Abstract
Circular RNAs (circRNAs) have emerged as an important new class of genomic regulatory molecules contributing to the development of various diseases, but their relevance to the development and progression of hypertension remains largely unknown. A major impediment to begin studying circRNAs in rat models of inherited hypertension is that the rat as a valuable model of human diseases lags far behind the mouse and human in providing knowledge on circRNAs. In this study, a genome-wide circRNA profiling was performed from four rat strains that are widely used in hypertension research: the Dahl salt-sensitive rat (S), the Dahl salt-resistant rat (R), the spontaneously hypertensive rat (SHR), and the Wistar Kyoto rat (WKY). Combined hybridization data obtained from these four strains allowed for the identification of 12,846 circRNAs as being expressed in the rat kidneys. Out of these, 318 and 110 circRNAs were differentially expressed with a fold change > 1.5 (P < 0.05) in S vs. R and SHR vs. WKY, respectively. Among these circRNAs, circRNA/microRNA interaction was predicted since circRNAs are known as microRNA sponges to sequester microRNAs. Several circRNAs were further validated by quantitative real-time PCR. To our knowledge, our study is the primary report of profiling circRNAs in renal tissue and illustrates that circRNAs could be candidate genetic factors controlling blood pressure.
Keywords: circular RNAs, hypertension, rat, renal, blood pressure
genomic “dark matter,” or regions that are not coding for proteins, was initially considered as transcriptional noise but has since been largely found to be functional, due to the identification of several classes of noncoding RNA. A large number of noncoding genetic variants play important roles in human diseases (13, 35). Linkage and mapping studies combined with genome-wide association studies and whole-genome sequencing data using rat models of cardiovascular and renal diseases suggest that noncoding genomic regions are associated with genetically complex diseases such as hypertension (1, 9, 25). Also, noncoding RNAs, such as microRNAs (miRNAs) and long noncoding RNAs, were previously reported to be associated with cardiovascular and renal functions (8, 19).
Circular RNAs (circRNAs) are a relatively new class of noncoding RNA. CircRNAs have a stable structure and are characterized by a covalently closed loop formed by special back-splicing events (17). To date, thousands of highly expressed circRNAs have been identified in mammals and distinct functions of circRNAs have been reported. For example, circRNAs could function as miRNA sponges with miRNA response elements (MREs) to sequester miRNAs and subsequently terminate the posttranscriptional control of their target genes (10, 29). In addition to functioning as miRNA sponges, circRNAs have multiple functions as they are known to facilitate intracellular miRNA transport (11), sequester RNA-binding proteins (17) and also directly target and regulate messenger RNA (mRNA) through imperfect base pairing (12).
CircRNAs are implicated in various diseases, such as cancer (30) and neurological diseases (28), but the involvement of circRNAs in hypertension remains largely unknown. Recently, two clinical reports (21, 34) demonstrated the association of circRNAs with hypertension, indicating the need to assess whether circRNAs represent inherited elements contributing to blood pressure regulation. To do so, in this study, a renal circRNA transcriptome analysis was performed in 4 rat genetic models of hypertension: the Dahl salt-sensitive rat (S), the Dahl salt-resistant rat (R), the spontaneously hypertensive rat (SHR), and the Wistar Kyoto rat (WKY). The transcriptome analysis resulted in the discovery of 12,846 rat renal circRNAs, and the identification of differentially expressed circRNAs between normotensive and hypertensive rats. Interestingly, several circRNAs validated by quantitative (q)RT-PCR were located within blood pressure quantitative trait loci (BP QTLs). To our knowledge, our study is the primary report of profiling circRNAs in renal tissue and serves as a basis to study the role of circRNAs as candidates for cardiovascular and renal diseases.
MATERIALS AND METHODS
Tissue collection and RNA isolation.
All animal procedures and protocols described in this study were approved by the University of Toledo Animal Care and Use Committee, and all methods were performed in accordance with the Guide for the Care and Use of Laboratory Animals. All rats used in the study were from stocks maintained in our animal facility at our institution. All rats were weaned at 28–30 days of age and fed with a low-salt diet (TD 7034, Harlan Teklad). Male S, R, SHR, and WKY rats (57 days old, n = 3 rats per group) were euthanized by CO2 inhalation and dissected for collection of kidneys. The kidney samples were snap-frozen on dry ice and stored at −80°C until further processing. Total tissue RNA was extracted using the TRIzol reagent (Life Technologies) according to the manufacturer's protocol. RNA quantity and quality were determined using a NanoDrop ND-1000. RNA integrity was assessed by electrophoresis on a denaturing agarose gel.
Sample preparation and array hybridization.
Arraystar Rat Circular RNA Microarray (www.arraystar.com) was used for microarray experiments. The microarray contained a total of 14,145 distinct circRNA probes to detect rat circRNAs including 1,668 mouse circRNA orthologs and 179 human circRNA orthologs. The sample preparation and microarray hybridization were performed based on Arraystar’s standard protocols. Briefly, total RNA was digested with RNase R (Epicenter) to remove linear RNAs and enrich circRNAs. The enriched circRNAs were amplified and transcribed into fluorescent cRNA using a random priming method according to the Arraystar Super RNA Labeling protocol. The labeled cRNAs were hybridized onto the Arraystar Rat CircRNA Array (8x15K) and incubated for 17 h at 65°C in an Agilent hybridization oven. After the slides were washed, the arrays were scanned with the Agilent Scanner G2505C for data collection and analysis.
CircRNA microarray analysis.
Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Quantile normalization of raw data and subsequent data processing were conducted using the R software limma package. Low-intensity filtering was performed after quantile normalization of the raw data and the circRNAs that at least three out of 12 samples have flags in “P” or “M” were defined as being expressed in rat renal tissue and retained for further analyses (Supplementary Table S1). (The online version of this article contains supplemental material.) Differentially expressed circRNAs with statistical significance (P value < 0.05) between two groups were identified using fold change (FC) cut-off (FC > 1.5) or through the volcano plot filtering. Hierarchical clustering was performed to assess the distinguishable circRNA expression pattern among samples. The circRNA/microRNA interaction and their MREs were predicted with miRNA target prediction software from Arraystar, which is based on TargetScan (6) and miRanda (24).
The gene expression data collected through this study is deposited in National Center for Biotechnology Information's Gene Expression Omnibus (GEO; Cheng X and Joe B, 2016) and is accessible through GEO series accession number GSE92669.
Validation of circRNAs by qRT-PCR.
Total RNA was extracted from kidney tissues using the TRIzol reagent (Life Technologies) according to the manufacturer's protocol. cDNA was obtained through reverse transcription with SuperScript III (Invitrogen) using random primers. qRT-PCR was performed on the StepOnePlus Real-Time PCR system (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems). Each experimental group consisted of three biological replicates, each of which had three individual technical replicates. The expression levels of circRNAs relative to the housekeeping gene Pgk1 were calculated by the 2−ΔΔCT method. All the primer sequences are provided in Supplementary Table S2.
Bioinformatics analysis for miRNA target and function prediction.
The five miRNAs for rno_circRNA_006016 predicted from the circRNA/miRNA interaction were identified from the above analysis. The target genes of these miRNAs were predicted by miRDB (32, 33). A circRNA-miRNA-gene interaction network was generated using Cytoscape (27). Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using Database for Annotation, Visualization and Integrated Discovery (14, 15).
Statistical analysis.
An unpaired t-test was performed to identify differential circRNA expression between any two groups in the microarray analysis. The significance of qRT-PCR validation was performed by the Student’s t-test, and data are presented as means ± SEs. A P value < 0.05 was considered as statistically significant.
RESULTS
CircRNA expression profiling.
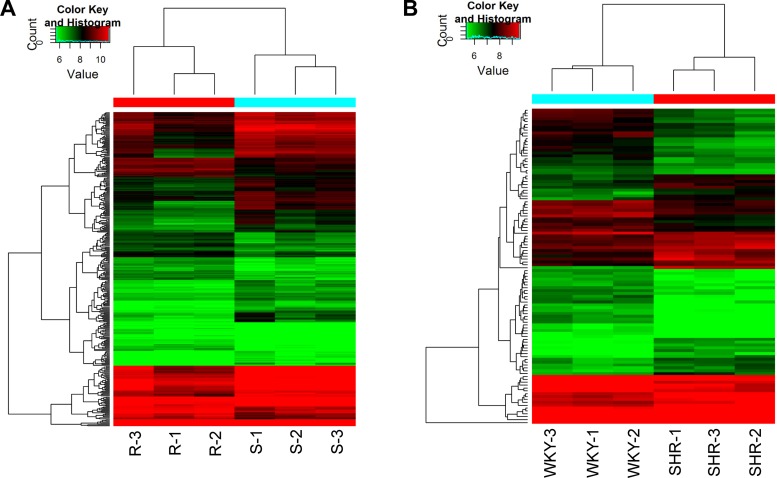
Box plots showed similar distributions of normalized intensities for all the samples in the microarray data set (Fig. 1). Renal transcriptome analysis indicated that a number of circRNAs were differentially expressed between normotensive and hypertensive rat strains. Volcano plots of the up- and downregulated circRNAs in the hypertensive rat strains compared with the normotensive rat strains are shown in Fig. 2. Variations in circRNA expression between normotensive R rats and hypertensive S rats are shown in Fig. 2A. Similarly, variations in circRNA expression between normotensive WKY rats and hypertensive SHR rats are shown in Fig. 2B. Specifically, by using a P value cut-off 0.05 and an FC cut-off 1.5, heat maps revealed hierarchical clustering of differential circRNA expression between normotensive and hypertensive rat strains (Fig. 3). Figure 3A shows the differential circRNA expression between normotensive R rats and hypertensive S rats. Figure 3B shows the differential circRNA expression between normotensive WKY rats and hypertensive SHR rats.
Fig. 1.
Box plots show the distribution of expression levels for all samples in the circular (circ)RNA microarray data set. R-1, R-2, and R-3 are 3 individual Dahl salt-resistant rats (R). S-1, S-2, and S-3 are 3 individual Dahl salt-sensitive rats (S). WKY-1, WKY-2, and WKY-3 are 3 individual Wistar Kyoto rats (WKY). SHR-1, SHR-2, and SHR-3 are 3 individual spontaneously hypertensive rats (SHR).
Fig. 2.
Volcano plots showing the significant up- and downregulated circRNAs in the hypertensive rats compared with the normotensive rats. A: Dahl salt-sensitive rats (S) vs. Dahl salt-resistant rats (R). B: spontaneously hypertensive rats (SHR) vs. Wistar Kyoto rats (WKY). The horizontal green line represents a P value with the cut-off 0.05. All the gray and red squares above this horizontal green line represent the differentially expressed circRNAs with statistical significance (P < 0.05). The vertical green lines mark the limits for fold-change with the cut-off value of 1.5, whereby the red squares outside of the area between the 2 vertical green lines represent the circRNAs with > 1.5-fold-change, and the gray squares between the 2 vertical green lines represent the circRNAs with < 1.5-fold-change.
Fig. 3.
Heat maps of differentially expressed circRNAs between normotensive and hypertensive rats. A: Dahl salt-sensitive rats (S) vs. Dahl salt-resistant rats (R). B: spontaneously hypertensive rats (SHR) vs. Wistar Kyoto rats (WKY). The differentially expressed circRNAs were selected with a P value cut-off 0.05 and a fold-change cut-off 1.5. CircRNA expression level is shown as a function of color, with lower expression in green and higher expression in red. R-1, R-2, and R-3 are 3 individual R rats. S-1, S-2, and S-3 are 3 individual S rats. WKY-1, WKY-2, and WKY-3 are 3 individual WKY rats. SHR-1, SHR-2, and SHR-3 are 3 individual SHR rats.
Summary of differential circRNA expression.
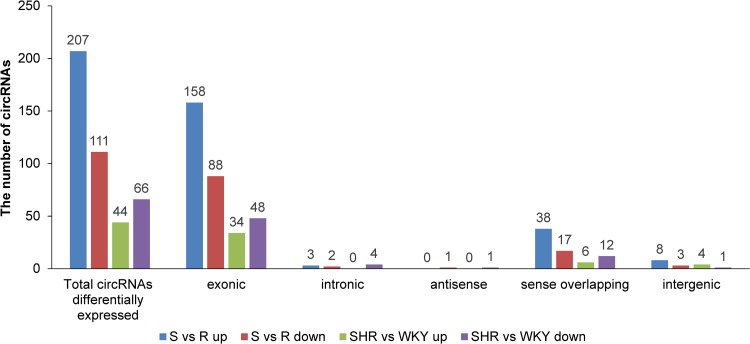
The number of differentially expressed circRNAs was summarized according to the different circRNA subcategories (Fig. 4). A total of 207 and 111 circRNAs were upregulated and downregulated, respectively, in hypertensive S rats compared with normotensive R rats. In contrast, a total number of 44 and 66 were upregulated and downregulated in hypertensive SHR rats compared with normotensive WKY rats, respectively. Interestingly, most differentially expressed circRNAs were located in the exonic regions, followed by sense overlapping regions (Fig. 4). Only a few differentially expressed circRNAs were observed belonging to intronic, antisense, and intergenic groups (Fig. 4). All the differentially expressed circRNAs in each group and their detailed information, including MREs for each circRNA, are provided in Supplementary Table S3–S6.
Fig. 4.
Differentially expressed circRNAs between normotensive and hypertensive rats. The figure represents the number of circRNAs differentially expressed according to the microarray analysis. Based on the location and direction on the genome, circRNAs are subcategorized into exonic, intronic, antisense, sense overlapping, and intergenic. S vs. R up indicates circRNAs were upregulated in Dahl salt-sensitive rats (S) compared with Dahl salt-resistant rats (R). S vs. R down indicates circRNAs were downregulated in S compared with R. SHR vs. WKY up indicates circRNAs were upregulated in spontaneously hypertensive rats (SHR) compared with Wistar Kyoto rats (WKY). SHR vs. WKY down indicates circRNAs were downregulated in SHR compared with WKY.
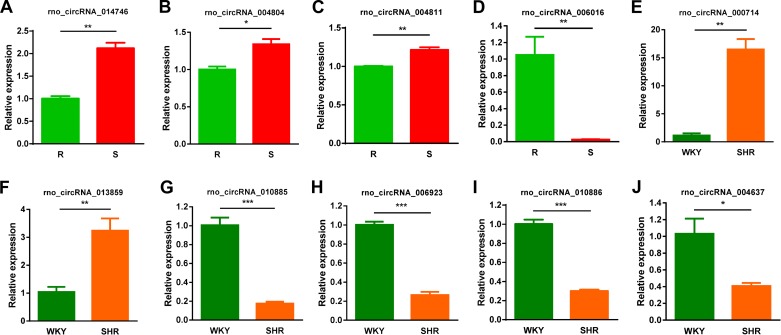
Validation of differentially expressed circRNAs by qRT-PCR.
Ten differentially expressed circRNAs shown in the microarray analysis were further validated by qRT-PCR (Fig. 5), and qRT-PCR amplified circRNA products were further confirmed using gel electrophoresis (Supplementary Fig. S1). The details of these 10 circRNAs are provided in Supplementary Table S7. Interestingly, six of these 10 circRNAs are located within BP QTL reported in the Rat Genome Database (Supplementary Table S7).
Fig. 5.
Validation of differentially expressed circRNAs in the microarray study by quantitative RT-PCR. R, Dahl salt-resistant rat; S, Dahl salt-sensitive rat; WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat. *P < 0.05, **P < 0.01, ***P < 0.001.
CircRNA-miRNA-gene network and pathway prediction.
Since the qRT-PCR result showed that rno_circRNA_006016 was highly downregulated (>30 FC) in hypertensive S rats compared with normotensive R rats and rno_circRNA_006016 is also located within BP QTL (Supplementary Table S7), we used it for further functional analysis. The top five predicted miRNA targets of rno_circRNA_006016 were rno-miR-297, rno-miR-466b-5p, rno-miR-423-5p, rno-miR-3573-5p, and rno-miR-185-5p (Supplementary Table S4). For better visualization, only the top 30 predicted genes targeted by each miRNA were included in the circRNA-miRNA-gene network (Fig. 6A). Interestingly, rno-miR-423-5p and rno-miR-3573-5p shared most of their target genes, and Slc20a2 was the predicted target of 3 miRNAs (Fig. 6A). Several target genes were also found to be common between rno-miR-297 and rno-miR-466b-5p (Fig. 6A). To gain further insights into the functions of rno_circRNA_006016, we analyzed the top 10 significant biological processes (GO:BP) and KEGG pathways using all five miRNA target genes (Fig. 6, B and C). Surprisingly, rno_circRNA_006016 showed a strong relationship with blood pressure regulation according to the GO:BP and KEGG analysis, which will be further discussed below.
Fig. 6.
Targeted circRNA-micro (mi)RNA-gene network and functional pathway analysis for rno_circRNA_006016. A: the circRNA-miRNA-gene network based on bioinformatics analysis. Only top 30 miRNA target genes for each miRNA are shown in the network. B: the top 10 significant Gene Ontology for Biological Processes (GO:BP) were predicted by using all the 5 miRNA target genes. C: the top 10 significant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were predicted by using all 5 miRNA target genes. “Enrichment Score” = “−log10(P value)” and P value < 0.05 was considered as statistically significant.
DISCUSSION
CircRNAs have emerged as an important class of noncoding RNAs characterized by their stable structure with a covalently closed loop (17). CircRNAs are demonstrated to play an important role in various diseases, such as cancer (30) and neurological diseases (28), but to date, there are only few reports of circRNA profiling in cardiovascular diseases (5, 7, 31); however, knowledge regarding their involvement in hypertension remains largely unknown.
To study the genetics of hypertension and other cardiovascular and renal diseases, rats have been the models of choice. Studies focused on identifying genetic factors linked to blood pressure regulation point to both coding and noncoding segments as candidate loci. However, to study noncoding genes in the rat, lack of annotations for several classes of regulatory RNAs poses to be an impediment. CircRNAs are a relatively newer class of regulatory RNAs that have been little studied in the rat. This is a problem for addressing the question of whether circRNAs are implicated in the etiology of hypertension in rat models. Therefore, in our current study, a genome-wide survey was conducted for circRNAs in the four rat strains widely used in hypertension research: the S, the R, the SHR, and the WKY strains, and 12,846 circRNAs were detected to be expressed in rat renal tissue (Supplementary Table S1). Renal circRNA expression profiles showed that a number of circRNAs were differentially expressed between normotensive and hypertensive rats (Figs. 2–4). To our knowledge, our study serves as the primary report demonstrating the existence of circRNAs in renal tissues. Moreover, our study serves as a basis to further explore the role of these newly characterized circRNAs in cardiovascular and renal diseases in general and hypertension in particular.
The data collected in this study indicate that a smaller number of the circRNAs were similarly differential between both the comparisons of S vs. R and SHR vs. WKY. For example, rno_circRNA_016002 and rno_circRNA_008772 were upregulated in both hypertensive rat strains compared with normotensive rat strains, which perhaps indicates that these two circRNAs may mediate physiological functions independent of their genomic background. However, a larger number and quite different circRNAs were differentially expressed in S vs. R compared with SHR vs. WKY (Fig. 4). This observation suggests that the S and SHR models of hypertension are perhaps influenced by different circRNAs functioning to influence the development of hypertension in these two strains.
CircRNAs are known to function as miRNA sponges with MREs to absorb miRNAs and subsequently mediate the posttranscription of miRNA target genes (10, 29). For example, the circRNA ciRS-7 contains multiple miRNA-7 binding sites, whereby it could act as an miRNA-7 sponge to inhibit the function of miRNA-7 (18). MiRNA-7 plays an important role in various pathways leading to cancer and neurological diseases (36, 37). Thus, ciRS-7 probably serves as a crucial factor engaging in tumorigenesis and neuron functions by absorbing miRNA-7. Therefore, the data collected with the circRNA/miRNA interaction in our current study point to the possibility of similar mechanisms in the context of hypertension and other cardiovascular and renal diseases. Importantly, all the differentially expressed rat circRNAs and their MREs discovered in our renal transcriptome analysis collectively serve as a platform to be examined for relevance to hypertension (Supplementary Table S3–S6).
As a technical note, it is worth documenting that validating circRNAs has an important technical challenge that is not posed by other forms of noncoding RNA that are not circular. While the microarray contains oligos, validating the detected signals by real-time PCR for forms of RNA that are not linear but are circular may have physical constraints and problems as we noted in our studies. To validate the differentially expressed circRNAs by qRT-PCR, we designed divergent, or “outward facing,” primers for a given circular target sequence. The amplicon amplified by divergent primers spans the circular junction to distinguish the linear counterpart of the circRNA. Initially, we designed the divergent primers targeting outside the circular junction, but we found off-target PCR-amplified products, which prevent further qRT-PCR validation. For example, by sequencing the off-target product of rno_circRNA_016550, we found that the circular junction was unexpectedly formed by the repeat of the same part of the same exon, suggesting that the same circRNA may have multiple isoforms (data not shown). Therefore, to improve the specificity of the divergent primers, we designed the forward or reverse primer targeting the circular junction and the specificity of divergent primers was improved. By this approach, 10 differentially expressed circRNAs were successfully validated by qRT-PCR (Fig. 5, Supplementary Fig. S1, Supplementary Table S7). However, it still seems that circRNAs are more difficult to be PCR amplified compared with linear transcripts because the rate of success for PCR amplification was only ~45% according to our current study.
To gain further insights into the mechanism of circRNAs in regulating blood pressure, we chose rno_circRNA_006016 for functional analysis since our qRT-PCR result indicated that rno_circRNA_006016 was highly downregulated (>30-FC) in hypertensive S rats compared with normotensive R rats and rno_circRNA_006016 is also located within BP QTL (Supplementary Table S7). Interestingly, different rno_circRNA_006016 targeted miRNAs shared common target genes (Fig. 6A), suggesting that a circRNA may target a specific downstream mechanism by simultaneously mediating multiple miRNAs which may commonly regulate same genes responsible for this mechanism. For example, Slc20a2 was the common predicted target of rno-miR-423-5p, rno-miR-3573-5p and rno-miR-297 (Fig. 6A). Renal Slc20a2, also named Pit-2, was reported to mediate the process of dietary potassium deficiency (3), and a low intake of potassium may increase blood pressure, suggesting an important role of Slc20a2, which may be upstream mediated through circRNA-miRNA interaction, in blood pressure control. The GO:BP analysis revealed several significant biological processes contributing to blood pressure control, such as small GTPase-mediated signal transduction (23), ion transmembrane transport (2), and regulation of N-methyl-D-aspartate selective glutamate receptor activity (26) (Fig. 6B). Moreover, the KEGG analysis also showed several significant pathways related to hypertension (Fig. 6C). For example, components in mitogen-activated protein kinase (MAPK) signaling pathway have been considered as biomarkers of hypertension (22). Wnt signaling pathway could regulate blood pressure by downregulating a GSK-3β-mediated pathway (4). Moreover, the ErbB signaling pathway and Rap1 signaling pathway were both demonstrated to play important roles in cardiovascular and renal diseases, such as hypertension (16, 20). Overall, all these results suggest that rno_circRNA_006016 could function as an important genetic factor for controlling blood pressure through circRNA-miRNA-gene interaction.
Perspectives
Our current study serves as a first-generation catalog to profile the poorly characterized class of noncoding RNA called circRNAs in the context of hypertension. The study reports renal expression data from commonly used hypertensive and relatively normotensive rat models, whereby it serves as a reference for future studies of circRNAs and their functions not only for blood pressure regulation, but for cardiovascular and renal diseases, for which these models serve as experimental tools.
GRANTS
This work was supported by National Heart Lung and Blood Institute Grant HL-020176 to B. Joe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C. and B.J. conceived and designed research; X.C. performed experiments; X.C. analyzed data; X.C. and B.J. interpreted results of experiments; X.C. prepared figures; X.C. drafted manuscript; X.C. and B.J. edited and revised manuscript; X.C. and B.J. approved final version of manuscript.
REFERENCES
- 1.Atanur SS, Diaz AG, Maratou K, Sarkis A, Rotival M, Game L, Tschannen MR, Kaisaki PJ, Otto GW, Ma MC, Keane TM, Hummel O, Saar K, Chen W, Guryev V, Gopalakrishnan K, Garrett MR, Joe B, Citterio L, Bianchi G, McBride M, Dominiczak A, Adams DJ, Serikawa T, Flicek P, Cuppen E, Hubner N, Petretto E, Gauguier D, Kwitek A, Jacob H, Aitman TJ. Genome sequencing reveals loci under artificial selection that underlie disease phenotypes in the laboratory rat. Cell 154: 691–703, 2013. doi: 10.1016/j.cell.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker EH. Ion channels and the control of blood pressure. Br J Clin Pharmacol 49: 185–198, 2000. doi: 10.1046/j.1365-2125.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Miyamoto K, Barry NP, Levi M. Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol 297: F350–F361, 2009. doi: 10.1152/ajprenal.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng PW, Chen YY, Cheng WH, Lu PJ, Chen HH, Chen BR, Yeh TC, Sun GC, Hsiao M, Tseng CJ. Wnt signaling regulates blood pressure by downregulating a GSK-3β-mediated pathway to enhance insulin signaling in the central nervous system. Diabetes 64: 3413–3424, 2015. doi: 10.2337/db14-1439. [DOI] [PubMed] [Google Scholar]
- 5.Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, Li X, Yang BB. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 38: 1402–1412, 2017. doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 6.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol 5: R1, 2003. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng HH, Li R, Su YM, Xiao J, Pan M, Cai XX, Ji XP. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS One 11: e0151753, 2016. doi: 10.1371/journal.pone.0151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopalakrishnan K, Kumarasamy S, Mell B, Joe B. Genome-wide identification of long noncoding RNAs in rat models of cardiovascular and renal disease. Hypertension 65: 200–210, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan K, Saikumar J, Peters CG, Kumarasamy S, Farms P, Yerga-Woolwine S, Toland EJ, Schnackel W, Giovannucci DR, Joe B. Defining a rat blood pressure quantitative trait locus to a <81.8 kb congenic segment: comprehensive sequencing and renal transcriptome analysis. Physiol Genomics 42A: 153–161, 2010. doi: 10.1152/physiolgenomics.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 495: 384–388, 2013. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 11.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J 30: 4414–4422, 2011. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J 32: 923–925, 2013. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrdlickova B, de Almeida RC, Borek Z, Withoff S. Genetic variation in the non-coding genome: Involvement of micro-RNAs and long non-coding RNAs in disease. Biochim Biophys Acta 1842: 1910–1922, 2014. doi: 10.1016/j.bbadis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, Artamonov MV, Maas JE, Szabo A, Zhang DX, Auchampach JA, Mattson DL, Somlyo AV, Chrzanowska-Wodnicka M. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler Thromb Vasc Biol 34: 1486–1494, 2014. doi: 10.1161/ATVBAHA.114.303678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA 20: 1829–1842, 2014. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukiw WJ. Circular RNA (circRNA) in Alzheimer’s disease (AD). Front Genet 4: 307, 2013. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 58: 1093–1098, 2011. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa R, Hirooka Y, Nishihara M, Ito K, Sunagawa K. Neuregulin-1/ErbB signaling in rostral ventrolateral medulla is involved in blood pressure regulation as an antihypertensive system. J Hypertens 29: 1735–1742, 2011. doi: 10.1097/HJH.0b013e32834937d6. [DOI] [PubMed] [Google Scholar]
- 21.Miao R, Wang Y, Wan J, Leng D, Gong J, Li J, Liang Y, Zhai Z, Yang Y. Microarray expression profile of circular RNAs in chronic thromboembolic pulmonary hypertension. Medicine (Baltimore) 96: e7354, 2017. doi: 10.1097/MD.0000000000007354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocaranza MP, Jalil JE. Mitogen-activated protein kinases as biomarkers of hypertension or cardiac pressure overload. Hypertension 55: 23–25, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141960. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension 48: 534–540, 2006. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 24.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13: 271–282, 2012. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 25.Pillai R, Waghulde H, Nie Y, Gopalakrishnan K, Kumarasamy S, Farms P, Garrett MR, Atanur SS, Maratou K, Aitman TJ, Joe B. Isolation and high-throughput sequencing of two closely linked epistatic hypertension susceptibility loci with a panel of bicongenic strains. Physiol Genomics 45: 729–736, 2013. doi: 10.1152/physiolgenomics.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyatin VF, Tatarnikov VS. NMDA receptors of A5 area in the regulation of blood pressure and respiratory activity during hypoxia in rats. Bull Exp Biol Med 159: 420–423, 2015. doi: 10.1007/s10517-015-2980-z. [DOI] [PubMed] [Google Scholar]
- 27.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao Y, Chen Y. Roles of circular RNAs in neurologic disease. Front Mol Neurosci 9: 25, 2016. doi: 10.3389/fnmol.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, Califano A. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147: 370–381, 2011. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Nazarali AJ, Ji S. Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am J Cancer Res 6: 1167–1176, 2016. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 37: 2602–2611, 2016. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 32.Wang X. Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics 32: 1316–1322, 2016. doi: 10.1093/bioinformatics/btw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res 43, D1: D146–D152, 2015. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu N, Jin L, Cai J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clin Exp Hypertens 39: 454–459, 2017. doi: 10.1080/10641963.2016.1273944. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Lupski JR. Non-coding genetic variants in human disease. Hum Mol Genet 24, R1: R102–R110, 2015. doi: 10.1093/hmg/ddv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Tao Y, Zhou Y, Qin N, Chen C, Tian D, Xu L. MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int 15: 103, 2015. doi: 10.1186/s12935-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener 11: 28, 2016. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]