Abstract
Advances in our ability to identify lymphatic endothelial cells and differentiate them from blood endothelial cells have led to important progress in the study of lymphatic biology. Over the past decade, preclinical and clinical studies have shown that there are changes to the lymphatic vasculature in nearly all lung diseases. Efforts to understand the contribution of lymphatics and their growth factors to disease initiation, progression, and resolution have led to seminal findings establishing critical roles for lymphatics in lung biology spanning from the first breath after birth to asthma, tuberculosis, and lung transplantation. However, in other diseases, it remains unclear if lymphatics are part of the overall lung remodeling process or real contributors to disease pathogenesis. The goal of this Translational Review is to highlight some of the advances in our understanding of the role(s) of lymphatics in lung disease and shed light on the critical needs and unanswered questions that might lead to novel translational applications.
Keywords: lymphatics, lymphangiogenesis, lung disease, edema, hyaluronan
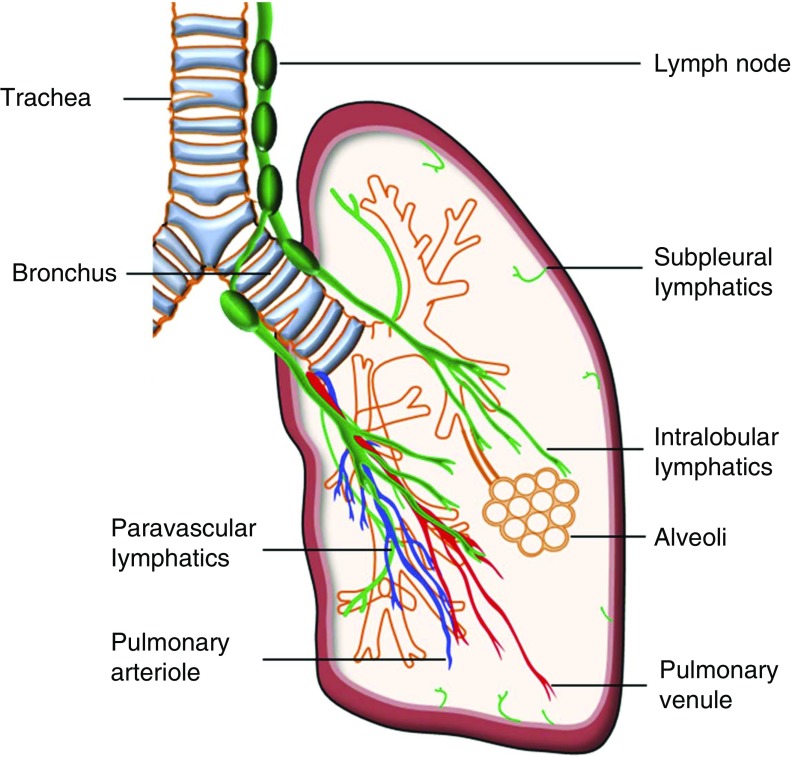
The pulmonary lymphatic system is comprised of an extensive network of vascular structures as well as tissue containing cells that are essential for immunity and the body’s defense against pathogenic material. Pulmonary lymphatic vessels are composed of a single lymphatic endothelial cell (LEC) wall with a discontinuous basement membrane, and serve as a transportation conduit for antigens and antigen-presenting cells from peripheral tissue to lymph nodes, as well as for the clearance of interstitial fluid (1). In healthy lungs, lymphatics run parallel to the major airways and respiratory bronchioles, and they also exist in close proximity to the intralobular arterioles and small veins. The number and size of lymphatics decrease significantly in the interalveolar walls, with 3.6–19% of alveoli associated with a lymphatic structure (2). In addition to the lymphatics in the lung, there is a network of subpleural lymphatics, which drains lymph from the surface of the lung (Figure 1).
Figure 1.
Schematic of lymphatic distribution in the healthy human lung. Lymphatics in the lung accompany the major airways and respiratory bronchioles, and they are also present near the intralobular arterioles and small veins. In addition, there is a network of subpleural lymphatics, which are distributed beneath pulmonary pleura. Under physiological conditions, lymphatic vessels generally do not extend to the distal alveolar spaces.
The relatively recent identification of a few important LEC markers has expanded our understanding of lymphatic biology in health and disease; Prospero-related homeodomain transcription factor (Prox) 1 is a transcription factor that is a master regulator of lymphatic lineage and is required for the differentiation of venous endothelial cells into LECs and the formation of lymphatics during embryonic development. Lymphatic vessel hyaluronan receptor (LYVE)-1 is a CD44 homolog that serves as a membrane-bound hyaluronic acid–binding site that is located on the surface of the lymphatic endothelium. Vascular endothelial growth factor (VEGF) receptor (VEGFR)-3 is a surface tyrosine kinase receptor for the lymphangiogeneic growth factors, VEGF-C and VEGF-D. Finally, podoplanin (T1α) and especially its epitope, D2-40 (3). Collectively, these markers have played essential roles in expanding current knowledge regarding lymphatic development and the role lymphatics play in various disease processes, including several affecting the pulmonary system. The suitability of these markers in identifying lung lymphatics is summarized in Table 1. Mechanisms of lymphangiogenesis have been extensively reviewed elsewhere (4). The focus of this Translational Review is to highlight some of the advances in our understanding of lymphatics and lymphangiogenesis in lung diseases, and to identify some of the critical unanswered questions.
Table 1.
Lymphatic Markers and Their Utility in Identifying Lymphatics in Lung Tissue
| Lymphatic Markers in Lung Tissue | Mouse | Human | Comment |
|---|---|---|---|
| Prox1 | Excellent marker to differentiate LECs from blood endothelial cells. Prox1 would stain neuroendocrine cells (51) | Excellent marker to differentiate LECs from blood endothelial cells | Nuclear stain, making detailed analysis of tissue more difficult |
| LYVE-1 | Largely LECs, but can stain microvascular endothelial cells (52) and some CD68+ macrophages (31) | Largely LECs, but can stain microvascular endothelial cells and some CD68+ macrophages (23) | Important to combine LYVE-1 staining with another marker, such as Prox1, to identify lung lymphatics |
| Podoplanin | Strong staining of epithelial cells (53) | Excellent marker in human lung tissue, especially D2-40 epitope (21, 23) | |
| VEGFR-3 | LECs, and can stain epithelial cells and macrophages as well | LECs, and can stain epithelial cells and macrophages as well (54) | Dual staining with podoplanin or Prox1 to accurately identify LECs |
| Chemokine (C-C motif) ligand 21 | LECs, and can stain high endothelial venules (55) | LECs, and can stain CD45-negative myofibroblast-like cells (56) | Not a specific marker for LECs |
| Chemokine decoy receptor D6 | LECs and hematopoietic cells (57) | LECs, and can stain hematopoietic cells (57) | Not a specific marker for LECs |
Definition of abbreviations: LEC, lymphatic endothelial cell; LYVE, lymphatic vessel hyaluronan receptor; Prox, Prospero-related homeodomain transcription factor; VEGFR, vascular endothelial growth factor receptor.
Lymphatics Are Critical for the First Breath and for Lung Development
During embryonic development, lymphatics originate from venous endothelial cells under the influence of transcription factors and regulatory proteins, including VEGF-C, Prox1, and collagen- and calcium-binding epidermal growth factor domain-1 with additional well established reliance on signaling through the tyrosine kinase receptor, VEGFR-3 (5). Pulmonary lymphatics appear to play a critical role in lung development in preparation for birth and in newborn viability. Collagen- and calcium-binding epidermal growth factor domain 1−/− and VEGFR-3kd/kd (kinase dead) mice lacking the ability to form lymphatics do not survive beyond birth with the immediate development of respiratory failure, despite having what appears to be otherwise normally developed pulmonary parenchyma. In one study (6), respiratory failure did not correlate with the size of the chylous effusion, and it remains unclear if lymphatic abnormalities affected the development of other organs. However, the lack of lung lymphatics resulted in diminished clearance of interstitial fluid, preventing expansion of the lung tissue in the immediate postnatal period. These findings clearly link lymphatic development with proper postnatal pulmonary mechanical function, and may offer new insights into respiratory failure in premature infants (6). It is important to note in this context that there is perhaps a threshold effect, because VEGF-D−/− mice exhibit decreased lung lymphatics, but normal lung development (7). More recently, studies have shown that VEGF-D may also play critical roles in lung vascular development. For instance, VEGF-D−/− mice are protected from the effects of hyperoxia (8), and the mutation in human VEGF-D (V118M) leads to aberrant protein dimerization, resulting in increased angiogenesis and worsening oxygen diffusion (9).
Further evidence of the devastating consequences of abnormalities in pulmonary lymphatic development is seen in the case of pulmonary lymphangiectasia. This condition is characterized by dilated saccular lymphatics with the clinical sequelae of respiratory distress, cyanosis, and, at times, both chylous and nonchylous pleural effusions (10). Relatively little is known regarding the pathogenesis and mechanisms leading to pulmonary lymphangiectasia. Recent evidence generated from a double-transgenic mouse with a doxycycline activating (Tet-On) Clara cell secretory protein promoter, resulting in enhanced VEGF-C expression, showed that pathologic changes with increased VEGF-C expression included the formation of dilated sheets of lymphatics associated with respiratory failure, pleural effusions, and chylothorax, a clinicopathological picture similar to that in humans with pulmonary lymphangiectasias (10). The response to VEGF-C induction varied in severity with the most pronounced findings and highest mortality in newborn mice exposed to doxycycline starting at Embryonic Day 15.5. Once pulmonary lymphangiectasia was present, there was not a meaningful response to the withdrawal of VEGF-C overexpression or the inhibition of VEGFR-2 and VEGFR-3 (10). The role of VEGF-C in human pulmonary lymphangiectasia remains unclear.
In addition, generalized lymphatic anomaly and Gorham-Stout disease are both characterized by systemic lymphatic malformations that can affect the lung and present with chylothorax (11). In one study, these anomalies have been associated with increased lung lymphatic area compared with healthy controls (0.6 versus 3.5%). Furthermore, the percentage of lymphatic vessels with robustly proliferating LECs was significantly higher than control subjects, and markedly more elevated in the pediatric compared with the adult population (11).
Fate of the Lymphatic Vasculature in Lung Disease
Asthma
Asthma is an inflammatory disease characterized by reversible, and at times irreversible, airflow obstruction and pulmonary symptoms of variable severity. Chronic inflammation in patients with asthma leads to mucosal edema, subepithelial fibrosis, and alterations in the extracellular matrix (12). Animal models of asthma, as well as human studies, have demonstrated increased angiogenesis and up-regulation of known proangiogenic factors, such as VEGF (13). However, there have been very few investigations into lymphatic involvement in asthma.
Experimental models of chronic airway inflammation using Mycoplasma pulmonis have demonstrated robust evidence of both angiogenesis and lymphangiogenesis under the influence of elevated growth factors, including VEGF-C and VEGF-D (14). Blockade of VEGFR3 resulted in reduced lymphangiogenesis, but not angiogenesis (14). An additional consequence of VEGFR3 blockade was increased mucosal edema with reduced regional lymph node enlargement, implicating diminished lymphatics in airflow obstruction. Interestingly, elimination of the offending pathogen (M. pulmonis) with antimicrobials resulted in a regression of angiogenic changes, but persistence of newly formed lymphatics (14).
TNF-α levels increase within 3 days after M. pulmonis infection, which precedes both new blood and lymphatic vessel formation (15). Blockade of TNF-α activity through the use of either an anti–TNF-α antibody or using TNFR−/− mice resulted in dampened angiogenesis and lymphangiogenesis after M. pulmonis exposure (15). Dexamethasone therapy also diminished the expression of both TNF-α and IL-1β, a proinflammatory cytokine, after M. pulmonis infection, but only if given at the initiation of the infectious period (16). When dexamethasone treatment was delayed for 14 days after the initial infection, both TNF-α and IL-1β expression continued to rise, similar to untreated, but infected, control animals (16). Blood vessel remodeling was, however, reversed with delayed dexamethasone therapy, but, despite limiting further lymphangiogenesis, lymphatic structural changes persisted that had occurred during the initial 14 days after infection (16).
In a mouse model of allergic airway disease, Th2 cells, which secrete IL-4 and IL-13 in response to allergen stimulation, were found to be potent inhibitors of lymphangiogenesis in vitro and in vivo, through Janus kinase 1 and signal transducer and activator of transcription 6–dependent pathways, leading to down-regulation of Prox1 and decreased lymphangiogenesis (17). Similarly, Th1 cells, limit both angiogenesis and lymphangiogenesis through secretion of IFN-γ (18). The exact roles that prolymphangiogenic and antilymphangiogenic factors play in acute or chronic airway inflammation have yet to be completely characterized.
Data gained from patients with asthma support diminished lymphangiogenesis. Postmortem tissue analysis demonstrated lymphatics in close proximity to the mucosal surface of both large and small airways in addition to smooth muscle proliferation and fibrotic changes (12). However, despite elevated levels of VEGF-C and VEGF-D, lymphatic density was diminished compared with control subjects (12), suggesting that, on balance, antilymphangiogeneic factors are more preponderant in asthma.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction with variable elements of airway and pulmonary parenchymal remodeling due to recurrent exposure, most typically to cigarette smoke (19). Very little is known about the role lymphatics play in the pathogenesis of COPD. Previous examination of human lung tissue identified increased lymphatic density based upon LYVE-1 and D2-40 staining in subjects with COPD (20). Another study evaluating lung tissue that was obtained from patients with COPD (stages I–IV), smokers without COPD, and nonsmoker control subjects identified the most pronounced increase in lymphatic formation within the alveolar parenchyma of patients with stage IV disease (21). Although not as pronounced, lymphangiogenesis appeared to also occur in the bronchiolar and arterial walls of patients with severe disease as compared with healthy control subjects, but the changes were proportional to the level of surrounding tissue remodeling (21). Furthermore, expressions of chemokine (C-C motif) ligand 21 and lymphatic and lymphatic chemokine scavenger receptor D6 were up-regulated, suggesting enhanced lymphatic transport of activated immune cells in COPD (21). Despite this clinical evidence, little is known about the role of lymphatic vessels in COPD pathogenesis and how modulation of lymphangiogenesis might affect progression of disease.
Interstitial Lung Disease
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by fibrotic alterations in the lung architecture that substantially disrupt the lungs’ capacity for ventilation and gas exchange (22). In IPF lung tissue sections, increased alveolar lymphangiogenesis and lymphatic area correlates with worsening disease severity (23). Image quantification demonstrated that increasing lymphatic density was associated with increased organizing and fibrotic collagen as well as progressive physiologic dysfunction, as determined by decreased forced vital capacity and carbon monoxide diffusing capacity (24).
In a mouse model of radiation-induced lung fibrosis, lymphatic vessel density decreased 1 week after ionizing radiation and preceded the development of fibrosis at week 16 (25), suggesting potential roles for the lymphatic system in disease pathogenesis. In the bleomycin model, at 28 days, lymphatic density was similar between treated and untreated mice. However, it is shown in the bleomycin-induced fibrosis model that platelet-derived growth factor receptor (PDGFR)-β–positive mural cells block lymphatic vessels from draining macromolecules, especially hyaluronan (26), a critical molecule in lung injury and repair. These two models of experimental fibrosis suggest that impairment of lung lymphatics could be key to the development of fibrosis. This apparent discrepancy in lymphatic changes between observations in human fibrotic lung disease and animal models is not completely understood, but could potentially be explained by the change of lymphatic function. It is possible that, even though lymphatic density is increased in human disease, these newly formed lymphatics are not functional, either through the presence of PDGFR+ mural cells (26) or because these lymphatics are interrupted and not in a continuum (27). However, in an IPF clinical trial, imatinib—a PDGFR inhibitor—failed to improve survival or lung function, raising questions about the importance of these observations in the pathogenesis of human pulmonary fibrosis (28). Nevertheless, further studies are needed to completely characterize the contribution of the lymphatic vasculature to fibrotic lung diseases.
Lung Transplant
Lung transplantation is the only therapy for irreversible terminal pulmonary disease. Despite improvement in surgical techniques and advances in immunomodulatory therapy, acute allograft rejection, the single most important risk factor for the development of chronic lung rejection, still affects roughly 30% of lung transplant recipients (29).
The role of lymphangiogenesis in the setting of solid organ transplantation, including lung transplantation, remains unclear. Blocking lymphangiogenesis is traditionally believed to limit the innate and adaptive immune response to donor tissue (1). In transplanted lung tissue, patients with histological evidence of acute allograft rejection displayed significantly increased density of Prox1-positive lymphatic vessels (30). In addition, blocking lymphangiogenesis through the use of VEGF-C/D–neutralizing VEGFR-3 IgG Fc fusion protein chimera, anti–VEGFR-3, or VEGFR-3 gene knockout appeared to decrease both acute and chronic rejection in a rat model for cardiac transplantation, resulting in improved graft survival (23). Importantly, it should be noted that the lack of surgical lymphatic anastomosis at the time of graft implantation greatly impairs fluid homeostasis and trafficking of macromolecules and immune cells.
However, there is also evidence supportive of lymphangiogenesis in promoting allograft survival. We have recently provided evidence that density of pulmonary lymphatic vessels decreased in the setting of acute allograft rejection in a mouse model of orthotopic, single-lung transplantation, and stimulation of lymphangiogenesis with the delivery of VEGF-C156S, a selective VEGFR-3 agonist, attenuates allograft rejection (31). Mechanistically, we determined that low–molecular weight hyaluronic acid accumulation, which has previously been reported to promote inflammation and chronic allograft rejection (32), is mitigated by an increase in lymphangiogenesis (31), suggesting that therapeutically induced lymphatic regeneration could be a viable option to combat rejection response in the lung grafts.
Tuberculosis
Mycobacterium tuberculosis (MTB) infection characteristically results in granumolatous structural changes in affected tissue, including the lungs (33). CD11b+ macrophages dominate the cellular makeup of granulomas, resulting in elevated levels of VEGF-C and, subsequently, perigranulomatous lymphatic proliferation after both high-dose intraperitoneal bacillus Calmette-Guerin injection and aerosolized MTB infection (34). Granulomas resulting from localized pulmonary MTB infection, however, demonstrated a 50% increase in LYVE-1+ lymphatic density as compared with affected liver tissue after systemic bacillus Calmette-Guerin exposure. In addition, the inhibition of lymphangiogenesis in both infectious models resulted in diminished T cell proliferation, suggesting a critical role for lymphangiogenesis in the adaptive immune response.
Extrapulmonary lymphatic infection with MTB is common. Until recently, the role of lymphatics in MTB infection has largely been believed to be related to immune cell and pathogen transport. However, podoplanin+/LYVE-1+ LECs isolated from lymph nodes or extranodal lymphatics have been shown to harbor MTB (35). In vitro, human LECs internalize MTB through the mannose receptor with subsequent intracellular bacterial replication. In contrast to MTB infection in myeloid cells, which become necrotic after bacterial internalization, human LECs survived with heightened bacterial burden. Alteration in LEC response to infection was initiated by IFN-γ, resulting in induction of autophagy and nitric oxide production, mitigating MTB proliferation (35). Lymphatics appear to play a clear role in the cellular response to mycobacterial infection in tissue, but the available evidence also supports LECs as critical for the persistence of infection.
In human subjects with active or latent TB, serum VEGF-C concentrations, in addition to proangiogenic those of VEGF-A and VEGFR-2, were significantly higher in patients with more extensive pulmonary involvement as compared with those with limited pulmonary involvement, with a further decline in concentrations in the latent TB cohort (36). Furthermore, there was also a direct correlation between the level of circulating VEGF-A, VEGF-C, VEGFR-2, and bacterial burden (36).
Sarcoidosis
Sarcoidosis is a systemic disease characterized by noncaseating granuloma formation with a predilection for lung tissue and associated lymphatic structures. Pulmonary manifestations include a variety of parenchymal radiographic changes, including both nodular and reticular abnormalities, as well as hilar lymphadenopathy. Extranodal lymphatics are also affected by granulomatous structural changes (37). Serum VEGF and VEGF-C levels are significantly higher in patients with pulmonary sarcoidosis, and granulomas are implicated as a critical source of elevated VEGF and VEGF-C (38). Tubular structures that have been described as irregular have been identified around sarcoid granulomas, with the suggestion that they may represent newly formed lymphatics (37). However, these irregular tubular structures express VEGFR-2 as opposed to VEGFR-3, generating much debate over their classification and function (38). Regardless, what is known about the immunologic contribution to sarcoid pathogenesis, including the interaction between alveolar macrophages and Th1 cells under antigen provocation (39, 40), supports the necessity of structures allowing for the egress of inflammatory cells from peripheral granulomas to nodal tissue, particularly in advanced disease.
Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a rare lung disease characterized by the proliferation of cells with mutations in the tuberous sclerosis complex 1 or 2 gene. There are a number of different lymphatic abnormalities, such as lymphangioleiomyomas and chylous effusions, in LAM (41). Furthermore, LAM cell clusters, consisting of LAM cells surrounded by LECs, are thought to metastasize using the lymphatic system (41). Aberrant lymphangiogenesis in LAM is driven by increased VEGF-D levels, which are present in approximately 70% of patients with LAM, and are a biomarker for diagnosis and therapeutic response (42). Although LAM cells are thought to be the source of VEGF-D, recent evidence suggests an important contribution of circulating mononuclear cells (43).
Mechanisms Driving Lymphangiogenesis in Lung Diseases
Postnatal lymphatic development occurs primarily through sprouting of new vessels from existing lymphatic vessels (44). LECs have been visualized as developing intracellular vacuoles that appear to coalesce with vacuoles of adjacent cells to form larger compartments and hollow vascular structures (45). The stimuli necessary to drive lymphangiogenesis are mostly well characterized in animal models of persistent airway and lung inflammation, where cells from the bronchus-associated lymphoid tissue generate VEGF-C and VEGF-D, leading to enhanced lymphangiogenesis (14, 46; reviewed in Reference 47). In addition, other chemokines and cytokines have been shown to contribute to lymphangiogenesis stimulation, such as TNF-α and IL1-β, or inhibition, such as IL4 and IL13, as discussed previously here.
Another mechanism that could potentially drive lymphangiogenesis is resident or circulating lymphatic endothelial progenitor cells. Mounting evidence suggests the presence of circulating progenitor LECs, which could contribute to postnatal lymphangiogenesis during health and disease (48). Although many surface markers have been used to identify these progenitor cells, their direct contribution to lymphangiogenesis in experimental models of lung disease has, to date, not been shown.
Critical Unanswered Questions
Studies investigating animal models of lung disease and human disease have shown dysregulation of the lymphatic vasculature (Table 2). Although it has been established that lymphatics and lymphangiogenesis play important role(s) in animal models of acute and chronic airways inflammation and in acute lung allograft rejection, the role(s) of the lymphatics, and their potential contribution to other parenchymal- and airways-related lung disease, remain poorly understood. Future preclinical studies using selective modulators of lymphangiogenesis (inducers, such as VEGF-C156S, or inhibitors, such as VEGFR-3–neutralizing antibodies) will evaluate the beneficial or harmful effects of lymphatics in various respiratory diseases. Studies addressing the following issues are of critical importance:
-
•
What is the fate of the lymphatic vasculature in lung diseases? Changes in the lymphatic vasculature and LECs in lung diseases are not completely understood. Beyond descriptive analysis of lymphatic vessel density changes during disease processes, the remodeling of lymphatic vessels and cell-specific changes in LECs have never been characterized. Are these cells similar to “normal” lung LECs? If LECs are different from normal LECs, which genes, pathways, or networks are differentially regulated? Do these differences translate into important disease-related therapeutic applications? Strategies to isolate and study these cells with novel sequencing platforms are needed to better understand lymphangiogenesis in lung disease.
-
•
Findings from animal models of lung fibrosis suggest dysfunction of the lymphatic vasculature; however, conclusive evidence proving that modulating lymphangiogenesis would lead to a phenotypic change with improvement in lung fibrosis is missing. In this regard, nintedanib, the recently approved drug to treat pulmonary fibrosis, is a potent pan–tyrosine kinase inhibitor. Its targets include many of the receptors that drive angiogenesis and lymphangiogenesis—in addition to epithelial cell homeostasis (49). It is intriguing to hypothesize that some of the beneficial effects could be due to its effects on the endothelium.
-
•
In animal models of chronic airway inflammation (M. pulmonis), TB, and lymphangiectasia, induction of lymphangiogenesis is irreversible, in marked contrast to the reversible angiogenesis response. Whether the irreversibility of newly formed lymphatic vessels contributes to disease pathogenesis or the persistence of disease is a subject that requires intense investigation.
-
•
Noninvasive means of assessing lymphatic function in normal and diseased lungs are nonexistent. Most current techniques depend on wet-to-dry weight of the lungs to evaluate fluid retention, or on the rate of dye clearance to determine lymphatic drainage. Further strategies using transit time ultrasonic flow meters might help us understand the lymphatic function in a more direct manner. Furthermore, advances in intravital microscopy and introduction of reporter mice with fluorescent lung lymphatics have made it possible to directly monitor lymphatic in the lung parenchyma (50). Developing less invasive methods to image lung lymphatics will constitute a major advance in the field.
-
•
High-throughput screens of existing drugs and small molecules to identify targets that modulate lymphangiogenesis could accelerate translational applications of preclinical findings.
Table 2.
Summary of Human and Animal Clinical and Experimental Observations Regarding Lymphatic Vessel Formation and Their Role in Disorders Affecting the Lungs
| Clinical and Experimental Findings | |
|---|---|
| Embryonic development | |
| Humans | Increased saccular dilation in pulmonary lymphangiectasia (7) |
| Animals | Increased dilated lymphatics with similar phenotype to human pulmonary lymphangiectasia due to embryonic VEGF-C overexpression (7) |
| Decreased lymphatic vessel density due to deletion of Prox1, VEGF-C, CCBE-1, or VEGFR-3 (4, 5) with increased perinatal morbidity | |
| Asthma | |
| Humans | Decreased lymphatic vessel density (8) |
| Animals | Decreased lymphatic vessel density with blockade of VEGFR-3 (10), TNF-α blockade (11), dexamethasone administration (decreasing TNF-α and IL-1β) (11), Th2 secretion of IL-4 and IL-13 through the JAK1 and STAT6 pathways (13), Th1 secretion of IFN-γ (14) |
| COPD | |
| Humans | Increased lymphatic vessel density (15, 16) |
| Interstitial lung disease | |
| Humans | Increased lymphatic vessel density (19, 20) |
| Animals | Newly formed lymphatic vessels after radiation exposure regress with subsequent development of pulmonary fibrosis (21) |
| Unchanged lymphatic vessel density with bleomycin exposure, but evidence of impaired lymphatic vessel function (22) | |
| Lung transplant | |
| Humans | Increased lymphatic vessel density in the setting of acute rejection (22) |
| Animals | VEGF-C induced lymphangiogenesis-attenuated allograft rejection through improved hyaluronan clearance (24) |
| Tuberculosis | |
| Humans | Increased serum VEGF-C (29) |
| Animals | Increased lymphatic vessel density in localized pulmonary tuberculosis infection (27) |
| LECs harbor Mycobacterium tuberculosis (28) |
Definition of abbreviations: CCBE, collagen- and calcium-binding epidermal growth factor domain; COPD, chronic obstructive pulmonary disease; JAK, Janus kinase; LEC, lymphatic endothelial cell; Prox, Prospero-related homeodomain transcription factor; STAT, signal transducer and activator of transcription; Th, T helper cell; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Conclusions
Over the past decade, much progress has been made in understanding lymphatic biology (Figure 2) and changes of the lymphatic vessels in lung disease and, in some instances, their contribution to disease pathogenesis. It is now clear that lymphatics play critical roles in lung disease, and that these changes go beyond just remodeling. Further studies, focused on understanding how modulation of lymphangiogenesis could lead to enhanced outcome in preclinical models, could have potential translational applications. Identifying off-the-shelf modulators of lymphangiogenesis might offer translational opportunities for novel therapeutic startegies.
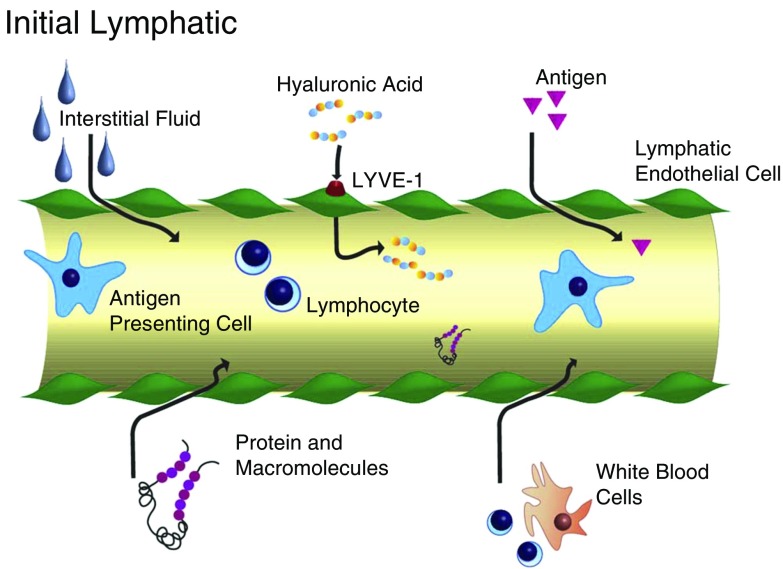
Figure 2.
Schematic of an initial lymphatic. Hyaluronic acid is transported via lymphatic vessel hyaluronan receptor (LYVE)-1, whereas interstitial fluid, proteins, macromolecules, white blood cells, and antigens enter initial lymphatics through valve-like openings between lymphatic endothelial cells.
Footnotes
This work was supported in part by National Institutes of Health/National Heart, Lung, and Blood Institute grants 5T32HL007633-30 (B.S.) and by R01-HL130275-01A1 (S.E.-C.).
Originally Published in Press as DOI: 10.1165/rcmb.2016-0290TR on April 26, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Kambouchner M, Bernaudin JF. Intralobular pulmonary lymphatic distribution in normal human lung using D2-40 antipodoplanin immunostaining. J Histochem Cytochem. 2009;57:643–648. doi: 10.1369/jhc.2009.953067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baluk P, McDonald DM. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 6.Jakus Z, Gleghorn JP, Enis DR, Sen A, Chia S, Liu X, Rawnsley DR, Yang Y, Hess PR, Zou Z, et al. Lymphatic function is required prenatally for lung inflation at birth. J Exp Med. 2014;211:815–826. doi: 10.1084/jem.20132308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25:2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Paquet-Fifield S, Harris NC, Roufail S, Turner DJ, Yuan Y, Zhang YF, Fox SB, Hibbs ML, Wilkinson-Berka JL, et al. VEGF-D promotes pulmonary oedema in hyperoxic acute lung injury. J Pathol. 2016;239:152–161. doi: 10.1002/path.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey E, Cui Y, Casey A, Stoler JM, Ai X, Ma D, Handin R, Sliz P, Vargas SO, El-Chemaly SY. Pulmonary vasculopathy associated with FIGF gene mutation. Am J Pathol. 2017;187:25–32. doi: 10.1016/j.ajpath.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao LC, Testini C, Tvorogov D, Anisimov A, Vargas SO, Baluk P, Pytowski B, Claesson-Welsh L, Alitalo K, McDonald DM. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res. 2014;114:806–822. doi: 10.1161/CIRCRESAHA.114.303119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori M, Dictor M, Brodszki N, López-Gutiérrez JC, Beato M, Erjefält JS, Eklund EA. Pulmonary and pleural lymphatic endothelial cells from pediatric, but not adult, patients with Gorham-Stout disease and generalized lymphatic anomaly, show a high proliferation rate. Orphanet J Rare Dis. 2016;11:67. doi: 10.1186/s13023-016-0449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebina M. Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution; beyond thickening of airway smooth muscle layers. Allergol Int. 2008;57:165–174. doi: 10.2332/allergolint.O-07-497. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 14.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao LC, Baluk P, Feng J, McDonald DM. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK, Lee SH. Th2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun. 2015;6:6196. doi: 10.1038/ncomms7196. [DOI] [PubMed] [Google Scholar]
- 18.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 20.Hardavella G, Tzortzaki EG, Siozopoulou V, Galanis P, Vlachaki E, Avgousti M, Stefanou D, Siafakas NM. Lymphangiogenesis in COPD: another link in the pathogenesis of the disease. Respir Med. 2012;106:687–693. doi: 10.1016/j.rmed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Mori M, Andersson CK, Graham GJ, Löfdahl CG, Erjefält JS. Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respir Res. 2013;14:65. doi: 10.1186/1465-9921-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 23.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci USA. 2009;106:3958–3963. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara AR, Cosgrove GP, Janssen WJ, Huie TJ, Burnham EL, Heinz DE, Curran-Everett D, Sahin H, Schwarz MI, Cool CD, et al. Increased lymphatic vessel length is associated with the fibroblast reticulum and disease severity in usual interstitial pneumonia and nonspecific interstitial pneumonia. Chest. 2012;142:1569–1576. doi: 10.1378/chest.12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Wilder J, Rietz C, Gigliotti A, Tang X, Shi Y, Guilmette R, Wang H, George G, Nilo de Magaldi E, et al. Radiation-induced impairment in lung lymphatic vasculature. Lymphat Res Biol. 2014;12:238–250. doi: 10.1089/lrb.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meinecke AK, Nagy N, Lago GD, Kirmse S, Klose R, Schrödter K, Zimmermann A, Helfrich I, Rundqvist H, Theegarten D, et al. Aberrant mural cell recruitment to lymphatic vessels and impaired lymphatic drainage in a murine model of pulmonary fibrosis. Blood. 2012;119:5931–5942. doi: 10.1182/blood-2011-12-396895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebina M, Shibata N, Ohta H, Hisata S, Tamada T, Ono M, Okaya K, Kondo T, Nukiwa T. The disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis. Lymphat Res Biol. 2010;8:199–207. doi: 10.1089/lrb.2010.0008. [DOI] [PubMed] [Google Scholar]
- 28.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR Imatinib–IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 29.Liu K, Vergani A, Zhao P, Ben Nasr M, Wu X, Iken K, Jiang D, Su X, Fotino C, Fiorina P, et al. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol. 2014;51:300–310. doi: 10.1165/rcmb.2013-0362OC. [DOI] [PubMed] [Google Scholar]
- 30.Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, Passlick B, Geissler HJ. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90:406–411. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D’Agostino E, Goldberg HJ, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest. 2015;125:4255–4268. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, Kelly FL, Huang H, Kreisel D, Palmer SM, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189:556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 34.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol. 2015;185:432–445. doi: 10.1016/j.ajpath.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner TR, de Souza Carvalho-Wodarz C, Repnik U, Russell MR, Borel S, Diedrich CR, Rohde M, Wainwright H, Collinson LM, Wilkinson RJ, et al. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J Clin Invest. 2016;126:1093–1108. doi: 10.1172/JCI83379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar NP, Banurekha VV, Nair D, Babu S. Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLoS One. 2016;11:e0146318. doi: 10.1371/journal.pone.0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kambouchner M, Pirici D, Uhl JF, Mogoanta L, Valeyre D, Bernaudin JF. Lymphatic and blood microvasculature organisation in pulmonary sarcoid granulomas. Eur Respir J. 2011;37:835–840. doi: 10.1183/09031936.00086410. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita M, Mouri T, Niisato M, Kowada K, Kobayashi H, Chiba R, Satoh T, Sugai T, Sawai T, Takahashi T, et al. Heterogeneous characteristics of lymphatic microvasculatures associated with pulmonary sarcoid granulomas. Ann Am Thorac Soc. 2013;10:90–97. doi: 10.1513/AnnalsATS.201209-078OC. [DOI] [PubMed] [Google Scholar]
- 39.Prasse A, Zissel G, Lützen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 40.Zissel G, Prasse A, Müller-Quernheim J. Immunologic response of sarcoidosis. Semin Respir Crit Care Med. 2010;31:390–403. doi: 10.1055/s-0030-1262208. [DOI] [PubMed] [Google Scholar]
- 41.Glasgow CG, El-Chemaly S, Moss J. Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:196–206. doi: 10.1183/09059180.00009311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, et al. MILES Trial Group. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1:445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Y, Steagall WK, Lamattina AM, Pacheco-Rodriguez G, Stylianou M, Kidambi P, Stump B, Golzarri F, Rosas IO, Priolo C, et al. Aberrant SYK kinase signaling is essential for tumorigenesis induced by TSC2 inactivation. Cancer Res. 2017;77:1492–1502. doi: 10.1158/0008-5472.CAN-16-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 45.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 46.Baluk P, Adams A, Phillips K, Feng J, Hong YK, Brown MB, McDonald DM. Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am J Pathol. 2014;184:1577–1592. doi: 10.1016/j.ajpath.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Kröber SM, Greinix H, Rosenmaier A, Karlhofer F, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 49.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 50.Hong M, Jung E, Yang S, Jung W, Seong YJ, Park E, Bramos A, Kim KE, Lee S, Daghlian G, et al. Efficient assessment of developmental, surgical and pathological lymphangiogenesis using a lymphatic reporter mouse and its embryonic stem cells. PLoS One. 2016;11:e0157126. doi: 10.1371/journal.pone.0157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGovern S, Pan J, Oliver G, Cutz E, Yeger H. The role of hypoxia and neurogenic genes (Mash-1 and Prox-1) in the developmental programming and maturation of pulmonary neuroendocrine cells in fetal mouse lung. Lab Invest. 2010;90:180–195. doi: 10.1038/labinvest.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol. 2003;285:H1917–H1938. doi: 10.1152/ajpheart.00983.2002. [DOI] [PubMed] [Google Scholar]
- 53.Vanderbilt JN, Dobbs LG. Characterization of the gene and promoter for RTI40, a differentiation marker of type I alveolar epithelial cells. Am J Respir Cell Mol Biol. 1998;19:662–671. doi: 10.1165/ajrcmb.19.4.3121. [DOI] [PubMed] [Google Scholar]
- 54.Takizawa H, Kondo K, Fujino H, Kenzaki K, Miyoshi T, Sakiyama S, Tangoku A. The balance of VEGF-C and VEGFR-3 mRNA is a predictor of lymph node metastasis in non-small cell lung cancer. Br J Cancer. 2006;95:75–79. doi: 10.1038/sj.bjc.6603209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, Buckley CD, Montecucco C, Pitzalis C. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol. 2007;171:1549–1562. doi: 10.2353/ajpath.2007.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKimmie CS, Fraser AR, Hansell C, Gutiérrez L, Philipsen S, Connell L, Rot A, Kurowska-Stolarska M, Carreno P, Pruenster M, et al. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J Immunol. 2008;181:3353–3363. doi: 10.4049/jimmunol.181.5.3353. [DOI] [PubMed] [Google Scholar]