Abstract
IL-25 and IL-4 signaling in the setting of infection or allergic responses can drive Type 2 inflammation. IL-25 requires the IL-17 receptor B (IL-17Rb) to mediate signaling through nuclear factor κ B (NF-κB) transcriptional activation. Despite the known coexistence of these two cytokines in the Type 2 inflammatory environment, collaborative signaling between the IL-4 and IL-25 axes is poorly explored. Here we demonstrate IL-4 induction of both IL-25 and IL-17Rb protein in human lung tissue culture, primary alveolar macrophages, and the THP-1 monocytic cell line. IL-4 treatment triggers gene transcription for both IL-25 and IL-17Rb but does not alter the receptor mRNA stability. Genetic antagonism of the IL-4 second messenger, signal transducer and activator of transcription 6 (STAT6), with small interfering RNA (siRNA) blunts IL-17Rb mRNA induction by IL-4. IL-25 induces signaling through the canonical NF-κB pathway, and STAT6 or NF-κB signaling inhibitors prevent IL-17Rb expression. Blockade of IL-25 with monoclonal antibody suppresses NF-κB activation after IL-4 treatment, and IL-4–mediated induction of IL-17Rb is suppressed by IL-25 siRNA. IL-25 and IL-17Rb promoter regions harbor putative NF-κB and STAT6 consensus sites, and chromatin immunoprecipitation identified these transcription factors in complex with the IL-17Rb 5′ untranslated region. In bronchoalveolar lavage RNA preparations, IL-25 and IL-17Rb mRNA transcripts are increased in asthmatics compared with healthy control subjects, and IL-25 transcript abundance correlates strongly with IL-4 mRNA levels. Thus, these results indicate that IL-4 signaling up-regulates the IL-25 axis in human monocytic cells, and that IL-25 may provide autocrine signals in monocytes and macrophages to sustain IL-17Rb expression and predispose to alternative activation.
Clinical Relevance
This work provides new mechanistic insight into a relatively unexplored receptor (IL-17Rb) and its molecular regulation in innate immune responses.
In T-helper type 2 (Th2)-associated disease states such as allergic asthma, IL-4 signaling plays a key role in the immune response by inducing eosinophilia, airway hyperresponsiveness, mucin expression, and granulocyte activation, thereby serving as a target for biologic therapeutics (1). IL-4 activation of IL-4R and Janus kinase 3 (JAK3) leads to phosphorylation and dimerization of signal transducer and activator of transcription 6 (STAT6). Phosphorylated Stat6 (p-Stat6) migrates to the nucleus to bind IL-4–responsive gene promoters and drive the Th2 inflammatory cascade (2). The IL-25 cytokine (also known as IL-17E) is also implicated in eliciting a Th2-mediated immune response via activation of NF-κB through tumor necrosis factor receptor associated factor 6 (TRAF-6) activation (3, 4). IL-25 and IL-17Rb signaling has been described in epithelial cells (5) and T cells. Allergen challenge promotes expression of IL-25 and IL-17Rb in serum and whole lungs, and IL-17Rb is detected within a myeloid cell population (6). The importance of IL-25 signaling is underscored by the fact that animals deficient in IL-17Rb fail to mount a robust Type 2 or Th2 cytokine response (6). T cells are known to up-regulate IL-17Rb in response to IL-25, suggesting a feed-forward cellular response that can drive Th2 differentiation (7). Although monocytic cells and tissue macrophages are known to be IL-4 sensitive, their expression of IL-25 and IL-17Rb is not well characterized. We hypothesized that IL-4 is important for molecular regulation of the IL-25 and IL-17Rb signaling axis in monocytic cells, and that the mechanistic cross talk between these factors may be important for monocytic cell differentiation in Type 2 inflammation signaling.
In human studies, patients with asthma have been shown to display higher levels of IL-25 than normal, and allergen exposure was shown to increase IL-17Rb+ cells (8, 9). Furthermore, allergen exposure was found to induce IL-17Rb on dendritic cells (DCs) in asthmatics, but not nondiseased subjects (10). Thus, there are mounting data suggesting that the IL-25 signaling axis is pivotal in the development of Type 2 immunity in airways. Because monocytic cells do not express antigen receptors, the link between allergen exposure and IL-17Rb induction is not mechanistically established. In patients with allergic asthma, more Th2 cells are present in the blood and airways (11), and cells rapidly produce IL-4 after antigen exposure (12). In the present study, we characterized induction of the IL-25/IL-17Rb axis by the IL-4 cytokine in human lung tissue, primary alveolar macrophages, and cells from the monocytic THP1 cell line. We observed that IL-4 rapidly induced IL-25 cytokine and IL-17Rb via increased gene transcription, an effect that depended on STAT6 activation by IL-4. Beyond the early IL-4 and STAT6 signals, we observed sustained activation of IL-17Rb transcription, which was mediated by NF-κB activation and nuclear translocation. This later phase of gene activation seems to be mediated by IL-25 ligation of IL-17Rb, as blocking IL-25 signaling with an antibody or small interfering RNA (siRNA) reduced IL-4–dependent IL-17Rb induction. This study demonstrates that IL-4 can cooperatively stimulate human monocytes and macrophages with IL-25 to sustain an IL-17Rb+–activated phenotype with a feed-forward autocrine receptor induction of IL-17Rb by IL-25.
Materials and Methods
Cell Cultures and Reagents
Immortalized human bronchial epithelial Beas-2B cells and THP1 monocytic cells were purchased from the ATCC (Bethesda, MD). BEAS-2B cells were maintained in HITES medium supplemented with 10% FBS. THP1 cells were grown in RPMI medium with 10% FBS. All cells were kept in a 37°C incubator with 5% CO2 as described previously (13). Alveolar macrophage primary cells were obtained by bronchoscopy of human donor lungs (see below) using an Ambu bronchoscope and sterile PBS. Bronchoalveolar lavage (BAL) samples were strained through a 100 μg filter before centrifugation at 1,000 rpm for 15 min and resuspended in RPMI with 5% serum and colistin and amphotericin B in addition to standard supplements. Anti-IL-17Rb antibody was obtained from R&D Systems (Minneapolis, MN). p-STAT6, STAT6, and nucleolin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). IκB kinase (IKK), p-IKK, IƙB, and p-IƙB antibodies were obtained from Cell Signaling (Danvers, MA). IL-25–blocking antibody and recombinant IL-25 and IL-4 were obtained from R&D Systems. Buffering chemicals and phosphatase inhibitor mixtures were obtained from Sigma (St. Louis, MO). Nuclear isolation for chromatin immunoprecipitation (ChIP) and QIAprep Spin Miniprep kits were obtained from Qiagen (Hilden, Germany). Talon metal affinity resin was obtained from Clontech (Mountain View, CA). All materials used in the experiments were of the highest grade and commercially available.
Human Tissues
Donor human lungs not accepted for transplant were obtained through the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents. Donor medical records were deidentified and IRB approval was not required to access these tissues. Organs were considered appropriate for study if there was no evidence of parenchymal lung disease, gas exchange was within normal limits before harvest, and organs could be processed with < 6 h cold ischemic time. Bronchoscopy was performed as described above for alveolar macrophages. For tissue slices, localized lesions (e.g., solitary nodules) were avoided, and single lung segments were dissected and warmed in a 37°C water bath for 30 min. Low-melting-point agarose (2%) in PBS (Invitrogen Ultrapure) was maintained at 37°C. The lung segments were filled with agarose by instillation into airways via syringe with an 18-gauge cannula and inspected for appropriate expansion, followed by airway clamping and packing in ice for 30 min or until the agarose had set. Tissue was cut to block size (2 cm × 1 cm × 1 cm) and sliced in ice-cold saline with a vibratome (Leica VT 1200) at a slice thickness of 300 μm. Uniform slices were sectioned into 1 cm × 1 cm sections and cultured in RPMI containing penicillin-streptomycin and amphotericin B without serum in 12-well dishes at 37°C in a tissue incubator with 5% CO2. The medium was changed after 2 h and experiments were performed in 1 ml of medium after overnight incubation. Slices were treated with 50 ng/ml of IL-4 for 4 h and then homogenized for immunoblotting and mRNA analysis.
Results
IL-4 Induces IL-25 and IL-17Rb in Human Lung Tissue, Alveolar Macrophages, and the THP1 Monocytic Cell Line
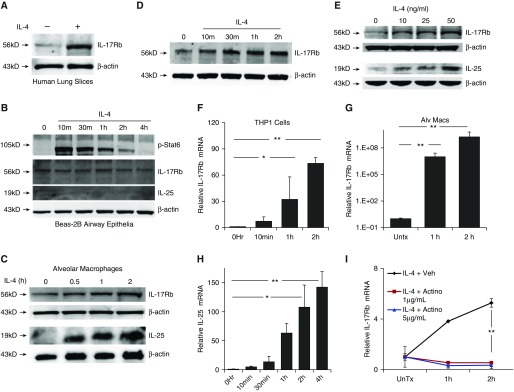
To evaluate induction of the IL-25 axis in lung tissue by IL-4, we treated human lung slices in an organ culture system with 50 ng/ml recombinant IL-4 for 4 h and observed robust IL-17Rb induction as an ∼56 kD protein (Figure 1A). To determine whether this tissue IL-4 response was cell-type specific, we evaluated cellular responses from a human airway epithelial cell line BEAS-2B and alveolar macrophages. Although the BEAS-2B epithelial cells responded to IL-4 with appropriate phosphorylation of the ∼110 kD STAT6 (p-Stats6) second messenger, very little baseline or induced expression of IL-17Rb or IL-25 was seen after 4 h of IL-4 treatment (Figure 1B). Human alveolar macrophages from BAL, however, showed increased expression of both IL-25 cytokine (∼18 kD) levels and IL-17Rb (Figure 1C). Likewise, monocytic (THP1) cells displayed time-dependent IL-17Rb induction after IL-4 stimulation (Figure 1D). IL-25 was also induced in a concentration-wise fashion after IL-4 treatment in these myeloid-like cells (Figure 1E). Quantitative PCR showed significant increases in expression of IL-25 and IL-17Rb mRNA after IL-4 exposure in both THP1 cells (Figures 1F and 1H) and IL-17Rb in alveolar macrophages (Figure 1G). These effects of Il-4 were not seen in Beas-2B cells (data not shown). To assess the underlying mechanisms, cells were treated with actinomycin D to inhibit mRNA synthesis and exposed to IL-4. In these studies, the IL-4–stimulated induction of IL-17Rb mRNA was abolished, suggesting that new IL-17Rb transcripts resulted from increased gene transcription rather from an extended half-life of abundant mRNAs (Figure 1H).
Figure 1.
IL-4 induces IL-25 and IL-17 receptor B (IL-17Rb). (A) Human lung slices were incubated with IL-4 (50 ng/ml) for 4 h before immunoblotting for IL-17Rb. (B) Beas-2B cells treated with IL-4 (50 ng/ml) were harvested at the indicated time points and assessed by immunoblotting for signal transducer and activator of transcription 6 (STAT6) phosphorylation at Tyr641 (p-Stat6), IL-17Rb, and IL-25 cytokine. (C) Human alveolar macrophages (alv macs) treated with IL-4 (50 ng/ml) induce expression of IL-17Rb and IL-25 compared to untreated (Untx). (D and E) THP1 cells increase IL-17Rb in response to IL-4 over time (D, 50 ng/ml IL-4) and varying concentrations of IL-4 (E, 4 h exposure). (F and G) IL-17Rb mRNA is induced with IL-4 treatment over 2 h in THP1 cells (F) and in human alveolar macrophages (G) by quantitative PCR. (H) THP cells also produce IL-25 mRNA in response to IL-4. (I) THP1 cells were treated with actinomycin D (Actino) at the indicated concentrations for 30 min before IL-4 (50 ng/ml) and IL-17Rb mRNA was assayed using quantitative PCR. Data are representative of at least three experiments performed on different days. *P < 0.05 and **P < 0.01 by two-sided t test (G and I) or ANOVA (F and H). veh, vehicle.
IL-4 Induction of IL-17Rb Requires STAT6 Activation
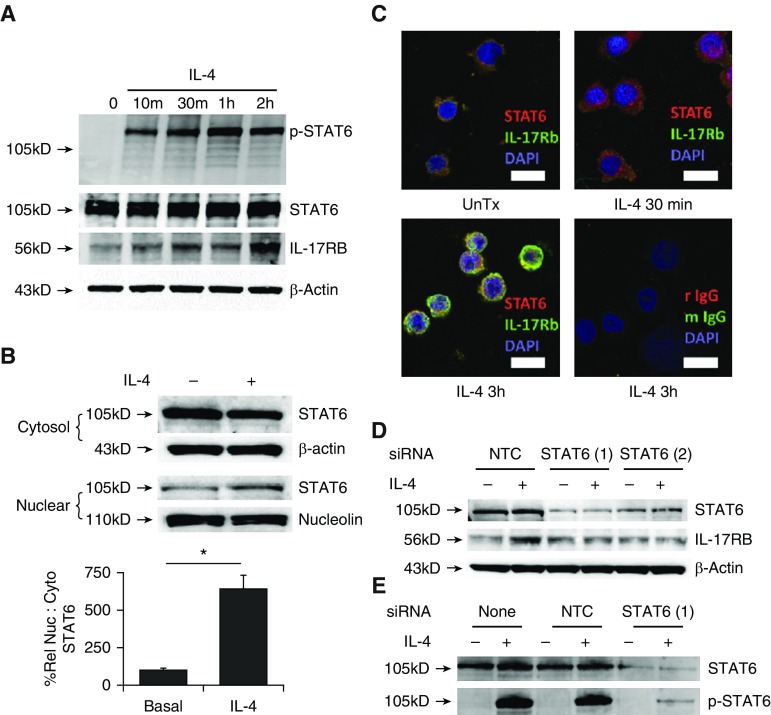
We next evaluated the necessity of STAT6 signaling for the induction of IL-17Rb in THP1 cells. With IL-4 treatment, there was rapid activation of STAT6 with phosphorylation of tyrosine 461 (Figure 2A), which preceded the appearance of IL-17Rb. In a subpopulation of cells, a fraction of STAT6 was trafficked to the nucleus within 30 min (Figures 2B and 2C, arrows), and we observed by immunoblotting (Figure 2A) and fluorescence microscopy (Figure 2C) that IL-17Rb protein was highly expressed in THP1 cells 3 h after IL-4 treatment. IL-17Rb staining was not exclusive to the membrane during this induction, presumably because it was being translated and trafficked at these time points. Cellular depletion of STAT6 using two separate siRNA constructs effectively blocked IL-4–driven induction of IL-17Rb by IL-4 (Figure 2D) by reducing the cellular mass of total and phosphorylated STAT6 (Figure 2E).
Figure 2.
STAT6 activation is required for IL-4 induction of IL-17Rb. (A) IL-4 (50 ng/ml) treatment induces rapid phosphorylation of Tyr641 on Stat6 with no change in the levels of total Stat6 and an increase in IL-17Rb levels. (B) Nuclear and cytosolic cell preparations were immunoblotted for STAT6 after 2 h of IL-4 treatment. The relative nuclear to cytosolic (%Rel Nuc:Cyto) STAT6 band intensity corrected for loading is graphed below. (C) Fluorescence micrographs (scale bar, 10 μm; IgG stain control as indicated) showing nuclear localization of STAT6 in some cells 30 min after IL-4 treatment, and increased IL-17Rb expression 3 h after IL-4 exposure. (D and E) Cells transfected with one of two separate siRNAs for STAT6 exhibit a substantially blunted STAT6 abundance and IL-17Rb response to IL-4 (50 ng/ml for 4 h) compared to nontargeting control (NTC) siRNA (D), with decreased p-STAT6 signal after IL-4 treatment (E). Blots are representative of three experiments performed on different days; images are representative of two separate experiments. *P < 0.05 by two-sided t test. siRNA, small interfering RNA.
NF-κB Activation Potentiates IL-17Rb Expression
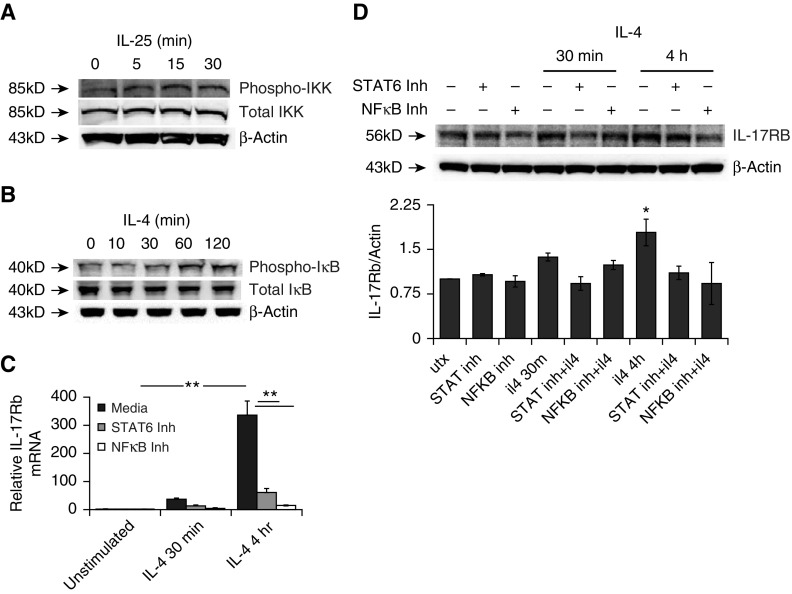
Because IL-4 stimulation induced both IL-25 and its cognate receptor in THP cells, we next tested whether the NF-κB signal response was elicited by IL-25 and after IL-4 treatment. IKK phosphorylates IκB to liberate the p65 subunit of NF-κB for nuclear translocation and signaling. IL-25 was sufficient to induce activation of IKK in THP1 cells, and we observed that IKK was rapidly phosphorylated after IL-25 exposure (Figure 3A). We also observed that IκB, the negative regulator of NF-κB, was phosphorylated after IL-4 stimulation (Figure 3B, upper blot). This phosphorylation of IκB led to its degradation (Figure 3B, middle panel). To determine whether this activation of NF-κB impacted transcription of IL-17Rb, we used small-molecule antagonists for either STAT6 (AS1517499) (14) or NF-κB (BAY11–7082) (15) and evaluated the cellular response to IL-4 treatment. Both inhibitors significantly suppressed induction of IL-17Rb mRNA (Figure 3C) and protein (Figure 3D).
Figure 3.
NF-κB signaling augments IL-4 induction of IL-17Rb. (A) Treatment with IL-25 activates IκB kinase (IKK). (B) The IκB inhibitor of NF-κB is phosphorylated and degraded after IL-4 treatment (50 ng/ml). (C and D) A 30-min pretreatment of cells with inhibitors of STAT6 (AS1517499) or NF-κB (Bay-11–0782), each at 10 μM, prevented the induction of IL-17Rb mRNA (C) and protein (D). Blots are representative of three experiments performed on different days, and the graph represents an aggregate of three experiments with *P < 0.05 or **P < 0.01 by ANOVA against untreated cells (utx) and other 4-h conditions. Inh, inhibitor; NF-κB, nuclear factor κ B.
IL-25 Signaling Is Required to Sustain IL-17Rb Expression
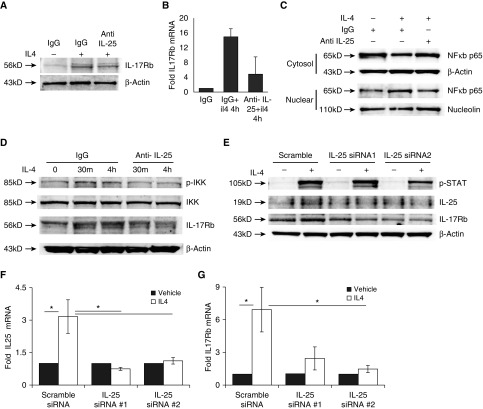
Because IL-4 induces IL-25 expression and NF-κB signaling is required for sustained IL-17Rb expression, we evaluated whether IL-25 itself might generate this second signal needed to reinforce its receptor abundance. When THP1 cells or macrophages were pretreated with monoclonal antibody (mAb) to IL-25 for 1 h before IL-4 treatment, we observed a distinct reduction in both IL-17Rb protein induction (Figure 4A) and mRNA (Figure 4B). We next evaluated cells for nuclear translocation of the NF-κB subunit p65 (also known as RELA) with IL-4 treatment and observed that p65 nuclear abundance was increased after IL-4 treatment, an effect that was blocked by the IL-25 antibody (Figure 4C). Accordingly, IL-25 blockade caused a decrease in IKK phosphorylation 30 min and 4 h after IL-4 treatment (Figure 4D). Although IL-17Rb levels were similarly induced after 30 min of IL-4, the abundance of IL-17Rb elicited at the 4 h time point was significantly reduced when the IL-25 blocking antibody was present (Figure 4D). To corroborate the effects observed with the IL-25 antibody, we used two separate constructs of siRNA targeting IL-25, and again observed that IL-4 failed to induce robust IL-17Rb expression at 4 h when the cells were treated with siRNA, despite intact STAT6 phosphorylation responses (Figure 4E). These siRNA constructs likewise suppressed IL-4 induction of both IL-25 (Figure 4F) and IL-17Rb (Figure 4G) mRNA expression. Of note, IL-4 induction of IL-25 transcript expression was also dependent upon STAT6 and NF-κB (Figure E1 in the online supplement). Taken together, these results indicate that IL-4 induced IL-25, and IL-17Rb expression was initiated by STAT6 and potentiated by subsequent IL-25–dependent NF-κB activation.
Figure 4.
IL-25 is required for sustained induction of IL-17Rb by IL-4. THP1 cells were incubated with isotype control IgG or a blocking antibody to IL-25 (1 μg/ml) for 1 h before IL-4 treatment (50 ng/ml, 4 h). IL-17Rb induction was then evaluated by immunoblotting (A) or mRNA analysis (B). Antagonism of IL-25 prevented induction of IL-17Rb. (C) Nuclear migration of the NF-κB p65 subunit (RELA) is increased at 4 h after IL-4 treatment (50 ng/ml), and this effect is blocked by IL-25 blockade. (D) Phosphorylation of IKK is increased after IL-4 treatment (50 ng/ml) but reduced by IL-25 blockade, and IL-17Rb is less potently induced by IL-4 when the IL-25–blocking antibody is present. (E–G) Cells transfected with one of two siRNAs for IL-25 and exposed to IL-4 (4 h, 50 ng/ml) do not show induction of IL-25 or IL-17Rb protein expression (E) or mRNA (F and G) compared with scrambled transfected controls. Blots are representative of three experiments performed on different days. *P < 0.05 by ANOVA compared with scrambled siRNA-transfected cells treated with IL-4.
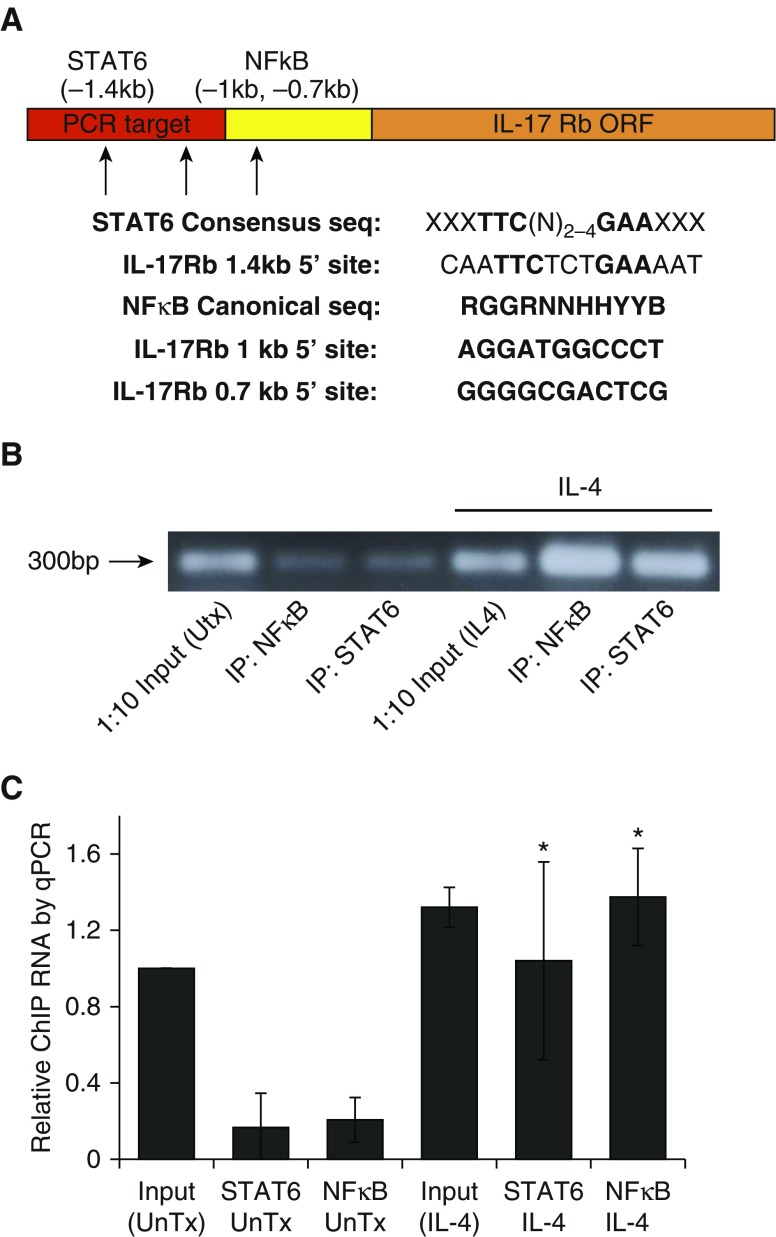
STAT6 and NF-κB Associate with the Promoter for IL17Rb in Response to IL-4
To evaluate the specific association of transcription factors STAT6 and NF-κB RELA protein with the 5′ untranslated region of the IL-17Rb open reading frame gene, we evaluated the primary sequence of the promoter to identify putative binding sites for NF-κB (16) and STAT6 (17). We identified one consensus match for STAT6 binding and two putative NF-κB binding domains (Figure 5A). The IL-25 promoter also possesses consensus sequences for these transcription factors (Figure E2). We performed ChIP with antibodies to NF-κB or STAT6 after a 4-h treatment with IL-4, followed by PCR amplification of a small (225 bp) amplicon from the IL-17Rb promoter that contains the consensus STAT6 site and is within 700 bp of both of the NF-κB sites identified. Figure 5B shows an amplification of this 300 bp fragment from PCR reactions with whole nuclear genomic DNA (Input, which is diluted 1:10) or DNA preparations after ChIP for NF-κB and STAT6 without and with IL-4 treatment for 4 h.
Figure 5.
IL-4 induces STAT6 and NF-κB association with the IL-17Rb promoter. (A) Promoter map upstream of the IL-17Rb open reading frame (ORF) with consensus and actual sequences for STAT6 and NF-κB. (B) Electrophoresis showing a PCR product (300 bp) using template DNA from control nuclear preparations (lane 1, Input, diluted 1:10), chromatin immunoprecipitation (ChIP) of NF-κB (lane 2) or STAT6 (lane 3), control nuclear preparations from IL-4–treated cells (lane 4), and ChIP after IL-4 treatment (lanes 5 and 6). (C) Quantitative PCR evaluation of the IL-17Rb promoter fragment after IP for STAT6 or NF-κB in control cells and IL-4–treated (50 ng/ml) cells. The gel image is representative of three ChIP experiments, and the graph represents an aggregate from quantitative PCR for three biologically separate ChIP experiments. *P < 0.05 by ANOVA against ChIP samples from untreated cells. ORF, open reading frame.
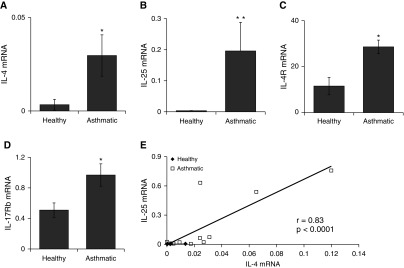
IL-25 and IL-17Rb Transcripts Are Increased in Asthmatic Airway Specimens
To evaluate whether the link among Type 2 inflammation, IL-4, and the IL-25 signal axis is intact in human subjects with asthma, we analyzed RNA preparations from BAL samples from healthy control subjects or patients with a clinical diagnosis of asthma (clinical data for these subjects are presented in Table E1). The mean transcript levels for IL-25, IL-17Rb, IL-4, and IL-4Ra1 were increased in the asthmatics compared with healthy controls (Figures 6A–6D). When we evaluated gene associations for each of the transcripts tested, we found a strong correlation between the levels of IL-25 and IL-4 mRNA (Figure 6E). We observed no significant association between other genes (data not shown), and stratification of the asthmatics into mild/moderate and severe phenotypes did not provide sufficient statistical power to resolve differences between any of the measured transcripts. When we evaluated the abundance of cytokine transcripts for an IL-4 and IL-25 correlation with the oral corticosteroid dose among the five subjects taking oral corticosteroids, we found a trend toward an inverse correlation that was not significant (data not shown). These data suggest that within airways, the IL-25 signaling axis is up-regulated in a subset of asthmatics compared with healthy controls, and that IL-4 and IL-25 induction may be linked.
Figure 6.
IL-25 signaling axis transcripts are increased in bronchoalveolar lavage (BAL) from subjects with asthma. (A–D) RNA preparations from BAL fluid from control subjects with asthma and healthy control subjects were assayed for IL-4, IL-4Ra, IL-25, and IL-17Rb transcripts by quantitative PCR. *P < 0.05 and **P < 0.01 by Mann–Whitney nondirectional U test. In E, the transcript abundances of IL-4 and IL-25 cytokines from all subjects are normally distributed data that display a significant pairwise correlation.
Discussion
In this study, we observed in alveolar macrophages and monocytes that IL-4 signaling induced coordinate expression of the cytokine IL-25 and its IL-17Rb receptor. The data suggest that in a feed-forward signaling loop, IL-25 strengthens the IL-4 signal to sustain IL-17Rb expression in these cells, maintaining signaling capacity for the Type 2 cytokine IL-25.
The best characterization to date of IL-17Rb expression on myeloid cells in airways was achieved by Petersen and colleagues in granulocytic cells, which the authors termed type 2 myeloid (T2M) cells (6). These T2M cells (which are CD11b+ and Gr1 mid, with a subset being IL-17Rb+) were instrumental in the cockroach allergen response in a 2-wk airway hypersensitivity model. Genetic deletion of IL-17Rb significantly lessened allergen sensitivity in these mice, and the phenotype was reconstituted with airway adoptive transfer of wild-type T2M cells. These IL-17Rb+ T2M cells responded robustly to IL-25, with subsequent activation of the IL-4 promoter. However, the authors did not investigate the mechanistic basis for the induction of IL-17Rb in the T2M cell population.
Our study shows a converse but consistent biological relationship between these two cytokines, where IL-4 can rapidly induce new IL-25 and IL-17Rb expression on myeloid cells that appear to have little or no IL-17Rb before exposure. We interpret these findings collectively to suggest that in the absence of interfering cytokine signals, either IL-4 or IL-25 can induce feed-forward expression of both cytokine axes and reinforce Type 2 inflammation in the larger cellular milieu. Similar biology has been demonstrated for the differentiation of naive T cells toward Th2 cells by IL-4 and IL-25 acting in concert (7). In our studies, the response by BAL cells (mostly alveolar macrophages) was more robust but congruent with effects seen in the THP1 monocytes, perhaps because the lung tissue environment has primed alveolar cells for such responses (18).
In this study, we found a positive association between IL-25 and IL-4 transcript abundances from unprovoked BAL samples from several asthmatics, whereas BAL isolated from other asthmatics and healthy controls had nearly undetectable levels of transcripts for either cytokine. Our observations are consistent with other human studies highlighting the importance of IL-25 signaling, with reports of increased serum IL-25 and expression of IL-17Rb on cells from patients with asthma. In a recent clinical study, IL-17Rb expression was evaluated on DCs isolated from blood and sputum samples several days after subjects were challenged with inhaled allergen (10). Both myeloid and plasmacytoid DCs showed IL-17Rb expression 24 h after allergen exposure, and the myeloid DCs showed a strong correlation between IL-17Rb and other markers of Th2 and asthma phenotypes (16). It is worth remarking that in our study we detected these Type 2 cytokine transcripts in some patients with severe asthma, whereas other patients had no detectable amount of mRNA for these factors, pointing to the broad diversity of asthma endotypes within the spectrum of inflammatory obstructive lung disease (19).
Precisely how the cells mentioned in the above studies initiate and sustain the expression of IL-17Rb is not fully understood; however, the current study fills in some gaps in our understanding of the relevant pathways. It is conceivable that antigen receptors (either IgE or receptors on Th2 cells) react to allergen exposure by releasing cytokines (e.g., IL-4, IL-13, and IL-25) with transcriptional activation of the IL-17Rb gene through the coordinated activities of Stat6 and NF-κB factors (as shown here) to induce a stable population of IL-17Rb+ myeloid cells. These cells could then propagate Type 2 immune pathology, as previously reported in animal studies and humans with Type 2–driven illness. Interestingly, our results demonstrate that IL-4 robustly up-regulates IL-17Rb mRNA, exceeding the induction by IL-4 at steady-state protein levels. This observation suggests a more complex, distinct mode of IL-4 regulation at both the IL-17Rb transcript and protein levels. Possible explanations for these effects include a discordance in transcript versus protein stability in the cell, more inefficient protein translation compared with rates of mRNA synthesis, and altered protein degradation before membrane trafficking. In addition, protein detection is critically dependent on antibody affinity with exposure of epitopes that may be masked depending on post-translational modifications and subcellular location of individual substrates.
Signal amplification is a common theme in immunobiology as systems of cells and cytokines organize themselves into a coordinated response, and autocrine macrophage stimulation seems to play a role in these systems (20). In a previous study, the IL-17A signaling axis was shown to display a similar interplay with the IL-6 signaling axis in an autoimmune arthritis model, such that both cytokines were required for a full phenotype and robust expression of the downstream genes KC and macrophage inflammatory protein (21). That study similarly invoked a dual-transcription factor model, with STAT3 and NF-κB synergistically participating in the transcriptomic inflammatory response, analogous to the observations we made in the current study with STAT6 and NF-κB. Coordinated gene expression in response to pathogen and tissue signals is a driver of cell polarization and identity, and dissection of the interplay of cellular responses to multiple inputs remains an interesting area of ongoing study.
Footnotes
This work was supported by a Merit Review Award from the U.S. Department of Veterans Affairs (R.K.M.), an American Lung Association Biomedical Research Grant (N.M.W.), National Institutes of Health K08 grant HL126135 (N.M.W.), R01 grants HL096376 (R.K.M.), HL097376 (R.K.M.), HL081784 (R.K.M.), and P01HL114453 (R.K.M.).
Author Contributions: Study conception and design: N.M.W., R.K.M., M.R., and S.W.; analysis and interpretation: N.M.W., R.K.M., and S.W.; drafting of the manuscript for important intellectual content: N.M.W. and R.K.M.; execution of experiments: N.M.W., S.M.K., Q.G., J.L., A.H., and J.T.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0316OC on April 19, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2:66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med. 2001;193:1087–1096. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012;18:751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, Cameron L. Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor. Immun Inflamm Dis. 2015;3:455–468. doi: 10.1002/iid3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Noguchi E, Imoto Y, Nanatsue K, Takeshita K, Shibasaki M, Arinami T, Fujieda S. Upregulation of IL17RB during natural allergen exposure in patients with seasonal allergic rhinitis. Allergol Int. 2011;60:87–92. doi: 10.2332/allergolint.10-OA-0230. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Mobini R, Fang Y, Barrenäs F, Zhang H, Xiang Z, Benson M. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. 2010;40:1194–1202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 10.Tworek D, Smith SG, Salter BM, Baatjes AJ, Scime T, Watson R, Obminski C, Gauvreau GM, O’Byrne PM. IL-25 receptor expression on airway dendritic cells after allergen challenge in subjects with asthma. Am J Respir Crit Care Med. 2016;193:957–964. doi: 10.1164/rccm.201509-1751OC. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 12.Leonard C, Tormey V, Burke C, Poulter LW. Allergen-induced cytokine production in atopic disease and its relationship to disease severity. Am J Respir Cell Mol Biol. 1997;17:368–375. doi: 10.1165/ajrcmb.17.3.2797. [DOI] [PubMed] [Google Scholar]
- 13.Chen BB, Mallampalli RK. Masking of a nuclear signal motif by monoubiquitination leads to mislocalization and degradation of the regulatory enzyme cytidylyltransferase. Mol Cell Biol. 2009;29:3062–3075. doi: 10.1128/MCB.01824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009;41:516–524. doi: 10.1165/rcmb.2008-0163OC. [DOI] [PubMed] [Google Scholar]
- 15.Melisi D, Chiao PJ. NF-κB as a target for cancer therapy. Expert Opin Ther Targets. 2007;11:133–144. doi: 10.1517/14728222.11.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Wong D, Teixeira A, Oikonomopoulos S, Humburg P, Lone IN, Saliba D, Siggers T, Bulyk M, Angelov D, Dimitrov S, et al. Extensive characterization of NF-κB binding uncovers non-canonical motifs and advances the interpretation of genetic functional traits. Genome Biol. 2011;12:R70. doi: 10.1186/gb-2011-12-7-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50:87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 20.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]