Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder that affects reproductive-age women. Hyperandrogenemia is present in a significant fraction (~80%) of women with PCOS. Increased prevalence of cardiometabolic risk factors is frequently observed in PCOS women. The present review aims to highlight the key role of androgens in mediating the negative cardiometabolic profile observed in PCOS women.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women, affecting 5–26% of them during their reproductive years (12, 62). PCOS is the most common cause of androgen excess in young women (7). Definition and diagnosis of PCOS remain controversial since three different criteria currently coexist (6, 103, 126). Each criteria is based on a different combination of the following characteristics: hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. The lack of consensus in PCOS definition and diagnosis has caused multiple heterogeneous phenotypes to be included under a broad definition umbrella (91). Furthermore, the etiology and the pathogenesis of PCOS is unknown at present.
Although women with PCOS frequently seek medical attention due to infertility, menstrual dysfunction, or excessive male pattern hair growth (hirsutism), much attention has recently been focused on several metabolic derangements, such as obesity, insulin resistance, increases in blood pressure, metabolic syndrome, fatty liver, sleep apnea, dyslipidemia, and endothelial dysfunction that affect a disproportionate percentage of PCOS women compared with normal-cycling women (55, 98, 100, 113).
Hyperandrogenemia is considered a sine qua non component for the diagnosis of PCOS criteria sponsored by the Androgen Excess Society (6) and National Institutes of Health (NIH) (126), but not for the Rotterdam criteria (103). More recently, the Rotterdam criteria for PCOS diagnosis was endorsed by the NIH at an NIH-sponsored workshop with the caveat that the PCOS phenotype should be clearly stated (91). Nevertheless, multiple unbiased epidemiological studies have shown that hyperandrogenemia is present in ~80% of PCOS-diagnosed cases (71). Interestingly, PCOS women with elevated androgen levels have a worse cardio-metabolic profile compared with women with PCOS with normal levels of androgens (6, 16, 54, 58, 97, 122). Whether and how androgens mediate the cardiometabolic derangements in PCOS women are unknown.
Does Hyperandrogenism Mediate the Increase in Cardiometabolic Risk Factors in PCOS?
As mentioned above, hyperandrogenism is considered a hallmark of PCOS by the Androgen Excess Society and NIH guidelines (6, 126). Androgens can originate from the ovaries in most PCOS women, although in 20–30% of them, excess androgens are produced by the adrenal gland. Plasma levels of total and free testosterone, free dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), and androstenedione are significantly elevated in PCOS (7, 48). Testosterone and DHT are the most potent androgens; however, despite the fact that androstenedione, DHEA, and DHEAS have significantly less androgenic potency, they circulate at higher concentrations in plasma (56) and act as a reservoir that can be converted to testosterone and estrogens in various tissues, such as the fat and the skin (6, 59). In PCOS patients, plasma levels of testosterone are moderately elevated ~1.5-fold compared with control women. Enhancement in the steroidogenic activity of subcutaneous adipose tissue of women with PCOS has been demonstrated in some recent studies (94). Furthermore, the concentrations of androstenedione, DHEA, and testosterone in female adipose tissues are several fold higher than in plasma (11, 21, 34, 110). Despite these findings, the roles of the local intra-adipose androgens in mediating increases in blood pressure and cardiometabolic risk factors in women with PCOS are unknown.
Insulin resistance is considered to have a fundamental role in mediating the increased cardiometabolic risk factors present in women with PCOS (91). Insulin resistance is present in 50–90% of patients with PCOS (86). There is also an association between insulin resistance and androgens in PCOS. Androgen levels are positively correlated with hyperinsulinemia in PCOS women. Insulin can stimulate androgen production directly by the ovaries (93) and indirectly by centrally stimulating luteinizing hormone release (86). Women with insulin receptor mutations, and thus high levels of insulin, develop severe hyperandrogenemia (85). Hyperinsulinemia also contributes to hyperandrogenemia by decreasing the hepatic synthesis of sex hormone binding globulin (SHBG), leading to increased free androgen levels (22). Several mechanisms have been suggested to cause insulin resistance in women with PCOS, with obesity considered the primary culprit. However, insulin resistance is often observed even in lean patients with PCOS. These data suggest that insulin resistance may play a key role in the pathophysiology and be an aggravating factor of the hyperandrogenemia in PCOS women.
Several epidemiological studies have shown a positive correlation between the plasma levels of androgens and blood pressure (18), obesity (36), insulin resistance (8), and endothelial dysfunction (98) in PCOS women. Furthermore, reduction of testosterone is associated with improvement in dyslipidemia (23), endothelial dysfunction, body weight, and, as noted above, insulin resistance in PCOS women (3). Despite the strong evidence linking plasma androgen levels and cardiometabolic disturbances in PCOS women, whether and how androgens cause such negative cardiometabolic effects remains unclear.
Endothelin-1 is a potent vasoactive agent that mediates vasoconstriction and vasodilation via endothelin type A (ETA-R) and endothelin type B (ETB-R) receptors, respectively. Insights into the mechanisms by which androgens may promote endothelial dysfunction in PCOS women came from elegant studies performed by Wenner et al. This group demonstrated that women with PCOS have lower ETB-R-mediated vasodilation in skin compared with BMI-matched control subjects (119). In follow-up studies, this group demonstrated that suppression of the chronic elevation of testosterone improves microvascular function in PCOS women (118). In summary, these data demonstrate the key role of testosterone in mediating endothelial dysfunction in PCOS women.
Oral contraceptives (OCPs) are the first-line treatment option for hyperandrogenemia and irregular menstrual periods in PCOS. OCPs reduce circulating testosterone and androgen precursor levels by suppression of luteinizing hormone and stimulation of SHBG, leading to a decrease in free testosterone plasma levels. Whether long-term use of oral contraceptive agents can modify cardiovascular morbidity and mortality in PCOS is currently unknown. Moreover, some studies suggest that OCPs can exacerbate hypertension in women and are associated with a twofold increase in the risk of cardiovascular diseases in the general female population (4). Exogenous administration of estrogens can stimulate the production of angiotensinogen in the liver of female rats (60, 61), which one may speculate could lead to higher levels of angiotensin II (Ang II), a potent vasoconstrictor that could contribute to the increase in blood pressure. Moreover, OCPs are contra-indicated for smokers due to the higher risk of cardiovascular diseases in this population (112). OCPs do not seem to affect glucose and insulin homeostasis in the general population (114). However, the effect of OCPs on PCOS women remains controversial, mainly due to heterogeneity of the studies, as shown in a recent meta-analysis (44). Furthermore, several clinical studies have suggested that the use of OCPs may aggravate insulin resistance and worsen hyperglycemia in obese women with PCOS (2, 83, 89, 90). When OCP therapy is chosen, a low dose of estrogens should be preferred. The long-term impact of OCPs in glucose and insulin homeostasis in PCOS women remains unknown. Combined OCPs increase the risk of venous thromboembolism in the general population; and this adverse reaction is influenced by age, smoking, and obesity (26). Although the ideal progestins to use in PCOS are those with the lowest androgenic profile, such as chlormadinone and drospirenone, these steroids may induce a higher number of venous thrombosis events and may be contra-indicated in patients with severe obesity (39). Moreover, clinical studies have shown that PCOS women have higher incidence of venous thromboembolism, but data regarding the effect of OCPs have been contradictory (10, 96). The effect of OCPs in the lipid profile of PCOS women has proven puzzling. A meta-analysis report has shown that, although OCPs have a beneficial effect by increasing high-density lipoprotein cholesterol (HDL-C), they also present a detrimental effect by increasing triglycerides (44). On the other hand, a retrospective cross-sectional cohort study of 1,297 PCOS women did not show an OCP effect in the lipid profiles among current users, ever users and never users (82). Prospective randomized controlled long-term clinical trials analyzing the effect of OCPs on cardiovascular morbidity and mortality in PCOS women, obese and lean, are lacking and desperately needed.
Androgen receptor blockers are used in the management of hirsutism in PCOS women. Androgen receptor blockers are indicated if hirsutism is refractory to OCPs and insulin sensitizers. In the U.S., the androgen receptor blocker most commonly used in the clinic is spironolactone (39). However, spironolactone is also a progesterone and mineralocorticoid receptor blocker. Blockade of the mineralocorticoid receptor causes a diuretic effect that potentially can cause serious side effects such as hyperkalemia and hypotension (39). The recommendation is that patients have their potassium levels frequently checked and, for these patients, increase water and salt intake during hot weather. Usually, a high dose of spironolactone is necessary to block the androgen receptor, which potentially may lead to higher incidence of undesired side effects. Further research is needed to address the effect of androgen receptor blockers in the management of the cardiometabolic derangements in PCOS women. Other potent anti-androgens are flutamide and cyproterone acetate, although both present challenges. On one hand, cyproterone acetate is not currently available in the U.S. On the other hand, flutamide use has been associated with severe hepatoxicity and is not FDA-approved for use in PCOS women. More efficient and specific androgen receptor blockers should positively impact the management of the cardiometabolic risk factors in women with PCOS.
The prevalence of Type 2 diabetes mellitus in the U.S. is 10 times higher among young women with PCOS compared with age-matched, normal-cycling women (28). Current guidelines for the treatment of cardiometabolic risk factors in PCOS recommend weight loss and insulin sensitizer agents such as metformin (39, 40). Weight loss in PCOS women ameliorates some of the cardiovascular risk factors, as in non-PCOS women. However, weight loss is very difficult to sustain with time, probably due to resetting of the basal metabolic rate (35). Since insulin resistance is present in obese and lean women with PCOS, it has been proposed to be the key factor in mediating the negative cardiovascular risk profile observed in PCOS subjects. Metformin has been used for years to treat insulin resistance in PCOS women. Systematic reviews and meta-analysis have shown that metformin decreases fasting glucose and insulin, blood pressure, and low-density lipoprotein cholesterol (73, 111). However, long-term prospective randomized controlled trials assessing the effect of metformin on long-term cardiovascular benefit to PCOS women by decreasing cardiovascular morbidity and mortality are not available at present. Even in the absence of PCOS, metformin reduces the risk of progression from insulin resistance to diabetes in only 30% of patients (63). Metformin can lower the level of testosterone; thereby any beneficial effect in PCOS women may be due to lowering their androgen levels (92).
Although women with PCOS have a higher prevalence of cardiovascular risk factors and surrogates of cardiovascular diseases, such as carotid intima-media thickness (64) and coronary artery calcification (19), whether PCOS women have a higher rate of cardiovascular mortality has not as yet been demonstrated. A 10-yr follow-up study of postmenopausal women with or without clinical features of PCOS from the Women's Ischemia Syndrome Evaluation (WISE) study showed that PCOS was not a predictor of fatal and non-fatal cardiovascular events (81). However, larger prospective long-term follow-up studies are necessary to answer the question of whether the diagnosis of PCOS carries a higher risk of morbidity and mortality due to cardiovascular diseases.
Hypertension is a major risk factor for cardiovascular disease and mortality (66, 70). Several clinical studies have shown that increased blood pressure or the incidence of hypertension is significantly increased in PCOS women (17, 30, 47, 72, 88, 117, 120). Moreover, the higher prevalence of hypertension in PCOS women was observed across multiple ethnic groups (17, 72). Recently, a large case-control study including 1,550 PCOS women and 447 control women showed that PCOS women present both systolic and diastolic blood pressure increases (99). The mechanisms that are involved in the higher blood pressure in PCOS are uncertain, and whether the mechanisms differ for individual ethnic groups is also unclear.
In summary, whether PCOS is linked to a higher rate of cardiovascular mortality is unknown at present and should be answered with better powered studies that include larger populations of women with PCOS. What is clear, however, is that women with PCOS frequently have hypertension or elevated blood pressure. The mechanism(s) underling the increase in blood pressure in PCOS remains unclear.
Cardiometabolic Features in the Hyperandrogenemic Female Rat: A Model of PCOS
There are multiple experimental animal models of PCOS. A particularly useful model in which to study the cardiometabolic effects of hyperandrogenemia in PCOS is the one reported by Manneras and colleagues produced by administration of the non-aromatizable androgen DHT to pre-pubertal, 4-wk-old female rats. DHT for 90 days causes morphological and histological changes in the ovaries, estrous cycle disruption, and insulin resistance similar to those observed in women with PCOS (77). Using this animal experimental model, we also reported that DHT administration causes a mild but consistent increase of blood pressure (10 mmHg) measured by radiotelemetry in freely moving conscious animals (123). Furthermore, this increase in blood pressure is associated with increases in food intake, subsequent obesity, insulin resistance, dyslipidemia, renal injury, systemic inflammation, and activation of the intrarenal renin angiotensin system (RAS) and the sympathetic nervous system (78, 123). Although these data strongly suggest that hyperandrogenemia in female rats may directly increase blood pressure in the PCOS model, the exact mechanism(s) by which androgens exhibit this deleterious effect remains unclear. Moreover, the presence of insulin resistance and obesity in this model, as in PCOS women, most likely further worsens the negative cardiovascular risk factor profile.
Possible Mechanisms by Which Androgens Increase Blood Pressure in PCOS
Insulin Resistance
Insulin resistance and hyperandrogenemia are the cardinal features of PCOS (107). Women with insulin receptor mutations, and thus high levels of insulin, develop severe hyperandrogenemia (85). Insulin can stimulate androgen production directly by the ovaries (93) and indirectly by centrally stimulating luteinizing hormone release (86). Insulin resistance is present in 50–90% of patients with PCOS (86), independent of obesity. Hyperinsulinemia has been postulated to link obesity and hypertension via the antinatriuretic actions of insulin. Pioneer work by Hall and colleagues showed several years ago that infusion of insulin can increase blood pressure in rats by 5–8 mmHg, but not in dogs (13, 14, 42). One caveat to those experiments is that plasma glucose values were normal; therefore, one could speculate that insulin per se is not enough to cause significant hypertension in the absence of elevated glucose levels. In addition, those experiments were performed in male animals.
Although a significant percentage of women with PCOS are obese, some of them are not. By the same token, insulin resistance is exacerbated by obesity in PCOS, but insulin resistance presents in lean PCOS women as well (86, 107). These observations may suggest that androgens can cause insulin resistance per se in females, independent of obesity. Furthermore, Huirliman and colleages recently showed that the presence of endothelial dysfunction, hyperinsulinemia, and insulin resistance develops in pair-fed DHT-treated female rats, suggesting an obesity-independent mechanism (49). Currently, whether insulin is the principal factor triggering the cardiometabolic abnormalities of PCOS remains unclear.
Renin Angiotensin System
Women with PCOS have hyper-reninemia that positively correlates with hyperandrogenemia (51). Furthermore, telmisartan, an angiotensin type 1 receptor (AT1R) antagonist, significantly reduces blood pressure elevation in PCOS patients (52). Androgen replacement in castrated male rats increases renin and angiotensinogen synthesis (124). Androgens also mediate a portion of the salt-sensitive hypertension in Dahl salt-sensitive rats (124). If renin enzyme activity is below Vmax, as has been shown in both humans and rats, then an increase in angiotensinogen will cause an increase in the conversion of angiotensinogen to angiotensin I, leading to an increase in Ang II production, since renin, not angiotensin-converting enzyme (ACE), is the rate-limiting enzyme for Ang II production (74). We found that, in the hyperandrogenemic female rat, renal angiotensinogen is upregulated, suggesting that the intrarenal RAS is activated in this model (123). Whether activation of the RAS plays a role in mediating hypertension in PCOS women is unknown.
AT1R blockers or ACE inhibitors are widely used as antihypertensive drugs. Women should be advised about the potential teratogenic and fetotoxic risks of ACE inhibitors or AT1R blockers if they become pregnant. Novel and tissue-selective RAS inhibitors that do not cross the placental barrier are warranted to ameliorate the increases in blood pressure in women with PCOS in the future.
Sympathetic Nervous System
PCOS women have an activation of the sympathetic nervous system (105, 109). The increase in sympathetic drive and impaired endothelial function seems to be independent of obesity and metabolic disturbances (65). There is also evidence that the renal sympathetic nervous system may also be upregulated. Schlaich and colleagues reported that radiofrequency renal nerve ablation reduced the blood pressure in two hypertensive young women with PCOS (105). We have recently shown that blood pressure elevation in hyperandrogenemic female rats is due, in part, to activation of the sympathetic nervous system, renal nerves, and central melanocortin-4-receptor (MC4R). Hall and colleagues reported that obesity-related hypertension and the concomitant sympathetic activation are mediated, in part, by the MC4R in the brain (24, 25, 43). We found that MC4R receptor expression in the brain was significantly upregulated in the PCOS model compared with normal rats, and blockade of the MC4R reduced their blood pressure (78). Targeting the MC4R could be a novel therapeutic tool to ameliorate the cardiometabolic risk factors in PCOS.
20-HETE
The role of 20-hydroxyeicosatetraeonic acid (20-HETE) to modulate blood pressure is well known (101, 102). 20-HETE has differential effects on the kidney, depending on the location of synthesis. In the renal microvasculature, 20-HETE is prohypertensive, acting as a vasoconstrictor, whereas in the renal tubules, 20-HETE attenuates Na+ reabsorption and thus is antihypertensive (121). We recently reported that, in the PCOS model, cytochrome P450 (CYP) ω-hydroxylase isoform CYP4A2, which is involved in 20-HETE biosynthesis, is upregulated and is associated with increases in endogenous renal microvascular 20-HETE. Furthermore, DHT did not increase MAP in 20-HETE-deficient Dahl SS female rats, whereas DHT did increase mean arterial pressure in Dahl SR females, suggesting that an intact 20-HETE system capable of upregulation contributes to the elevated blood pressure in PCOS model. This effect seems to be specific to the presence of CYP4A2 ω-hydroxylase, since lack of this enzyme prevented the DHT-mediated pressor response in CYP4A2-null female rats. Taken together, these data suggest that 20-HETE contributes to the DHT-mediated elevated blood pressure in PCOS model and that the CYP4A2 isoform may be at least partially responsible for the increase in blood pressure. These data also suggest that metabolites of arachidonic acid constitute a novel pathway that may be involved in mediating blood pressure elevation in PCOS.
Incretin System and Other Novel Mechanisms
Several other mechanisms have been proposed to mediate the metabolic abnormalities observed in PCOS women. Glucagon-like peptide-1 (GLP-1) is an incretin that potentiates the food-mediated release of insulin, leading to a decrease in plasma glucose levels, delaying gastric emptying, and exerting satiety effects that assist with weight control. Recently, a small clinical trial showed that administration of GLP-1 receptor agonists caused significant improvement in insulin resistance, decreases in blood pressure, and improvement in the abnormalities of the estrous cycle in PCOS women (53). Similar beneficial effects of GLP-1 were observed in DHT-treated females rats, as administration of the long-acting GLP-1 agonist, liraglutide, to DHT-treated rats significantly improved insulin resistance and decreased blood pressure (46). These results suggest that GLP-1 treatment could improve DHT-induced metabolic and blood pressure abnormalities associated with PCOS. Whether the incretin system interacts with or modulates androgens is unknown, but, if so, it is also possible that incretin modulators may constitute a novel therapeutic tool to ameliorate the cardiometabolic abnormalities observed in PCOS women.
Women with PCOS also have an inverse association between vitamin D status and metabolic disturbances. A recent study demonstrated that vitamin D deficiency is prevalent in women with PCOS, with 60–70% of them having low levels of 25-OH vitamin D (84). Further research and adequately powered randomized controlled clinical trials of vitamin D supplementation and its effects on the cardiometabolic risk factors observed in women affected by PCOS are necessary to elucidate the endocrine role of vitamin D in PCOS.
Nesfatin-1 peptide was discovered in 2006 in the rat hypothalamus and is described as a centrally acting anorexigenic peptide. Nesfatin-1 is derived from nucleobindin 2 (NUCB2) and is released from several tissues, including forebrain, hindbrain, brain stem, spinal cord, and adipose tissues (106). In addition to its effects on food intake, nesfatin-1 exerts cardiovascular and hypertensive effects, and causes insulin resistance in several animal models (95, 108, 125). Several studies have shown that nesfatin-1 is associated with increased body mass index (BMI), insulin resistance, inflammation, hypertension, and PCOS (1, 104, 108, 125). Whether and how androgens may regulate nesfatin-1 in PCOS remains unknown.
Obesity as a Critical Factor in the Clinical Manifestations of PCOS
In the U.S., up to 80% of PCOS women are obese (28, 69), a percentage much higher than observed in the general population. Furthermore, obese PCOS subjects are characterized by worsened hyperandrogenemic and metabolic states compared with normal-weight women with PCOS (33, 87). Moreover, weight loss by diet or bariatric surgery reduces the level of androgens and is associated with improvement in both metabolic and reproductive abnormalities in PCOS (31, 57).
Testosterone has a differential metabolic effect in adipose tissue in women vs. men. For example, in men, low levels of testosterone are associated with increases in obesity and visceral adiposity (15, 41), whereas in women with PCOS, there is a positive correlation between circulating levels of testosterone and obesity (41, 67, 116). In women with PCOS, the distribution of adipose tissue is abnormal (80). There have been conflicting reports as to whether women with PCOS have increased or decreased amounts of visceral fat (5, 32, 33, 76). More recently, it has been shown that the amount of subcutaneous adipose tissue is significantly increased with PCOS (9, 27, 29). The role that increases in subcutaneous adipose tissue play in mediating obesity, insulin resistance, and hypertension in PCOS is unclear. Obesity has a major impact on the incidence and clinical manifestations of PCOS (68). Also, the environment, with diet being probably the main factor, plays a major role in the pathogenesis of the syndrome, since women with PCOS in the U.S. generally have a higher body mass index (BMI) than their European or Asian counterparts. In a large cohort of women with PCOS in the U.S., ~60% of the patients studied were obese (BMI of >30 kg/m2), and 20% were severely obese (BMI of >40 kg/m2) (38). Moreover, there is a positive relationship between plasma androgen levels, obesity, and insulin resistance in women with PCOS. Weight loss, the first-line clinical recommendation for women with PCOS, is associated with improvement of infertility, improvement in metabolic derangement, and reductions in hyperandrogenism (31, 57). Exercise, independent of weight loss, may have a beneficial effect on insulin resistance, dyslipidemia, and visceral obesity in PCOS, and patients must be counseled about the beneficial effects of exercise in the management of PCOS cardiometabolic risk factors (50). The beneficial effect of exercise in PCOS seems not to be related to the type, frequency, or length of exercise, suggesting that any amount of exercise may be helpful (45).
Moreover, serum androgens are positively correlated with BMI, not only in PCOS, but also in obese subjects without PCOS (115). Weight loss via lifestyle modification, pharmacotherapy, or bariatric surgery in women with PCOS decreases androgen levels (31, 57). The concentrations of androstenedione, DHEA, and testosterone in female adipose tissue are several-fold higher than in plasma (11, 21, 34, 110). However, the role of the local intra-adipose androgen in mediating the negative cardiometabolic profile in women with PCOS is unknown at present.
Summary and Open Questions
PCOS is the most common endocrine disorder that affects young women. Unfortunately, a retrospective birth cohort community study has shown that ~70% of young PCOS women are undiagnosed and not aware of this medical condition (79). Moreover, a study of recently diagnosed PCOS women showed that half of them need to consult with three or more health care professionals, and for one-third of them, it took >2 yr to have a PCOS diagnosis established (37). The standard pharmacological approach in PCOS women is oral contraceptives and insulin-sensitizing agents. Oral contraceptives are the first-line medical treatment for many women with PCOS. Estrogens can have negative cardiovascular effects, especially thromboembolic effects, and their use is not recommended for women above the age of 35. Thus, currently, therapeutic options to treat PCOS-associated cardiovascular risk factors are limited (75). Androgen blockers are rarely used to ameliorate the negative cardiometabolic profile frequently observed in PCOS women. These issues underscore how critical it is to understand how androgens mediate the increased risks in cardiovascular diseases in women with PCOS to provide better therapeutic options to these patients.
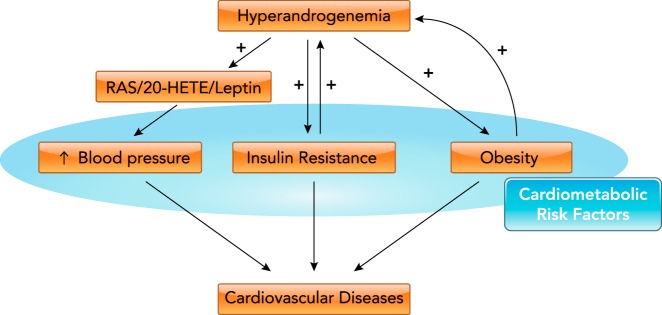
Whether and how hyperandrogenemia causes obesity, insulin resistance, increases in blood pressure, and endothelial dysfunction in PCOS and the interplay between these cardiovascular risk factors in PCOS are unclear (FIGURE 1). Increased blood pressure, insulin resistance, and obesity can have a major impact in long-term development of cardiovascular diseases and negatively impact health status. Mechanistically, androgen induced insulin resistance, activation of vasoconstrictors such as angiotensin II, endothelin-1, and 20-HETE, and activation of sympathetic nervous system, all of which can mediate the negative cardiovascular profile observed in PCOS women. New agents such as GLP-1 analogs, melanocortin 4 receptor (MC4R) agonists, and specific RAS blockers may constitute attractive agents to ameliorate the metabolic abnormalities in PCOS.
FIGURE 1.
Potential mechanisms by which hyperandrogenemia mediates the negative cardiometabolic risk factor profile exhibited in PCOS
In PCOS women, hyperandrogenemia increase cardiometabolic risk factors such as obesity, insulin resistance, and increased blood pressure by means of activation of the renin-angiotensin system (RAS), 20-HETE, and leptin systems, which ultimately leads to cardiovascular diseases.
Although PCOS is the most common cause of androgen excess in women, several other clinical conditions in women are associated with increased levels of androgens, such as congenital adrenal hyperplasia, ovarian tumors, Cushing syndrome, testosterone supplementation, some seizure medications, female-to-male transgender, and menopause. To what extent individuals with these conditions have increased cardiometabolic risk factors as in PCOS women remains to be elucidated. A better understanding of the physiological and pathophysiological roles of androgens in cardiovascular and metabolic function in women is paramount at present.
Acknowledgments
This work was supported by American Heart Association Grants 0830239N (L.L.Y.C.) and 12SDG8980032 (D.G.R.), Endocrine Fellows Foundation Endocrine Research Grant (L.L.Y.C.), and National Institutes of Health grants R21 DK-113500 (D.G.R.), R01 HL-66072 (J.F.R.), P01 HL-51971 (J.F.R.), and P20 GM-121334 (J.F.R. and L.L.Y.C.).
No conflicts of interest, financial or otherwise, are declared by the authors.
Author contributions: L.L.Y.C., D.G.R., and J.F.R. drafted manuscript; L.L.Y.C., D.G.R., and J.F.R. edited and revised manuscript; L.L.Y.C., D.G.R., and J.F.R. approved final version of manuscript.
References
- 1.Ademoglu EN, Gorar S, Carlıoglu A, Yazıcı H, Dellal FD, Berberoglu Z, Akdeniz D, Uysal S, Karakurt F. Plasma nesfatin-1 levels are increased in patients with polycystic ovary syndrome. J Endocrinol Invest 37: 715–719, 2014. doi: 10.1007/s40618-014-0089-2. [DOI] [PubMed] [Google Scholar]
- 2.Adeniji AA, Essah PA, Nestler JE, Cheang KI. Metabolic effects of a commonly used combined hormonal oral contraceptive in women with and without polycystic ovary syndrome. J Womens Health (Larchmt) 25: 638–645, 2016. doi: 10.1089/jwh.2015.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiri M, Golsorkhtabaramiri M, Esmaeilzadeh S, Ghofrani F, Bijani A, Ghorbani L, Delavar MA. Effect of metformin and flutamide on anthropometric indices and laboratory tests in obese/overweight PCOS women under hypocaloric diet. J Reprod Infertil 15: 205–213, 2014. [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo LF, de Matos Soeiro A, Fernandes JL, Pesaro AE, Serrano CV Jr. Coronary artery disease in women: a review on prevention, pathophysiology, diagnosis, and treatment. Vasc Health Risk Manag 2: 465–475, 2006. doi: 10.2147/vhrm.2006.2.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arpaci D, Gurkan Tocoglu A, Yilmaz S, Ergenc H, Tamer A, Keser N, Gunduz H. The relationship between epicardial fat tissue thickness and visceral adipose tissue in lean patients with polycystic ovary syndrome. J Ovarian Res 8: 71, 2015. doi: 10.1186/s13048-015-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society . Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91: 4237–4245, 2006. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 89: 453–462, 2004. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 8.Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond) 15, Suppl 6: s72–s76, 2015. doi: 10.7861/clinmedicine.15-6-s72. [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 10.Bird ST, Hartzema AG, Brophy JM, Etminan M, Delaney JA. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ 185: E115–E120, 2013. doi: 10.1503/cmaj.120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borg W, Shackleton CH, Pahuja SL, Hochberg RB. Long-lived testosterone esters in the rat. Proc Natl Acad Sci USA 92: 1545–1549, 1995. doi: 10.1073/pnas.92.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31: 2841–2855, 2016. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 13.Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Hypertension during chronic hyperinsulinemia in rats is not salt-sensitive. Hypertension 19, Suppl: I83–I89, 1992. doi: 10.1161/01.HYP.19.1_Suppl.I83. [DOI] [PubMed] [Google Scholar]
- 14.Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA, Hall JE. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens 4: 164–168, 1991. doi: 10.1093/ajh/4.2.164. [DOI] [PubMed] [Google Scholar]
- 15.Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl 16: 146–152, 2014. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cakir E, Doğan M, Topaloglu O, Ozbek M, Cakal E, Vural MG, Yeter E, Delibasi T. Subclinical atherosclerosis and hyperandrogenemia are independent risk factors for increased epicardial fat thickness in patients with PCOS and idiopathic hirsutism. Atherosclerosis 226: 291–295, 2013. doi: 10.1016/j.atherosclerosis.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Chang AY, Oshiro J, Ayers C, Auchus RJ. Influence of race/ethnicity on cardiovascular risk factors in polycystic ovary syndrome, the Dallas Heart Study. Clin Endocrinol (Oxf) 85: 92–99, 2016. doi: 10.1111/cen.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442–1447, 2007. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 19.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF II, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88: 2562–2568, 2003. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 20.Dalmasso C, Maranon R, Patil C, Moulana M, Romero DG, Reckelhoff JF. 20-HETE and CYP4A2 ω-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol 311: F71–F77, 2016. doi: 10.1152/ajprenal.00458.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deslypere JP, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab 61: 564–570, 1985. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 22.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33: 981–1030, 2012. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab 83: 2699–2705, 1998. doi: 10.1210/jcem.83.8.5041. [DOI] [PubMed] [Google Scholar]
- 24.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life 65: 692–698, 2013. doi: 10.1002/iub.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol 305: R359–R368, 2013. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domecq JP, Prutsky G, Mullan RJ, Sundaresh V, Wang AT, Erwin PJ, Welt C, Ehrmann D, Montori VM, Murad MH. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab 98: 4646–4654, 2013. doi: 10.1210/jc.2013-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41: 1257–1266, 1992. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 28.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22: 141–146, 1999. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 29.Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 82: 1147–1153, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod 16: 556–560, 2001. doi: 10.1093/humrep/16.3.556. [DOI] [PubMed] [Google Scholar]
- 31.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 90: 6364–6369, 2005. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 32.Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, Dumesic DA, Azziz R. Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab 98: 1541–1548, 2013. doi: 10.1210/jc.2012-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97: 28–38.e25, 2012. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Fehér T, Bodrogi L. A comparative study of steroid concentrations in human adipose tissue and the peripheral circulation. Clin Chim Acta 126: 135–141, 1982. doi: 10.1016/0009-8981(82)90029-8. [DOI] [PubMed] [Google Scholar]
- 35.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 24: 1612–1619, 2016. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 26: 883–896, 2002. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 37.Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 102: 604–612, 2017. doi: 10.1210/jc.2016-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, Sieve L. Obesity and extreme obesity, manifest by ages 20-24 years, continuing through 32-41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 122: 206–212, 2005. doi: 10.1016/j.ejogrb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES) . American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: Guide to the best practices in the evaluation and treatment of Polycystic Ovary Syndrome-Part 1. Endocr Pract 21: 1291–1300, 2015. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 40.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society . American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: Guide to the best practices in the evaluation and treatment of Polycystic Ovary Syndrome-Part 2. Endocr Pract 21: 1415–1426, 2015. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 41.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17: 247–256, 2010. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- 42.Hall JE, Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA. Chronic intrarenal hyperinsulinemia does not cause hypertension. Am J Physiol 260: F663–F669, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum Reprod 26: 191–201, 2011. doi: 10.1093/humrep/deq301. [DOI] [PubMed] [Google Scholar]
- 45.Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 17: 171–183, 2011. doi: 10.1093/humupd/dmq045. [DOI] [PubMed] [Google Scholar]
- 46.Hoang V, Bi J, Mohankumar SM, Vyas AK. Liraglutide improves hypertension and metabolic perturbation in a rat model of polycystic ovarian syndrome. PLoS One 10: e0126119, 2015. doi: 10.1371/journal.pone.0126119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holte J, Gennarelli G, Berne C, Bergh T, Lithell H. Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod 11: 23–28, 1996. doi: 10.1093/oxfordjournals.humrep.a019028. [DOI] [PubMed] [Google Scholar]
- 48.Huang A, Brennan K, Azziz R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril 93: 1938–1941, 2010. doi: 10.1016/j.fertnstert.2008.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurliman A, Keller Brown J, Maille N, Mandala M, Casson P, Osol G. Hyperandrogenism and insulin resistance, not changes in body weight, mediate the development of endothelial dysfunction in a female rat model of polycystic ovary syndrome (PCOS). Endocrinology 156: 4071–4080, 2015. doi: 10.1210/en.2015-1159. [DOI] [PubMed] [Google Scholar]
- 50.Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab 96: E48–E56, 2011. doi: 10.1210/jc.2010-0828. [DOI] [PubMed] [Google Scholar]
- 51.Jaatinen TA, Matinlauri I, Anttila L, Koskinen P, Erkkola R, Irjala K. Serum total renin is elevated in women with polycystic ovarian syndrome. Fertil Steril 63: 1000–1004, 1995. doi: 10.1016/S0015-0282(16)57537-2. [DOI] [PubMed] [Google Scholar]
- 52.Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, Kocjan T. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: case series. Croat Med J 48: 864–870, 2007. doi: 10.3325/cmj.2007.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 170: 451–459, 2014. doi: 10.1530/EJE-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, Cuthbertson DJ. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab 97: 3709–3716, 2012. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 55.Kargili A, Karakurt F, Kasapoglu B, Derbent A, Koca C, Selcoki Y. Association of polycystic ovary syndrome and a non-dipping blood pressure pattern in young women. Clinics (Sao Paulo) 65: 475–479, 2010. doi: 10.1590/S1807-59322010000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keefe CC, Goldman MM, Zhang K, Clarke N, Reitz RE, Welt CK. Simultaneous measurement of thirteen steroid hormones in women with polycystic ovary syndrome and control women using liquid chromatography-tandem mass spectrometry. PLoS One 9: e93805, 2014. doi: 10.1371/journal.pone.0093805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36: 105–111, 1992. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim MJ, Lim NK, Choi YM, Kim JJ, Hwang KR, Chae SJ, Park CW, Choi DS, Kang BM, Lee BS, Kim T, Park HY. Prevalence of metabolic syndrome is higher among non-obese PCOS women with hyperandrogenism and menstrual irregularity in Korea. PLoS One 9: e99252, 2014. doi: 10.1371/journal.pone.0099252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirschner MA, Bardin CW. Androgen production and metabolism in normal and virilized women. Metabolism 21: 667–688, 1972. doi: 10.1016/0026-0495(72)90090-X. [DOI] [PubMed] [Google Scholar]
- 60.Klett C, Ganten D, Hellmann W, Kaling M, Ryffel GU, Weimar-Ehl T, Hackenthal E. Regulation of hepatic angiotensinogen synthesis and secretion by steroid hormones. Endocrinology 130: 3660–3668, 1992. doi: 10.1210/endo.130.6.1597163. [DOI] [PubMed] [Google Scholar]
- 61.Klett C, Hellmann W, Hackenthal E, Ganten D. Modulation of tissue angiotensinogen gene expression by glucocorticoids, estrogens, and androgens in SHR and WKY rats. Clin Exp Hypertens 15: 683–708, 1993. doi: 10.3109/10641969309041637. [DOI] [PubMed] [Google Scholar]
- 62.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83: 3078–3082, 1998. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 63.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakhani K, Constantinovici N, Purcell WM, Fernando R, Hardiman P. Internal carotid artery haemodynamics in women with polycystic ovaries. Clin Sci (Lond) 98: 661–665, 2000. doi: 10.1042/cs0980661. [DOI] [PubMed] [Google Scholar]
- 65.Lambert EA, Teede H, Sari CI, Jona E, Shorakae S, Woodington K, Hemmes R, Eikelis N, Straznicky NE, De Courten B, Dixon JB, Schlaich MP, Lambert GW. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clin Endocrinol (Oxf) 83: 812–819, 2015. doi: 10.1111/cen.12803. [DOI] [PubMed] [Google Scholar]
- 66.Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension . Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513–1518, 2008. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 67.LaZovic G, Radivojevic U, Milicevic S, Spremovic S. Influence of adiposity on leptin, LH and androgen levels in lean, overweight and obese PCOS patients. Int J Fertil Womens Med 52: 82–88, 2007. [PubMed] [Google Scholar]
- 68.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med 30: 496–506, 2012. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84: 165–169, 1999. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 70.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 71.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106: 6–15, 2016. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 91: 1357–1363, 2006. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 73.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 327: 951–953, 2003. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luther RR, Stein HH, Glassman HN, Kleinert HD. Renin inhibitors: specific modulators of the renin-angiotensin system. Arzneimittelforschung 39: 1–5, 1989. [PubMed] [Google Scholar]
- 75.Mahalingaiah S, Diamanti-Kandarakis E. Targets to treat metabolic syndrome in polycystic ovary syndrome. Expert Opin Ther Targets 19: 1561–1574, 2015. doi: 10.1517/14728222.2015.1101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 96: E304–E311, 2011. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 77.Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148: 3781–3791, 2007. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 78.Maranon R, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol 308: R708–R713, 2015. doi: 10.1152/ajpregu.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25: 544–551, 2010. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 80.Martínez-Bermejo E, Luque-Ramírez M, Escobar-Morreale HF. Obesity and the polycystic ovary syndrome. Minerva Endocrinol 32: 129–140, 2007. [PubMed] [Google Scholar]
- 81.Merz CN, Shaw LJ, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-DeHoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Cardiovascular disease and 10-year mortality in postmenopausal women with clinical features of polycystic ovary syndrome. J Womens Health (Larchmt) 25: 875–881, 2016. doi: 10.1089/jwh.2015.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mes-Krowinkel MG, Louwers YV, Mulders AG, de Jong FH, Fauser BC, Laven JS. Influence of oral contraceptives on anthropomorphometric, endocrine, and metabolic profiles of anovulatory polycystic ovary syndrome patients. Fertil Steril 101: 1757–65.e1, 2014. doi: 10.1016/j.fertnstert.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 83.Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care 30: 471–478, 2007. doi: 10.2337/dc06-0618. [DOI] [PubMed] [Google Scholar]
- 84.Mishra S, Das AK, Das S. Hypovitaminosis D and associated cardiometabolic risk in women with PCOS. J Clin Diagn Res 10: BC01–BC04, 2016. doi: 10.7860/JCDR/2016/19407.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moller DE, Cohen O, Yamaguchi Y, Assiz R, Grigorescu F, Eberle A, Morrow LA, Moses AC, Flier JS. Prevalence of mutations in the insulin receptor gene in subjects with features of the type A syndrome of insulin resistance. Diabetes 43: 247–255, 1994. doi: 10.2337/diab.43.2.247. [DOI] [PubMed] [Google Scholar]
- 86.Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab 81: 2854–2864, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16: 347–363, 2010. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 88.Morgan CL, Jenkins-Jones S, Currie CJ, Rees DA. Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab 97: 3251–3260, 2012. doi: 10.1210/jc.2012-1690. [DOI] [PubMed] [Google Scholar]
- 89.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab 85: 3161–3168, 2000. doi: 10.1210/jcem.85.9.6792. [DOI] [PubMed] [Google Scholar]
- 90.Nader S, Riad-Gabriel MG, Saad MF. The effect of a desogestrel-containing oral contraceptive on glucose tolerance and leptin concentrations in hyperandrogenic women. J Clin Endocrinol Metab 82: 3074–3077, 1997. doi: 10.1210/jcem.82.9.4192. [DOI] [PubMed] [Google Scholar]
- 91.National Institutes of Health Evidence-based methodology workshop on polycystic ovary syndrome. Bethesda, MD: National Institutes of Health, 2012. [Google Scholar]
- 92.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 335: 617–623, 1996. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 93.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83: 2001–2005, 1998. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 94.O’Reilly M, Gathercole L, Capper F, Arlt W, Tomlinson J. Effect of insulin on AKR1C3 expression in female adipose tissue: in-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet 385, Suppl 1: S16, 2015. doi: 10.1016/S0140-6736(15)60331-2. [DOI] [PubMed] [Google Scholar]
- 95.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 96.Okoroh EM, Hooper WC, Atrash HK, Yusuf HR, Boulet SL. Is polycystic ovary syndrome another risk factor for venous thromboembolism? United States, 2003-2008. Am J Obstet Gynecol 207: 377.e1–377.e8, 2012. doi: 10.1016/j.ajog.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, Macut D. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod 27: 541–549, 2012. doi: 10.1093/humrep/der418. [DOI] [PubMed] [Google Scholar]
- 98.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103: 1410–1415, 2001. doi: 10.1161/01.CIR.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 99.Pinola P, Puukka K, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, Stener-Victorin E, Lindén Hirschberg A, Ravn P, Skovsager Andersen M, Glintborg D, Mellembakken JR, Ruokonen A, Tapanainen JS, Morin-Papunen LC. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril 107: 788–795.e2, 2017. doi: 10.1016/j.fertnstert.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 100.Rizzo M, Berneis K, Spinas G, Rini GB, Carmina E. Long-term consequences of polycystic ovary syndrome on cardiovascular risk. Fertil Steril 91, Suppl: 1563–1567, 2009. doi: 10.1016/j.fertnstert.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 101.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 102.Roman RJ, Maier KG, Sun CW, Harder DR, Alonso-Galicia M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol 27: 855–865, 2000. doi: 10.1046/j.1440-1681.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 103.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81: 19–25, 2004. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Sahin FK, Sahin SB, Ural UM, Cure MC, Senturk S, Tekin YB, Balik G, Cure E, Yuce S, Kirbas A. Nesfatin-1 and vitamin D levels may be associated with systolic and diastolic blood pressure values and hearth rate in polycystic ovary syndrome. Bosn J Basic Med Sci 15: 57–63, 2015. doi: 10.17305/bjbms.2015.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlaich MP, Straznicky N, Grima M, Ika-Sari C, Dawood T, Mahfoud F, Lambert E, Chopra R, Socratous F, Hennebry S, Eikelis N, Böhm M, Krum H, Lambert G, Esler MD, Sobotka PA. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 29: 991–996, 2011. doi: 10.1097/HJH.0b013e328344db3a. [DOI] [PubMed] [Google Scholar]
- 106.Stengel A, Mori M, Taché Y. The role of nesfatin-1 in the regulation of food intake and body weight: recent developments and future endeavors. Obes Rev 14: 859–870, 2013. doi: 10.1111/obr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 28: 777–784, 2013. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 108.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun 391: 1039–1042, 2010. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 109.Sverrisdóttir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 110.Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids 63: 319–321, 1998. doi: 10.1016/S0039-128X(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 111.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev (5): CD003053, 2012. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed] [Google Scholar]
- 112.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, van der Graaf Y, Rosendaal FR. Oral contraceptives and the risk of myocardial infarction. N Engl J Med 345: 1787–1793, 2001. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 113.Tock L, Carneiro G, Togeiro SM, Hachul H, Pereira AZ, Tufik S, Zanella MT. Obstructive sleep apnea predisposes to nonalcoholic Fatty liver disease in patients with polycystic ovary syndrome. Endocr Pract 20: 244–251, 2014. doi: 10.4158/EP12366.OR. [DOI] [PubMed] [Google Scholar]
- 114.Troisi RJ, Cowie CC, Harris MI. Oral contraceptive use and glucose metabolism in a national sample of women in the united states. Am J Obstet Gynecol 183: 389–395, 2000. doi: 10.1067/mob.2000.105909. [DOI] [PubMed] [Google Scholar]
- 115.Valderhaug TG, Hertel JK, Nordstrand N, Dale PO, Hofsø D, Hjelmesæth J. The association between hyperandrogenemia and the metabolic syndrome in morbidly obese women. Diabetol Metab Syndr 7: 46, 2015. doi: 10.1186/s13098-015-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, O’Rourke RW, Roberts CT Jr. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology 153: 3100–3110, 2012. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vrbíková J, Cífková R, Jirkovská A, Lánská V, Platilová H, Zamrazil V, Stárka L. Cardiovascular risk factors in young Czech females with polycystic ovary syndrome. Hum Reprod 18: 980–984, 2003. doi: 10.1093/humrep/deg218. [DOI] [PubMed] [Google Scholar]
- 118.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol 589: 4671–4679, 2011. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 52: 595–600, 2000. doi: 10.1046/j.1365-2265.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 121.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wiltgen D, Spritzer PM. Variation in metabolic and cardiovascular risk in women with different polycystic ovary syndrome phenotypes. Fertil Steril 94: 2493–2496, 2010. doi: 10.1016/j.fertnstert.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 123.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 8: 103–115, 2011. doi: 10.1016/j.genm.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642–R1647, 2010. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome, edited by Dunaif A, Givens JR, Haseltine FP, Merrian GR. Boston: Blackwell Scientific Publications, 1992, p. 377–384. [Google Scholar]