Abstract
Urban particulate matter (UPM) air pollution and vitamin D deficiency are detrimentally associated with respiratory health. This is hypothesized to be due in part to regulation of IL-17A, which UPM is reported to promote. Here, we used a myeloid dendritic cell (DC)-memory CD4+ T cell co-culture system to characterize UPM-driven IL-17A+ cells, investigate the mechanism by which UPM-primed DCs promote this phenotype, and address evidence for cross-regulation by vitamin D. CD1c+ myeloid DCs were cultured overnight with or without a reference source of UPM and/or active vitamin D (1,25[OH]2D3) before they were co-cultured with autologous memory CD4+ T cells. Supernatants were harvested for cytokine analysis on Day 5 of co-culture, and intracellular cytokine staining was performed on Day 7. UPM-primed DCs increased the proportion of memory CD4+ T cells expressing the T helper 17 cell (Th17)-associated cytokines IL-17A, IL-17F, and IL-22, as well as IFN-γ, granulocyte–macrophage colony-stimulating factor, and granzyme B. Notably, a large proportion of the UPM-driven IL-17A+ cells co-expressed these cytokines, but not IL-10, indicative of a proinflammatory Th17 profile. UPM-treated DCs expressed elevated levels of il23 mRNA and increased secretion of IL-23p40. Neutralization of IL-23 in culture reduced the frequency of IL-17A+IFN-γ+ cells without affecting cell proliferation. 1,25(OH)2D3 counteracted the UPM-driven DC maturation and inhibited the frequency of IL-17A+IFN-γ+ cells, most prominently when DCs were co-treated with the corticosteroid dexamethasone, while maintaining antiinflammatory IL-10 synthesis. These data indicate that UPM might promote an inflammatory milieu in part by inducing an IL-23–driven proinflammatory Th17 response. Restoring vitamin D sufficiency may counteract these UPM-driven effects without obliterating important homeostatic immune functions.
Keywords: air pollution, vitamin D, Th17, IL-23, corticosteroids
Clinical Relevance
Urban particulate matter air pollution and vitamin D deficiency are both detrimentally associated with airway function. This research describes an IL-23–dependent pathway by which urban particulate matter promotes a proinflammatory T helper 17 cell response, which is predicted to exacerbate respiratory diseases, and the capacity of vitamin D to oppose this effect. This supports the concept that restoring vitamin D sufficiency will help to counteract the detrimental properties of air pollution to maintain immune homeostasis.
Asthma is a complex and heterogeneous disease that is estimated to affect more than 300 million people worldwide. It is typically well controlled with β2-receptor agonists and corticosteroids. However, 5–10% of asthmatics have particularly poorly controlled severe disease that is frequently associated with a neutrophilic infiltrate alongside elevated levels of IL-17A, the prototypical cytokine of T helper 17 (Th17) cells, throughout the airways (1). Chronic obstructive pulmonary disease (COPD), another respiratory disease that is predicted to be the third leading cause of mortality by 2020, is also characterized by elevated levels of IL-17A in the bronchial submucosa and peripheral blood (2). Homeostatically, Th17 cells are nevertheless essential for protection against bacteria and fungi such as Klebsiella pneumoniae and Candida albicans at mucosal surfaces.
Th17 cells are not a homogeneous population, and a subset of proinflammatory Th17.1 cells has been described that drives experimental autoimmune encephalomyelitis in mice (3, 4). Upon T cell receptor stimulation, Th17.1 cells differentiate in the presence of IL-23 to co-express the Th17-associated cytokines IL-17A, IL-17F, and IL-22 with Th1-associated IFN-γ, but not the antiinflammatory mediator IL-10. Zielinski and colleagues showed that human C. albicans-specific Th17 cells produced both IL-17A and IFN-γ, but not IL-10, upon restimulation (5). Beyond Th1 and Th17 cytokines, the pathogenicity of IL-23–driven Th17 cells appears to be dependent on granulocyte-macrophage colony-stimulating factor (GM-CSF), such that in the absence of GM-CSF, autoimmune neuroinflammation does not develop in mice (6, 7). Ramesh and colleagues further phenotyped human proinflammatory Th17.1 cells by culturing CD4+ T cells in the presence of anti-CD3, anti-CD28, and IL-23 (8). These cells were identified as CCR6+CXCR3hiCCR4loCCR10−CD161+, transiently expressing c-Kit and stably expressing multi-drug resistance type 1 (MDR1). This is of interest because levels of both IL-17A and IFN-γ are heightened in severe and steroid refractory asthma (9–11), and MDR1+ proinflammatory Th17 cells were found to be refractory to a range of corticosteroids (8).
The increase in the prevalence of chronic respiratory diseases over recent decades highlights a key role for environmental factors in disease development and progression (12). In particular, there is a substantial body of epidemiological data that detrimentally link both poor air quality and vitamin D deficiency to asthma and COPD (13, 14). Exposure to elevated concentrations of ambient particulate matter (PM) has been associated with asthma exacerbations as well as increased hospitalization and medication usage, with emerging evidence supporting a role in the initiation of asthma (15). Importantly, previous epidemiological studies have shown that air pollution–triggered asthma exacerbations occur with a 2- to 5-day lag, suggesting that the pollution itself may play an indirect role, possibly by perturbing lymphocyte responses rather than by directly inducing bronchoconstriction or immediately triggering innate immune responses (16). Consistent with this view, a reduction in PM-induced airway hyperresponsiveness and mucus cell hyperplasia was reported in Rag1−/− mice that lack lymphocytes (17).
Vitamin D deficiency has also been associated with the increased incidence and severity of many respiratory diseases, including asthma, as reviewed in detail elsewhere (14, 18). The majority of vitamin D is generated when UV-B radiation photolyses 7-dehydrocholesterol in the skin into vitamin D3. Vitamin D3 can then be converted into the active form of vitamin D, namely, 1,25(OH)2D3, by the enzyme CYP27B1, which is present in the kidneys and various peripheral immune cells. Although statistically significant associations between respiratory diseases and both air pollution and vitamin D deficiency have been reported (as discussed above), the underlying molecular mechanisms by which these environmental factors influence pathophysiology are not fully understood. Furthermore, although it has been proposed that vitamin D may counteract the detrimental effects of UPM, how it achieves this remains to be fully elucidated.
In vivo, the inhaled PM and desorbed components are presented to the immune system in part by interdigitating myeloid dendritic cells (DCs) that line the airways and lung parenchyma. The DCs can then traffic to the mesenteric lymph node and modulate effector T cell function, or can act more locally to promote airway inflammation (19). Whereas UPM has been reported to promote the maturation of DCs (19), vitamin D has been shown independently to have the opposite effect, acting to promote a tolerogenic DC phenotype (20). Within the lung, memory CD4+ T cells are extremely abundant and therefore any modulation of these cells by UPM-primed DCs may play an important role in air pollution–induced disease exacerbations (21), an issue that we sought to address in the current study.
In this study, we aimed to phenotype the cytokine profile of human memory CD4+ IL-17A+ T cells generated after co-culture with UPM-pretreated CD1c+ myeloid DCs, and specifically to identify whether UPM promoted a proinflammatory Th17 phenotype, as well as the DC-derived signals that might drive such a response. Vitamin D has been shown independently and repeatedly to downregulate Th17 responses both in vitro and in vivo (14). We therefore further addressed whether 1,25(OH)2D3 could counteract any effects of UPM in promoting a potentially pathogenic Th17 response.
Materials and Methods
Cell Isolation
Peripheral blood mononuclear cells were isolated from healthy donors by means of density centrifugation (Axis-Shield, Birmingham, UK) (REC 14/LO/1699) and CD1c+ DCs were positively selected (> 98% CD11c+HLA-DR+; Miltenyi Biotec, Bisley, UK). Unlabeled peripheral blood mononuclear cell were frozen overnight in RPMI-1640 (Invitrogen, Paisley, UK) containing 40% FCS and 10% DMSO, and then CD4+CD45RO+ cells were isolated by negative selection (> 98.5% CD4+CD45RO+; Miltenyi Biotec). The cells were resuspended in RPMI-1640 supplemented with 10% human AB serum (PAA Laboratories, Oxford, UK), 2 mM L-glutamine, and 50 mg/ml gentamycin. Inferior turbinate tissue was donated by patients undergoing turbinate resection (REC 12/LO/1931), and CD1a+ DCs were positively isolated as previously described (22) (> 95% CD11c+HLA-DR+ excluding debris).
Cell Culture
DCs (1 × 104/ml) were cultured in a U-bottomed 96-well plate for 20 hours with 50 ng/ml recombinant human GM-CSF (rhGM-CSF) ± 5 μg/ml NIST (3% methanol vehicle control [VC]), 100 nM 1,25(OH)2D3 (0.01% DMSO VC; BIOMOL Research Laboratories, Exeter, UK), 100 nM dexamethasone (Sigma Aldrich, Gillingham, UK), and/or 2 μg/ml anti-IL-23p19 or the relevant isotype control (R&D Systems, Abingdon, UK) unless otherwise stated. DC supernatants were harvested after 20 hours and 2 × 105 autologous memory CD4+ T cells were added. Five days later, supernatants were harvested and cells were transferred to a 48-well plate with 10 IU/ml recombinant human IL-2 (Eurocetus, Harefield, UK) for a further 2 days.
Here, “NIST” refers to a standard reference source of total UPM (SRM-1648a) from the National Institute of Standards and Technology. SRM-1648a was prepared from UPM previously collected in St. Louis, Missouri, over a 1-year period and was resuspended in 3% methanol to prevent the oxidation of aryl hydrocarbons. Particles ranged from 1.35 to 30.1 μm in diameter (mean 5.85 μm) and comprised a combination of polycyclic aromatic hydrocarbons, polychlorinated biphenyl congeners, and chlorinated pesticides (23).
Surface Staining
DCs were harvested using 2 mM EDTA in PBS containing 2% FCS and then incubated on ice with different antibodies (CD40-FITC, ILT1-PE, ILT3-APC, and CCR7-PE [5C3, 24, ZM4.1, and G043H7, respectively; BioLegend, London, UK]; and CD83-APC, HLA-DR-PerCP, ILT2-PECy7, and ILT4 Alexa Fluor 647 [HB15e, L243, GHI/75, and 287219, respectively; BD Biosciences, Oxford, UK]). The DCs were washed and assessed for fluorescence using an NxT Attune flow cytometer (ThermoFisher, Wilmington, DE).
Cell Proliferation
Before culturing, T cells were labeled with 5 μM CellTrace Violet (ThermoFisher). Cell proliferation was assessed on Day 7 of co-culture by loss of fluorescence intensity.
Intracellular Cytokine Staining
Cells were stimulated at 37°C with 50 ng/ml phorbol myristate acetate and 500 ng/ml ionomycin for 1 hour, and 2 μM monensin was added for a further 4 hours. Cells were surface stained with CD4-PerCP (SK3; BD Biosciences) and Zombie Aqua (BioLegend) ± CD161-BV412 (DX12; BD Biosciences) and MDR1-PerCPeFluor710 (UIC2; eBioscience) before they were fixed and permeabilized (Fix/Perm kit; BD Biosciences). Samples were then incubated with the following antibodies: IL-17A-APC and IL-22-eFluor450 (eBio64DEC17 and 22URTI, respectively; eBioscience); IFN-γ-FITC, GM-CSF-PE, IL-10-PE, IL-13-PE, and granzyme B Alexa Fluor 700 (4S.B3, BVD2-21C11, JES3-9D7, JES10-5A2, and GB-11, respectively; BD Biosciences); and IL-17F Alexa Fluor 488 and TNF-α-FITC (Poly5166 and MAb11, respectively; BioLegend).
Cytometric Bead Array
Cytometric bead array (BD Biosciences) was employed to measure the concentration of cytokines within supernatants in accordance with the manufacturer’s instructions.
qRT-PCR
RNA was isolated from Qiazol-lysed cells using an miRNeasy Mini Kit (Qiagen, Crawley, UK). mRNA was converted to cDNA by using RevertAid Reverse Transcriptase and complementary reagents. qRT-PCR was performed in triplicate on a ViiA7 system using TaqMan probes (ThermoFisher).
Data Analysis
Flow-cytometry data were analyzed using FlowJo (Treestar Inc., version 10). Cumulative data were analyzed using Graphpad Prism version 6.00 for Windows. After the data were assessed for a Gaussian distribution, statistical analyses were performed as outlined in the figure legends.
Results
1,25(OH)2D3 Counteracts UPM-Driven Myeloid DC Maturation
CD1c+ DCs are the precursors of CD11b− and CD11b+ DCs that line the airways and lung parenchyma, respectively (24). Considering that both air pollution and vitamin D deficiency are associated with the incidence and severity of respiratory diseases, the impact of these factors on CD1c+ DC maturation and downstream memory T cell responses was investigated. CD1c+ DCs were cultured for 20 hours in the presence of the indicated concentration of 1,25(OH)2D3 and/or a reference source of UPM (NIST SRM-1648a, referred to as NIST), added at a concentration (5 μg/ml) that was determined to consistently stimulate DC maturation both here (Figure E1A in the online supplement) and in a previous study (25). rhGM-CSF (50 ng/ml) was added to all DC cultures as a substitute for the rhGM-CSF released by UPM-stimulated human bronchial epithelial cells (26, 27), an approach that was previously shown to enhance the CD1c+ DC maturation induced by NIST (28).
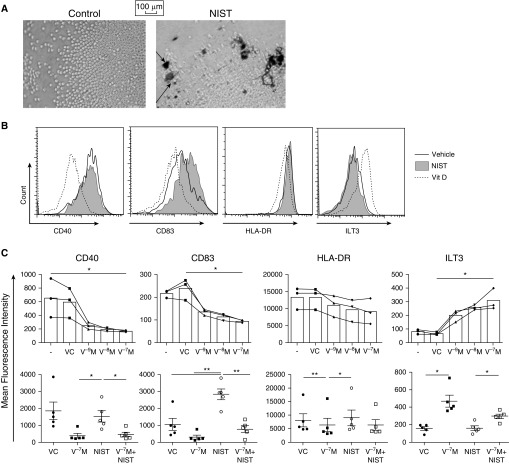
Figure 1A shows a magnified image of DCs clumping around the NIST particle agglomerates after 20 hours in culture, in contrast to the resting cells cultured in the presence of 3% methanol VC. DC surface staining was performed, and both representative histograms and cumulative data are shown (Figures 1B and 1C). The VC did not modulate expression of the surface markers assessed, but there was a 1,25(OH)2D3 dose-dependent downregulation in expression of CD40, CD83, and HLA-DR. This occurred alongside an upregulation in expression of the inhibitory receptor immunoglobulin-like transcript 3 (ILT3), as previously independently reported (29), but not of the ILT1, ILT2, or ILT4 molecules (Figure E2A).
Figure 1.
1,25(OH)2D3 counteracts urban particulate matter (UPM)-induced CD1c+ dendritic cell (DC) activation. Peripheral CD1c+ DCs cultured with 50 ng/ml recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF) alone (−) or in the presence of 10−9–10−7 M 1,25(OH)2D3 (V−xM), 5 μg/ml NIST, and/or a vehicle control (VC) for 20 hours. (A) Light-microscope images taken at ×25 magnification. Cell-surface staining was performed and the mean fluorescence intensity was determined; shown are representative histograms (B) and cumulative data (C; n = 3/5). Data were assessed by repeated-measures one-way ANOVA with Holm-Sidak’s multiple-comparisons test. *P ≤ 0.05, **P ≤ 0.01. ILT3, immunoglobulin-like transcript; NIST, standard reference source of UPM from the National Institute of Standards and Technology; Vit D, vitamin D.
In contrast, NIST dose-dependently increased expression of the maturation marker CD83 at the mRNA and protein level (Figure E1A), such that expression was significantly enhanced relative to both the VC and 1,25(OH)2D3 conditions (P < 0.01; Figure 1C). There was also a trend toward increased expression of the lymph node–homing receptor CCR7 on the NIST-treated DCs (Figure E2A). Expression of HLA-DR, CD40, and ILT3 was not consistently modulated by NIST treatment. Addition of 1,25(OH)2D3 to the culture with NIST significantly reduced expression of CD83 (P < 0.01) and CD40 (P < 0.05) relative to the NIST-only condition, whereas expression of ILT3 remained significantly elevated (P < 0.05; Figure 1C). Similarly, levels of IL-6 (P < 0.01) and TNF-α (P = 0.17) were elevated in NIST-treated DC culture supernatants after 20 hours (Figure E2B). In support of this, mRNA expression of il6 was dose-dependently elevated in NIST-primed DCs (Figure E1B). Treatment of CD1a+ DCs derived from nasal turbinates with NIST modestly increased expression of CD40 and CCR7 in a small sample size, and there was a trend toward elevated levels of IL-6 in cell culture supernatants (P = 0.0531; Figures E2C and E2D). 1,25(OH)2D3 alone had no effect, but appeared to counteract the elevated expression of CD40 and CCR7 as well as the augmented levels of IL-6 when added in combination with NIST.
UPM-Primed DCs Drive a Th17.1-Like Phenotype That Is Opposed by 1,25(OH)2D3
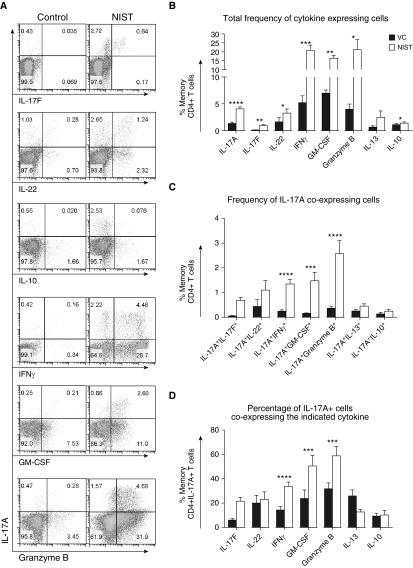
Considering the growing body of literature highlighting the existence of Th17 subsets and T-helper cell plasticity (8, 30), alongside studies showing a link between air pollution and IL-17A (25, 31), we aimed to phenotype NIST-driven IL-17A+ cells in greater detail. In addition to significantly increasing the frequency of cells expressing IL-17A and IFN-γ, co-culture of memory CD4+ T cells with NIST-primed CD1c+ DCs enhanced the proportion of cells expressing IL-17F, IL-22, GM-CSF, and granzyme B (Figures 2A and 2B). Notably, the frequency of memory CD4+ T cells co-expressing IL-17A with IFN-γ, GM-CSF, or granzyme B was significantly elevated in the NIST condition as compared with the VC when shown as a percentage of all memory CD4+ T cells (Figure 2C), or as a percentage of total memory CD4+ IL-17A+ T cells (Figure 2D). There was, however, no difference in the frequency of IL-17A+IL-13+ cells or IL-17A+IL-10+ cells between the conditions, and minimal IL-17A/IL-10 co-expression. Of note, lipopolysaccharide (LPS) concentrations were < 1 pg per well and the NIST-driven enhancement of IL-17A was double that induced by treating DCs with 10 μg/ml LPS (data not shown).
Figure 2.
UPM drives a putatively pathogenic T helper type 17 (Th17) cytokine response. Peripheral CD1c+ DCs were cultured with 50 ng/ml rhGM-CSF and either vehicle control (VC, black) or 5 μg/ml NIST (white) for 20 hours. Autologous memory CD4+ T cells were co-cultured for a further 5 days followed by a 2-day expansion in the presence of 10 IU/ml IL-2. The cells were then stimulated for 5 hours with phorbol myristate acetate (PMA) and ionomycin before intracellular cytokine expression was assessed. (A) Representative plots from independent experiments. (B–D) Cumulative data for the total frequency of cytokine-expressing cells (B), the frequency of IL-17A–co-expressing cells (C), and the percentage of IL-17A+ cells that co-expressed the indicated cytokine (D) (n = 5–7 except for IL-17A and IFN-γ [n = 21 and 16, respectively]). Data were assessed by paired t test comparing the vehicle and NIST; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
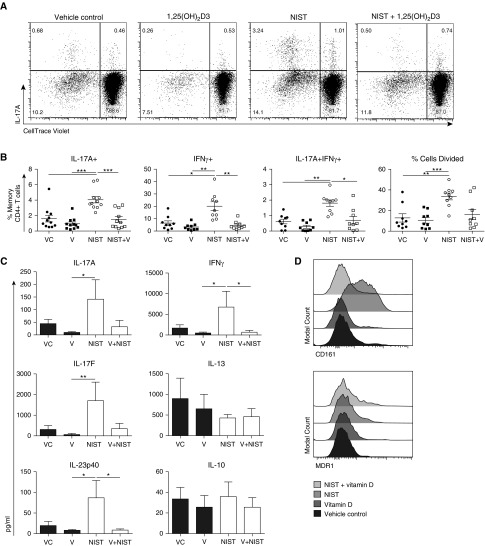
In contrast to NIST alone, addition of 1,25(OH)2D3 ± NIST reduced the frequency of IL-17A+ and IFN-γ+ cells as well as the percentage of IL-17A+IFN-γ+ cells (Figures 3A and 3B), a characteristic of proinflammatory Th17.1 cells. 1,25(OH)2D3 similarly overcame the NIST-driven enhancement in expression of IL-17F, IL-22, GM-CSF, and granzyme B (Figure E3A). Whereas NIST-primed DCs significantly increased memory CD4+ T cell proliferation, as previously reported (25), 1,25(OH)2D3 appeared able to counteract this (P = 0.05). However, there was no difference in the viability of memory CD4+ T cells co-cultured with DCs that had been pretreated with 1,25(OH)2D3 and/or NIST (data not shown).
Figure 3.
Pretreating CD1c+ DCs with 1,25(OH)2D3 counteracted the UPM-driven Th1/Th17 profile. Peripheral CD1c+ DCs were cultured with 50 ng/ml rhGM-CSF in the presence of 10−7 M 1,25(OH)2D3, 5 μg/ml NIST, and/or a vehicle control (VC) for 20 hours. Autologous CellTrace Violet-labeled memory CD4+ T cells were then added for a further 5 days, after which supernatants were harvested and the cells were expanded with 10 U/ml IL-2 for a further 2 days. The cells were stimulated for 5 hours with PMA and ionomycin before intracellular cytokine expression was assessed. Shown are representative dot plots (A) and cumulative data (B) for the percentage of cells that divided and the frequency of cells that expressed IL-17A and IFN-γ (n = 7–9; data assessed by repeated-measures one-way ANOVA with Tukey’s multiple-comparison test). MDR1, multidrug resistant protein 1; V, vitamin D (1,25[OH]2D3). (C) Cytometric bead array was employed to assess the concentration of cytokines in supernatants harvested on Day 5 of co-culture (n = 6; data assessed by Friedman’s test with Dunn’s multiple-comparisons test). (D) Surface expression of CD161 and MDR1 was assessed on Day 7 of co-culture (representative histogram, n = 2). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Results from analysis of secreted cytokines present in the culture supernatants reflected those obtained by intracellular cytokine staining (Figure 3C), with levels of IL-17A, IL-17F, IL-12/23p40, and IFN-γ being consistently elevated in the NIST-treated condition and reduced by co-incubation of DCs with 1,25(OH)2D3. Levels of IL-13 and IL-10 were variable and not consistently modulated by 1,25(OH)2D3 and/or NIST. In addition to cytokines, NIST-primed DCs also increased T cell surface expression of the lectin-like receptor CD161, which was opposed by 1,25(OH)2D3, with a more modest effect on expression of MDR1 (Figure 3D); both of these markers have been associated with Th17.1 cells in humans (8). The mRNA expression of relevant Th17-associated transcription factors was additionally screened after 48 hours of co-culture (data shown in Figure E3B). There was a trend toward increased expression of stat3, tbx21, mdr1, and irf4 in the NIST condition, with 1,25(OH)2D3 treatment opposing this effect (P < 0.05 for stat3, mdr1, and irf4; P = 0.073 for tbx21).
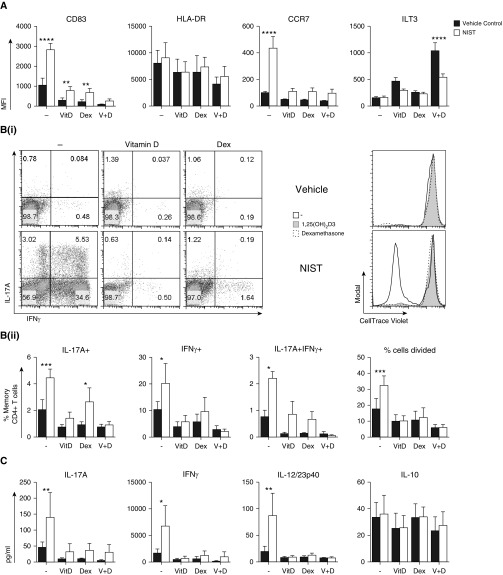
Dexamethasone Enhances the Capacity of Vitamin D to Dampen the NIST-Driven IL-17A+IFN-γ+ T Cell Response
Given previous evidence of complementary interactions between corticosteroids and vitamin D (14), we additionally examined a combination of these two factors in our cultures. As was the case with 1,25(OH)2D3, treating CD1c+ DCs with the synthetic corticosteroid dexamethasone was able to counteract the NIST-driven proinflammatory profile (Figure 4). Dexamethasone reduced expression of CD83 and CCR7 on CD1c+ DCs (Figure 4A). Addition of vitamin D to dexamethasone-treated DCs further suppressed CD83 expression even in the presence of NIST. Dexamethasone also further enhanced the 1,25(OH)2D3-mediated induction of ILT3, an effect that was opposed by NIST. Downstream, priming DCs with dexamethasone alone or in combination with 1,25(OH)2D3 counteracted the ability of NIST treatment to promote autologous memory CD4+ T cell proliferation and reduced the levels of IL-17A, IFN-γ, and IL-23p40 within the cell culture supernatants (Figures 4B and 4C). Notably, the combination of vitamin D and dexamethasone suppressed the NIST-induced IL-17A+IFN-γ+ memory CD4+ T cell response to the greatest extent.
Figure 4.
Pretreating CD1c+ DCs with 1,25(OH)2D3 and dexamethasone countered the UPM-driven proinflammatory profile. Peripheral CD1c+ DCs were cultured with 50 ng/ml rhGM-CSF in the presence of 10−7 M 1,25(OH)2D3 (VitD; V), 10−7 M dexamethasone (Dex; D), and/or 5 μg/ml NIST for 20 hours. (A) Cell-surface staining was performed and the mean fluorescence intensity was determined (n = 5). Autologous CellTrace Violet–labeled memory CD4+ T cells were added for a further 5 days and then supernatants were harvested for cytometric bead array (C; n = 6). (B) Cells were expanded with 10 U/ml IL-2 for 2 days, stimulated for 5 hours with PMA and ionomycin, and then assessed for intracellular cytokine expression (n = 5). Data were assessed by two-way ANOVA with Sidak’s multiple-comparisons test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
UPM Upregulates a Population of IL-17A+IFN-γ+ Cells in Part via Enhanced Endogenous IL-23
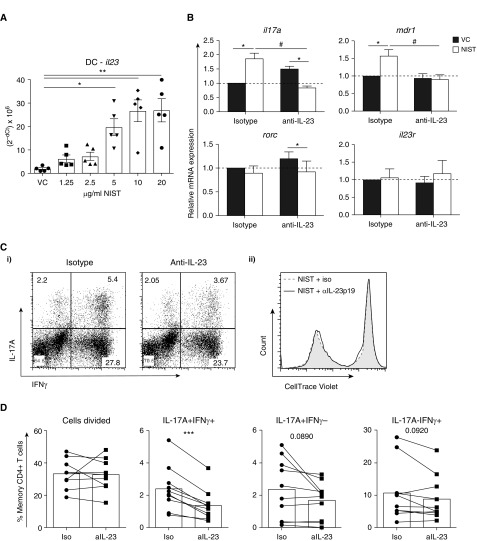
Given that NIST pretreatment of CD1c+ DC drove a phenotypically proinflammatory Th17.1-like profile with an increased frequency of IL-17A+IFN-γ+ cells, and levels of IL-12/23p40 were significantly upregulated in co-culture supernatants, we investigated the possibility that IL-23 plays a role as an intermediate. Significantly enhanced DC production of IL-23 was first confirmed at the mRNA levels over a NIST dose response (Figure 5A). Of note, il12 mRNA expression in DCs was undetectable by qRT-PCR (Figure E1C). A neutralizing antibody specific for IL-23p19 (which would thus inhibit IL-23, but not IL-12) or a relevant isotype control was then added throughout the culture period. As Figure 5B shows, NIST-primed DCs significantly increased expression of il17a and mdr1 mRNA after 48 hours of co-culture in the isotype condition, in agreement with the protein data in Figure 3, but this was significantly reduced by addition of anti-IL-23 into the culture, whereas expression of il10 was unaffected. Similarly, at the protein level, although anti-IL-23p19 had no effect on NIST-driven cell division, it significantly reduced the frequency of IL-17A+IFN-γ+ cells in particular (P < 0.001; Figures 5C and 5D), with a more modest effect on the IL-17A (P = 0.089) and IFN-γ (P = 0.092) single-positive populations.
Figure 5.
UPM acts via IL-23 to drive the synthesis of IL-17A+IFN-γ+ memory CD4+ T cells. Peripheral CD1c+ DCs were cultured with 50 ng/ml rhGM-CSF in the presence of 10−7 M 1,25(OH)2D3, 1.25–20 μg/ml NIST (5 μg/ml unless stated) and/or a vehicle control (VC) for 20 hours. (A) DC pellets were harvested and RNA was isolated for qRT-PCR; il23 gene expression is shown normalized to the 18s endogenous control (data assessed by Friedman’s test with Dunn’s multiple-comparisons test). (B–D) Autologous CellTrace Violet–labeled memory CD4+ T cells were added after 20 hours of DC priming; an isotype control or 2 μg/ml anti-IL-23p19 was added throughout the culture. (B) After 48 hours of co-culture, cells were harvested, RNA was isolated, and qRT-PCR was performed to assess mRNA expression relative to the isotype and VC condition (n = 5; data assessed by two-way ANOVA with Sidak’s multiple-comparisons test; *P < 0.05 between VC and NIST; #P < 0.05 between isotype and anti-IL-23). (C and D) On Day 5 of co-culture, supernatants were harvested and the cells were expanded with 10 U/ml IL-2 for a further 2 days. They were then stimulated for 5 hours with PMA and ionomycin, and assessed for intracellular cytokine expression. Shown are representative plots (C) and cumulative data (D) (n = 8; data assessed by a two-tailed paired t-test, **P = 0.01; ***P ≤ 0.001). Iso, isotype; Rorc, the transcription factor retinoic acid receptor related orphan receptor C.
Discussion
The current study demonstrates that pretreatment of human myeloid DCs with a common reference source of UPM (NIST) alters their maturation state, resulting in the expansion of a population of memory CD4+ T cells possessing a proinflammatory Th17.1-like phenotype. These cells are characterized by the co-expression of IL-17A with IFN-γ, GM-CSF, and granzyme B, and are predicted to drive exacerbations of respiratory diseases. Our data indicate a central role for NIST-induced IL-23 synthesis by myeloid DC in driving this proinflammatory Th17 response. An additional novel feature of these data is the evidence that UPM significantly increased expression of GM-CSF and the serine protease granzyme B, specifically enriching the proportion of cells that co-expressed these cytokines with IL-17A (Figure 2). Granzyme B can be released from the granules of cytotoxic T cells and is traditionally thought of as mediating apoptosis of target cells, but it can also stimulate proinflammatory cytokine release and drive extracellular matrix remodeling (32). To date, co-expression of IL-17A and granzyme B by CD4+ T cells has been implicated predominantly in neuroinflammation (33), but elevated levels of granzyme B have been associated with various diseases, including autoimmune conditions, type I diabetes, and asthma. Collectively, these data suggest that UPM, via actions on the antigen-presenting-cell compartment, promotes a Th17 population with a potentially pathogenic phenotype.
In contrast to the effects of NIST, vitamin D reduced both CD1c+ DC priming and the subsequent proinflammatory memory T cell response when added alone and in combination with NIST, and instead promoted a more tolerogenic phenotype (Figure 1). Although the individual effects of NIST (19) and 1,25(OH)2D3 (20) on DC maturation have previously been described, the capacity of 1,25(OH)2D3 to oppose certain proinflammatory properties of NIST when added in combination is a novel and important finding given that these two environmental factors co-exist. Furthermore, the synthetic corticosteroid dexamethasone was similarly capable of reducing the expression of NIST-driven maturation markers on CD1c+ DCs, both alone and more prominently when added in combination with 1,25(OH)2D3 (Figure 4). Most significantly, 1,25(OH)2D3 counteracted the induction of the maturation marker CD83 on CD1c+ DCs, as well as the heightened frequency of IL-17A+IFN-γ+ memory CD4+ T cells induced by NIST-primed CD1c+ DCs. 1,25(OH)2D3 also reduced expression of the inflammatory cytokines GM-CSF, granzyme B, IL-17F, and IL-22 (Figure E3). These results might help to explain previous observations: for example, one study reported that vitamin D–insufficient children in Puerto Rico living close to a major road, and therefore to traffic-related air pollution, have an elevated risk of severe asthma exacerbations (34). Notably, in the European Study of Cohorts for Air Pollution Effects, the relative effect of air pollution on health outcomes differed between cohorts, with Scandinavian groups often being more sensitive despite having lower levels of ambient PM (35). This may be due to geographical differences in PM composition and/or intrinsic variations in population, which warrant further study. For example, reduced UVR exposure in Scandinavian countries would lower circulating levels of vitamin D, which, as we show here, counteracts certain potentially pathogenic properties of PM.
The fact that priming DCs with a source of UPM increased the frequency of IL-17A+ cells concurs with previous studies in mice (31, 36) and humans (25, 31, 37) that identified links between air pollution and IL-17A, albeit in distinct experimental settings. However, in this study, we extend that observation to show that NIST-primed DCs also enhanced the frequency of cells expressing Th17-associated cytokines IL-17F and IL-22, as well as IFN-γ, GM-CSF, and granzyme B. Critically, a large proportion of the NIST-driven IL-17A+ cells in this study co-expressed IL-17F, IL-22, IFN-γ, GM-CSF, and granzyme B, but not immunoregulatory IL-10 (Figure 2), a phenotype that is indicative of a putatively pathogenic Th17 cell (Th17.1) (3, 4, 6, 7). Furthermore, NIST-primed DCs increased T cell expression of MDR1 and CD161 in co-culture (Figure 3D), both of which have been associated with a proinflammatory Th17 phenotype in humans (8). This subset of Th17 cells has been shown to drive autoimmune conditions in a GM-CSF–dependent manner in mice; however, their functional role and in vivo existence in humans is less clear. Although Th17/Th1 cells have been identified in humans, predominantly in the periphery, inflamed joints, and the gut (5, 8, 38), functional analyses and detailed phenotyping of these cells have been understandably limited to date.
However, production of both IL-17A and IFN-γ was shown to correlate inversely with lung function after corticosteroid therapy in steroid-refractory asthmatics (39), and GM-CSF was found to be elevated even during the asymptomatic stage of asthma (40). Moreover, proinflammatory MDR1+ human Th17 cells reportedly are resistant to a range of corticosteroids (8), a characteristic of both severe asthma and COPD as well as various autoimmune conditions. Recently, a study performed using the murine house dust mite model of asthma found that both dexamethasone and anti-IL-17A were required to alleviate diesel exhaust particle–induced corticosteroid-refractory asthma (41). However, we observed that when we used the in vitro co-culture system described here, priming of CD1c+ DCs from healthy donors in the presence of dexamethasone dampened the resultant NIST-driven proliferation and IL-17A/IFN-γ memory CD4+ T cell profile response, with no effect on the levels of IL-10 (Figure 4). This conforms with data showing that dexamethasone-treated, monocyte-derived DCs possess a stable tolerogenic phenotype (42). Nonetheless, using various in vitro and ex vivo experimental systems, both our group (9, 39) and others (43) observed that corticosteroids acting directly on human T cells failed to inhibit IL-17A production and enhanced levels of IL-10, highlighting the importance of the context in which corticosteroids are administered.
Considering the phenotype of the NIST-driven IL-17A+ cells and the fact that il23 mRNA expression was significantly upregulated in DCs alongside enhanced levels of IL-12/23p40 in culture supernatants, we hypothesized that NIST acts via enhanced IL-23 activity to promote the proinflammatory Th17 response. Indeed, specific neutralization of IL-23 impaired the NIST-driven IL-17A and IFN-γ response while maintaining levels of IL-10 and not affecting cell proliferation, most significantly targeting IL-17A+IFN-γ+ cells (Figure 5). Importantly, as was true for vitamin D, anti-IL-23p19 did not obliterate all of the IL-17A+IFN-γ+ cells, which concurs with another study in which only a subset of CCR6+ CXCR3+ Th17/Th1 cells were IL-23 responsive (44). We therefore identify a novel pathway whereby NIST drives enhanced IL-23 production by CD1c+ DCs, which promotes a putatively pathogenic Th17 cell response in co-culture. The molecular mechanisms by which NIST acts on DCs to induce IL-23 are not fully understood. However, previous work has shown that NIST-primed DCs promote an effector memory CD4+ T cell response in a manner that is dependent on HLA-DR (25), but the effects are not due to LPS contamination (data not shown). We speculate that NIST might modify self-antigens that stimulate the DCs and/or that exogenous antigens such as viruses, bacteria, and allergens are adsorbed to the NIST to drive the observed effects. Studies to address this important issue are ongoing.
1,25(OH)2D3 significantly reduced the concentration of IL-12/23p40 when added in combination with NIST, as well as the resultant Th17 response promoted by the NIST-pretreated DCs (Figure 3). However, addition of exogenous recombinant IL-23 was unable to overcome the effect (data not shown), suggesting that 1,25(OH)2D3 is also likely to act through other mechanisms to dampen Th17 responses, although there may well be redundancy in the system. Indeed, in vivo, 1,25(OH)2D3 is thought to act through several immunoregulatory mechanisms to dampen Th17 responses (14). ILT3, which was shown here to be upregulated by 1,25(OH)2D3 (Figure E3B), may play a role in dampening the proinflammatory response, since it has been reported to reduce the synthesis of IL-17A and IFN-γ in mice (45). In contrast, interferon regulatory factor 4, which contains vitamin D response elements (46), promotes Th17 cell differentiation in mice (47) and was downregulated by 1,25(OH)2D3 at the mRNA level, with NIST having the reciprocal effect (Figure E3B).
From a therapeutic perspective, we believe that the reported capacity of vitamin D to dampen but not obliterate adaptive Th17 responses is critical. It seems highly plausible that such effects act alongside the well-documented capacity of vitamin D to act on structural and innate cells to promote antimicrobial pathways (14). Vitamin D has also been shown to counteract other potentially detrimental properties of UPM beyond the data presented here. For example, vitamin D can protect epithelial cells against oxidative stress (48), a major consequence of PM exposure that has been implicated in asthma, and oppose the induction of airway inflammation (49). Of particular relevance in the context of corticosteroid-refractory disease, vitamin D can overcome oxidative stress–induced impairment in the nuclear translocation of ligand-bound receptors such as the glucocorticoid receptor (50).
This research further elucidates the mechanisms that are likely to contribute to the epidemiological associations between vitamin D deficiency, air pollution, and respiratory diseases. Priming of CD1c+ DCs with NIST increased IL-23 synthesis, driving a phenotypically proinflammatory and potentially pathogenic Th17 profile. However, addition of active vitamin D, alone or in combination with corticosteroids, was able to counteract some of the effects of NIST while maintaining the levels of IL-10, supporting the notion that restoring vitamin D sufficiency may help to control inflammatory diseases and counteract certain negative effects of air pollution. This might be particularly true in subgroups of individuals, such as vitamin D–deficient individuals and those who are regularly exposed to high levels of air pollution.
Acknowledgments
Acknowledgments
We thank David F. Richards and Beverley Rodger for technical support throughout this project.
Footnotes
E.H.M. was supported by the Medical Research Council, and P.E.P. was a Wellcome Trust clinical training research fellow.
Author Contributions: E.H.M., P.E.P., N.C.M., and C.M.H. designed the study and wrote the manuscript. E.H.M. and T.-R.H. performed experiments. E.C. supplied nasal turbinate samples. I.M. and F.J.K. provided advice throughout and donated the pollution samples.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0409OC on May 2, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 2.Cosmi L, Liotta F, Annunziato F. Th17 regulating lower airway disease. Curr Opin Allergy Clin Immunol. 2016;16:1–6. doi: 10.1097/ACI.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 6.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 7.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132:297–304.e3. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, Ryanna K, Xystrakis E, Bush A, Saglani S, Hawrylowicz CM. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69:508–515. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- 11.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104:1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma. 2016 GINA Report, Global Strategy for Asthma Management and Prevention. 2016 [accessed 2017 May 23]. Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/
- 13.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin. Exp. Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 14.Mann EH, Chambers ES, Pfeffer PE, Hawrylowicz CM. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Ann N Y Acad Sci. 2014;1317:57–69. doi: 10.1111/nyas.12410. [DOI] [PubMed] [Google Scholar]
- 15.Adar SD, Filigrana PA, Clements N, Peel JL. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Environ Health Rep. 2014;1:258–274. doi: 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SY, Peel JL, Hannigan MP, Dutton SJ, Sheppard L, Clark ML, Vedal S. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120:1094–1099. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118:640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax. 2012;67:1018–1020. doi: 10.1136/thoraxjnl-2012-202139. [DOI] [PubMed] [Google Scholar]
- 19.Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 2010;184:426–432. doi: 10.4049/jimmunol.0902564. [DOI] [PubMed] [Google Scholar]
- 20.Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology. 2016;148:227–236. doi: 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faith A, McDonald J, Peek E, Richards D, Caulfield J, Chevretton E, Roberts D, Lee T, Corrigan C, Hawrylowicz C. Functional plasticity of human respiratory tract dendritic cells: GM-CSF enhances T(H)2 development. J Allergy Clin Immunol. 2005;116:1136–1143. doi: 10.1016/j.jaci.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Standards & Technology. Standard Reference Material 1648a. 2015 [accessed 2016 Aug 19]. Available from: https://www-s.nist.gov/srmors/certificates/1648A.pdf.
- 24.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 25.Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, Lee TH. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory Th1, Th2, and Th17 effector cells. Am J Respir Cell Mol Biol. 2016;54:250–262. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol. 2006;176:7431–7437. doi: 10.4049/jimmunol.176.12.7431. [DOI] [PubMed] [Google Scholar]
- 27.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, Bogunovic M, Gautier EL, Miller J, Leboeuf M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews NC, Faith A, Pfeffer P, Lu H, Kelly FJ, Hawrylowicz CM, Lee TH. Urban particulate matter suppresses priming of T helper type 1 cells by granulocyte/macrophage colony-stimulating factor-activated human dendritic cells. Am J Respir Cell Mol Biol. 2014;50:281–291. doi: 10.1165/rcmb.2012-0465OC. [DOI] [PubMed] [Google Scholar]
- 29.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204.e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annoni R, Silva LF, Nussbaumer-Ochsner Y, van Schadewijk A, Mauad T, Hiemstra PS, Rabe KF. Increased expression of granzymes A and B in fatal asthma. Eur Respir J. 2015;45:1485–1488. doi: 10.1183/09031936.00213814. [DOI] [PubMed] [Google Scholar]
- 33.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosser F, Brehm JM, Forno E, Acosta-Pérez E, Kurland K, Canino G, Celedón JC. Proximity to a major road, vitamin D insufficiency, and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2014;190:1190–1193. doi: 10.1164/rccm.201408-1568LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, Bellisario V, Buschka A, Bono R, Brunekreef B, et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE) Environ Health Perspect. 2015;123:613–621. doi: 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45:977–983. doi: 10.1165/rcmb.2011-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obregon C, Graf L, Chung KF, Cesson V, Nicod LP. Nitric oxide sustains IL-1β expression in human dendritic cells enhancing their capacity to induce IL-17-producing T-cells. PLoS One. 2015;10:e0120134. doi: 10.1371/journal.pone.0120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulissen SM, van Hamburg JP, Davelaar N, Vroman H, Hazes JM, de Jong PH, Lubberts E. CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther. 2015;17:344. doi: 10.1186/s13075-015-0800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136:628–637.e4. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CK, Choi J, Callaway Z, Iijima K, Volcheck G, Kita H. Increases in airway eosinophilia and a th1 cytokine during the chronic asymptomatic phase of asthma. Respir Med. 2010;104:1436–1443. doi: 10.1016/j.rmed.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt EB, Khurana Hershey GK. A combination of dexamethasone and anti-IL-17A treatment can alleviate diesel exhaust particle-induced steroid insensitive asthma. J Allergy Clin Immunol. 2016;138:924–928.e2. doi: 10.1016/j.jaci.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 43.Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, Zhou H, Wang E, Tsang JS, Nussenblatt R CHI Consortium. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:23002. doi: 10.1038/srep23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bordon Y. T cells: spotting the troublemakers. Nat Rev Immunol. 2014;14:64–65. doi: 10.1038/nri3610. [DOI] [PubMed] [Google Scholar]
- 45.Chang CC, Liu Z, Vlad G, Qin H, Qiao X, Mancini DM, Marboe CC, Cortesini R, Suciu-Foca N. Ig-like transcript 3 regulates expression of proinflammatory cytokines and migration of activated T cells. J Immunol. 2009;182:5208–5216. doi: 10.4049/jimmunol.0804048. [DOI] [PubMed] [Google Scholar]
- 46.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, He D, Yin C, Tan J. Inhibition of interferon regulatory factor 4 suppresses Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Scand J Immunol. 2015;82:345–351. doi: 10.1111/sji.12334. [DOI] [PubMed] [Google Scholar]
- 48.Peng X, Vaishnav A, Murillo G, Alimirah F, Torres KE, Mehta RG. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J Cell Biochem. 2010;110:1324–1333. doi: 10.1002/jcb.22646. [DOI] [PubMed] [Google Scholar]
- 49.Golden GA, Wyatt TA, Romberger DJ, Reiff D, McCaskill M, Bauer C, Gleason AM, Poole JA. Vitamin D treatment modulates organic dust-induced cellular and airway inflammatory consequences. J Biochem Mol Toxicol. 2013;27:77–86. doi: 10.1002/jbt.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan N, Luo G, Yang X, Cheng Y, Zhang Y, Wang X, Wang X, Xie T, Li G, Liu Z, et al. 25-Hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS One. 2014;9:e111599. doi: 10.1371/journal.pone.0111599. [DOI] [PMC free article] [PubMed] [Google Scholar]