Abstract
Pure nicotine impairs macrophage killing of Mycobacterium tuberculosis (MTB), but it is not known whether the nicotine component in cigarette smoke (CS) plays a role. Moreover, the mechanisms by which nicotine impairs macrophage immunity against MTB have not been explored. To neutralize the effects of nicotine in CS extract, we used a competitive inhibitor to the nicotinic acetylcholine receptor (nAChR)—mecamylamine—as well as macrophages derived from mice with genetic disruption of specific subunits of nAChR. We also determined whether nicotine impaired macrophage autophagy and whether nicotine-exposed T regulatory cells (Tregs) could subvert macrophage anti-MTB immunity. Mecamylamine reduced the CS extract increase in MTB burden by 43%. CS extract increase in MTB was also significantly attenuated in macrophages from mice with genetic disruption of either the α7, β2, or β4 subunit of nAChR. Nicotine inhibited autophagosome formation in MTB-infected THP-1 cells and primary murine alveolar macrophages, as well as increased the intracellular MTB burden. Nicotine increased migration of THP-1 cells, consistent with the increased number of macrophages found in the lungs of smokers. Nicotine induced Tregs to produce transforming growth factor-β. Naive mouse macrophages co-cultured with nicotine-exposed Tregs had significantly greater numbers of viable MTB recovered with increased IL-10 production and urea production, but no difference in secreted nitric oxide as compared with macrophages cocultured with unexposed Tregs. We conclude that nicotine in CS plays an important role in subverting macrophage control of MTB infection.
Keywords: tuberculosis, nicotine, macrophage, cigarette smoke, host immunity
Clinical Relevance
Cigarette smoke exposure increases risk for tuberculosis (TB), as evinced by epidemiological studies and cell and animal models. The role of nicotine is less well characterized. This current work shows that nicotine impairs macrophage control of TB by various mechanisms.
The geographic overlap of cigarette smoking prevalence and the number of latent and active tuberculosis (TB) cases is striking (1). Epidemiologic, human cellular, and murine studies indicate that cigarette smoke (CS) exposure increases the risk of developing TB (2–8). Mathematical modeling estimated that, between 2010 and 2050, 18 million more TB cases and 40 million more TB deaths will occur as a result of CS exposure; furthermore, more aggressive smoking control could save 27 million individuals from TB-related death by 2050 (9). Another analysis determined that smoking cessation could potentially eliminate 16% of the world’s TB cases (10).
Smokeless tobacco products—including nicotine-containing smoking cessation aids, chew tobacco, snuff, snus, electronic cigarettes, and various other smokeless inhalational devices—are increasingly used as “safe” alternatives to cigarettes. The Centers for Disease Control estimates that over 300 million people in more than 70 countries regularly use smokeless tobacco (11). Because nicotine is a potent immunosuppressant, if it is even partly responsible for the aforementioned link between CS and TB, then the increasing use of smokeless nicotine could have similar consequences. We previously reported that macrophages incubated with nicotine had two- to fivefold more Mycobacterium tuberculosis (MTB) burden than control macrophages (7). van Zyl-Smit and coworkers (8) showed that nicotine significantly inhibited IL-10 production, but also showed a trend toward reduced TNF-α production in monocyte-derived human macrophages infected with Mycobacterium bovis bacillus Calmette-Guerin. However, it has not been shown that the nicotine component found in CS extract—commonly used in research—is responsible, at least in part, for the ability of CS to impair macrophage killing of MTB. Furthermore, the mechanism(s) by which nicotine sabotages macrophage control of MTB is not known. Because nicotine has been shown to enhance T regulatory cell (Treg) function, and Tregs may impair host immunity against MTB (12), we also determined whether nicotine-exposed Tregs affect macrophage control of MTB.
Materials and Methods
Materials
The monocytic human cell line, THP-1, and mouse macrophage cell line, RAW 264.7 (TIB-71), were obtained from the American Type Culture Collection (Rockville, MD). MTB H37Rv was obtained from Dr. Martin Voskuil at University of Colorado Anschutz Medical Campus (Aurora, CO). 3R4F research cigarettes (University of Kentucky, Lexington, KY) were used to prepare the CS extract as previously described (13). Mice with genetic disruption for the α7 (14) or β4 (15) subunit of the nicotinic acetylcholine receptor (nAChR) were originally obtained from Dr. Arthur Beaudet (Baylor College of Medicine, Houston, TX), and the β2-nAChR subunit knockout was obtained from Dr. Marina Picciotto (Yale University, New Haven, CT) (16). All three knockouts were bred onto a C57BL/6J background at the Institute of Behavioral Genetics, University of Colorado Boulder (Boulder, CO); the strain identification for α7−/−, β2−/−, and β4−/− are B6.129S7-Chrna7tm1BAY, B6.129P2-Chrnb2tm1JPC, and B6.129S7-Chrnb4tm1 mdb, respectively. The forkhead box P3-enhanced green fluorescent protein (FoxP3-EGFP) reporter mice (B6.Cg-FoxP3tm2Tch/J) were purchased from Jackson Laboratory (Bar Harbor, ME) (17). All other key reagents are listed in the Materials and Methods in the online supplement.
For derivation of murine bone marrow–derived macrophages (BMM), isolation of murine alveolar macrophages, [125I]epibatidine binding assay, CS extract and nicotine stimulation, MTB infection of macrophages, Western immunoblotting, autophagosome detection, NF-κB p65 activation assay, cell viability assay, cytokine assay, and arginase activity assay, see the supplemental Materials and Methods in the online supplement.
Isolation of Primary Murine GFP-Foxp3 Tregs
Spleens from FoxP3-EGFP reporter mice were harvested aseptically and splenocyte cell suspensions were kept sterile throughout. Splenocytes were negatively enriched for CD4+ cells using the CD4 Negative Selection kit (cat. no. 130-104-454; Miltenyi Biotec, San Diego, CA), according to manufacturer’s instructions, and then stained with anti-CD4 efluor 450 (clone RM4-5; eBiosciences, Waltham, MA). Cells were sorted on a MoFlo XDP Cell Sorter (Beckman Coulter, Brea, CA) for the GFP+CD4+ population (Tregs) (Figure E1).
Migration Assay
THP-1 medium (0.5 ml) containing 10% FBS was added to the lower compartment of each transwell (Transwell Permeable Supports, 6.5-mm diameter polycarbonate inserts with 5.0-μm pore size; Corning Inc., Corning, NY). THP-1 cell suspensions were prepared using 5 × 105 cells/ml in serum-free THP-1 medium alone or with MTB (multiplicity-of-infection of 5), nicotine (1 μg/ml), or MTB plus nicotine (0.1 and 1 μg/ml). A 100-μl aliquot of each cell suspension solution was added to the upper compartment of each transwell and incubated at 37°C and 5% ambient CO2 for 48 hours. The migrated cells in the lower compartment were centrifuged, fixed with 4% paraformaldehyde, and stained with EMD Millipore Hemcolor Rapid Staining kit (Fisher Scientific, Waltham, MA). The number of migrated cells was quantified by microscopy (200×), counting at least 100 fields in each experiment.
Statistical Analysis
Data are presented as the mean (±SEM) of three independent experiments, unless otherwise indicated. Group means were compared by repeated-measures ANOVA using Fisher’s least significance difference test or by two-way ANOVA with Bonferroni’s post hoc test.
Results
Antagonism of nAChR Abrogates CS Extract Increase in MTB Burden in Macrophages
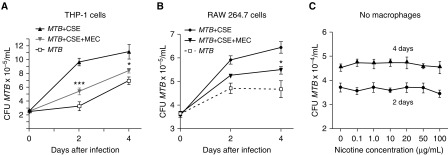
We previously found that addition of pure nicotine to human macrophages resulted in a significant increase in recovered MTB (7). To determine if the nicotine component in CS extract also has this effect, THP-1 cells were incubated with 10% CS extract with or without 50 μM mecamylamine (MEC), an nAChR antagonist that competitively inhibits nicotine from binding to its receptor. Whereas CS extract increased the burden of MTB in THP-1 cells, MEC attenuated this increase by 43% after 2 days of infection (Figure 1A). Similar effects were seen in RAW 264.7 mouse macrophages (Figure 1B). Although there was a modest decrease in macrophage viability with time (up to 4 days of culture), nicotine at 0.1 and 1 μg/ml had no significant effect on the proliferation of THP-1 cells, RAW 264.7 cells, or BMM (Figure E2). There was greater decrement in cell proliferation by 10% CS extract, but it was still greater than 80% of controls even after 4 days of incubation (Figure E3). MEC (20 and 50 μM) alone (in the absence of CS extract) had no effect on the recovery of MTB after 2 and 4 days of infection in THP-1 cells, RAW 264.7 cells, and BMM (Figure E4). MEC (50 μM) also did not alter the viability of THP-1 cells compared with CS extract exposure alone (data not shown). These findings demonstrate that the nicotine component in CS extract plays a significant role in undermining macrophage control of MTB. To determine whether nicotine directly affects the growth of MTB, MTB was cultured in 7H9 liquid medium alone or with nicotine at 0.1–100 μg/ml at 37°C for 2 and 4 days, and MTB was then quantified. As shown in Figure 1C, nicotine alone (in the absence of macrophages) had no effect on the viability or growth of MTB.
Figure 1.
Nicotine component in cigarette smoke (CS) extract plays a role in increasing the burden of Mycobacterium tuberculosis (MTB) in human and mouse macrophages. (A) THP-1 cells were infected with MTB, MTB + CS extract (CSE), or MTB + CSE + mecamylamine (MEC) for 1 hour (Day 0) and 2 and 4 days, and bacterial load determined. (B) RAW 264.7 murine macrophages were infected with MTB, MTB + CSE, or CS + MTB + MEC for 1 hour (Day 0) and 2 and 4 days, and bacterial load determined. (C) Nicotine, at indicated concentrations, was incubated with MTB for 2 and 4 days and MTB quantified. Data shown are the mean (±SEM) of three independent experiments. *P < 0.05, ***P < 0.001 compared with MTB + CSE.
Nicotine Impairment of Macrophages Depends on Binding to Specific nAChR
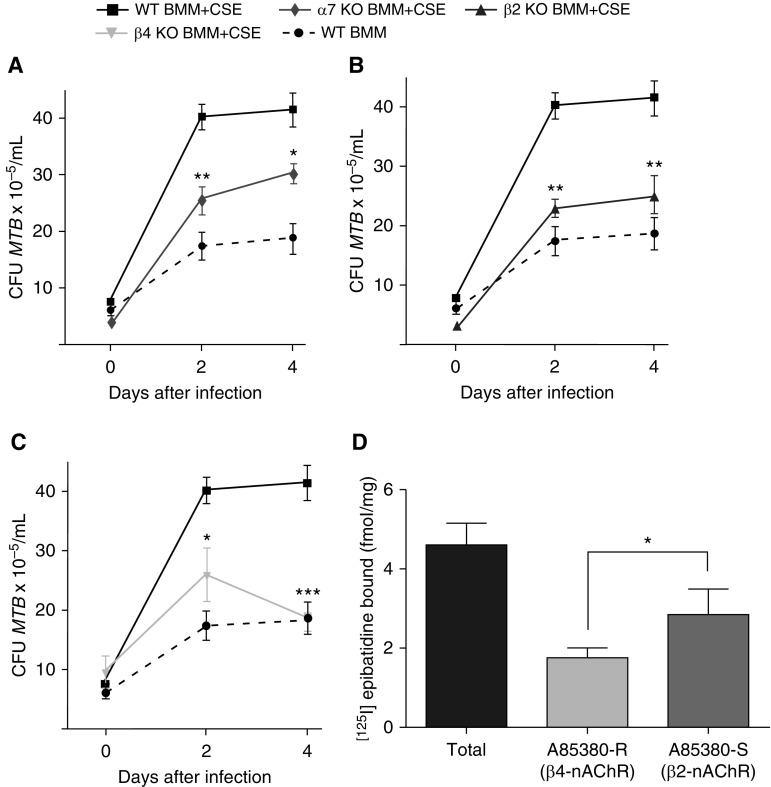
To corroborate that nicotine binding to nAChR subverts macrophage activity against MTB, we infected BMM from wild-type C57BL/6 mice and from mice with genetic disruption of the α7 subunit of nAChR in the presence of 10% CS extract. Although CS extract increased the amount of MTB recovered from wild-type BMM, there was 38% less viable MTB from BMM lacking α7-nAChR at Day 2 and 30% less by Day 4 after infection compared with similarly treated wild-type BMM (Figure 2A). Similarly, there was significantly less viable MTB recovered from CS extract–exposed BMM from mice with genetic disruption for either the β2 or β4 subunit of nAChR compared with wild-type BMM exposed to CS extract (Figures 2B and 2C).
Figure 2.
Macrophages with genetic disruption of α7, β2, or β4 subunit of nicotinic acetylcholine receptor (nAChR) attenuate the immunosuppressive effect of nicotine. Bone marrow–derived macrophages (BMM) of wild-type (WT) C57BL/6 and (A) α7-nAChR knockout (KO) mice, (B) β2-nAChR KO mice, and (C) β4-nAChR KO mice were incubated with 10% CSE for 1 hour before infection with MTB H37Rv. At 1 hour (Day 0) and 2 and 4 days after infection, intracellular MTB was quantified. Data shown are the mean (±SEM) of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT BMM + CSE. (D) [125I]epibatidine binding to detect β2 and β4 subunits of the nAChR on WT BMM. R, resistant; S, sensitive. Data shown are the mean (±SEM) of four independent experiments. *P < 0.05.
By 4 days after MTB infection, absence of the β4 subunit of nAChR (β4−/−) on the BMM showed greater reversal of the impaired macrophage control of MTB infection induced by nicotine compared with α7−/− or β2−/− BMM, albeit the differences were modest (Figure 2). To verify the expression of β2- and β4-containing nAChR in BMM, we performed a binding assay using [125I]epibatidine in the presence and absence of A85380, a competitive inhibitor to β2-containing nAChR (i.e., β2-containing nAChR is A85380 sensitive, whereas β4-containing nAChR is A85380 resistant). We found that BMM express modest levels of both β2- and β4-containing nAChRs (i.e., 2.84 ± 0.65 fmol/mg and 1.76 ± 0.25 fmol/mg, respectively; Figure 2D). We conclude that nAChRs are important in mediating the immunosuppressive effect of CS extract on macrophages against MTB.
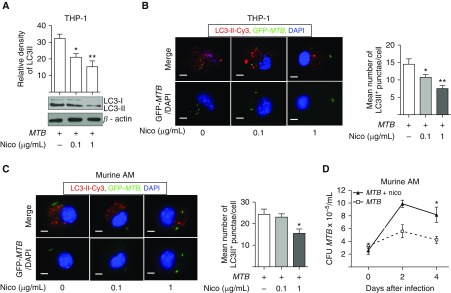
Nicotine Inhibits Autophagy in MTB-Infected THP-1 Cells and Primary Murine Alveolar Macrophages
Autophagy is an effector mechanism used by host cells to help eliminate intracellular MTB (18). Alveolar macrophages from smokers are defective in the autophagic process (19); thus, we determined whether nicotine inhibits autophagy in the context of MTB infection. We first determined qualitatively the presence of autophagy by detecting for the presence of microtubule-associated protein light chain-3-II (LC3-II), a protein critical in the formation of the autophagic membrane (20). THP-1 cells were preincubated with 0.1 or 1 μg/ml nicotine for 1 hour and then infected with MTB for 18 hours. Whole-cell lysates were prepared and the proteins separated by SDS-PAGE were immunoblotted to detect LC3-I and LC3-II isoforms. Compared with MTB infection alone for 18 hours, nicotine significantly decreased LC3-II expression in a dose-dependent fashion (Figure 3A). The number of LC3-II–positive autophagosomes in MTB-infected cells was also quantified after immunocytofluorescent staining, showing that nicotine decreased the number of LC3-II–positive punctae (autophagosomes) per cell (Figure 3B).
Figure 3.
Nicotine inhibits autophagosome formation in MTB-infected macrophages. (A) THP-1 cells were infected with MTB alone or with 0.1 and 1 μg/ml of nicotine (Nico) for 18 hours, whole-cell lysates prepared, proteins separated by SDS-PAGE, and immunoblotted for microtubule-associated protein light chain-3-I (LC3-I) and LC3-II. (B) Representative immunocytofluorescence of LC3-II–Cy3+ autophagosomes in THP-1 cells infected with GFP-MTB alone or with 0.1 and 1 μg/ml nicotine. To quantify the number of autophagosomes per cell, the numbers of LC3-II–Cy3+ punctae were counted from at least 100 random cells/well in duplicate wells for each condition. Data shown are the mean (±SEM) of three independent experiments. (C) Representative immunocytofluorescence of LC3-II–Cy3+ autophagosomes in murine alveolar macrophages (AMs) infected with GFP-MTB alone or with 0.1 and 1 μg/ml nicotine. Quantitation of the number of autophagosomes per cell is as described in B. Data shown are the mean (±SEM) of three independent experiments. (D) Murine AMs were infected with MTB alone or with 1 μg/ml nicotine. At 1 hour and 2 and 4 days after infection, macrophage-associated MTB was quantified. Data shown are the mean (±SEM) of two independent experiments performed in duplicates. Scale bars: 5 μm. *P < 0.05, **P < 0.01.
We next examined the effects of nicotine on host immunity against MTB in primary murine alveolar macrophages, because (α7)5-nAChR has been found in murine macrophages (21–23). To first determine if murine alveolar macrophages also contain β2- and β4-containing nAChR, we assayed [125I]epibatidine binding, but were unable to obtain consistently reliable results (data not shown). Nevertheless, nicotine reduced the number of autophagosomes (Figure 3C) and increased MTB burden in primary murine alveolar macrophages (Figure 3D).
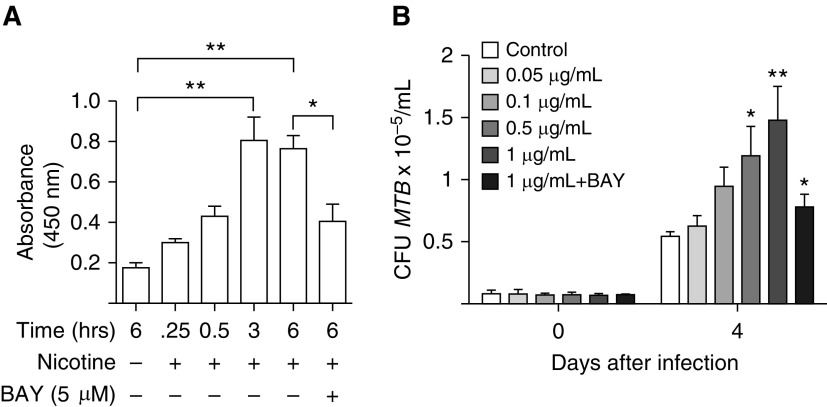
Nicotine Induces NF-κB Activation
Because we previously showed that NF-κB activation inhibits both apoptosis and autophagy in MTB-infected human macrophages, inhibiting their ability to control the infection (24), we determined whether nicotine itself induces NF-κB activation. THP-1 cells were cultured with 1 μg/ml nicotine for 0.25, 0.5, 3, and 6 hours and p65 subunit–containing NF-κB binding to its DNA consensus sequence was quantified. As shown in Figure 4A, nicotine induced p65 subunit–containing NF-κB binding and incubation with the IκBα kinase inhibitor, BAY 11-7082, significantly inhibited binding. Furthermore, inhibition of NF-κB activation significantly abrogated the nicotine-mediated increase in CFU in THP-1 cells (Figure 4B).
Figure 4.
Nicotine induces NF-κB activation in THP-1 cells. (A) NF-κB (p65) activation was quantified basally in THP-1 cells and after incubation with 1 μg/ml nicotine at the indicated time points. The cells were also incubated with 1 μg/ml nicotine plus the IκBα kinase inhibitor, BAY 11-7082 (BAY), for 6 hours, and p65 subunit–containing NF-κB binding determined. (B) THP-1 cells were incubated with various concentrations of nicotine (0.05 to 1 μg/ml) and infected with MTB. In separate wells, THP-1 cells were also incubated with 1 μg/ml nicotine, 5 μM BAY 11-7082, and infected with MTB. CFU was determined after 1 hour (Day 0) and 4 days after infection. Data shown are the mean (±SEM) of three independent experiments. *P < 0.05, **P < 0.01 compared with control cells with no nicotine exposure.
Nicotine Induces Tregs to Produce Transforming Growth Factor-β
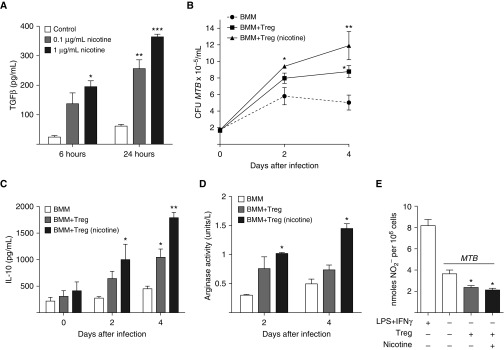
Nicotine increases the suppressive activity of Tregs (25), and because Tregs are capable of skewing macrophages toward the alternatively activated, immunosuppressive (M2) phenotype (12), it is plausible that nicotine may deactivate macrophages against MTB indirectly through activation of Tregs. To validate that nicotine activates Tregs, we obtained Tregs from the spleens of transgenic mice expressing FoxP3-EGFP, incubated the cells with 0.1 and 1 μg/ml of nicotine for 6 and 24 hours, and quantified the transforming growth factor (TGF)-β concentration in the supernatant. As shown in Figure 5A, nicotine induced TGF-β production from Tregs.
Figure 5.
Nicotine induces T regulatory cells (Tregs) to produce transforming growth factor (TGF)-β, and nicotine-exposed Tregs impair macrophage activity against MTB infection. (A) Tregs isolated from FoxP3-EGFP reporter mice were incubated in medium alone or medium containing 0.1 and 1 μg/ml nicotine for 6 and 24 hours, and TGF-β levels measured in the supernatant. *P < 0.05, **P < 0.01, ***P < 0.001 compared with respective controls. (B) BMM from FoxP3-EGFP mice were cultured alone or cocultured with unexposed or nicotine-exposed Tregs at a ratio of 100 BMM:1 Tregs, and then infected with MTB. Intracellular MTB was quantified at 1 hour and 2 and 4 days after infection. (C) IL-10 concentration in the supernatant and (D) arginase activity—calculated from the urea assay—in whole-cell lysates were measured in the MTB-infected cell cultures. (E) Nitric oxide (NO2−) concentration was measured in the supernatant of LPS + IFN-γ–stimulated BMM, MTB-infected BMM, MTB-infected BMM + unexposed Tregs, and MTB-infected BMM + nicotine-exposed Tregs. *P < 0.05, **P < 0.01 compared with BMM alone infected with MTB. Data shown are the mean (±SEM) of three independent experiments.
Nicotine-Exposed Tregs Impair Macrophage Control of MTB Infection
To determine whether nicotine-exposed Tregs impair macrophage control of MTB infection, we isolated Tregs from the spleens of FoxP3-EGFP mice and cultured them in medium alone or medium containing 1 μg/ml nicotine for 18 hours. BMM were also derived from the same mice. BMM, alone and cocultured with unexposed or nicotine-exposed Tregs at a ratio of 100 BMM:1 Treg, were then infected with MTB and bacterial load determined 1 hour, 2 days, and 4 days later. At 4 days after infection, there was significantly more intracellular MTB recoverable when BMM were cultured with Tregs than BMM alone (Figure 5B). Moreover, Tregs exposed to nicotine further increased the number of MTB recovered. Concomitant measurement of IL-10 (Figure 5C) and urea (reported as arginase activity; Figure 5D) showed the greatest increase in the BMM plus nicotine–exposed Treg cell cultures. Although nitric oxide (NO) levels (measured as NO2−) were decreased in cell culture supernatants of BMM plus Tregs as compared with BMM alone, there was no difference in NO2− measured between BMM cocultured with unexposed or nicotine-exposed Tregs (Figure 5E).
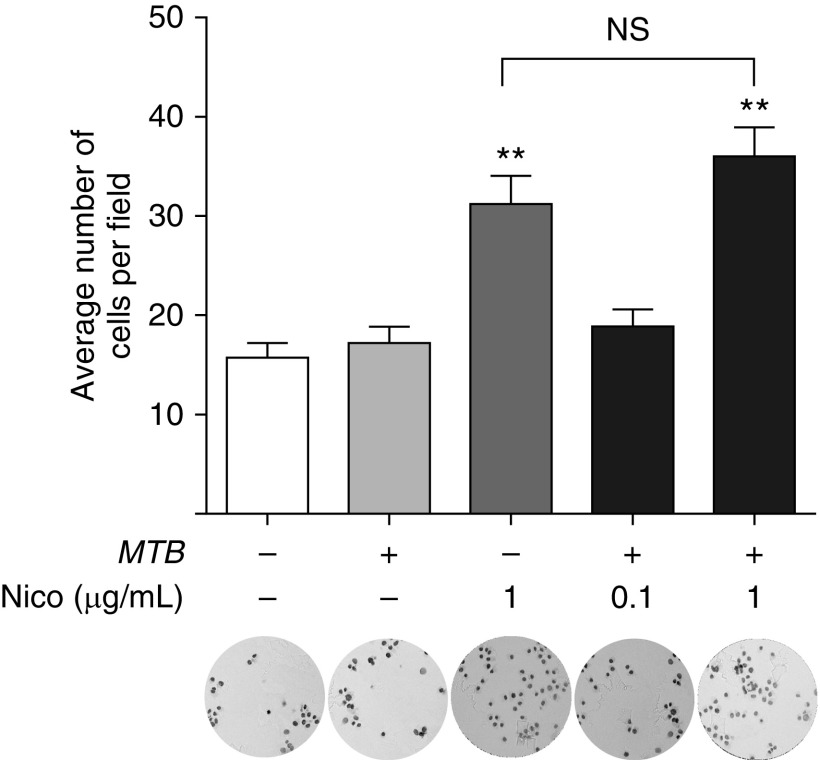
The Effects of Nicotine and MTB on Migration of THP-1 Cells
Smokers are more susceptible to TB, despite having more immune cells in their lungs. In the case of macrophages—the predominant cell type found in the lavage fluids of smokers—one possible mechanism is increased migration from the peripheral blood. Another mechanism, not necessarily mutually exclusive, is reduced migration of differentiated macrophages that were derived from blood monocytes that entered the lungs or those that originated from progenitor cells that traveled to the lungs from the yolk sac during embryogenesis (26). To begin to address whether nicotine plays a role in the lung leukocytosis seen in smokers, we examined the effects of nicotine on the migration of undifferentiated THP-1 cells. Whereas MTB alone did not increase cell migration, nicotine (1 μg/ml) significantly increased their movement (Figure 6). In asking whether there is synergy of MTB and nicotine in increasing migration, THP-1 cells were incubated with MTB along with 0.1 and 1 μg/ml nicotine and migration assay performed. Nicotine at 0.1 μg/ml combined with MTB did not affect THP-1 cell migration compared with unstimulated controls. Similarly, there was no further augmentation in migration with addition of MTB to 1 μg/ml nicotine compared with the same concentration of nicotine alone (Figure 6).
Figure 6.
Nicotine enhances migration of THP-1 cells. THP-1 cells were cultured with MTB, nicotine, or both for 48 hours, and the number of cells that migrated to the lower compartments of the transwells were stained and counted. The microscopic fields shown are representative of the corresponding conditions. The results are expressed as mean (±SD) of two independent experiments. **P < 0.01 compared with control cells (open bar). NS, not significant.
Discussion
Nicotine is found mainly in the roots and leaves of the nightshade family of plants, notably in relatively high concentrations in the tobacco plant Nicotiana tabacum. It is among the most thoroughly studied components of CS. Nicotine is immunosuppressive, as it is known to: (1) inhibit peripheral blood mononuclear cell production of IL-12, TNF-α, and IFN-γ (27); (2) inhibit the function and proliferation of T cells (28); (3) suppress the production of the antimicrobial peptide, cathelicidin (29); (4) activate the immunosuppressive cholinergic pathway (30); (5) inhibit dendritic cell activation of Th1 cells (30, 31); (6) enhance the immunosuppressive capacity of Tregs (25); (7) inhibit granuloma formation (32); and (8) antagonize apoptosis, an effector mechanism known to kill intracellular MTB in macrophages (33). We previously showed that nicotine impaired the ability of THP-1 cells and primary human alveolar macrophages in controlling MTB infection (7). Using a pharmacologic inhibitor to nAChRs as well as BMM from mice with genetic disruption of specific subunits of nAChR, we found that the nicotine component of CS extract plays a significant role in impairing macrophage inhibition of MTB proliferation. Although we found no significant effect of nicotine on macrophage proliferation, others showed that nicotine induced DNA damage in a bronchial epithelial cell line, although it required at least 1 mM (162 μg/ml) of nicotine, which is roughly 160 to 1,600 times the nicotine concentration used in our studies; furthermore, despite inducing DNA damage, nicotine had no effect on apoptosis in these epithelial cells (34). In addition, we found that nicotine alone (without macrophages) had no effect on the viability of MTB, consistent with previous reports showing that nicotine had no significant effect on the growth of various bacteria (35); furthermore, the minimum inhibitory concentration for nicotine on various strains of MTB ranged from 600 to 1,000 μg/ml, or approximately 1,000- to 10,000-fold greater nicotine concentration than we used in our macrophage studies (36).
Although measured nicotine concentrations in the venous blood of smokers vary from 4 to 72 ng/ml (37), the nicotine concentrations we chose (0.1 and 1 μg/ml) in our in vitro macrophage cultures are physiologically appropriate, because the nicotine level measured in venous blood significantly underestimates the amount of nicotine exposure of tissues due to several factors. First, nicotine in arterial blood is roughly 10-fold higher than that of venous blood; indeed, it is estimated that the amount of nicotine found in arterial blood after smoking one cigarette is equivalent to that found in venous blood after smoking 20 cigarettes (38). The significance of this phenomenon—likely due to the first-pass metabolism of nicotine through the liver—is that tissues are perfused with much higher concentrations of nicotine than what is routinely measured in (venous) blood. Second, except for adipose tissue, most tissues, including the lungs, have great affinity for nicotine, resulting in tissue levels that are several fold greater than that of blood (39). Finally, nicotine that is acquired by inhalation of CS or nicotine vapor would be expected to have significantly higher local nicotine concentrations in the lung parenchyma, due to both direct exposure and nicotine being highly permeable through cell membranes; indeed, the nicotine concentration in saliva and gingival crevicular fluid after smoking one cigarette was found to be approximately 2 and 6 μg/ml, respectively (40). In addition, MTB-infected macrophages were incubated for up to 4 days, and nicotine can be degraded by cytochrome P450 2A6, uridine diphosphate–glucuronosyltransferase, and flavin-containing mono-oxygenase, enzymes which have been found in various primary monocytes and macrophages, as well as monocytic cell lines (41). It is important to note that, although blood nicotine concentrations achieved in vapers of electronic cigarettes are generally lower than smokers, there is great overlap in the blood nicotine levels achieved, depending on the nicotine concentration of the vaping solution used as well as the puff frequency, depth, and duration; for example, in one investigation in which study subjects were asked to inhale 10 puffs of a vaping solution containing 18 mg nicotine/ml for the first 5 min and then ad libitum for the next 60 minutes, the mean venous nicotine concentration at the end of the 65-minute exposure period was approximately 25 ng/ml, a level that is well within the range seen in smokers (42).
nAChR are cation channels comprised of five subunits arranged around a central pore. There are 11 identified neuronal nAChR subunits. Various arrangements of different subunits result in an array of pentamers that comprise the complete nAChR. The predominant nAChR subtypes are homomeric α7-nAChRs and heteromeric α4β2*- and α3β4*-nAChRs (the asterisk indicates other potential subunits). Upon binding to its endogenous ligand acetylcholine—but also exogenous nicotine—there is often an increase in cytosolic calcium, resulting in the homeostatic function of acetylcholine-mediated neurotransmission, but also activation of calcium-dependent kinases and gene regulation. In contrast, nicotine has also been shown to attenuate ATP-induced release of calcium from intracellular stores into the cytoplasm (43), indicating that the effect of nAChR activation on intracellular calcium is both cell type and nAChR subtype dependent. nAChRs are also present on macrophages, consistent with the aforementioned immunomodulatory effects of nicotine (44). We found that, by preventing nicotine binding to nAChR—either by a nonselective pharmacologic inhibitor to nAChR or by use of macrophages from mice with genetic disruption of the α7, β2, or β4 subunit of nAChR, which represent all three major classes of neuronal nAChR—significantly attenuated CS extract-induced increase in MTB burden in macrophages. Although there was partial abrogation of the CS extract–induced increase in CFU with the α7nAChR−/− and β2-nAChR−/− BMM, there was greater reversal of the effects of CS extract with BMM missing the β4-nAChR subunit. Although nicotine is lipophilic and capable of entering cells by passing directly through the plasma membrane, these results indicate that nicotine binding to nAChR is an important mechanism by which nicotine impairs macrophage immunity against MTB. MEC alone did not affect the ability of THP-1 cells to control MTB; although this finding is not surprising given the absence of nicotine (or acetylcholine) in these experimental cell cultures, methyllycaconitine—a relatively specific antagonist of (α7)5-nAChR—has been shown to increase cytosolic calcium in Jurkat T cells, and thus, paradoxically behaving more like an nAChR agonist rather than its canonical function as a nAChR antagonist (45). Therefore, predicting any potential anti-TB effect of nAChR antagonists in vivo cannot be made based on its salutary effects in macrophages, as other cell types may be differentially affected.
Using [125I]epibatidine binding in the absence and presence of the β2-selective compound A85380, we demonstrated that wild-type murine BMM do express both β2- and β4-containing nAChR at low but detectable levels. However, because we used only a single concentration of [125I]epibatidine, we cannot say if the level of these nAChR subtypes differs in BMM. Regardless, to the best of our knowledge, these data represent the first demonstration that BMM express β2- and β4-nAChRs. Other studies have reported the expression of various nAChR subunits in macrophages via PCR or immunocytochemistry, but none of these studies determined whether the subunits formed ligand binding sites (46). Although the current study established that BMM do express both β2- and β4-nAChRs, the specific composition of the β2- or β4-containing nAChRs expressed in BMM remains to be determined. Others have shown that primary murine and rat alveolar macrophages possess nAChR (21–23, 43). By RT-PCR, various nAChR subunits have been found on rat alveolar macrophages, most notably, α9, α10, β1, and β2, but not α7 (43). Experiments with a recombinant mouse in which cells of the α7 lineage are labeled with the yellow fluorescent protein (α7Cre:YFP mice) revealed that 20% of the CD11c+ alveolar macrophages possessed α7-nAChR (21, 22). Our findings, that nicotine also impaired the ability of alveolar macrophage to control MTB infection, would also corroborate these findings.
One mechanism by which nicotine impairs macrophage control of MTB is via inhibition of autophagy. Nicotine induces NF-κB activation (47), and we corroborated this in the current study. Because activated NF-κB inhibits autophagy (24), nicotine-induced NF-κB likely mediates nicotine inhibition of autophagy. These findings are entirely consistent with the observation that alveolar macrophages of smokers have a defect in the autophagic process (19). Nicotine-induced NF-κB activation with subsequent inhibition of apoptosis is likely another mechanism by which nicotine inhibits macrophage effector function against MTB.
Nicotine also has the capacity to inhibit production of proinflammatory cytokines necessary for host defense against microbes (48). Matsunaga and coworkers (48) found that, in MH-S murine alveolar macrophages, nicotine increased the replication of intracellular Legionella pneumophila while simultaneously decreasing IL-6, IL-12, and TNF-α production, but had no effect on IL-10 expression. Although it may seem counterintuitive that nicotine induces NF-κB and yet inhibits proinflammatory cytokine expression, inhibition of post-transcriptional events—in large part through induction of an array of microRNAs (miRNA)—has been shown to be the mechanism for the antiinflammatory effects of nicotine (30, 49–51). The precise mechanisms by which nicotine-induced miRNAs inhibit cytokine protein production is complex, dependent on the stimuli, the cytokines induced, the miRNA involved, and direct or indirect inhibition of cytokine production. Indirect inhibition by nicotine of cytokine expression via specific miRNA may involve inhibition of the translation of TNF-α–converting enzyme mRNA, preventing the conversion of pro–TNF-α to TNF-α; another mechanism is miRNA inhibition of the translation of signal transducer and activator of transcription 3 (STAT3) mRNA, preventing the transcription of IL-6 (50). Furthermore, such intricate and indirect control is consistent with the hypothesis that nicotine-induced miRNA inhibition of inflammatory responses finely attenuates inflammatory responses rather than completely inhibiting them (50, 52). However, a completely different mechanism by which nicotine and other similar agonists—specifically, those that bind to nAChRs that contain the α7, α9, and/or α10 subunits—could reduce inflammation is via inhibition of ATP-P2X7 receptor activation of inflammasomes in human and rat monocytes, preventing the maturation of pro–IL-1β to IL-1β (53).
Unexposed Tregs cocultured with macrophages also increased recovery of viable MTB compared with MTB-infected macrophages alone, indicating that nonspecific priming of Tregs is capable of suppressing macrophages. Coculture of unexposed macrophages with nicotine-exposed Tregs resulted in an even greater recovery of MTB as well as increased levels of IL-10 and urea in the culture supernatant. These findings indicate that, in addition to its direct immunosuppressive effect on macrophages, nicotine also activates Tregs that then inhibit macrophage activity against MTB; this indirect nicotine inhibition of anti-MTB macrophage activity may occur, in part, due to biasing of macrophages to the M2 phenotype as evinced by greater urea and IL-10 production. This finding is consistent with the ability of Tregs to induce macrophages to the alternatively activated phenotype (12). In addition, although the ability of Tregs to reduce NO production by BMM would lend credence to M2 macrophage activation, there was no further reduction of NO produced in BMM cocultured with nicotine-exposed Tregs; one possible reason for this finding is that the sensitivity of the Griess assay to detect difference in NO2− may have decreased at low levels of NO2−. Although Vassallo and colleagues (54) found that CS extract directly suppressed dendritic cell function, resulting in preferential induction of Th2 cells, the effects of CS extract on the dendritic cells were not exclusively mediated by nicotine. The ability of nicotine and CS to increase cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and Foxp3 expression on T cells is one intracellular mechanism by which nicotine differentiates and activates Tregs (25, 55), inducing them to produce IL-10 and TGF-β (25) and suppressing macrophage immunity against MTB (12, 56, 57). These studies also imply that CD4+ T cells possess nAChRs, although the precise isoforms are not known. Although we found increased levels of TGF-β in the supernatant of Treg cultures stimulated with nicotine, others did not find such an effect (25). A possible reason for this discrepancy is that, whereas Wang and colleagues (25) isolated Tregs gating for CD4+ and CD25+ cells, we isolated the Tregs from Foxp3-EGFP reporter mice, with the latter method expected to yield a purer population of Tregs. In an in vivo model of acute pancreatitis, nicotine also appeared to increase TGF-β production from Tregs (58). Interestingly, others have shown that smokers’ alveolar macrophages—in a reciprocal fashion—are more likely to drive naive T cells toward the Treg phenotype (5).
Nicotine can also promote phenotypic expression of other Th cell types that can deleteriously impact host immunity against MTB. Specifically, nicotine has been shown to promote a Th2 adaptive immune response, and this may be due, in part, to nicotine binding to nAChRs that contain the α4 subunit on undifferentiated CD4+ T cells (59, 60).
Although it is a seeming paradox that smokers are more susceptible to TB despite having significantly increased numbers of macrophages in their lungs, experimental data indicate that CS-exposed macrophages are impaired in their ability to control MTB. The enhanced chemotaxis observed with nicotine suggests that increased monocyte migration from the peripheral blood is one plausible mechanism for the lung leukocytosis of smokers, although it does not rule out the possibility that CS may also increase local differentiation of lung stem cells into macrophages and/or decrease migration of differentiated macrophages. However, our finding of nicotine-induced increase in macrophage migration makes the latter hypothesis less tenable.
In summary, we found that nicotine is able to directly impair macrophages in controlling an MTB infection as well as indirectly via activation of Tregs. Whether other components of CS, such as reactive oxygen intermediates and acrolein, play a role in suppressing anti-TB immunity remains to be seen.
Acknowledgments
Acknowledgments
The authors are grateful to the Potts Memorial Foundation (Hudson, NY), the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Washington, DC), and the National Institutes of Health (Bethesda, MD) for their generosity in helping to fund this work. They also thank Ella Kushner at National Jewish Health (Denver, CO) for her expertise in isolating the murine T regulatory cells.
Footnotes
This work was supported by the Potts Memorial Foundation, the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development grant 1 I01 CX001452-01A1 (E.D.C.), and National Institutes of Health grant R21 AI121605-01.
Author Contributions: conception and design—X.B., J.A.S., and E.D.C.; analysis and interpretation—X.B., J.A.S., A.B., C.A.Z., M.P., P.M., and E.D.C.; drafting the manuscript for important intellectual content—X.B., J.A.S., A.B., M.P., and E.D.C.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0270OC on April 11, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pai M, Mohan A, Dheda K, Leung CC, Yew WW, Christopher DJ, Sharma SK. Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther. 2007;5:385–391. doi: 10.1586/14787210.5.3.385. [DOI] [PubMed] [Google Scholar]
- 2.Bishwakarma R, Kinney WH, Honda JR, Mya J, Strand MJ, Gangavelli A, Bai X, Ordway DJ, Iseman MD, Chan ED. Epidemiologic link between tuberculosis and cigarette/biomass smoke exposure: limitations despite the vast literature. Respirology. 2015;20:556–568. doi: 10.1111/resp.12515. [DOI] [PubMed] [Google Scholar]
- 3.Chan ED, Kinney WH, Honda JR, Bishwakarma R, Gangavelli A, Mya J, Bai X, Ordway DJ. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis (Edinb) 2014;94:544–550. doi: 10.1016/j.tube.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Kong Y, Barnes PF, Huang FF, Klucar P, Wang X, Samten B, Sengupta M, Machona B, Donis R, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun. 2011;79:229–237. doi: 10.1128/IAI.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Leary SM, Coleman MM, Chew WM, Morrow C, McLaughlin AM, Gleeson LE, O’Sullivan MP, Keane J. Cigarette smoking impairs human pulmonary immunity to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2014;190:1430–1436. doi: 10.1164/rccm.201407-1385OC. [DOI] [PubMed] [Google Scholar]
- 6.Shaler CR, Horvath CN, McCormick S, Jeyanathan M, Khera A, Zganiacz A, Kasinska J, Stampfli MR, Xing Z. Continuous and discontinuous cigarette smoke exposure differentially affects protective Th1 immunity against pulmonary tuberculosis. PLoS One. 2013;8:e59185. doi: 10.1371/journal.pone.0059185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shang S, Ordway D, Henao-Tamayo M, Bai X, Oberley-Deegan R, Shanley C, Orme IM, Case S, Minor M, Ackart D, et al. Cigarette smoke increases susceptibility to tuberculosis—evidence from in vivo and in vitro models. J Infect Dis. 2011;203:1240–1248. doi: 10.1093/infdis/jir009. [DOI] [PubMed] [Google Scholar]
- 8.van Zyl-Smit RN, Binder A, Meldau R, Semple PL, Evans A, Smith P, Bateman ED, Dheda K. Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages. Thorax. 2014;69:363–370. doi: 10.1136/thoraxjnl-2013-204229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu S, Stuckler D, Bitton A, Glantz SA. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343:d5506. doi: 10.1136/bmj.d5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zyl-Smit R, Dheda K. Partners in crime: the deadly synergy of tuberculosis and tobacco smoke? Mycobact Dis. 2012;2:e111. [Google Scholar]
- 11.Centers for Disease Control and Prevention & National Center Cancer Institute. Web-based Newsroom Release. More than 300 million people in at least 70 countries use smokeless tobacco. 2014 Dec 15 [accessed 2015 Mar 20]. Available from: https://www.cdc.gov/media/releases/2014/p1215-smokeless-tobacco.html.
- 12.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tollefson AK, Oberley-Deegan RE, Butterfield KT, Nicks ME, Weaver MR, Remigio LK, Decsesznak J, Chu HW, Bratton DL, Riches DW, et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic Biol Med. 2010;49:1937–1946. doi: 10.1016/j.freeradbiomed.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoli M, Léna C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using β2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enioutina EY, Myers EJ, Tvrdik P, Hoidal JR, Rogers SW, Gahring LC. The nicotinic receptor α7 impacts the mouse lung response to LPS through multiple mechanisms. PLoS One. 2015;10:e0121128. doi: 10.1371/journal.pone.0121128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gahring LC, Enioutina EY, Myers EJ, Spangrude GJ, Efimova OV, Kelley TW, Tvrdik P, Capecchi MR, Rogers SW. Nicotinic receptor α7 expression identifies a novel hematopoietic progenitor lineage. PLoS One. 2013;8:e57481. doi: 10.1371/journal.pone.0057481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wongtrakool C, Grooms K, Ping XD, Rivera H, Ward J, Roser-Page S, Roman J, Brown LA, Gauthier TW. In utero nicotine exposure promotes M2 activation in neonatal mouse alveolar macrophages. Pediatr Res. 2012;72:147–153. doi: 10.1038/pr.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai X, Feldman NE, Chmura K, Ovrutsky AR, Su WL, Griffin L, Pyeon D, McGibney MT, Strand MJ, Numata M, et al. Inhibition of nuclear factor-κB activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS One. 2013;8:e61925. doi: 10.1371/journal.pone.0061925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao GJ, Dong N, Sheng ZY. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 2010;335:553–561. doi: 10.1124/jpet.110.169961. [DOI] [PubMed] [Google Scholar]
- 26.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, et al. Tissue-resident macrophages originate from yolk-sac–derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallquist N, Hakki A, Wecker L, Friedman H, Pross S. Differential effects of nicotine and aging on splenocyte proliferation and the production of Th1- versus Th2-type cytokines. Proc Soc Exp Biol Med. 2000;224:141–146. doi: 10.1046/j.1525-1373.2000.22412.x. [DOI] [PubMed] [Google Scholar]
- 28.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83:148–156. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 29.Radek KA, Elias PM, Taupenot L, Mahata SK, O’Connor DT, Gallo RL. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe. 2010;7:277–289. doi: 10.1016/j.chom.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 31.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchet M-R, Israël-Assayag E, Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med. 2004;169:903–909. doi: 10.1164/rccm.200210-1154OC. [DOI] [PubMed] [Google Scholar]
- 33.Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis. 2007;12:1927–1943. doi: 10.1007/s10495-007-0102-8. [DOI] [PubMed] [Google Scholar]
- 34.Ginzkey C, Stueber T, Friehs G, Koehler C, Hackenberg S, Richter E, Hagen R, Kleinsasser NH. Analysis of nicotine-induced DNA damage in cells of the human respiratory tract. Toxicol Lett. 2012;208:23–29. doi: 10.1016/j.toxlet.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Cogo K, Montan MF, Bergamaschi CdeC, D Andrade E, Rosalen PL, Groppo FC. In vitro evaluation of the effect of nicotine, cotinine, and caffeine on oral microorganisms. Can J Microbiol. 2008;54:501–508. doi: 10.1139/w08-032. [DOI] [PubMed] [Google Scholar]
- 36.Allen BW, Mitchison DA. Nicotine and mycobacteria. J R Soc Med. 1983;76:893–894. doi: 10.1177/014107688307601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell MAH, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. BMJ. 1980;280:972–976. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 39.Urakawa N, Nagata T, Kudo K, Kimura K, Imamura T. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. Int J Legal Med. 1994;106:232–236. doi: 10.1007/BF01225411. [DOI] [PubMed] [Google Scholar]
- 40.Ryder MI, Fujitaki R, Lebus S, Mahboub M, Faia B, Muhaimin D, Hamada M, Hyun W. Alterations of neutrophil L-selectin and CD18 expression by tobacco smoke: implications for periodontal diseases. J Periodontal Res. 1998;33:359–368. doi: 10.1111/j.1600-0765.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 41.Jin M, Earla R, Shah A, Earla RL, Gupte R, Mitra AK, Kumar A, Kumar S. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV+ smokers. J Neuroimmune Pharmacol. 2012;7:289–299. doi: 10.1007/s11481-011-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers) Sci Rep. 2015;5:11269. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikulski Z, Hartmann P, Jositsch G, Zasłona Z, Lips KS, Pfeil U, Kurzen H, Lohmeyer J, Clauss WG, Grau V, et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir Res. 2010;11:133. doi: 10.1186/1465-9921-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galvis G, Lips KS, Kummer W. Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J Mol Neurosci. 2006;30:107–108. doi: 10.1385/JMN:30:1:107. [DOI] [PubMed] [Google Scholar]
- 45.Razani-Boroujerdi S, Boyd RT, Dávila-García MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express α7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- 46.St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc É, Morley BJ, Hao J, Simard AR. Nicotinic acetylcholine receptors modulate bone marrow-derived pro-inflammatory monocyte production and survival. PLoS One. 2016;11:e0150230. doi: 10.1371/journal.pone.0150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley-Weber CL, Dvorakova K, Crowley C, Bernstein H, Bernstein C, Garewal H, Payne CM. Nicotine increases oxidative stress, activates NF-κB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: relevance to colon carcinogenesis. Chem Biol Interact. 2003;145:53–66. doi: 10.1016/s0009-2797(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 48.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 49.Reale M, Di Bari M, Di Nicola M, D’Angelo C, De Angelis F, Velluto L, Tata AM. Nicotinic receptor activation negatively modulates pro-inflammatory cytokine production in multiple sclerosis patients. Int Immunopharmacol. 2015;29:152–157. doi: 10.1016/j.intimp.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF, Liu X. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013;23:1270–1283. doi: 10.1038/cr.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taki FA, Pan X, Lee MH, Zhang B. Nicotine exposure and transgenerational impact: a prospective study on small regulatory microRNAs. Sci Rep. 2014;4:7513. doi: 10.1038/srep07513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 53.Hecker A, Küllmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, et al. Phosphocholine-modified macromolecules and canonical nicotinic agonists inhibit ATP-induced IL-1β release. J Immunol. 2015;195:2325–2334. doi: 10.4049/jimmunol.1400974. [DOI] [PubMed] [Google Scholar]
- 54.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol. 2005;175:2684–2691. doi: 10.4049/jimmunol.175.4.2684. [DOI] [PubMed] [Google Scholar]
- 55.Hao J, Simard AR, Turner GH, Wu J, Whiteaker P, Lukas RJ, Shi FD. Attenuation of CNS inflammatory responses by nicotine involves α7 and non-α7 nicotinic receptors. Exp Neurol. 2011;227:110–119. doi: 10.1016/j.expneurol.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor β. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Zheng YS, Wu ZS, Zhang LY, Ke L, Li WQ, Li N, Li JS. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas. 2015;44:500–506. doi: 10.1097/MPA.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 59.Nordman JC, Muldoon P, Clark S, Damaj MI, Kabbani N. The α4 nicotinic receptor promotes CD4+ T-cell proliferation and a helper T-cell immune response. Mol Pharmacol. 2014;85:50–61. doi: 10.1124/mol.113.088484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Petro TM. The effect of nicotine on murine CD4 T cell responses. Int J Immunopharmacol. 1996;18:467–478. doi: 10.1016/s0192-0561(96)00054-9. [DOI] [PubMed] [Google Scholar]