The molecular mechanisms of preeclampsia are unclear, making it difficult to predict, prevent, or manage the pregnancy-associated disorder. This study showed that placental ischemia, possibly through the release of TNF-α, causes increases in the levels of matrix metalloproteinase (MMP)-1 and MMP-7, which could alter collagen deposition and cause inadequate uteroplacental and vascular remodeling in hypertension in pregnancy. The data suggest that targeting MMP-1 and MMP-7 and their upstream modulators, such as TNF-α, could provide a new approach in the management of hypertension in pregnancy and preeclampsia.
Keywords: aorta, placenta, preeclampsia, remodeling, uterus, tumor necrosis factor-α
Abstract
Preeclampsia is a pregnancy-related disorder manifested as maternal hypertension in pregnancy (HTN-Preg) and fetal growth restriction. Placental ischemia could be an initiating event that leads to abnormal vascular and uteroplacental remodeling in HTN-Preg; however, the molecular targets and intermediary mechanisms involved are unclear. We tested the hypothesis that placental ischemia could target vascular and uteroplacental matrix metalloproteinases (MMPs) through an inflammatory cytokine-mediated mechanism. MMP levels and distribution were measured in the aorta, uterus, and placenta of normal pregnant (Preg) rats and pregnant rats with reduced uterine perfusion pressure (RUPP). Maternal blood pressure was higher and the litter size and pup weight were lower in RUPP compared with Preg rats. Gelatin zymography showed prominent uterine MMP-2 and MMP-9 activity that was dependent on the amount of loaded protein. At saturating protein loading, both gelatin and casein zymography revealed two additional bands corresponding to MMP-1 and MMP-7 that were greater in the aorta, uterus, and placenta of RUPP compared with Preg rats. Western blots and immunohistochemistry confirmed increased MMP-1 and MMP-7 in the aorta, uterus, and placenta of RUPP versus Preg rats. The levels of MMP-1 and MMP-7 substrate collagen type I were greater in tissues of RUPP compared with Preg rats. In organ culture, TNF-α increased MMP-1 and MMP-7 in the aorta, uterus, and placenta of Preg rats, and a TNF-α antagonist prevented the increases in MMPs in tissues of RUPP rats. Thus, placental ischemia, possibly through TNF-α, increases vascular and uteroplacental MMP-1 and MMP-7, which, in turn, alter collagen deposition and cause inadequate tissue remodeling in HTN-Preg. Cytokine antagonists may reverse the increase in MMP-1 and MMP-7 expression/activity and, in turn, restore proper vascular and uteroplacental remodeling in HTN-Preg and preeclampsia.
NEW & NOTEWORTHY The molecular mechanisms of preeclampsia are unclear, making it difficult to predict, prevent, or manage the pregnancy-associated disorder. This study showed that placental ischemia, possibly through the release of TNF-α, causes increases in the levels of matrix metalloproteinase (MMP)-1 and MMP-7, which could alter collagen deposition and cause inadequate uteroplacental and vascular remodeling in hypertension in pregnancy. The data suggest that targeting MMP-1 and MMP-7 and their upstream modulators, such as TNF-α, could provide a new approach in the management of hypertension in pregnancy and preeclampsia.
normal pregnancy is associated with significant hemodynamic, vascular, and uteroplacental changes. In the maternal circulation, increases in heart rate, plasma volume, and cardiac output together with a decrease in vascular resistance maintain adequate blood supply to different tissues with a minimal change in blood pressure (BP). Also, during pregnancy, the uterus gradually enlarges to provide adequate space for the fetus to grow. Additionally, the placenta undergoes marked development, and cytotrophoblasts progressively invade the spiral arteries in the decidua to maintain adequate blood and nutrient supply to the developing fetus (46, 63). These changes in maternal vascular and uteroplacental tissues involve significant alterations in the structure and function of the maternal vasculature as well as the uterus and placenta (48, 90).
Preeclampsia, a pregnancy-related disorder that complicates 5–8% of pregnancies, is manifested as hypertension (HTN) in pregnancy (HTN-Preg), with occasional proteinuria and edema (7, 83). If not adequately managed, preeclampsia could lead to eclampsia, with severe HTN and convulsions (32, 65, 77). Preeclampsia is also often associated with restricted fetal development and intrauterine growth retardation (IUGR), causing preterm pregnancies and premature labor (79, 89). Preeclampsia is a major cause of maternal and fetal morbidity and mortality, but the pathophysiological mechanisms are not clearly understood. Induction of placental ischemia by reducing uteroplacental perfusion pressure (RUPP) in late pregnant rats shows some of the characteristics of preeclampsia, including HTN-Preg and IUGR (3, 17, 27, 32). Similarly, rat models of gestational hypoxia show preeclampsia-like manifestations (100), supporting the view that placental ischemia/hypoxia could be an initiating event in HTN-Preg (17, 28, 32). However, the tissue targets and the intermediary mechanisms linking placental ischemia to the vascular and uteroplacental changes in HTN-Preg have not been clearly identified.
Placental ischemia could lead to the release of bioactive factors in the maternal circulation including proinflammatory cytokines such as TNF-α (7, 36, 37, 83). Circulating TNF-α levels are greater in preeclamptic than normal pregnant women (12, 57, 69), and plasma levels and CD4+ T cell production of TNF-α are greater in RUPP compared with normal pregnant rats (16, 36, 92). Infusion of TNF-α during pregnancy causes HTN and proteinuria in baboons (86), rats (5), and mice (11). TNF-α plays a role in multiple cytoskeletal and inflammatory disorders, and identifying its tissue targets in HTN-Preg is an area of topical research interest (15, 36).
Matrix metalloproteinases (MMPs) are zinc-dependent proteases that degrade different components of the extracellular matrix (ECM) and play a role in the remodeling of various tissues and organs (41, 56). The MMP family includes collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (71). MMP-2 (gelatinase A) and MMP-9 (gelatinase B) play a role in endometrial tissue remodeling during the estrous and menstrual cycles and during pregnancy (55, 88, 99). We have previously shown that MMP-2 and MMP-9 are upregulated in the aorta and myometrium during pregnancy in rats, supporting their role in pregnancy-related vascular and uterine remodeling (18, 95). Although some studies have shown an increase in circulating levels of MMP-2 and MMP-9 (23), other studies have shown a decrease in MMP-9 in preeclamptic versus normal pregnant women (56). We have reported that MMP-2 and MMP-9 levels are reduced in the aorta, uterus, and placenta of RUPP rats and suggested that low MMP levels would favor excess collagen deposition and affect smooth muscle growth and spiral arteries remodeling (41). However, gelatinases mainly degrade collagen type IV and only partially degrade collagen type I, suggesting that other MMPs are involved in vascular and uteroplacental remodeling during pregnancy. Consistent with this paradigm, MMP-1 is expressed in cytotrophoblasts of the placenta and decidua and may play a role in trophoblast invasion (21). Also, MMP-7 is expressed in the uterus and could play a role in endometrial tissue remodeling during estrous and menstrual cycles and during pregnancy (80). However, the vascular and uteroplacental expression and activity of MMP-1 and MMP-7 during HTN-Preg are less clear. Also, the effects of these MMPs on collagen deposition during pregnancy have not been examined. Furthermore, although inflammatory cytokines such as TNF-α can modify placental expression of adhesion molecules (36) and play a role in preeclampsia (12, 57, 69), the effects of TNF-α on MMP expression/activity are unclear.
The present study was designed to test the hypothesis that alterations of vascular and uteroplacental MMP-1 and MMP-7 and the protein substrate collagen are important molecular targets during placental ischemia-induced HTN-Preg through a potential intermediary mechanism involving inflammatory cytokines. We used the aorta, uterus, and placenta from normal pregnant and RUPP rats to investigate whether 1) MMP-1 and MMP-7 levels are altered in RUPP compared with normal pregnant rats, 2) whether the changes in vascular and uteroplacental MMPs affect the downstream target and protein substrate collagen, and 3) whether cytokines such as TNF-α could function as an intermediary upstream modulator of MMP expression/activity during HTN-Preg.
MATERIALS AND METHODS
Animals.
Timed-pregnant (gestational day 11) Sprague-Dawley rats (12 wk of age) from Charles River Laboratories (Wilmington, MA) were maintained on ad libitum standard rat chow and tap water in 12:12-h light-dark cycle. On gestational day 14, pregnant rats allocated to the RUPP group were anesthetized by inhalation of isoflurane, the abdominal cavity was opened, and a silver clip (0.203-mm inner diameter) was placed around the lower abdominal aorta above the iliac bifurcation, as previously described (6, 9, 13, 17). This procedure reduces uterine perfusion pressure in the gravid rat by ~40% (22). Since compensation of blood flow to the placenta occurs through an adaptive increase in ovarian blood flow, a silver clip (0.1-mm inner diameter) was also placed on the main uterine branches of both the right and left ovarian arteries. Rats in the normal pregnant group (Preg rats) were sham operated. RUPP rats in which the clipping procedure resulted in maternal death or total resorption of the fetuses were excluded from the data analyses. All procedures were performed in accordance with the National Institutes of Health Guide for the Care of Laboratory Animal Welfare Act and were approved by the Animal Care and Use Committee at the Brigham and Women’s Hospital.
Tissue preparation.
On gestational day 19, BP was measured via a polyethylene-50 carotid arterial catheter connected to a pressure transducer as previously described (53). Rats were then euthanized by inhalation of CO2, the thoracic and abdominal cavities were opened, and the thoracic aorta and gravid uterus were excised and placed in Krebs solution. The gravid uterus was cut open, the placentae and pups were separated and gently blotted between filter papers, and the litter size and individual pup wet weight were recorded. The aorta, uterus, and placenta were then cut into 5-mm-wide segments, and experiments were performed on 8−12 tissue segments from each rat from 4−6 rats/group. Some aortic, uterine, and placental segments from Preg rats were incubated in the presence of TNF-α (0.1 μg/ml, R&D Systems, Minneapolis, MN) with or without a TNF-α antagonist (p75TNFR:Fc, 0.1 μg/ml, Amgen) for 48 h in organ culture medium. The TNF-α antagonist is a soluble TNF-α receptor decoy that binds TNF-α and renders it inactive (45, 68). Also, some uterine, placental, and aortic segments from RUPP rats were incubated with the TNF-α antagonist (0.1 μg/ml) for 48 h in organ culture medium. The culture medium was changed and fresh TNF-α and/or TNF-α antagonist was added every 24 h.
Gelatin zymography.
Aortic, uterine, and placental segments were homogenized using a 2-ml tight-fitting homogenizer (Kontes Glass, Vineland, NJ) and homogenization buffer (without DTT) containing 20 mM MOPS, 4% SDS, 10% glycerol, 1.2 mM EDTA, 0.02% BSA, 5.5 μM leupeptin, 5.5 μM pepstatin, 2.15 μM aprotinin and 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride. The homogenate was centrifuged at 10,000 g for 10 min, the supernatant was collected, and protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA). Tissue homogenate was subjected to electrophoresis on 8% SDS polyacrylamide gel containing 0.1% gelatin (Sigma, St. Louis, MO). The gel was then incubated in a zymogram renaturing buffer containing 2.5% Triton X-100 (Sigma) with gentle agitation for 30 min at room temperature. The gel was then equilibrated in a zymogram developing buffer (pH 6.7) containing 50 mM Tris base, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij35 (Fisher Scientific, Pittsburgh, PA), and 1 μM ZnCl2 (Sigma) for 30 min at room temperature and then incubated in the zymogram developing buffer at 37°C for 16 h. The gel was stained with 0.5% Coomassie blue R-250 (Sigma) for 30 min and then destained with a coomassie R-250 destaining solution [methanol-acetic acid-water (50:10:40)]. Areas corresponding to MMP gelatinolytic activity appeared as clear bands against a dark blue background. The bands were analyzed by optical densitometry and ImageJ software (National Institutes of Health, Bethesda, MD), and the integrated protease activity was measured as pixel intensity × mm2 normalized to actin intensity, as previously described (18, 95).
Casein zymography.
Aortic, uterine, and placental tissue homogenate was subjected to electrophoresis on an 8% SDS polyacrylamide gel containing increasing concentrations of casein (0.2%, 0.4%, 0.6%, 0.8%, 1%, and 2%, Sigma). The zymograms were then developed, stained, destained, and analyzed as described for gelatin zymography.
Western blots.
Aortic, uterine, and placental segments were homogenized using a 2-ml tight-fitting homogenizer (Kontes Glass) and homogenization buffer containing 20 mM MOPS, 4% SDS, 10% glycerol, 2.3 mg DTT, 1.2 mM EDTA, 0.02% BSA, 5.5 μM leupeptin, 5.5 μM pepstatin, 2.15 μM aprotinin, and 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride. The homogenate was centrifuged at 10,000 g for 10 min, the supernatant was collected, and protein concentration was determined using a protein assay kit (Bio-Rad). Protein extracts (20 μg) were combined with an equal volume of 2× Laemmli loading buffer, boiled for 5 min, and size fractionated by electrophoresis on 8% SDS polyacrylamide gels. Proteins were transferred from the gel to a nitrocellulose membrane by electroblotting. Membranes were incubated in 5% nonfat dry milk in PBS-Tween for 1 h and then overnight at 4°C with polyclonal rabbit anti-MMP-1 (1:1,000, no. 2583988, Abcam), anti-MMP-7 (1:1,000, sc-30071, Santa Cruz Biotechnology, Dallas, TX), or anti-collagen type I antibody (1:1,000, sc-28657, Santa Cruz Biotechnology). Membranes were washed three times for 15 min each in PBS-Tween and then incubated with horseradish peroxidase-conjugated secondary antibody (1:3,000, Bio-Rad) for 2 h. Immunoreactive bands were detected using ECL Western blotting detection reagent (GE Healthcare Bio-Sciences, Piscataway, NJ). Membranes were stripped in stripping buffer and subsequently reprobed with β-actin antibody (1:8,000, A1978, Sigma). Equal amounts of sample protein were loaded onto the gels, and equal exposure times were used for the processing and development of the Western blots. Data were analyzed by optical densitometry and ImageJ software. Densitometry values represent the pixel intensity normalized to β-actin to correct for loading as previously described (18, 95).
Histology and immunohistochemistry.
Tissues from Preg and RUPP rats were cryopreserved in Tissue-Tek 4583 optimal cutting temperature compound (Fisher Scientific) and stored at −80°C. Cross-sectional 6-μm-thick cryosections from the middle segment of the aorta, uterus, and placenta were placed on glass slides and prepared for staining with hematoxylin and eosin; stained sections were coded and labeled in a blinded fashion.
To determine the tissue distribution of MMP-1 and MMP-7, cryosections of the aorta, uterus, and placenta were thawed and fixed in ice-cold acetone for 30 min. Endogenous peroxidase was quenched in 1.5% H2O2 solution for 30 min, and nonspecific binding was blocked in 10% horse serum for 30 min. Tissue sections were incubated with polyclonal MMP-1 (1:100) or MMP-7 (1:100) antibody. After being rinsed with PBS, tissue sections were incubated with biotinylated anti-rabbit secondary antibody, rinsed with PBS, then incubated with avidin-labeled peroxidase (VectaStain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Positive labeling was visualized using diaminobenzadine and appeared as brown spots. Negative control slides were run simultaneously with no primary antibody. Specimens were counterstained with hematoxylin for 40 s, rinsed with PBS, topped with cytoseal 60, and covered with slide coverslips.
Images were acquired on a Nikon microscope using the same microscope magnification, light intensity, and camera gain. Images of the aorta were acquired using bright-field and ×4 or ×40 objectives, and images of the uterus and placenta were acquired with ×4 objective. Because the rat uterus is large, 16−20 picture frames of sequential parts of the uterine tissue section were acquired using the ×4 objective and inserted as individual raw images in Powerpoint. The image frames were aligned to produce a reconstructed image of the uterus, and individual images were then grouped and saved as a composite JPG image of the whole reconstituted uterine tissue section as previously described (41, 49). A similar image acquisition and reconstitution protocol was followed for the rat placenta. Images of tissue sections were analyzed using ImageJ software.
Outlines of the tissue exterior and interior were used to define the whole tissue area and lumen area, respectively, and the wall area was calculated as whole tissue area – lumen area.
The total number of pixels in the tissue wall image was defined, and the number of brown spots (pixels) corresponding to MMP-1 or MMP-7 was then counted and presented as a percentage of total pixels. The number of pixels in the specific vascular layer (intima, media, and adventitia) was also defined and transformed into the area in mm2 using a calibration bar. The number of brown spots (pixels) representing MMP-1 or MMP-7 in each vascular layer was then counted and presented as the number of pixels per mm2 as previously described (18, 49, 85).
Solutions and drugs.
Krebs solution was used for tissue dissection and contained the following (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2 and bubbled with 95% O2-5% CO2, pH 7.4. TNF-α antagonist (p75TNFR:Fc) was a generous gift from Amgen. The tissue culture medium used to pretreat the tissues with TNF-α or TNF-α antagonist was composed of minimum essential medium supplemented with penicillin, streptomycin, and amphotericin B (Invitrogen, Grand Island, NY). PBS contained (in mM) 137 NaCl, 2.7 KCl, 8 Na2HPO4, and 2 KH2PO4 at pH 7.4. All other chemicals were of reagent grade or better.
Statistical analysis.
Experiments were conducted on the aorta, uterus, and placenta isolated from four to six different rats per group (Preg vs. RUPP rats), and cumulative data are presented as means ± SE, with the n value representing the number of rats per group. Data were analyzed and plotted using Prism (v.5.01, GraphPad software, San Diego, CA). Data were first analyzed using ANOVA. When a statistical difference was observed, data were further analyzed using Bonferroni’s post hoc correction. Student’s unpaired t-test was used for comparison of two means. Differences were statistically significant when P < 0.05.
RESULTS
Maternal measurements on gestational day 19 showed that BP was significantly higher in RUPP (131.1 ± 2.9 mmHg) compared with Preg rats (92.7 ± 3.1 mmHg). Plasma levels of TNF-α were greater in RUPP (78.6 ± 0.8 pg/ml) compared with Preg rats (28.2 ± 15.50 pg/ml). Fetal measurements also showed that the litter size (number of pups) was significantly less in RUPP (8 ± 1) compared with Preg rats (12 ± 1), and that the individual pup weight was significantly reduced in RUPP (1.84 ± 0.14 g) compared with Preg rats (2.27 ± 0.10 g), as previously described (41).
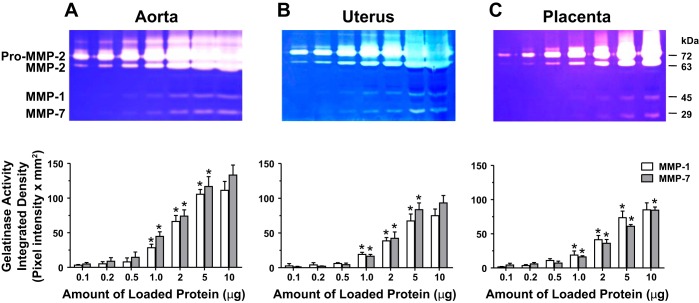
We have previously performed gelatin zymography analysis on aortic, uterine, and placenta tissue homogenate from Preg rats and showed that the intensity of proteolytic bands corresponding to pro-MMP-2, MMP-2, pro-MMP-9, and MMP-9 was dependent on the amount of loaded protein (41). In this study, gelatin zymography on aortic, uterine, and placenta tissue homogenate from RUPP rats showed concentration-dependent increases in the intensity of the pro-MMP-2 and MMP-2 bands at loading protein amount from 0.1 to 0.2 to 0.5 μg and clearly discernible bands at 1 and 2 μg protein. Further increases in loaded protein to 5 and 10 μg showed further increases in proteolytic activity, and the MMP-2 bands became almost saturated (Fig. 1). Careful examination of the zymograms revealed additional bands at ~45 kDa, corresponding to MMP-1, and ~29 kDa, corresponding to MMP-7. The intensity of the MMP-1 and MMP-7 bands was dependent on the amount of loaded protein being undetectable at 0.1, 0.2, and 0.5 μg, clearly discernible at 1 and 2 μg, and prominent at 5 μg protein. Further increases in loaded protein to 10 μg showed insignificant increases in MMP-1 proteolytic activity in the aorta, uterus, and placenta or MMP-7 in the aorta and uterus of RUPP rats (Fig. 1). Because 5 μg loaded protein produced clearly discernible MMP-1 and MMP-7 bands, all further gelatin zymography experiments comparing the aorta, uterus, and placenta of Preg and RUPP rats were performed using 5 μg protein for loading.
Fig. 1.
Concentration-dependent gelatinase activity in the aorta, uterus, and placenta of pregnant rats with reduced uterine perfusion pressure (RUPP). Aortic (A), uterine (B), and placental tissue strips (C) from RUPP rats were homogenized and prepared for gelatin zymography analysis using different amounts of loaded protein (0.1–10 µg). The proteolytic bands corresponding to pro-matrix metalloproteinase (MMP)-2 and MMP-2 as well as MMP-9 were not analyzed as they were the topic of a previous report (41). The densitometry values of the proteolytic bands corresponding to MMP-1 and MMP-7 are presented as pixel intensity × mm2. Bar graphs represent means ± SE; n = 4/group. *MMP-1 or MMP-7 activity at a specific amount of loaded protein was significantly different (P < 0.05) from the preceding protein amount.
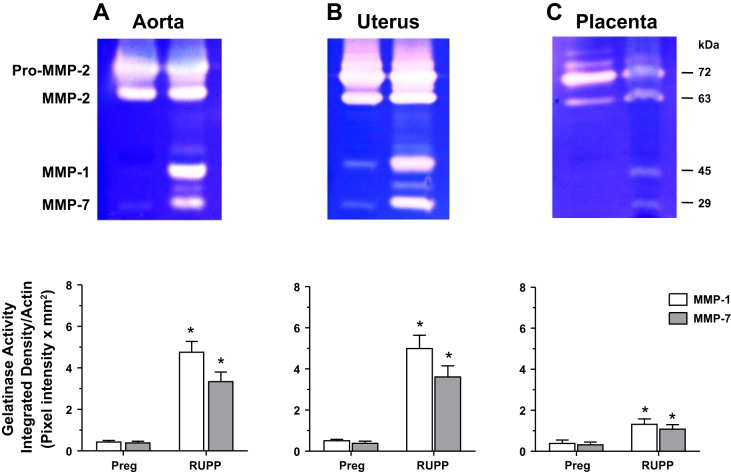
At 5 μg loaded protein, the gelatinase activity corresponding to pro-MMP-2 and MMP-2 was almost saturated, and no difference in the intensity of MMP-2 could be observed in the aorta (Fig. 2A) or uterus (Fig. 2B) of RUPP versus Preg rats. Consistent with our previous report (41), the MMP-2 bands appeared to be reduced in the placenta of RUPP versus Preg rats (Fig. 2C). Importantly, the MMP-1 and MMP-7 bands were undetectable in the aorta and placenta and barely detectable in the uterus of Preg rats (Fig. 2). In contrast, the MMP-1 and MMP-7 bands were very prominent in the aorta (Fig. 2A) and uterus (Fig. 2B) and significantly greater in the placenta (Fig. 2C) of RUPP versus Preg rats.
Fig. 2.
Aortic, uterine, and placental MMP-1 and MMP-7 gelatinase activity in normal pregnant (Preg) and RUPP rats. Equal protein amount (5 μg) of tissue homogenates from the aorta (A), uterus (B), and placenta (C) of Preg and RUPP rats were prepared for gelatin zymography analysis. At 5 µg loaded protein amount, the proteolytic bands corresponding to pro-MMP-2 and MMP-2 in the aorta and uterus were saturated, and no differences could be detected between the groups. The proteolytic bands corresponding to pro-MMP-2 and MMP-2 in the placenta appeared to be reduced in RUPP versus Preg rats, confirming our previous report (41). The densitometry values of the proteolytic bands corresponding to MMP-1 and MMP-7 are presented as pixel intensity × mm2 and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 6/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
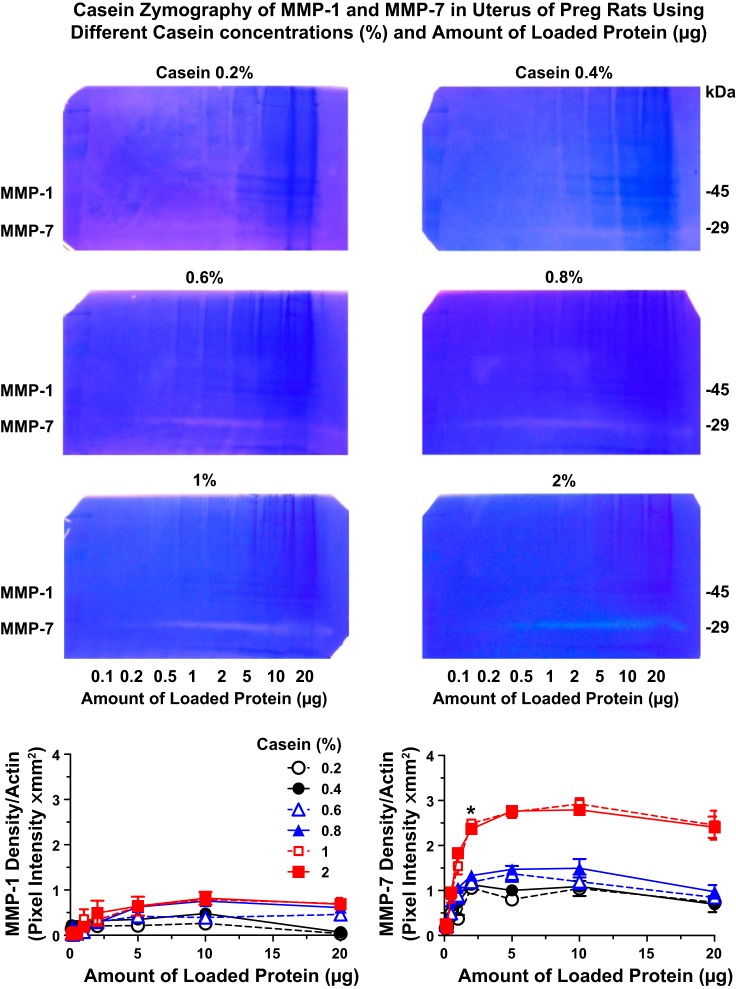
Because gelatin may not be the best substrate for MMP-1 and MMP-7, we repeated the zymography experiments using the other MMP substrate, casein (84). Because of the difficulty in dissolving casein in the buffer, we performed preliminary casein zymography experiments using increasing concentrations of casein in the gel (0.2%, 0.4%, 0.6%, 0.8%, 1%, and 2%) and increasing amounts of loaded protein (0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 µg) from uterine tissue homogenate of Preg rats. MMP-1 and MMP-7 activities were more difficult to detect using casein zymography (Fig. 3) compared with gelatin zymography (Fig. 2), suggesting that casein is less prone to degradation by MMPs compared with gelatin. The intensity of the caseinolytic bands increased with increasing casein concentrations in the gel and reached a maximum at 1% casein (Fig. 3). Further increases in casein concentration to 2% did not cause further increases in the intensity of the caseinolytic bands. The caseinolytic bands corresponding to MMP-1 and MMP-7 were also dependent on the amount of loaded protein, being undetectable at 0.1, 0.2, 0.5, and 1 µg and prominent at 2 µg protein, and further increases in protein amount to 5, 10, and 20 µg did not cause further increases in the intensity of the caseinolytic band. Because 1% caein and 2 μg protein produced detectable MMP-1 bands and clearly discernible MMP-7 bands, all further casein zymography experiments comparing the aorta, uterus, and placenta of Preg and RUPP rats were performed using 1% casein in the gel and 2 μg protein for loading.
Fig. 3.
Concentration-dependent caseinase activity in the uterus of Preg rats. Uterine tissue strips from Preg rats were homogenized and prepared for casein zymography analysis using different concentrations of casein in gel (0.2%, 0.4%, 0.6%, 0.8%, 1%, and 2%) as well as different amounts of loaded protein (0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 µg). The densitometry values of the proteolytic bands corresponding to MMP-1 and MMP-7 are presented as pixel intensity × mm2. Data points represent means ± SE; n = 4/group. *MMP-1 or MMP-7 activity at a specific amount of loaded protein was significantly different (P < 0.05) from the activity at the preceding protein amount.
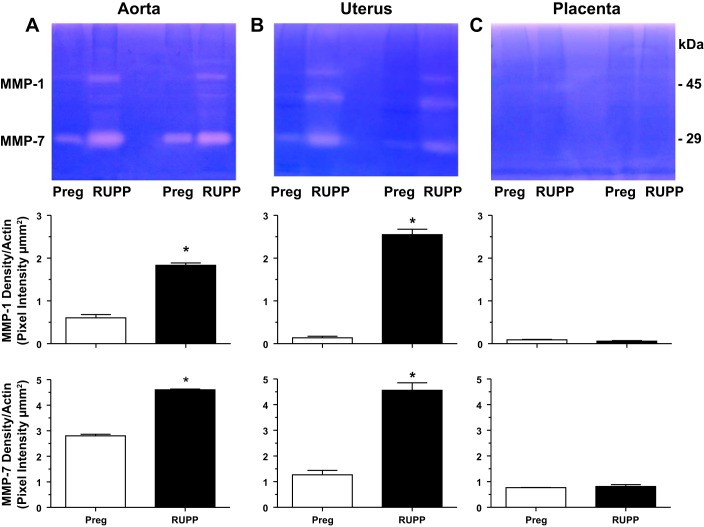
Casein zymography revealed that the activity of MMP-1 and MMP-7 was increased in the aorta and uterus of RUPP versus Preg rats (Fig. 4). An additional band slightly below MMP-1 could be detected in the uterus and was also enhanced in RUPP compared with Preg rats. The nature of this band is not clear at the present time but may represent an additional form or degradation product of MMP-1 or a different MMP; this needs to be further examined in future experiments. The MMP-1 and MMP-7 caseinolytic bands were not easily detectable in the placenta compared with the aorta and uterus and were not different in RUPP versus Preg rats (Fig. 4), suggesting a reduced role in the placenta and placental remodeling.
Fig. 4.
Aortic, uterine, and placental MMP-1 and MMP-7 caseinase activity in Preg and RUPP rats. Equal protein amount (2 μg) of tissue homogenates from the aorta (A), uterus (B), and placenta (C) of Preg and RUPP rats were prepared and run in duplicates for casein zymography analysis. The densitometry values of the proteolytic bands corresponding to MMP-1 and MMP-7 are presented as pixel intensity × mm2 and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 4/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
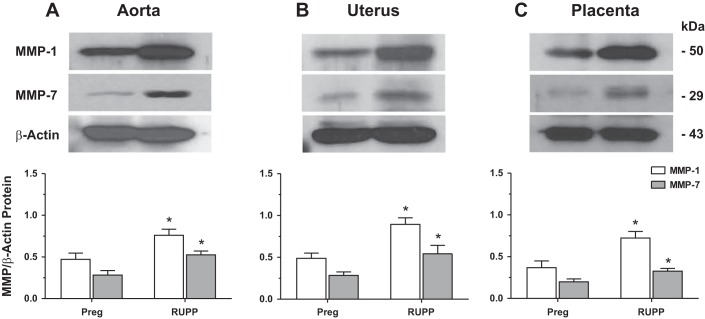
Western blot analysis using specific antibodies to MMP-1 and MMP-7 confirmed detectable bands corresponding to MMP-1 at 50 kDa and MMP-7 at 29 kDa in the aorta, uterus, and placenta of Preg rats. The protein levels of MMP-1 and MMP-7 were significantly increased in aorta, uterus, and placenta of RUPP versus Preg rats (Fig. 5).
Fig. 5.
Protein amount of aortic, uterine, and placental MMP-1 and MMP-7 in Preg and RUPP rats. Tissue homogenates of the aorta (A), uterus (B), and placenta (C) of Preg and RUPP rats were prepared for Western blot analysis using antibodies to MMP-1 (1:1,000) and MMP-7 (1:1,000). Immunoreactive bands corresponding to MMP-1 and MMP-7 were analyzed by optical densitometry and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 4/group. *Measurements in RUPP rats were significantly different (P < 0.05) from corresponding measurements in Preg rats.
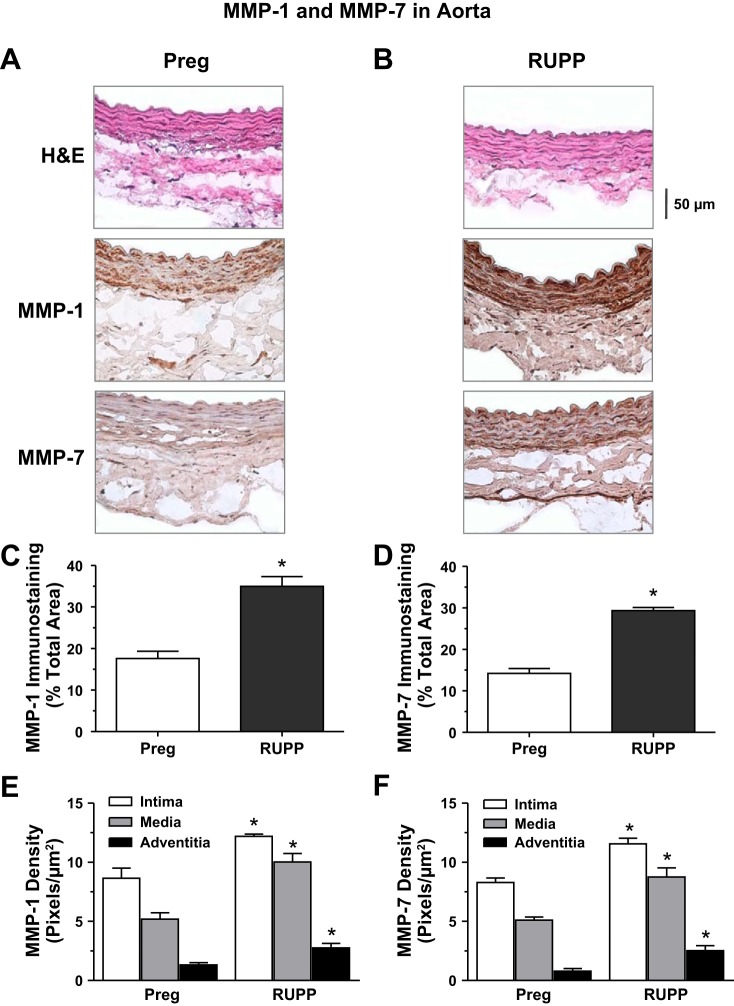
We examined the pregnancy-associated changes in tissue distribution of MMP-1 and MMP-7. Immunohistochemistry revealed detectable immunostaining for MMP-1 and MMP-7 in tissue sections of the aorta of Preg rats. Total MMP-1 and MMP-7 immunostaining was increased in RUPP versus Preg rats. Also, the distribution of MMP-1 and MMP-7 was greater in the intima, media, and adventitia of the aorta of RUPP versus Preg rats (Fig. 6).
Fig. 6.
Distribution of MMP-1 and MMP-7 in the aorta of Preg and RUPP rats. Cryosections (6 μm) of the aorta of Preg (A) and RUPP rats (B) were prepared for hematoxylin and eosin (H&E) staining or immunohistochemical staining using MMP-1 or MMP-7 antibodies (1:100). For MMP immunostaining, the total number of pixels in the tissue section wall was first defined and the number of brown spots (pixels) was then counted and presented as a percentage of the total wall area (C and D). The number of pixels in the specific vascular layer (intima, media, and adventitia) was also defined and transformed into the area in mm2 using a calibration bar. The number of brown spots (pixels) representing MMP-1 and MMP-7 in each vascular layer was then counted and presented as number of pixels per mm2 (E and F). Total magnification: ×400. Bar graphs represent means ± SE; n = 4−6/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
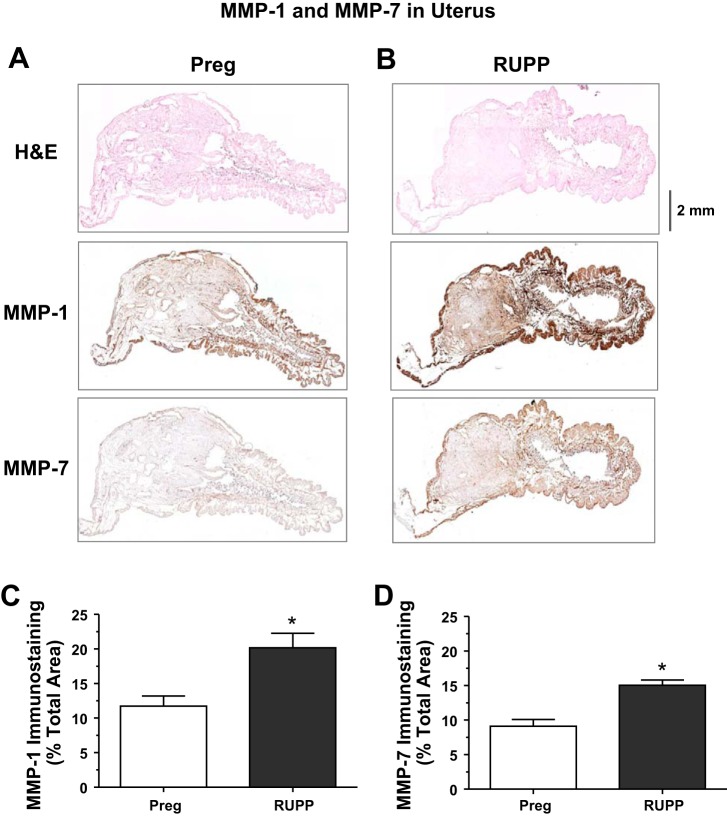
Because the pregnant rat uterus and placenta are large, only a small portion of the tissue section could be imaged when using a ×4 objective. Also, the uterine wall thickness was not homogeneous (Fig. 7). To circumvent these difficulties, and to perform quantitative analysis in the whole tissue section, 16−20 picture frames of sequential parts of the uterine tissue section were acquired using the ×4 objective, and the composite image of the whole uterine section was reconstituted and analyzed. Similar procedure was followed to obtain composite image of the whole placenta tissue section.
Fig. 7.
Distribution of MMP-1 and MMP-7 in the uterus of Preg and RUPP rats. Cryosections (6 μm) of the uterus of Preg (A) and RUPP rats (B) were prepared for H&E staining or immunohistochemical staining using MMP-1 or MMP-7 antibodies (1:100). Because the rat uterus is large, 10−16 picture frames of sequential parts of the uterine tissue section were acquired using a ×4 objective and inserted as individual raw images in Powerpoint. The image frames were aligned and then grouped and saved as a composite JPG image of the whole reconstituted uterine tissue section for image analysis using ImageJ software. For MMP immunostaining, the total number of pixels in the tissue section wall was first defined and the number of brown spots (pixels) was then counted and presented as a percentage of the total wall area (C and D). Total magnification: ×40. Bar graphs represent means ± SE; n = 4−6/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
Immunohistochemical analysis revealed MMP-1 and MMP-7 brown staining in uterine tissue sections of Preg and RUPP rats. Quantitative image analysis showed that the brown immunostaining for MMP-1 and MMP-7 was greater in the uterus of RUPP compared Preg rats (Fig. 7).
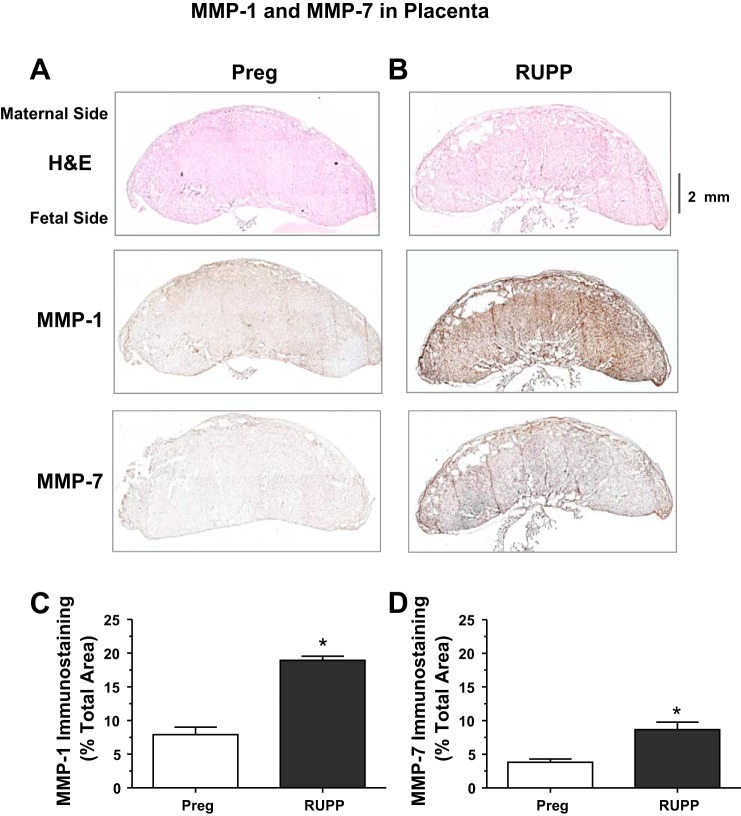
A similar immunohistochemistry protocol showed that MMP-1 and MMP-7 immunostaining was increased in the placenta of RUPP versus Preg rats (Fig. 8).
Fig. 8.
Distribution of MMP-1 and MMP-7 in the placenta of Preg and RUPP rats. Cryosections (6 μm) of the placenta of Preg (A) and RUPP rats (B) were prepared for H&E or immunohistochemical staining using MMP-1 or MMP-7 antibodies (1:100). Because the rat placenta is large, 10−16 picture frames of sequential parts of the placental tissue section were acquired using a ×4 objective and the composite image of the whole placental tissue section was reconstituted and analyzed using ImageJ software. For MMP immunostaining, the total number of pixels in the tissue section image was first defined and the number of brown spots (pixels) was then counted and presented as a percentage of the total area (C and D). Total magnification: ×40. Bar graphs represent means ± SE; n = 4−6/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
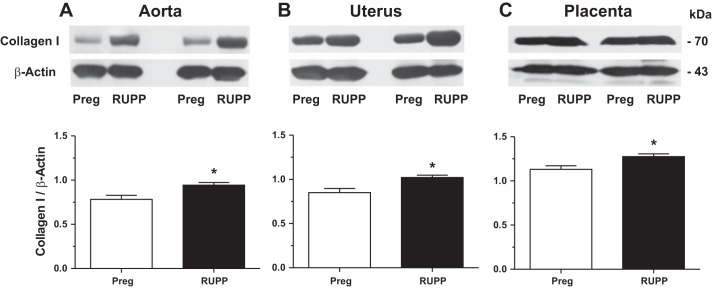
We examined the potential downstream target substrates of MMP-1 and MMP-7. Western blots revealed that collagen type I was increased in the aorta, uterus, and placenta of RUPP versus Preg rats (Fig. 9).
Fig. 9.
Protein levels of aortic, uterine, and placental collagen type I in Preg and RUPP rats. Tissue homogenates of the aorta (A), uterus (B), and placenta (C) of Preg and RUPP rats were prepared and run in duplicate for Western blot analysis using antibody to collagen type I (1:1,000). Immunoreactive bands corresponding to collagen were analyzed by optical densitometry and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 4/group. *Measurements in RUPP rats were significantly different (P < 0.05) from the corresponding measurements in Preg rats.
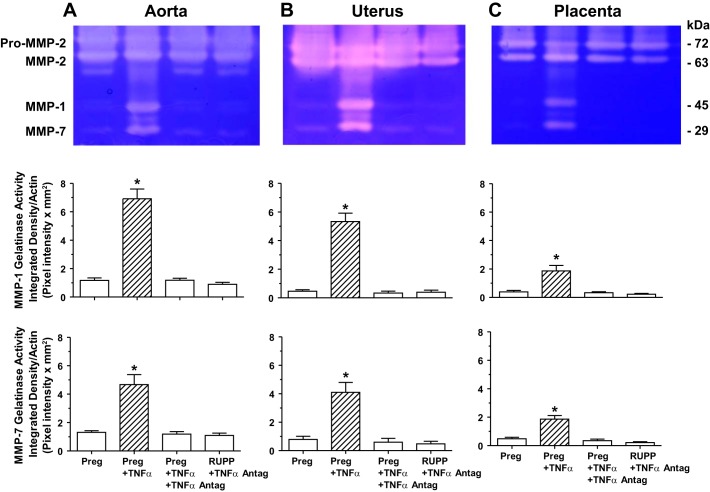
We tested the effects of cytokines as potential upstream modulators of MMPs. Gelatin zymography showed that the gelatinase activity corresponding to MMP-1 and MMP-7 was significantly increased in the aorta (Fig. 10A), uterus (Fig. 10B), and placenta (Fig. 10C) isolated from Preg rats and treated with TNF-α (0.1 µg/ml) for 48 h in organ culture. In tissues of Preg rats treated with TNF-α plus the TNF-α antagonist (0.1 µg/ml) for 48 h, the MMP-1 and MMP-7 gelatinase activity was not significantly different from that in control nontreated tissues of Preg rats (Fig. 10). Also, in contrast with the observed significant increases in MMP-1 and MMP-7 gelatinolytic activity in RUPP versus control Preg rats (see Fig. 2) in the isolated aorta, uterus, and placenta of RUPP rats treated with TNF-α antagonist for 48 h, the MMP-1 and MMP-7 gelatinase activity was not significantly different from that in control Preg rats (Fig. 10). Of note, at 5 µg and almost saturating loading protein, gelatin zymography did not appear to show differences in pro-MMP-2 and MMP-2 activity in the aorta, uterus, and placenta of the different groups.
Fig. 10.
Effect of the cytokine TNF-α and TNF-α antagonist on gelatinase activity of aortic, uterine, and placental MMP-1 and MMP-7 in Preg and RUPP rats. Aortic (A), uterine (B), and placental strips (C) of Preg rats were treated with TNF-α (0.1 μg/ml) with or without TNF-α antagonist (0.1 μg/ml), and tissues of RUPP rats were treated with TNF-α antagonist for 48 h in organ culture. Tissue homogenates were prepared and loaded at 5 µg protein amount for gelatin zymography analysis. At 5 µg amount of loaded protein, the proteolytic bands corresponding to pro-MMP-2 and MMP-2 were saturated, and no differences could be detected between the treatment groups. The densitometry values of the proteolytic bands corresponding to MMP-1 and MMP-7 are presented as pixel intensity × mm2 and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 6/group. *Significantly different (P < 0.05) from corresponding measurements in control nontreated tissues of Preg rats.
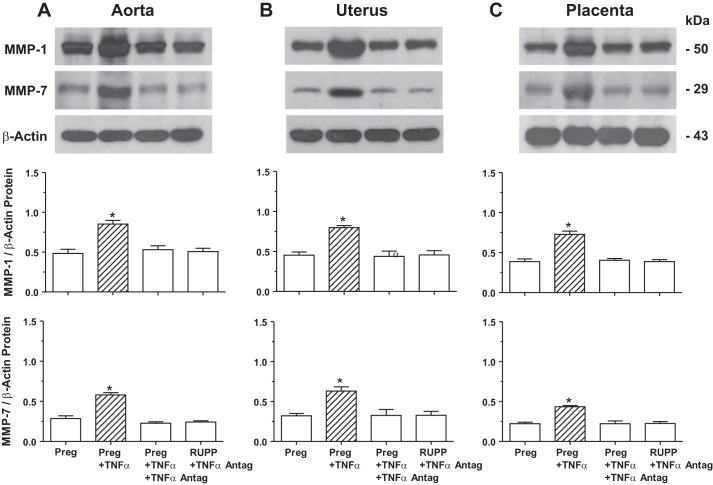
Western blot analysis showed that the protein level of MMP-1 and MMP-7 was significantly increased in the aorta (Fig. 11A), uterus (Fig. 11B), and placenta (Fig. 11C) isolated from Preg rats and treated with TNF-α (0.1 µg/ml) in organ culture for 48 h. In tissues of Preg rats treated with TNF-α plus TNF-α antagonist (0.1 µg/ml) for 48 h, no significant increase in the amount of MMP-1 or MMP-7 was observed (Fig. 11). Also, in contrast with the observed significant increases in the amount of MMP-1 and MMP-7 in RUPP versus control Preg rats (see Fig. 3), in the isolated aorta, uterus, and placenta of RUPP rats treated with TNF-α antagonist for 48 h, the levels of MMP-1 and MMP-7 were not significantly different from those in control Preg rats (Fig. 11).
Fig. 11.
Effect of the cytokine TNF-α and TNF-α antagonist on protein amount of aortic, uterine, and placental MMP-1 and MMP-7 in Preg and RUPP rats. Tissue homogenates of the aorta (A), uterus (B), and placenta (C) of Preg rats were treated with TNF-α (0.1 μg/ml) with or without TNF-α antagonist (0.1 μg/ml), and tissues of RUPP rats were treated with TNF-α antagonist for 48 h in organ culture. Tissues were homogenized and prepared for Western blot analysis using antibody to MMP-1 (1:1,000) or MMP-7 (1:1,000). Immunoreactive bands corresponding to MMP-1 and MMP-7 were analyzed by optical densitometry and normalized to β-actin to correct for loading. Bar graphs represent means ± SE; n = 6/group. *Significantly different (P < 0.05) from corresponding measurements in control nontreated tissues of Preg rats.
DISCUSSION
The main findings of this study are 1) MMP-1 and MMP-7 levels are increased in the aorta, uterus, and placenta of RUPP versus Preg rats; 2) the MMP substrate collagen type I is increased in the aorta, uterus, and placenta of RUPP versus Preg rats; and 3) the cytokine TNF-α increases MMP-1 and MMP-7 in the aorta, uterus, and placenta of Preg rats, whereas a TNF-α antagonist reverses the enhancing effects of TNF-α on MMPs in tissues of Preg rats and brings the levels of MMP-1 and MMP-7 in tissues of RUPP rats to levels similar to those in control Preg rats.
Normal pregnancy is associated with significant vascular and uteroplacental remodeling to accommodate the hemodynamic and metabolic demands of mother and fetus (7, 83). MMPs are major regulators of tissue remodeling during pregnancy. Evidence suggests that the gelatinases MMP-2 and MMP-9 are involved in ECM remodeling and trophoblast invasion of the spiral arteries. MMP-2 is the main collagenolytic enzyme in the umbilical cord artery (7), and serum MMP-9 levels are elevated in normal pregnant women (56). Also, our previous study (18) has shown increases in MMP-2 and MMP-9 in the aorta and myometrium during the course of pregnancy.
Animal models of HTN-Preg have provided mechanistic insights on the pathophysiological changes in preeclampsia (6, 17, 19, 43, 62). Studies in the late pregnant sheep, dog, rabbit, and rat have shown that RUPP induces some of the manifestations of preeclampsia including HTN-Preg and IUGR (6, 17, 32). Consistent with previous reports (4, 29, 41, 53, 102), BP was increased and the litter size and individual pup weight were decreased in RUPP versus Preg rats, making the RUPP rat a consistent model to study the mechanisms of HTN-Preg.
In search for the molecular mechanisms underlying the vascular and uteroplacental changes in preeclampsia, we have hypothesized possible changes in MMPs. We examined the aorta as representative of the vascular changes in the maternal circulation, the uterus, which undergoes expansive remodeling to accommodate the growing fetus, and the placenta, which provides blood and nutrient supply to the developing fetus. We have previously reported that MMP-2 and MMP-9 levels are reduced in the aorta, uterus, and placenta of RUPP versus Preg rats and suggested that low MMP levels may cause excessive collagen deposition, affect smooth muscle growth, and decrease remodeling of spiral arteries (41). Although some studies have shown an increase in circulating MMP-2 and MMP-9 in preeclamptic versus normal pregnant women (23), other studies have shown a decrease in serum MMP-9 in preeclampsia (56). Also, in first trimester trophoblasts, suppression of MMP-9 expression inhibits their invasive capability, supporting a role of MMP-9 in modulating trophoblast invasion (97). Although these reports support changes in MMP-2 and MMP-9 in preeclampsia, they also raise important questions regarding whether other MMPs could be modulated during normal pregnancy and HTN-Preg, the putative target substrates downstream of the changes in MMPs, the potential structural and functional changes that could be influenced by MMPs, and the factors that may act upstream of MMPs and link RUPP to changes in MMP levels.
In addition to gelatinases, the MMP family includes collagenases, stromelysins, matrilysins, membrane-type-MMPs, and other MMP subtypes that could influence tissue remodeling and other cellular functions (44, 71). In the present study, careful examination of the gelatin zymograms showed additional gelatinolytic activities that appear to correspond to MMP-1 and MMP-7 because 1) the gelatinolytic bands ran at 45 and 29 kDa, which correspond to reported molecular weight of MMP-1 and MMP-7, respectively (44, 71); 2) the MMP-1 and MMP-7 gelatinolytic activity was detected only at a high amount of loaded protein, consistent with reports that gelatin may not be an ideal substrate for MMP-1 (44, 71, 84); 3) zymography using the other MMP substrate casein demonstrated caseinolytic bands at molecular weights corresponding to MMP-1 and MMP-7; 4) Western blots showed specific immunoreactive bands with MMP-1 and MMP-7 antibodies; and 5) immunohistochemical analysis confirmed detectable MMP-1 and MMP-7 immunostaining in the aorta, uterus, and placenta of Preg rats. These observations are consistent with reports that MMP-1 is expressed in cytotrophoblasts of the placenta and decidua (21), and MMP-7 could be involved in uterine tissue remodeling during pregnancy (80).
The present gelatin zymography, casein zymography, Western blots, and immunohistochemistry experiments suggest that the levels of MMP-1 and MMP-7 are increased in the aorta and uterus of RUPP versus Preg rats. Also, MMP-1 and MMP-7 immunostaining was augmented in the aortic intima, media, and adventitia of RUPP rats, consistent with reports that vascular cells are a major source of MMPs (40, 82). We speculate that the increased abundance of MMP-1 and MMP-7 is likely associated with increases in their activity. The changes in MMP-1 and MMP-7 were less clear in the placenta compared with the aorta or uterus, suggesting specific changes in MMP activity in the placenta compared with other maternal tissues. This is supported by our previous report (18) of tissue-specific changes in MMP activity in the placenta compared with the aorta and uterus of late-pregnant versus mid-pregnant rats. Nevertheless, the observed increases in MMP-1 and MMP-7 in tissues of RUPP rats support a role of other MMPs in the vascular and uteroplacental remodeling in HTN-Preg.
In search for the downstream targets of MMPs that could influence tissue remodeling, we investigated possible changes in MMP substrates. MMPs degrade different substrates including gelatin, collagen, and other proteins (33, 71, 91). In line with our report of decreased MMP-2 and MMP-9, we have previously shown an increase in total collagen content with no change in elastin content in the aorta, uterus, and placenta of RUPP versus Preg rats (41). Because MMPs facilitate cell growth and migration by promoting proteolysis of the ECM, we have proposed that the decreased MMP-2 and MMP-9 and consequently the increased collagen deposition in RUPP tissues could impede smooth muscle cell growth, proliferation, and migration and, in turn, interfere with uteroplacental tissue invasion and remodeling and could also decrease the blood vessel’s plasticity and, in turn, contribute to increased vascular resistance and HTN (41). It is important to note that collagen has 18 types and different subtypes (26). Gelatinases mainly degrade collagen type IV, although MMP-2 can also degrade collagen types I, II, III, V, VII, X, and XI and MMP-9 can degrade collagen types V, VII, X, and XIV (33, 71, 91). Thus, although MMP-2 is a gelatinase, it can also function as interstitial collagenase, acting much like MMP-1 but in a weaker manner (58). Accordingly, MMP-2 can degrade collagen in two stages: interstitial collagenase-like degradation followed by gelatinolysis promoted by the fibronectin-like domain (2). MMP-9 can also function as a collagenase, where it binds the α2-chains of collagen type IV with high affinity even when it is inactive and makes the substrate readily available and then as a gelatinase (61). The present study showed specific increases in collagen type I levels in the aorta, uterus, and placenta of RUPP versus Preg rats. The increases in collagen type I may contribute to excessive collagen deposition, vascular rigidity, and uteroplacental growth restrictive remodeling in RUPP rats. However, if collagen type I is a primary substrate of MMP-1 and possibly MMP-7 (44, 71), one would expect that an increase in MMP-1 and MMP-7 would lead to a decrease in collagen type I in RUPP rats, which is opposite from the present results. One possibility is that collagen turnover is a dynamic process, and an increase in the collagenolytic activity of MMP-1 and MMP-7 could trigger a feedforward compensatory mechanism to upregulate and provide additional supply of the substrate collagen type I. Another possibility is that collagen type I turnover is controlled by other MMPs and proteases, and although an increase in MMP-1 and MMP-7 would decrease collagen type I, a decrease in other proteases may increase collagen type I. This is consistent with our previous report (41), which showed that a decrease in MMP-2 and MMP-9 was associated with an increase in total aortic and uteroplacental collagen content. Another possibility is that collagen type I may not be the ideal substrate for MMP-1 and MMP-7, and the expression of other collagen subtypes in vascular and uteroplacental tissues of HTN-Preg rats should be examined in future studies.
In addition to their proteolytic effects on ECM, MMPs may affect vascular and uterine function and the mechanisms of smooth muscle contraction. We have previously shown that MMP-2 and MMP-9 cause relaxation of the precontracted rat aorta (14) and uterus (95) and induce vasodilation in the rat inferior vena cava via hyperpolarization and activation of K+ channels (70, 72). Other studies have shown that MMP-2 and MMP-9 may increase the production of vasoconstrictor peptides such as endothelin-1 and decrease vasodilator peptides such as adrenomedullin (24, 59), leading to endothelial dysfunction and imbalance between vasodilator and constrictor factors. MMP-2 may also enhance big endothelin-1-induced constriction in mesenteric vessels of RUPP rats (1). We have previously suggested that a decrease in vascular MMP-2 and MMP-9 could lead to increased vascular contraction and HTN-Preg, and a decrease in uterine MMP-2 and MMP-9 could lead to increased uterine contraction and premature labor (41, 95). Other studies have shown that MMP-1 may enhance vascular contraction. In omental vessels of pregnant women, MMP-1 causes vasoconstriction and enhances reactivity to ANG II via an endothelium-dependent protease-activated receptor and endothelin-1 pathway (60). We and other groups have shown that vascular contraction is enhanced in RUPP rats (17, 53, 102), and the observed increase in vascular MMP-1 and MMP-7 may contribute to increased vasoconstriction and BP in HTN-Preg rats.
We also examined the potential upstream mechanisms that could link RUPP-induced placental ischemia to the changes in MMP levels. Placental ischemia may enhance the release of inflammatory cytokines such as TNF-α and IL-6 (35, 36, 98). Circulating TNF-α levels are increased in preeclamptic vs. pregnant women (12, 57, 69), although placental levels of TNF-α may not be different in preeclampsia compared with pregnancy (67). LIGHT, a member of the TNF superfamily, is also increased in preeclampsia and may contribute to placental damage and ischemia (93). Experimental studies have shown that plasma levels and CD4+ T cell production of TNF-α are increased in RUPP versus Preg rats (16, 36, 92). Also, infusion of TNF-α during late pregnancy causes HTN and proteinuria in baboons (86), rats (5), and mice (11), and infusion of LIGHT increases BP, induces the expression of endothelin-1 and the antiangiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1), and causes proteinuria in Preg mice (93). TNF-α may contribute to the vascular and uteroplacental changes in HTN-Preg by increasing vascular permeability, fibroblast proliferation, and lymphocyte activation and promoting the production of IL-6 and IL-8 (20, 78). TNF-α may also downregulate endothelial nitric oxide synthase (8, 96) and mitochondrial biogenesis, leading to mitochondrial dysfunction, oxidative stress, and increased ROS (81). However, in most in vivo cytokine infusion experiments it is difficult to determine if the observed effects are caused directly by the cytokine or involve a cascade of other mediators and pathways, and therefore the direct targets of cytokines have not been clearly defined. Specifically, little information is available on whether TNF-α could target tissue MMPs during pregnancy. The present study supports a role of TNF-α as a potential upstream mechanism linking placental ischemia to the increase in vascular and uteroplacental MMP-1 and MMP-7 in HTN-Preg because 1) treatment of the aorta, uterus, and placenta of Preg rats with TNF-α increased the levels of MMP-1 and MMP-7; 2) the TNF-α-induced increases in MMP-1 and MMP-7 were specific, as treatment of tissues of Preg rats with TNF-α plus a TNF-α antagonist did not cause detectable changes in MMP-1 and MMP-7 levels; and 3) in contrast with the observed increase in the levels of MMP-1 and MMP-7 in tissues of RUPP versus Preg rats, in tissues of RUPP rats treated with a TNF-α antagonist the levels of MMP-1 and MMP-7 were not increased and were not significantly different from those in control Preg rats. These findings are consistent with reports that TNF-α may modify the expression of adhesion molecules in placental vessels (36) and may contribute to abnormal MMP production in preeclampsia (64). The findings are also in agreement with reports that that blockade of TNF-α with etanercept reduced BP in RUPP rats and that human umbilical vein endothelial cells treated with serum from RUPP rats that received the TNF-α blocker produced less endothelin-1 compared with cells that were treated with serum from RUPP rats that did not receive the cytokine antagonist (36).
Other observations and considerations include the following. First, MMP-1 is expressed in cytotrophoblasts and syncytiotrophoblasts of the placenta and decidua and may play a role in trophoblast invasion of spiral arteries. Some studies have shown low levels of MMP-1 in the umbilical cord blood, placenta, and decidua of preeclamptic versus normal pregnant women; the low MMP-1 levels are correlated with the severity of preeclampsia (21). Also, cultured human decidual microvascular endothelial cells from preeclamptic pregnancies express lower levels of MMP-1 than those from normal pregnancies (25). However, studies in omental vessels of pregnant women have shown that MMP-1 causes vasoconstriction and enhances reactivity to ANG II via an endothelium-dependent protease-activated receptor and endothelin-1 pathway and suggested a role of MMP-1 in vasoconstriction and the pathogenesis of preeclampsia (60). With regard to MMP-7, studies have suggested that it may play a role in endometrial tissue remodeling during the menstrual cycle and pregnancy (80). Also, the expression of MMP-7 by extravillous trophoblast cells, especially those close to the spiral arteries, was reduced in preeclamptic patients (76). Measurements of the levels of MMP-1 and MMP-7 should be carefully interpreted in the context of differences in species (human vs. animals), specimens examined (plasma vs. placenta, uterus, and aorta), and type of tissues or cells examined (native vs. cultured). Second, MMPs comprise a large family of at least 28 proteolytic enzymes (33, 71, 91). Although we examined MMP-2 and MMP-9 in a previous study and the changes in MMP-1 and MMP-7 in the present study, other MMPs have been detected in the aorta, uterus, and placenta, and the changes in these MMPs in HTN-Preg need to be examined. For instance, trophoblast- and vascular smooth muscle-derived MMP-12 has been suggested to mediate proteolysis and uterine spiral artery remodeling during pregnancy (30). Importantly, MMP activity could be influenced by other MMP activators and inhibitors. In effect, some MMPs may cleave other pro-MMPs, and membrane-type 1-MMP is a key activator of pro-MMP-2 (33, 66, 71, 91). Also, tissue inhibitors of metalloproteinases (TIMPs) are endogenous modulators of MMP activity (33, 56, 66, 71, 91). Some studies have shown increases or no change in TIMP-1 and TIMP-2 in the circulation and umbilical cord serum of preeclamptic versus normal pregnant women (21, 23, 56). Other studies have shown increases in TIMP-1 and TIMP-3 in preeclamptic patients (101), and the changes in TIMPs in HTN-Preg need to be further examined. Third, in the present study, aortic, uterine, and placental MMPs were measured on gestational day 19, and the progressive changes in MMPs during the course of pregnancy, their effects on BP and placental and fetal development, and their reversal in the postpartum period need to be examined. It should be noted that, while plasma levels of MMPs may show increase during pregnancy (54), MMPs are released from different maternal tissues, and plasma MMP levels often reflect global changes in multiple tissues. In the present study, we examined three pregnancy-relevant tissues and showed increases in aortic, uterine, and placental MMP-1 and MMP-7 in HTN-Preg rats. The changes in MMPs expression/activity and their effects on the structure and function of other tissues, e.g., the small resistance vessels, which significantly affect BP (52, 53), need to be examined. Fourth, in addition to TNF-α, IL-6 is a proinflammatory cytokine that may be elevated in preeclampsia (57, 74). RUPP rats show increased plasma levels and higher CD4+ T cell production of IL-6 (16, 92), and chronic infusion of IL-6 in late-pregnant rats leads to HTN, proteinuria (35), enhanced vascular contraction, and reduced endothelium-dependent relaxation via the nitric oxide-cGMP pathway (62). IL-1β is another proinflammatory cytokine that could contribute to the inflammatory response and disrupts endothelial cells in preeclampsia, and monocytes of preeclamptic women produce more IL-1β than those of normal pregnant women (50). Importantly, while cytokines affect MMP levels, MMPs could increase cytokines or promote their effects on ROS, causing a feedforward cycle in preeclampsia (65). Finally, placental ischemia during pregnancy may cause the release of antiangiogenic and vasoactive factors such as sFlt-1, ROS, and hypoxia-inducible factors, which could cause vascular and uteroplacental dysfunction in HTN-Preg (19, 27, 28, 37, 39, 62, 77). Preeclamptic women show imbalance between the proangiogenic factors VEGF and platelet-derived growth factor as well as the antiangiogenic factor sFlt-1 (28, 31, 34, 38, 47, 51, 73, 75, 87, 94). Also, placental ischemia in RUPP rats is associated with increased serum sFlt-1 (27) and soluble endoglin (28). The factors released during preeclampsia may work synergistically. TNF-α may work in concert with other inflammatory cytokines to increase endothelin-1 levels and cause HTN in RUPP rats (36). TNF-α may also act in synergy with sFlt-1 to promote a pro-inflammatory state and endothelial dysfunction. In human umbilical vein endothelial cells, treatment with TNF-α and sFlt-1 causes an increase in the adhesion molecules ICAM and VCAM and markers of endothelial dysfunction such as von Willebrand factor and endothelin-1 (15). Interestingly, sFlt-1, ROS, and hypoxia-inducible factors may affect MMP expression/activity (10, 41, 42), and studying their potential interaction with cytokines in modulating MMP expression/activity in HTN-Preg should be further examined.
In conclusion, HTN-Preg is associated with increased aortic, uterine, and placental levels of MMP-1 and MMP-7 and increased tissue content of the MMP substrate collagen type I. Given that collagen is an important component of the vascular and uteroplacental architecture and is one of the substrates of MMP-1 and MMP-7, the data suggest a possible role of MMPs in vascular and uteroplacental remodeling in HTN-Preg. Cytokines such as TNF-α increase MMP-1 and MMP-7 levels in the aorta, uterus, and placenta of Preg rats, and a TNF-α antagonist reverses the effects of TNF-α in tissues of Preg rats and brings the levels of MMP-1 and MMP-7 in RUPP rats to levels similar to those in control Preg rats. Targeting MMP-1 and MMP-7 and their potential upstream modulators such as TNF-α could provide a new approach in the management of HTN-Preg and preeclampsia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65998, HL-98724, and HL-111775; and The Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD-60702.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.L., N.C., K.M.M., and R.A.K. conceived and designed research; W.L., N.C., and K.M.M. performed experiments; W.L., N.C., K.M.M., and R.A.K. analyzed data; W.L., N.C., K.M.M., and R.A.K. interpreted results of experiments; W.L., N.C., K.M.M., and R.A.K. prepared figures; W.L., N.C., M.Q.M., K.M.M., and R.A.K. edited and revised manuscript; W.L., N.C., M.Q.M., K.M.M., and R.A.K. approved final version of manuscript; R.A.K. drafted manuscript.
ACKNOWLEDGMENTS
W. Li was a visiting scholar from Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei Province, China, and a recipient of scholarship from the China Scholarship Council. N. Cui was a visiting scholar from the Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China, and a recipient of scholarship from the China Scholarship Council. M. Q. Mazzuca was a recipient of an American Heart Association Postdoctoral Fellowship (Founders Affiliate). K. Mata was a visiting scholar from the Department of Pathology, Faculty of Medicine of Ribeirão Preto, University of São Paulo, and a recipient of a fellowship from the Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo Research Foundation, São Paulo, Brazil. We thank Tachianna Griffiths, Rachel Kana, and Emily Jiang for their assistance in preparing the tissue samples as well as the image acquisition and analysis.
REFERENCES
- 1.Abdalvand A, Morton JS, Bourque SL, Quon AL, Davidge ST. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 61: 488–493, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00055. [DOI] [PubMed] [Google Scholar]
- 2.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270: 5872–5876, 1995. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 290: R1–R10, 2006. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001. doi: 10.1161/01.HYP.38.3.742. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. doi: 10.1016/S0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 6.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 7.Ali SM, Khalil RA. Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert Opin Ther Targets 19: 1495–1515, 2015. doi: 10.1517/14728222.2015.1067684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso J, Sánchez de Miguel L, Montón M, Casado S, López-Farré A. Endothelial cytosolic proteins bind to the 3′ untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha. Mol Cell Biol 17: 5719–5726, 1997. doi: 10.1128/MCB.17.10.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CM, Lopez F, Zhang HY, Shirasawa Y, Pavlish K, Benoit JN. Mesenteric vascular responsiveness in a rat model of pregnancy-induced hypertension. Exp Biol Med (Maywood) 231: 1398–1402, 2006. doi: 10.1177/153537020623100813. [DOI] [PubMed] [Google Scholar]
- 10.Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am J Physiol Cell Physiol 298: C679–C692, 2010. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- 11.Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental regulation of inflammation and hypoxia after TNF-α infusion in mice. Am J Reprod Immunol 74: 407–418, 2015. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- 12.Cakmak M, Yilmaz H, Bağlar E, Darcin T, Inan O, Aktas A, Celik HT, Ozdemir O, Atalay CR, Akcay A. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clin Exp Hypertens 38: 137–142, 2016. doi: 10.3109/10641963.2015.1060993. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Khalil RA. Differential [Ca2+]i signaling of vasoconstriction in mesenteric microvessels of normal and reduced uterine perfusion pregnant rats. Am J Physiol Regul Integr Comp Physiol 295: R1962–R1972, 2008. doi: 10.1152/ajpregu.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg 40: 1001–1010, 2004. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res 89: 671–679, 2011. doi: 10.1093/cvr/cvq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R884–R891, 2015. doi: 10.1152/ajpregu.00154.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000. doi: 10.1161/01.HYP.35.1.367. [DOI] [PubMed] [Google Scholar]
- 18.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol 86: 734–747, 2013. doi: 10.1016/j.bcp.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-α-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 282: R390–R399, 2002. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 20.De Cesaris P, Starace D, Riccioli A, Padula F, Filippini A, Ziparo E. Tumor necrosis factor-αinduces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J Biol Chem 273: 7566–7571, 1998. doi: 10.1074/jbc.273.13.7566. [DOI] [PubMed] [Google Scholar]
- 21.Deng CL, Ling ST, Liu XQ, Zhao YJ, Lv YF. Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Exp Ther Med 9: 992–998, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eder DJ, McDonald MT. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hyper Hyper Preg 6: 431–451, 1987. doi: 10.3109/10641958709023492. [DOI] [Google Scholar]
- 23.Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens 5: 205–208, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Patron C, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1-32]. FASEB J 15: 2230–2240, 2001. doi: 10.1096/fj.01-0178com. [DOI] [PubMed] [Google Scholar]
- 25.Gallery ED, Campbell S, Arkell J, Nguyen M, Jackson CJ. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res 57: 340–346, 1999. doi: 10.1006/mvre.1998.2142. [DOI] [PubMed] [Google Scholar]
- 26.Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55: 1531–1546, 2003. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9: 147–160, 2002. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 30.Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knöfler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol 177: 2103–2115, 2010. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 145: 4835–4837, 2004. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 32.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 33.Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets 14: 287–324, 2013. [PMC free article] [PubMed] [Google Scholar]
- 34.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 46: 1077–1085, 2005. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 35.LaMarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res 2011: 59–64, 2011. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-αblockade. Hypertension 52: 1161–1167, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-α. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 38.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension 51: 982–988, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–485, 2007. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 40.Li HX, Kong FJ, Bai SZ, He W, Xing WJ, Xi YH, Li GW, Guo J, Li HZ, Wu LY, Wang R, Yang GD, Tian Y, Xu CQ. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol Cell Biochem 362: 115–122, 2012. doi: 10.1007/s11010-011-1133-6. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol 89: 370–385, 2014. doi: 10.1016/j.bcp.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg 53: 764–773, 2011. doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci 303: 233–240, 1992. doi: 10.1097/00000441-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 44.MacColl E, Khalil RA. Matrix metalloproteinases as regulators of vein structure and function: implications in chronic venous disease. J Pharmacol Exp Ther 355: 410–428, 2015. doi: 10.1124/jpet.115.227330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahe E, Descamps V. [Anti-TNF alpha in dermatology]. Ann Dermatol Venereol 129: 1374–1379, 2002. [PubMed] [Google Scholar]
- 46.Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev 64: 540–582, 2012. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 48.Mandala M, Osol G. Physiological remodelling of the maternal uterine circulation during pregnancy. Basic Clin Pharmacol Toxicol 110: 12–18, 2012. doi: 10.1111/j.1742-7843.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 49.Mata KM, Li W, Reslan OM, Siddiqui WT, Opsasnick LA, Khalil RA. Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy. Am J Physiol Heart Circ Physiol 309: H1679–H1696, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matias ML, Romão M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araújo JP Jr, Peraçoli JC, de Oliveira L, Peraçoli MT. Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia. PLoS One 10: e0129095, 2015. doi: 10.1371/journal.pone.0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca2+]i in mesenteric microvessels of pregnant rats. Br J Pharmacol 169: 1335–1351, 2013. doi: 10.1111/bph.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension 64: 632–643, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG 111: 931–939, 2004. doi: 10.1111/j.1471-0528.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 55.Mishra B, Kizaki K, Koshi K, Ushizawa K, Takahashi T, Hosoe M, Sato T, Ito A, Hashizume K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its related extracellular matrix degrading enzymes in the endometrium during estrous cycle and early gestation in cattle. Reprod Biol Endocrinol 8: 60, 2010. doi: 10.1186/1477-7827-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, Guidi GC. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal 23: 88–92, 2009. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno-Eutimio MA, Tovar-Rodríguez JM, Vargas-Avila K, Nieto-Velázquez NG, Frías-De-León MG, Sierra-Martinez M, Acosta-Altamirano G. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. BioMed Res Int 2014: 413249, 2014. doi: 10.1155/2014/413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573, 2006. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Nascimento RA, Mendes G, Possomato-Vieira JS, Gonçalves-Rizzi VH, Kushima H, Delella FK, Dias-Junior CA. Metalloproteinase inhibition protects against reductions in circulating adrenomedullin during lead-induced acute hypertension. Basic Clin Pharmacol Toxicol 116: 508–515, 2015. doi: 10.1111/bcpt.12337. [DOI] [PubMed] [Google Scholar]
- 60.Nugent WH, Mishra N, Strauss JF III, Walsh SW. Matrix metalloproteinase 1 causes vasoconstriction and enhances vessel reactivity to angiotensin ii via protease-activated receptor 1. Reprod Sci 23: 542–548, 2016. doi: 10.1177/1933719115607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson MW, Toth M, Gervasi DC, Sado Y, Ninomiya Y, Fridman R. High affinity binding of latent matrix metalloproteinase-9 to the α2(IV) chain of collagen IV. J Biol Chem 273: 10672–10681, 1998. doi: 10.1074/jbc.273.17.10672. [DOI] [PubMed] [Google Scholar]
- 62.Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension 43: 434–444, 2004. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- 63.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 30: 317–329, 2012. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets 14: 325–334, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 208: 224–233, 2013. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pascual G, Rodríguez M, Gómez-Gil V, Trejo C, Buján J, Bellón JM. Active matrix metalloproteinase-2 upregulation in the abdominal skin of patients with direct inguinal hernia. Eur J Clin Invest 40: 1113–1121, 2010. doi: 10.1111/j.1365-2362.2010.02364.x. [DOI] [PubMed] [Google Scholar]
- 67.Peixoto AB, Araujo E Jr, Ribeiro JU, Rodrigues DB, Castro EC, Caldas TM, Rodrigues V Jr. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med 29: 75–79, 2016. [DOI] [PubMed] [Google Scholar]
- 68.Peng T, Lu X, Lei M, Moe GW, Feng Q. Inhibition of p38 MAPK decreases myocardial TNF-alpha expression and improves myocardial function and survival in endotoxemia. Cardiovasc Res 59: 893–900, 2003. doi: 10.1016/S0008-6363(03)00509-1. [DOI] [PubMed] [Google Scholar]
- 69.Pinheiro MB, Gomes KB, Ronda CR, Guimarães GG, Freitas LG, Teixeira-Carvalho A, Martins-Filho OA, Dusse LM. Severe preeclampsia: association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine 71: 232–237, 2015. doi: 10.1016/j.cyto.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res 159: 755–764, 2010. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg 45: 373–380, 2007. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1 (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26: 563–573, 2005. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Ramma W, Buhimschi IA, Zhao G, Dulay AT, Nayeri UA, Buhimschi CS, Ahmed A. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis 15: 333–340, 2012. doi: 10.1007/s10456-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension 50: 137–142, 2007. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 76.Reister F, Kingdom JC, Ruck P, Marzusch K, Heyl W, Pauer U, Kaufmann P, Rath W, Huppertz B. Altered protease expression by periarterial trophoblast cells in severe early-onset preeclampsia with IUGR. J Perinat Med 34: 272–279, 2006. doi: 10.1515/JPM.2006.052. [DOI] [PubMed] [Google Scholar]
- 77.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem 8: 204–226, 2010. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E. Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes, and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci USA 92: 5808–5812, 1995. doi: 10.1073/pnas.92.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 46: 1243–1249, 2005. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 80.Roy SC, Ghosh J. Dynamic in vivo changes in the activities of gelatinases, matrix metalloproteinases (MMPs), and tissue inhibitor of metalloproteinases (TIMPs) in buffalo (Bubalus bubalis) uterine luminal fluid during estrous cycle and early pregnancy. Mol Reprod Dev 77: 944–953, 2010. doi: 10.1002/mrd.21240. [DOI] [PubMed] [Google Scholar]
- 81.Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol 5: 372, 2014. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo KW, Lee SJ, Kim YH, Bae JU, Park SY, Bae SS, Kim CD. Mechanical stretch increases MMP-2 production in vascular smooth muscle cells via activation of PDGFR-β/Akt signaling pathway. PLoS One 8: e70437, 2013. doi: 10.1371/journal.pone.0070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol 95: 211–226, 2015. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 38: 73–83, 2005. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 85.Stennett AK, Qiao X, Falone AE, Koledova VV, Khalil RA. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol Heart Circ Physiol 296: H745–H755, 2009. doi: 10.1152/ajpheart.00861.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56: 192–199, 2011. doi: 10.1016/j.cyto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 88: 5555–5563, 2003. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 88.Ulbrich SE, Meyer SU, Zitta K, Hiendleder S, Sinowatz F, Bauersachs S, Büttner M, Fröhlich T, Arnold GJ, Reichenbach HD, Wolf E, Meyer HH. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol Cell Endocrinol 332: 48–57, 2011. doi: 10.1016/j.mce.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valdés G, Corthorn J. Review: The angiogenic and vasodilatory utero-placental network. Placenta 32, Suppl 2: S170–S175, 2011. doi: 10.1016/j.placenta.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 92.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 63: 595–606, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolf M, Shah A, Lam C, Martinez A, Smirnakis KV, Epstein FH, Taylor RN, Ecker JL, Karumanchi SA, Thadhani R. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol 193: 16–22, 2005. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 95.Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab 303: E55–E70, 2012. doi: 10.1152/ajpendo.00553.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 73: 205–209, 1993. doi: 10.1161/01.RES.73.1.205. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y, Wang L, Liu T, Guan H. MicroRNA-204 suppresses trophoblast-like cell invasion by targeting matrix metalloproteinase-9. Biochem Biophys Res Commun 463: 285–291, 2015. doi: 10.1016/j.bbrc.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 98.Zárate A, Saucedo R, Valencia J, Manuel L, Hernández M. Early disturbed placental ischemia and hypoxia creates immune alteration and vascular disorder causing preeclampsia. Arch Med Res 45: 519–524, 2014. doi: 10.1016/j.arcmed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Qi C, Lin J. Enhanced expressions of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factors (VEGF) and increased microvascular density in the endometrial hyperplasia of women with anovulatory dysfunctional uterine bleeding. Fertil Steril 93: 2362–2367, 2010. doi: 10.1016/j.fertnstert.2008.12.142. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J, Xiao D, Hu Y, Wang Z, Paradis A, Mata-Greenwood E, Zhang L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 62: 599–607, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu J, Zhong M, Pang Z, Yu Y. Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum Dev 90: 657–664, 2014. doi: 10.1016/j.earlhumdev.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol 311: R505−R521, 2016. doi: 10.1152/ajpregu.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]