Abstract
Objective
Microbial invasion of the fetus due to intra-amniotic infection can lead to a systemic inflammatory response characterized by elevated concentrations of cytokines in the umbilical cord plasma/serum. Clinical chorioamnionitis represents the maternal syndrome often associated with intra-amniotic infection, although other causes of this syndrome have been recently described. The objective of this study was to characterize the umbilical cord plasma cytokine profile in neonates born to mothers with clinical chorioamnionitis at term, according to the presence or absence of bacteria and/or intra-amniotic inflammation.
Materials and Methods
A cross-sectional study was conducted, including patients with clinical chorioamnionitis at term (n=38; cases) and those with spontaneous term labor without clinical chorioamnionitis (n=77; controls). Women with clinical chorioamnionitis were classified according to the results of amniotic fluid culture, broad-range polymerase chain reaction coupled with electrospray ionization mass spectrometry (PCR/ESI-MS), and amniotic fluid interleukin (IL)-6 concentrations into three groups: 1) no intra-amniotic inflammation; 2) intra-amniotic inflammation without detectable microorganisms; or 3) microbial-associated intra-amniotic inflammation. Fetal inflammatory response syndrome (FIRS) was defined as an umbilical cord plasma IL-6 concentration >11 pg/mL. The umbilical cord plasma concentrations of 29 cytokines were determined with sensitive and specific V-PLEX immunoassays. Nonparametric statistical methods were used for analysis, adjusting for a false discovery rate of 5%.
Results
1) Neonates born to mothers with clinical chorioamnionitis at term (considered in toto) had significantly higher median umbilical cord plasma concentrations of IL-6, IL-12p70, IL-16, IL-13, IL-4, IL-10, and IL-8, but significantly lower interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) concentrations than neonates born to mothers with spontaneous term labor without clinical chorioamnionitis; 2) neonates born to mothers with clinical chorioamnionitis at term but without intra-amniotic inflammation had higher concentrations of IL-6, IL-12p70, IL-13, IL-4, IL-5, and IL-8 but lower IFN-γ than neonates not exposed to clinical chorioamnionitis, suggesting that maternal fever in the absence of intra-amniotic inflammation leads to a change in the fetal cytokine network; 3) there were significant, positive correlations between maternal and umbilical cord plasma IL-6 and IL-8 concentrations (IL-6: Spearman’s correlation=0.53; p<0.001; IL-8: Spearman’s correlation=0.42; p<0.001), consistent with placental transfer of cytokines; 4) an elevated fetal plasma IL-6 (> 11pg/mL), the diagnostic criterion for FIRS, was present in 21% of cases (8/38), and all of these neonates were born to mothers with proven intra-amniotic infection; and 5) FIRS was associated with a high concentration of umbilical cord plasma IL-8, IL-10, and monocyte chemoattractant protein (MCP)-1.
Conclusions
Neonates born to mothers with clinical chorioamnionitis at term had higher concentrations of umbilical cord plasma cytokines than those born to mothers without clinical chorioamnionitis. Even neonates exposed to clinical chorioamnionitis but not to intra-amniotic inflammation had elevated concentrations of multiple cytokines, suggesting that intrapartum fever alters the fetal immune response.
Keywords: biomarker, chemokine, fetal inflammatory response syndrome (FIRS), funisitis, interleukin (IL)-6, interleukin (IL)-8, intra-amniotic infection/inflammation, monocyte chemoattractant protein (MCP)-1, neonatal sepsis, umbilical cord plasma
Introduction
Clinical chorioamnionitis is a syndrome characterized by a maternal systemic inflammatory response [1] and often attributed to intra-amniotic infection [2–14]. Neonates born to mothers with clinical chorioamnionitis are at risk for sepsis [15–25], meconium aspiration syndrome [15, 26–30], neonatal encephalopathy [31–35], long-term neurodevelopmental disabilities including cognitive impairment [36–39] and cerebral palsy [22, 33, 40–46] as well as neonatal death [24, 47–50].
A growing body of evidence suggests that a maternal systemic inflammatory response can have powerful effects on the fetus [51–54]. For example, maternal systemic inflammation (sterile or induced by microorganisms) during critical windows of pregnancy may predispose to serious adverse infant outcomes [51–92], including autism spectrum disorders [52, 73, 74, 77, 79, 81–83, 85–89, 92–94] and schizophrenia [55, 56, 58, 60–64, 66, 67, 69–74, 76–78, 80, 85, 92]. Intrapartum fever is associated with an increased risk for cerebral palsy (odds ratio = 9.3; 95% confidence interval: 2.7–31) [41]. Moreover, recent evidence suggests that maternal systemic inflammatory response can affect other target organs, such as the lung [95–103]. The emerging picture is that early exposure to maternal systemic inflammation may predispose to multiple organ injury, whose clinical manifestations may occur only in infancy or adulthood.
Clinical chorioamnionitis at term (a state of maternal systemic inflammation [1]), offers a unique opportunity to examine the relationship between maternal systemic inflammation (with or without intra-amniotic inflammation) and the fetal systemic immune response as reflected by the peripheral concentrations of cytokines in humans. The purpose of this study was to determine the cytokine profile in the fetal peripheral circulation after exposure to systemic maternal inflammation.
Materials and Methods
Study population
A cross-sectional study was conducted that included patients with clinical chorioamnionitis at term (n=38; cases) and those with spontaneous term labor without clinical chorioamnionitis (n=77; controls). Inclusion and exclusion criteria for the study population were previously reported [1, 3]. These patients have been included in previous communications focusing on clinical chorioamnionitis at term [2, 3]. The number of cases is slightly different among the studies; this is due to the availability of samples.
All patients provided written informed consent, and the use of biological specimens, as well as clinical and ultrasound data for research purposes, was approved by the Institutional Review Boards of NICHD, Wayne State University, and the Sótero del Río Hospital, Santiago, Chile.
The clinical definitions, microbiological studies, and the determination of cytokines/chemokines have been previously described [1–3, 13]. Fetal inflammatory response syndrome (FIRS) is defined as an umbilical cord blood IL-6 concentration >11 pg/mL [104–113].
Sample collection and cytokine immunoassays
Umbilical cord blood samples were collected immediately after delivery from both cases and controls, and then placed into tubes containing EDTA, centrifuged for 10 minutes at 4°C, and stored at −70°C. Laboratory personnel were blinded to clinical diagnosis. The umbilical cord plasma concentrations of the following 29 cytokines were determined with sensitive and specific V-PLEX immunoassays (Meso Scale Discovery, Gaithersburg, MD, USA): [Pro-inflammatory cytokines: interferon gamma (IFN-γ), interleukin (IL)-1α, IL-1β, IL-2, IL-6, IL-7, IL-12p70, IL-12/IL-23p40, IL-15, IL-16, IL-17α, tumor necrosis factor (TNF)-α, TNF-β, vascular endothelial growth factor (VEGF), granulocyte macrophage colony-stimulating factor (GM-CSF); anti-inflammatory cytokines: IL-4, IL-5, IL-10, IL-13; and chemokines: IL-8, thymus and activation-regulated chemokine (TARC), eotaxin, eotaxin-3, macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α, MIP-1β, monocyte chemoattractant protein (MCP)-1, MCP-4, C-X-C motif chemokine 10 (CXCL-10) or IFN-γ-induced protein 10 (IP-10)].
Briefly, 50 µL of each umbilical cord blood sample were dispensed into separate wells of the plates and incubated for 2 hours with vigorous shaking at room temperature. The samples and calibrators were discarded, and the plates were washed three times with phosphate-buffered saline and 0.05% Tween-20 (Meso Scale Discovery), followed by an addition of 25 µL of the 1× Detection Antibody Solution (Meso Scale Discovery) into each well. Plates were then incubated for 2 hours with vigorous shaking at room temperature. The detection antibody was removed, and the plates were washed three times. To each well were added 150 µL of 2× Read Buffer T (Meso Scale Discovery), and the signals were read by the SECTOR® Imager 2400 (Meso Scale Discovery). Standard curves were generated, and the assay values of the samples were interpolated from the curves. The assay characteristics are described in the Supplementary Table. The coefficient of variation was less than 15% for 19 of the 29 analytes. For samples with concentrations below the limits of detection, missing values were replaced with 99% of the lowest detectable concentration.
Statistical analysis
For demographic data analysis, the Kolmogorov-Smirnov test was used to test whether the distribution of continuous variables was normal. Chi-square and Fisher’s exact tests were used for comparisons of proportions. Kruskal-Wallis and Mann-Whitney U tests were used to compare median concentrations of analytes between and among groups. Statistical analysis of demographics data was performed using SPSS 19 (IBM Corp, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
Comparison of analyte concentrations determined by multiplex assay was restricted to the analytes that were detected in a number of samples larger than one-half of the size of the smallest group. Statistical analysis was performed using the Wilcoxon rank-sum test and the R statistical environment [114]. Nominal p values were adjusted using the Benjamini & Hochberg method [115], controlling the false discovery rate at 5%.
Results
Characteristics of the study population
A total of 38 cases (patients with clinical chorioamnionitis at term) and 77 controls (patients with spontaneous term labor without clinical chorioamnionitis) were included in the study. Descriptive characteristics of the study population are displayed in Table 1. The patients in this study represent a subset included in previous reports [1, 3]. A description of the microorganisms identified in the amniotic fluid [2] and the concentration of cytokines in maternal plasma [1] and amniotic fluid [3], have been reported elsewhere.
Table 1.
Characteristics of the study population
| Term in labor (n=77) |
Clinical chorioamnionitis at term (n=38) |
P value | |
|---|---|---|---|
| Maternal age (years) | 25 (20.5–30.5) | 20.5 (18–25) | 0.003 |
| Body mass index (kg/m2) | 23.9 (21.0–25.9) | 23.8 (21.6–24.8) | 0.96 |
| Amniotic fluid glucose (mg/dL) | NA | 9 (9–9) | |
| Amniotic fluid white blood cell (cell/mm3) | NA | 41.5 (5–468.7) | |
| Gestational age at amniocentesis and delivery (weeks) | 39.6 (38.9–40.5) | 39.9 (38.9–40.8) | 0.46 |
| Birthweight (grams) | 3400 (3175–3650) | 3500 (3210–3775) | 0.34 |
| Suspected neonatal sepsis | 1.3% (1/77) | 34.2% (13/38) | <0.001 |
| Fetal inflammatory response syndrome (FIRS) | NA | 21.1% (8/38) | − |
| Acute inflammatory lesions of placenta | 0% (0/0) | 55.3% (21/38) | − |
Data are presented as % (n), median (interquartile range).
Acute inflammatory lesions of placenta: acute histologic chorioamnionitis and/or acute funisitis.
NA: results were not available.
When classified according to the presence or absence of intra-amniotic inflammation and microorganisms [by amniotic fluid cultures and PCR/ESI-MS (broad-range PCR coupled with electrospray ionization mass spectrometry)], 57.9% (22/38) of cases had microbial-associated intra-amniotic inflammation, 18.4% (7/38) had intra-amniotic inflammation without detectable microorganisms, and 23.7% (9/38) had no evidence of intra-amniotic inflammation. About one-half of the patients with clinical chorioamnionitis at term [55.3% (21/38)] had acute inflammatory lesions of the placenta, whereas no patients in the control group had such lesions. The frequency of suspected neonatal sepsis was significantly higher in the group with clinical chorioamnionitis than in the control group [34.2% (13/38) vs. 1.3% (1/77); p<0.001]. Approximately 70% (9/13) of neonates with suspected neonatal sepsis were born to mothers with intra-amniotic inflammation. All neonates with suspected sepsis had negative blood cultures. The diagnosis of neonatal sepsis was based on clinical signs and laboratory tests, such as a white blood cell count and C-reactive protein (CRP). of the patients with clinical chorioamnionitis at term, 21% (8/38) had neonates with FIRS. All of these neonates were exposed to microbial-associated intra-amniotic inflammation (also termed “intra-amniotic infection”).
Umbilical cord plasma cytokine concentrations
Clinical chorioamnionitis vs. spontaneous term labor without clinical chorioamnionitis
The median (interquartile range: IQR) cytokine concentrations in umbilical cord plasma between cases and controls are displayed in Table 2. Neonates born to mothers with clinical chorioamnionitis at term had significantly higher median umbilical cord plasma concentrations of IL-6, IL-12p70, IL-16, IL-13, IL-4, IL-10, and IL-8 than those with spontaneous term labor without clinical chorioamnionitis, with a fold-change difference in median concentrations that ranged from 1.39 to 6.0. IL-12p70 was the cytokine with the highest fold change (fold change = 6). Median umbilical cord plasma IFN-γ and TNF-β concentrations were significantly lower in patients with clinical chorioamnionitis at term than in the controls [fold differences in median: IFN-γ=0.65 (p=0.00004), TNF-β=0.72 (p=0.04)] (Table 2).
Table 2.
Umbilical cord plasma cytokine and chemokine concentrations in term in labor vs. clinical chorioamnionitis at term
| Analytes (pg/mL) |
Term in labor Median (IQR) (n=77) |
Clinical Chorioamnionitis at term Median (IQR) (n=38) |
Fold change | Adjusted P value |
|---|---|---|---|---|
| Pro-inflammatory cytokines | ||||
| IL-12p70 | 0.02 (0.02–0.1) | 0.12 (0.1–0.16) | 6.00 | 0.00001 |
| IL-6 | 0.55 (0.32–0.82) | 1.92 (1.16–3.55) | 3.48 | <0.00001 |
| IL-16* | 288.61 (223.88–406.54) | 402.6 (294.1–610.95) | 1.39 | 0.02 |
| IFN-γ | 2.9 (2.26–3.65) | 1.89 (1.48–2.64) | 0.65 | 0.00004 |
| TNF-β | 0.23 (0.16–0.33) | 0.17 (0.12–0.25) | 0.72 | 0.04 |
| IL-1β | 0.38 (0.18–0.79) | 0.38 (0.12–1.4) | 1.01 | 0.97 |
| IL-17α | 1.32 (1.07–1.71) | 1.34 (0.1–1.75) | 1.02 | 0.95 |
| TNF-α | 2.8 (2.4–3.1) | 2.88 (2.49–3.32) | 1.04 | 0.73 |
| IL-2 | 0.06 (0.01–0.14) | 0.12 (0.01–0.18) | 1.92 | 0.26 |
| IL-12/IL-23p40 | 645.63 (537.97–815.11) | 618.83 (480.67–871.85) | 0.96 | 0.77 |
| IL-15 | 1.23 (0.92–1.52) | 1.43 (0.87–2.67) | 1.16 | 0.1 |
| IL-1α | 0.92 (0.73–1.15) | 0.84 (0.69–1.1) | 0.91 | 0.72 |
| GM-CSF | 0.15 (0.09–0.22) | 0.05 (0.02–0.24) | 0.33 | 0.26 |
| IL-7 | 1.24 (0.73–2.21) | 1.13 (0.75–3.58) | 0.91 | 0.95 |
| VEGF | 45.16 (18.52–103.61) | 45.91 (20.21–98.51) | 1.02 | 0.77 |
| Anti-inflammatory cytokines | ||||
| IL-13 | 0.17 (0.17–0.54) | 0.87 (0.55–1.21) | 5.14 | <0.00001 |
| IL-4 | 0.01 (0–0.02) | 0.03 (0.02–0.04) | 2.50 | 0.00001 |
| IL-10 | 0.28 (0.2–0.38) | 0.42 (0.27–1.04) | 1.48 | 0.009 |
| IL-5 | 0.51 (0.37–0.65) | 0.58 (0.34–0.72) | 1.13 | 0.47 |
| Chemokines | ||||
| IL-8 | 2.50 (1.63–3.93) | 6.69 (3.84–10.48) | 2.67 | <0.00001 |
| MIP-1α | 11.97 (9.83–14.37) | 11.47 (10–13.6) | 0.96 | 0.97 |
| Eotaxin | 64.73 (48.03–81.72) | 56.93 (39.19–70.37) | 0.88 | 0.26 |
| MCP-4 | 51.94 (42.32–73.82) | 49.11 (27.56–89.94) | 0.95 | 0.88 |
| MCP-1 | 60 (44.78–67.54) | 58.78 (48.06–80.69) | 0.98 | 0.76 |
| MDC | 1124.23 (931.33–1311.54) | 1028.62 (834.1–1347.12) | 0.91 | 0.33 |
| Eotaxin-3 | 17.8 (12.25–-22.92) | 14.27 (6.27–27.13) | 0.8 | 0.68 |
| CXCL-10 (IP-10) | 84.82 (66.27–114.91) | 109.82 (66.68–151.54) | 1.29 | 0.31 |
| TARC | 98.97 (64.29–194.61) | 113.93 (70.44–276.82) | 1.15 | 0.24 |
| MIP-1β | 94.77 (73.29–120.46) | 76.39 (63.93–112.93) | 0.81 | 0.26 |
IL-16 has pro- and anti-inflammatory properties. The units of all analytes are pg/mL. Values in bold font indicate that the results are significant.
CXCL-10: C-X-C motif chemokine 10; GM-CSF: granulocyte macrophage colony-stimulating factor; IFN-γ: interferon gamma; IL: interleukin; IP-10: interferon gamma-induced protein 10; IQR: interqurtile range; MCP: monocyte chemoattractant protein; MDC: macrophage-derived chemokine; MIP: macrophage inflammatory protein; TARC: thymus and activation-regulated chemokine; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
Clinical chorioamnionitis without intra-amniotic inflammation
Neonates born to mothers with clinical chorioamnionitis but without intra-amniotic inflammation had significantly higher median umbilical plasma concentrations of IL-12p70, IL-6, IL-4, IL-5, IL-8, and IL-13 than neonates born to mothers with spontaneous labor at term and without clinical chorioamnionitis (Figure 1). The fold-change difference in their median concentrations ranged from 1.9 to 6 (Table 3). In contrast, the median umbilical cord plasma concentrations of IFN-γ and MIP-1β were significantly lower.
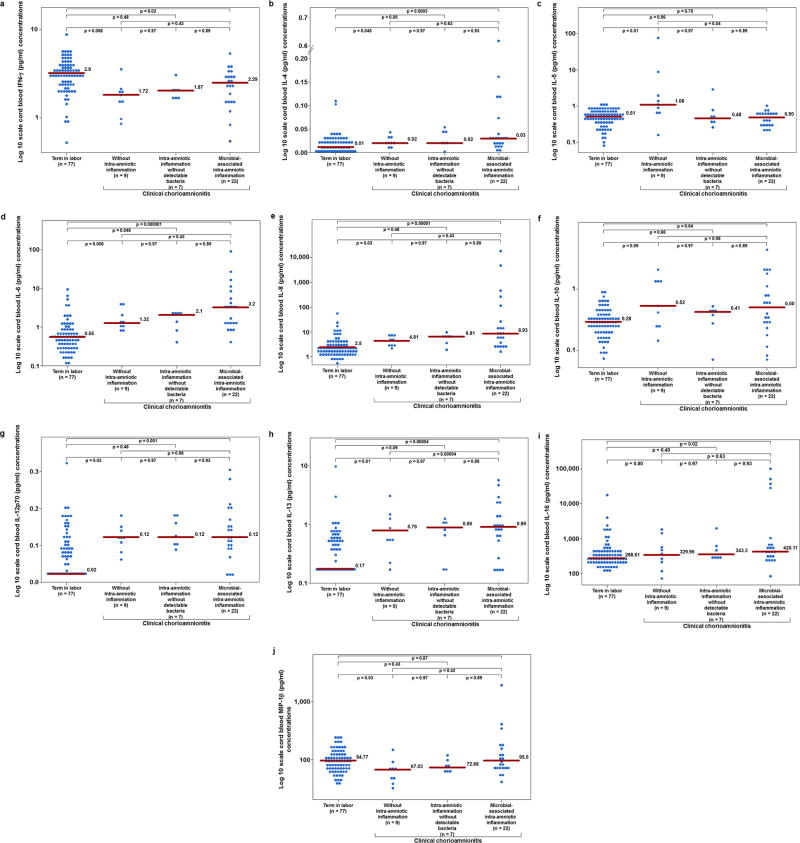
Figure 1.
The umbilical cord plasma concentrations of cytokines and chemokines in patients at term in labor (controls) (n=77), with clinical chorioamnionitis without intra-amniotic inflammation (n=9), with clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria (n=7), and with clinical chorioamnionitis with microbial-associated intra-amniotic inflammation (n=22).
a: The median umbilical cord plasma concentrations of interferon (IFN)-γ are 2.9 pg/mL (term in labor), 1.72 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 1.87 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 2.29 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
b: The median umbilical cord plasma concentrations of interleukin (IL)-4 are 0.01 pg/mL (term in labor), 0.02 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 0.02 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 0.03 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
c: The median umbilical cord plasma concentrations of interleukin (IL)-5 are 0.51 pg/mL (term in labor), 1.08 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 0.48 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 0.50 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
d: The median umbilical cord plasma concentrations of interleukin (IL)-6 are 0.55 pg/mL (term in labor), 1.32 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 2.1 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 3.2 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
e: The median umbilical cord plasma concentrations of interleukin (IL)-8 are 2.5 pg/mL (term in labor), 4.81 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 6.81 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 8.93 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
f: The median umbilical cord plasma concentrations of interleukin (IL)-10 are 0.28 pg/mL (term in labor), 0.52 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 0.41 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 0.50 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
g: The median umbilical cord plasma concentrations of interleukin (IL)-12p70 are 0.02 pg/mL (term in labor), 0.12 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 0.12 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 0.12 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
h: The median umbilical cord plasma concentrations of interleukin (IL)-13 are 0.17 pg/mL (term in labor), 0.79 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 0.86 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 0.89 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
i: The median umbilical cord plasma concentrations of interleukin (IL)-16 are 288.61 pg/mL (term in labor), 329.96 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 343.3 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 420.11 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
j: The median umbilical cord plasma concentrations of macrophage inflammatory protein (MIP)-1β are 94.77 pg/mL (term in labor), 67.03 pg/mL (clinical chorioamnionitis without intra-amniotic inflammation), 72.68 pg/mL (clinical chorioamnionitis with intra-amniotic inflammation without detectable bacteria), and 95.5 pg/mL (clinical chorioamnionitis with microbial-associated intra-amniotic inflammation).
Table 3.
Umbilcal cord plasma cytokine and chemokine concentrations in the subgroups of clinical chorioamnionitis and term in labor
| Analytes (pg/mL) |
Term in labor (Controls) (n=77) |
Clinical chorioamnnionitis at term (n=38) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without intra-amniotic inflammation (n=9) |
With intra-amniotic inflammation without detectable microorganisms (n=7) |
With microbial-associated intra-amniotic inflammation (n=22) |
|||||||||||
| Median (IQR) |
Median (IQR) |
Fold change (compa red to term in labor) |
Adjusted P- value*(co mpared to term in labor) |
Median (IQR) | Fold change (compare d to term in labor) |
Adjusted P- value (compared to term in labor) |
Adjusted P- value (compared to without intra- amniotic inflammation) |
Median (IQR) |
Fold change (compa red to term in labor) |
Adjusted P- value (compared to term in labor) |
Adjusted P-value (compared to without intra- amniotic inflammati on) |
Adjusted P- value (compared to with intra- amniotic inflammation without demonstrable microorganis ms) |
|
| Pro-inflammatory cytokines | |||||||||||||
| IL-12p70 | 0.02 (0.02–0.1) | 0.12 (0.11–0.14) | 6.00 | 0.03 | 0.12 (0.1–0.17) | 6.00 | 0.048 | 0.97 | 0.12 (0.09–0.17) | 6.00 | 0.001 | 0.98 | 0.93 |
| IL-6 | 0.55 (0.32–0.82) | 1.32 (1.12–2.09) | 2.40 | 0.008 | 2.1 (1.17–2.25) | 3.82 | 0.048 | 0.97 | 3.2 (1.33–5.37) | 5.81 | <0.00001 | 0.43 | 0.89 |
| IFN-γ | 2.9 (2.26–3.65) | 1.72 (1.37–1.81) | 0.59 | 0.008 | 1.87 (1.62–1.95) | 0.64 | 0.048 | 0.97 | 2.29 (1.48–2.73) | 0.79 | 0.03 | 0.43 | 0.89 |
| IL-16 | 288.61 (223.88–406.54) | 329.96 (232.68–517.24) | 1.14 | 0.80 | 343.3 (313.84–503.11) | 1.19 | 0.40 | 0.97 | 420.11 (304.97–637.82) | 1.46 | 0.02 | 0.63 | 0.93 |
| TNF-α | 2.78 (2.39–3.14) | 3.06 (1.8–3.33) | 1.10 | 0.94 | 2.72 (2.7–3.3) | 0.98 | 0.76 | 0.97 | 2.91 (2.45–3.19) | 1.04 | 0.73 | 0.98 | 0.89 |
| IL-2 | 0.06 (0.01–0.14) | 0.16 (0.11–0.28) | 2.67 | 0.07 | 0.14 (0.06–0.15) | 2.33 | 0.76 | 0.97 | 0.1 (0.01–0.15) | 1.58 | 0.73 | 0.43 | 0.89 |
| IL-15 | 1.23 (0.92–1.52) | 2.48 (1.34–2.74) | 2.02 | 0.08 | 1.49 (0.92–2.18) | 1.21 | 0.70 | 0.97 | 1.4 (0.8–2.37) | 1.14 | 0.41 | 0.83 | 0.93 |
| IL-17α | 1.32 (1.07–1.71) | 1.34 (0.82–1.45) | 1.02 | 0.45 | 1.27 (1.16–1.54) | 0.96 | 0.96 | 0.97 | 1.43 (0.93–1.9) | 1.08 | 0.73 | 0.63 | 0.89 |
| TNF-β | 0.23 (0.16–0.33) | 0.17 (0.15–0.21) | 0.74 | 0.12 | 0.14 (0.13–0.21) | 0.61 | 0.40 | 0.97 | 0.18 (0.12–0.31) | 0.78 | 0.38 | 0.98 | 0.93 |
| IL-12/IL-23p40 | 645.63 (537.97–815.11) | 515.93 (474.91–782.58) | 0.80 | 0.53 | 627.35 (583.53–965.06) | 0.97 | 0.76 | 0.97 | 651.52 (336.43–933.05) | 1.01 | 0.73 | 0.98 | 0.89 |
| IL-1α | 0.92 (0.73–1.15) | 0.78 (0.7–0.88) | 0.85 | 0.50 | 0.83 (0.73–0.94) | 0.9 | 0.76 | 0.97 | 0.89 (0.7–1.22) | 0.97 | 1.00 | 0.65 | 0.89 |
| GM-CSF | 0.15 (0.09–0.22) | 0.02 (0.02–0.23) | 0.13 | 0.50 | 0.04 (0.02–0.11) | 0.27 | 0.31 | 1.00 | 0.08 (0.02–0.28) | 0.50 | 0.66 | 0.87 | 0.89 |
| IL-7 | 1.24 (0.73–2.21) | 0.82 (0.73–1.39) | 0.66 | 0.50 | 1.12 (0.72–2.58) | 0.9 | 0.89 | 0.97 | 1.35 (0.81–8.13) | 1.09 | 0.50 | 0.43 | 0.89 |
| IL-1β | 0.38 (0.18–0.79) | 0.14 (0.06–0.38) | 0.37 | 0.12 | 0.22 (0.19–0.76) | 0.58 | 0.89 | 0.97 | 0.59 (0.15–2.76) | 1.55 | 0.41 | 0.39 | 0.89 |
| VEGF | 45.16 (18.52–103.61) | 45.4 (14.01–59.87) | 1.00 | 0.80 | 30.72 (22.41–63.07) | 0.68 | 0.89 | 1.00 | 57.93 (28.19–138.75) | 1.28 | 0.47 | 0.63 | 0.89 |
| Anti-inflammatory cytokines | |||||||||||||
| IL-13 | 0.17 (0.17–0.54) | 0.79 (0.54–1.22) | 4.69 | 0.01 | 0.86 (0.41–1.05) | 5.11 | 0.09 | 0.97 | 0.89 (0.6–1.48) | 5.26 | 0.00004 | 0.89 | 0.89 |
| IL-4 | 0.01 (0–0.02) | 0.02 (0.02–0.03) | 2.00 | 0.045 | 0.02 (0.02–0.04) | 2 | 0.09 | 0.97 | 0.03 (0.02–0.04) | 3 | 0.0003 | 0.63 | 0.93 |
| IL-5 | 0.51 (0.37–0.65) | 1.08 (0.72–1.89) | 2.12 | 0.01 | 0.48 (0.39–0.64) | 0.94 | 0.96 | 0.97 | 0.50 (0.31–0.65) | 0.97 | 0.75 | 0.04 | 0.89 |
| IL-10 | 0.28 (0.2–0.38) | 0.52 (0.25–1.38) | 1.86 | 0.09 | 0.41 (0.32–0.44) | 1.46 | 0.66 | 0.97 | 0.50 (0.27–1.04) | 1.77 | 0.04 | 0.98 | 0.89 |
| Chemokines | |||||||||||||
| IL-8 | 2.50 (1.63–3.93) | 4.81 (3.82–6.56) | 1.92 | 0.03 | 6.81 (5.16–7.19) | 2.72 | 0.048 | 0.97 | 8.93 (3.57–26.09 | 3.57 | 0.00001 | 0.43 | 0.89 |
| MIP-1β | 94.77 (73.29–120.46) | 67.03 (46.18–70.18) | 0.71 | 0.03 | 72.68 (62.24–89.07) | 0.77 | 0.43 | 0.97 | 95.5 (68.24–141.3) | 1.01 | 0.87 | 0.20 | 0.89 |
| CXCL-10 (IP-10) | 84.82 (66.27–114.91) | 107.04 (37.79–141.01) | 1.26 | 0.99 | 117.05 (78.85–135.4) | 1.38 | 0.66 | 0.97 | 98.99 (70.17–155.14) | 1.17 | 0.41 | 0.63 | 0.93 |
| MCP-1 | 60 (44.78–67.54) | 59.63 (33.22–79.21) | 0.99 | 0.64 | 53.25 (51.52–62.79) | 0.89 | 0.83 | 1.00 | 61.62 (50.68–127.63) | 1.03 | 0.41 | 0.43 | 0.89 |
| Eotaxin-3 | 17.8 (12.25–22.92) | 12.57 (10.95–22.5) | 0.71 | 0.53 | 19.5 (16.12–23.37) | 1.1 | 0.89 | 0.97 | 13.42 (4.25–31.77) | 0.75 | 0.66 | 0.98 | 0.89 |
| MIP-1α | 11.97 (9.83–14.37) | 11.59 (9.84–13.34) | 0.97 | 0.80 | 10.84 (9.51–12.34) | 0.91 | 0.76 | 0.97 | 12.08 (10.23–13.97) | 1.01 | 0.67 | 0.77 | 0.89 |
| TARC | 98.97 (64.29–194.61) | 164.06 (75.37–212) | 1.66 | 0.53 | 196.14 (100.6–283.75) | 1.98 | 0.55 | 0.97 | 108.64 (68.33–419.86) | 1.1 | 0.41 | 0.98 | 0.89 |
| MDC | 1124.23 (931.33–1311.54) | 959.09 (864.9–1227.79) | 0.85 | 0.53 | 1156.54 (953.28–1262.82) | 1.03 | 0.96 | 0.97 | 981.61 (834.1–1397.83) | 0.87 | 0.41 | 0.98 | 0.89 |
| MCP-4 | 51.94 (42.32–73.82) | 29.51 (27.53–35.29) | 0.57 | 0.11 | 61.01 (24.07–76.94) | 1.17 | 0.9 | 1.00 | 57.87 (42.57–99.73) | 1.11 | 0.56 | 0.43 | 0.89 |
| Eotaxin | 64.73 (48.03–81.72) | 61.54 (50.91–69.26) | 0.95 | 0.53 | 52.82 (46.66–69.6) | 0.82 | 0.76 | 0.97 | 56.49 (37.92–78.75) | 0.87 | 0.41 | 0.98 | 0.93 |
IL-16 has pro- and anti-inflammatory properties. The units of all analytes are pg/mL. Values in bold font indicate significant results.
CXCL-10: C-X-C motif chemokine 10; GM-CSF: granulocyte macrophage colony-stimulating factor; IFN-γ: interferon gamma; IL: interleukin; IP-10: interferon gamma-induced protein 10; IQR: interqurtile range; MCP: monocyte chemoattractant protein; MDC: macrophage-derived chemokine; MIP: macrophage inflammatory protein; TARC: thymus and activation-regulated chemokine; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
Clinical chorioamnionitis with intra-amniotic inflammation without demonstrable bacteria
Neonates born to mothers with clinical chorioamnionitis at term and intra-amniotic inflammation (amniotic fluid IL-6 concentration ≥2.6 ng/mL) without detectable microorganisms had significantly higher median umbilical cord plasma concentrations of IL-12p70 (fold change 6), IL-6 (fold change 3.8), and IL-8 (fold change 2.7) but lower IFN-γ concentrations than neonates not exposed to clinical chorioamnionitis (Table 3).
Clinical chorioamnionitis with microbial-associated intra-amniotic inflammation
Neonates born to mothers with intra-amniotic infection had significantly higher median umbilical cord plasma concentrations of IL-12p70, IL-6, IL-16, IL-13, IL-4, IL-10, and IL-8 than neonates not exposed to clinical chorioamnionitis. The fold change ranged from 1.46 to 6 (Table 3).
Clinical chorioamnionitis with and without FIRS
All neonates with FIRS were born to mothers who had microbial-associated intra-amniotic inflammation or intra-amniotic infection. The median umbilical cord plasma concentrations of IL-6, IL-8, IL-10, and MCP-1 were significantly higher in neonates with FIRS than in those without FIRS, with fold-change differences in the median that ranged from 2.02 to 6.69 (Table 4 and Figure 2). Umbilical cord plasma IL-6 and IL-8 had the highest fold-change differences in the median [6.69 and 4.33, respectively (Table 4)].
Table 4.
Umbilcal cord plasma cytokine and chemokine concentrations in neonates with FIRS and without FIRS born to mothers with clinical chorioamnionitis at term
| Analytes (pg/mL) |
Neonates Born without FIRS Median (IQR) (n=30) |
Neonates Born with FIRS Median (IQR) (n=8) |
Fold change | Adjusted P value |
|---|---|---|---|---|
| Pro-inflammatory cytokines | ||||
| IL-6 | 1.46 (1.12–2.27) | 9.77 (5.37–20.49) | 6.69 | 0.002 |
| IL-12p70 | 0.12 (0.1–0.15) | 0.14 (0.1–0.17) | 1.17 | 0.76 |
| IL-16* | 394.71 (299.01–620.64) | 435.23 (266.18–9422.55) | 1.1 | 0.96 |
| IFN-γ | 1.78 (1.47–2.35) | 2.61 (2.03–2.93) | 1.47 | 0.38 |
| TNF-β | 0.15 (0.12–0.23) | 0.26 (0.18–0.32) | 1.7 | 0.38 |
| IL-1β | 0.21 (0.09–0.64) | 1.35 (0.49–7.88) | 6.43 | 0.15 |
| IL-17α | 1.35 (1.07–1.74) | 1.36 (0.81–1.8) | 1.004 | 0.96 |
| TNF-α | 2.82 (2.44–3.29) | 2.97 (2.58–3.28) | 1.05 | 0.82 |
| IL-2 | 0.1 (0.01–0.16) | 0.18 (0.11–0.26) | 1.8 | 0.38 |
| IL-12/IL-23p40 | 610.32 (502.52–813.43) | 677.61 (285.39–901.52) | 1.11 | 0.96 |
| IL-15 | 1.4 (0.87–2.53) | 2 (1.24–6.76) | 1.43 | 0.51 |
| IL-1α | 0.84 (0.7–1.11) | 0.85 (0.54–1.52) | 1.01 | 0.96 |
| GM-CSF | 0.02 (0.02–0.2) | 0.15 (0.04–0.36) | 7.58 | 0.38 |
| IL-7 | 1.1 (0.73–1.59) | 6.98 (0.96–16.33) | 6.34 | 0.38 |
| VEGF | 43.03 (18.97–66.87) | 87.71 (25.05–454.06) | 2.04 | 0.46 |
| Anti-inflammatory cytokines | ||||
| IL-10 | 0.37 (0.24–0.6) | 1.47 (0.66–2.1) | 3.96 | 0.04 |
| IL-13 | 0.79 (0.28–1.01) | 1.47 (0.78–3.39) | 1.85 | 0.33 |
| IL-4 | 0.02 (0.02–0.03) | 0.03 (0.03–0.08) | 1.5 | 0.38 |
| IL-5 | 0.57 (0.33–0.89) | 0.52 (0.36–0.66) | 0.9 | 0.68 |
| Chemokines | ||||
| IL-8 | 5.02 (3.22–8.51) | 21.76 (11.02–1283.3) | 4.33 | 0.02 |
| MCP-1 | 53.92 (44.95–61.82) | 108.77 (72.23–172.57) | 2.02 | 0.047 |
| MIP-1α | 11.22 (9.84–13.32) | 13.34 (11.73–17.32) | 1.19 | 0.37 |
| Eotaxin | 56.13 (44.74–69.38) | 53.86 (32.5–81.94) | 0.96 | 0.96 |
| MCP-4 | 43.25 (27.53–72.92) | 90.88 (59.82–236.68) | 2.1 | 0.38 |
| MDC | 989.07 (864.9–1227.79) | 979.36 (764.73–1606.51) | 0.99 | 0.96 |
| Eotaxin-3 | 14.11 (10.95–25.39) | 13.33 (4.17–133.17) | 0.94 | 0.96 |
| CXCL-10 (IP-10) | 107.04 (65.12–150.1) | 116.8 (72.7–465.04) | 1.09 | 0.43 |
| TARC | 108.32 (68.8–212) | 373.74 (99.85–637.92) | 3.45 | 0.43 |
| MIP-1β | 70.18 (61.17–98.05) | 111.01 (70.43–200.1) | 1.58 | 0.33 |
IL-16 has pro- and anti-inflammatory properties. The units of all analytes are pg/mL. Values in bold font indicate significant results.
CXCL-10: C-X-C motif chemokine 10; FIRS: fetal inflammatory response syndrome; GM-CSF: granulocyte macrophage colony-stimulating factor; IFN-γ: interferon gamma; IL: interleukin; IP-10: interferon gamma-induced protein 10; IQR: interquartile range; MCP: monocyte chemoattractant protein; MDC: macrophage-derived chemokine; MIP: macrophage inflammatory protein; TARC: thymus and activation-regulated chemokine; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
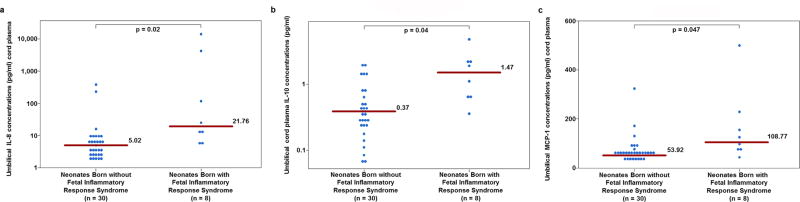
Figure 2.
The umbilical cord plasma concentrations of cytokines and chemokines in neonates born with (n=8) and without (n=30) fetal inflammatory response syndrome.
a: The median umbilical cord plasma concentrations of interleukin (IL)-8 are 5.02 pg/mL in neonates born without fetal inflammatory response syndrome and 21.76 pg/mL in neonates born with fetal inflammatory response syndrome.
b: The median umbilical cord plasma concentrations of interleukin (IL)-10 are 0.37 pg/mL in neonates born without fetal inflammatory response syndrome and 1.47 pg/mL in neonates born with fetal inflammatory response syndrome.
c: The median umbilical cord plasma concentrations of monocyte chemoattractant protein (MCP)-1 are 53.92 pg/mL in neonates born without fetal inflammatory response syndrome and 108.77 pg/mL in neonates born with fetal inflammatory response syndrome.
The relationship between the concentrations of umbilical cord and maternal plasma IL-6
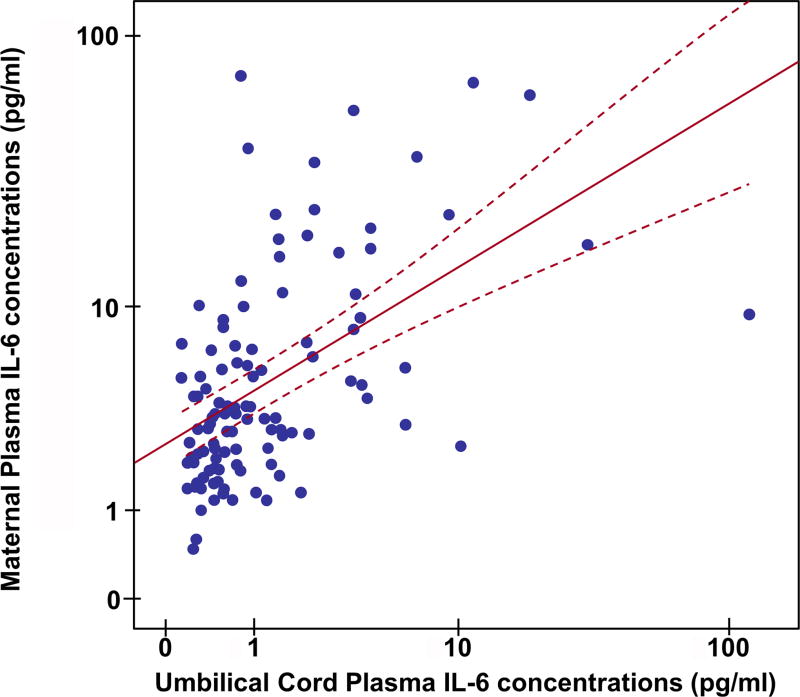
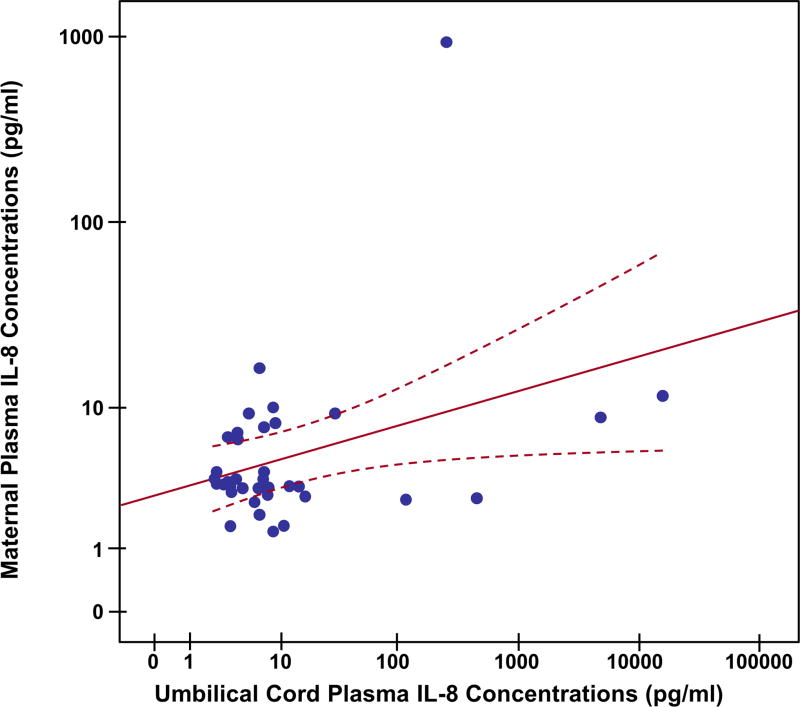
IL-6, a major cytokine involved in the host response against infection and tissue injury, is believed to cross the placenta [116]; therefore, we examined the relationship of IL-6 between the maternal and umbilical cord circulations. Median (IQR) IL-6 concentrations were significantly higher in maternal plasma than in umbilical cord plasma for both cases and controls [control: maternal plasma: 2.53 (1.64–4.08) pg/mL vs. umbilical cord plasma: 0.55 (0.32–0.82) pg/mL; p ≤ 0.0001; cases: maternal plasma: 10.94 (5.36–22.08) pg/mL vs. umbilical cord plasma: 1.92 (1.16–3.55) pg/mL; p ≤ 0.001]. There was a positive correlation between maternal and umbilical cord plasma IL-6 and IL-8 concentrations (IL-6: Spearman’s correlation=0.53; p<0.001; IL-8: Spearman’s correlation=0.42; p<0.001) (Figures 3 and 4).
Figure 3.
Umbilical cord and maternal plasma interleukin (IL)-6 scatter diagram with linear regression line (red line). Dashed line indicates the confidence interval of mean. There is a positive correlation between maternal and umbilical cord plasma IL-6 concentrations (Spearman’s correlation=0.53; p<0.001).
Figure 4.
Umbilical cord and maternal plasma interleukin (IL)-8 scatter diagram with linear regression line (red line). Dashed line indicates the confidence interval of mean. There is a positive correlation between maternal and umbilical cord plasma IL-8 concentrations (Spearman’s correlation=0.42; p<0.001).
The supplementary material contains scatterplots of maternal plasma concentrations of cytokines among different groups (Supplementary Figures 1–5).
Discussion
Principal findings of the study
1) Neonates born to mothers with clinical chorioamnionitis at term (considered in toto) had significantly higher median umbilical cord plasma concentrations of IL-6, IL-12p70, IL-16, IL-13, IL-4, IL-10, and IL-8, but significantly lower IFN-γ and TNF-α concentrations than neonates born to mothers with spontaneous term labor without clinical chorioamnionitis; 2) neonates born to mothers with clinical chorioamnionitis but without intra-amniotic inflammation had higher concentrations of IL-6, IL-12p70, IL-13, IL-4, IL-5, and IL-8, but lower IFN-γ, than neonates not exposed to clinical chorioamnionitis, suggesting that maternal fever in the absence of intra-amniotic inflammation leads to a change in the fetal cytokine network; 3) there were significant, positive correlations between maternal and umbilical cord plasma IL-6 and IL-8 concentrations (IL-6: Spearman’s correlation=0.53; p<0.001; IL-8: Spearman’s correlation=0.42; p<0.001), consistent with the placental transfer of cytokines; 4) an elevated fetal plasma IL-6 (FIRS; IL-6 > 11 pg/mL), the diagnostic criterion for FIRS, was present in 21% (8/38) of patients with clinical chorioamnionitis at term, and all of these neonates were born to mothers with proven intra-amniotic infection; and 5) FIRS was associated with a high concentration of umbilical cord plasma IL-8, IL-10, and MCP-1.
Maternal systemic inflammation: an underappreciated cause of developmental disorders
Normal pregnancy is a state of physiologic intravascular inflammation in which there is activation of the innate immune system and suppression of the adaptive immune response to paternal antigens [117–123]. The latter is thought to promote a tolerogenic state favoring the survival of the placental and fetal semi-allograft [121, 124–132]. Activation of the innate limb of the immune response is thought to protect mother and fetus against infection [118]. Infection during pregnancy is known to lead to an exaggerated systemic intravascular inflammatory response with increased concentrations of cytokines [118, 133, 134]. This occurs in the context of both bacterial and viral infections. It is now clear that mothers who have infections during pregnancy are at increased risk for maternal death [135–138]. This has been the case in the pandemics of influenza [139–149] and, more recently, Ebola [150–153] and has been attributed to a cytokine storm that occurs when pregnant women are exposed to microorganisms [154–156].
Maternal inflammation during pregnancy can also have an effect on fetal brain development [51–89, 92, 157]. A solid body of evidence now indicates that infants of mothers who experienced viral infections during pregnancy are at increased risk of both schizophrenia [55, 56, 58, 60–64, 66, 67, 69–72, 74, 76–78, 80, 85, 92] and autism spectrum disorders [52, 73, 74, 77, 79–83, 85–87, 92, 93]. Systemic infection is thought to induce the production of cytokines, in particular, IL-6, which has direct effects on the placenta by inducing the activation of Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3) [158]. IL-6 can cross the placenta [116, 158–161], have direct effects in multiple target organs of the fetus, including the brain [73, 158, 162, 163], and can induce microglial activation, astrogliosis, and synaptic pruning [164–166]. This is thought to be the basis for the predisposition to schizophrenia and autism. Even short-lived fever, such as intrapartum fever or acute histologic chorioamnionitis, has been associated with an increased risk for cerebral palsy at the age of three in both term and preterm neonates [167]. The mechanism whereby intrapartum fever leads to the development of brain injury [31, 40, 41, 43, 45, 101, 167–181] during labor has been a subject of recent investigation. Fever is mediated by pyrogenic cytokines [182–184] (i.e. IL-1 [184–192], IL-2 [193–195], IL-6 [196–200], and TNF (195, 201–204]) and can induce the production of multiple other cytokines/chemokines as well as generate a strong pro-inflammatory state. The effects of maternal systemic inflammation in the human fetal immune system have not been adequately studied. Clinical chorioamnionitis at term represents a unique model to examine the effect of maternal systemic inflammation in the presence or absence of intra-amniotic inflammation with or without bacteria. This study aimed to examine the effect of such maternal systemic inflammatory response on the fetal inflammatory response gauged by the concentrations of circulating fetal cytokines.
The human fetal cytokine profile after exposure to maternal systemic inflammation
The clinical signs of chorioamnionitis at term represent evidence of a systemic maternal inflammatory response [1]. In this study, IL-12p70 and IL-13 had a fold-change difference in the median of greater than 5. IL-12p70 is mainly produced by dendritic cells [205–217], monocytes [205, 211–214, 216–218], macrophages [205, 211, 212, 214–218], and neutrophils [213, 219–221]. This pro-inflammatory cytokine can induce the production of IFN-γ [207, 212, 214, 215, 222–228]. An elevation of circulating IL-12p70 has been reported in patients with sepsis [229–231], and preterm delivery < 35 weeks with severe histologic chorioamnionitis [228]. Our findings are consistent with these results.
IL-13 is produced by activated Th2 cells [232–234], mast cells [233, 235], natural killer cells [233, 236], dendritic cells [237], and alveolar macrophages [238, 239]. This cytokine has anti-inflammatory properties that are thought to result from downregulation of the expression of pro-inflammatory cytokines such as IL-1α, IL-1β, TNF-α, IFN-γ, IL-6, IL-8, IL-10, and IL-12 [232, 233, 239–242]. In murine models of sepsis, IL-13 reduces the inflammatory response by suppression of pro-inflammatory cytokines and chemokines in tissues such as the liver, lung, and kidney [239, 242–246]. In addition, an increase in circulating IL-13 concentrations is associated with sepsis in both adults [234, 247] and children [248, 249]. Therefore, it is not surprising that clinical chorioamnionitis at term is associated with a significant elevation in the umbilical cord plasma concentrations of IL-12p70 and IL-13.
Several studies have demonstrated the potential value of umbilical cord plasma concentrations of IL-6 [105, 250–277] and IL-8 [257, 260–262, 267, 268, 276, 278, 279] for the identification of intra-amniotic infection and early-onset neonatal sepsis. Our findings are consistent with those of Lencki et al, who reported that neonates born to mothers with clinical chorioamnionitis had higher umbilical cord plasma concentrations of IL-6, but not IL-1β and soluble IL-2 receptor, than those without this condition [280].
An increase in the concentrations of umbilical cord anti-inflammatory cytokines may reflect a host response mounted to counteract the effect of pro-inflammatory cytokines [281–283]. Collectively, clinical chorioamnionitis at term is associated with upregulation of several umbilical cord plasma pro- and anti-inflammatory cytokine/chemokine concentrations.
The fetal systemic cytokine profile after exposure to maternal systemic inflammation in the absence of intra-amniotic inflammation
An important finding of this study is that fetal exposure to a maternal systemic inflammatory response in the absence of intra-amniotic inflammation is associated with an elevation of multiple cytokines, consistent with a pro-inflammatory state in the human fetus. The precise mechanisms responsible for this observation remain to be elucidated. IL-6 can cross the placenta [116, 159, 161, 284] as well as induce an acute phase response in the fetus [104, 105, 111, 113, 285]; therefore, it is possible that some of the findings reported herein occur in response to the transplacental passage of cytokines and chemokines. However, some cytokines can exert a direct effect in the placenta and modulate its inflammatory response [158]. In mice, maternal immune activation via viral mimic (double-stranded RNA) induces the production of IL-6 in the placenta [158]. Maternally derived IL-6 can engage the JAK/STAT3 pathway, which results in the upregulation of acute phase proteins, such as suppressor of cytokine signaling 3 (SOCS-3), and in the downregulation of placental growth hormone production [158]. These changes in endocrine factors could have effects on fetal development (including neurodevelopment). For example, we have reported changes in fetal plasma cortisol in patients with fetal systemic inflammation [286], and similar observations have been reported by Gravett et al. in the Rhesus monkey model of intrauterine infection [287, 288]. The effects of glucocorticoids in fetal programming of multiple organ systems (including the brain) are well-established [289–297]. Interestingly, maternal immune activation induces long-term changes in the composition of the gut microbiota and subsequent alteration in the gastrointestinal physiology of the offspring [298–302].
Collectively, our findings, along with the observations from aforementioned experimental studies, support the view of maternal immune activation (even without intra-amniotic inflammation) and its subsequent effects on fetal immune development.
The fetal cytokine profile in the context of intra-amniotic inflammation with or without bacteria
Clinical chorioamnionitis with intra-amniotic inflammation with or without detectable bacteria in the amniotic cavity is characterized by elevated concentrations of several cytokines in the amniotic fluid [3] and maternal circulation [1]. In this study, a fetal systemic inflammatory response, reflected by changes in cytokine concentrations, was also observed. The main cytokines upregulated in the circulation of fetuses exposed to intra-amniotic inflammation were IL-12p70, IL-6, IL-13, IL-4, and IL-8. Umbilical cord plasma IL-6 and IL-8 concentrations were higher in neonates born to mothers with intra-amniotic inflammation with or without detectable bacteria than in neonates of mothers without clinical chorioamnionitis. Moreover, we and others have reported the occurrence of a fetal systemic inflammatory response in preterm labor with intact membranes [104, 113, 285, 303] as well as preterm PROM [108, 304].
Umbilical cord plasma cytokines could be of maternal, placental, or fetal origin [305]. For example, IL-6 has been identified in neonatal blood mononuclear cells [254], trophoblasts [306, 307], and/or decidual cells[308, 309] as well as maternal cells that have crossed the placenta [310]. The observation that IL-6 concentrations are higher in the umbilical artery than in the umbilical vein is consistent with fetal production of IL-6 [105, 255, 256]. The long-term consequences of early exposure of the human fetus to systemic inflammation need to be explored.
The cytokine profile of neonates born with a fetal systemic inflammatory response syndrome
In this study, all neonates with FIRS were born to mothers with microbial-associated intra-amniotic inflammation (intra-amniotic infection). Umbilical cord plasma IL-8, IL-10, and MCP-1 concentrations were significantly associated with FIRS. Our findings are consistent with those of Mestan et al, who used multiplex immunoassays and reported that, among the 27 cord blood biomarkers examined, the concentrations of IL-1β, IL-6, and IL-8 were associated with the presence of funisitis (the magnitude of association was stronger for IL-6 and IL-8 than for IL-1β) [268].
It is well-established that FIRS is associated with the impending onset of labor [303], multi-systemic involvement and high risk of short- and long-term complications [104–107, 260, 276, 311–321]. This condition is defined by an elevation of the umbilical cord plasma concentration of IL-6 [104–113, 322], although changes in other cytokine concentrations, such as IL-10 [321, 323], granulocyte-colony-stimulating factor [111], IL-1β [313], soluble TNF receptors-1 and −2 [324, 325], TNF-α [313], IL-8 [107], IL-19 [326], and CRP [317], have been reported. An exaggerated and uncontrolled inflammatory response may be detrimental to the fetus by leading to multiple organ involvement including the skin [327–329], heart [304, 330, 331], lung [332–339], eyes [340], kidneys [341], adrenal glands [286], hematologic system [110, 111, 342], thymus [343–345], and central nervous system [167, 313, 314, 318, 346–355]. Reports from the laboratories of Newnham and Jobe have demonstrated that these changes can be experimentally produced after exposure to endotoxin [336, 356–361]. Altogether, these data suggest that neonates born to mothers with intra-amniotic infection or inflammation in the context of clinical chorioamnionitis at term are at increased risk for FIRS.
Strengths and limitations
The major strength of this study is that, by assessing the state of inflammation of the amniotic cavity, we could study the effect of maternal systemic inflammation in the presence or absence of intra-amniotic inflammation. We used both cultivation and molecular microbiologic techniques to identify microorganisms in the amniotic cavity; therefore, the diagnosis of microbial invasion is based on state-of-the-art techniques. Limitations are related to the sample size in the three subgroups of patients with clinical chorioamnionitis at term. However, this is the only study which examines umbilical cord cytokines in term gestations with chorioamnionitis in reference to the microbial and inflammatory state of the amniotic cavity.
Conclusions
Neonates born to mothers with clinical chorioamnionitis at term had higher concentrations of umbilical cord plasma cytokines than those not exposed to clinical chorioamnionitis. Even neonates of mothers with clinical chorioamnionitis without intra-amniotic inflammation had elevated concentrations of multiple cytokines, suggesting that intrapartum fever alters the fetal immune response. In addition, intra-amniotic infection is associated with the presence of FIRS. Umbilical cord plasma IL-6, IL-8, IL-10, and MCP-1 are the major cytokines involved in FIRS in the context of clinical chorioamnionitis at term. The observations reported herein provide insight into the fetal immune response in patients with clinical chorioamnionitis at term.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
References
- 1.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44:77–98. doi: 10.1515/jpm-2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 5.MacVicar J. Chorioamnionitis. Clin Obstet Gynecol. 1970;13:272–90. doi: 10.1097/00003081-197006000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol. 1977;1:71–7. [PubMed] [Google Scholar]
- 7.Hollander D. Diagnosis of chorioamnionitis. Clin Obstet Gynecol. 1986;29:816–25. doi: 10.1097/00003081-198612000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–8. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 993(36):795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman SG, Gelber SE. Evidence for the clinical management of chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:46–50. doi: 10.1016/j.siny.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44:23–32. doi: 10.1515/jpm-2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoder PR, Gibbs RS, Blanco JD, Castaneda YS, St Clair PJ. A prospective, controlled study of maternal and perinatal outcome after intra-amniotic infection at term. Am J Obstet Gynecol. 1983;145:695–701. doi: 10.1016/0002-9378(83)90575-6. [DOI] [PubMed] [Google Scholar]
- 16.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87:188–94. doi: 10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 17.Ladfors L, Tessin I, Mattsson LA, Eriksson M, Seeberg S, Fall O. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34-42 weeks. J Perinat Med. 1998;26:94–101. doi: 10.1515/jpme.1998.26.2.94. [DOI] [PubMed] [Google Scholar]
- 18.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103:e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 19.Rouse DJ, Landon M, Leveno KJ, Leindecker S, Varner MW, Caritis SN, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191:211–6. doi: 10.1016/j.ajog.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Volante E, Moretti S, Pisani F, Bevilacqua G. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med. 2004;16(Suppl 2):13–6. doi: 10.1080/14767050410001727116. [DOI] [PubMed] [Google Scholar]
- 21.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200(372):e1–6. doi: 10.1016/j.ajog.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 23.de Sam Lazaro S, Cheng Y, Snowden J, Pereira L, Aziz N, Caughey A. Does the neonatal impact of chorioamnionitis in the setting of PPROM vary depending on degree of prematurity? J Obstet Gynecol. 2013;208:S314. [Google Scholar]
- 24.Uyemura A, Ameel B, Caughey A. Outcomes of chorioamnionitis in term pregnancies. Am J Obstet Gynecol. 2014;210:S215. [Google Scholar]
- 25.Garcia-Munoz Rodrigo F, Galan Henriquez GM, Ospina CG. Morbidity and mortality among very-low-birth-weight infants born to mothers with clinical chorioamnionitis. Pediatr Neonatol. 2014;55:381–6. doi: 10.1016/j.pedneo.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991;164:859–62. doi: 10.1016/0002-9378(91)90529-z. [DOI] [PubMed] [Google Scholar]
- 27.Wen TS, Eriksen NL, Blanco JD, Graham JM, Oshiro BT, Prieto JA. Association of clinical intra-amniotic infection and meconium. Am J Perinatol. 1993;10:438–40. doi: 10.1055/s-2007-994625. [DOI] [PubMed] [Google Scholar]
- 28.Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome. An update. Pediatr Clin North Am. 1998;45:511–29. doi: 10.1016/s0031-3955(05)70025-0. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Bacteria and endotoxin in meconium-stained amniotic fluid at term: could intra-amniotic infection cause meconium passage? J Matern Fetal Neonatal Med. 2014;27:775–88. doi: 10.3109/14767058.2013.844124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Secreted phospholipase A2 is increased in meconium-stained amniotic fluid of term gestations: potential implications for the genesis of meconium aspiration syndrome. J Matern Fetal Neonatal Med. 2014;27:975–83. doi: 10.3109/14767058.2013.847918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson SJ, Alessandri LM, Badawi N, Burton PR, Pemberton PJ, Stanley F. Predictors of neonatal encephalopathy in full-term infants. Br Med J. 1995;311:598–602. doi: 10.1136/bmj.311.7005.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willoughby RE, Jr, Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol. 2002;29:603–21. doi: 10.1016/s0095-5108(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg H, Wennerholm UB, Savman K. Sequelae of chorioamnionitis. Curr Opin Infect Dis. 2002;15:301–6. doi: 10.1097/00001432-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Cooke R. Chorioamnionitis, maternal fever, and neonatal encephalopathy. Dev Med Child Neurol. 2008;50:9. doi: 10.1111/j.1469-8749.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 35.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol. 2008;50:19–24. doi: 10.1111/j.1469-8749.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 36.Versland LB, Sommerfelt K, Elgen I. Maternal signs of chorioamnionitis: persistent cognitive impairment in low-birthweight children. Acta Paediatr. 2006;95:231–5. doi: 10.1080/08035250500352151. [DOI] [PubMed] [Google Scholar]
- 37.Burd I, Brown A, Gonzalez JM, Chai J, Elovitz MA. A mouse model of term chorioamnionitis: unraveling causes of adverse neurological outcomes. Reprod Sci. 2011;18:900–7. doi: 10.1177/1933719111398498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med. 2014;42:731–43. doi: 10.1515/jpm-2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. J Am Med Assoc Pediatr. 2014;168:137–47. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eastman NJ, Deleon M. The etiology of cerebral palsy. Am J Obstet Gynecol. 1955;69:950–61. doi: 10.1016/0002-9378(55)90094-6. [DOI] [PubMed] [Google Scholar]
- 41.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. J Am Med Assoc. 1997;278:207–11. [PubMed] [Google Scholar]
- 42.Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–9. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. J Am Med Assoc. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 44.Nelson KB. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev. 2002;8:146–50. doi: 10.1002/mrdd.10037. [DOI] [PubMed] [Google Scholar]
- 45.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. J Am Med Assoc. 2003;290:2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 46.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):387–92. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- 47.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165(4 Pt 1):955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 48.Moyo SR, Hagerstrand I, Nystrom L, Tswana SA, Blomberg J, Bergstrom S, et al. Stillbirths and intrauterine infection, histologic chorioamnionitis and microbiological findings. Int J Gynaecol Obstet. 1996;54:115–23. doi: 10.1016/0020-7292(96)02705-1. [DOI] [PubMed] [Google Scholar]
- 49.Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J Perinatol. 2014;34:611–5. doi: 10.1038/jp.2014.81. [DOI] [PubMed] [Google Scholar]
- 50.Mendez-Figueroa H, Abramovici A, O’Neil AE, Dahlke J, Pedroza C, Chauhan S. Chorioamnionitis without and with neonatal sepsis: newborn and infant outcomes. Am J Obstet Gynecol. 2015;212:S318–19. [Google Scholar]
- 51.Patterson PH. Modeling autistic features in animals. Pediatr Res. 2011;69(5 Pt 2):34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–90. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–26. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- 55.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bulletin. 2000;26:287–95. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 56.Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–15. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 57.Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, et al. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry. 2002;7:633–40. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- 58.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–8. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 59.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–89. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 61.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch General Psychiatry. 2004;61:774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 62.Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatric Res. 2004;38:335–45. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–73. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 64.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–47. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–23. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–9. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 67.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–2. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–88. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–54. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 70.Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–7. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–7. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 72.Penner JD, Brown AS. Prenatal infectious and nutritional factors and risk of adult schizophrenia. Expert Rev Neurother. 2007;7:797–805. doi: 10.1586/14737175.7.7.797. [DOI] [PubMed] [Google Scholar]
- 73.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–15. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 75.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Research. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PloS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–23. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–7. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 80.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252:75–82. doi: 10.1016/j.jneuroim.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 83.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447–54. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry. 2012;2:e98. doi: 10.1038/tp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–57. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 86.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109:12776–81. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19:259–64. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325, e1–e10. doi: 10.1016/j.ajog.2013.10.882. [DOI] [PubMed] [Google Scholar]
- 91.Horvath B, Lakatos F, Toth C, Bodecs T, Bodis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med. 2014;42:441–7. doi: 10.1515/jpm-2013-0186. [DOI] [PubMed] [Google Scholar]
- 92.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75:332–41. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 94.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009;197:210–8. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 95.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–5. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jobe AH, Kallapur SG. Chorioamnionitis, surfactant, and lung disease in very low birth weight infants. J Pediatr. 2010;156:3–4. doi: 10.1016/j.jpeds.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68:210–5. doi: 10.1203/PDR.0b013e3181e9c556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol. 2010;30(Suppl):S21–30. doi: 10.1038/jp.2010.96. [DOI] [PubMed] [Google Scholar]
- 100.Collins JJ, Kuypers E, Nitsos I, Jane Pillow J, Polglase GR, Kemp MW, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2012;303:L778–87. doi: 10.1152/ajplung.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014;32:56–67. doi: 10.1055/s-0033-1361823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolfs TG, Kramer BW, Thuijls G, Kemp MW, Saito M, Willems MG, et al. Chorioamnionitis-induced fetal gut injury is mediated by direct gut exposure of inflammatory mediators or by lung inflammation. Am J Physiol Gastrointest Physiol. 2014;306:G382–93. doi: 10.1152/ajpgi.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maneenil G, Kemp MW, Kannan PS, Kramer BW, Saito M, Newnham JP, et al. Oral, nasal and pharyngeal exposure to lipopolysaccharide causes a fetal inflammatory response in sheep. PloS One. 2015;10:e0119281. doi: 10.1371/journal.pone.0119281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 105.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–82. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 106.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 107.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 108.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40:19–32. doi: 10.1515/JPM.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaiworapongsa T, Romero R, Berry SM, Hassan SS, Yoon BH, Edwin S, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med. 2011;39:653–66. doi: 10.1515/JPM.2011.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vaisbuch E, Romero R, Gomez R, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2011;24:391–6. doi: 10.3109/14767058.2010.507294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Romero R, Soto E, Berry SM, Hassan SS, Kusanovic JP, Yoon BH, et al. Blood pH and gases in fetuses in preterm labor with and without systemic inflammatory response syndrome. J Matern Fetal Neonatal Med. 2012;25:1160–70. doi: 10.3109/14767058.2011.629247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Available at: http://www.R-project.org/ [Google Scholar]
- 115.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 116.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–50. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 117.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20:114–8. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 118.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 119.Szekeres-Bartho J. Immunological relationship between the mother and the fetus. Int Rev Immunol. 2002;21:471–95. doi: 10.1080/08830180215017. [DOI] [PubMed] [Google Scholar]
- 120.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 121.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–6. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 122.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Betz AG. Immunology: tolerating pregnancy. Nature. 2012;490:47–8. doi: 10.1038/490047a. [DOI] [PubMed] [Google Scholar]
- 124.Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993;10:85–102. [PubMed] [Google Scholar]
- 125.Koch CA, Platt JL. Natural mechanisms for evading graft rejection: the fetus as an allograft. Springer Semin Immunopathol. 2003;25:95–117. doi: 10.1007/s00281-003-0136-0. [DOI] [PubMed] [Google Scholar]
- 126.Koch CA, Platt JL. T cell recognition and immunity in the fetus and mother. Cell Immunol. 2007;248:12–7. doi: 10.1016/j.cellimm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Makrigiannakis A, Karamouti M, Drakakis P, Loutradis D, Antsaklis A. Fetomaternal immunotolerance. Am J Reprod Immunol. 2008;60:482–96. doi: 10.1111/j.1600-0897.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 128.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–36. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 129.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J reprod Immunol. 2010;63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Erlebacher A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol Metab. 2010;21:428–34. doi: 10.1016/j.tem.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85:71–5. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 132.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 133.Gotsch F, Romero R, Espinoza J, Kusanovic JP, Mazaki-Tovi S, Erez O, et al. Maternal serum concentrations of the chemokine CXCL10/IP-10 are elevated in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2007;20:735–44. doi: 10.1080/14767050701511650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chaiworapongsa T, Romero R, Gotsch F, Kusanovic JP, Mittal P, Kim SK, et al. Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. J Matern Fetal Neonatal Med. 2010;23:167–78. doi: 10.3109/14767050903067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 136.Barton JR, Sibai BM. Severe sepsis and septic shock in pregnancy. Obstet Gynecol. 2012;120:689–706. doi: 10.1097/AOG.0b013e318263a52d. [DOI] [PubMed] [Google Scholar]
- 137.Acosta CD, Knight M. Sepsis and maternal mortality. Curr Opin Obstet Gynecol. 2013;25:109–16. doi: 10.1097/GCO.0b013e32835e0e82. [DOI] [PubMed] [Google Scholar]
- 138.Edwards SE, Grobman WA, Lappen JR, Winter C, Fox R, Lenguerrand E, et al. Modified obstetric early warning scoring systems (MOEWS): validating the diagnostic performance for severe sepsis in women with chorioamnionitis. Am J Obstet Gynecol. 2015;212:536. doi: 10.1016/j.ajog.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 139.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Louie JK, Acosta M, Jamieson DJ, Honein MA. California Pandemic Working G. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 141.Hewagama S, Walker SP, Stuart RL, Gordon C, Johnson PD, Friedman ND, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis. 2010;50:686–90. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 142.Tamma PD, Steinhoff MC, Omer SB. Influenza infection and vaccination in pregnant women. Expert Rev Respir Med. 2010;4:321–8. doi: 10.1586/ers.10.26. [DOI] [PubMed] [Google Scholar]
- 143.Centers for Disease and Control Prevention. Maternal and infant outcomes among severely ill pregnant and postpartum women with 2009 pandemic influenza A (H1N1) - United States, April 2009-August 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1193–6. [PubMed] [Google Scholar]
- 144.Dede FS, Celen S, Bilgin S, Ure G, Ozcan AO, Buzgan T, et al. Maternal deaths associated with H1N1 influenza virus infection in Turkey: a whole-of-population report. Br J Obstet Gynecol. 2011;118:1216–22. doi: 10.1111/j.1471-0528.2011.03002.x. [DOI] [PubMed] [Google Scholar]
- 145.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–8. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 146.Zhang PJ, Li XL, Cao B, Yang SG, Liang LR, Gu L, et al. Clinical features and risk factors for severe and critical pregnant women with 2009 pandemic H1N1 influenza infection in China. BMC Infect Dis. 2012;12:29. doi: 10.1186/1471-2334-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu SL, Wang J, Yang XH, Chen J, Huang RJ, Ruan B, et al. Pandemic influenza A(H1N1) 2009 virus in pregnancy. Rev Med Virol. 2013;23:3–14. doi: 10.1002/rmv.1712. [DOI] [PubMed] [Google Scholar]
- 148.Doyle TJ, Goodin K, Hamilton JJ. Maternal and neonatal outcomes among pregnant women with 2009 pandemic influenza A(H1N1) illness in Florida, 2009-2010: a population-based cohort study. PloS One. 2013;8:e79040. doi: 10.1371/journal.pone.0079040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.da Silva AA, Ranieri TM, Torres FD, Vianna FS, Paniz GR, Sanseverino PB, et al. Impact on pregnancies in south Brazil from the influenza A (H1N1) pandemic: cohort study. PloS One. 2014;9:e88624. doi: 10.1371/journal.pone.0088624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obst Gynecol. 2014;124:1005–10. doi: 10.1097/AOG.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 151.Pines A. Ebola in women - what the Ob-Gyns should know. Climacteric. 2015;18:103. [PubMed] [Google Scholar]
- 152.Hayden EC. Maternal health: Ebola’s lasting legacy. Nature. 2015;519:24–6. doi: 10.1038/519024a. [DOI] [PubMed] [Google Scholar]
- 153.Kitching A, Walsh A, Morgan D. Ebola in pregnancy: risk and clinical outcomes. Br J Obstet Gynecol. 2015;122:287. doi: 10.1111/1471-0528.13286. [DOI] [PubMed] [Google Scholar]
- 154.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol. 2010;2010:378472. doi: 10.1155/2010/378472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumar SR, Biswas M, Elankumaran S. Pandemic H1N1 influenza A virus induces a potent innate immune response in human chorionic cells. Viral Immunol. 2014;27:129–37. doi: 10.1089/vim.2013.0093. [DOI] [PubMed] [Google Scholar]
- 156.Periolo N, Avaro M, Czech A, Russo M, Benedetti E, Pontoriero A, et al. Pregnant women infected with pandemic influenza A(H1N1)pdm09 virus showed differential immune response correlated with disease severity. J Clin Virol. 2015;64:52–8. doi: 10.1016/j.jcv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 157.Armstrong-Wells J, Donnelly M, Post MD, Manco-Johnson MJ, Winn VD, Sebire G. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol. 2015;212(212):e1–9. doi: 10.1016/j.ajog.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–15. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goetzl L, Evans T, Rivers J, Suresh MS, Lieberman E. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–8. doi: 10.1067/mob.2002.127135. [DOI] [PubMed] [Google Scholar]
- 160.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–12. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 161.Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–51. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 162.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hsiao E, Patterson PH. Maternal immune activation evokes IL-6-dependent downstream signalling in the placenta and fetal brain, Program No. 436.19. Society of Neuroscience; Chicago, IL (online): 2009. http://www.abstractsonline.com/plan/ViewAbstract.aspx?cKey=81071b23-f04a-4cdd-a18e-5cf3ac68e9a4&mID=2285&mKey=%7b081F7976-E4CD-4F3DA0AF-E8387992A658%7d&sKey=a2ad0b98-37f4-4f47-bfe9-539766622a18. [Google Scholar]
- 164.Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–9. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- 165.Conroy SM, Nguyen V, Quina LA, Blakely-Gonzales P, Ur C, Netzeband JG, et al. Interleukin-6 produces neuronal loss in developing cerebellar granule neuron cultures. J Neuroimmunol. 2004;155:43–54. doi: 10.1016/j.jneuroim.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 166.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 167.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 168.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O’Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. Br Med J. 1998;317:1554–8. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lieberman E, Eichenwald E, Mathur G, Richardson D, Heffner L, Cohen A. Intrapartum fever and unexplained seizures in term infants. Pediatrics. 2000;106:983–8. doi: 10.1542/peds.106.5.983. [DOI] [PubMed] [Google Scholar]
- 170.Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105(1 Pt 1):8–13. doi: 10.1542/peds.105.1.8. [DOI] [PubMed] [Google Scholar]
- 171.Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. Br J Obstet Gynecol. 2001;108:594–7. doi: 10.1111/j.1471-0528.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 172.Petrova A, Demissie K, Rhoads GG, Smulian JC, Marcella S, Ananth CV. Association of maternal fever during labor with neonatal and infant morbidity and mortality. Obstet Gynecol. 2001;98:20–7. doi: 10.1016/s0029-7844(01)01361-8. [DOI] [PubMed] [Google Scholar]
- 173.Dammann O, Drescher J, Veelken N. Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol. 2003;45:148–51. doi: 10.1017/s001216220300029x. [DOI] [PubMed] [Google Scholar]
- 174.Previc FH. Prenatal influences on brain dopamine and their relevance to the rising incidence of autism. Med Hypotheses. 2007;68:46–60. doi: 10.1016/j.mehy.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 175.Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol. 2008;198(49):e1–6. doi: 10.1016/j.ajog.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 176.Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–8. e1. doi: 10.1016/j.jpeds.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–72. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–94. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- 179.Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ, Lieberman E. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129:e447–54. doi: 10.1542/peds.2010-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kuypers E, Jellema RK, Ophelders DR, Dudink J, Nikiforou M, Wolfs TG, et al. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PloS One. 2013;8:e81644. doi: 10.1371/journal.pone.0081644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dell’Ovo V, Rosenzweig J, Burd I, Merabova N, Darbinian N, Goetzl LM. An animal model for chorioamnionitis at term. Am J Obstet Gynecol [Epub ahead of print] 2015 doi: 10.1016/j.ajog.2015.05.007. [182] Dinarello CA, Bunn PA Jr. Fever. Semin Oncol. 1997;24:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–22. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 183.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–49. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 184.Sobrado J, Moldawer LL, Bistrian BR, Dinarello CA, Blackburn GL. Effect of ibuprofen on fever and metabolic changes induced by continuous infusion of leukocytic pyrogen (interleukin 1) or endotoxin. Infect Immun. 1983;42:997–1005. doi: 10.1128/iai.42.3.997-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sobrado J, Moldawer LL, Bistrian BR, Dinarello CA, Blackburn GL. Effect of ibuprofen on fever and metabolic changes induced by continuous infusion of leukocytic pyrogen (interleukin 1) or endotoxin. Infect Immun. 1983;42:997–1005. doi: 10.1128/iai.42.3.997-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dinarello CA. Interleukin-1. Rev Infect Dis. 1984;6:51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- 187.Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260(2 Pt 2):R453–7. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 188.Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270(1 Pt 1):E91–5. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- 189.Gourine AV, Rudolph K, Tesfaigzi J, Kluger MJ. Role of hypothalamic interleukin-1beta in fever induced by cecal ligation and puncture in rats. Am J Physiol. 1998;275(3 Pt 2):R754–61. doi: 10.1152/ajpregu.1998.275.3.R754. [DOI] [PubMed] [Google Scholar]
- 190.Lundkvist J, Sundgren-Andersson AK, Tingsborg S, Ostlund P, Engfors C, Alheim K, et al. Acute-phase responses in transgenic mice with CNS overexpression of IL-1 receptor antagonist. Am J Physiol. 1999;276(3 Pt 2):R644–51. doi: 10.1152/ajpregu.1999.276.3.R644. [DOI] [PubMed] [Google Scholar]
- 191.Boneberg EM, Hartung T. Febrile temperatures attenuate IL-1 beta release by inhibiting proteolytic processing of the proform and influence Th1/Th2 balance by favoring Th2 cytokines. J Immunol. 2003;171:664–8. doi: 10.4049/jimmunol.171.2.664. [DOI] [PubMed] [Google Scholar]
- 192.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nedwin GE, Svedersky LP, Bringman TS, Palladino MA, Jr, Goeddel DV. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985;135:2492–7. [PubMed] [Google Scholar]
- 194.Lotze MT, Matory YL, Rayner AA, Ettinghausen SE, Vetto JT, Seipp CA, et al. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986;58:2764–72. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 195.Mier JW, Vachino G, van der Meer JW, Numerof RP, Adams S, Cannon JG, et al. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clinical Immunol. 1988;8:426–36. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- 196.Ueno Y, Takano N, Kanegane H, Yokoi T, Yachie A, Miyawaki T, et al. The acute phase nature of interleukin 6: studies in Kawasaki disease and other febrile illnesses. Clin Exp Immunol. 1989;76:337–42. [PMC free article] [PubMed] [Google Scholar]
- 197.LeMay LG, Vander AJ, Kluger MJ. Role of interleukin 6 in fever in rats. Am J Physiol. 1990;258(3 Pt 2):R798–803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- 198.Coceani F, Lees J, Mancilla J, Belizario J, Dinarello CA. Interleukin-6 and tumor necrosis factor in cerebrospinal fluid: changes during pyrogen fever. Brain Res. 1993;612:165–71. doi: 10.1016/0006-8993(93)91657-e. [DOI] [PubMed] [Google Scholar]
- 199.Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J Exp Med. 1996;183:311–6. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localized inflammation in rats. J Physiol. 2000;526(Pt 3):653–61. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–50. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakamura H, Seto Y, Motoyoshi S, Kadokawa T, Sunahara N. Recombinant human tumor necrosis factor causes long-lasting and prostaglandin-mediated fever, with little tolerance, in rabbits. J Pharmacol Exp Ther. 1988;245:336–41. [PubMed] [Google Scholar]
- 203.Morimoto A, Sakata Y, Watanabe T, Murakami N. Characteristics of fever and acute-phase response induced in rabbits by IL-1 and TNF. Am J Physiol. 1989;256(1 Pt 2):R35–41. doi: 10.1152/ajpregu.1989.256.1.R35. [DOI] [PubMed] [Google Scholar]
- 204.Kawasaki H, Moriyama M, Ohtani Y, Naitoh M, Tanaka A, Nariuchi H. Analysis of endotoxin fever in rabbits by using a monoclonal antibody to tumor necrosis factor (cachectin) Infect Immun. 1989;57:3131–5. doi: 10.1128/iai.57.10.3131-3135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 206.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 207.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 208.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Ecp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, et al. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–23. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 211.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–78. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 212.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 213.Braun MC, Kelsall BL. Regulation of interleukin-12 production by G-protein-coupled receptors. Microbes Infect. 2001;3:99–107. doi: 10.1016/s1286-4579(00)01357-5. [DOI] [PubMed] [Google Scholar]
- 214.Esche C, Shurin MR, Lotze MT. IL-12. In: Oppenheim JJ, Feldmann M, Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine reference: a compendium of cytokines and other mediators of host defense. Massachusetts: Academic Press; 2001. pp. 187–201. [Google Scholar]
- 215.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 216.Albrecht I, Tapmeier T, Zimmermann S, Frey M, Heeg K, Dalpke A. Toll-like receptors differentially induce nucleosome remodelling at the IL-12p40 promoter. EMBO Rep. 2004;5:172–7. doi: 10.1038/sj.embor.7400078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang S, Wang Q. Factors determining the formation and release of bioactive IL-12: regulatory mechanisms for IL-12p70 synthesis and inhibition. Biochem Biophys Rese Comm. 2008;372:509–12. doi: 10.1016/j.bbrc.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 218.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cassatella MA, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 220.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–21. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 221.Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–89. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Germann T, Gately MK, Schoenhaut DS, Lohoff M, Mattner F, Fischer S, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. EurJ Immunol. 1993;23:1762–70. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 225.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–9. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Aste-Amezaga M, D’Andrea A, Kubin M, Trinchieri G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell Immunol. 1994;156:480–92. doi: 10.1006/cimm.1994.1192. [DOI] [PubMed] [Google Scholar]
- 228.Gargano JW, Holzman C, Senagore P, Thorsen P, Skogstrand K, Hougaard DM, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J reprod Immunol. 2008;79:100–10. doi: 10.1016/j.jri.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hazelzet JA, Kornelisse RF, van der Pouw Kraan TC, Joosten KF, van der Voort E, van Mierlo G, et al. Interleukin 12 levels during the initial phase of septic shock with purpura in children: relation to severity of disease. Cytokine. 1997;9:711–6. doi: 10.1006/cyto.1997.0215. [DOI] [PubMed] [Google Scholar]
- 230.Sherwin C, Broadbent R, Young S, Worth J, McCaffrey F, Medlicott NJ, et al. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol. 2008;25:629–36. doi: 10.1055/s-0028-1090585. [DOI] [PubMed] [Google Scholar]
- 231.Jekarl DW, Kim JY, Lee S, Kim M, Kim Y, Han K, et al. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clin Chem Lab Med. 2015;53:575–81. doi: 10.1515/cclm-2014-0607. [DOI] [PubMed] [Google Scholar]
- 232.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 233.McKenzie ANJ, Matthews DJ. IL-13. In: Oppenheim JJ, Feldmann M, Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine reference a compendium of cytokines and other mediators of host defense. Massachusetts: Academic Press; 2001. pp. 203–11. [Google Scholar]
- 234.Collighan N, Giannoudis PV, Kourgeraki O, Perry SL, Guillou PJ, Bellamy MC. Interleukin 13 and inflammatory markers in human sepsis. Br J Surg. 2004;91:762–8. doi: 10.1002/bjs.4521. [DOI] [PubMed] [Google Scholar]
- 235.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–80. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hoshino T, Winkler-Pickett RT, Mason AT, Ortaldo JR, Young HA. IL-13 production by NK cells: IL-13-producing NK and T cells are present in vivo in the absence of IFN-gamma. J Immunol. 1999;162:51–9. [PubMed] [Google Scholar]
- 237.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 238.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–5. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 239.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Strieter RM, et al. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J Immunol. 2000;164:2738–44. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- 240.Doherty TM, Kastelein R, Menon S, Andrade S, Coffman RL. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–60. [PubMed] [Google Scholar]
- 241.de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 242.Muchamuel T, Menon S, Pisacane P, Howard MC, Cockayne DA. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: correlation with down-modulation of TNF-alpha, IFN-gamma, and IL-12 production. J Immunol. 1997;158:2898–903. [PubMed] [Google Scholar]
- 243.Nicoletti F, Mancuso G, Cusumano V, Di Marco R, Zaccone P, Bendtzen K, et al. Prevention of endotoxin-induced lethality in neonatal mice by interleukin-13. Eur J Immunol. 1997;27:1580–3. doi: 10.1002/eji.1830270639. [DOI] [PubMed] [Google Scholar]
- 244.Baumhofer JM, Beinhauer BG, Wang JE, Brandmeier H, Geissler K, Losert U, et al. Gene transfer with IL-4 and IL-13 improves survival in lethal endotoxemia in the mouse and ameliorates peritoneal macrophages immune competence. Eur J Immunol. 1998;28:610–5. doi: 10.1002/(SICI)1521-4141(199802)28:02<610::AID-IMMU610>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 245.Steinhauser ML, Hogaboam CM, Matsukawa A, Lukacs NW, Strieter RM, Kunkel SL. Chemokine C10 promotes disease resolution and survival in an experimental model of bacterial sepsis. Infect Immun. 2000;68:6108–14. doi: 10.1128/iai.68.11.6108-6114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX, Liu MH. Protective effect of Ulinastatin against murine models of sepsis: inhibition of TNF-alpha and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol. 2012;64:543–7. doi: 10.1016/j.etp.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 247.Socha LA, Gowardman J, Silva D, Correcha M, Petrosky N. Elevation in interleukin 13 levels in patients diagnosed with systemic inflammatory response syndrome. Intensive Care Med. 2006;32:244–50. doi: 10.1007/s00134-005-0020-6. [DOI] [PubMed] [Google Scholar]
- 248.Sikora JP, Chlebna-Sokol D, Krzyzanska-Oberbek A. Proinflammatory cytokines (IL-6, IL-8), cytokine inhibitors (IL-6sR, sTNFRII) and anti-inflammatory cytokines (IL-10, IL-13) in the pathogenesis of sepsis in newborns and infants. Arch Immunol Ther Exp. 2001;49:399–404. [PubMed] [Google Scholar]
- 249.Blanco-Quiros A, Casado-Flores J, Garrote Adrados JA, Moro MN, Anton JA, Sanz EA. Interleukin-13 is involved in the survival of children with sepsis. Acta Paediatr. 2005;94:1828–31. doi: 10.1111/j.1651-2227.2005.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 250.Miller LC, Isa S, LoPreste G, Schaller JG, Dinarello CA. Neonatal interleukin-1 beta, interleukin-6, and tumor necrosis factor: cord blood levels and cellular production. J Pediatr. 1990;117:961–5. doi: 10.1016/s0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- 251.Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics. 1994;93:54–8. [PubMed] [Google Scholar]
- 252.Lehrnbecher T, Schrod L, Kraus D, Roos T, Martius J, von Stockhausen HB. Interleukin-6 and soluble interleukin-6 receptor in cord blood in the diagnosis of early onset sepsis in neonates. Acta Paediatr. 1995;84:806–8. doi: 10.1111/j.1651-2227.1995.tb13761.x. [DOI] [PubMed] [Google Scholar]
- 253.Gunn L, Hardiman P, Tharmaratnam S, Lowe D, Chard T. Measurement of interleukin-1 alpha and interleukin-6 in pregnancy-associated tissues. Reprod Fertil Dev. 1996;8:1069–73. doi: 10.1071/rd9961069. [DOI] [PubMed] [Google Scholar]
- 254.Singh B, Merchant P, Walker CR, Kryworuchko M, Diaz-Mitoma F. Interleukin-6 expression in cord blood of patients with clinical chorioamnionitis. Pediatr Res. 1996;39:976–9. doi: 10.1203/00006450-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 255.Smulian JC, Bhandari V, Campbell WA, Rodis JF, Vintzileos AM. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med. 1997;6:254–9. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<254::AID-MFM2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 256.Smulian JC, Campbell WA, Vintzileos AM, Rodis JF. Correlation between umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1. J Matern Fetal Med. 1997;6:67–70. doi: 10.1002/(SICI)1520-6661(199703/04)6:2<67::AID-MFM1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 257.Berner R, Niemeyer CM, Leititis JU, Funke A, Schwab C, Rau U, et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–77. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 258.Weimann E, Rutkowski S, Reisbach G. G-CSF, GM-CSF and IL-6 levels in cord blood: diminished increase of G-CSF and IL-6 in preterms with perinatal infection compared to term neonates. J Perinat Med. 1998;26:211–8. doi: 10.1515/jpme.1998.26.3.211. [DOI] [PubMed] [Google Scholar]
- 259.Perenyi A, Johann-Liang R, Stavola JJ. Assessment of cord blood IL-6 levels as an indicator of neonatal sepsis. Am J Perinatol. 1999;16:525–30. doi: 10.1055/s-1999-7282. [DOI] [PubMed] [Google Scholar]
- 260.Santana C, Guindeo MC, Gonzalez G, Garcia-Munoz F, Saavedra P, Domenech E. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr. 2001;90:1176–81. doi: 10.1080/080352501317061602. [DOI] [PubMed] [Google Scholar]
- 261.Dollner H, Vatten L, Linnebo I, Zanussi GF, Laerdal A, Austgulen R. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate. 2001;80:41–7. doi: 10.1159/000047118. [DOI] [PubMed] [Google Scholar]
- 262.Dollner H, Vatten L, Halgunset J, Rahimipoor S, Austgulen R. Histologic chorioamnionitis and umbilical serum levels of proinflammatory cytokines and cytokine inhibitors. Br J Obstet Gynecol. 2002;109:534–9. [PubMed] [Google Scholar]
- 263.Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110:673–80. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- 264.Smulian JC, Bhandari V, Vintzileos AM, Shen-Schwarz S, Quashie C, Lai-Lin YL, et al. Intrapartum fever at term: serum and histologic markers of inflammation. Am J Obstet Gynecol. 2003;188:269–74. doi: 10.1067/mob.2003.11. [DOI] [PubMed] [Google Scholar]
- 265.Hatzidaki E, Gourgiotis D, Manoura A, Korakaki E, Bossios A, Galanakis E, et al. Interleukin-6 in preterm premature rupture of membranes as an indicator of neonatal outcome. Acta Obstet Gynecol Scand. 2005;84:632–8. doi: 10.1111/j.0001-6349.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 266.Tasci Y, Dilbaz B, Uzmez Onal B, Caliskan E, Dilbaz S, Doganci L, et al. The value of cord blood interleukin-6 levels for predicting chorioamnionitis, funisitis and neonatal infection in term premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2006;128:34–9. doi: 10.1016/j.ejogrb.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 267.Veleminsky M, Jr, Stransky P, Veleminsky MSr, Tosner J. Relationship of IL-6, IL-8, TNF and sICAM-1 levels to PROM, pPROM, and the risk of early-onset neonatal sepsis. Neuro Endocrinol Lett. 2008;29:303–11. [PubMed] [Google Scholar]
- 268.Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22:379–87. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cancelier AC, Petronilho F, Reinke A, Constantino L, Machado R, Ritter C, et al. Inflammatory and oxidative parameters in cord blood as diagnostic of early-onset neonatal sepsis: a case-control study. Pediatr Crit Care Med. 2009;10:467–71. doi: 10.1097/PCC.0b013e318198b0e3. [DOI] [PubMed] [Google Scholar]
- 270.Hassanein SM, El-Farrash RA, Hafez HM, Hassanin OM, Abd El Rahman NA. Cord blood interleukin-6 and neonatal morbidities among preterm infants with PCR-positive Ureaplasma urealyticum. J Matern Fetal Neonatal Med. 2012;25:2106–10. doi: 10.3109/14767058.2012.678435. [DOI] [PubMed] [Google Scholar]
- 271.Cernada M, Badia N, Modesto V, Alonso R, Mejias A, Golombek S, et al. Cord blood interleukin-6 as a predictor of early-onset neonatal sepsis. Acta Paediatr. 2012;101:e203–7. doi: 10.1111/j.1651-2227.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 272.Cobo T, Kacerovsky M, Andrys C, Drahosova M, Musilova I, Hornychova H, et al. Umbilical cord blood IL-6 as predictor of early-onset neonatal sepsis in women with preterm prelabour rupture of membranes. PloS One. 2013;8:e69341. doi: 10.1371/journal.pone.0069341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kacerovsky M, Cobo T, Andrys C, Musilova I, Drahosova M, Hornychova H, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2013;26:795–801. doi: 10.3109/14767058.2013.765404. [DOI] [PubMed] [Google Scholar]
- 274.Prashant A, Vishwanath P, Kulkarni P, Sathya Narayana P, Gowdara V, Nataraj SM, et al. Comparative assessment of cytokines and other inflammatory markers for the early diagnosis of neonatal sepsis-a case control study. PloS One. 2013;8:e68426. doi: 10.1371/journal.pone.0068426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sorokin Y, Romero R, Mele L, Iams JD, Peaceman AM, Leveno KJ, et al. Umbilical cord serum interleukin-6, C-reactive protein, and myeloperoxidase concentrations at birth and association with neonatal morbidities and long-term neurodevelopmental outcomes. Am J Perinatol. 2014;31:717–26. doi: 10.1055/s-0033-1359723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nishimaki S, Shima Y, Sato M, An H, Kadota K, Yokota S. Postnatal changes of cytokines in premature infants with or without funisitis. J Matern Fetal Neonatal Med. 2014;27:1545–9. doi: 10.3109/14767058.2013.867321. [DOI] [PubMed] [Google Scholar]
- 277.Su H, Chang S, Han CM, Wu KY, Li MC, Huang CY, et al. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and metaanalysis. J Perinatol. 2014;34:268–74. doi: 10.1038/jp.2013.186. [DOI] [PubMed] [Google Scholar]
- 278.Shimoya K, Matsuzaki N, Taniguchi T, Okada T, Saji F, Murata Y. Interleukin-8 level in maternal serum as a marker for screening of histological chorioamnionitis at term. Int J Gynecol Obstet. 1997;57:153–9. doi: 10.1016/s0020-7292(97)02891-9. [DOI] [PubMed] [Google Scholar]
- 279.Berner R, Tuxen B, Clad A, Forster J, Brandis M. Elevated gene expression of interleukin-8 in cord blood is a sensitive marker for neonatal infection. Eur J Pediatr. 2000;159:205–10. doi: 10.1007/s004310050051. [DOI] [PubMed] [Google Scholar]
- 280.Lencki SG, Maciulla MB, Eglinton GS. Maternal and umbilical cord serum interleukin levels in preterm labor with clinical chorioamnionitis. Am J Obstet Gynecol. 1994;170(5 Pt 1):1345–51. doi: 10.1016/s0002-9378(94)70154-7. [DOI] [PubMed] [Google Scholar]
- 281.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 282.Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012;22:R733–40. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–8. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci. 2007;1107:118–28. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- 285.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–52. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179:1107–14. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 287.Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol. 1996;174:1725–31. doi: 10.1016/s0002-9378(96)70203-x. discussion 31–3. [DOI] [PubMed] [Google Scholar]
- 288.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182:1404–13. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 289.Barja-Fidalgo C, Souza EP, Silva SV, Rodrigues AL, Anjos-Valotta EA, Sannomyia P, et al. Impairment of inflammatory response in adult rats submitted to maternal undernutrition during early lactation: role of insulin and glucocorticoid. Inflamm Res. 2003;52:470–6. doi: 10.1007/s00011-003-1207-3. [DOI] [PubMed] [Google Scholar]
- 290.Theogaraj E, John CD, Dewar A, Buckingham JC, Smith SF. The long-term effects of perinatal glucocorticoid exposure on the host defence system of the respiratory tract. J Pathol. 2006;210:85–93. doi: 10.1002/path.2017. [DOI] [PubMed] [Google Scholar]
- 291.Rodriguez JS, Zurcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204:545.e1–10. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Long NM, Shasa DR, Ford SP, Nathanielsz PW. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol. 2012;207:203.e1–8. doi: 10.1016/j.ajog.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sominsky L, Fuller EA, Bondarenko E, Ong LK, Averell L, Nalivaiko E, et al. Functional programming of the autonomic nervous system by early life immune exposure: implications for anxiety. PloS One. 2013;8:e57700. doi: 10.1371/journal.pone.0057700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Long NM, Smith DT, Ford SP, Nathanielsz PW. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol. 2013;209:353, e1–9. doi: 10.1016/j.ajog.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Long NM, Ford SP, Nathanielsz PW. Multigenerational effects of fetal dexamethasone exposure on the hypothalamicpituitary-adrenal axis of first- and second-generation female offspring. Am J Obstet Gynecol. 2013;208:217, e1–8. doi: 10.1016/j.ajog.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Almgren M, Schlinzig T, Gomez-Cabrero D, Gunnar A, Sundin M, Johansson S, et al. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: implications for future health? Am J Obstet Gynecol. 2014;211(502):e1–8. doi: 10.1016/j.ajog.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 297.Yu HR, Kuo HC, Chen CC, Sheen JM, Tiao MM, Chen YC, et al. Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factoralpha production decrease. Immunology. 2014;143:651–60. doi: 10.1111/imm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249–54. doi: 10.1016/j.ajog.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 299.Romero R, Korzeniewski SJ. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am J Obstet Gynecol. 2013;208:243–6. doi: 10.1016/j.ajog.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Braback L, Lowe A, Hjern A. Elective cesarean section and childhood asthma. Am J Obstet Gynecol. 2013;209:496. doi: 10.1016/j.ajog.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 301.Cho CE, Norman M. Reply: To PMID 22939691. Am J Obstet Gynecol. 2013;209:496–7. doi: 10.1016/j.ajog.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 302.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 304.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–57. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 305.Jarvis JN, Deng L, Berry SM, Romero R, Moore H. Fetal cytokine expression in utero detected by reverse transcriptase polymerase chain reaction. Pediatr Res. 1995;37(4 Pt 1):450–4. doi: 10.1203/00006450-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 306.Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Invest. 1995;73:384–92. [PubMed] [Google Scholar]
- 307.Steinborn A, von Gall C, Hildenbrand R, Stutte HJ, Kaufmann M. Identification of placental cytokine-producing cells in term and preterm labor. Obstet Gynecol. 1998;91:329–35. doi: 10.1016/s0029-7844(97)00680-7. [DOI] [PubMed] [Google Scholar]
- 308.Kameda T, Matsuzaki N, Sawai K, Okada T, Saji F, Matsuda T, et al. Production of interleukin-6 by normal human trophoblast. Placenta. 1990;11:205–13. doi: 10.1016/s0143-4004(05)80266-8. [DOI] [PubMed] [Google Scholar]
- 309.Matsuzaki N, Taniguchi T, Shimoya K, Neki R, Okada T, Saji F, et al. Placental interleukin-6 production is enhanced in intrauterine infection but not in labor. Am J Obstet Gynecol. 1993;168(1 Pt 1):94–7. doi: 10.1016/s0002-9378(12)90892-3. [DOI] [PubMed] [Google Scholar]
- 310.Liechty KW, Koenig JM, Mitchell MD, Romero R, Christensen RD. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin-1. Pediatr Res. 1991;29:1–4. doi: 10.1203/00006450-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 311.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165(4 Pt 1):813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 312.Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol. 1994;171:912–21. doi: 10.1016/s0002-9378(94)70058-3. [DOI] [PubMed] [Google Scholar]
- 313.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 314.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response,and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 315.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 316.Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32:623–9. doi: 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- 317.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85–90. doi: 10.1080/jmf.14.2.85.90. [DOI] [PubMed] [Google Scholar]
- 318.Mittendorf R, Montag AG, MacMillan W, Janeczek S, Pryde PG, Besinger RE, et al. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol. 2003;188:1438–4. doi: 10.1067/mob.2003.380. discussion 44–6. [DOI] [PubMed] [Google Scholar]
- 319.Arad I, Ergaz Z. The fetal inflammatory response syndrome and associated infant morbidity. Isr Med Assoc J. 2004;6:766–9. [PubMed] [Google Scholar]
- 320.Murthy V, Kennea NL. Antenatal infection/inflammation and fetal tissue injury. Best Pract Res Clin Obstet Gynaecol. 2007;21:479–89. doi: 10.1016/j.bpobgyn.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 321.Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154:39–43. e3. doi: 10.1016/j.jpeds.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 322.Stampalija T, Romero R, Korzeniewski SJ, Chaemsaithong P, Miranda J, Yeo L, et al. Soluble ST2 in the fetal inflammatory response syndrome: in vivo evidence of activation of the anti-inflammatory limb of the immune response. J Maternal Fetal Neonatal Med. 2013;26:1384–93. doi: 10.3109/14767058.2013.784258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wirbelauer J, Seidenspinner S, Thomas W, Kunzmann S, Speer CP. Funisitis is associated with increased interleukin-10 gene expression in cord blood mononuclear cells in preterm infants < / = 32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2011;155:31–5. doi: 10.1016/j.ejogrb.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 324.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–7. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 325.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–30. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 326.Savasan ZA, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Xu Y, et al. Interleukin-19 in fetal systemic inflammation. J Matern Fetal Neonatal Med. 2012;25:995–1005. doi: 10.3109/14767058.2011.605917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Tolllike receptors in epidermal keratinocytes. Histopathology. 2006;49:506–14. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kemp MW, Saito M, Kallapur SG, Jobe AH, Keelan JA, Li S, et al. Inflammation of the fetal ovine skin following in utero exposure to Ureaplasma parvum. Reprod Sci. 2011;18:1128–37. doi: 10.1177/1933719111408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhang L, Saito M, Jobe A, Kallapur SG, Newnham JP, Cox T, et al. Intra-amniotic administration of E coli lipopolysaccharides causes sustained inflammation of the fetal skin in sheep. Reprod Sci. 2012;19:1181–9. doi: 10.1177/1933719112446079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–6. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 331.Letti Muller AL, Barrios Pde M, Kliemann LM, Valerio EG, Gasnier R, Magalhaes JA. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet Gynecol. 2010;36:26–31. doi: 10.1002/uog.7584. [DOI] [PubMed] [Google Scholar]
- 332.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–5. [PubMed] [Google Scholar]
- 333.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest. 1997;99:2992–9. doi: 10.1172/JCI119494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 335.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 336.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Ame J Obstet Gynecol. 2000;182:401–8. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- 337.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79:205–9. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 338.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mittendorf R, Covert R, Montag AG, elMasri W, Muraskas J, Lee KS, et al. Special relationships between fetal inflammatory response syndrome and bronchopulmonary dysplasia in neonates. J Perinat Med. 2005;33:428–34. doi: 10.1515/JPM.2005.076. [DOI] [PubMed] [Google Scholar]
- 340.Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–8. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 342.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–20. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 343.De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr. 1999;135:384–6. doi: 10.1016/s0022-3476(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 344.Toti P, De Felice C, Stumpo M, Schurfeld K, Di Leo L, Vatti R, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000;31:1121–8. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- 345.Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006;194:153–9. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 346.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 347.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 348.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 349.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–6. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 350.Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J Obstet Gynaecol Can. 2002;24:705–9. doi: 10.1016/s1701-2163(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 351.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. Br J Obstet Gynecol. 2003;110(Suppl 20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 352.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2205;(Suppl 1):16–8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 353.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 354.Andrews WW, Cliver SP, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt AI, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198:466, e1–e11. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–71. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186:1062–8. doi: 10.1067/mob.2002.122293. [DOI] [PubMed] [Google Scholar]
- 357.Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham JP, Jobe AH. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol. 2002;187:1059–65. doi: 10.1067/mob.2002.126296. [DOI] [PubMed] [Google Scholar]
- 358.Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Obstet Gynecol. 2002;165:805–11. doi: 10.1164/ajrccm.165.6.2108053. [DOI] [PubMed] [Google Scholar]
- 359.Moss TJ, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189:1771–6. doi: 10.1016/s0002-9378(03)00810-x. [DOI] [PubMed] [Google Scholar]
- 360.Kallapur SG, Nitsos I, Moss TJ, Kramer BW, Newnham JP, Ikegami M, et al. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol. 2005;288:L966–74. doi: 10.1152/ajplung.00389.2004. [DOI] [PubMed] [Google Scholar]
- 361.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–7. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.