This research is noteworthy because it combines and links, through the 5-HT7 receptor, an in vitro observation (venorelaxation) with in vivo events (venodilation and fall in blood pressure). This supports the idea that splanchnic venodilation plays a role in blood pressure regulation.
Keywords: blood pressure, imaging, pharmacology, veins, SB-269970, serotonin
Abstract
Serotonin [5-hydroxytryptamine (5-HT)] causes relaxation of the isolated superior mesenteric vein, a splanchnic blood vessel, through activation of the 5-HT7 receptor. As part of studies designed to identify the mechanism(s) through which chronic (≥24 h) infusion of 5-HT lowers blood pressure, we tested the hypothesis that 5-HT causes in vitro and in vivo splanchnic venodilation that is 5-HT7 receptor dependent. In tissue baths for measurement of isometric contraction, the portal vein and abdominal inferior vena cava relaxed to 5-HT and the 5-HT1/7 receptor agonist 5-carboxamidotryptamine; relaxation was abolished by the 5-HT7 receptor antagonist SB-269970. Western blot analyses showed that the abdominal inferior vena cava and portal vein express 5-HT7 receptor protein. In contrast, the thoracic vena cava, outside the splanchnic circulation, did not relax to serotonergic agonists and exhibited minimal expression of the 5-HT7 receptor. Male Sprague-Dawley rats with chronically implanted radiotelemetry transmitters underwent repeated ultrasound imaging of abdominal vessels. After baseline imaging, minipumps containing vehicle (saline) or 5-HT (25 μg·kg−1·min−1) were implanted. Twenty-four hours later, venous diameters were increased in rats with 5-HT-infusion (percent increase from baseline: superior mesenteric vein, 17.5 ± 1.9; portal vein, 17.7 ± 1.8; and abdominal inferior vena cava, 46.9 ± 8.0) while arterial pressure was decreased (~13 mmHg). Measures returned to baseline after infusion termination. In a separate group of animals, treatment with SB-269970 (3 mg/kg iv) prevented the splanchnic venodilation and fall in blood pressure during 24 h of 5-HT infusion. Thus, 5-HT causes 5-HT7 receptor-dependent splanchnic venous dilation associated with a fall in blood pressure.
NEW & NOTEWORTHY This research is noteworthy because it combines and links, through the 5-HT7 receptor, an in vitro observation (venorelaxation) with in vivo events (venodilation and fall in blood pressure). This supports the idea that splanchnic venodilation plays a role in blood pressure regulation.
serotonin [5-hydroxytryptamine (5-HT)] was discovered in the cardiovascular system (22, 37) but is best known for its actions in the gastrointestinal system and in the central nervous system (27). Exogenously administered 5-HT causes complex time- and dose-dependent changes in blood pressure and other hemodynamic variables (27, 55, 57), but it is unclear whether endogenous 5-HT exerts significant effects on overall cardiovascular system function under normal or pathophysiological conditions.
The ability of 5-HT to lower blood pressure, when given acutely (<1-h duration), has been recognized for decades (10, 15, 50, 52). In fact, those that discovered 5-HT were likely the first to demonstrate that acutely infused 5-HT resulted in a fall in blood pressure [dogs and humans (37)]. In contrast to these studies, we have been interested in identifying the mechanisms by which 5-HT lowers blood pressure when infused chronically (24 h or more). There are two reasons motivating this interest. First, we and others have shown that chronic administration of serotonergic agonists or the 5-HT precursor 5-hydroxytryptophan (5-HTP) can produce a sustained fall in blood pressure in hypertensive animals, potentially suggesting a novel approach to antihypertensive drug therapy (10, 17, 18, 29, 33). Second, 5-HT is handled dynamically by the body. An elevation in free circulating levels of 5-HT can occur in a number of different ways: greater release from enterochromaffin cells, less uptake or more release from platelets, and less uptake and/or more release from serotonergic neurons (27). There are thus conditions in which 5-HT could exert a significant effect on the regulation of blood pressure and systemic hemodynamics.
In attempting to understand the effects on blood pressure of 5-HT under chronic (≥24 h) conditions, we showed that a 1-wk infusion of 5-HT (25 μg·kg−1·min−1) produced a sustained fall in blood pressure in conscious, unrestrained sham rats. This same infusion significantly reduced the hypertension of deoxycorticosterone acetate (DOCA)-salt rats (17). The 5-HT-induced fall in blood pressure was dose dependent (49), occurred in male and female animals (12), and was maintained over a longer 30-day administration (14). This chronic fall in blood pressure is qualitatively consistent with the fall in blood pressure that was observed when low doses of 5-HT are given acutely (minutes) (15, 50, 52). Use of fluorescent microspheres to measure changes in blood flow supported a focus in splanchnic tissue because 5-HT selectively increased splanchnic blood flow relative to vehicle-infused rats (41). We have eliminated two logical targets for reducing blood pressure in the splanchnic vasculature. First, 5-HT does not appear to inhibit the sympathetic nervous system in the splanchnic bed (11, 13). Second, 5-HT does not stimulate a relaxation of isolated arteries >250 μm in diameter, including the thoracic aorta, abdominal aorta, superior mesenteric artery, or mesenteric resistance artery (13).
Rather, we discovered that 5-HT directly relaxed the isolated superior mesenteric vein (SMV) through activation of the 5-HT7 receptor (3, 56). Others have shown that the acute fall in blood pressure seen during 5-HT infusion is due to activation of 5-HT7 receptors (50). We tested the hypothesis that 5-HT will relax splanchnic veins in vitro and cause dilation of splanchnic veins in vivo by 5-HT7 receptor activation. If splanchnic venodilation is one mechanism by which 5-HT causes a chronic fall in blood pressure, then the blood pressure fall and splanchnic venodilation should be attenuated by 5-HT7 receptor blockade. We use a combination of pharmacology, physiology, and a newly developed ultrasound technique to test this hypothesis.
MATERIALS AND METHODS
Animals
The Michigan State University Institutional Animal Care and Use Committee approved all protocols used in this study. Male Sprague-Dawley rats (200–350 g, Charles River Laboratories, Portage, MI) were used and were housed in a temperature-controlled room (22°C) with 12:12-h light-dark cycles. Animals were given standard chow and distilled water ad libitum and housed in standard cages with stainless steel wire lids and containing environmental enrichment (e.g., Bed-r’Nest). The rats used were randomized to in vitro and in vivo groups and further randomized within each experimental group (e.g., vehicle- and drug-treated groups). Each n value represents data that came from one animal. When samples were pooled from several animals, this was noted.
Materials
ACh chloride, forskolin, 5-HT creatinine sulfate, norepinephrine (NE) hydrochloride, and phenylephrine hydrochloride were obtained from Sigma Chemical (St. Louis, MO). SB-269970 and 5-carboxamidotryptamine (5-CT) maleate were purchased from Tocris (R&D, Minneapolis, MN) or Abcam (Cambridge, MA). Endothelin (ET)-1(1–21) was purchased from Bachem (Torrance, CA).
In Vitro Experiments
Tissue preparation for isometric contraction.
Naïve rats were anesthetized with pentobarbital (60–80 mg/kg ip) and vessels were dissected. The vessels studied were taken from the same rats such that an appropriate intra-animal comparison could be made. All dissections took place under a stereomicroscope and in a Silastic-coated dish filled with physiological salt solution (PSS) containing (in mM) 130.00 NaCl, 4.70 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 14.80 NaHCO3, 5.50 dextrose, 0.03 CaNa2EDTA 0.03, and 1.60 CaCl2 (pH 7.2). The portal vein (PV) exiting the liver was dissected away from the liver, the thoracic (above diaphragm) vena cava was removed, and the abdominal inferior vena cava (Ab IVC) was removed with the abdominal aorta. The Ab IVC was dissected away from the abdominal aorta. Once isolated, vessels were guided onto the stabilizing wire, cleaned of fat, and used in one of the protocols described below. The endothelium was left intact as validated by a ≥50% relaxation to ACh in PGF2α-contracted tissues.
Tissue bath measurement of isometric contraction.
Cleaned vessels were cut into rings (~3 mm wide) for the measurement of isometric contractile force. Rings were mounted in warmed (37°C) and aerated (95% O2-5% CO2) tissue baths (30 ml PSS) on Grass isometric transducers (FT03, Grass Instruments, Quincy, MA) connected to an ADInstruments PowerLab (ADInstruments, Colorado Springs, CO). Tissues were placed under optimal resting tension [PV: 300 mg and thoracic vena cava (TVC) and Ab IVC: 1,000 mg] and equilibrated for 1 h before an initial challenge with a maximal concentration of NE (10−5 M). These tensions were determined in experiments for each tissue that allowed us to determine the applied tension that generated a maximum active response. Initial contractions to NE were as follows: PV = 394.6 ± 98.6 mg, Ab IVC = 366.0 ± 41.6 mg, and TVC = 53.7 ± 6.1 mg. After this challenge, tissues were washed until tone returned to baseline. Preliminary experiments determined that ET-1 caused a stable contraction in vessels. The ET-1 concentration used was submaximal, achieving ~40–50% of maximal ET-1-induced contraction. Once ET-1-induced contraction was stable (~12–15 min after addition), increasing concentrations of the serotonin receptor agonist 5-HT or 5-CT (54a) were added cumulatively (10−10–10−5 M). Only one agonist was examined in each tissue. In some experiments, vehicle or the 5-HT7 receptor antagonist SB-269970 was added 45 min before the addition of ET-1. Tissues that did not relax to agonist were incubated with the adenylate cyclase stimulator forskolin (10−5 M) to determine the relaxing potential of the vessel.
Western blot analysis.
Vessels were cleaned, frozen, and ground into a powder using a mortar and a pestle. Two to three of the same type of vessels were pooled from multiple rats for each lane upon finding that use of tissue from one animal provided insufficient material to be able to detect the 5-HT7 receptor. Homogenization buffer [125 mM Tris (pH 6.8), 4% SDS, 20% glycerol, 0.5 mM PMSF, 1 mM orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin] was added, and the homogenates were vortexed, sonicated for five cycles, and centrifuged. Supernatants were collected, and protein concentration was determined with the BCA protein kit (catalog no. BCA1, Sigma Chemical). SDS-PAGE separation of proteins in tissue homogenates (50 μg) was performed, and proteins were transferred to polyvinylidene difluoride membranes. Positive controls [rat brain for the 5-HT7 receptor (50 μg)] were run in parallel lanes along with molecular weight markers. Blots were incubated overnight at 4°C with 5-HT7 primary antibody (catalog no. ab13898, Abcam, 1:1,000), washed three times with Tris-buffered saline, and incubated with secondary antibody [catalog no. 7074, anti-rabbit horseradish peroxidase (HRP)-linked IgG, Cell Signaling Technology, Danvers, MA, 1:1,000). The same blots were reprobed for smooth muscle α-actin (primary antibody: mouse, catalog no. 113200, EMD Chemicals/Calbiochem, Gibbstown, NJ, 1:2,000; secondary antibody, anti-mouse HRP-linked IgG, GE Healthcare Life Sciences, Marlborough, MA, 1:1,000) to compare protein loading. Blots were developed using species-specific HRP-conjugated secondary antibodies and ECL reagents (GE Healthcare Life Sciences, Piscataway, NJ) on a Kodak X-OMAT Film Processor 2000A. Exposures were 16 s for α-actin and 24 min for the 5-HT7 receptor. This receptor antibody was validated previously using brain homogenates as a positive control and visualization of a band consistent with the expected molecular weight (56).
In Vivo Experiments
Telemetry, pump implantation, and ultrasound experiments.
telemeters.
Radiotelemeter transmitters (C40 model, Data Sciences, Minneapolis, MN) with attached catheters with pressure-sensing tips were implanted subcutaneously as previously described (17). Rats recovered 5 days postoperatively, and then 3 days of baseline cardiovascular measurements were made. Mean arterial pressure, pulse pressure, and heart rate were recorded for 10 s every 10 min throughout the duration of the study. On the final baseline day, rats underwent baseline ultrasound imaging of the PV, Ab IVC, abdominal aorta, and SMV (described below).
pump implantation.
Osmotic pumps (model 2ML1, Alzet, Durect, Cupertino, CA) containing either 5-HT (at a concentration calculated to deliver 25 μg·kg−1·min−1) or vehicle [1% ascorbate (antioxidant) in sterile saline, pH balanced to 7.4–7.6] were implanted subcutaneously between the scapulae in anesthetized rats [isoflurane (1–2%)]. In some experiments, osmotic pumps were removed under isoflurane anesthesia. Pumps were weighed before and after the experiments as a validation of pump function.
ultrasound imaging.
More details on this technique have been reported elsewhere (40). Rats [isoflurane anesthetized (1–2%)] were positioned supine on a warmed platform (Vevo 2100 Imaging System, Visualsonics, Toronto, ON, Canada). Ultrasound gel was applied to each front and hind paw to obtain measures of heart rate, respiration, and ECG. Warmed ultrasound gel was applied to the abdominal skin, just below the xiphoid process, to couple the transducer (21-MHz probe, MS250) before imaging in B-mode. Two separate real-time images were scanned at 25 frames/s: 1) an image at the location of the PV exiting the liver, which provides images of the abdominal aorta, Ab IVC, and PV; and 2) an image at the level just below the branching of the PV toward the spleen via the splenic vein, which provides images of the SMV, splenic vein, Ab IVC, and aorta for an arterial comparison. Each session took ~10 min/rat, minimizing the amount of time each animal was under anesthesia.
Three separate in vivo experiments were performed. All included radiotelemetry-implanted male Sprague-Dawley rats as described above.
Experiments.
experiment 1: 5-ht infusion alone (vessel diameter and hemodynamics).
After baseline imaging, rats were randomized into two treatment groups. They were implanted with osmotic mini-pumps (2ML1, Alzet) containing either 5-HT (25 μg·kg−1·min−1; group 1) or vehicle [1% ascorbate (antioxidant); group 2]. Imaging was performed 24 h after pump implantation, pumps were removed, and imaging was repeated 1 wk after pump removal. Pressures (mean, systolic, and diastolic) and heart rate were continuously recorded in a conscious state throughout this study except during imaging.
experiment 2: 5-ht infusion with sb-269970 blockade (hemodynamics only).
All animals were implanted with an indwelling femoral vein catheter and placed in tether jackets. Animals were randomized into two treatment groups. Intravenous infusion of SB-269970 (2 mg·kg−1·h−1; group 1) or saline (group 2) was started 1 h before the implantation of 5-HT osmotic pumps (25 µg·kg−1·min−1, 2ML1, Alzet). Infusion was performed because of the short half-life of SB-269970 (25). Infusion was continued for the subsequent 24 h in the presence of 5-HT-containing osmotic pump. SB-269970 and saline infusion were stopped while 5-HT infusion continued, and blood pressure was measured for an additional 24 h.
experiment 3: 5-ht infusion with sb-269970 blockade (vessel diameter and hemodynamics).
After baseline images of noted vessels, all animals were implanted with an indwelling femoral vein catheter and placed in tether jackets. Animals were randomized and placed into one of three treatment groups: 5-HT + saline (group 1), 5-HT + SB-269970 (group 2), and saline + saline (control; group 3). Animals were implanted with either a 5-HT (25 µg·kg−1·min−1) or saline osmotic pump and given an intravenous infusion of either SB-269970 or saline. The same surgical protocol and experimental paradigm as in experiment 2 (above) were used. Venous diameters and cardiovascular parameters were collected at baseline and at 24 h after 5-HT infusion for all three groups.
Data Analyses
Quantitative data are reported as means ± SE for n number of animals. For isometric contractile measures, relaxation is reported as a percentage of the initial contraction to a half-maximal concentration of ET-1; values of absolute contraction to NE and ET-1 (in mg) are reported in materials and methods. Agonist potencies were calculated using a nonlinear regression (curve fit) within GraphPad Prism 6.0 (La Jolla, CA) and are reported as –logEC50 values (in M); they were calculated only when maximums could be achieved. Maximums are reported as the maximal effect achieved. Images of Western blots were processed through Photoshop (CC 2014) and were not modified from the original acquisition. Values for Western blot analyses were desensitized in ImageJ (1.47v, National Institutes of Health), and data are reported as arbitrary densitometry units relative to α-actin densitometry units. Data are reported as pooled samples/n values (total number of lanes) for the total number of animals used.
For measurements made on ultrasound images, the same person, who has considerable experience in the use of the Vevo Imaging system and was blinded to the nature of the infusion group, analyzed each vessel diameter (40). All measurements were controlled for respiration and cardiac cycles. Vessel diameters and coefficient of variation parameters are reported as the maximum percent change from baseline. Baseline values are shown in Tables 1 and 2.
Table 1.
Baseline physiological parameters and venous diameters of the vehicle and 5-HT groups before pump implantation for the animals shown in Fig. 5
| Parameter | Vehicle Group | 5-HT Group |
|---|---|---|
| Number of animals | 9 | 9 |
| Mean arterial pressure, mmHg | 101.40 ± 2.10 | 96.80 ± 2.10 |
| Systolic pressure, mmHg | 117.20 ± 3.70 | 119.50 ± 1.80 |
| Diastolic pressure, mmHg | 88.40 ± 2.30 | 82.50 ± 1.20* |
| Heart rate, beats/min | 389.00 ± 22.00 | 379.00 ± 8.00 |
| Vessel diameters | ||
| Superior mesenteric vein, mm | 1.85 ± 0.04 | 1.81 ± 0.05 |
| Portal vein, mm | 2.13 ± 0.06 | 2.08 ± 0.03 |
| Abdominal inferior vena cava, mm | 3.53 ± 0.13 | 3.16 ± 0.12 |
| Abdominal aorta, mm | 2.14 ± 0.04 | 2.20 ± 0.05 |
Values are means ± SE. 5-HT, 5-hydroxytrypamine (serotonin).
Significantly different from vehicle measure (P < 0.05).
Table 2.
Baseline physiological parameters and venous diameters of 5-HT + saline, 5-HT + SB-269970, and saline + saline groups before pump implantation for the animals shown in Fig. 7
| 5-HT + Saline Group | 5-HT + SB-269970 Group | Saline + Saline Group | |
|---|---|---|---|
| Number of animals | 6 | 6 | 4 |
| Mean arterial pressure, mmHg | 102.00 ± 3.30 | 102.20 ± 2.90 | 99.70 ± 0.70 |
| Systolic blood pressure, mmHg | 121.50 ± 3.70 | 126.80 ± 3.40 | 120.10 ± 0.60 |
| Diastolic blood pressure, mmHg | 87.70 ± 3.80 | 88.80 ± 1.90 | 84.20 ± 1.00 |
| Heart rate, beats/min | 394.00 ± 8.00 | 394.00 ± 13.00 | 392.00 ± 12.00 |
| Vessel diameters | |||
| Superior mesenteric vein, mm | 1.80 ± 0.05 | 1.80 ± 0.07 | 1.80 ± 0.03 |
| Portal vein, mm | 2.00 ± 0.04 | 2.10 ± 0.09 | 2.10 ± 0.05 |
| Abdominal inferior vena cava, mm | 2.80 ± 0.02 | 3.30 ± 0.25 | 3.00 ± 0.27 |
| Abdominal aorta, mm | 2.30 ± 0.06 | 2.30 ± 0.03 | 2.10 ± 0.02 |
Values are means ± SE.
Statistical Analysis
For in vitro measures, t-tests to compare responses at a maximum concentration of agonist were used. For densitometry, a Grubs test (extreme studentized deviate) was performed to determine whether the most extreme value was a significant outlier. Multiple tissues went in to making samples for each lane, and we report both the number of total animals and lanes (n) for the Western blot experiments. For in vivo measures, statistical analysis was performed using paired two-tailed t-tests between two groups. Repeated-measures ANOVA was used when comparing pressure or vessel diameter values from baseline (GraphPad Prism 6). In all cases, a P value of <0.05 was considered significant.
RESULTS
5-HT7 Receptors Are Expressed in Splanchnic Veins
We first investigated 5-HT7 receptor expression in two splanchnic veins (PV and Ab IVC) and one nonsplanchnic vein (TVC) using Western blot analyses. We have previously used this antibody, validated by the detection of brain protein at the appropriate molecular weight (56). Each vessel type was pooled from three animals such that the lanes shown in Fig. 1A represent a total of nine animals (3 lanes × 3 animals from which the 3 different veins were taken). The 5-HT7 receptor was robustly expressed in the Ab IVC, moderately expressed in the PV, and less so in the TVC. Because of the variability in 5-HT7 receptor expression observed in the PV and TVC, this experiment was repeated again for the PV and TVC. Figure 1B shows data from these two separate experiments, with 5-HT7 receptor expression reported as a percentage of α-actin expression. One statistical outlier was observed in both PV and IVC groups. This point for each group was removed to make the bar graphs shown in Fig. 1B. These bars represent a total of 22, 22, and 9 rats for the PV, TVC, and Ab IVC, respectively. There were no significant differences among these values.
Fig. 1.
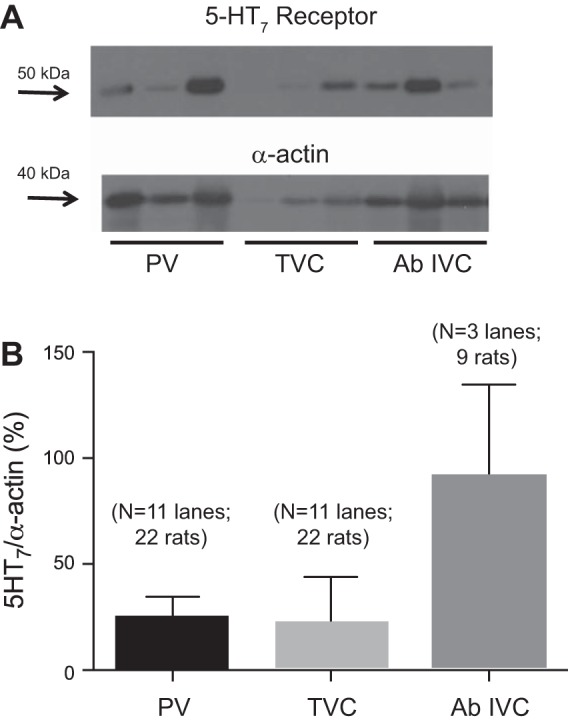
A: Western analyses of the 5-HT7 receptor in homogenates of the portal vein (PV), thoracic (above diaphragm) vena cava (TVC), and abdominal inferior vena cava (Ab IVC) from the same set of 9 animals (3 animals/lane, 3 lanes). The blot was reprobed with α-actin. B: densitometry of all nonoutlying values for the 5-HT7 receptor signal relative to α-actin expression. Bars represent means ± SE for the number of lanes and animals specified on the graph.
Isolated Splanchnic Veins Relax to 5-HT and 5-CT
Veins, contracted to ET-1, relaxed to cumulative additions of 5-HT. The maximal relaxant efficacy of 5-HT was as follows (in percent ET-1 contraction remaining): Ab IVC = 64.3 ± 16.6, PV = 79.5 ± 18.7, and no relaxation in the TVC (Fig. 2A). All tissues relaxed completely to the adenylate cyclase stimulator forskolin (10 μM). At high concentrations of 5-HT (1 μM), contraction was observed, consistent with the interaction of 5-HT with the 5-HT2A receptor in smooth muscle (56). The potency of 5-HT in relaxing the veins was not calculated because of the biphasic nature of these curves. In comparison, 5-CT was more efficacious compared with 5-HT. 5-CT has a high affinity for the 5-HT7 receptor and, compared with 5-HT, considerably lower affinity for the 5-HT2A receptors that mediate contraction in most vessels (54a).
Fig. 2.
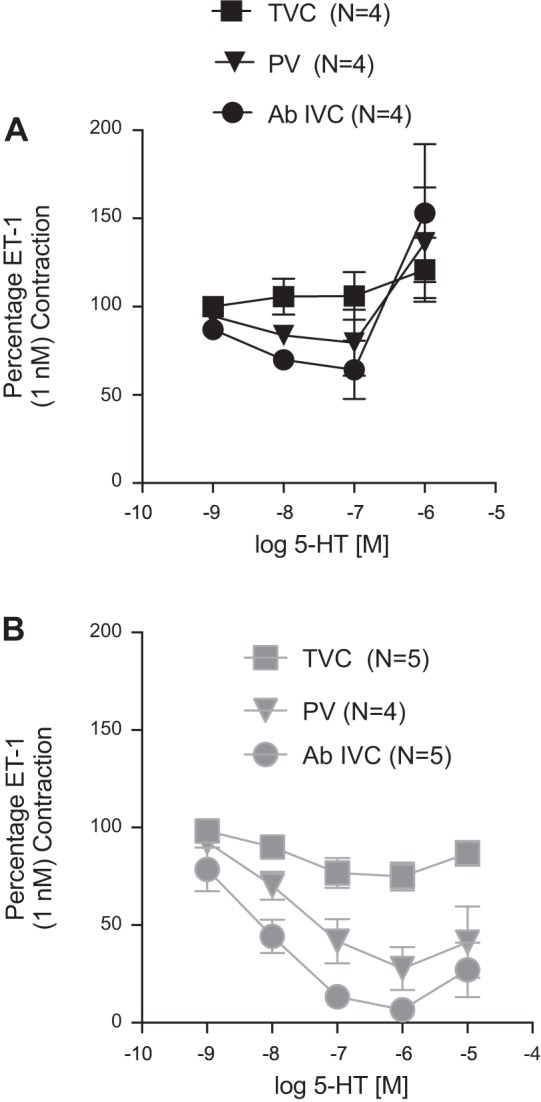
Relaxation of the endothelin (ET)-1-contracted isolated thoracic VC (above diaphragm; TVC), PV, and Ab IVC to 5-HT (A) and 5-carboxamidotryptamine (5-CT; B). The magnitude of venous contraction to 1 nM ET-1 was PV = 279.1 ± 44.6 mg, Ab IVC = 206.2 ± 54.7 mg, and TVC = 66.1 ± 7.0 mg. Points represent means ± SE for the number of animals in parentheses. Veins were taken from the same animals for intravessel comparison of sensitivity to each agonist.
The maximum relaxant efficacy of 5-CT was as follows (percent ET-1 contraction remaining): Ab IVC (6.6 ± 4.5) > PV (27.8 ± 11.2) >> TVC (74.9 ± 7.2) (Fig. 2B). The potency of 5-CT [−logEC50 (in M)] was calculated for the Ab IVC (8.22 ± 0.42), PV (7.88 ± 0.28), and TVC (8.10 ± 0.80). The 5-HT7 receptor antagonist SB-269970 blocked 5-CT-induced relaxation in the isolated Ab IVC and PV (Fig. 3, A and B, respectively), and contraction consistent with 5-HT2A receptor activation was observed at high concentrations just as it was for 5-HT (above). The TVC was not tested because of the low efficacy of relaxation to 5-HT and 5-CT. Collectively, these experiments support the ability of serotonergic agonists to directly relax isolated splanchnic veins through activation of the 5-HT7 receptor.
Fig. 3.
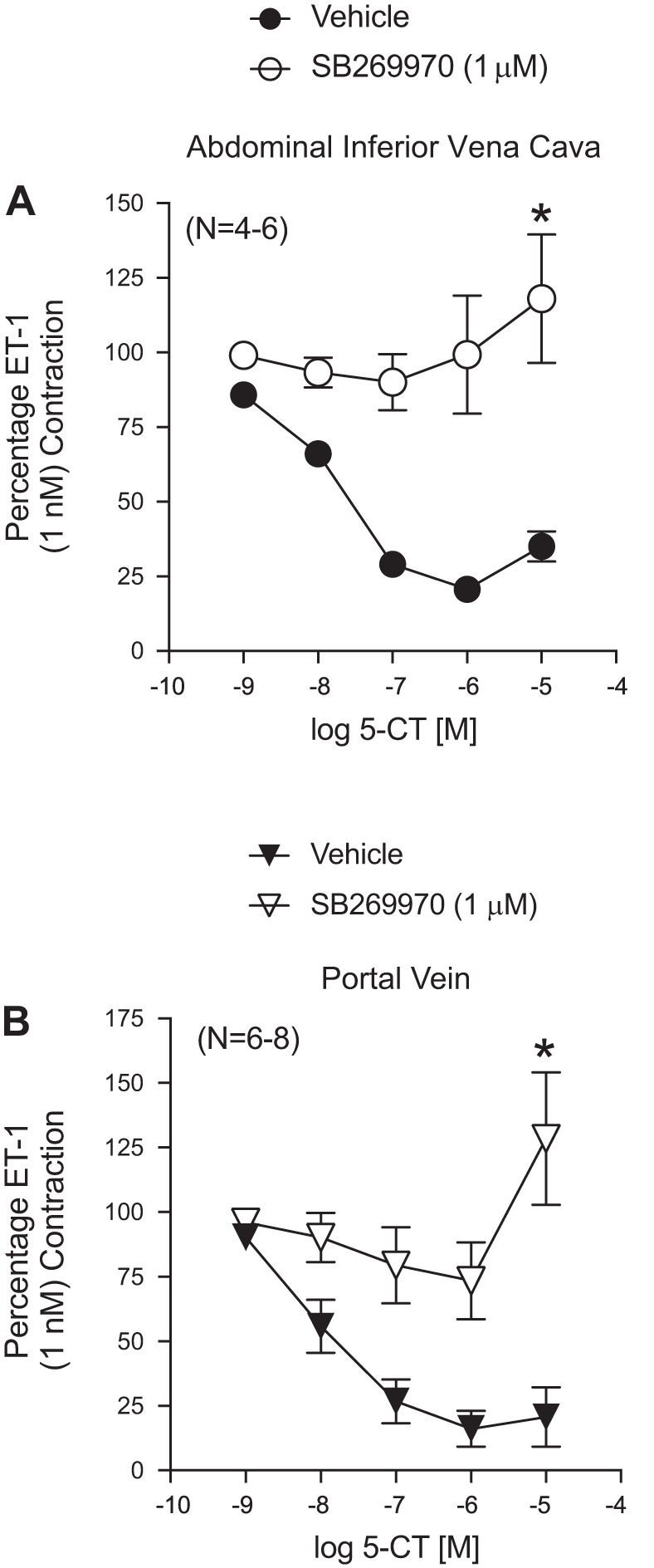
Ability of the 5-HT7 receptor antagonist SB-269960 to antagonize 5-CT-induced relaxation in the isolated Ab IVC (A) and PV (B). Points represent means ± SE for the number of animals in parentheses. These veins were taken from the same animals for intra-arterial comparison. *Significant difference vs. vehicle-exposed tissues at the maximum agonist concentration tested (P < 0.05).
5-HT Infusion Results in Splanchnic Venodilation In Vivo
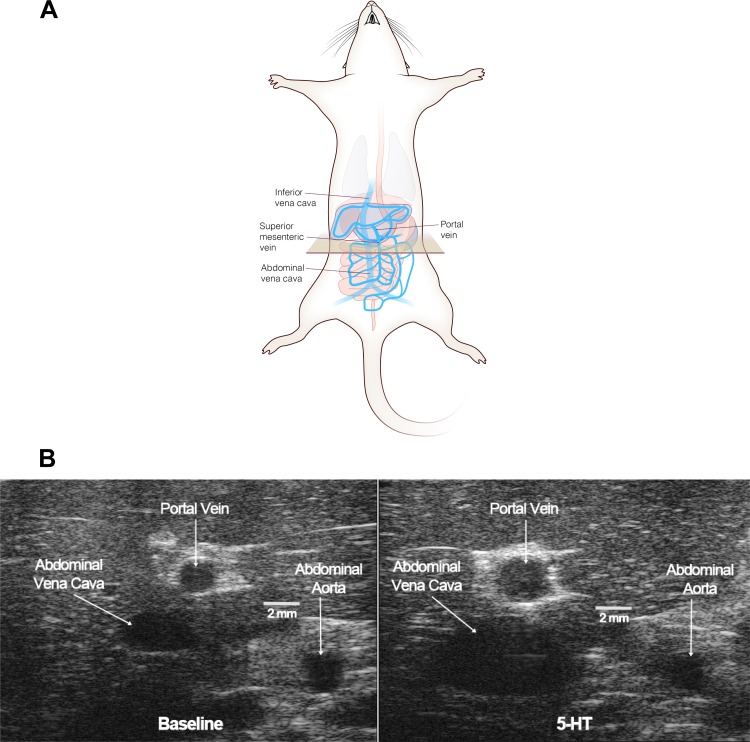
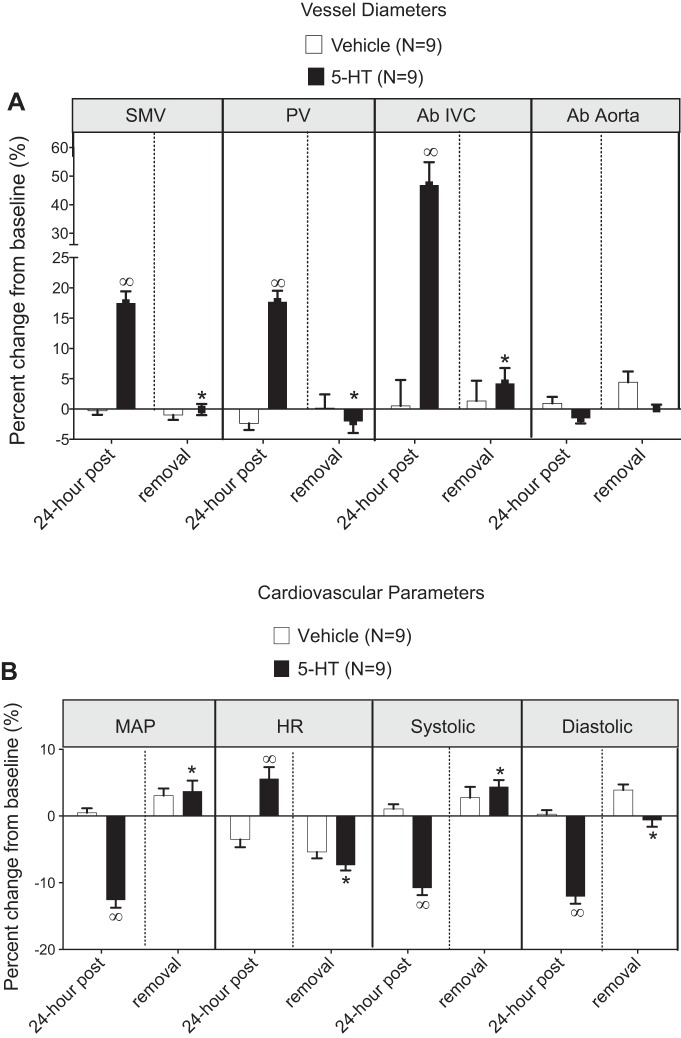
Radiotelemetery-implanted rats were randomly assigned to one of two groups: one group that received an osmotic pump with vehicle and the other group that received 5-HT. Baseline physiological parameters (venous diameters, mean arterial blood pressure, heart rate, systolic blood pressure, and diastolic blood pressure) before pump implantation are shown in Table 1. Parameters were similar between these two groups except for diastolic blood pressure, which was lower in the 5-HT group compared with the vehicle group. Figure 4A shows the first position of ultrasound visualization (tan box); the box was moved 2 mm caudally for imaging the SMV. Figure 4B shows representative ultrasound images comparing venous diameter before (left) and after (right) 24 h of 5-HT administration. The vessel diameters and physiological parameters of rats given vehicle or 5-HT-containing pumps are shown in Fig. 5 at 24 h after implantation and 1 wk after pump removal.
Fig. 4.
A: diagram of ultrasound wand/transducer placement for image procurement using the Vevo2100. The tan box shows the initial position of the plane imaged by the ultrasound probe. The drawing was done by Chris McKee. B: representative B-mode ultrasound images taken at baseline (left) and 24 h after 5-HT (right) in the same rat. Horizontal lines quantify size.
Fig. 5.
Change in vessel diameter (A) and physiological parameters (B) with infusion of vehicle (open bars) and 5-HT (solid bars). Bars represent means ± SE for the number of animals indicated in parentheses (n). *Statistical differences between 5-HT after 24 h and 5-HT reversal. ∞Significant differences between vehicle and 5-HT after 24 h.
Venous diameter increased with 5-HT infusion by over 17% in all veins, with the Ab IVC being particularly responsive (>40% change; Fig. 5A). Additionally, the SMV, which we have published as having 5-HT7 receptors that relax to 5-CT (56), demonstrated in vivo dilation to 5-HT. In contrast, the abdominal aorta demonstrated no change in internal diameter with 5-HT infusion (−1.59 ± 0.86%). This artery was investigated as an arterial comparison to the venous vessels. In parallel, blood pressures (mean, systolic, and diastolic) were reduced (−12.58 ± 1.17%), whereas heart rate was modestly elevated compared, with their baseline values and versus vehicle-infused rats (Fig. 5B). These measures were taken during the morning when the gastrointestinal system exhibited naturally low peristaltic activity, given that 5-HT increases the prokinetic action of the intestine (27).
Splanchnic Venous Dilation Is Mediated Through the 5-HT7 Receptor
Our final experiment was to determine whether the splanchnic venodilation and fall in blood pressure caused by 5-HT were both mediated by the 5-HT7 receptor. This was done using the 5-HT7 receptor antagonist SB-269970. In validation experiments, this dosing protocol for SB-269970 abolished the acute hypotension caused by 5-CT administered intravenously (1 μg/kg 5-CT: vehicle = −17.3 ± 2.2 mmHg from baseline and SB-269970 = −1.1 ± 1.4 mmHg, n = 5–8, P < 0.05).
Figure 6A shows mean arterial blood pressure, demonstrating a significant fall in blood pressure in those animals that received 5-HT plus vehicle infusion but not in those that received 5-HT plus SB-269970. Removal of SB-269970 infusion from animals that still received 5-HT resulted in a fall in blood pressure. Figure 6B shows a comparison of the magnitude of fall in blood pressure to 5-HT within 24 h of infusion in these two groups of rats. SB-269970 significantly attenuated the fall in blood pressure to 5-HT compared with vehicle-infused rats (13.3 ± 2.4 to 3.0 ± 1.5 mmHg).
Fig. 6.
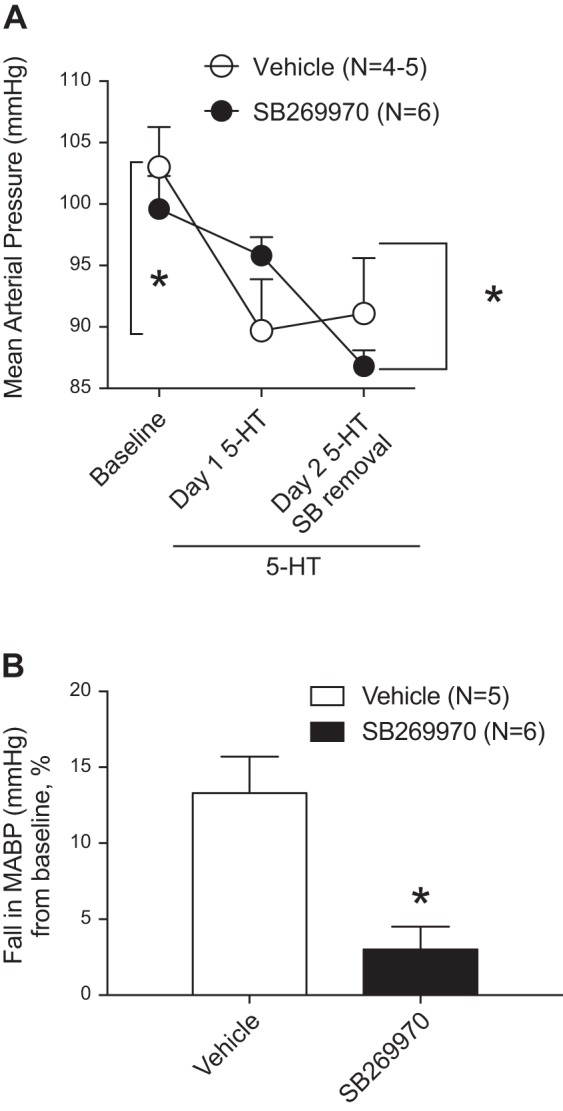
Mean arterial blood pressure (A) and magnitude of the fall in blood pressure (percent baseline; B) in rats infused with SB-269970 or vehicle before pump implantation with 5-HT (25 μg·kg−1·min−1). Measures of blood pressure were done 24 h after pump implantation and then again after SB-269970 removal. Points/bars represent means ± SE for the number of animals in parentheses. *Statistical differences between the two bracketed bars.
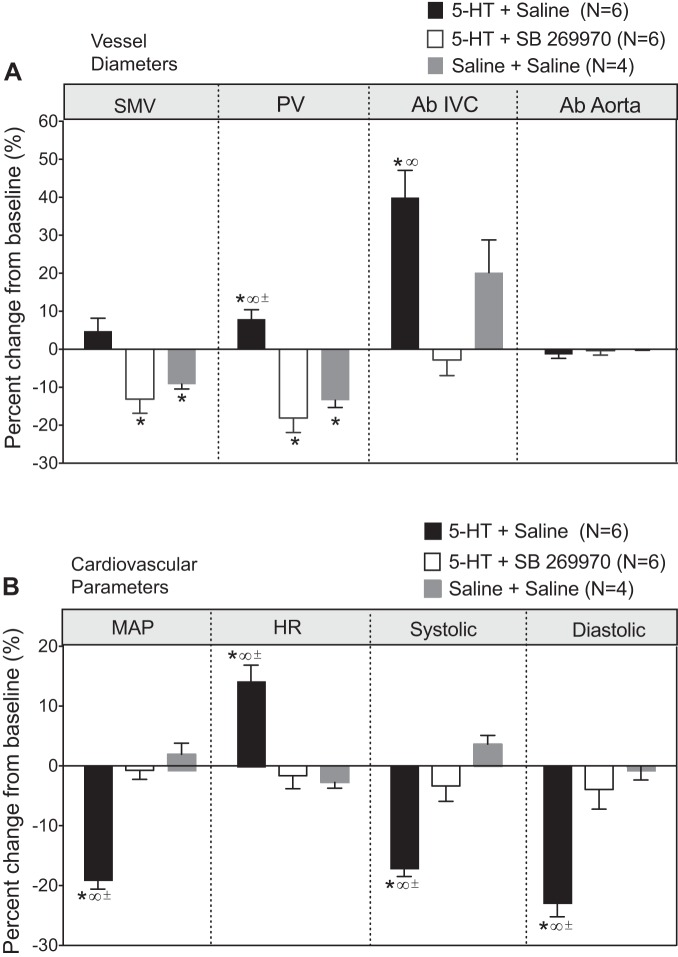
We performed an additional experiment to investigate the association between 5-HT7 receptor-mediated effects on blood pressure and venous dimensions within our infusion model. This was, unlike the above two in vivo experiments, done without reversal/removal of infusion. Table 2 shows the venous diameters and baseline cardiovascular parameters for the three groups of animals used. Splanchnic venous diameters are shown in Fig. 7A. The control (saline + saline; gray bars) animals demonstrated small, variable changes in vessel diameter in the SMV, PV, and Ab IVC with no change in the abdominal aorta. 5-HT infusion alone caused a modest venodilation in the SMV and a significant venodilation in the PV (7.9 ± 2.4%) and Ab IVC (39.9 ± 7.2%), consistent with data shown in Fig. 2. Infusion of SB-269970 along with 5-HT abolished venodilation in these splanchnic vessels. Figure 7B shows the cardiovascular parameters of these same rats. Blood pressure (mean, systolic, and diastolic) and heart rate in control rats (saline + saline) did not change during the course of this experiment. 5-HT alone (5-HT + saline) reduced blood pressures (mean: −18.6 ± 2.0%) and elevated heart rate, consistent with the data shown in Fig. 2. SB-269970 abolished the 5-HT-induced reduction in blood pressure and elevation in heart rate (Fig. 7B), in line with the results of the experiments shown in Fig. 6. Thus, 5-HT7 receptor blockade prevented both 5-HT-induced splanchnic venous dilation and hypotension.
Fig. 7.
5-HT-induced changes in vessels diameters (A) and cardiovascular parameters (B) in rats infused with SB-269970 or vehicle before pump implantation with 5-HT (25 μg·kg−1·min−1). Measures of blood pressure were done 24 h after pump implantation. Saline + saline animals were used as controls for infusion of vehicles that carried both 5-HT and SB-269970. Points/bars represent means ± SE for the number of animals in parentheses. *Significant from own baseline, ∞significant compared with 5-HT + SB-269970, ±significant compared with saline + saline.
DISCUSSION
Veins Versus Arteries
Splanchnic veins (and the abdominal vena cava) relaxed to serotonergic agonists, both in vitro and in vivo, in a manner dependent on activation of the 5-HT7 receptor. Our focus on the splanchnic venous system, in attempting to understand the hemodynamic effects of chronic 5-HT infusion, was predicated on several previous findings. First, the SMV relaxed to 5-HT at a lower concentration of 5-HT than arteries contracted to 5-HT (11, 56). A number of different veins relax directly to 5-HT [goat pulmonary vein (7), sheep pulmonary vein (8, 59), rat jugular vein (19), guinea pig jugular vein (24), pig pial veins (28), monkey jugular vein (32), pig vena cava (46, 53), and rabbit facial vein (54)]. In contrast, the human saphenous vein contracted to 5-HT (39), whereas the cremaster postcapillary venules showed significant macromolecular leakage in hypercholesterolemic rats (47). Thus, a majority of studies have supported venous relaxation to 5-HT through activation of the 5-HT7 receptor.
Second, the TVC (nonsplanchnic, central compartment) had the smallest relaxation (efficacy) to both 5-HT and 5-CT and low 5-HT7 receptor expression (Figs. 1 and 2). 5-HT7 receptor protein was, however, detected in some samples but did not mediate agonist-induced relaxation. There is precedent for a 5-HT receptor being present but not active in modifying vascular tone in the 5-HT2B receptor expressed in arteries from normotensive rats (2). This contrasts with the 5-HT7 receptor expression in splanchnic veins and the serotonergic agonist-induced relaxation that occurs in the splanchnic veins. At least in the rat, there appears to be a relative selective functioning of the 5-HT7 receptor in splanchnic (and abdominal) veins. We cannot state that this response is unique to these veins, as the only other area we investigated is the thoracic compartment.
Third and finally, we have been unable to show direct arterial relaxation to 5-HT; relaxant receptors do exist in arteries but in vessels not normally thought of as regulating blood pressure [e.g., 5-HT2B and 5-HT7 receptors in the pig pulmonary artery (30), dog coronary artery (9), and canine cerebral arteries (51)]. Published work conflicts as to whether 5-HT relaxes small arterioles, vessels clearly important in blood pressure regulation (31, 43–45, 48, 54a, 58). Although not addressed in the present study, relaxation of arterioles must remain another possible mechanism by which 5-HT reduces blood pressure and exerts other hemodynamic effects.
Venodilation In Vivo
Given the ability of 5-HT to relax veins via the 5-HT7 receptor and earlier evidence that acute 5-HT-induced hypotension is caused by 5-HT7 receptor activation (15, 50), we hypothesized that venodilation contributes to the chronic depressor response of 5-HT. That idea was strongly supported, but not proven, by the finding reported here that blockade of 5-HT7 receptors with SB-269970 virtually eliminated the fall in blood pressure normally seen during chronic 5-HT infusion as well as the accompanying splanchnic venodilation. Although 5-HT has been previously shown to increase vascular capacitance during acute administration in dogs (26), consistent with a venodilator action in vivo, no studies have investigated venous responses during chronic infusion. It should be noted, however, that other agents that are relatively selective venodilators can lower blood pressure chronically (34). Furthermore, it is known that directly reducing splanchnic venous capacitance in humans can result in sustained increases in blood pressure (36). The detection of venodilation through the deeper splanchnic system was limited in that we did not examine small vessels (<250 μm in diameter) in the imaging or in vitro experiments, and thus we cannot state whether all veins of the splanchnic circulation relax to 5-HT. The importance of the splanchnic venous system in humans with regard to regulating blood pressure was recently supported by Okamoto et al. (36). In this study, the authors demonstrated that a servo-controlled abdominal binder was as effective as the standard of care, midodrine, in the treatment of orthostatic hypotension. The mechanism by which this worked is to prevent the splanchnic venous pooling believed to contribute to orthostatic hypotension.
Understanding venous circulation regulation is important because it is a physiological partner of the arterial circulation, and their integrative function assures cardiovascular homeostasis (20, 38). Regulation of arterial pressure is accomplished via integrated control of total peripheral resistance and cardiac output; the former is achieved mainly by contraction of small arteries and arterioles and the latter by regulation of cardiac pumping ability and venous return to the heart. Overall, venous return to the heart is largely dependent on the tone of smooth muscle in the small veins and venules of the splanchnic bed (23). Constriction of these vessels reduces vascular capacitance (blood storage) and redistributes “unstressed” blood volume into the central circulation, thereby augmenting cardiac output. These facts suggest that splanchnic venodilation would be expected to lower blood pressure by reducing cardiac output, which is not the hemodynamic pattern that we observed in our model (6, 13). We cannot resolve this dilemma at this time, but it is possible that autoregulation or other adaptive responses convert an initial 5-HT-induced fall in cardiac output to a reduction in vascular resistance.
There are several limitations to consider in our in vivo work. First, all the measures made with the Vevo 2100 ultrasound system were done in anesthetized rats. We may have underestimated the venodilation caused by 5-HT given that blood pressure is lowered by anesthesia. However, in this protocol, 5-HT still caused a fall in blood pressure, meaning there was a signal to measure in the anesthetized rat. Second, we do not know of any specific interaction of 5-HT with isoflurane that could affect the outcome of these experiments. Third, our tethered animals in the chronic pharmacology experiments showed a smaller fall (~13 mmHg) to 5-HT than in nontethered animals (~22 mmHg). Tethered animals are stressed, and this was also reflected in the modest venoconstriction observed in the animals shown in Fig. 7A.
5-HT in Cardiovascular Physiology
An important question is whether endogenous 5-HT could produce active venodilation in the splanchnic and abdominal regions under physiological or pathophysiological circumstances. Circulating levels of 5-HT are at a sufficient concentration to activate the 5-HT7 receptor, for which 5-HT has subnanomolar affinity (3). Specifically, we detected 2–10 ng/ml of free 5-HT in normal rats (17), levels also within the range observed in human platelet free plasma (35). Thus, it is possible that venous 5-HT7 receptors are activated under normal conditions, and this idea could be pursued in future studies. In hypertension, our and other studies have shown that basal levels of circulating 5-HT (nonplatelet) are elevated in rodents and humans (17, 57). Specifically, in the DOCA-salt model, the basal level of 5-HT was 24.9 ± 5.06 ng/ml, significantly higher than the sham at 2.7 ± 0.29 ng/ml free 5-HT. This raises the possibility that an active 5-HT7 receptor-mediated venodilator mechanism may abrogate high blood pressure; this is also of interest for future work. A recent finding in human subjects is potentially relevant here: advanced heart failure was found to be associated with elevated circulating levels of 5-HT where human heart failure is a chronic disease indisputably influenced by the venous system (42).
There are several independent lines of investigation that make wanting to understand the mechanism(s) of a chronic 5-HT-induced hypotension compelling. Page and colleagues (37) identified 5-HT as a molecule that could lower blood pressure in the hypertensive human, but the mechanisms were not uncovered; 5-HT given chronically can reduce blood pressure in the experimentally hypertensive rat (17). Importantly, administration of the 5-HT precursor 5-HTP acutely and chronically (12 days) reduced blood pressure of normal male Sprague-Dawley, spontaneously hypertensive, and Dahl salt-sensitive rats (4, 10, 16, 18, 29). Fregly et al. (21) demonstrated that chronic treatment with 5-HTP prevented the development of DOCA-salt hypertension but described no mechanism for how this occurred. Although we have not investigated splanchnic venodilation in hypertensive rats, the intent is to determine whether a 5-HT7 receptor-related mechanism contributes to reduced blood pressure (17) and thus could be a potential therapeutic approach. The present study is the first significant positive and directional lead in our understanding of at least one mechanism(s) of 5-HT-induced reduction in blood pressure at a tissue and whole animal level, and it lays the foundation for moving forward into studies in hypertension.
In summary, these findings support the ability of 5-HT infusion to act as a splanchnic venodilator both in vitro and in vivo. Venodilation was accompanied by a fall in blood pressure, and both 5-HT-induced venodilation and hypotension were blocked by the 5-HT7 receptor antagonist SB-269970.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-107495 (to S. Watts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.M.S., T.K.-B., and S.W.W. conceived and designed research; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., and S.W.W. performed experiments; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., and S.W.W. analyzed data; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., G.D.F., and S.W.W. interpreted results of experiments; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., and S.W.W. prepared figures; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., G.D.F., and S.W.W. edited and revised manuscript; B.M.S., H.O., T.K.-B., E.S.D., J.M.T., G.D.F., and S.W.W. approved final version of manuscript; S.W.W. drafted manuscript.
REFERENCES
- 1.Altura BM. Sex and estrogens and responsiveness of terminal arterioles to neurohypophyseal hormones and catecholamines. J Pharmacol Exp Ther 193: 403–412, 1975. [PubMed] [Google Scholar]
- 2.Banes AK, Watts SW. Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension. BMC Pharmacol 3: 12, 2003. doi: 10.1186/1471-2210-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268: 23422–23426, 1993. [PubMed] [Google Scholar]
- 4.Baron A, Riesselmann A, Fregly MJ. Reduction in the elevated blood pressure of Dahl salt-sensitive rats treated chronically with l-5-hydroxytryptophan. Pharmacology 42: 15–22, 1991. doi: 10.1159/000138763. [DOI] [PubMed] [Google Scholar]
- 5.Brengelmann GL. A critical analysis of the view that right atrial pressure determines venous return. J Appl Physiol 94: 849–859, 2003. doi: 10.1152/japplphysiol.00868.2002. [DOI] [PubMed] [Google Scholar]
- 7.Chand N. 5-Hydroxytryptamine induces relaxation of goat pulmonary veins: evidence for the noninvolvement of M and D-tryptamine receptors. Br J Pharmacol 72: 233–237, 1981. doi: 10.1111/j.1476-5381.1981.tb09118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocks TM, Arnold PJ. 5-Hydroxytryptamine (5-HT) mediates potent relaxation in the sheep isolated pulmonary vein via activation of 5-HT4 receptors. Br J Pharmacol 107: 591–596, 1992. doi: 10.1111/j.1476-5381.1992.tb12788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing DJ, Zgombick JM, Nelson DL, Cohen ML. LY215840, a high-affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery. J Pharmacol Exp Ther 277: 1560–1566, 1996. [PubMed] [Google Scholar]
- 10.Dalton DW, Feniuk W, Humphrey PP. An investigation into the mechanisms of the cardiovascular effects of 5-hydroxytryptamine in conscious normotensive and DOCA-salt hypertensive rats. J Auton Pharmacol 6: 219–228, 1986. doi: 10.1111/j.1474-8673.1986.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 11.Darios ES, Barman SM, Orer HS, Morrison SF, Davis RP, Seitz BM, Burnett R, Watts SW. 5-Hydroxytryptamine does not reduce sympathetic nerve activity or neuroeffector function in the splanchnic circulation. Eur J Pharmacol 754: 140–147, 2015. doi: 10.1016/j.ejphar.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick Davis R, Linder AE, Watts SW. Lack of the serotonin transporter (SERT) reduces the ability of 5-hydroxytryptamine to lower blood pressure. Naunyn Schmiedebergs Arch Pharmacol 383: 543–546, 2011. doi: 10.1007/s00210-011-0622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis RP, Pattison J, Thompson JM, Tiniakov R, Scrogin KE, Watts SW. 5-hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol 12: 4, 2012. doi: 10.1186/1471-2210-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RP, Szasz T, Garver H, Burnett R, Tykocki NR, Watts SW. One-month serotonin infusion results in a prolonged fall in blood pressure in the deoxycorticosterone acetate (DOCA) salt hypertensive rat. ACS Chem Neurosci 4: 141–148, 2013. doi: 10.1021/cn300114a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vries P, De Visser PA, Heiligers JPC, Villalón CM, Saxena PR. Changes in systemic and regional haemodynamics during 5-HT7 receptor-mediated depressor responses in rats. Naunyn Schmiedebergs Arch Pharmacol 359: 331–338, 1999. doi: 10.1007/PL00005359. [DOI] [PubMed] [Google Scholar]
- 16.Ding XR, Stier CT Jr, Itskovitz HD. Serotonin and 5-hydroxytryptophan on blood pressure and renal blood flow in anesthetized rats. Am J Med Sci 297: 290–293, 1989. doi: 10.1097/00000441-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther 325: 1031–1038, 2008. doi: 10.1124/jpet.108.136226. [DOI] [PubMed] [Google Scholar]
- 18.Echizen H, Freed CR. Long-term infusion of l-5-hydroxytryptophan increases brain serotonin turnover and decreases blood pressure in normotensive rats. J Pharmacol Exp Ther 220: 579–584, 1982. [PubMed] [Google Scholar]
- 19.Ellis ES, Byrne C, Murphy OE, Tilford NS, Baxter GS. Mediation by 5-hydroxytryptamine2B receptors of endothelium-dependent relaxation in rat jugular vein. Br J Pharmacol 114: 400–404, 1995. doi: 10.1111/j.1476-5381.1995.tb13240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink GD. Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension 53: 307–312, 2009. doi: 10.1161/HYPERTENSIONAHA.108.119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fregly MJ, Lockley OE, Sumners C. Chronic treatment with l-5-hydroxytryptophan prevents the development of DOCA-salt-induced hypertension in rats. J Hypertens 5: 621–628, 1987. doi: 10.1097/00004872-198710000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Göthert M. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part I. General background and discovery of serotonin as a basis for 5-HT receptor identification. Pharmacol Rep 65: 771–786, 2013. doi: 10.1016/S1734-1140(13)71059-4. [DOI] [PubMed] [Google Scholar]
- 23.Greenway CV. Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc 42: 1678–1684, 1983. [PubMed] [Google Scholar]
- 24.Gupta P. An endothelial 5-HT receptor that mediates relaxation in guinea-pig isolated jugular vein resembles the 5-HT1D subtype. Br J Pharmacol 106: 703–709, 1992. doi: 10.1111/j.1476-5381.1992.tb14398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol 130: 539–548, 2000. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedberg PA, Rutlen DL. Influence of serotonin on total intravascular capacity in the anaesthetized dog. Acta Physiol Scand 139: 561–568, 1990. doi: 10.1111/j.1748-1716.1990.tb08959.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer D, Clarke DE, Fozard JR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification for 5-hydroxytryptamine receptors. Pharmacol Rev 46: 157–203, 1994. [PubMed] [Google Scholar]
- 28.Ishine T, Bouchelet I, Hamel E, Lee TJ. Serotonin 5-HT7 receptors mediate relaxation of porcine pial veins. Am J Physiol Heart Circ Physiol 278: H907–H912, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Itskovitz HD, Werber JL, Sheridan AM, Brewer TF, Stier CT Jr. 5-Hydroxytryptophan and carbidopa in spontaneously hypertensive rats. J Hypertens 7: 311–315, 1989. doi: 10.1097/00004872-198904000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Jähnichen S, Glusa E, Pertz HH. Evidence for 5-HT2B and 5-HT7 receptor-mediated relaxation in pulmonary arteries of weaned pigs. Naunyn Schmiedebergs Arch Pharmacol 371: 89–98, 2005. doi: 10.1007/s00210-004-1006-6. [DOI] [PubMed] [Google Scholar]
- 31.Kurita H, Ernberg M, Tominaga K, Alstergren P, Kopp S. Effect of 5-hydroxytryptamine-2 and alpha-adrenergic receptor antagonists on the 5-hydroxytryptamine-induced decrease in rabbit masseter muscle blood flow. Arch Oral Biol 44: 651–656, 1999. doi: 10.1016/S0003-9969(99)00058-8. [DOI] [PubMed] [Google Scholar]
- 32.Leung E, Walsh LK, Pulido-Rios MT, Eglen RM. Characterization of putative 5-HT7 receptors mediating direct relaxation in Cynomolgus monkey isolated jugular vein. Br J Pharmacol 117: 926–930, 1996. doi: 10.1111/j.1476-5381.1996.tb15282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linden AS, Desmecht DJ, Amory H, Beduin JM, Lekeux PM. Cardiovascular response to exogenous serotonin in healthy calves. Am J Vet Res 57: 731–738, 1996. [PubMed] [Google Scholar]
- 34.Miller RR, Fennell WH, Young JB, Palomo AR, Quinones MA. Differential systemic arterial and venous actions and consequent cardiac effects of vasodilator drugs. Prog Cardiovasc Dis 24: 353–374, 1982. doi: 10.1016/0033-0620(82)90019-6. [DOI] [PubMed] [Google Scholar]
- 35.Monaghan PJ, Brown HA, Houghton LA, Keevil BG. Measurement of serotonin in platelet depleted plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2163–2167, 2009. doi: 10.1016/j.jchromb.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, Robertson D, Biaggioni I. Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension 68: 418–426, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page IH, McCubbin JW. The variable arterial pressure response to serotonin in laboratory animals and man. Circ Res 1: 354–362, 1953. doi: 10.1161/01.RES.1.4.354. [DOI] [PubMed] [Google Scholar]
- 38.Reddi BA, Carpenter RH. Venous excess: a new approach to cardiovascular control and its teaching. J Appl Physiol 98: 356–364, 2005. doi: 10.1152/japplphysiol.00535.2004. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LB, Radic ZS, O’Donohoe MK, Mikat EM, McCann RL, Hagen PO. Saphenous vein endothelium-dependent relaxation in patients with peripheral vascular disease. Ann Vasc Surg 6: 425–432, 1992. doi: 10.1007/BF02006997. [DOI] [PubMed] [Google Scholar]
- 40.Seitz BM, Krieger-Burke T, Fink GD, Watts SW. Serial measurement of splanchnic vein diameters in rats. Front Pharmacol 7: 116, 2016. doi: 10.3389/fphar.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitz BM, Watts SW. Serotonin-induced hypotension is mediated by a decrease in intestinal vascular resistance. Pharmacologia 5: 50–54, 2014. doi: 10.5567/pharmacologia.2014.50.54. [DOI] [Google Scholar]
- 42.Selim AM, Sarswat N, Kelesidis I, Iqbal M, Chandra R, Zolty R. Plasma serotonin in heart failure: possible marker and potential treatment target. Heart Lung Circ 26: 442−449, 2017. doi: 10.1016/j.hlc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Sellke FW, Dai HB. Responses of porcine epicardial venules to neurohumoral substances. Cardiovasc Res 27: 1326–1332, 1993. doi: 10.1093/cvr/27.7.1326. [DOI] [PubMed] [Google Scholar]
- 44.Sellke FW, Wang SY, Friedman M, Harada K, Edelman ER, Grossman W, Simons M. Basic FGF enhances endothelium-dependent relaxation of the collateral-perfused coronary microcirculation. Am J Physiol Heart Circ Physiol 267: H1303–H1311, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Struyker-Boudier HA, le Noble JL, Le Noble FA, Messing MW, van Essen H. Hypertension, the microcirculation and serotonin. Clin Physiol Biochem 3: 28–39, 1990. [PubMed] [Google Scholar]
- 46.Sumner MJ, Feniuk W, Humphrey PP. Further characterization of the 5-HT receptor mediating vascular relaxation and elevation of cyclic AMP in porcine isolated vena cava. Br J Pharmacol 97: 292–300, 1989. doi: 10.1111/j.1476-5381.1989.tb11953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuschke DA, Joshua IG, Miller FN. Comparison of early microcirculatory and aortic changes in hypercholesterolemic rats. Arterioscler Thromb 11: 154–160, 1991. doi: 10.1161/01.ATV.11.1.154. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi R, Sakai T, Furuyama Y, Kondo Y, Inoue CN, Onuma S, Iinuma K. The vasocontractive action of norepinephrine and serotonin in deep arterioles of rat cerebral gray matter. Tohoku J Exp Med 190: 129–142, 2000. doi: 10.1620/tjem.190.129. [DOI] [PubMed] [Google Scholar]
- 49.Tan T, Watts SW, Davis RP. Drug delivery, enabling technology for drug discovery and development. IPRECIO micro infusion pump: programmable, refillable and implantable. Front Pharmacol 2: 44, 2011. doi: 10.3389/fphar.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terrón JA. Role of 5-HT7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 121: 563–571, 1997. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terrón JA, Falcón-Neri A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br J Pharmacol 127: 609–616, 1999. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terrón JA, Sánchez-Maldonado C, Martínez-García E. Pharmacological evidence that 5-HT(1B/1D) receptors mediate hypotension in anesthetized rats. Eur J Pharmacol 576: 132–135, 2007. doi: 10.1016/j.ejphar.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Trevethick MA, Feniuk W, Humphrey PP. 5-Hydroxytryptamine-induced relaxation of neonatal porcine vena cava in vitro. Life Sci 35: 477–486, 1984. doi: 10.1016/0024-3205(84)90240-6. [DOI] [PubMed] [Google Scholar]
- 54.Tsuru H, Nakai S, Uchiyama T, Teranishi Y. Endothelium-independent relaxant effect of 5-hydroxytryptamine (5-HT) on the isolated rabbit facial vein. J Smooth Muscle Res 34: 101–110, 1998. doi: 10.1540/jsmr.34.101. [DOI] [PubMed] [Google Scholar]
- 54a.University of North Carolina. PDSP Ki Database. Test ligand: 5-CT. http://kidbdev.med.unc.edu/databases/pdsp.php. [27 June 2017].
- 55.Watts SW. Oh, the places you’ll go! My many colored serotonin (apologies to Dr. Seuss). Am J Physiol Heart Circ Physiol 311: H1225–H1233, 2016. doi: 10.1152/ajpheart.00538.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts SW, Darios ES, Seitz BM, Thompson JM. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3: e00103, 2015. doi: 10.1002/prp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilmoth FR, Harris PD, Miller FN. Differential serotonin responses in the skeletal muscle microcirculation. Life Sci 34: 1135–1141, 1984. doi: 10.1016/0024-3205(84)90084-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Dyer DC, Fiscus RR, Grujic Z, Wang X. 5-Hydroxytryptamine induces endothelium-independent relaxations of sheep pulmonary vein: role of cyclic nucleotide. Eur J Pharmacol 280: 335–337, 1995. doi: 10.1016/0014-2999(95)00285-S. [DOI] [PubMed] [Google Scholar]