Abstract
Purpose
Inter-species transplantation, xenotransplantation, is becoming a realistic strategy to solve the organ shortage crisis. Here we focus on seminal publications that have driven research in xenotransplantation, as well as recently published literature and future endeavors.
Recent Findings
Advances in gene editing technology have allowed for the efficient production of multi-transgenic porcine donors leading improved xenograft survival in baboons, up to 2-years following heterotopic heart xenotransplantation and from weeks to several months following life-supporting kidney xenotransplanation. As technology evolves, additional challenges have arisen, including the development of proteinuria, early graft loss associated with porcine CMV, disparities in organ growth between donors and recipients as well as high-dose continuous immunosuppression requirements. To address these issues, our laboratory developed a tolerance-inducing protocol which has allowed for >6 months survival of a life-supporting kidney with further approaches currently underway to address the challenges mentioned above.
Summary
Our recent findings, reviewed in this article, led us to develop methods to overcome obstacles, which, in conjunction with the work of others, are promising for future clinical applications of xenotransplantation.
Introduction
Kidney transplantation is now a generally accepted management strategy for various end-stage diseases due to hypertension, diabetes, autoimmune disorders, genetic disorders and infectious etiologies. In 2016, despite over 19,000 kidney transplants performed in the United States (1), and the number of patients that were added to the waitlist far exceeded the number that ultimately went on to transplantation. As of February 23rd, 2017 there are over 98,000 patients listed for a kidney transplant in the United States, of which over 38,000 patients were removed for a variety of clinical reasons, including 4,747 (12.35%) patients removed for being too sick to transplant, and 4,270 (11.11%) patients being removed because they died while awaiting a transplant (1). Given these discrepancies, alternative clinical strategies must be developed to resolve this organ shortage crisis.
One potential option could be to regenerate organs or develop functional organs de novo. Previous studies have shown that allogeneic hepatocytes and Islets of Langerhans developed de novo can support the life of the recipients (2–4), and as a result there has been significant interest in the generation of these tissues through advanced tissue engineering techniques. However, the function of these engineered tissues has been limited (5, 6). Recently, techniques for reprogramming adult tissues through gene manipulation that induce pluriopotent stem cells (iPS) have spawned interest in organ regeneration (7–9). The group at the Massachusetts General Hospital (MGH) has reported that when rat hearts were decellularized with detergents and reseeded with cardiac or endothelial cells, investigators were successful in producing an engineered heart with about 2% of function observed in an adult heart (10). While such technologies are new and innovative, and may provide an alternative source of allogeneic organs in the future, they have yet to yield fully functional life-supporting solid organs in a large animal model.
Another option to increase the supply of organs is to introduce interspecies transplantation, otherwise known as xenotransplantation. Recent improvements through the use of the CRISPR/CAS9 technology have markedly improved the efficiency of multiple gene manipulations in the donor (11, 12) and the use of these new multi-transgenic alpha-1,3-galactosyltransferase knockout (GalT-KO) pigs has demonstrated marked prolongation of porcine renal xenograft survival from days to greater than six months in a life-supporting model (13), and for >2 years in a heterotopic non-life-supporting cardiac xenograft model (14). Xenotransplantation is now becoming a more realistic strategy to solve the organ shortage crisis and in this review we have focused on both the seminal publications that have driven research in xenotransplantation, in addition to the most recently published work and future endeavors.
I. The Use of GalT-KO Pigs Overcame Hyperacute Rejection
Swine have generally been considered the best match for potential human xenotransplantation because of their size, their favorable breeding characteristics, and well-established known genetic profile (15). However, until the development of α-1,3-galactosyltransferase gene knockout pigs (GalT-KO) pigs in 2002 (16–18), xenotransplantation was not feasible due to hyperacute rejection caused by a glycoprotein antigen constitutively expressed on the surface of swine cells to which humans (and new world monkeys) have preformed anti-pig natural antibodies (19–23). Using these newly developed GalT-KO donors, in 2002 the author’s team performed the world’s first pig-to-baboon renal xenotransplantation and demonstrated the successful prevention of hyperacute rejection of xenogeneic renal grafts (23).
II. Strategies to Prevent T-cell Mediated Rejection
A. Xenogeneic T-cell Responses
Due to the intensity of antibody mediated hyperacute rejection across xenogeneic barriers, researchers were initially unable to ascertain the degree of xenogeneic anti-donor T-cell responses to vascularized donor organs. There was early speculation that the xenogeneic T-cell response would be less severe than an allogeneic response, because of discordant MHCs. Early investigations into T-cell responses exploited the human-anti-mouse model (25). These studies revealed a defective human CD4 T-cell anti-murine major histocompatibility complex (MHC) class II molecule interaction, effectively eliminating T-cell activation via the direct pathway (25). These results suggested that if humoral mechanisms could be overcome, cell-mediated rejection would be a minor obstacle.
However, studies done in the early 1990’s at the authors’ laboratory (26, 27), and others (28), demonstrated that the direct pathway of activation did exist in the pig-to-human model. Our laboratory has demonstrated that human T-cells responded to xeno-MHC antigens as they did to allo-MHC antigens in mixed lymphocyte reaction (MLR) assays. In addition, human-anti-pig T-cell responses appeared to share similar antigen presenting cell (APC) requirements for stimulators (direct pathway) or responders (indirect pathway); and the majority of the primary human-anti-pig xeno-response was directed toward porcine MHC class II antigens and involved interactions with human CD4 accessory molecules (26). These data indicated that the human-anti-porcine T-cell response was similar, and could be stronger than allogeneic barriers, in strength. In addition, activation of CD4 T-cells provide T-cell help to B-cells and NK-cells as well as CD8 T-cell killer progenitors (25). Therefore, anti-pig specific strategies directed at inhibition of the direct pathway as well as the indirect pathway must be included for successful xenotransplantation between pigs and primates.
B. The Importance of Inducing Tolerance for Successful Life-supporting Xenogeneic Renal Transplantation
In initial studies in 2002, we achieved survival of up to 34-days for life-supporting GalT-KO pig-to-baboon renal xenotransplantation using immunosuppressive regimens that included anti-thymocyte globulin (ATG) and anti-CD154 mAb (24). Meanwhile, in 2005, Zhong et al., reported maximum survival of only 16-days using GalT-KO kidneys. Even with the use of heavy immunosuppression in these protocols, rejected GalT-KO grafts showed severe cellular and humoral rejection (28). The glomeruli and peritubular capillaries of these rejected xenografts stained brightly for IgG, suggesting T-cell help allows class switching from IgM to IgG with specificity for porcine antigens. Due to recipients developing cellular rejection despite heavy chronic immunosuppression, these results suggest that small numbers of T-cells can initiate both cellular and humoral responses. This data is consistent with reports by Korsgren and colleagues which indicated that even very small numbers of T-cells were sufficient to initiate rejection of porcine islets by macrophages in T-cell deficient rodents (29). The level of immunosuppression needed to control these T-cell responses and prolong xenograft survival has been associated with prohibitive morbidity and mortality. Additionally, these regimens have not yielded survival that would merit transition to clinical trials. These results do however provide compelling rationale to pursue a clinically applicable tolerance strategy that would potentially avoid the high level of immunosuppression employed in current xenotransplantation protocols.
C. Strategies to Overcome Xenogeneic T-cell Responses: Vascularized Thymic Transplantation
In an attempt to control the powerful T-cell response, many strategies, aimed at various targets of the immune response, have been tested. Due to the fact that immunosuppression alone is prohibitively morbid, the authors believe that a strategy for xenogeneic tolerance induction is imperative for successful xenotransplantation. Transplantation tolerance, which uses the body’s own immune system to reteach T-cells to suppress the anti-donor response, was first attempted by the author using the simultaneous transplantation of a donor pig thymus. Since T-cell education occurs in the thymus and because the thymus is known to play an integral role in self-tolerance and in tolerance across allogeneic barriers, thymic transplantation is thought to lead to tolerance via a central mechanism, much like the education of T-cells in a normal developing child.
Thymic tissue, as opposed to vascularized thymic grafts, was first demonstrated to induce xenograft tolerance in a pig-to-mouse model by Sykes et al. through the transplantation of fetal thymic tissue directly transplanted under the renal capsule in T-cell depleted, thymectomized mice. These results demonstrated that murine recipients of porcine thymic tissue developed donor-specific unresponsiveness in MLR assays and went on to subsequently accept skin grafts from the porcine thymic donors (30–32). In an effort to extend these exciting results to our pig-to-primate model, it was first necessary to demonstrate that thymic transplantation was technically possible in a large animal model, such as the pig. Our initial attempts to implant minced thymic tissue in a similar manner to the mouse model failed, with evidence of graft rejection by days 15–30 (33). We hypothesized that ischemic damage to the transplanted thymus during revascularization led to an active immunologic response and we reasoned that thymic tissue needed to be transplanted as a vascularized organ. Furthermore, we hypothesized that during the tenuous period of revascularization, unless complete T-cell depletion was achieved, which is very difficult in large animals, thymic tissue is rejected before it has the ability to induce tolerance.
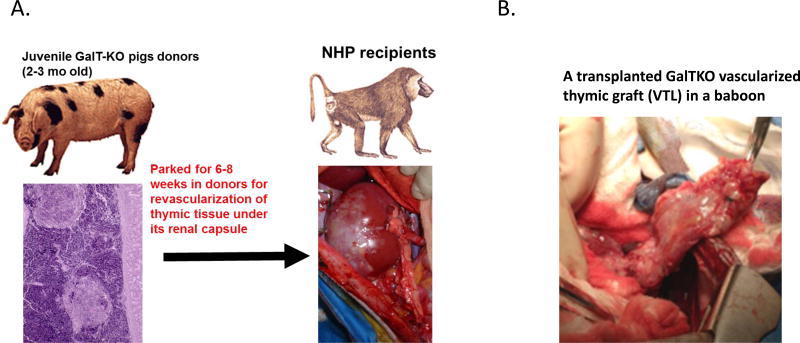
In an effort to eliminate the damage that occurs during the revascularization period after allogeneic or xenogeneic transplantation, Yamada et al. have developed two methods for vascularized thymic transplantation in miniature swine (34, 35); First is the preparation of a composite "thymokidney" in which autologous thymic tissue is allowed to engraft for a period of 1–2 months under the donor’s own kidney capsule before allogeneic transplantation (Fig. 1 A). Second is the “Yamada VTL procedure”, which is the transplantation of an isolated vascularized thymic lobe (VTL) (Fig. 1 B). Using these two novel techniques, we demonstrated that vascularized thymic tissue can successfully induce tolerance and support thymopoiesis across fully allogeneic barriers in MGH miniature swine (36). In follow-up studies when kidneys and non-vascularized thymic tissue from the same donor were transplanted as separate grafts, recipients rejected the non-vascularized allogeneic thymic grafts within four weeks and kidney grafts were rejected within the second post-operative month. In contrast, recipients of thymus with simultaneous kidneys (thymokidney) or heart (heart plus VTL), or kidneys following vascularized thymus lobe transplant, all survived long-term with stable graft function and in-vitro donor-specific unresponsiveness (37–40).
Figure 1.
(A) Preparation of thymokidney (TK) in a juvenile GalTKO pig and transplantation of a GalTKO TK in a baboon. (B) A transplanted GalTKO vascularized thymic lobe graft (VTL) in a baboon.
Based on these encouraging results, the author’s laboratory has extended this vascularized thymic transplant strategy to the pig-to-baboon model of renal xenotransplantation. Initial studies in 2002, which utilized GalT-KO kidneys and thymic swine donors, led to prolongation of graft survival up to 83-days while GalT-KO kidneys without vascularized thymic grafts were rejected by 34-days (24).
III. Persistent Proteinuria Despite T-cell Unresponsiveness
Our group has previously demonstrated that the co-transplantation of vascularized thymic grafts with kidneys from the same GalT-KO donors had prolonged functional life-supporting renal grafts in baboons. These baboons demonstrated in vitro evidence of donor-specific tolerance with development of early baboon thymopoiesis in the vascularized pig thymic grafts, suggesting that these recipients were on a path toward to tolerance. However, the majority of these animals developed significant proteinuria as early as post-operative day (POD) 2, despite relatively normal appearing glomeruli and normal renal function (41–43). These data strongly suggested that development of proteinuria is not initiated by antibody-mediated rejection.
We found that the histologic findings and clinical conditions observed were remarkably similar to the clinical entity of Minimal Change Disease observed in children (42, 44). The development of this nephrotic syndrome leads to significant edema and increases the risk of renal damage, bacterial infections, and thrombosis. It deserves to be mentioned that although thrombosis and infections are quite frequent complications in xenotransplantation (41), the fact that these complications can result from nephrotic syndrome provides an additional rationale to identify the etiologic mechanism(s) driving the nephrotic response (45).
Approach to prevent development of proteinuria
A. Rituximab Prevents Pig Podocyte Disruption in an SMPDL-3b-Dependent Manner and Delays the Development of Proteinuria Following Xenogeneic Thymokidney Transplantation
We have recently reported that rituximab, when administered in the perioperative period, protects sphingomyelin phosphodiesterase acid like 3b (SMPDL-3b)/sphingomyelinase activity on porcine podocytes, which in turn delays the development of proteinuria (43). Since podocytes are the primary cells within the glomerulus that are responsible for preventing proteinuria, we initially studied the mechanism of post-xenotransplantation proteinuria by developing a technique for the culture of pig podocytes, as confirmed by staining with anti-nephrin and anti-podocin antibodies. Using this porcine podocyte culture, we have found that (i) SMPDL-3b/sphingomyelinase expression on porcine podocytes played an essential role in initiating proteinuria and (ii) Rituximab (anti-CD20 antibody) binds to porcine SMPDL-3b in glomeruli of the kidney grafts, and thereby prevents damage from baboon preformed anti-pig natural antibodies or anti-porcine soluble factors. In an effort to test the effect of Rituximab in vivo, six baboons received Rituximab in the peri-transplant period (treated group), versus eighteen baboons that underwent GalT-KO thymokidney transplantation, but without Rituximab administration in the peri-transplant period (control group). Animals in both groups received Rituximab 2-weeks prior to transplantation to deplete CD20+ B-cells. Peripheral B-cell counts during the pre- and peri-transplant periods, as well as following transplantation were not significantly different between the two groups. The onset of 2+ post-transplant proteinuria was markedly delayed in the treated group compared to the control group (p=0.004). Most of the baboons in the control group developed >2+ proteinuria within 2 days following transplantation (1.39 ± 0.61 days), while in the treated group the average time of onset of 2+ proteinuria was >12.50 ± 5.54 days. To our knowledge, this is the first time Rituximab has been shown to prevent pig podocyte disruption in an SMPDL-3b-dependent manner and consequently delayed the development of proteinuria following xenogeneic GalT-KO kidney transplantation in non-human primates (NHP) (43). However, since this effect lasts only two to three weeks, additional strategies are required for long lasting resolution.
B. Upregulation of CD80 Plays an Important Role and a Potential Therapy of CTLA4-Ig to Prevent Proteinuria
Podocytes, the primary cells within the glomerulus that are responsible for preventing proteinuria, have recently been shown to have characteristics of antigen-presenting dendritic cells (46). One of the antigens that podocytes expresses following activation is CD80, which is a receptor normally expressed by dendritic cells, that can act as a second signal for T-cell activation (47). Recently, urinary levels of CD80 were reported to be extremely high in a patient who developed minimal change-like nephrotic syndrome following allogeneic stem cell transplantation (48). Moreover, minimal change disease in children is also associated with high levels of CD80 in the urine in association with CD80 expression in glomerular podocytes in renal biopsies (49, 50).
In recent studies our group has also found that the nephrotic syndrome associated with xenograft (‘xenograft nephropathy’) is also associated with induction of CD80 expression in podocytes. In particular, we found that the nephrotic syndrome in baboons with pig xenografts have (i) increased urinary CD80 excretion that precedes the development of proteinuria. The urinary CD80 appears to be of both baboon and porcine origan. (ii) CD80 was found to be expressed in glomeruli by immunostaining in biopsies of baboons with nephrotic syndrome; and (iii) multiple doses of CTLA4-Ig therapy added on top of an anti-CD40L-based regimen resulted in a marked reduction in proteinuria with significantly improved survival compared to baboons treated with the anti-CD40L-based regimen without the CTLA4-Ig therapy (Yamada K and Johnson R, manuscript in preparation). By minimizing proteinuria with this modified protocol, we have recently reported that stable renal function was maintained over 6 months using a GalT-KO pig thymokidney without additional gene modification in a baboon recipient (51). The 193-day life-supporting kidney functional graft is, thus far, the longest survival of GalT-KO kidneys without additional gene modification in baboons. More recently, our studies have been focusing on gene(s) that are responsible for the development of proteinuria.
C. Recent Progress Using Multi-Transgenes
The Emory group has most recently reported >133-days survival of GalTKO/hDAF kidneys in rhesus monkeys (n=2) that had low preformed natural antibodies without development of proteinuria (13). However, Pintore et al. reported that proteinuria and presence of low molecular weight proteins were consistently found in urine after kidney transplantation in a multitrangenic, including human decay accerlating factor (hDAF), GalT-KO pig-to-cynomologus model (52). In addition, we have a case of an hDAF/GalT-KO-to-cynomolgus monkey renal transplant using an anti-CD40L based regimen without vascularized thymic graft which developed proteinuria following kidney transplantation similar to baboon recipients of GalT-KO kidneys (Yamada et al., manuscript in preparation). Therefore, although it is not conclusive, recipient strains might be involved in absence of proteinuria using GaT-KO with only an hDAF alone transgenic pig donors in the rhesus macaque model (13).
Recent improvements in gene manipulation, especially through the use of the CRISPR/CAS9 technology have markedly improved the efficiency of multiple gene manipulations (11, 12). Further, the use of these new multi-transgenic GalT-KO pigs has demonstrated marked prolongation of porcine xenograft survival, especially with the use of CD46/human thrombomodulin (hTBM) GalT-KO heterotopic hearts following pig-to-baboon transplantation, where graft survival surpassed 2 years (14). Therefore, multiple transgenic GalTKO pig donors may improve renal graft survivals by overcoming immunologic barriers associated with species incompatibility. However, although the use of the CRISPR/CAS9 technology has made the development of new lines of GalT-KO swine far easier than previously thought, the genes responsible for development of proteinuria has not been address by others. Recent data have found that incompatibility of a porcine CD47-baboon signal regulatory protein α (SIRP-α), which is an interspecies ligand-receptor interaction, induces activation of macrophages and phagocytosis in xenogeneic combinations (52–56). Immune activation of the porcine podocyte leads to expression of CD80 which potentially downregulates SIRP-α and SMPDL-3b. We are currently investigating a role of hCD47 on development of proteinuria in vitro as well as in vivo using transgenic hCD47-GalT-KO pigs (57). Our preliminary results demonstrate that the addition of hCD47 and hDAF to GalT-KO pigs almost eliminated proteinuria following thymokidney transplantation in baboons (Yamada et al., manuscript in preparation).
IV. Donor factors
A. The Need for pCMV Negative GalT-KO Donors
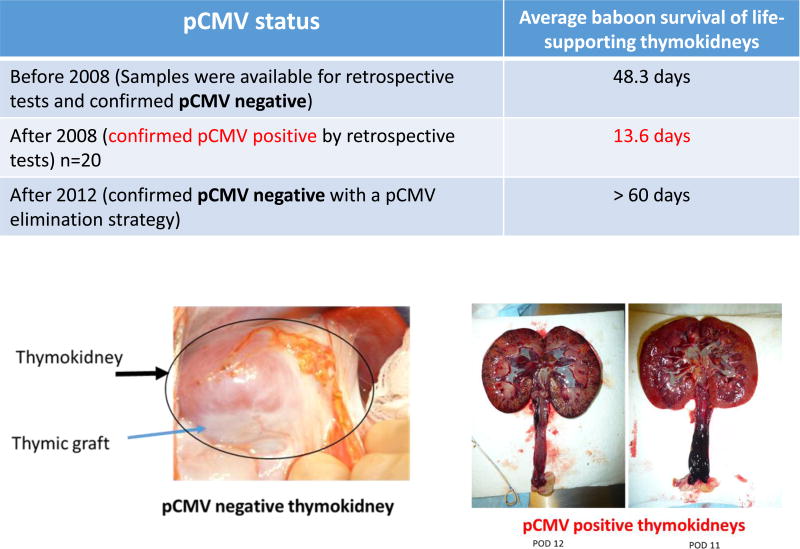
To our knowledge, our laboratory has the largest series of life-supporting renal xenografts using GalT-KO swine in baboons. Of note, there was a three year period after 2008 in which renal xenograft survival was decreased markedly (<3 weeks), even with thymic co-transplantation (58). We investigated potential causes of this early loss and found a strong association of porcine cytomegalovirus (pCMV) infection in GalT-KO porcine kidneys with decreased graft survival (58, 59). We subsequently tested for pCMV in donor splenocytes and/or in the explanted kidney grafts in 53 cases. In all instances with a pCMV+ donor (n=20), early graft loss was observed (Fig. 2). Moreover, our study indicated that pCMV transmission could be avoided by cesarean delivery of offspring even in the presumed presence of latent pCMV infection in the pregnant sow. Notably, elimination of pCMV restored life-supporting renal xenografts and baboon survivals to an average of >60 days (Fig. 2). These results indicate that elimination of pCMV from the graft prevents xenograft activation and thus a pCMV-free donor is essential for successful pig-to-primate renal xenotransplantation (58, 59). More recently data from our colleagues investigating pig-to-baboon liver xenotransplantation also demonstrated that recipients of porcine livers from pCMV− donors survived for 25-days while livers from pCMV+ pigs were lost much earlier (60).
Figure 2.
PCMV induced the early loss of life-supporting kidneys.
(Left bottom) A life-supporting TK graft from a pCMV negative donor (>60 days). (Right bottom) Rejected TKs from pCMV+ donors.
B. Donor Strain Should be Considered because of Potential Growth Discrepancies
Recent improvements in survival following pig-to-NHP transplantation suggest that solid organ xenotransplantation may be feasible for clinical trials. However, growth of porcine organs in NHP following transplantation is poorly understood. Previous reports by Soin et al., in which survival to 48-days following pig-to-NHP xenotransplantation was achieved, have demonstrated that porcine kidney xenografts in NHP grew in the first two weeks following transplantation, at which point further growth ceased and growth plateaued in three of six cases (61). In the remaining three pigs, graft size increased due to acute vascular rejection, however, two animals in which graft size plateaued, acute cellular rejection was also present. Therefore, the growth of organs from growth-curve mismatched combinations remained unclear.
In order to address this issue more clearly, we have recently investigated whether kidneys from donors that naturally grow faster than recipients grow following the donors’ growth curve or the recipients’ growth curve (52). With long follow up periods, our data suggest that intrinsic factors within the transplanted organ are largely involved in graft growth following transplantation in both allogeneic and xenogeneic transplant models. We have recently reported that (i) if the ratio of the pig kidney volume to the recipient animal's body weight was greater than 25 cm3/kg there appeared to be a deleterious effect on renal function across xenogeneic barrier between pig and baboons and (ii) allogeneic kidney graft size and percentage increase of kidney volume/body weight from outbred Yorkshire swine in miniature swine increased three times more than those of grafts from miniature swine to miniature swine at 12 weeks post-transplant, and (iii) rapid growth of kidneys and lungs across allogeneic and xenogeneic models caused compartment syndrome that resulted in ischemic damage in solid organs (52). These results suggest that intrinsic factors are responsible, at least in part, for the growth of donor organs and that this property should be taken into consideration for growth-curve mismatched transplants, especially for life-supporting organs transplanted into a limited recipient space. Although wild type pigs are generally used for production of GalT-KO or multi-transgenic GalT-KO pigs because of its productivity, these pigs grow much faster and can reach sizes >300kg. Miniature swine, instead, are generally considered the best match for potential human xenotransplantation partly because of their size, but also because of largely known genetic profile (17, 18, 24, 62).
V. Clinical application
Which should be the first organ transplanted across a xenogeneic barrier? Among the most likely candidates are: 1) kidney; 2) heart; 3) liver; 4) lung; and 5) pancreas/islets. Each has its proponents and detractors. Short of extracorporeal membrane oxygenation, one cannot live without a heart and/or lungs. Thus, an alternative source of hearts or lungs is an enticing solution to end stage heart or lung disease. For this reason, some have suggested that xenotransplantation could potentially be used as a “bridge” to allotransplantation, rather than destination therapy. However, others argue that the use of a xenogeneic organ as a bridge does not solve the problem of organ shortage, but rather increases the number of people on the waiting list. Unlike end-stage kidney disease, end stage liver disease cannot be treated with dialysis. Regarding xenotransplantation of the liver and lungs, some have argued that the complex natural function of the liver (e.g. production of enzymes, coagulation factors, complement components, etc.) makes it unlikely that a xenograft would function properly across a species disparity. Despite this, we have recently made significant progress in heart and kidneys, as well as livers, where survival up to 29-days has been achieved (Vagefi et al, manuscript in preparation) and lungs where survival up to one week following pig-to-baboon xenotransplantation (Yamada et al, manuscript in preparation).
In favor of the kidney being the first organ to be transplanted across a xenogeneic barrier is the fact that failure of the transplant would not necessarily be fatal, as the patient could presumably be put back on dialysis. The availability of living donor transplants and the fact that renal failure is treatable by dialysis, argue against its adoption. However, numerous patients on the waiting lists for kidneys are highly sensitized to potential donors (i.e. high panel reactive antibody, or PRA)(63). We have previously reported that allosensitization does not increase the risk of xenoreactivity to GalT-KO miniature swine in patients on the transplantation waiting list. We have tested a panel of 88 human serum samples from patients awaiting cadaveric renal allotransplantation for reactivity against human, standard miniature swine and GalT-KO peripheral blood lymphocytes (PBL) and cultured endothelial cells (64). We found no correlation between the degree of anti-human panel reactive antibodies (PRA) and xenoreactivity against either standard or GalT-KO miniature swine. These results suggest that highly allosensitized patients awaiting renal transplants do not appear to be at an increased risk of xenosensitization over their non-sensitized cohorts, and could therefore be candidates for xenotransplantation using GalT-KO swine donors (64).
Finally, it is important to note that a tolerance strategy approach that was described earlier in this review is a reasonable approach to clinical renal xenotransplantation due to the ability to use donor thymic cells (either as a vascularized composite allograft, or as a combined “thymokidney” approach) (24, 34–40, 51) as well as bone marrow cells (57, 65, 66) from the SLA identical donors using MGH inbred gene modified donors (17, 18. 24, 61) to help allow for the induction of tolerance. The latter concept being critical if xenotransplantation is to become a reality in humans because of the potential need for several different immunosuppressive agents to allow for successful graft acceptance in humans.
Acknowledgments
The authors would like to thank Dr. David H. Sachs for his helpful advice and review of this manuscript. Our research described in this review article was supported by NIH grant P01 AI045897.
Abreactions
- APC
antigen presenting cell
- ATG
anti-thymocyte globulin
- GalT-KO
alpha-1,3-galactosyltransferase knockout
- iPS
induced pluriopotent stem cells
- MGH
Massachusetts General Hospital
- MLR
mixed lymphocyte reaction
- MHC
major histocompatibility complex
- NHP
non-uman primates
- PBL
peripheral blood lymphocytes
- pCMV
porcine cytomegalo virus
- POD
post-operative day
- PRA
panel reactive antibodies
- SMPDL-3b
sphingomyelin phosphodiesterase acid like 3b
- Tg
transgenic
- SLA
swine leukocyte antigens
- VTL
vascularized thymic lobe
References
- 1.https://www.unos.org/data/transplant-trends/#transplants_by_organ_type+year+2016
- 2.Kobayashi N. Cell Transplant. 2008;17(1–2):3–9. doi: 10.3727/000000008783907099. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Sevigny J, Robson SC, Waelkens E, Csizmadia E, Smith RN, Lemmens R. Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem. 2000;275:5640–5647. doi: 10.1074/jbc.275.8.5640. [DOI] [PubMed] [Google Scholar]
- 5.Cho IS, Kim J, Seo HY, Lim DH, Hong JS, Park YH, et al. Cloning and characterization of microRNAs from porcine skeletal muscle and adipose tissue. Mol Biol Rep. 2010;37:3567–74. doi: 10.1007/s11033-010-0005-6. [DOI] [PubMed] [Google Scholar]
- 6.Ophir E, Eidelstein Y, Afik R, Bachar-Lustig E, Reisner Y. Induction of tolerance to bone marrow allografts by donor-derived host nonreactive ex vivo-induced central memory CD8 T cells. Blood. 2010;115:2095–2104. doi: 10.1182/blood-2009-10-248716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- **11.Cowan PJ. The use of CRISPR/Cas associated technologies for cell transplant applications. Curr Opin Organ Transplant. 2016 Oct;21(5):461–6. doi: 10.1097/MOT.0000000000000347. This review article summarized recent developments in the use of the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) genome editing system for cell transplant applications, ranging from transplantation of corrected autologous patient stem cells, to the treatment of inherited diseases, as well as the tailoring of donor pigs for cell xenotransplantation. [DOI] [PubMed] [Google Scholar]
- 12.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24(3):372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplant. 2015;22:221–230. doi: 10.1111/xen.12166. This article reported long-term survival of rhesus macaques transplanted with galactose-α1,3-galactose knockout/CD55 transgenic pig kidneys (>133 and >126 days) that had low-titer levels of preformed natural antibodies and were treated with an anti-CD154-based regimen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ***14.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3rd, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. This article reported the median (298 days) and longest (945 days) graft survival of heterotopically transplanted GTKO.hCD46.hTBM pig hearts in baboons. To date, it represents the longest report of a cardiac xenograft survival in a non-human primate and it is a step towards a life-supporting orthotopic cardiac xenotransplantation model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs DH, Galli C. Curr Opin Organ Transplant. 2009;14:148–153. doi: 10.1097/mot.0b013e3283292549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai L, Kolber-Simonds D, Park K, Cheong H, Greenstein JL, Im G, et al. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 20.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Proc Natl Acad Sci U S A. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galili U. Springer Semin. Immunopathol. 1993;15:155–171. doi: 10.1007/BF00201098. [DOI] [PubMed] [Google Scholar]
- 22.Galili U, Gregory CR, Morris RE. Transplant Proc. 1996;28:567–568. [PubMed] [Google Scholar]
- 23.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. J Biol Chem. 1998;263:17755–17762. [PubMed] [Google Scholar]
- 24.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of a-1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 25.Moses RD, Pierson RN, Winn HJ, Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. J Exp Med. 1990;172:567–575. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K, Seebach JD, DerSimonian H, Sachs DH. Human anti-pig T-cell mediated cytotoxicity. Xenotransplant. 1996;3:179–187. [Google Scholar]
- 27.Seebach JD, Yamada K, McMorrow IM, Sachs DH, DerSimonian H. Xenogeneic human anti-pig cytotoxicity mediated by activated natural killer cells. Xenotransplant. 1996;3:188–197. [Google Scholar]
- 27.Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benda B, Karlsson-Parra A, Ridderstad A, Korsgren O. Transplantation. 1996;62:1207–1211. doi: 10.1097/00007890-199611150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Nature Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Barbosa JI, Zhao Y, Barth R, Zhao G, Arn JS, Sachs DH, Sykes M. Transplantation. 2001;72:1223–1231. doi: 10.1097/00007890-200110150-00007. [DOI] [PubMed] [Google Scholar]
- 32.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. J. Immunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 33.Haller GW, Esnaola N, Yamada K, Wu A, Shimizu A, Hansen A, et al. J Immunol. 1999;163:3785–3792. [PubMed] [Google Scholar]
- 34.Yamada K, Shimizu A, Ierino FL, Utsugi R, Barth R, Esnaola N, et al. Transplantation. 1999;68:1684–1692. doi: 10.1097/00007890-199912150-00011. [DOI] [PubMed] [Google Scholar]
- 35.LaMattina JC, Kumagai N, Barth RN, Yamamoto S, Kitamura H, Moran SG, et al. Transplantation. 2002;73:826–831. doi: 10.1097/00007890-200203150-00032. [DOI] [PubMed] [Google Scholar]
- 36.Kamano C, Vagefi PA, Kumagai N, Yamamoto S, Barth RN, LaMattina JC, et al. Proc Natl Acad Sci U S A. 2004;101:3827–3832. doi: 10.1073/pnas.0306666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K, Shimizu A, Utsugi R, Ierino FL, Gargollo P, Haller GW, et al. J Immunol. 2000;164:3079–3086. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Vagefi PA, Utsugi R, Kitamura H, Barth RN, LaMattina JC, et al. Transplantation. 2003;76:530–536. doi: 10.1097/01.TP.0000080608.42480.E8. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto S, Teranishi K, Kamano C, Samelson-Jones E, Arakawa H, Nobori S, et al. Transplantation. 2006 Feb 27;81(4):607–13. doi: 10.1097/01.tp.0000198735.17555.f1. [DOI] [PubMed] [Google Scholar]
- 39.Nobori S, Shimizu A, Okumi M, Samelson-Jones E, Griesemer A, Hirakata A, et al. Proc Natl Acad Sci U S A. 2006;103:19081–19086. doi: 10.1073/pnas.0605159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobori S, Samelson-Jones E, Shimizu A, Hisashi Y, Yamamoto S, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;81(1):26–35. doi: 10.1097/01.tp.0000200368.03991.e0. [DOI] [PubMed] [Google Scholar]
- 41.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunological reviews. 2014;258(1):241–58. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB. Pathologic characteristics of transplanted kidney xenografts. Journal of the American Society of Nephrology. 2012;23(2):225–35. doi: 10.1681/ASN.2011040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. Journal of the American Society of Nephrology. 2014;25(4):737–44. doi: 10.1681/ASN.2013040363. The authors examined the role of SMPDL-3b on post-transplant proteinuria following a GalT-KO pig-to-baboon kidney transplantation model, both in vitro and in vivo, and demonstrated for the first time that the prevention of pig podocyte disruption relies on an SMPDL-3b–dependent mechanism and can inhibit the early development of proteinuria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20(6):765–71. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 45.Harris RC, Ismail N. Extrarenal complications of the nephrotic syndrome. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1994;23(4):477–97. doi: 10.1016/s0272-6386(12)80369-6. [DOI] [PubMed] [Google Scholar]
- 46.Goldwich A, Burkard M, Olke M, Daniel C, Amann K, Hugo C, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. Journal of the American Society of Nephrology. 2013;24(6):906–16. doi: 10.1681/ASN.2012020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–7. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huskey J, Rivard C, Myint H, Lucia S, Smith M, Shimada M, et al. Minimal change disease in graft versus host disease: a podocyte response to the graft? Clin Nephrol 2012. Clin Nephrol. 2013 Dec;80(6):469–73. doi: 10.5414/CN107420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. Journal of the American Society of Nephrology. 2009;20(2):260–6. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78(3):296–302. doi: 10.1038/ki.2010.143. [DOI] [PubMed] [Google Scholar]
- ***51.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, et al. Role of intrinsic (graft) vs. extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. American journal of transplantation. American Journal of Transplantation. 2017 Jan 24; doi: 10.1111/ajt.14210. [Epub ahead of print] Authors demonstrated that baboon recipients of GalT-KO kidney co-transplanted with vascularized thymic grafts (thymokidney) without additional gene modification markedly prolonged renal graft survival without immunologic sensitization up to 193 days. In addition, authors examined renal function of growth-rate mismatched combinations in both xenogeneic and allogeneic transplant models. They found that intrinsic factors are responsible, at least in part, for growth of donor organs and that this property should be taken into consideration for growth-curve-mismatched transplants, especially for life-supporting growth mismatched organ transplants (pig-to-non hunman primates/primate xenotransplantation) and organs transplanted into a limited recipient space (intrathoracic space). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pintore L, Paltrinieri S, Vadori M, Besenzon F, Cavicchioli L, De Benedictis GM, et al. Clinicopathological findings in non-human primate recipients of porcine renal xenografts: quantitative and qualitative evaluation of proteinuria. Xenotransplant. 2013;20:449–457. doi: 10.1111/xen.12063. [DOI] [PubMed] [Google Scholar]
- 53.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science (New York, NY) 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 54.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol. 2011;8(4):285–288. doi: 10.1038/cmi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Yang YG. Innate cellular immunity and xenotransplantation. Curr Opin Organ Transplant. 2012;17(2):162–167. doi: 10.1097/MOT.0b013e328350910c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ***57.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation. 2017;101(2):316–321. doi: 10.1097/TP.0000000000001267. Authors reported markedly prolonged survival of donor swine skin xenografts in baboons.by using mobilized peripheral blood hematopoietic cells from transgenic swine expressing human CD47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **58.Yamada K, Tasaki M, Sekijima M, Wilkinson RA, Villani V, Moran SG, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014 Aug 27;98(4):411–8. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **59.Sekijima M, Waki S, Sahara H, Tasaki M, Wilkinson RA, Villani V, et al. Results of life-supporting galactosyltransferase knockout kidneys in cynomolgus monkeys using two different sources of galactosyltransferase knockout Swine. Transplantation. 2014 Aug 27;98(4):419–26. doi: 10.1097/TP.0000000000000314. References 58 and 59 demonstrated porcine cytomegalovirus may be responsible for early loss of GalT-KO swine kidney xenografts in two research centers (back-to-back publications), and Ref 58 showed data indicating transmission of pCMV to swine offspring can be avoided by C-section delivery and scrupulous isolation of donor animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60.Shah JA, Navarro-Alvarez N, DeFazio M, Rosales IA, Elias N, Yeh H, et al. A Bridge to Somewhere: 25-day Survival After Pig-to-Baboon Liver Xenotransplantation. Ann Surg. 2016;263(6):1069–71. doi: 10.1097/SLA.0000000000001659. The authors demonstrated 25-day survival after GalTKO pig-to-baboon liver xenotransplantation which is thus far a longest survival of xeno liver graft in large animal xeno transplant model, and also showed importance of pCMV free grafts in prolonged pig liver xeno graft survival. [DOI] [PubMed] [Google Scholar]
- 61.Sonin B, Ostlie D, Cozzi E, Smith KG, Bradley JR, Vial C, et al. Growth of porcine kidneys in their native and xenograft environment. Xenotransplant. 2000;7(2):96–100. doi: 10.1034/j.1399-3089.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 62.Sachs DH. The pig as a xenograft donor. Pathologie-biologie. 1994;42(3):217–9. [PubMed] [Google Scholar]
- 63.Duquesnoy RJ, Howe J, Takemoto S. HLAmatchmaker: a molecularly based algorithm for histocompatibility determination. IV. An alternative strategy to increase the number of compatible donors for highly sensitized patients. Transplantation. 2003;75:889–897. doi: 10.1097/01.TP.0000055097.58209.83. [DOI] [PubMed] [Google Scholar]
- 64.Wong BS, Yamada K, Okumi M, Weiner J, O'Malley PE, Tseng YL, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82(3):314–9. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 65.Abe M, Qi J, Sykes M, Yang YG. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood. 2002;99(10):3823–9. doi: 10.1182/blood.v99.10.3823. [DOI] [PubMed] [Google Scholar]
- *66.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. American Journal of Transplantation. 2015 Apr;15(4):974–83. doi: 10.1111/ajt.13070. Authors showed high incidence of xenogenic bone marrow engraftment and persistence of chimerism in a pig-to-baboon model using their unique strategy of intra-bone bone marrow transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]