Our findings demonstrate that folic acid ingestion improves blood flow via enhanced vascular conductance in the exercising skeletal muscle of aged humans. These findings provide evidence for the therapeutic use of folic acid to improve skeletal muscle blood flow, and perhaps exercise and functional capacity, in human primary aging.
Keywords: aging, exercise hyperemia, vascular conductance
Abstract
Skeletal muscle blood flow is attenuated in aged humans performing dynamic exercise, which is due, in part, to impaired local vasodilatory mechanisms. Recent evidence suggests that folic acid improves cutaneous vasodilation during localized and whole body heating through nitric oxide-dependent mechanisms. However, it is unclear whether folic acid improves vasodilation in other vascular beds during conditions of increased metabolism (i.e., exercise). The purpose of this study was to test the hypothesis that folic acid ingestion improves skeletal muscle blood flow in aged adults performing graded handgrip and plantar flexion exercise via increased vascular conductance. Nine healthy, aged adults (two men and seven women; age: 68 ± 5 yr) performed graded handgrip and plantar flexion exercise before (control), 2 h after (acute, 5 mg), and after 6 wk (chronic, 5 mg/day) folic acid ingestion. Forearm (brachial artery) and leg (superficial femoral artery) blood velocity and diameter were measured via Duplex ultrasonography and used to calculate blood flow. Acute and chronic folic acid ingestion increased serum folate (both P < 0.05 vs. control). During handgrip exercise, acute and chronic folic acid ingestion increased forearm blood flow (both conditions P < 0.05 vs. control) and vascular conductance (both P < 0.05 vs. control). During plantar flexion exercise, acute and chronic folic acid ingestion increased leg blood flow (both P < 0.05 vs. control), but only acute folic acid ingestion increased vascular conductance (P < 0.05 vs. control). Taken together, folic acid ingestion increases blood flow to active skeletal muscle primarily via improved local vasodilation in aged adults.
NEW & NOTEWORTHY Our findings demonstrate that folic acid ingestion improves blood flow via enhanced vascular conductance in the exercising skeletal muscle of aged humans. These findings provide evidence for the therapeutic use of folic acid to improve skeletal muscle blood flow, and perhaps exercise and functional capacity, in human primary aging.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/folic-acid-and-exercise-hyperemia-in-aging/.
the arterial vasculature undergoes profound changes across the human lifespan. This “vascular aging” includes structural remodeling, in addition to alterations in local vascular control mechanisms (21, 34, 50, 71, 72). Ultimately, these vascular changes contribute to malperfusion of active skeletal muscle (e.g., limited exercise hyperemia), which reduces functional and exercise capacity, independent of changes in cardiac function (21). Importantly, attenuated functional and exercise capacities directly increase the risk for cardiovascular morbidity and mortality (1, 4, 5, 17, 26, 33).
Augmented sympathetic vasoconstriction (i.e., impaired functional sympatholysis) and an altered release of endothelium-derived local vasodilator and vasoconstrictor substances are the primary factors contributing to malperfusion of skeletal muscle associated with human primary aging (21, 29). These changes not only influence regional or bulk blood flow (31, 43, 46) but can also impair the distribution of oxygenated blood within exercising skeletal muscle (39). Reduced availability of endothelium-derived nitric oxide is one mechanism by which exercise hyperemia is attenuated with primary aging. In a seminal study, Schrage et al. (58) reported that the contribution of nitric oxide to exercise hyperemia is reduced by ~45% with primary aging, an effect that likely occurs secondary to increased vascular oxidative stress, inflammation, and the reduced availability of essential substrates and cofactors necessary for optimal endothelial nitric oxide synthase activity (12, 31, 67). Interestingly, synthetic analogs of tetrahydrobiopterin (BH4), a critical cofactor necessary for optimal endothelial nitric oxide synthase activity, improve nitric oxide-mediated vasodilation in the microcirculation of middle-aged and aged adults (23, 60, 62). However, the extraordinary expense and limited availability of synthetic BH4 analogs available for human use precludes its mainstream therapeutic use. Recent evidence suggests that nitric oxide-dependent vasodilation in the cutaneous microvasculature of aged adults is improved through local administration (via microdialysis) and chronic ingestion of folic acid (61), an essential vitamin that may improve the vasodilatory response through a direct interaction with endothelial nitric oxide synthase and/or an indirect restoration of BH4 availability (28, 65). Additionally, chronic folic acid ingestion improved macrovascular dilator function (assessed via flow-mediated dilation) in young female athletes with vascular dysfunction (24, 25). Folic acid supplementation could, therefore, represent an alternative therapy to improve nitric oxide-dependent vasodilation and increase skeletal muscle blood flow in aged adults. However, it remains unclear whether the vascular benefits of folic acid extend to noncutaneous vascular beds during conditions of elevated metabolism, such as exercise.
Therefore, the purpose of this study was to test the hypothesis that acute and chronic folic acid ingestion improves skeletal muscle blood flow in aged adults performing graded handgrip and plantar flexion exercise via heightened vasodilation (i.e., improved vascular conductance in active skeletal muscle). Furthermore, we hypothesized that the exercise-induced dilation of the brachial artery, an indirect measure of nitric oxide availability, would be increased with acute and chronic folic acid ingestion. The dilator response during plantar flexion exercise was not assessed given the negligible conduit vessel dilation observed during isolated leg exercise (52, 71). To determine the systemic vasodilatory effects of folic acid, we used isolated arm and leg exercise models given the known heterogeneities in vascular control and function between limbs (40, 42, 45, 48, 49, 54).
METHODS
Subjects
Subject physical characteristics and fasting blood data are shown in Table 1. Written informed consent was obtained from all subjects subsequent to a verbal and written briefing of all experimental procedures. This study and consent were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas and was performed in accordance with the principles outlined in the Declaration of Helsinki. Nine recreationally active adults (two men and seven women), aged 60–80 yr, participated in this study. All subjects were free from known cardiometabolic disease and were deemed healthy following the completion of an indepth medical history questionnaire and a resting 12-lead electrocardiogram. Subjects were required to abstain from caffeine, supplements (i.e., multivitamin and vitamin D), alcohol, and exercise for 24 h before the study. Subjects were required to abstain from over-the-counter (e.g., NSAIDs and antihistamines) or prescription medications (i.e., levothyroxine and pravastatin) at the time of the study. Additionally, subjects reported to the laboratory after an overnight fast. All aged women were postmenopausal (18 ± 4 yr from cessation of menstruation) and not currently on hormone replacement therapy.
Table 1.
Subject characteristics
| Age, yr | 68 ± 5 |
| Height, cm | 165 ± 5 |
| Weight, kg | 70 ± 8 |
| Body mass index, kg/m | 26 ± 3 |
| Heart rate, beats/min | 66 ± 7 |
| Mean arterial pressure, mmHg | 84 ± 6 |
| MVC, kg | 22 ± 7 |
| Cholesterol, mg/dl | 186 ± 29 |
| Triglycerides, mg/dl | 89 ± 36 |
| HDL, mg/dl | 57 ± 15 |
| LDL, mg/dl | 110 ± 27 |
| Glucose, mg/dl | 82 ± 4 |
Values are expressed as means ± SD. MVC, maximal voluntary handgrip contraction; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Experimental Approach
Subjects reported to the laboratory at 9:00 AM. Subsequent to obtaining a fasting blood sample, subjects consumed a standardized breakfast (oatmeal raisin walnut Clif Bar and a Boost nutritional beverage containing 90 µg folic acid). Dietary time control trials indicate that this breakfast does not increase serum folate measured 1 h after consumption. Maximal voluntary handgrip contraction was then determined as the average of three maximal contractions using a handgrip dynamometer (Stoelting, Chicago, IL). After instrumentation and a ~25-min rest period, subjects performed three stages of graded handgrip exercise. Subjects then rested for ~20 min before performing three stages of graded plantar flexion exercise. After a brief recovery period, subjects ingested 5 mg folic acid (Bio-Tech Pharmacal, Fayetteville, AR) and then rested quietly for 2 h before repeating handgrip and plantar flexion exercise protocols (acute folic acid ingestion trial). A second sample of venous blood was obtained immediately before the second bout of handgrip and plantar flexion exercise for the determination of serum folate. Folic acid is absorbed largely in the proximal portion of the small intestine and has a time to peak concentration of 1–2 h and a half-life of 2–5 h (7, 41, 64, 69). A hemodynamic time-control trial was not performed, as Richards et al. (53) previously demonstrated, using a nearly identical experimental approach, that central and peripheral exercising hemodynamics are similar across this time frame in aged adults. Room temperature was maintained at ~23°C.
Subsequent to the completion of the acute folic acid ingestion trial, subjects ingested 5 mg folic acid daily for the next 6 wk (chronic folic acid ingestion trial). After this period, subjects returned to the laboratory and completed the same handgrip and plantar flexion exercise protocols. A final sample of venous blood was obtained before the consumption of the standardized breakfast and before exercise for the determination of serum folate. The final dose of folic acid was taken at least 12 h before blood sampling and completion of the final exercise trial. The dose and duration of folic acid ingestion were chosen on the basis of prior reports suggesting that this pharmacological approach increases circulating folate and improves endothelial function in primary aging, hyperhomocysteinemia, and coronary artery disease (2, 14, 15, 61).
Exercise Protocols and Measurements
Handgrip and plantar flexion exercise.
Graded (3, 6, and 9 kg) intermittent isometric handgrip exercise was performed using a handgrip dynamometer, and graded (5, 9, and 14 kg) dynamic plantar flexion exercise was performed using a custom-built ergometer. Exercise stages were 4 min, with 1-min rest separating each stage to prevent fatigue, although this was not confirmed experimentally. Handgrip and plantar flexion exercise was performed using a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min). Subjects were provided with real-time visual (handgrip only) and audio feedback to ensure correct contraction timing.
Central hemodynamics.
Arterial blood pressure was measured from the left arm using an automated sphygmomanometer (Tango+, SunTech Medical, Raleigh, NC). Beat-by-beat arterial blood pressure was measured noninvasively by finger photoplethysmography, and stroke volume was estimated via Modelflow (Nexfin, Edwards Life Sciences, Irvine, CA). Heart rate was monitored via electrocardiogram (Solar 8000i, GE Healthcare, Milwaukee, WI) interfaced with a cardiotachometer (CWE, Ardmore, PA).
Blood flow.
Duplex ultrasonography was used to measure simultaneously blood velocity and diameter in the brachial and superficial femoral arteries. Velocity and diameter measurements were made proximal to the brachial artery bifurcation and distal to the common femoral artery bifurcation. All velocity measurements were made using a linear-array transducer (11 MHz, Phillips iE33, Andover, MA) operating with an insonation angle of 60° and a Doppler sample volume that encompassed the entire vessel lumen. The ultrasound system was interfaced with a computer running custom audio-recording software (DUC2) to capture blood velocity (8, 16, 36, 37, 55, 56). Throughout each exercise trial, the ultrasound transducer was manually held in place to ensure consistent measurements of velocity and diameter. All ultrasound measurements were performed by the same sonographer (S. A. Romero). Additionally, an outline of the ultrasound transducer was made on the skin to ensure consistent placement across time (acute folic acid ingestion trial only).
Serum folate.
Venous blood was sampled before exercise for the three conditions of control, acute folic acid ingestion, and chronic folic acid ingestion. Venous blood was collected into a Vacutainer and centrifuged within 10 min at 1,950 g. Serum was kept at 4°C until analysis, which was performed within 24 h. Serum folate was quantified using a chemiluminescent microparticle folate binding protein assay (ARCHITECT folate assay, Abbott Laboratories, Abbott Park, IL).
Data and Statistical Analyses
Central hemodynamics were collected at 50 Hz and averaged across the final minute of each stage of exercise using commercially available software (Biopac MP150, Santa Barbara, CA). Blood velocities were determined from the Doppler ultrasound audio recordings using a custom intensity-weighted algorithm (DUC2) subsequent to demodulation of forward and reverse Doppler frequencies (9, 56). Brachial artery diameter was determined using custom-edge detection and wall-tracking software (3, 70). Leg movement associated with plantar flexion exercise precluded the use of wall-tracking software for the determination of vessel diameter. Therefore, ultrasound calipers were used to determine manually superficial femoral artery diameter. All (arm and leg) blood velocities and brachial artery diameter were averaged across the final minute of each stage of exercise. Superficial femoral artery end-diastolic diameter was measured in triplicate across several (3–5) cardiac cycles in the final minute of each stage of exercise. Blood flow was calculated as the cross-sectional area of the imaged artery multiplied by mean blood velocity and reported in milliliters per minute. Vascular conductance was calculated by dividing blood flow by mean arterial pressure and then multiplying by 100 and expressed as milliliters per minute per 100 mmHg. Shear rate was calculated by multiplying 8 by the quotient of blood velocity and vessel diameter and expressed as s−1. Recent evidence suggests that exercise-induced dilation of the brachial artery is highly dependent on nitric oxide (68, 73). Therefore, we examined exercise-induced brachial artery dilation during handgrip exercise as an indirect measure of nitric oxide availability. This response was not examined given the negligible conduit vessel dilation observed during isolated leg exercise (52, 71).
Our primary outcome variables were analyzed using a mixed-model ANOVA with repeated measures (JMP Pro 12, SAS Institute, Cary, NC). Planned comparisons were used to examine specific condition-exercise stage interactions. General interactions were examined using Tukey’s post hoc procedure. For the relative assessment of exercise-induced brachial artery dilation, shear rate was entered into the model as a covariate to account for this dilatory stimulus. Significance was set at P ≤ 0.05. Data are reported as means ± SE unless stated otherwise (e.g., SD in Table 1).
RESULTS
Serum Folate
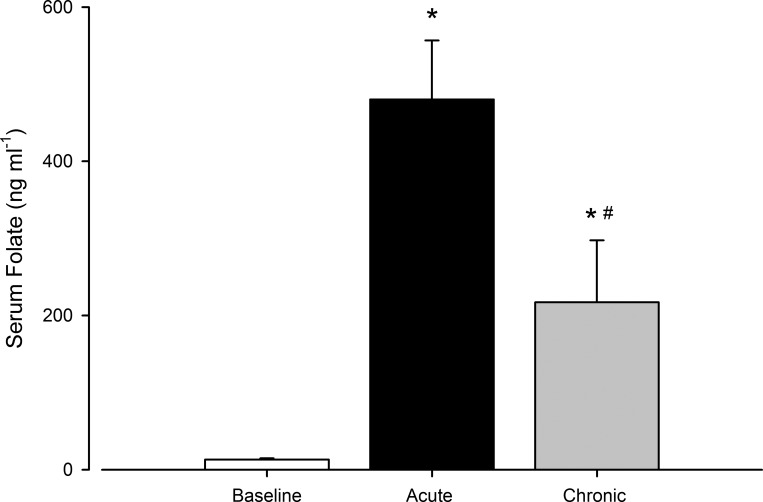
Serum folate is shown in Fig. 1. Compared with control, serum folate increased with acute and chronic folic acid ingestion (both P < 0.05). Relative to acute ingestion, serum folate was lower after chronic ingestion (P < 0.05).
Fig. 1.
Serum folate concentrations at baseline and following acute and chronic folic acid ingestion. Open bar, baseline control; black bar, acute folic acid ingestion; gray bar, chronic folic acid ingestion. *P < 0.05 vs. baseline; #P < 0.05 vs. acute folic acid ingestion.
Resting Hemodynamics
Resting hemodynamics are shown in Table 2. Heart rate was greater for chronic folic acid ingestion compared with acute folic acid ingestion (P = 0.05). Cardiac output tended to be greater for control (P = 0.08) and chronic folic acid ingestion (P = 0.06) compared with acute folic acid ingestion. Leg blood flow tended to be greater with chronic folic acid ingestion compared with acute folic acid ingestion (P = 0.08). Likewise, leg vascular conductance did not differ between control and acute folic acid ingestion (P = 0.1) but tended to be greater with chronic folic acid ingestion (P = 0.08 vs. acute folic acid ingestion).
Table 2.
Resting hemodynamics
| Control | Acute Folic Acid Ingestion |
Chronic Folic Acid Ingestion |
|
|---|---|---|---|
| Heart rate, beats/min | 66 ± 2 | 62 ± 2 | 68 ± 3* |
| Mean arterial pressure, mmHg | 84 ± 2 | 89 ± 3 | 85 ± 3 |
| Cardiac output, l/min | 5.1 ± 0.3 | 4.4 ± 0.3 | 5.2 ± 0.4 |
| Stroke volume, ml | 75 ± 3 | 69 ± 3 | 78 ± 5 |
| Brachial artery diameter, cm | 0.36 ± 0.01 | 0.35 ± 0.01 | 0.36 ± 0.01 |
| Forearm blood flow, ml/min | 44 ± 5 | 43 ± 2 | 46 ± 7 |
| Forearm vascular conductance, ml·min−1·100 mmHg−1 | 53 ± 6 | 49 ± 3 | 53 ± 7 |
| Superficial femoral artery Diameter, cm | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.62 ± 0.02 |
| Leg blood flow, ml/min | 125 ± 9 | 113 ± 11 | 131 ± 12 |
| Leg vascular conductance, ml·min−1·100 mmHg−1 | 147 ± 13 | 126 ± 12 | 149 ± 15 |
Values are expressed as means ± SE.
P = 0.05 vs. acute folic acid ingestion.
Exercising Hemodynamics
Handgrip exercise.
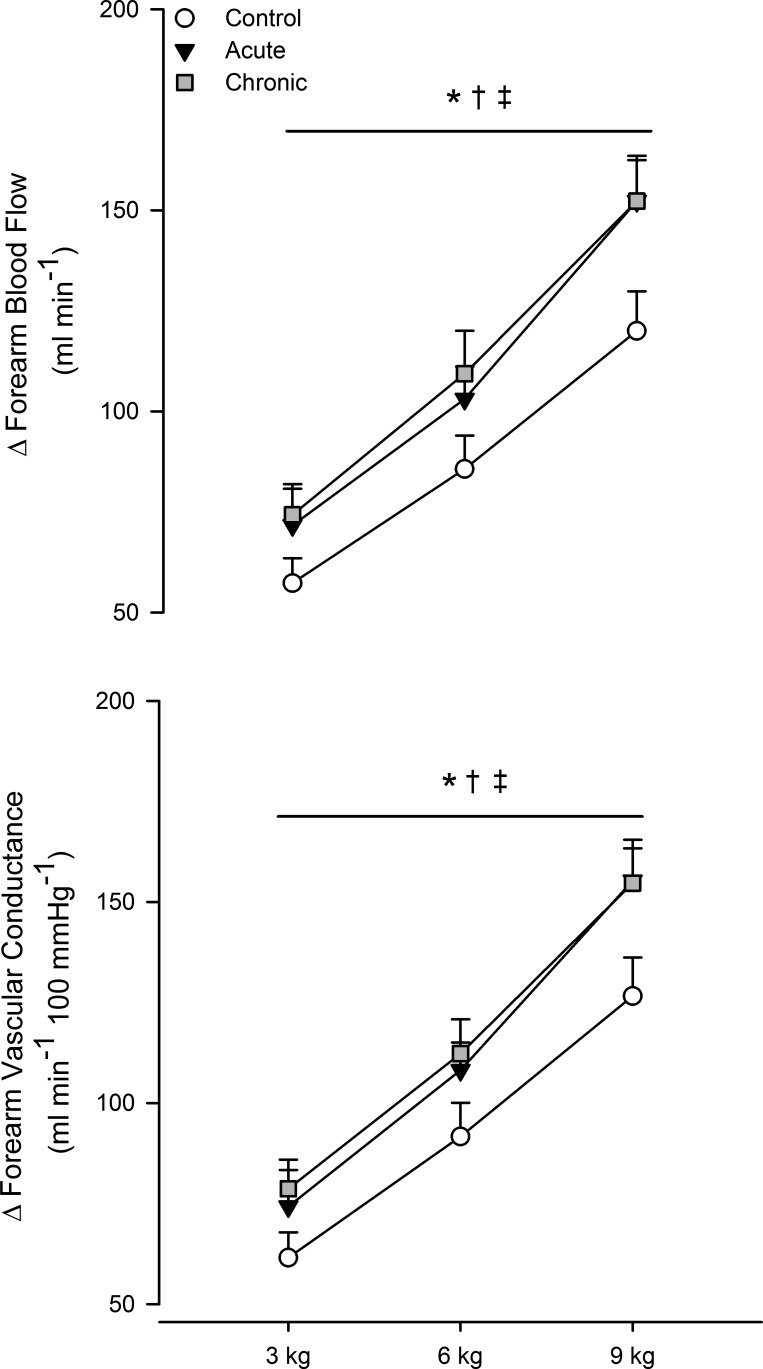
Central hemodynamic responses to hand grip exercise are shown in Table 3, and forearm hemodynamics are shown in Figs. 2 and 3. As expected, forearm blood flow (main effect of exercise, P < 0.05) and vascular conductance (main effect of exercise, P < 0.05) progressively increased during exercise for all three conditions. The increase in forearm blood flow was greater during exercise with acute and chronic folic acid ingestion (both P < 0.05 vs. control). Likewise, the increase in forearm vascular conductance was greater during exercise with acute and chronic folic acid ingestion (both P < 0.05 vs. control). Compared with the control condition (3 kg: 102 ± 10 ml/min, 6 kg: 130 ± 13 ml/min, and 9 kg: 164 ± 14 ml/min), absolute blood flow was greater during handgrip exercise with acute (3 kg: 115 ± 9 ml/min, 6 kg: 146 ± 9 ml/min, and 9 kg: 195 ± 12 ml/min, P < 0.05 vs. control) and chronic (3 kg: 120 ± 11 ml/min, 6 kg: 156 ± 12 ml/min, and 9 kg: 198 ± 12 ml/min, P < 0.05 vs. control) folic acid ingestion.
Table 3.
Central hemodynamics during handgrip exercise
| Control |
Acute Folic Acid Ingestion |
Chronic Folic Acid Ingestion |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 kg | 6 kg | 9 kg | 3 kg | 6 kg | 9 kg | 3 kg | 6 kg | 9 kg | |
| ΔHeart rate, beats/min | 3 ± 1 | 5 ± 1 | 6 ± 1 | 3 ± 1 | 5 ± 1 | 6 ± 1 | 2 ± 1 | 3 ± 2 | 4 ± 1 |
| ΔMean arterial pressure, mmHg | 4 ± 1 | 5 ± 1 | 7 ± 1* | 3 ± 1 | 4 ± 1 | 6 ± 1* | 4 ± 2 | 7 ± 2 | 9 ± 3* |
| ΔCardiac output, l/min | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.2 |
| ΔStroke volume, ml | 1 ± 1† | −1 ± 2† | 0 ± 1† | 2 ± 1 | 5 ± 2 | 2 ± 1 | 1 ± 1† | 0 ± 2† | −1 ± 2† |
Values are expressed as means ± SE.
Main effect of exercise intensity, P < 0.05 vs. 3 kg;
main effect of condition, P ≤ 0.05 vs. acute folic acid ingestion.
Fig. 2.
Change from baseline in forearm blood flow (top) and change in vascular conductance (bottom) during graded handgrip exercise. Open circles, control; black circles, acute folic acid ingestion; gray circles, chronic folic acid ingestion. *P < 0.05 for main effect of acute folic acid ingestion vs. control; †P < 0.05 for main effect of chronic folic acid ingestion vs. control; ‡P < 0.05 for main effect of exercise intensity.
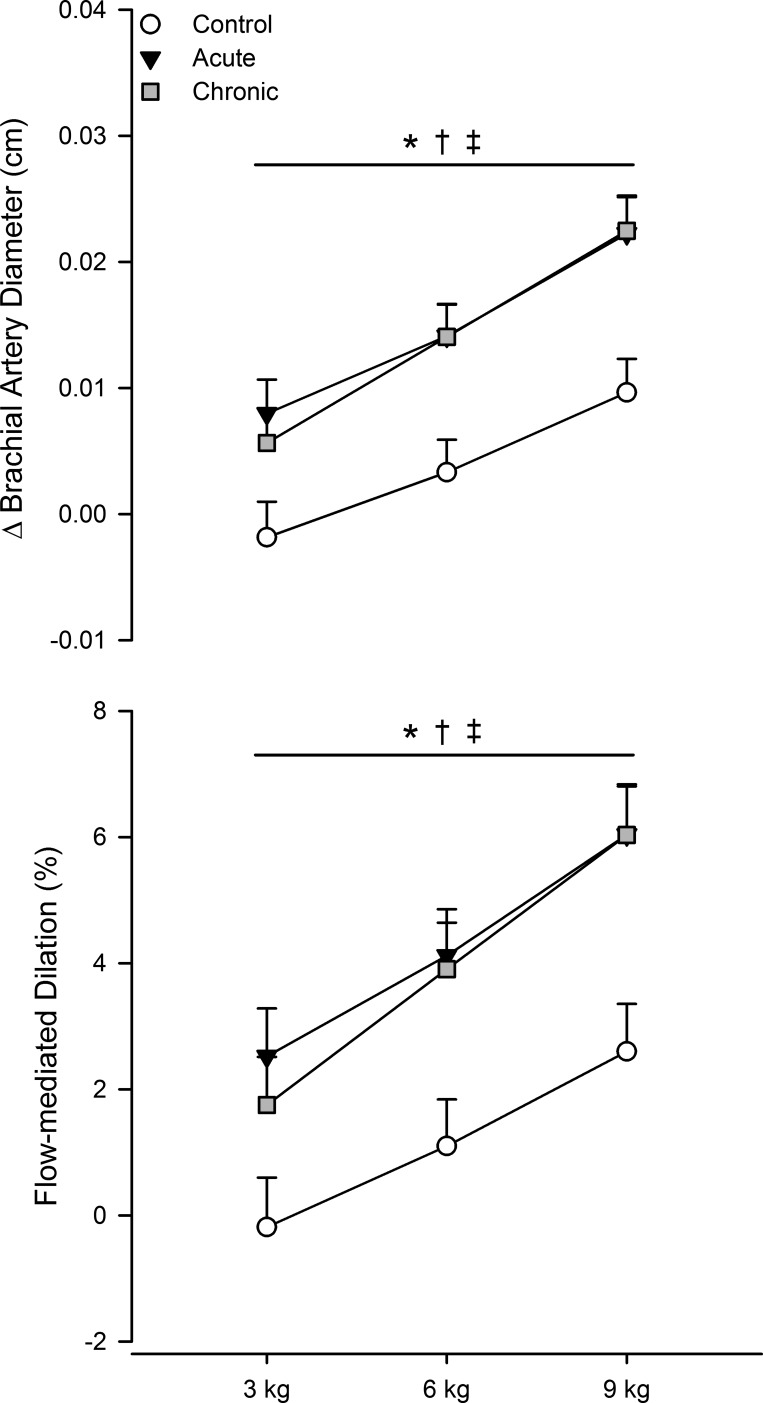
Fig. 3.
Absolute change from baseline in exercise-induced brachial artery diameter (top) and relative flow-mediated dilatory response after correcting for shear stimulus via analysis of covariance (bottom). Open circles, control; black circles, acute folic acid ingestion; gray circles, chronic folic acid ingestion. *P < 0.05 for main effect of acute folic acid ingestion vs. control; †P < 0.05 for main effect of chronic folic acid ingestion vs. control; ‡P < 0.05 for main effect of exercise intensity.
Shear rate increased progressively throughout exercise for all conditions (main effect of exercise, P < 0.05), which induced a progressive dilation of the brachial artery (main effect of exercise, P < 0.05). Compared with control (317 ± 26 s−1), the average increase in shear rate during exercise was greater with acute (359 ± 30 s−1) and chronic folic acid ingestion (355 ± 27 s−1, both P < 0.05 vs. control). The increase in brachial artery diameter during exercise was greater with acute and chronic folic acid ingestion (both P < 0.05 vs. control; Fig. 3). When expressed as a relative change (i.e., flow-mediated dilation) and after adjusting (via analysis of covariance) for shear rate stimulus, the brachial artery dilatory response was increased with acute and chronic folic acid ingestion (both P < 0.05 vs. control; Fig. 3).
Plantar flexion exercise.
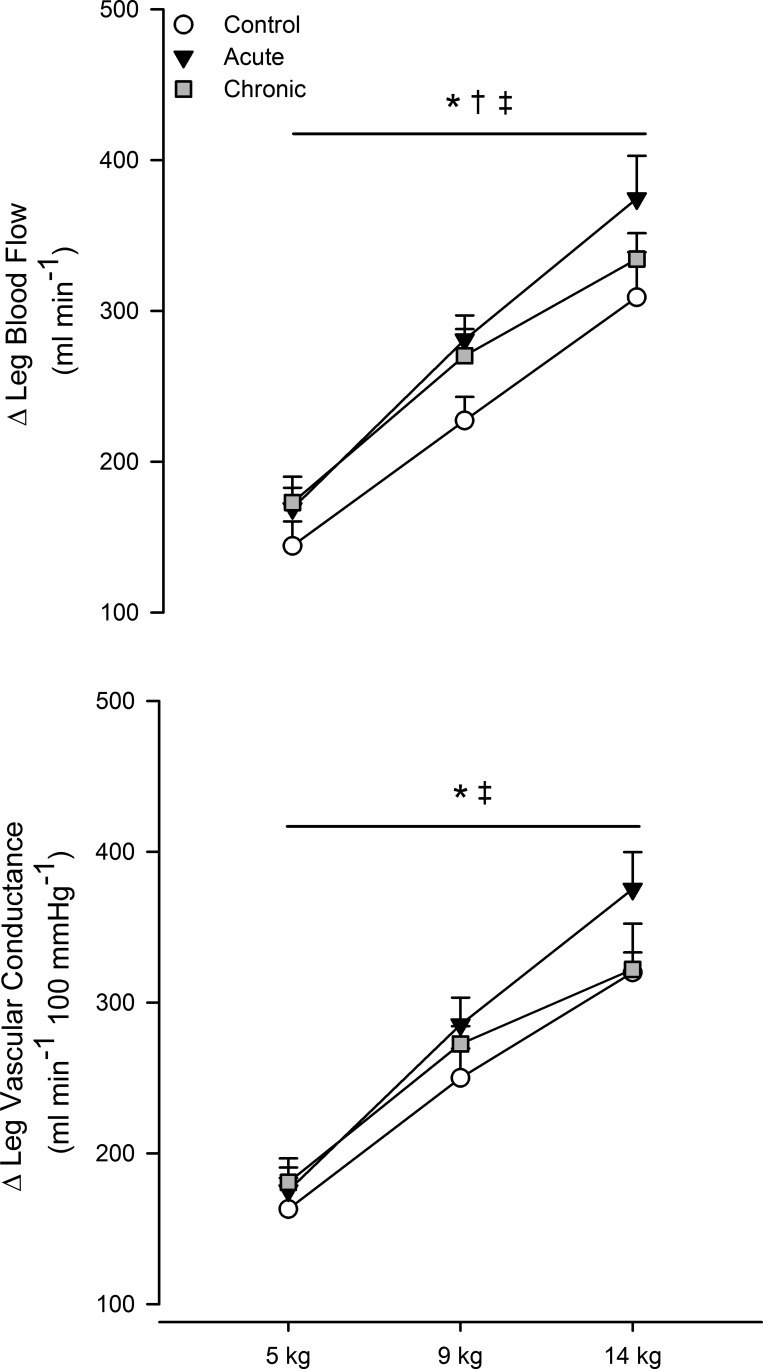
Central hemodynamic responses to plantar flexion exercise are shown in Table 4, and leg hemodynamics are shown in Fig. 4. Leg blood flow and vascular conductance increased with workload (main effect of exercise, P < 0.05). The magnitude of the increase in leg blood flow (P < 0.05 vs. control) and vascular conductance (P < 0.05 vs. control) was greater during exercise with acute folic acid ingestion. A greater increase in leg blood flow during exercise persisted after chronic folic acid ingestion (P < 0.05 vs. control). However, in contrast to acute folic acid ingestion, this response was not mediated by improved vascular conductance (P = 0.3). Compared with the control condition (5 kg: 262 ± 22 ml/min, 9 kg: 352 ± 22 ml/min, and 14 kg: 434 ± 33 ml/min), absolute blood flow was greater during plantar flexion exercise with acute (5 kg: 282 ± 20 ml/min, 9 kg: 394 ± 19 ml/min, and 14 kg: 488 ± 33 ml/min, P < 0.05 vs. control) and chronic (5 kg: 304 ± 24 ml/min, 9 kg: 401 ± 25 ml/min, and 14 kg: 466 ± 12 ml/min, P < 0.05 vs. control) folic acid ingestion.
Table 4.
Central hemodynamics during plantar flexion exercise
| Control |
Acute Folic Acid Ingestion |
Chronic Folic Acid Ingestion |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 kg | 9 kg | 14 kg | 5 kg | 9 kg | 14 kg | 5 kg | 9 kg | 14 kg | |
| ΔHeart rate, beats/min | 3 ± 1 | 5 ± 1* | 9 ± 1† | 4 ± 1 | 6 ± 1* | 9 ± 1† | 4 ± 1‡ | 7 ± 1*‡ | 10 ± 1†‡ |
| ΔMean arterial pressure, mmHg | 2 ± 1 | 3 ± 1 | 7 ± 1*† | 3 ± 1 | 5 ± 1 | 6 ± 1*† | 3 ± 1‡ | 6 ± 1‡ | 9 ± 1*†‡ |
| ΔCardiac output, l/min | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1* | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.2* | 0.2 ± 0.1§ | 0.3 ± 0.1§ | 0.4 ± 0.1*§ |
| ΔStroke volume, ml | 2 ± 1 | 1 ± 1 | 0 ± 1 | 1 ± 1 | 3 ± 2 | 2 ± 2 | −1 ± 1‡§ | −1 ± 1‡§ | −4 ± 1‡§ |
Values are expressed as means ± SE.
Main effect of exercise intensity, P < 0.05 vs. 5 kg;
main effect of exercise intensity, P < 0.05 vs. 9 kg;
main effect of condition, P ≤ 0.05 vs. control;
main effect of condition, P ≤ 0.05 vs. acute folic acid ingestion.
Fig. 4.
Change from baseline in leg blood flow (top) and change in vascular conductance (bottom) during graded plantar flexion exercise. Open circles, control; black circles, acute folic acid ingestion; gray circles, chronic folic acid ingestion. *P < 0.05 for main effect of acute folic acid ingestion vs. control; †P < 0.05 for main effect of chronic folic acid ingestion vs. control; ‡P < 0.05 for main effect of exercise intensity.
DISCUSSION
The purpose of this study was to test the hypothesis that acute and chronic folic acid ingestion improves skeletal muscle blood flow in aged adults performing graded handgrip and plantar flexion exercise. In agreement with our hypothesis, we found that acute and chronic folic acid ingestion improved forearm blood flow during graded handgrip exercise, a response mediated by improved vasodilation within exercising skeletal muscle (i.e., augmented vascular conductance). The mechanism responsible for this improvement appears to be improved nitric oxide availability, given that exercise-induced dilation of the brachial artery was similarly improved with folic acid ingestion. We also found that acute and chronic folic acid ingestion improved leg blood flow during graded plantar flexion exercise. Elevated leg blood flow with acute folic acid ingestion was mediated by improved vascular conductance, whereas an increase in perfusion pressure was responsible for the improvement associated with chronic folic acid ingestion, given an absence of an effect of chronic folic acid on vascular conductance.
Mechanism/s of Improved Skeletal Muscle Blood Flow
Prior work has demonstrated that exercise-induced dilation of the brachial artery is nitric oxide dependent and is attenuated with primary aging (68, 73). We found that folic acid induces an upward shift of this response (absolute and relative change; Fig. 3) throughout handgrip exercise, which suggests that folic acid increased nitric oxide availability. Along these lines, we speculate that folic acid functions as a proxy for naturally occurring BH4, thereby improving nitric oxide availability. Folic acid or more specifically its active metabolite, 5-methyltetrahydrofolate (5-MTHF), can directly bind (at the BH4 docking site) to endothelial nitric oxide synthase in a manner analogous to that of naturally occurring BH4 (28). Thus, with folic acid ingestion, endothelial nitric oxide synthase transitions from its monomeric uncoupled form, which produces superoxide, to its dimeric coupled form, which catalyzes the formation of nitric oxide from l-arginine and molecular oxygen (18, 74, 75).
Several indirect pathways exist by which folic acid could improve nitric oxide availability (35, 63). First, 5-MTHF can augment the synthesis of BH4 from its oxidized form [dihydrobiopterin (BH2)] by upregulating activity of the enzyme dihydrofolate reductase (10, 11). Second, 5-MTHF-mediated stabilization of BH4 and/or improved efficacy of BH4 to prevent nitric oxide synthase uncoupling could improve nitric oxide availability (38, 65). Third, 5-MTHF scavenging of superoxide could attenuate endothelial nitric oxide synthase uncoupling. However, the capacity of 5-MTHF is far below that which is considered efficacious in scavenging superoxide in vivo (65). Interestingly, Trinity et al. (67) recently demonstrated that the intra-arterial infusion of ascorbate improved brachial artery dilation and skeletal muscle blood flow in aged humans performing handgrip exercise. However, they found that direct and indirect measures of oxidative stress were not reduced during exercise despite a high-dose antioxidant infusion, leading them to conclude that their results can be explained, in part, through improved BH4-dependent signaling (22, 27). Finally, previous findings suggest that, in patients with hyperhomocysteinemia and vascular disease, folic acid ingestion can reduce circulating concentrations of the potent vasoconstrictor endothelin-1 (19). Given the recent observations that endothelin-1-mediated vasoconstriction is augmented in aged humans performing exercise, we speculate that it is possible that folic acid ingestion can directly improve vasodilatory signaling in aging by attenuating circulating endothelin-1 or indirectly through an endothelin-1 nitric oxide interaction (6).
Folic Acid Ingestion: Do Duration and Dose Matter?
The vast majority of investigations examining vascular responses to folic acid ingestion have used experimental paradigms with a chronic intervention. However, our results and others (61, 66) suggest that acute folic acid administration is efficacious, provided that the administration route is direct (e.g., intradermal) and/or the dose is high. With respect to the latter, the dose used in our study (5 mg) is well above the daily intake of 350 µg/day recommended for healthy adults (59). However, it should be noted that folic acid ingestion, even in high doses, such as that used in our study, is considered safe and poses no toxicity risk (38). It is unclear at present whether a lower dose of folic acid or whether increased consumption of food-derived folates would be equally advantageous.
Arm Versus Leg Exercise
We found that acute and chronic folic acid ingestion improved forearm and leg blood flow during graded exercise. However, the improvement in leg blood flow observed with chronic folic acid ingestion was not mediated by improved vasodilation. It is unclear at present why this response differs from the arm and why it differs (within the leg) from acute folic acid ingestion. This discrepancy may be related to the amount of circulating folate and the threshold concentration necessary to improve vasodilatory signaling within a given limb. Relative to baseline, serum folate increased with chronic ingestion but was approximately half of the concentration attained with acute ingestion. Despite this difference, forearm vascular conductance remained elevated (vs. control) to a similar extent between conditions. Given the known heterogeneities in vascular control and function between limbs (13, 40, 71), it is possible that the threshold concentration necessary to improve vasodilation in the leg is greater than that in the arm.
Experimental Considerations
Several experimental considerations warrant discussion. First, our study did not include a young cohort. However, our intention was not to determine whether folic acid ingestion will “rescue” skeletal muscle blood flow, thereby minimizing age-related differences during exercise, but rather to examine whether folic acid ingestion improves skeletal muscle blood flow in aged adults. Second, we lack a more direct measure of improved nitric oxide availability, such as a venous metabolite draining from the active skeletal muscle or via the pharmacological inhibition of nitric oxide production. Furthermore, our indirect measure of nitric oxide availability was assessed in a large conducting vessel, the results of which may not extend to the resistance vasculature. Third, it is well documented that age-related decrements in exercise hyperemia are more pronounced in women (44, 47, 50, 51). Thus, given the unbalanced n between sexes, it is possible that our observations are driven predominantly by women with little contribution from men. Fourth, it is possible that the small amount of folic acid contained in the standardized breakfast could have influenced our results, particularly those for the control exercise trial. However, dietary time control trials indicate that serum folate does not increase 1 h after consumption of the standardized breakfast. Fully controlling for folic acid intake is further complicated given that we did not track dietary history before the initial visit or throughout 8 wk of daily folic acid ingestion. Although we recognize that we cannot fully account for folic acid intake, on the basis of normal baseline serum folate concentrations measured for the control condition, we can reason that folic acid intake was normal and likely within daily requirements. Fifth, without inclusion of a blinded placebo condition, it is possible that the observed findings, though unlikely, could have been influenced by the participants and not as a direct effect of folic acid ingestion. Finally, it is possible that our findings, regarding chronic folic acid ingestion, could merely be an extension of the acute response, despite evaluating participants at least 12 h after ingestion of the final folic acid tablet. However, our findings of a lack of improved vasodilation in the leg after chronic folic acid ingestion would argue against this possibility. Nevertheless, this does not diminish our conclusions regarding the efficacy of folic acid. Indeed, it could be reasoned that if the primary goal is to improve skeletal muscle blood flow in aged adults performing exercise, then the present data suggest that the timing in which folic acid is ingested is irrelevant and chronic use is unnecessary.
Perspectives
Human primary aging is associated with a significant decline in physical activity and exercise capacity, which increases the risk for cardiovascular disease and mortality (1, 4, 5, 26, 33, 57). Aerobic capacity is highly dependent on the adequate delivery and distribution of O2 and nutrients to the active skeletal muscle. As such, it stands to reason that the folic acid-mediated improvement in skeletal muscle blood flow could improve oxygen utilization and exercise tolerance in aged humans, an effect that could ultimately reduce the cardiovascular disease risk by increasing functional and aerobic capacity. Moreover, preserving and/or improving endothelial health/function through folic acid ingestion could decrease cardiovascular disease risk independent of its effect on issues related to exercise tolerance (20, 30). Taken together, various mechanisms exist by which folic acid could improve cardiovascular health and improve quality of life in human primary aging. These remain exciting avenues for future research.
Summary
Vascular changes associated with human primary aging contribute to malperfusion of skeletal muscle during dynamic exercise. In this study, we examined the efficacy of folic acid ingestion to improve skeletal muscle blood flow via improved vascular conductance (i.e., vasodilation) in aged adults performing exercise. Our findings demonstrate that folic acid ingestion improves blood flow primarily via enhanced vascular conductance in the aging skeletal muscle vasculature. These findings provide evidence for the therapeutic use of folic acid to improve skeletal muscle blood flow in human primary aging.
GRANTS
This research was funded by National Institute of General Medical Sciences Grants GM-068865 and GM-117693.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.R., D.G., and C.G.C. conceived and designed research; S.A.R., D.G., A.N.A., G.M., K.K., M.F.J., M.N.C., and C.G.C. performed experiments; S.A.R. analyzed data; S.A.R. and C.G.C. interpreted results of experiments; S.A.R. prepared figures; S.A.R. drafted manuscript; S.A.R., D.G., A.N.A., G.M., K.K., M.F.J., M.N.C., and C.G.C. edited and revised manuscript; S.A.R., D.G., A.N.A., G.M., K.K., M.F.J., M.N.C., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects who cheerfully participated in this research study. We also thank Dr. John R. Halliwill for the development and use of DUC2 for Doppler ultrasound acquisition and analysis. We also thank Naomi Kennedy, RN, for her assistance with the study.
REFERENCES
- 1.Aspenes ST, Nilsen TI, Skaug E-A, Bertheussen GF, Ellingsen Ø, Vatten L, Wisløff U. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc 43: 1465–1473, 2011. doi: 10.1249/MSS.0b013e31820ca81c. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy MF, McDowell IFW, Ramsey MW, Brownlee M, Newcombe RG, Lewis MJ. Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur J Clin Invest 29: 659–662, 1999. doi: 10.1046/j.1365-2362.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 3.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW III, Paffenbarger RSJ Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300: R1288–R1295, 2011. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- 7.Brunton L, Chabner B, Knollman B. Goodman & Gilman’s Pharmocological Basis of Therapeutics (12th ed.). New York: McGraw-Hill, 2011. [Google Scholar]
- 8.Buck TM, Romero SA, Ely MR, Sieck DC, Abdala PM, Halliwill JR. Neurovascular control following small muscle-mass exercise in humans. Physiol Rep 3: e12289, 2015. doi: 10.14814/phy2.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck TM, Sieck DC, Halliwill JR. Thin-beam ultrasound overestimation of blood flow: how wide is your beam? J Appl Physiol 116: 1096–1104, 2014. doi: 10.1152/japplphysiol.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalupsky K, Kračun D, Kanchev I, Bertram K, Görlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal 23: 1076–1091, 2015. doi: 10.1089/ars.2015.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88, 2011. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 14.Doshi SN, McDowell IF, Moat SJ, Lang D, Newcombe RG, Kredan MB, Lewis MJ, Goodfellow J. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol 21: 1196–1202, 2001. doi: 10.1161/hq0701.092000. [DOI] [PubMed] [Google Scholar]
- 15.Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, Goodfellow J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 105: 22–26, 2002. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 16.Ely MR, Romero SA, Sieck DC, Mangum JE, Luttrell MJ, Halliwill JR. A single dose of histamine-receptor antagonists prior to downhill running alters markers of muscle damage and delayed onset muscle soreness. J Appl Physiol 122: 631−641, 2016. doi: 10.1152/japplphysiol.00518.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erikssen G, Liestøl K, Bjørnholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet 352: 759–762, 1998. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 18.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottsäter A, Forsblad J, Mattiasson I, Lindgärde F. Decreasing plasma endothelin-1 and unchanged plasma neopterin during folate supplementation in hyperhomocysteinemia. Int Angiol 21: 158–164, 2002. [PubMed] [Google Scholar]
- 20.Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105: 766–768, 2008. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 23.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Hoch AZ, Lynch SL, Jurva JW, Schimke JE, Gutterman DD. Folic acid supplementation improves vascular function in amenorrheic runners. Clin J Sport Med 20: 205–210, 2010. doi: 10.1097/JSM.0b013e3181df59f4. [DOI] [PubMed] [Google Scholar]
- 25.Hoch AZ, Papanek P, Szabo A, Widlansky ME, Gutterman DD. Folic acid supplementation improves vascular function in professional dancers with endothelial dysfunction. PM R 3: 1005–1012, 2011. doi: 10.1016/j.pmrj.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloszy JO, Kohrt WM. Exercise. In: Handbook of Physiology. Bethesda, MD: Am. Physiol. Soc., 1995, section 11, chapt. 24, p. 633–666. [Google Scholar]
- 27.Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 275: 17399–17406, 2000. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 28.Hyndman ME, Verma S, Rosenfeld RJ, Anderson TJ, Parsons HG. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol Heart Circ Physiol 282: H2167–H2172, 2002. doi: 10.1152/ajpheart.00935.2001. [DOI] [PubMed] [Google Scholar]
- 29.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakka TA, Venäläinen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med 330: 1594–1554, 1994. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 34.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Forstermann U. Pharmacological prevention of eNOS uncoupling. Curr Pharm Des 20: 3595–3606, 2014. doi: 10.2174/13816128113196660749. [DOI] [PubMed] [Google Scholar]
- 36.Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol 563: 633–642, 2005. doi: 10.1113/jphysiol.2004.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCord JL, Halliwill JR. H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J Appl Physiol (1985) 101: 1693–1701, 2006. doi: 10.1152/japplphysiol.00441.2006. [DOI] [PubMed] [Google Scholar]
- 38.Moens AL, Vrints CJ, Claeys MJ, Timmermans J-P, Champion HC, Kass DA. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol 294: H1971–H1977, 2008. doi: 10.1152/ajpheart.91503.2007. [DOI] [PubMed] [Google Scholar]
- 39.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen P, Boskovic R, Yazdani P, Kapur B, Vandenberghe H, Koren G. Comparing folic acid pharmacokinetics among women of childbearing age: single dose ingestion of 1.1 versus 5 MG folic acid. Can J Clin Pharmacol 15: e314–e322, 2008. [PubMed] [Google Scholar]
- 42.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 43.Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590: 5361–5370, 2012. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- 45.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol 92: 2105–2113, 2002. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 46.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 47.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- 48.Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol 98: 193–202, 2005. doi: 10.1152/japplphysiol.00704.2004. [DOI] [PubMed] [Google Scholar]
- 49.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc 38: 1819–1828, 2006. doi: 10.1249/01.mss.0000230340.79247.52. [DOI] [PubMed] [Google Scholar]
- 50.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- 51.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Râdegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383–1388, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Richards JC, Crecelius AR, Larson DG, Dinenno FA. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am J Physiol Heart Circ Physiol 309: H360–H368, 2015. doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridout SJ, Parker BA, Proctor DN. Age and regional specificity of peak limb vascular conductance in women. J Appl Physiol 99: 2067–2074, 2005. doi: 10.1152/japplphysiol.00825.2005. [DOI] [PubMed] [Google Scholar]
- 55.Romero SA, Ely MR, Sieck DC, Luttrell MJ, Buck TM, Kono JM, Branscum AJ, Halliwill JR. Effect of antioxidants on histamine receptor activation and sustained postexercise vasodilatation in humans. Exp Physiol 100: 435–449, 2015. doi: 10.1113/EP085030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro- and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol 312: H89–H97, 2017. doi: 10.1152/ajpheart.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 328: 533–537, 1993. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 58.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab 3: 211–223, 2002. doi: 10.2174/1389200024605163. [DOI] [PubMed] [Google Scholar]
- 60.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol 115: 972–978, 2013. doi: 10.1152/japplphysiol.00481.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond) 129: 159–167, 2015. doi: 10.1042/CS20140821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol 112: 791–797, 2012. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 75: 61–70, 2017. doi: 10.1093/nutrit/nuw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern SJ, Matok I, Kapur B, Koren G. A comparison of folic acid pharmacokinetics in obese and nonobese women of childbearing age. Ther Drug Monit 33: 336–340, 2011. doi: 10.1097/FTD.0b013e318219407a. [DOI] [PubMed] [Google Scholar]
- 65.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res 86: 1129–1134, 2000. doi: 10.1161/01.RES.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 66.Tawakol A, Migrino RQ, Aziz KS, Waitkowska J, Holmvang G, Alpert NM, Muller JE, Fischman AJ, Gewirtz H. High-dose folic acid acutely improves coronary vasodilator function in patients with coronary artery disease. J Am Coll Cardiol 45: 1580–1584, 2005. doi: 10.1016/j.jacc.2005.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Zhao J, Richardson RS. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol 310: H765–H774, 2016. doi: 10.1152/ajpheart.00817.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trinity JD, Wray DW, Witman MAH, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305: R893–R899, 2013. doi: 10.1152/ajpregu.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willems FF, Boers GH, Blom HJ, Aengevaeren WR, Verheugt FW. Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br J Pharmacol 141: 825–830, 2004. doi: 10.1038/sj.bjp.0705446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Wray DW, Richardson RS. Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc 38: 1804–1810, 2006. doi: 10.1249/01.mss.0000230342.86870.94. [DOI] [PubMed] [Google Scholar]
- 72.Wray DW, Richardson RS. ‘Fine-tuning’ blood flow to the exercising muscle with advancing age: an update. Exp Physiol 100: 589–602, 2015. doi: 10.1113/EP085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y-M, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Janssens SP, Wingler K, Schmidt HH, Moens AL. Modulating endothelial nitric oxide synthase: a new cardiovascular therapeutic strategy. Am J Physiol Heart Circ Physiol 301: H634–H646, 2011. doi: 10.1152/ajpheart.01315.2010. [DOI] [PubMed] [Google Scholar]