Abstract
Background
Prediction of neonatal respiratory morbidity may be useful to plan delivery in complicated pregnancies. The limited predictive performance of the current diagnostic tests together with the risks of an invasive procedure restricts the use of fetal lung maturity assessment.
Objective
The objective of this study was to evaluate the performance of quantitative ultrasound texture analysis of the fetal lung (quantusFLM) to predict neonatal respiratory morbidity in preterm and early-term (<39.0 weeks) deliveries.
Study Design
This was a prospective multicenter study conducted in 20 centers worldwide. Fetal lung ultrasound images were obtained at 25.0-38.6 weeks of gestation within 48 hours of delivery, stored in Digital Imaging and Communication in Medicine format, and analyzed with quantusFLM. Physicians were blinded to the analysis. At delivery, perinatal outcomes and the occurrence of neonatal respiratory morbidity, defined as either respiratory distress syndrome or transient tachypnea of the newborn, were registered. The performance of the ultrasound texture analysis test to predict neonatal respiratory morbidity was evaluated.
Results
A total of 883 images were collected, but 17.3% were discarded because of poor image quality or exclusion criteria, leaving 730 observations for the final analysis. The prevalence of neonatal respiratory morbidity was 13.8% (101/730). The quantusFLM predicted neonatal respiratory morbidity with a sensitivity, specificity, and positive and negative predictive values of 74.3% (75/101), 88.6% (557/629), 51.0% (75/147), and 95.5% (557/583), respectively. Accuracy was 86.5% (632/730), and the positive and negative likelihood ratios were 6.5 and 0.3, respectively.
Conclusion
The quantusFLM predicted neonatal respiratory morbidity with an accuracy similar to that previously reported for other tests with the advantage of being a non-invasive technique.
Keywords: amniocentesis, amniotic fluid analysis, biomarker, computational methods, diagnostic indices, fetal lung maturity, neonatal respiratory morbidity, predictive values, quantitative texture analysis, respiratory distress syndrome, sonography, transient tachypnea, ultrasound
Introduction
Neonatal respiratory morbidity (NRM) due to either respiratory distress syndrome or transient tachypnea of the newborn is the most common complication in infants born preterm and early term (<39 weeks) [1-3]. Assessment of fetal lung maturity for the prediction of NRM may be relevant, particularly after 34 weeks of gestation, when the risk of NRM ranges from 5% to 20%, to better assess the risk/benefit ratio of elective delivery in late pregnancy complications [4-6] and/or with the use of corticosteroids [7, 8]. In current clinical practice, the evaluation of the risk of NRM relies on the study of different components of the amniotic fluid that requires an amniocentesis [9, 10].
Prediction of fetal lung maturity using fetal ultrasound has long been proposed as a non- invasive alternative to amniocentesis [11, 12]. Several approaches using computer analysis of fetal lung ultrasound images have been attempted over the last 25 years, including gray-scale measurements [13, 14], lung tissue motion [15, 16], or the relationship between image features of fetal lung versus placental or liver tissue [17]. These studies generally showed a good correlation with NRM, but the diagnostic accuracy was insufficient for clinical use. However, over recent years, image resolution of fetal ultrasound and computer image processing has evolved immensely. Quantitative texture analysis is a powerful technique that can be used to extract information from medical images and to quantify tissue changes not visible to the human eye, allowing the training of computer programs that may predict clinical events [18, 19]. Earlier studies reported that texture analysis can be applied to fetal lung ultrasound images and to correlate with both gestational age [20] and the results of fetal lung maturity testing of the amniotic fluid [21]. In a recent single-center study, we tested software based on quantitative texture analysis of the fetal lung (quantusFLM) trained to predict NRM. The software achieved a predictive accuracy similar to that commonly reported for fetal lung maturity testing of the amniotic fluid [22].
Herein, we report the results of a large multicenter study designed to evaluate the performance of quantusFLM to predict NRM. Fetal lung ultrasound images were obtained for analysis within 48 hours of delivery in a large cohort of pregnancies at 25.0-38.6 weeks of gestation. Neonatal respiratory outcomes were prospectively recorded and the performance of the software to predict NRM was analyzed.
Material and Methods
This was a prospective multicenter study involving 20 centers. Patients were recruited from June 2011 to December 2014. Eligible cases included pregnancies between 25.0 and 38.6 weeks of gestation and for which an ultrasound was obtained within 48 hours of delivery. Cases were considered non-eligible if corticosteroids were used for lung maturity between the ultrasound and delivery, when the maternal body mass index was ≥ 35 kg/m2, and when fetuses had known congenital malformations. Furthermore, neonates with the following conditions were excluded: neonatal sepsis, an umbilical artery pH <7.00, hemodynamic failure, symptomatic anemia (hemoglobin <12 mg/dL), a postnatal diagnosis of structural or chromosomal abnormalities, and meconium aspiration. These conditions could directly predispose or lead to NRM, irrespective of lung maturity.
Ultrasound images were obtained following a detailed acquisition protocol. Briefly, an axial section of the fetal thorax at the level of the four-chamber cardiac view was magnified by adjusting the depth, but not the zoom option, until the thorax occupied about two-thirds of the screen, avoiding obvious acoustic shadows from the fetal ribs (Figure 1A). Images were acquired without any type of post-processing manipulation such as smoothing, color Doppler, or any calipers or pointers. The use of tissue harmonic imaging and adjustment of image settings such as gain, frequency, and time-gain compensation were left to the discretion of the ultrasound operator performing the ultrasound scan.
Figure 1. Fetal lung image acquisition and delineation.
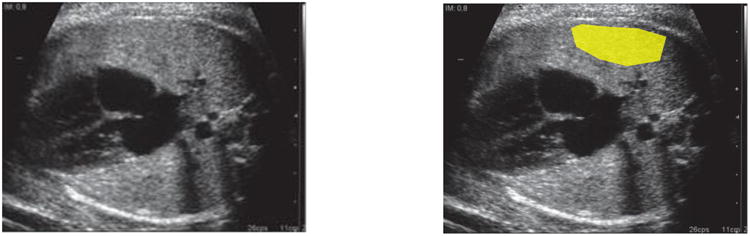
A) Lateral axial transverse section of the fetal thorax at the level of the 4-chamber section of the fetal heart. B) Region of interest delineated.
Before starting recruitment, each center submitted a minimum of five ultrasound images of the fetal lung that were reviewed by imaging engineers (E.B.-C. and A.P.-M.), according to this acquisition protocol, to ensure that quality criteria were fulfilled. If not, further images were requested. All study images were collected and stored in the original Digital Imaging and Communication in Medicine (DICOM) format and sent to the coordinator via a file transfer protocol. The DICOM scans were anonymized, removing all information related to the patient. To track the scan, a new random number was generated for each new image. Lung images for the study were then inspected for image quality control by the engineer's team and discarded if one or more of the requirements previously mentioned were not fulfilled. Images passing the quality criteria were then loaded via the Internet through restricted access to the commercial software web site and delineated using the quantusFLM web interface (www.quantusflm.com; Transmural Biotech, Barcelona, Spain). Delineations were performed either by the same clinicians acquiring the images at each participating center or by research clinicians at the coordinating center. Delineation of the region of interest included the largest possible area of the fetal lung proximal to the transducer, avoiding the heart and great vessels (Figure 1B). The web software contained an automatic filter to accept the delineation only when at least 400 pixels were included. Delineated ultrasound images were then analyzed automatically with quantusFLM. Features of the software used by quantusFLM have been described in detail elsewhere [22]. The software contains algorithms that analyze the textural patterns of the delineated area in the ultrasound image. These algorithms have been “trained” by means of a machine learning approach to estimate the probability of NRM, using hundreds of cases of fetal lung ultrasound images in which the occurrence of NRM was known. The software used in this study utilizes different sequences of texture features adapted to gestational age ranges [16]. Therefore, gestational age in weeks was not used to calculate any a priori risk of NRM but to decide the specific algorithm used to calculate the probability of NRM. The software used in this study provided categorical results, i.e., either “high” or “low” risk for NRM.
For each recruited case, the centers prospectively recorded the maternal baseline characteristics and the neonatal outcomes in a database purposely designed for this study. Anonymized clinical information from each case was submitted to the coordinator through a customized file transfer protocol and stored in a database available only to the clinical researchers of this project (M.P. and T.C.), who confirmed eligibility criteria and the absence of exclusion criteria for each case. Analysis of the neonatal clinical information was supervised by a neonatologist (F.B.). The study protocol was approved by the coordinator's Institutional Review Board (2011/6291, 2013/8892). Patients included in the study received care in the participating institutions and were enrolled in a specific protocol for the evaluation of fetal lung maturity, in studies involving the use of fetal ultrasound, or in studies for which ultrasound was used as part of the clinical management approved by the local review boards. All patients included gave written informed consent for the use of ultrasound images and perinatal data. None of the observations here reported has been previously used in another study.
The primary clinical outcome of the study was NRM, including respiratory distress syndrome or transient tachypnea of the newborn. Respiratory distress syndrome was defined based on clinical criteria, including grunting, nasal flaring, tachypnea, and chest wall retraction, or the need for supplemental oxygen together with typical chest radiography findings and admission to the neonatal intensive care unit for respiratory support [2]. Transient tachypnea of the newborn was diagnosed based on early respiratory distress (isolated tachypnea, rare grunting, and minimal retraction) and a chest X-ray showing hyperaeration of the lungs and prominent pulmonary vascular patterns [23].
The performance of quantusFLM to predict NRM was analyzed by the clinical researchers of this project (M.P., T.C.) by matching quantitative ultrasound analysis and clinical outcome. Descriptive statistical methods were used to summarize the distribution of all the variables; for continuous variables, mean and standard deviation values were obtained; and for categorical variables, frequencies and percentages were reported. Descriptive statistics were performed with R language (R Foundation for Statistical Computing, Vienna, Austria, 2015; https://www.R-project.org).
Results
A total of 883 cases were recruited. Of these, 135 (15.3%) were excluded after image quality control and 18 (2.0%) were excluded because of one or more clinical exclusion criteria (42/164, 25.6%, in the 25.0-33.6 weeks of gestation group; and 111/566, 19.6%, in the 34.0-38.6 weeks of gestation group), leaving a total of 730 images for analysis (Figure 2). The final number of cases included per center and the ultrasound equipment locally used are described in the supplementary material (Tables 1S and 2S). The clinical characteristics of the pregnant women enrolled in the study and the relevant conditions for which ultrasound was indicated are detailed in Table 1. The study included the following: 17 (2.5%) women at <28 weeks of gestation; 128 (18.7%) women at 28.0 to <34.0 weeks of gestation; 176 (25.7%) women at 34.0 to <37.0 weeks of gestation; and 364 (53.1%) women of ≥ 37.0 weeks of gestation. Perinatal and neonatal outcomes and the characteristics of the respiratory support are shown in Tables 2 and 3, respectively.
Figure 2. Flow chart of the eligible samples.
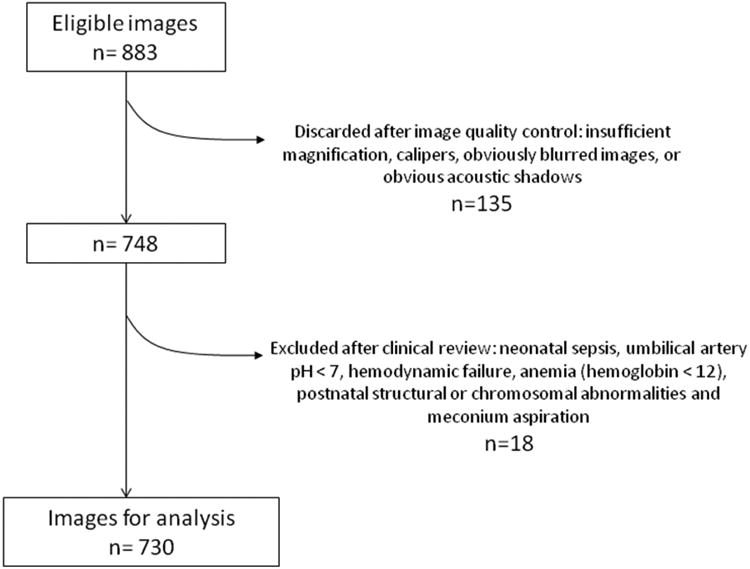
Table 1. Clinical characteristics of the women included in the study.
| GA range at scan, wks | |||
|---|---|---|---|
| Total (n = 685) | (25.0 —33.6) (n = 145) | (34.0 —38.6) (n = 540) | |
| Maternal age | 32.3 (5.8) | 31.4 (5.8) | 31.3 (5.8) |
| Nulliparity | 340 (49.6%) | 70 (48.3%) | 270 (50%) |
| Ethnicity | |||
| Caucasian | 400 (58.4%) | 93 (64.1%) | 307 (56.9%) |
| Black | 40 (5.8%) | 9 (6.2%) | 31 (5.7%) |
| Asian | 44 (6.4%) | 0 | 44 (8.2%) |
| Hispanic | 121 (17.7%) | 24 (16.6%) | 97 (18.0%) |
| Other | 53 (7.7%) | 18 (12.4 %) | 35 (6.5%) |
| Multiple pregnancy | 65 (9.5%) | 21 (14.5%) | 44 (8.1%) |
| Maternal or fetal relevant conditions | |||
| Preterm labor | 48 (7%) | 26 (17.9%) | 22 (4.1%) |
| PPROM | 158 (23.1%) | 70 (48.3%) | 88 (16.3%) |
| Preeclampsia | 116 (16.9%) | 40 (27.6%) | 76 (14.1%) |
| IUGR | 148 (21.6%) | 32 (22%) | 116 (21.5%) |
| Pre-gestational diabetes | 15 (2.2%) | 3 (2.1%) | 12 (2.2%) |
| Antepartum hemorrhage | 10 (1.5%) | 3 (2.1%) | 7 (1.3%) |
| Otherg* | 160 (23.4%) | 31 (21.4%) | 129 (23.9%) |
Data are represented as mean (SD) or n (%) when appropriate.
Hypothyroidism, hypertensive disorders, placenta previa, lupus, human immunodeficiency virus positive, assessment of fetal well-being, fetal presentation.
GA, gestational age; IUGR: intrauterine growth restriction; PPROM: preterm premature rupture of the membranes.
Table 2. Perinatal and neonatal outcomes of the newborns included in the study.
| Gestational age at scan, wks | |||
|---|---|---|---|
| Variables | Total (n = 730) | (25.0—33.6) (n = 164) | (34.0—38.6) (n = 566) |
| Gestational age at delivery (wks) | 36.0 (2.6) | 31.4 (2.2) | 37.2 (1.2) |
| Ultrasound-to-delivery lapse of time (d) | 0.6 (0.7) | 0.7 (0.7) | 0.6 (0.6) |
| Mode of delivery | |||
| Spontaneous vaginal delivery | 294 (40.3%) | 50 (30.5%) | 244 (43.1%) |
| Operative vaginal delivery | 48 (6.6%) | 4 (2.4%) | 44 (7.8%) |
| Non-elective cesarean section | 125 (17.1%) | 36 (22.0%) | 89 (15.7%) |
| Elective cesarean section | 263 (36.0%) | 74 (45.1%) | 189 (33.4%) |
| Birthweight (g) | 2517 (760) | 1554 (486) | 2796 (575) |
| Female gender | 365 (50.0%) | 70 (42.7%) | 295 (52.1%) |
| Apgar at 5 min < 7 | 10/729 (1.4%) | 7/163 (4.3%) | 3/566 (0.5%) |
| pH UA 7.00 to < 7.10 | 18/479 (3.8%) | 5/124 (4%) | 13/355 (3.7%) |
| Hyperbilirubinemia (phototherapy) | 152 (20.8%) | 86 (52.4%) | 66 (11.7%) |
| Other relevant conditions | |||
| Apnea | 20 (2.7%) | 20 (12.2%) | 0 |
| Bronchopulmonary dysplasia | 8 (1.1%) | 8 (4.9%) | 0 |
| Persistent pulmonary hypertension | 3 (0.4%) | 2 (1.2%) | 1 (0.2%) |
| Intraventricular hemorrhage (III or IV) | 3 (0.4%) | 3 (1.8%) | 0 |
| Necrotizing enterocolitis | 3 (0.4%) | 3 (1.8%) | 0 |
| Neonatal death < 28 d | 3 (0.4%) | 3 (1.8%) | 0 |
| NICU admission | 242 (33.2%) | 148 (90.2%) | 94 (16.6%) |
| Length of stay at NICU | 18.7 (19.5) | 25.5 (21.4) | 8.2 (9.0) |
| Discharged alive from NICU | 239/242 (98.8%) | 145/148 (98.0%) | 94/94 (100%) |
Data are represented as mean (SD) or n (%) when appropriate.
NICU: neonatal intensive care unit; UA: umbilical artery.
Table 3. Characteristics of the respiratory support and respiratory morbidity.
| Gestational Age at scan, wks | |||
|---|---|---|---|
| Characteristics | Total (n = 730) | (25.0—33.6) (n = 164) | (34.0—38.6) (n = 566) |
| Need for respiratory support (any) | 115 (15.8%) | 89 (54.3%) | 26 (4.6%) |
| Oxygen therapy ≥ 40% | 55 (7.5%) | 37 (22.6%) | 18 (3.2%) |
| CPAP | 117 (16%) | 94 (57.3%) | 23 (4.1%) |
| NIV/BPAP | 23 (3.2%) | 22 (13.4%) | 1 (0.2%) |
| Intubation required | 31 (4.3%) | 28 (17.1%) | 3 (0.5%) |
| Days of intubation (if any) | 6 (9.4) | 6.7 (9.9) | 1.8 (1.5) |
| High-frequency ventilation | 12 (1.6%) | 10 (6.1%) | 2 (0.4%) |
| Surfactant use | 34 (4.7%) | 32 (19.5%) | 2 (0.4%) |
| Doses of surfactant (if any) | 1.4 (0.7) | 1.4 (0.7) | 2 (1.4) |
| Neonatal respiratory morbidity | 101 (13.8%) | 72 (43.9%) | 29 (5.1%) |
Data are represented as mean (SD) or n (%) when appropriate.
CPAP: continuous positive airway pressure; NIV/BPAP: non-invasive ventilation/bi-level positive airway pressure.
The prevalence of NRM was 13.8% (101/730), of which 66.3% (67/101) were diagnosed with respiratory distress syndrome and 33.7% (34/101) with transient tachypnea of the newborn. All newborns diagnosed with respiratory distress syndrome were treated with at least one of the following: oxygen higher than 40%, continuous positive airway pressure, or non-invasive ventilation, high-frequency ventilation and an endotracheal tube for invasive ventilation, or surfactant use. The quantusFLM analysis predicted the occurrence of NRM with a sensitivity, specificity, and positive and negative predictive values of 75/101 (74.3%), 557/629 (88.6%), 75/147 (51.0%), and 557/583 (95.5%), respectively. Accuracy was 86.5% (632/730), and the positive and negative likelihood ratios were 6.5 and 0.3, respectively. The predictive performance stratified by gestational age is shown in Table 4.
Table 4. quantusFLM performance to predict neonatal respiratory morbidity.
| Gestational Age, wks | |||
|---|---|---|---|
| Characteristics | Total (n = 730) | (25.0—33.6) (n = 164) | (34.0—38.6) (n = 566) |
| Neonatal respiratory morbidity | 101 (13.8%) | 72 (43.9%) | 29 (5.1%) |
| True positives | 75 | 57 | 18 |
| True negatives | 557 | 67 | 490 |
| False positives | 72 | 25 | 47 |
| False negatives | 26 | 15 | 11 |
| Accuracy | 86.6% (632/730) | 75.6% (124/164) | 89.8% (508/566) |
| Sensitivity | 74.3% (75/101) | 79.2% (57/72) | 62.1% (18/29) |
| Specificity | 88.6% (557/629) | 72.8% (67/92) | 91.3% (490/537) |
| Positive predictive value | 51% (75/147) | 69.5% (57/82) | 27.7% (18/65) |
| Negative predictive value | 95.5% (557/583) | 81.7% (67/82) | 97.8% (490/501) |
| Positive likelihood ratio | 6.5 | 2.9 | 7.1 |
| Negative likelihood ratio | 0.3 | 0.3 | 0.4 |
Comment
Principal findings of the study
The main finding of this large multicenter study is that quantitative texture analysis of fetal lung ultrasound images predicted NRM with a similar accuracy to that of laboratory tests using amniotic fluid, which have reported sensitivities and specificities ranging from 74% to 89% and from 54% to 89% respectively [9, 24, 25], although a wide range of figures has been reported (Table 5 and Table 3S). Furthermore, the risk of respiratory neonatal morbidity observed in this study was similar to that reported in a recently published large cohort study of late preterm and early term infants (Table 4S).
Table 5. Summary of performance of invasive tests in amniotic fluid used to predict neonatal respiratory morbidity (summarized from Table 3S).
| Ac | Se | Sp | PPV | NPV | |
|---|---|---|---|---|---|
| quantusFLM | 86.5% | 74.3% | 88.6% | 51% | 95.5% |
| L/S | 81.6% | 74.6% | 82.5% | 34.1% | 96.4% |
| PG | 57.5% | 82.7% | 54.4% | 18.0% | 96.3% |
| LBC | 75.4% | 84.2% | 74.4% | 27.9% | 97.6% |
| TDxII | 78.7% | 88.5% | 77.7% | 28.5% | 98.5% |
Ac, accuracy; L/S, lecithin/sphingomyelin ratio; LBC, lamellar body count; NPV, negative predictive value; PG, phosphatidylglycerol; PPV, positive predictive value; Se, sensitivity; Sp, specificity; TDxII, surfactant/albumin ratio.
Results of the study in the context of other observations
Several attempts have been made to predict fetal lung maturity using ultrasound images. Serizawa and Maeda [13] and Maeda et al [14] compared the ultrasonic gray-level histogram width of the fetal lungs and liver, while Bhanu Prakash et al [17] compared the values for the fetal lungs to those of liver. La Torre et al [16] correlated several patterns of fetal breathing movements with fetal lung maturity tests, and Tekesin et al [26] evaluated the mean gray value of the fetal lungs. The accuracy identifying NRM in all these studies has ranged from 73% to 96%. However, no prospective studies have been conducted after them to validate the associations observed (Table 3S). The approach used in this study was different from previous attempts to non-invasively assess fetal lung maturity. The method used herein is based on the combination of texture extraction with machine learning methods, allowing the identification of texture patterns in the ultrasound image that correlate with the clinical outcome. This approach has been shown to be reliable and robust to small variations in the conditions of the image acquisition, including depth and changes in the gain of the image, and does not need other tissues with which to be compared (placenta, fetal liver…) [20]. Additionally, a previous pilot study reported on the ability of this non-invasive technology to predict NRM [22].
Clinical implications
Liggins and Howie [27] stated that the use of antenatal corticosteroids could enhance fetal lung maturity in preterm pregnancies; as a result, corticosteroid use is common practice with pregnancies up to 34 weeks of gestation [28-30]. Now, the question as to whether late preterm fetuses may benefit from such an intervention is on the rise.
The practice of testing for fetal lung maturity is extremely variable worldwide, being widely used in some areas and completely ignored in others. Estimation of fetal lung maturity might reduce the use of corticosteroids in late preterm deliveries (34 to 36 weeks of gestation), for which the risk of NRM is relevant but relatively low, ranging 10% to 20%. As recently shown, steroids decrease by one-third the occurrence of NRM in late preterm deliveries [8, 31- 34] and the number needed to treat to reduce one case of NRM in the circumstances described is 25 [8]. These findings have resulted in the publication of a Society for Maternal-Fetal Medicine statement on the use of antenatal corticosteroids in the late preterm period [35]; it recommends treatment under the strict inclusion criteria of the Antenatal Late Preterm Steriods study, while warning against overtreatment in those cases that do not meet the inclusion criteria. Even if mid and long-term follow-up of babies exposed to corticosteroids has shown no adverse effects or no benefits in some studies [36-39], antenatal corticosteroids might be associated with potential side effects related to overexposure later in life [40-42], particularly in those babies who will be delivered at term [43, 44]. A substantial proportion of fetuses treated with corticosteroids are delivered long after one week of the initial dose or even at term [45-50]. Rescue doses are debatable [51, 52], and the benefits and risks have to be evaluated when repeated doses are considered long after an initial course was given early in pregnancy [53-55] or if an early term elective cesarean delivery is planned [56]. Thus, strategies to define the target population are urged.
On the other hand, the fear of overtreatment has to be counterbalanced against the fact that restrictive messages may limit the use of corticosteroids in those cases for which the intervention has been proven of benefit and for which additional information from quantusFLM is of limited value (i.e. preterm delivery at <32 weeks of gestation). For instance, some data showed that, among cases with potential benefit, only 80% received one dose and 70% received two doses [57]. On the contrary, there are other studies reporting that a wide use of corticosteroids might not be of benefit in all countries [58].
All these aspects have been discussed in recent reviews; therefore, the issue remains controversial [59, 60]. It is in this context that the selection of a low-risk group for respiratory morbidity by a non-invasive tool might reduce exposure in a large number of pregnancies, avoiding the risks of overexposure in an unselected population and optimizing intervention in those cases for which it is needed.
Additionally, a common argument against testing for fetal lung maturity is that there is or is not a clear indication for elective preterm delivery; therefore, the results of fetal lung maturity besting would not be of help [4, 61]. This view might be challenged by studies reporting that about 23% of late-term deliveries had no clear indication for delivery [62] or that they were delivered after a “non-evidence-based” indication [63]. Therefore, a fraction of complicated pregnancies may fall within a gray zone, for which elective delivery may be considered as an option when there is not a strict indication according to clinical protocols or guidelines [64].In these cases, information about fetal lung maturity might be of help to plan delivery.
Likewise, access to advanced neonatal care is not readily available in all clinical settings, even in high-resource countries. In these circumstances, knowing the risks of respiratory morbidity with an acceptable accuracy might help clinicians and parents to make more balanced decisions and/or determine the most appropriate place for delivery [65]. Finally, among the reasons for avoiding fetal lung maturity testing may be the fear for complications of amniocentesis, reported to occur in 0.7% of cases [66, 67], as well as medical costs and/or maternal discomfort. This perception and, consequently, the attitude of physicians and parents seeking information about fetal lung maturity might be reconsidered if this information can be obtained with a non-invasive test.
Strengths and limitations
The results of this multicenter study are in line with those obtained in a previous smaller study for which the technology was prospectively and blindly evaluated at a single center for 144 patients [22]. These findings and the multicenter nature of the study support the fact, provided the quality criteria in the acquisition of the images are respected, that the test is robust and yields similar performances in different clinical settings, enhancing the likelihood that results are generalizable.
However, this study has some limitations. The method tested in this study uses an indirect approach to estimate lung maturity. By definition, prenatal prediction of NRM is hampered by the fact that the outcome is largely, but not exclusively, determined by the fetal lung maturity status. Thus, in circumstances such as neonatal sepsis, congenital anomalies potentially affecting lung function, or intrapartum hypoxic-ischemic events, newborns with normal lung maturity in utero may present respiratory impairment. Also, specific conditions such as fetal growth restriction, multiple pregnancy, diabetes, or premature rupture of the membranes were not analyzed separately. Differences in the performance of quantusFLM in these subgroups cannot be excluded and requires further research. On the other hand, the performance of the software for each specific gestational age was not assessed in this study because the algorithms were not designed to predict NRM for each specific gestational age. Future algorithms with one- or two- week gestational age intervals would be more precise, although whether this could improve the accuracy reported herein remains to be assessed. Regarding the mode of delivery, the rate of cesarean delivery was high, around 50%, because delivery had to occur within 48 hours of the image acquisition to meet inclusion criteria. Therefore, planned cesarean delivery might be over represented in our study population, although this rate could be comparable to some settings. According to clinical practice, elective and non-elective cesarean deliveries are more frequent in preterm pregnancies. Finally, despite the ultrasound image required to perform the test was an axial section of the thorax, considered to be a standard section, a relatively high number of images were eventually discarded because of the lack of compliance with the quality criteria requisites. This stresses the fact that obtaining a valid ultrasound axial section of the fetal thorax at late gestation might not always be straightforward, and, in particular cases, the test might require special care or training to ensure optimal image acquisition.
Conclusion
In summary, the results of this large multicenter study are consistent with the findings of a pilot study on the ability of a non-invasive technology to predict NRM from fetal lung ultrasound images [22]. The technology also showed accuracy similar to that of biochemical testing of the amniotic fluid, previously reported. Therefore, quantusFLM provides a non-invasive tool that might aid clinicians in the decision-making process.
Supplementary Material
Acknowledgments
The Fetal Lung Texture Team includes the following collaborators: Montse Palacio, Teresa Cobo, Marta López, Dulce Castro, Juan Pablo Piraquive, Juan Carlos Ramírez, Federico Migliorelli, Mónica Martínez-Terrón, Francesc Botet, Eduard Gratacós (BCNatal Hospital Clínic, Barcelona, Spain); Joan Sabrià, Silvia Ferrero Martínez, Dolores Gómez Roig (BCNatal Hospital Sant Joan de Déu, Barcelona, Spain); Elisenda Bonet-Carné, Àlvaro Pérez, Mara Domínguez, David Coronado (Transmural Biotech S.L., Barcelona, Spain); Jan Deprest, Jute Richter, Philip DeKoninck (University Hospitals Leuven, Belgium); Marian Kacerovsky, Ivana Musilova, Tomas Bestvina, Jan Maly, Zdenek Kokstein (University Hospital Hradec Kralove, Czech Republic); Bo Jacobsson, Lars Cedergren, Patricia Johansson, Panagiotis Tsiartas, Karin Sävman, Maria Hallingström (Sahlgrenska University Hospital/Ostra, Gothenburg, Sweden); Raúl García Posadas (Clínica del Prado. Medellín, , Colombia); Fernando Bugatto González, Maria Antonia Fajardo, Rocío Quintero Prado, Victoria Melero Jiménez, Isabel Benavente Fernández (Hospital Universitario Puerta del Mar, Cádiz, Spain); Ramon Santisteve Prat, Benjamín de la Barrera Correa, Elena Gómez Valencia, Raúl Martínez Rodríguez, Elionor Roma Mas (Hospital de Sant Joan de Déu de Manresa, Manresa, Spain); Àngels Vives Argilagós, Alejandra Rodríguez Veret, Esperanza García Cancela, Paloma Araujo Salinas (Consorci Sanitari de Terrassa, Terrassa, Spain); Mauro Parra-Cordero, Álvaro Sepúlveda-Martínez (University of Chile Hospital, Santiago de Chile, Chile); Edgar Hernández-Andrade, Roberto Romero, Hyunyoung Ahn (Perinatology Research Branch, Wayne, USA); José Luis Bartha, Eugenia Antolín (Hospital Universitario La Paz, Madrid, Spain); Pilar Carretero Lucena, Francisca Molina García, Noemí Jiménez Garrido, Carmen Contreras Tallón, Belén Morillo Antón (Hospital San Cecilio, Granada, Spain); George Yeo, Kai Lit Tan (KK Women's & Children's Hospital, Singapore); Rogelio Cruz (Children's Hospital of Querétaro, Querétaro, México); Jon Hyatt, Minke Burke, Ritu Mogra (Royal Prince Alfred Hospital, Sidney, Australia); Suseela Vavilala (Fernández Hospital, Hyderabad, India); J.Igor Iruretagoyena (Maternal Fetal Medicine Division. University of Wisconsin. Madison, Wisconsin); Juan Luis Delgado (Hospital ‘Virgen de la Arrixaca’, Murcia, Spain); Mauro Schenone (University of Tennessee Health Science Center, USA); Josep Vilanova, Neus Bons (Hospital Nostra Senyora de Meritxell, Andorra).
The work of Dr. Romero was supported by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Footnotes
Disclosure Statements: MP, TC, JD and EG have served as scientific advisors to Transmural Biotech. All other authors declare no conflicts of interest.
Condensation: Quantitative analysis of fetal lung texture predicted neonatal respiratory morbidity with an accuracy comparable to invasive tests assessing fetal lung maturity.
References
- 1.Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. American journal of obstetrics and gynecology. 2011;205:374, e1–e9. doi: 10.1016/j.ajog.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Consortium on Safe Labor. Hibbard JU, Wilkins I, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419–425. doi: 10.1001/jama.2010.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoder BA, Gordon MC, Barth WH., Jr Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814–822. doi: 10.1097/AOG.0b013e31816499f4. [DOI] [PubMed] [Google Scholar]
- 4.Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. American journal of obstetrics and gynecology. 2009;200:156, e1–e4. doi: 10.1016/j.ajog.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, Carrion V, Shelton J, et al. Adverse Neonatal Outcomes Associated With Early-Term Birth. JAMA Pediatr. 2013;167:1053–1059. doi: 10.1001/jamapediatrics.2013.2581. [DOI] [PubMed] [Google Scholar]
- 7.Porto AM, Coutinho IC, Correia JB, Amorim MM. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ. 2011;342:d1696. doi: 10.1136/bmj.d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311–1320. doi: 10.1056/NEJMoa1516783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besnard AE, Wirjosoekarto SAM, Broeze KA, Opmeer BC, Mol BWJ. Lecithin/sphingomyelin ratio and lamellar body count for fetal lung maturity: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2013;169:177–183. doi: 10.1016/j.ejogrb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists. Fetal lung maturity. ACOG Practice bulletin no. 97. Obstet Gynecol. 2008;112:717–726. doi: 10.1097/AOG.0b013e318188d1c2. [DOI] [PubMed] [Google Scholar]
- 11.Grannum PA, Berkowitz RL, Hobbins JC. The ultrasonic changes in the maturing placenta and their relation to fetal pulmonic maturity. Am J Obstet Gynecol. 1979;133:915–922. doi: 10.1016/0002-9378(79)90312-0. [DOI] [PubMed] [Google Scholar]
- 12.Harman CR, Manning FA, Stearns E, Morrison I. The correlation of ultrasonic placental grading and fetal pulmonary maturation in five hundred sixty-three pregnancies. Am J Obstet Gynecol. 1982;143:941–943. doi: 10.1016/0002-9378(82)90478-1. [DOI] [PubMed] [Google Scholar]
- 13.Serizawa M, Maeda K. Noninvasive fetal lung maturity prediction based on ultrasonic gray level histogram width. Ultrasound Med Biol. 2010;36:1998–2003. doi: 10.1016/j.ultrasmedbio.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, Utsu M, Yamamoto N, Serizawa M. Echogenicity of fetal lung and liver quantified by the grey-level histogram width. Ultrasound Med Biol. 1999;25:201–208. doi: 10.1016/s0301-5629(98)00160-4. [DOI] [PubMed] [Google Scholar]
- 15.Cosmi EV, Anceschi MM, Cosmi E, Piazze JJ, La Torre R. Ultrasonographic patterns of fetal breathing movements in normal pregnancy. Int J Gynecol Obstet. 2003;80:285–290. doi: 10.1016/s0020-7292(02)00384-3. [DOI] [PubMed] [Google Scholar]
- 16.La Torre R, Cosmi E, Anceschi MH, Piazze JJ, Piga MD, Cosmi EV. Preliminary report on a new and noninvasive method for the assessment of fetal lung maturity. J Perinat Med. 2003;31:431–434. doi: 10.1515/JPM.2003.067. [DOI] [PubMed] [Google Scholar]
- 17.Bhanu Prakash KN, Ramakrishnan AG, Suresh S, Chow TWP. Fetal lung maturity analysis using ultrasound image features. IEEE Trans Inf Technol Biomed. 2002;6:38–45. doi: 10.1109/4233.992160. [DOI] [PubMed] [Google Scholar]
- 18.Insana MF, Garra BS, Rosenthal SJ, Hall TJ. Quantitative ultrasonography. Med Prog Technol. 1988;15:141–153. [PubMed] [Google Scholar]
- 19.Sanz-Cortes M, Figueras F, Bonet-Carne E, et al. Fetal brain MRI texture analysis identifies different microstructural patterns in adequate and small for gestational age fetuses at term. Fetal Diagn Ther. 2013;33:122–129. doi: 10.1159/000346566. [DOI] [PubMed] [Google Scholar]
- 20.Cobo T, Bonet-Carne E, Martinez-Terron M, et al. Feasibility and reproducibility of fetal lung texture analysis by Automatic Quantitative Ultrasound Analysis and correlation with gestational age. Fetal Diagn Ther. 2012;31:230–236. doi: 10.1159/000335349. [DOI] [PubMed] [Google Scholar]
- 21.Palacio M, Cobo T, Martinez-Terron M, et al. Performance of an automatic quantitative ultrasound analysis of the fetal lung to predict fetal lung maturity. Am J Obstet Gynecol. 2012;207:504.e1–e.5. doi: 10.1016/j.ajog.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Bonet-Carne E, Palacio M, Cobo T, et al. Quantitative ultrasound texture analysis of fetal lungs to predict neonatal respiratory morbidity. Ultrasound Obstet Gynecol. 2015;45:427–433. doi: 10.1002/uog.13441. [DOI] [PubMed] [Google Scholar]
- 23.Taeusch HW, Ballard R, Gleason C. Avery's Diseases of the Newborn. 8th. Philadelphia PA: Elsevier Saunders; 2005. [Google Scholar]
- 24.Grenache DG, Gronowski AM. Fetal lung maturity. Clin Biochem. 2006;39:1–10. doi: 10.1016/j.clinbiochem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Wijnberger LDE, de Kleine M, Voorbij HAM, et al. Prediction of fetal lung immaturity using gestational age, patient characteristics and fetal lung maturity tests: a probabilistic approach. Arch Gynecol Obstet. 2010;281:15–21. doi: 10.1007/s00404-009-1033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekesin I, Anderer G, Hellmeyer L, Stein W, Kühnert M, Schmidt S. Assessment of fetal lung development by quantitative ultrasonic tissue characterization: a methodical study. Prenat Diagn. 2004;24:671–676. doi: 10.1002/pd.951. [DOI] [PubMed] [Google Scholar]
- 27.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 28.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–335. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health Consensus Statement. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. [Google Scholar]
- 30.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Yinon Y, Haas J, Mazaki-Tovi S, et al. Should patients with documented fetal lung immaturity after 34 weeks of gestation be treated with steroids? Am J Obstet Gynecol. 2012;207:222.e1–e4. doi: 10.1016/j.ajog.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Shanks A, Gross G, Shim T, Allsworth J, Sadovsky Y, Bildirici I. Administration of steroids after 34 weeks of gestation enhances fetal lung maturity profiles. Am J Obstet Gynecol. 2010;203:47.e1–e5. doi: 10.1016/j.ajog.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamath-Rayne BD, DeFranco EA, Marcotte MP. Antenatal steroids for treatment of fetal lung immaturity after 34 weeks of gestation: an evaluation of neonatal outcomes. Obstet Gynecol. 2012;119:909–916. doi: 10.1097/AOG.0b013e31824ea4b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Invest. 2010;70:95–99. doi: 10.1159/000295898. [DOI] [PubMed] [Google Scholar]
- 35.Society of Maternal-Fetal Medicine Publications Committee. SMFM statement: implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13–B15. doi: 10.1016/j.ajog.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental Outcome After a Single Course of Antenatal Steroids in Children Born Preterm: A Systematic Review and Meta-analysis. Obstet Gynecol. 2015;125:1385–1396. doi: 10.1097/AOG.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 37.Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331:665. doi: 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asztalos EV, Murphy KE, Willan AR, et al. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5) JAMA Pediatr. 2013;167:1102–1110. doi: 10.1001/jamapediatrics.2013.2764. [DOI] [PubMed] [Google Scholar]
- 39.Crowther CA, McKinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2015;7:CD003935. doi: 10.1002/14651858.CD003935.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiken CE, Fowden AL, Smith GC. Antenatal glucocorticoids prior to cesarean delivery at term. JAMA Pediatr. 2014;168:507–508. doi: 10.1001/jamapediatrics.2014.9. [DOI] [PubMed] [Google Scholar]
- 41.Crowther CA, Harding JE. Antenatal glucocorticoids for late preterm birth? N Engl J Med. 2016;374:1376–1377. doi: 10.1056/NEJMe1601867. [DOI] [PubMed] [Google Scholar]
- 42.Waffarn F, Davis EP. Effects of antenatal corticosteroids on the hypothalamic-pituitary- adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. Am J Obstet Gynecol. 2012;207:446–454. doi: 10.1016/j.ajog.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91:1415–1421. doi: 10.1111/aogs.12014. [DOI] [PubMed] [Google Scholar]
- 44.Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97:3538–3544. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 45.Boesveld M, Oudijk MA, Koenen SV, et al. Evaluation of strategies regarding management of imminent preterm delivery before 32 weeks of gestation: a regional cohort study among 1375 women in the Netherlands. Am J Obstet Gynecol. 2015;212:348.e1–e7. doi: 10.1016/j.ajog.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Mahony R, McKeating A, Murphy T, McAuliffe F, O'Herlihy C, Foley M. Appropriate antenatal corticosteroid use in women at risk for preterm birth before 34 weeks of gestation. BJOG. 2010;117:963–967. doi: 10.1111/j.1471-0528.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 47.Razaz N, Skoll A, Fahey J, Allen VM, Joseph KS. Trends in optimal, suboptimal, and questionably appropriate receipt of antenatal corticosteroid prophylaxis. Obstet Gynecol. 2015;125:288–296. doi: 10.1097/AOG.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 48.Melamed N, Shah J, Soraisham A, et al. Association Between Antenatal Corticosteroid Administration-to-Birth Interval and Outcomes of Preterm Neonates. Obstet Gynecol. 2015;125:1377–1384. doi: 10.1097/AOG.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 49.Adams TM, Kinzler WL, Chavez MR, Vintzileos AM. The timing of administration of antenatal corticosteroids in women with indicated preterm birth. Am J Obstet Gynecol. 2015;212:645.e1–e4. doi: 10.1016/j.ajog.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Wilms FF, Vis JY, Pattinaja DA, et al. Relationship between the time interval from antenatal corticosteroid administration until preterm birth and the occurrence of respiratory morbidity. Am J Obstet Gynecol. 2011;205:49.e1–e7. doi: 10.1016/j.ajog.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Davidson C, Monga M, Ellison D, Vidaeff A. Continuation of pregnancy after antenatal corticosteroid administration: opportunity for rescue? J Reprod Med. 2010;55:14–18. [PubMed] [Google Scholar]
- 52.Makhija NK, Tronnes AA, Dunlap BS, Schulkin J, Lannon SM. Antenatal corticosteroid timing: accuracy after the introduction of a rescue course protocol. Am J Obstet Gynecol. 2016;214:120.e1–e6. doi: 10.1016/j.ajog.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 53.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 54.Murphy KE, Hannah ME, Willan AR, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 55.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 56.Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662. doi: 10.1136/bmj.38547.416493.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrasekaran S, Srinivas SK. Antenatal corticosteroid administration: understanding its use as an obstetric quality metric. Am J Obstet Gynecol. 2014;210:143.e1–e7. doi: 10.1016/j.ajog.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 58.Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster- randomised trial. Lancet. 2015;385:629–639. doi: 10.1016/S0140-6736(14)61651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123:1067–1069. doi: 10.1111/1471-0528.13853. [DOI] [PubMed] [Google Scholar]
- 60.Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: what do we do now? Am J Obstet Gynecol. 2016;215:423–430. doi: 10.1016/j.ajog.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 61.American College of Obstetricians and Gynecologists. Medically indicated late-preterm and early-term deliveries. ACOG Committee opinion no. 560. Obstet Gynecol. 2013;121:908–910. doi: 10.1097/01.AOG.0000428648.75548.00. [DOI] [PubMed] [Google Scholar]
- 62.Reddy UM, Ko CW, Raju TNK, Willinger M. Delivery indications at late-preterm gestations and infant mortality rates in the United States. Pediatrics. 2009;124:234–240. doi: 10.1542/peds.2008-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gyamfi-Bannerman C, Fuchs KM, Young OM, Hoffman MK. Nonspontaneous late preterm birth: etiology and outcomes. Am J Obstet Gynecol. 2011;205:456.e1–e6. doi: 10.1016/j.ajog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Towers CV, Freeman RK, Nageotte MP, Garite TJ, Lewis DF, Quilligan EJ. The case for amniocentesis for fetal lung maturity in late-preterm and early-term gestations. Am J Obstet Gynecol. 2014;210:95–96. doi: 10.1016/j.ajog.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Damron DP. Fetal lung maturity testing. Am J Obstet Gynecol. 2014;211:184–185. doi: 10.1016/j.ajog.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 66.Zalud I, Janas S. Risks of third-trimester amniocentesis. J Reprod Med. 2008;53:45–48. [PubMed] [Google Scholar]
- 67.Gordon MC, Narula K, O'Shaughnessy R, Barth WH., Jr Complications of third-trimester amniocentesis using continuous ultrasound guidance. Obstet Gynecol. 2002;99:255–259. doi: 10.1016/s0029-7844(01)01715-x. [DOI] [PubMed] [Google Scholar]
- 68.Karcher R, Sykes E, Batton D, et al. Gestational age-specific predicted risk of neonatal respiratory distress syndrome using lamellar body count and surfactant-to-albumin ratio in amniotic fluid. Am J Obstet Gynecol. 2005;193:1680–1684. doi: 10.1016/j.ajog.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 69.Hagen E, Link JC, Arias F. A comparison of the accuracy of the TDx-FLM assay, lecithin-sphingomyelin ratio, and phosphatidylglycerol in the prediction of neonatal respiratory distress syndrome. Obstet Gynecol. 1993;82:1004–1008. [PubMed] [Google Scholar]
- 70.Russell JC, Cooper CM, Ketchum CH, et al. Multicenter evaluation of TDx test for assessing fetal lung maturity. Clin Chem. 1989;35:1005–1010. [PubMed] [Google Scholar]
- 71.Neerhof MG, Haney EI, Silver RK, Ashwood ER, Lee IS, Piazze JJ. Lamellar body counts compared with traditional phospholipid analysis as an assay for evaluating fetal lung maturity. Obstet Gynecol. 2001;97:305–309. doi: 10.1016/s0029-7844(00)01133-9. [DOI] [PubMed] [Google Scholar]
- 72.Haymond S, Luzzi VI, Parvin CA, Gronowski AM. A direct comparison between lamellar body counts and fluorescent polarization methods for predicting respiratory distress syndrome. Am J Clin Pathol. 2006;126:894–899. doi: 10.1309/8VXN5EM5L3831AT2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


