INH and RIF (Huiri et al. 2017). Despite the effectiveness of this treatment regimen, treatment‐related toxicity manifesting as hepatotoxicity, skin reactions, and gastrointestinal disturbances have been reported (Tostmann et al. 2008).
Reactive Oxygen Species (ROS) are produced in the presence of diseases or drugs, resulting in lipid peroxidation and oxidative stress (Zhai et al. 2008). Thus, a reduction in lipid peroxidation in tissue and increase in superoxide dismutase, catalase and glutathione activities would help to maintain cell integrity and control the increase in markers of oxidative stress (Hamza and Al‐Harbi 2015). Elevated hepatic, renal, hematological and antioxidant indices are important biomarkers in confirming toxicity and extent of organ damage.
The liver is implicated in drug‐induced toxicity as it plays an important role in the metabolism and excretion of many drugs, including antituberculosis drugs. Drug‐induced liver injury (DILI) causes acute and chronic disease, though the link between drugs and preexisting liver disease is complex, more so when symptoms develop in patients with liver disease during treatment (Teschke and Danan 2016). This problem is evident as many physicians attending to chronic liver disease patients are faced with determining if drug therapy is a risk that increases their patient's chances for drug‐induced liver injury (Teschke and Danan 2017). To properly establish cause and effect, it is appropriate to conduct an assessment of causality, using various criteria and methods, such as the Roussel Uclaf Causality Assessment Method (RUCAM) (Teschke and Danan 2016).
Drug‐induced toxicity can also cause clinical adverse effects, which can occur either among antitubercular medicines or between antitubercular medicines and other medicines (Farazi et al. 2014). This may extend or modify treatment, and cause drug resistance leading to treatment failure (Kaona et al. 2004) and death. Factors like age, sex, race, other drugs, breastfeeding and pregnancy (Alomar 2014) can increase the occurrence of adverse drug reactions. The very young and elderly people are more at risk of developing adverse drug reactions (ADRs) than other age groups (Pretorius et al. 2013), while the female gender has a higher risk of developing adverse drug reactions than males (Rademaker 2001).
The severity of antitubercular drug‐induced toxicity has led to the idea to use other drugs, which when co‐administered may prevent or significantly reduce toxicity. Methionine is an antioxidant that protects glutathione, the major antioxidant in human cells that protect against free radicals and toxic compounds. Vitamin B complex contains Vitamin B1 and B6, which have been reported to possess antioxidative properties (Hellmann and Mooney 2010; Alvarado and Navarro 2016). This study evaluated the modulatory effect of co‐administration of methionine and vitamin B‐complex on antitubercular drugs‐induced toxicity in tuberculosis patients.
Materials and Methods
This prospective study was conducted amongst 285 treatment‐naïve tuberculosis patients at the Chest clinics of Mainland Hospital, Yaba and General Hospital, Lagos in Lagos, Nigeria, after ethical approval and informed consent were obtained. Participants were allotted into the test (co‐administered antitubercular medicines with methionine and vitamin B‐complex) and control groups (administered antitubercular medicines only). Test group participants were placed on 4 tablets of methionine daily (250 mg each) via the oral route and 2 tablets of vitamin B‐complex orally daily for 6 months, representing the period of TB treatment. This was in addition to the combined antitubercular medicine regimen (all patients).
Blood samples were collected at initiation of treatment, at 2 months and 6 months, then hematological, biochemical and antioxidant parameters were analyzed according to standard protocols. Data on patients’ demographics and reported adverse drug reactions was also collected prospectively.
Data entry, coding, cleaning and analysis was done using SPSS version 20.0. Descriptive statistics was summarized using frequency, proportions, measures of central tendency and dispersion. Bivariate analysis such as chi‐square test was used to investigate the association between adverse drug reaction and selected variables. Logistic regression was further used to determine the factors that may be significantly associated with adverse drug reactions by the patients. Comparisons between the baseline and follow‐up data were performed, using the Mann Whitney U test. All tests were carried out at 5% level of significance.
Results
Demographic characteristics of research participants
Most of the participants were males (71.28%) versus females (28.72%) in the present study. Participants were aged between 18 to 65 years, with majority (36.84%) aged 31–40 years and 34.39% were aged 18–30. Majority was married (53.19%), singles made up 37.94% of participants, while widows/widowers were the least (3.55%) (Table 1). Study participants with a secondary school certificate were more (50.18%), than those with tertiary education (25.96%).
Table 1.
Demographic characteristics of research subjects
| Characteristics | Group | All patients n (%) | Control group n (%) | Test group n (%) | P‐value |
|---|---|---|---|---|---|
| Sex | Male | 201 (71.28) | 92 (45.77) | 109 (54.23) | 0.137 |
| Female | 81 (28.72) | 45 (55.56) | 36 (44.44) | ||
| Age (years) | 18–30 | 98 (34.39) | 49 (50.00) | 49 (50.00) | 0.008a |
| 31–40 | 105 (36.84) | 39 (37.14) | 66 (62.86) | ||
| 41–50 | 44 (15.44) | 23 (52.27) | 21 (47.73) | ||
| 50+ | 38 (13.33) | 26 (68.42) | 12 (31.58) | ||
| Marital status | Single | 107 (37.94) | 52 (48.6) | 55 (51.40) | 0.582 |
| Married | 150 (53.19) | 71 (47.33) | 79 (52.67) | ||
| Divorced | 15 (5.32) | 7 (46.67) | 8 (53.33) | ||
| Widow/widower | 10 (3.55) | 7 (70.00) | 3 (30.00) | ||
| Highest education | NFE/Primary | 68 (23.86) | 34 (50.00) | 34 (50.00) | 0.810 |
| Secondary | 143 (50.18) | 66 (46.15) | 77 (53.85) | ||
| Tertiary | 74 (25.96) | 37 (50.00) | 37 (50.00) |
Chi‐square test (significant). NFE, no formal education.
Effect of methionine and vitamin B‐complex on hepatic parameters in control and test groups at 2 and 6 months of antituberculosis drug treatment
At the end of 2 months of TB treatment, ALT, AST, ALP and total bilirubin were significantly (P < 0.001) lower in test group participants (Median: 8.4, 22.1, 64.7 and 3.1) compared to participants in the control group (Median: 74.95, 56.25, 85.4 and 8.25) (Table 2).
Table 2.
Effect of methionine and vitamin B‐complex on hepatic parameters in control and test groups at baseline, 2 and 6 months of antituberculosis drug treatment
| Parameter | Median (IQR) level Control (a) | Median (IQR) level Test (b) | P‐value | |
|---|---|---|---|---|
| Baseline | AST (U/L) | 22.80 (IQR: 15.25–32.10) | 35.00 (IQR: 25.25–49.60) | <0.0001a |
| ALT (U/L) | 9.90 (IQR: 5.7–16.83) | 14.80 (IQR: 7.40–27.10) | 0.008a | |
| ALP (U/L) | 65.60 (IQR: 54.85–90.58) | 82.70 (IQR: 68.80–99.55) | 0.002a | |
| T. Bilirubin (mmol/L) | 4.75 (IQR: 3.35–6.65) | 5.90 (IQR: 4.50–8.35) | 0.01a | |
| Month 2 | AST (U/L) | 56.25 (IQR: 47.20–63.95) | 22.1 (IQR: 15.10–32.80) | <0.001a |
| ALT (U/L) | 74.95 (IQR: 65.38–89.03) | 8.4 (IQR: 4.70–12.30) | <0.001a | |
| ALP (U/L) | 85.4 (IQR: 80.35–102.50) | 64.7 (IQR: 51.70–88.60) | <0.001a | |
| T. Bilirubin (mmol/L) | 8.25 (IQR: 5.48–15.08) | 3.1 (IQR: 2.50–4.00) | <0.001a | |
| Month 6 | AST (U/L) | 30.1 (IQR: 19.60–35.50) | 25.45 (IQR: 16.68–32.73) | 0.276 |
| ALT (U/L) | 17.2 (IQR: 5.30–70.10) | 8.45 (IQR: 4.70–30.75) | 0.183 | |
| ALP (U/L) | 71.35 (IQR: 56.28–83.03) | 68.5 (IQR: 58.30–113.00) | 0.370 | |
| T. Bilirubin (mmol/L) | 4.3 (IQR: 3.25–6.03) | 4.2 (IQR: 3.60–5.30) | 0.910 |
Mann Whitney U test (Significant); IQR, Interquartile range.
ALT, AST, ALP, and total bilirubin were not significant (P > 0.05) in the test group, relative to the control group participants at the end of 6 months of tuberculosis therapy (Table 2).
Change in hepatic function parameters from baseline at 2 and 3 months of antituberculosis treatment
A significant difference (P < 0.001) was observed in changes in AST, ALT, ALP and total bilirubin from baseline at 2 months between control and test group participants (Table 3). No difference was observed between these same parameters at 6 months compared to baseline. (Table 3).
Table 3.
Change in hepatic function parameters from baseline at 2 and 6 months of antituberculosis treatment
| Control group Median (IQR) (2 or 6 months value Minus baseline value) | Test group Median (IQR) (2 or 6 months value Minus baseline value) | P‐value | |
|---|---|---|---|
| Month 2 | |||
| AST (U/L) | 33.45 (18.73–42.88) | −12.6 (−30.2 to 4.7) | <0.001a |
| ALT (U/L) | 58.75 (48.68–73.98) | −12.5 (−17.6 to 6.5) | <0.001a |
| ALP (U/L) | 14.1 (−2.8 to 35.88) | −15.1 (−27.2 to 4.1) | <0.001a |
| T_Bil (mmol/L) | 3.2 (0–10.1) | −2.6 (−3.5 to 1.5) | <0.001a |
| Month 6 | |||
| AST (U/L) | 4.75 (−11.93−16.33) | −4.7 (−33.5 to 8.9) | 0.313 |
| ALT (U/L) | −0.5 (−10.6 to 25.4) | 10.8 (−11.6 to 55.3) | 0.531 |
| ALP (U/L) | 8.3 (−30.3–25.43) | −2.2 (−52.6 to 17.9) | 0.454 |
| T_Bil (mmol/L) | 0.15 (−2.78 to 1.3) | −1.1 (−3.7 to 0.5) | 0.918 |
A positive median value of hepatic parameter shows an increase from baseline value, while a negative value indicate a decrease from baseline value.
Mann Whitney U test (Significant); IQR, Interquartile range.
Effect of methionine and vitamin B‐complex on renal parameters between test and control groups at 2 and 6 months of anti‐TB therapy
Creatinine and urea levels (Median: 63.13 and 2.6) were significantly (P < 0.001) lower and albumin (Median: 37.1) significantly (P = 0.096) lower in the test participants, relative to control group participants at the end of 2 months of TB treatment (Table 4). Total protein was not significant (P > 0.05) in the test versus control group.
Table 4.
Effect of methionine and vitamin B‐complex on renal parameters between test and control groups at baseline, 2 and 6 months of anti‐TB therapy
| Parameter | Median (IQR) Control (a) | Median (IQR) Test (b) | P value a versus. b | |
|---|---|---|---|---|
| Baseline | Creatinine (mmol/L) | 65.50 (IQR: 54.58–78.14) | 74.31 (IQR: 63.76–88.38) | 0.003a |
| Urea (mmol/L) | 3.20 (IQR: 2.50–4.10) | 3.60 (IQR: 2.60–5.25) | 0.068 | |
| ALB (g/L) | 32.80 (IQR: 28.90–36.25) | 34.7 (IQR: 30.85–39.73) | 0.150 | |
| T.Protein | 74.60 (IQR: 64.85–79.35) | 77.30 (IQR: 71.58–81.73) | 0.014a | |
| Month 2 | Creatinine (mmol/L) | 94.25 (IQR: 81.53–136.24) | 63.13 (IQR: 50.40–73.13) | <0.001a |
| Urea (mmol/L) | 7.65 (IQR: 5.25–8.95) | 2.6 (IQR: 2.30–3.60) | <0.001a | |
| Alb (g/L) | 35 (IQR: 31.60–38.95) | 37.1 (IQR: 29.00–41.70) | 0.096a | |
| T. Protein (g/L) | 74.30 (IQR: 70.48–79.75) | 74.7 (IQR: 65.80–82.10) | 0.105 | |
| Month 6 | Creatinine (mmol/L) | 64.65 (IQR: 58.91–88.91) | 62.84 (IQR: 58.13–81.37) | 0.734 |
| Urea (mmol/L) | 3.25 (IQR: 2.88–3.95) | 3.2 (IQR: 2.60–4.10) | 0.688 | |
| Alb (g/L) | 34.9 (IQR: 30.08–40.98) | 36.5 (IQR: 33.10–40.60) | 0.423 | |
| T. Protein (g/L) | 74.7 (IQR: 68.85–79.65) | 71.2 (IQR: 69.80–80.10) | 0.721 |
Mann Whitney U test (Significant); IQR, Interquartile range.
There was no difference in levels of creatinine, urea, total protein and albumin in the test versus control group participants at the end of 6 months (Table 4).
Change in renal function parameters from baseline at 2 and 6 months of antituberculosis treatment
A significant difference was observed in changes in creatinine and urea from baseline at 2 and 6 months between control and test group participants (Table 5). No difference was observed between albumin and total protein in both test and control participants at 2 and 6 months compared to baseline (Table 5).
Table 5.
Change in renal function parameters from baseline at 2 and 6 months of antituberculosis treatment
| Control group (2 or 6 months value Minus baseline value) | Test group (2 or 6 months value Minus baseline value) | P value | |
|---|---|---|---|
| Month 2 | |||
| Creatinine (mmol/L) | 26.16 (12.75–74.83) | −22.34 (−33.5 to 2.88) | <0.001a |
| Urea (mmol/L) | 4.4 (2.25–5.45) | −1.5 (−3.4 to 0.8) | <0.001a |
| ALB (g/L) | 1.4 (−5.35 to 5.8) | 1.8 (−10.2 to 10) | 0.650 |
| T. protein (g/L) | 2.45 (−6.38 to 13.03) | 0.2 (−13.3 to 8.6) | 0.536 |
| Month 6 | |||
| Creatinine (mmol/L) | 1.65 (−13.99 to 15.42) | −7.58 (−28.7 to 4.53) | 0.051a |
| Urea (mmol/L) | 0.4 (−0.58 to 0.95) | −2 (−3.1 to 0.2) | 0.01a |
| ALB (g/L) | 0 (−6.15 to 6.28) | −0.5 (−6.3 to 7.2) | 0.875 |
| T. protein (g/L) | −0.35 (−9.2 to 5.78) | −3.2 (−10.3 to 2.6) | 0.986 |
Mann Whitney U test (Significant); IQR, Interquartile range.
A positive median value of hepatic parameter shows an increase from baseline value, while a negative value indicates a decrease from baseline value.
Effect of methionine and vitamin B‐complex on antioxidant indices at 2 and 6 months of antitubercular therapy
At the end of 2 and 6 months, GSH level was significantly (P = 0.005 and P < 0.001 respectively) elevated in the test group participants (median: 45.1 and 64.0) compared to the control (median: 34.7 and 20.4, respectively) (Fig. 1). At the end of 2 and 6 months, SOD level was significantly (P < 0.001) elevated in the test group participants (median: 145.8 and 223.4, respectively) compared to the control (median: 68.5 and 38.2) (Fig. 2). At the end of 2 and 6 months, there was no significant difference in CAT level (P > 0.05) between the test group participants and the control (Fig. 3). A significant (P < 0.001) difference was observed at baseline between control (median: 717.4) and test participants (median: 391.3). At the end of 2 and 6 months, malondialdehyde level was significantly (P < 0.001) lower in the test group participants (median: 2.3 and 1.3, respectively) compared to the control (median: 4.9 and 7.1, respectively) (Fig. 4).
Figure 1.
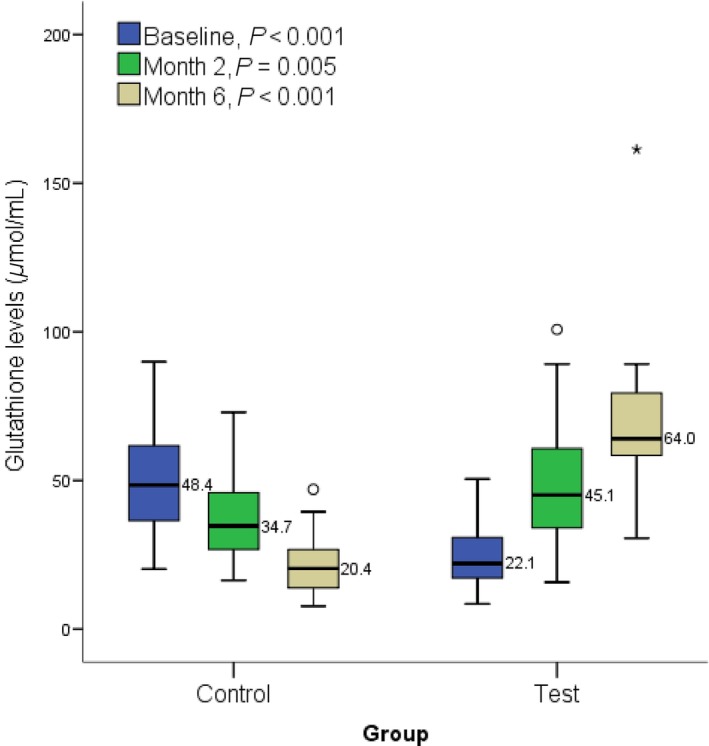
Showing effect of methionine and vitamin B‐complex on reduced glutathione levels at baseline, 2 and 6 months of TB treatment. Small circle “o” indicate Outliers or “out” values, while star “*” represent far out” or “Extreme values”.
Figure 2.
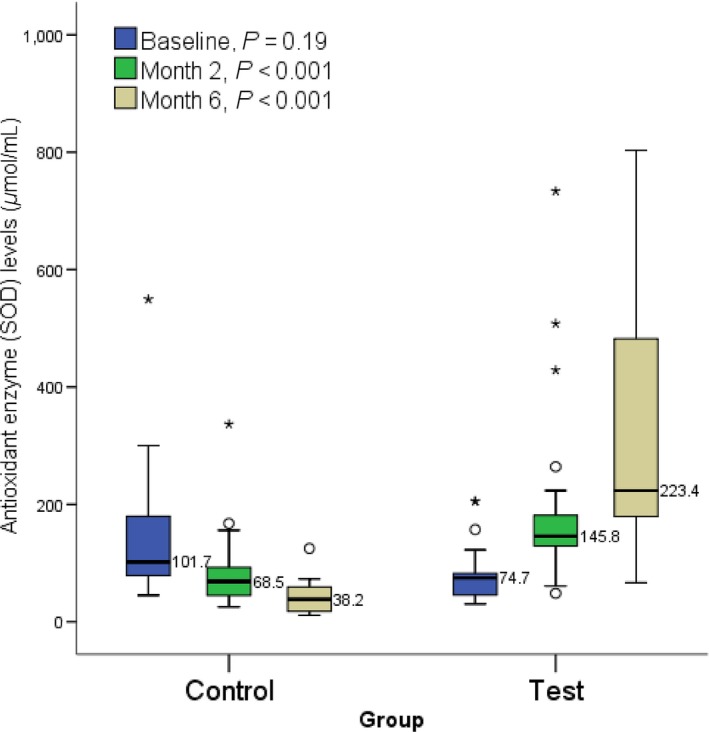
Showing effect of methionine and vitamin B‐complex on superoxide dismutase level at baseline, 2 and 6 months of TB treatment. Small circle “o” indicate Outliers or “out” values, while star “*” represent far out” or “Extreme values”
Figure 3.
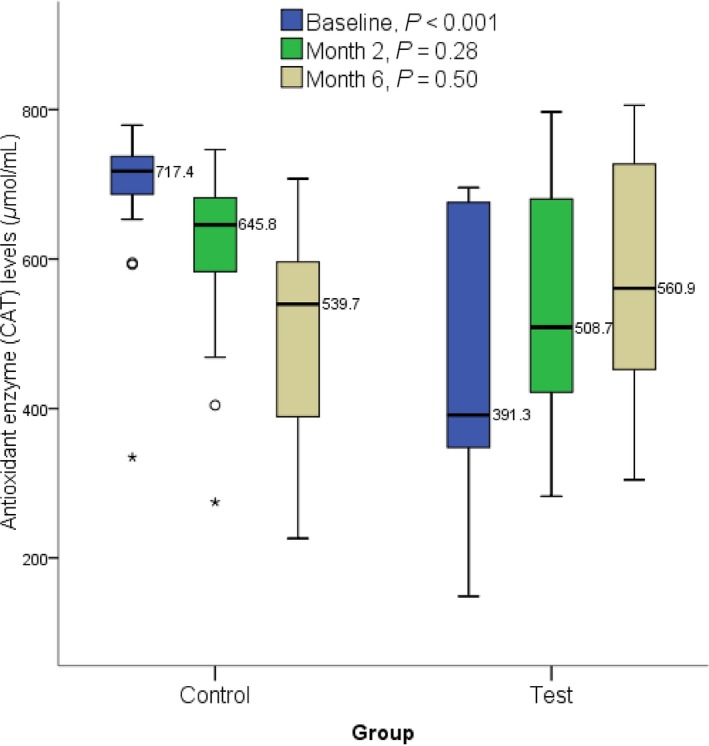
Effect of methionine and vitamin B‐complex on catalase levels at baseline, 2 and 6 months of TB treatment. Small circle “o” indicate Outliers or “out” values, while star “*” represent far out” or “Extreme values”.
Figure 4.
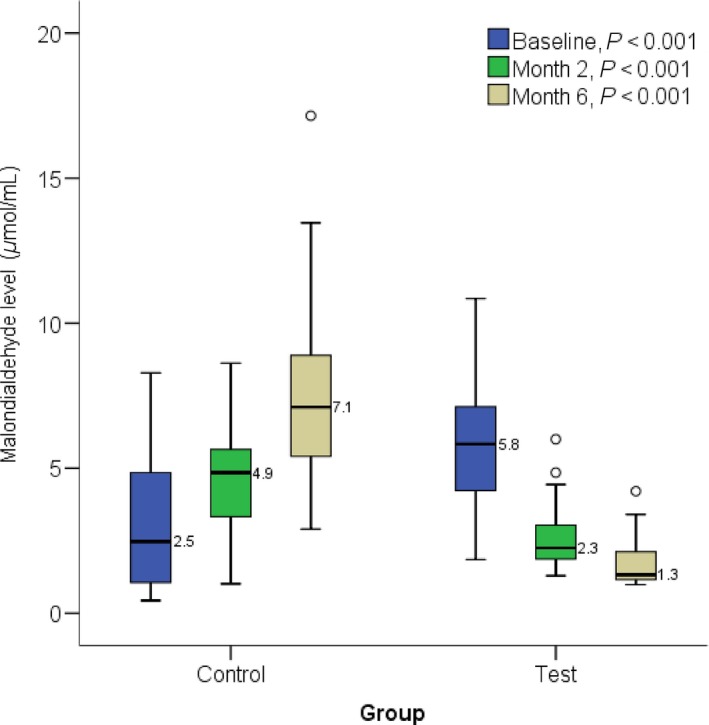
Showing effect of methionine and vitamin B‐complex on malondialdehyde levels in at 2 and 6 months of TB treatment. Small circle “o” indicate Outliers or “out” values, while star “*” represent far out” or “Extreme values”.
Effect of methionine and vitamin B‐complex on hematological indices at 2 and 6 months of antituberculosis treatment
A significant (P < 0.001) difference was observed in ESR between the control and test groups at 2 months of TB treatment (Table 6). WBC count was significantly (P = 0.013) lower in the test group, compared to participants in the control group. Lymphocyte count was significantly (P = 0.009) lower in the test group, compared to the control group participants.
Table 6.
Effect of methionine and vitamin B‐complex on hematological parameters at 2 months of antituberculosis treatment
| Control group a | Test group b | P‐value a versus b | |
|---|---|---|---|
| ESR (mm/h) | 45 (IQR: 35.00–60.00) | 32 (IQR: 26.00–38.00) | <0.001a |
| WBC (x109/L) | 5.2 (IQR: 4.15–6.75) | 4.41 (IQR: 3.30–5.86) | 0.013a |
| RBC (x1012/L) | 4.3 (IQR: 4.02–5.02) | 4.66 (IQR: 4.13–5.12) | 0.070 |
| HGB (g/dL) | 11.6 (IQR: 10.45–13.25) | 12.5 (IQR: 10.90–13.60) | 0.970 |
| PCV (x103 μL) | 36.2 (IQR: 33.30–40.25) | 38 (IQR: 33.80–41.90) | 0.117 |
| PLT (x103/L) | 293 (IQR: 231.50–405.50) | 275 (IQR: 211.00–380.00) | 0.358 |
| NEUT (×109/L) | 2.7 (IQR: 2.05–4.25) | 2.34 (IQR: 1.71–3.70) | 0.103 |
| NEUT (%) | 55.7 (IQR: 44.65–63.85) | 52.5 (IQR: 44.10–61.90) | 0.330 |
| LYMPH (%) | 33.2 (IQR: 24.85–44.45) | 34.6 (IQR: 24.90–45.40) | 0.825 |
| LYMPH (×109/L) | 1.8 (IQR: 1.33–2.30) | 1.5 (IQR: 1.20–2.05) | 0.009a |
| MCV (fL) | 81.4 (IQR: 76.50–89.25) | 81.9 (IQR: 76.40–88.30) | 0.982 |
| MCH (pg) | 26.8 (IQR: 24.50–28.70) | 27.2 (IQR: 24.40–28.70) | 0.993 |
| MCHC (g/dL) | 31.5 (IQR: 30.60–32.80) | 31.9 (IQR: 30.40–34.00) | 0.653 |
Mann Whitney U test (Significant); IQR, Interquartile range.
There was no difference in RBC, HGB, PCV, MCV, MCH, and MCHC were higher in test group participants compared to the control group participants. PLT and neutrophil count were not different in the test versus control group participants (Table 6).
Neutrophil count was significantly (P < 0.001) and PCV significantly (P = 0.037) higher while there was no difference in ESR, HGB, and MCV levels in the test group participants at 6 months of treatment compared to control (Table 7). Lymphocytes (%), RBC and MCHC were significantly (P = 0.008) and significantly (P = 0.045 and P = 0.01) lower in the test group participants at 6 months of treatment compared to control, while WBC, PLT, and MCH were not different in test versus control participants (Table 7).
Table 7.
Effect of methionine and vitamin B‐complex on hematological parameters at 6 months of antituberculosis treatment
| Control | Test | P‐value | |
|---|---|---|---|
| ESR (mm/h) | 23 (IQR: 19.00–29.00) | 32 (IQR: 23.00–41.50) | 0.622 |
| WBC (x109/L) | 4.05 (IQR: 3.55–5.20) | 3.92 (IQR: 2.76–5.30) | 0.259 |
| RBC (x1012/L) | 4.39 (IQR: 4.01–4.83) | 4.34 (IQR: 4.08–4.82) | 0.045a |
| HGB (g/dL) | 12.4 (IQR: 10.83–14.15) | 12.9 (IQR: 11.90–14.60) | 0.798 |
| PCV (x103 μL) | 37.85 (IQR: 35.43–45.40) | 38.4 (IQR: 35.00–42.20) | 0.037a |
| PLT (x103/L) | 265 (IQR: 180.25–329.75) | 228 (IQR: 190.00–304.00) | 0.320 |
| NEUT (×109/L) | 1.64 (IQR: 1.06–2.54) | 2.29 (IQR: 1.69–4.00) | <0.001a |
| NEUT (%) | 46.6 (IQR: 36.90–52.70) | 55.15 (IQR: 47.08–67.23) | 0.032a |
| LYMPH (%) | 43.4 (IQR: 26.20–52.20) | 30.65 (IQR: 23.25–42.40) | 0.008a |
| LYMPH (×109/L) | 1.9 (IQR: 1.36–2.90) | 1.32 (IQR: 0.98–2.00) | 0.624 |
| MCV (fL) | 84.7 (IQR: 78.80–93.60) | 88.2 (IQR: 81.25–91.30) | 0.140 |
| MCH (pg) | 29 (IQR: 26.80–30.80) | 27.9 (IQR: 25.75–29.80) | 0.698 |
| MCHC (g/dL) | 33.2 (IQR: 31.50–34.60) | 31.95 (IQR: 31.00–33.6) | 0.012a |
Mann Whitney U test (significant); IQR, Interquartile range.
Prevalence of adverse drug reactions in the different treatment groups
A significant (P < 0.001) difference was observed in the treatment groups, with fewer participants developing ADRs in the test group (32.40%) compared to the control (56.20%) (Table 8). Conversely, more test group participants (67.60%) did not develop ADRs than control group participants (43.80%).
Table 8.
Prevalence of adverse drug reactions in the treatment groups
| ADR reported | All patients | Control group A | Test group b | P‐value a versus b | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| No | 160 | 56.10 | 60 | 43.80 | 100 | 67.60 | <0.001a |
| Yes | 125 | 43.90 | 77 | 56.20 | 48 | 32.40 | |
Chi‐square test (significant).
Prevalence of different adverse drug effects between test and control groups
Comparing the test versus control, number of participants who developed generalized weakness (14. 9% vs. 35%) and tiredness (3.4% vs. 17.5%) were significantly (P < 0.001) lower; rash (3.4% vs. 13.9%) and headache (2.7% vs. 12.4%) were significantly (P = 0.001 and P = 0.002 respectively) lower, while loss of appetite (2.7% vs. 7.3%) was significantly (P = 0.099) lower in test participants compared to control (Table 9).
Table 9.
Prevalence of different adverse drug effects between test and control groups
| Type of ADR | Number (%) of patients | P value a versus b | |
|---|---|---|---|
| Control (a) | Test (b) | ||
| Weakness | 48 (35.00) | 22 (14.90) | <0.001a |
| Tiredness | 24 (17.50) | 5 (3.40) | <0.001a |
| Nausea and Vomiting | 42 (30.70) | 35 (23.60) | 0.183 |
| Dizziness | 29 (21.20) | 21 (14.20) | 0.122 |
| Rash | 19 (13.90) | 5 (3.40) | 0.001a |
| Abdominal pain | 17 (12.40) | 9 (6.10) | 0.064 |
| Headache | 17 (12.40) | 4 (2.70) | 0.002a |
| Fever | 8 (5.80) | 4 (2.70) | 0.242 |
| Loss of appetite | 10 (7.30) | 4 (2.70) | 0.099a |
| Dark urine | 19 (13.90) | 14 (9.50) | 0.245 |
| Death | 1 (0.70) | 0 (0.00) | 0.298 |
Chi‐square test (significant).
3.4% and 6.1% test group participants developed rashes and abdominal pain, respectively, compared to a higher percentage (13.9% and 12.4%) of control group subjects (Table 7). Headache, fever, and loss of appetite occurred in 2.7%, 2.7%, and 2.7% of test subjects, respectively, compared to 12.4%, 5.8%, and 7.3% in the control group subjects.
13.9% of subjects in the control group reported dark urine as opposed to 9.5% of test group subjects. Death occurred in 1 participant in the control group (Table 9).
Association of risk factors with hepatotoxicity at 2 months of antituberculosis therapy
A significant (P = 0.026) difference was observed between the control group participants (6.9%) that developed hepatotoxicity and test group participants (0%) (Table 10). A higher percentage of control (93.1%) and test (100%) did not develop hepatotoxicity. Out of the 4 participants who developed hepatotoxicity, 3 were aged 18–30 and 1 was between 31 and 40; 3 were males and 1 was a female; 1 was a smoker and 3 nonsmokers; 1 used hard drugs while 3 did not (Table 8). These risk factors (age, sex, alcohol, cigarette smoking and substance use) were not significantly associated with the development of ADRs in both control and test group participants (Table 10).
Table 10.
Association of risk factors with hepatotoxicity at 2 months of antituberculosis therapy
| Factors | Subgroup | Number (%) of patients | P‐value | |
|---|---|---|---|---|
| No hepatotoxicity | Hepatotoxicity | |||
| Treatment Group | Control | 54 (93.10) | 4 (6.90) | 0.026a |
| Test | 48 (100.00) | 0 (0.00) | ||
| Age (years) | 18–30 | 28 (90.32) | 3 (9.68) | 0.210 |
| 31–40 | 39 (97.50) | 1 (2.50) | ||
| 41–50 | 19 (100.00) | 0 (0.00) | ||
| 51–65 | 16 (100.00) | 0 (0.00) | ||
| Sex | Male | 78 (96.30) | 3 (3.70) | 0.917 |
| Female | 23 (95.83) | 1 (4.17) | ||
| Alcohol use | Yes | 43 (97.73) | 1 (2.27) | 0.717 |
| No | 55 (96.49) | 2 (3.51) | ||
| Smokes cigarette | Yes | 19 (95.00) | 1 (5.00) | 0.773 |
| No | 80 (96.39) | 3 (3.61) | ||
| Reported substance use | Yes | 8 (88.89) | 1 (11.11) | 0.245 |
| No | 90 (96.77) | 3 (3.23) | ||
Chi‐square test (Significant).
Association between some risk factors and other adverse drug reactions
There was no difference observed between males than females (46.77% vs. 38.27%) who developed ADRs, and between participants in the different age groups who developed ADRs, compared to those who did not. No statistical difference was observed between overweight and obese participants (58.82% and 66.67%, respectively) developed ADRs compared to those who did not (41.18% and 33.33%) (Table 11). There was no statistical difference in weight and ADRs observed in participants who were under weight and normal weight in the test group (36.59% and 42.37%) compared to control (63.41% and 57.63%) (Table 11).
Table 11.
Association between some risk factors and other adverse drug reactions
| Risk factor | Subgroup | No ADR {n(%)} | ADR {n(%)} | P‐value |
|---|---|---|---|---|
| Sex | Male | 107 (53.23) | 94 (46.77) | 0.194 |
| Female | 50 (61.73) | 31 (38.27) | ||
| Age (years) | 18–30 | 56 (57.14) | 42 (42.86) | 0.901 |
| 31–40 | 56 (53.33) | 49 (46.67) | ||
| 41–50 | 26 (59.09) | 18 (40.91) | ||
| 50+ | 22 (57.89) | 16 (42.11) | ||
| BMI (kg/m2) | <18.5 (under weight) | 52 (63.41) | 30 (36.59) | 0.293 |
| 18.5–24.99 (normal) | 68 (57.63) | 50 (42.37) | ||
| 25–29.9 (over weight) | 7 (41.18) | 10 (58.82) | ||
| Alcohol intake | Yes | 54 (47.37) | 60 (52.63) | 0.019a |
| No | 98 (61.64) | 61 (38.36) | ||
| Smokes cigarette | Yes | 22 (42.31) | 30 (57.69) | 0.027a |
| No | 132 (59.19) | 91 (40.81) | ||
| Reported substance use | Yes | 10 (41.67) | 14 (58.33) | 0.145 |
| No | 140 (57.14) | 105 (42.86) |
Chi‐square test (Significant); ADR, adverse drug reaction, BMI, body mass index.
A significant (P = 0.019) difference was observed amongst participants who consumed alcohol, with more of them (52.63%) developing ADRs compared to those that did not take alcohol (47.37%) (Table 11). Fewer people 38.36% that did not consume alcohol developed ADRs than those who did not develop any ADR. A significant (P = 0.027) difference was observed amongst participants who consumed smoked, with more of them (57.63%) developing ADRs compared to those that did not smoke (42.31%) (Table 11).
More participants who reported using hard drugs (58.33%) developed ADRs compared to those that did not use any hard drug (41.67%). 42. 86% of participants that did not use any hard drug developed ADRs, compared to those that did not develop any ADR (57.14%) (Table 11).
Logistic regression for factors influencing development of adverse drug reactions
The factors identified to be significantly associated with Adverse Drug Reaction in bivariate analysis (Table 11) were harvested and subjected to multivariate analysis. The dependent variable in Table 12 above is Adverse Drug Reaction status, a Yes‐or‐No outcome. Patients who take alcohol were about two times more likely (OR = 1.501, P = 0.173, 95% CI: 1.02, 3.3) to develop Adverse Drug Reaction than those who do not take alcohol. Also, patients who smoke have about forty percent increase in risk of developing Adverse Drug Reactions (OR = 1.371, P = 0.402, 95% CI: 0.656, 2.864) compared to those who do not smoke. The model was a good fit as Hosmer–Lemeshow goodness of fit was not significant (χ2 = 1.220, P = 0.543) (Table 12).
Table 12.
Logistic regression for factors influencing development of adverse drug reactions
| Variables | Odds ratio | SE | Wald statistic | P‐value | 95% CI |
|---|---|---|---|---|---|
| Alcohol intake | |||||
| Yes | 1.501 | 0.298 | 1.856 | 0.173 | (0.837, 2.692) |
| Noa | |||||
| Smoking status | |||||
| Yes | 1.371 | 0.376 | 0.704 | 0.402 | (0.656, 2.864) |
| Noa | |||||
Reference category.
Association between some risk factors and ADR by treatment group
More control group participants who consumed alcohol (75. 47%) and smoked cigarettes (78.57%) developed ADRs in a significant (P = 0.000) and significant (P = 0.010) manner compared to those who did not smoke (51.46%) or consume alcohol (44.16%). Participants in the control group who used hard drugs (73.33%) developed ADRs in an insignificant (P > 0.05) manner compared to those who did not (Table 13).
Table 13.
Association between some risk factors and ADR by treatment group
| Characteristic | Control | Test | |||||
|---|---|---|---|---|---|---|---|
| No ADR n(%) | ADR n(%) | P value | No ADR n(%) | ADR n(%) | P value | ||
| Alcohol intake | Yes | 13 (24.53) | 40 (75.47) | 0.000a | 41 (67.21) | 20 (32.79) | 0.945 |
| No | 43 (55.84) | 34 (44.16) | 54 (66.67) | 27 (33.33) | |||
| Smokes cigarette | Yes | 6 (21.43) | 22 (78.57) | 0.010a | 16 (66.67) | 8 (33.33) | 0.893 |
| No | 50 (48.54) | 53 (51.46) | 81 (68.07) | 38 (31.93) | |||
| Reported substance use | Yes | 4 (26.67) | 11 (73.33) | 0.195 | 6 (66.67) | 3 (33.33) | 0.937 |
| No | 50 (44.25) | 63 (55.75) | 89 (67.94) | 42 (32.06) | |||
Chi‐square test (Significant).
More test group participants who consumed alcohol (67.21%), smoked cigarettes (66.67%) and used hard drugs (66.67%) did not develop ADRs in an insignificant (P > 0.05) manner compared to those who developed ADRs (32.79%, 33.33% and 33.33%, respectively)(Table 13).
Discussion
Hepatic transaminases, renal, hematological and antioxidant indices are mostly affected by deleterious antitubercular drug‐induced toxicity in patients undergoing treatment. An elevation in AST and the liver‐specific ALT would, therefore, indicate leakage from injured tissues (Ozer et al. 2008), while an increase in ALP level occurs due to overproduction and release in blood (Ramaiah 2007). Supplementation with agents capable of modulating the harmful effects of these antitubercular medicines would be of immense benefit to patients undergoing treatment for tuberculosis. Achieving this would reduce often severe adverse effects, enhance compliance and ultimately improve treatment outcomes. Compared to participants on only the antitubercular medicines, administration of methionine and vitamin B‐complex (in the presence of antitubercular medicines) showed a significant (P < 0.001) decrease in total bilirubin and the liver enzymes‐ALT, AST, and ALP at the end of the intensive phase of treatment. This decrease can be attributed to the reported antioxidant activity of methionine and B‐complex, while the higher levels in the control group support the knowledge that antitubercular medicines, particularly rifampicin, induce hepatocellular injury and hyperbilirubinemia (Singh et al. 2016).
Tuberculosis and the liver are related in many ways, one of which is the direct hepatic involvement by the disease itself that can impair hepatic functions (Essop et al. 1984) and elevate indices like ALT, AST, and ALP. It is thus possible to infer that as the bacterial load reduced in the course of treatment (from the 2nd to the 6th month of treatment), the toxic effect of the infection reduced, allowing the hepatic and renal cells to recover and reduce elevated parameters. This is important, considering that the liver is a regenerative organ. According to the standard protocol of tuberculosis treatment in Nigeria, pyrazinamide and rifampicin (a known nephrotoxic (Manika et al. 2013) and hepatotoxic (Awodele et al. 2011)) agent, are not included in the continuation phase of treatment, as opposed to their use in the intensive phase (along with isoniazid and ethambutol). Thus, the assault on the liver is thus less, therefore improving the ability of the hepatic cells to regenerate. These reasons are likely responsible for recovery of many of the liver and renal function parameters with time in the control group.
Previous studies indicate a strong association between renal injury and oxidative stress in patients treated with antituberculosis drugs (Kwon et al. 2004; Schubert et al. 2010). This causes an elevation in creatinine, urea (Yanardag et al. 2005) and albumin and a decrease in total protein levels (Shabana et al. 2012). This correlates to results from this study when baseline values are compared to values at the end of 2 months of therapy in the treatment groups. Comparing the treatment groups at the end of 2 months showed a significant (P < 0.001) decrease in urea and creatinine and a significant (P = 0.096) increase in albumin in participants co‐administered methionine and vitamin B‐complex with the combined antitubercular medicines, indicating an amelioration of the deleterious effects of antitubercular agents. These parameters were similarly affected up to the end of 6 months of therapy but in an insignificant (P > 0.05) manner. Albumin has been reported to possess antioxidant properties (Roche et al. 2008), which makes it possible to postulate a potentiation of the antioxidant potential of albumin and methionine/vitamin B‐complex for this effect. In this present study, total protein was higher in the control group at the end of 6 months compared to test group participants, possibly due to higher globulin fraction relative to albumin fraction. This corresponds to the result of Shingdang et al. (2016), which showed higher globulin levels compared to albumin at the end of the continuation phase of TB treatment. Levels of globulin were, however, not evaluated in this present study.
Hematological changes associated with tuberculosis treatments have been reported in many parts of the world (Kassa et al. 2016). Results from this current study showed lower levels of RBC, HGB, PCV, and PLT in control group subjects at the end of the intensive phase of treatment, relative to test subjects. This suggests antitubercular drug‐induced deleterious effects on these indices, for example, isoniazid, which has been reported to cause a decrease in HGB synthesis (Ghosh et al. 2017). Methionine and vitamin B‐complex are therefore able to inhibit this effect by promoting HGB synthesis, hence its increase in test group subjects. PCV was observed in the present study to be higher in patients on combined antitubercular medicines, methionine and vitamin B‐complex, relative to those on antitubercular medicines alone. This is similar to the results of Bharti et al. (2017), who reported that propolis, an antioxidant, elevated both HGB and PCV following exposure to combined antitubercular medicines. It is reasonable to, therefore, state that methionine, which has been reported to have free radical scavenging activity, would produce a similar effect.
A previous study had reported that INH‐induced oxidative stress in red blood cells (RBCs) (Yilmaz et al. 2008), and also inhibits hem biosynthesis (Huang and Benz 2001). This corresponds to results from this present study, where the RBC counts were lower in participants on antitubercular medicines compared to those on methionine and vitamin B‐complex (in the presence of antiTB medicines). This modulatory role can be explained by the link between phosphatidylcholine, methionine and vitamin B12. Phosphatidylcholine, a major component of red blood cell membranes (Cooper and Hausman 2015), is synthesized from the metabolite of methionine, S‐Adenosyl‐L‐Methionine (SAMe). Vitamin B12 is used by methionine synthase to convert homocysteine into methionine (Seetharam and Li 2000). This is further converted to S‐Adenosyl‐L‐Methionine, which in turn synthesizes phosphatidyl choline. Administering methionine and vitamin B‐complex to TB patients on anti‐TB medicines would, therefore, help stabilize red blood cells leading to higher counts.
Amilo et al. (2013) stated that white blood cells (WBC) count tend to be higher among TB patients, compared to normal health patients, with neutrophils being implicated as the main contributor to the increase. During TB treatment, the bacterial load is expected to decrease as the antitubercular medicines clear the Mycobacterium tuberculosis. The white blood cells that would be required to fight the infection would therefore decrease. This observation was made in the present research. The WBC count was lower in patients taking methionine and vitamin B‐complex (in the presence of anti‐TB medicines), relative to patients administered the anti‐TB medicines alone. Tuberculosis may cause neutropenia (Koju et al. 2005), which corresponds to results of the present study. Exposure to methionine and vitamin B‐complex further decreased neutrophil, further confirming their modulatory role in antitubercular medicine induced toxicity.
Platelets, involved in the inflammatory and immunological response, have the capacity to release cytokines and chemokines, thus acting as immune regulators (Trzeciak‐Ryczek et al. 2013). The direct relationship between platelet and WBC is logical because when there is an immune response due to TB infection, platelets tend to increase. It is thus reasonable to state that treatment, which would see a reduction in the TB infective state, would result in a decrease in WBC and platelet counts.
Elevated ESR, an indicator of disease severity, was observed in our study at baseline. This correlates with other studies that also reported elevated ESR in tuberculosis patients (Hungund et al. 2012). At 2 months of commencing treatment, the modulatory effect of methionine and vitamin B‐complex reduced ESR levels in a significant (P < 0.001) manner, compared to patients on only the antitubercular medicines.
Oxidative stress caused by antitubercular medicines leads to the depletion of glutathione, the major endogenous antioxidant (Stine and Chalasani 2015), while isoniazid depletes vitamin B6 stores (Burda et al. 2007). As a result, methionine, a precursor of glutathione and vitamin B6 need to be supplemented from external sources. Vitamin B‐complex tablets serve as a source of vitamin B6 and vitamin B12 that is vital in the Methionine‐Homocysteine‐Folate‐B12 Cycle (Seetharam and Li 2000).
Antitubercular medicines like isoniazid produce toxic metabolites, acetyl isoniazid, and hydrazine, which inactivate catalase and superoxide dismutase to induce oxidative stress (Zhai et al. 2008). Following an injury to the liver caused by antitubercular medicines, endogenous antioxidant enzymes (CAT, SOD), and the nonenzymatic antioxidant glutathione (GSH) decrease while malondialdehyde (MDA), a marker of lipid peroxidation, increases (Liu et al. 2017); corresponding to results from the current research. However, in the test subjects, methionine and vitamin B‐complex co‐administered with the combination antitubercular medicines corrected these abnormalities compared to patients on only the antitubercular medicines. This modulation of antioxidant activity was obvious by a significant (P < 0.001) increase in GSH and SOD and a significant (P < 0.001) decrease in lipid peroxidation, similar to that reported by Anisimova et al. (2013). This observation was at the end of the intensive and continuation phases of treatment. The antioxidant activity of S‐Adenosyl‐L‐Methionine (SAMe) (a metabolite of methionine) is mainly due to its role as a precursor of GSH, the major endogenous antioxidant (Niedzwiecki et al. 2013). SAMe was found to be able to prevent and reverse hepatotoxicity associated with several drugs and increase GSH levels (Anstee and Day 2012).
Toxicity due to antitubercular medicines can manifest clinically as adverse effects (Blumberg et al. 2003). A significant (P < 0.001) decrease in the prevalence of ADRs in test group participants (56.2%) was observed in the present research, compared to control (32.4%). Rash was reported in 3.4% of patients co‐administered antitubercular medicines with methionine and vitamin B‐complex, compared to 13.9% on only antitubercular medicines. INH, RIF, PZA, and EMB have been reported to cause this adverse effect in tuberculosis patients (Kaswala 2013).
Males tend to engage more in smoking, use of alcohol consumption than females; all these are risk factors that increase their susceptibility to tuberculosis (Jeong et al. 2015). This could also be responsible for the observation in the present research where more males (46.77%) developed adverse drug reactions (ADRs) than females (38.27%). A significant interaction occurred between smoking (P = 0.027), alcohol use (P = 0.019) and adverse drug reactions in the present study, with smokers having about forty‐percent increase in risk of developing adverse drug reactions, compared to those who do not smoke, while patients who take alcohol regularly were about two times more likely to develop ADRs than those who do not take alcohol. This link between smoking, alcohol and adverse drug effects was similarly reported by (Chung‐Delgado et al. 2011).
In this present research, hepatotoxicity was observed in 6.9% of control group participants on only the antitubercular medicines, lower than 18.2% reported by Isa et al. (2016). Hepatotoxicity is caused by isoniazid and rifampicin in patients (Devarbhavi et al. 2010). One death was reported in the present study due to complications of tuberculosis and possibly, drug‐induced hepatotoxicity as a secondary cause.
Conclusion
Toxicity to hepatic, renal, and hematological indices and the antioxidant system, as well as adverse effects observed in patients exposed to antitubercular medicines during the 6‐month period of treatment was modulated by the combination of methionine and vitamin B‐complex tablets. This clearly indicates that such interventions could form part of new treatment strategies aimed at reducing adverse effects due to the antitubercular medicines and improve treatment outcomes in tuberculosis patients.
Disclosure
The authors declare that no conflict of interest exists in the course of conducting and funding this research. All authors had final decision regarding the manuscript and the decision to submit the findings for publication.
Acknowledgements
The authors acknowledge the support of Lagos State Health Services Commission, Mainland Hospital, Yaba and General Hospital, Lagos, Nigeria, for enabling access to patients, hospital records, personnel and office/workspace. Special thanks to Pharm. Isaac Abah of Jos University Teaching Hospital, Jos, Nigeria and Mr. O.J Akinsola of Department of Community Health and Primary care, University of Lagos, Nigeria, both of whom performed the statistical analysis. The immense contribution of our research assistants‐ Elizabeth Kuponiyi Oluwafunmi and Sobande Tolulope Oluwatosin is also acknowledged.
Amagon K. I., Awodele O., Akindele A. J.. Methionine and vitamin B‐complex ameliorate antitubercular drugs‐induced toxicity in exposed patients. Pharma Res Per, 5(5), 2017, e00360, https://doi.org/10.1002/prp2.360
References
- Alomar MJ (2014). Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J 22: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado AM, Navarro SA (2016). Complex B vitamins: physiology and therapeutic effect on pain. Am J Pharm Sci 4: 20–27. [Google Scholar]
- Amilo GI, Meludu SC, Ele PU, Ezechukwu C, Onyenekwe C, Chukwu MI (2013). Haematologic indices in pulmonary tuberculosis with or without HV Co‐infection in South Eastern Nigeria. Adv Life Sci Tech 11: 1–7. [Google Scholar]
- Anisimova SI, Donchenko GV, Parkhomenko YM, Kovalenko VM (2013). Mechanism of hepatoprotective action of methionine and composition “Metovitan” against a background of antituberculosis drugs administration to rats. Ukr Biochem J 85: 59–67. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Day CP (2012). S‐adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. J Hepatol 57: 1097–1109. [DOI] [PubMed] [Google Scholar]
- Awodele O, Agbaje EO, Adesina EA, Akintonwa A (2011). Hepatoprotective role of neutrosecR on hepatic damage induced by combination of zidovudine and combined anti‐tuberculous agents in rats. Tokai J Exp Clin Med 36: 31–36. [PubMed] [Google Scholar]
- Bharti U, Kumar NR, Kaur J (2017). Protective effect of bee propolis against anti‐tuberculosis drugs (rifampicin and isoniazid)‐induced haematological toxicity in Sprague Dawley rats. Asian J Pharm Clin Res 10: 188–190. [Google Scholar]
- Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. (2003). Treatment of tuberculosis. Am J Respir Crit Care Med 167: 603–662. [DOI] [PubMed] [Google Scholar]
- Burda AM, Sigg T, Haque D, Bardsley CH (2007). Inadequate pyridoxine stock and its effect on patient outcome. Am J Ther 14: 262–264. [DOI] [PubMed] [Google Scholar]
- Chung‐Delgado K, Revilla‐Montag A, Guillen‐Bravo S, Velez‐Segovia E, Soria‐Montoya A, Nuñez‐Garbin A, et al. (2011). Factors associated with anti‐tuberculosis medication adverse effects: a case‐control study in lima, Peru. PLoS ONE 6: e27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Hausman RE (2015). The Cell: a Molecular Approach. 7th ed. Sinauer Associates, Sunderland (MA). [Google Scholar]
- Devarbhavi H, Dierkhising R, Kremers WK (2010). Antituberculosis therapy drug‐induced liver injury and acute liver failure. Hepatol 52: 798–799. [DOI] [PubMed] [Google Scholar]
- Essop AR, Posen JA, Hodkinson JH, Segal I (1984). Tuberculosis hepatitis: a clinical review of 96 cases. QJ Med 53: 465–477. [PubMed] [Google Scholar]
- Farazi A, Sofian M, Jabbariasl M, Keshavarz S (2014). Adverse reactions to antituberculosis drugs in Iranian tuberculosis patients. Tuberc Res Treat 2014: 412893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Indra N, Jagadeesan G (2017). The ameliorating effect of Centella asiatica ethanolic extract on albino rats treated with isoniazid. J Basic Clin Physiol Pharmacol 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Hamza RZ, Al‐Harbi MS (2015). Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver with silymarin and Nigella sativa extract supplements. Asian Pac J Trop Biomed 5: 521–531. [Google Scholar]
- Hellmann H, Mooney S (2010). Vitamin B6: a molecule for human health? Mol 15: 442–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Benz EJ (2001). Posttranscriptional factors influencing the hemoglobin content of the red cell Pp. 146–173 in Steinberg M. H., Higgs D. and Nagel R. L., (eds). Disorders of hemoglobin: genetics, pathophysiology, and clinical management. Cambridge University Press Cambridge, United Kingdom. [Google Scholar]
- Huiri A, Xueqiong W, Zhongyuan W, Jing X, Shaohua Z, Kun W (2017). The clinical characteristics of anti‐tuberculosis drug induced liver injury in 2457 hospitalized patients with tuberculosis in China. Afr J Pharm Pharmacol 7: 710–714. [Google Scholar]
- Hungund BR, Sangolli SS, Bannur HB (2012). Blood and bone marrow findings in tuberculosis in adults‐A cross sectional study. AlAmeen J Med Sci 5: 362–366. [Google Scholar]
- Isa SE, Ebonyi AO, Shehu NY, Idoko P, Anejo‐Okopi JA, Simji G, et al. (2016). Antituberculosis drugs and hepatotoxicity among hospitalized patients in Jos, Nigeria. Int J Mycobacteriol 5: 21–26. [DOI] [PubMed] [Google Scholar]
- Jeong I, Park JS, Co YJ, Yoon H, Song J, Lee CT, et al. (2015). Drug‐induced hepatotoxicity of anti‐tuberculosis drugs and their serum levels. J Korean Med Sci 30: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaona FA, Tuba M, Siziya S, Sikaona L (2004). An assessment of factors contributing to treatment adherence and knowledge of TB transmission among patients on TB treatment. BMC Public Health 4: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa E, Enawgaw B, Gelaw A, Gelaw B (2016). Effect of anti‐tuberculosis drugs on haematological profiles of tuberculosis patients attending at University of Gondar Hospital Northwest Ethiopia. BMC Haematol 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaswala DH (2013). Drug rash with eosinophilia and systemic symptoms syndrome due to anti‐TB medication. J Family Med Prim Care 2: 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koju D, Rao BS, Shrestha B, Shakya R, Makaju R (2005). Occurrence of side effects from anti‐tuberculosis drugs in urban Nepalese population under DOTS treatment. Kathmandu University J Sci Eng Technol 1: 1–8. [Google Scholar]
- Kwon SH, Kim JH, Yang JO, Lee EY, Hong SY (2004). Ethambutol‐induced acute renal failure. Nephrol Dial Transplant 14: 1335–1336.Erratum in: Nephrology Dialysis Transplantation 2004;19:2160. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao M, Mi J, Chen H, Sheng L, Li Y (2017). Protective effect of bicyclol on anti‐tuberculosis drug induced liver injury in rats. Mol 22: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manika K, Tasiopoulou K, Vlogiaris L, Lada M, Papaemmanouil S, Zarogoulidis K, et al. (2013). Rifampicin‐associated acute renal failure and hemolysis: a rather uncommon but severe complication. Ren Fail 35: 1179–1181. [DOI] [PubMed] [Google Scholar]
- Mohajan HK (2015). Tuberculosis is a fatal disease among some developing countries of the world. Am J Infect Dis Microbiol 3: 18–31. [Google Scholar]
- Niedzwiecki MM, Hall MN, Liu X, Oka J, Harper KN, Slavkovich V, et al. (2013). Blood glutathione redox status and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Epigenetics 8: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S (2008). The current state of serum biomarkers of hepatotoxicity. Toxicol 245: 194–205. [DOI] [PubMed] [Google Scholar]
- Pretorius RW, Gataric G, Swedlund SK, Miller JR (2013). Reducing the risk of adverse drug events in older adults. Am Fam Phys 87: 331–336. [PubMed] [Google Scholar]
- Rademaker M (2001). Do women have more adverse drug reactions? Am J Clin Dermatol 2: 349–351. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK (2007). A toxicologist's guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45: 1551–1557. [DOI] [PubMed] [Google Scholar]
- Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008). The antioxidant properties of serum albumin. FEBS Lett 582: 1783–1787. [DOI] [PubMed] [Google Scholar]
- Schubert C, Bates WD, Moosa MR (2010). Acute tubulointerstitial nephritis related to antituberculous drug therapy. Clin Nephrol 14: 413–419. [DOI] [PubMed] [Google Scholar]
- Seetharam B, Li N (2000). Transcobalamin II and its cell surface receptor. Vitam Horm 59: 337–366. [DOI] [PubMed] [Google Scholar]
- Shabana MB, Ibrahim HM, Khadre SEM, Elemam MG (2012). Influence of rifampicin and tetracycline administration on some biochemical and histological parameters in albino rats. J Basic Appl Zool 65: 299–308. [Google Scholar]
- Shingdang J, Bot Y, Ojo O, Edeh O, Essien C, Bwende E, et al. (2016). Serum Albumin/Globulin ratio in Tuberculosis and HIV Patients any Relationship? Mycobact Dis 6: 199. [Google Scholar]
- Singh D, William C, Cho WC, Upadhyay G (2016). Drug‐Induced liver toxicity and prevention by herbal antioxidants: an overview. Front Physiol 6: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine JG, Chalasani N (2015). Chronic liver injury induced by drugs: a systematic review. Liver Int 35: 2343–2353. [DOI] [PubMed] [Google Scholar]
- Teschke R, Danan G (2016). Review article: diagnosis and management of drug‐induced liver injury (DILI) in patients with pre‐existing liver disease. Drug Saf 39: 729–744. [DOI] [PubMed] [Google Scholar]
- Teschke R, Danan G (2017). Drug‐induced liver injury: is chronic liver disease a risk factor and a clinical issue? Expert Opin Drug Metab Toxicol 13: 4. [DOI] [PubMed] [Google Scholar]
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R (2008). Antituberculosis drug‐induced hepatotoxicity: concise up‐to‐date review. J Gastroenterol Hepatol 23: 192–202. [DOI] [PubMed] [Google Scholar]
- Trzeciak‐Ryczek A, Tokarz‐Deptuła B, Deptuła W (2013). Platelets‐an important element of the immune system. Pol J Vet Sci 16: 407–413. [DOI] [PubMed] [Google Scholar]
- Yanardag H, Caner M, Gunes Y, Uygun S (2005). Acute hemolysis and oligoanuric acute renal failure caused by interrupted. Internet J Nephrol 2: 1–3. [Google Scholar]
- Yilmaz HR, Uz E, Gökalp O, Ozçelik N, Ciçek E, Ozer MK (2008). Protective role of caffeic acid phenethyl ester and erdosteine on activities of purine‐catabolizing enzymes and level of nitric oxide in red blood cells of isoniazid‐administered rats. Toxicol Ind Health 24: 519–524. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Lu SR, Lin Y, Yang QL, Yu B (2008). Oxidative stress potentiated by diallylsulfide, a selective CYP2E1 inhibitor, in isoniazid toxic effect on rat primary hepatocytes. Toxicol Lett 183: 95–98. [DOI] [PubMed] [Google Scholar]


