Abstract
Classical human astroviruses (HAstV) are the third most common cause of non-bacterial acute gastroenteritis. Due to the lack of routine molecular assays, novel HAstV are underdiagnosed and the magnitude of their contribution to clinical disease remains unknown. To better understand their prevalence and the susceptible patient profile, we conducted a comprehensive screening of novel and classical HAstV in stool and cerebrospinal fluid (CSF) samples collected for clinical care in a tertiary care hospital using a specially designed rRT-PCR panel for the detection of novel (MLB1-3 and VA1-4) and classical HAstV. Of the 654 stool samples, 20 were positive for HAstV, and the novel (n=10; 3 MLB1, 4 MLB2; 3 VA2) and classical (n=10) serotypes were equally prevalent. None of the 105 CSF samples were positive. Investigating the patient profile, we found a higher prevalence (P=0.0002) of both novel and classical HAstV in pediatric stool samples (3.4% and 3%, respectively) compared with adult stool samples (0.5% and 0.7%, respectively). Furthermore, all novel and classical HAstV-positive pediatric subjects were ≤four years old, demonstrating similar susceptible populations. Forty-five percent of positive patients were immunocompromised (novel: 40%, classical: 50%). A comparison of novel and classical HAstV-positive cases showed a lower viral load for novel HAstV (P=0.0007) with significantly more upper respiratory symptoms (70% of subjects; P=0.02); this observation may suggest a unique pathogenic pathway. This study confirms the clinical and epidemiological relevance of novel HAstV and identifies a target population in which routine screening may yield clinically valuable information.
Keywords: cerebrospinal fluid, classical human astroviruses, novel human astroviruses, rRT-PCR, stool, survey, tertiary care hospital
INTRODUCTION
Human astroviruses (HAstV) are non-enveloped, single-stranded RNA viruses of the Astroviridae family. Classical HAstV are a major cause of viral diarrhea1, 2 and affect mainly children,3, 4 the elderly5, 6, 7 and immunocompromised8, 9 subjects. In the immunocompromised population, severe digestive symptoms of long duration and potentially fatal disseminated infections have been reported.8, 9, 10 Classical HAstV are classified into eight distinct serotypes belonging to the Mamastrovirus (MAstV) 1 species. HAstV-1 is the most commonly detected serotype11 with a seroprevalence of up to 90% in children by the age of 5 years.12, 13
Recently, novel HAstV (MLB and VA) phylogenetically distant from classical HAstV have been identified in humans.14 Although the correlation between novel HAstV and diarrhea remains to be clarified,15, 16, 17, 18 they have been reported as the causal agents for meningitis and encephalitis, mainly in immunocompromised patients.19, 20, 21, 22, 23, 24 Furthermore, we recently detected novel HAstV VA1 in a nasopharyngeal swab specimen from a 13-month-old patient suffering from an acute respiratory disease of unknown etiology.25 However, the available studies offer limited information on the epidemiology and respective distribution of the novel HAstV known to infect humans. The current routine molecular assays only target the classical HAstV serotypes 1–8, and in the absence of appropriate diagnostic tools, novel HAstV remain largely underdiagnosed.
This study aims to investigate the epidemiological and clinical relevance of novel HAstV and the pediatric and adult patient profiles that they are most likely to affect. To this end, we designed a specific rRT-PCR panel to perform systematic and standardized screening for both novel and classical HAstV. Given the known gastrointestinal replication of classical HAstV and the increasing number of reports linking HAstV to unusual cases of meningoencephalitis, systematic and standardized screening of classical and novel HAstV was conducted on stool and cerebrospinal fluid (CSF) samples collected as part of routine clinical care and sent to our virology laboratory. HAstV prevalence and demographic, epidemiological and clinical data were analyzed systematically.
MATERIALS AND METHODS
Ethics statement
This study was approved by the research ethics committee of the University Hospitals of Geneva (project # 2016-01096).
Study design
Stool specimens were collected between 1 November 2014 and 31 October 2015 from pediatric (≤15-year-old) and adult patients (with or without diarrheal disorders) admitted or attending (in- and outpatient) the University Hospitals of Geneva (Geneva, Switzerland). These samples were obtained when viral screening was requested by the physician in charge and were then tested for the presence of classical and novel HAstV. In the event that several samples were collected from the same subject within a period of less than 30 days, a single sample (the earliest) was selected for analysis. Cerebrospinal fluid samples with a total leukocyte count of >5 cells/μL collected between 1 May 2015 and 31 July 2016 that were sent to the laboratory of virology for viral screening were included in the study when sufficient leftover volume was available. Stool and CSF samples were stored at −80 °C before analysis.
Design and validation of the specific rRT-PCR panel
A genetic alignment analysis was performed, comparing all classical and novel HAstV sequences available in GenBank up to August 2015. Based on this alignment, specific primers and probes were designed using Primer Express software v3.0 (Applied Biosystems, Rotkreuz, Switzerland). The nucleotide sequences of primers and probes are shown in Supplementary Table S1. For each rRT-PCR assay, standard curves and the lower limit of detection were assessed using 10-fold serial dilutions of specific RNA oligonucleotides (Microsynth, Balgach, Switzerland) consisting of the target sequences (Supplementary Table S1). The standard curve for the MLB1, MLB2, MLB2-3, VA1, VA2, VA3, VA4 and Hast rRT-PCR assays were obtained using RNA oligonucleotides corresponding to the GenBank accession numbers NC_011400, KT224358, NC_019028, NC_013060, GQ502193, JX857868, JX857869 and FJ755402 (HAstV-1), respectively.
Screening of novel and classical HAstV
Due to the diverse composition of stool, standardization of the re-suspension process is typically challenging. The method of re-suspension used in this study was optimized to ensure minimal impact on the viral load measurement. Thus, the re-suspension process is not considered to significantly influence the viral loads measured in this study. Briefly, 10 μL of solid stool (using a 10-μL loop) or 500 μL of liquid stool were resuspended in 5 mL of 1 × phosphate-buffered saline. After vortexing, an aliquot of 1 mL was stored at −70 °C.
The viral genome was extracted individually from 380 μL of CSF and 760 μL of phosphate-buffered saline-resuspended stool samples using the NucliSENS easyMAG (bioMérieux, Geneva, Switzerland) nucleic acid kit. As an internal control, 20 and 40 μL of standardized canine distemper virus were added to each CSF and stool sample, respectively, before extraction.26 Elutions were performed in 50 and 100 μL, respectively, for the CSF and stool samples. Overall, eight different rRT-PCR assays were needed for the intended screening. The MLB1, MLB2, MLB2-3, VA1, VA2, VA3, VA4 and an updated version of the Hast27 rRT-PCRs were performed using the one-step QuantiTect Probe RT-PCR Kit (Qiagen, Hombrechtikon, Switzerland) in a StepOne Plus instrument (Applied Biosystems) under the following cycling conditions: 50 °C for 30 min, 95 °C for 15 min, 45 cycles of 15 s at 94 °C and 1 min at 55 °C. For each rRT-PCR assay, RNA concentrations (RNA copies/mL of resuspended stool) were estimated by reporting Ct values on standard curves previously obtained from serial dilutions of specific RNA oligonucleotides containing the target sequences.
Classical HAstV typing
Extracted RNA from classical HAstV-positive stool samples were reverse transcribed with random hexamers (Roche, Indianapolis, IN, USA) using reverse transcriptase SuperScript II (Invitrogen, Carlsbad, CA, USA). A 449-bp region of the capsid protein was amplified using the primers Mon269 and Mon270 as previously described.28 Sequences were analyzed with the Geneious version 6.1.8 software platform and blasted in GenBank.
Data collection
A retrospective review of the medical records of the 20 patients with positive stool samples was performed to collect patient characteristics and clinical data.
Statistical analysis
Statistics were performed using Stata (StataCorp. 2015, College Station, TX, USA). For continuous variables, differences between groups were tested using the Mann–Whitney U-test. For categorical variables, differences between groups were tested using χ2 or Fisher’s exact test. A two-sided P-value of <0.05 was considered significant.
RESULTS
Prevalence of novel and classical HAstV in stool and CSF samples
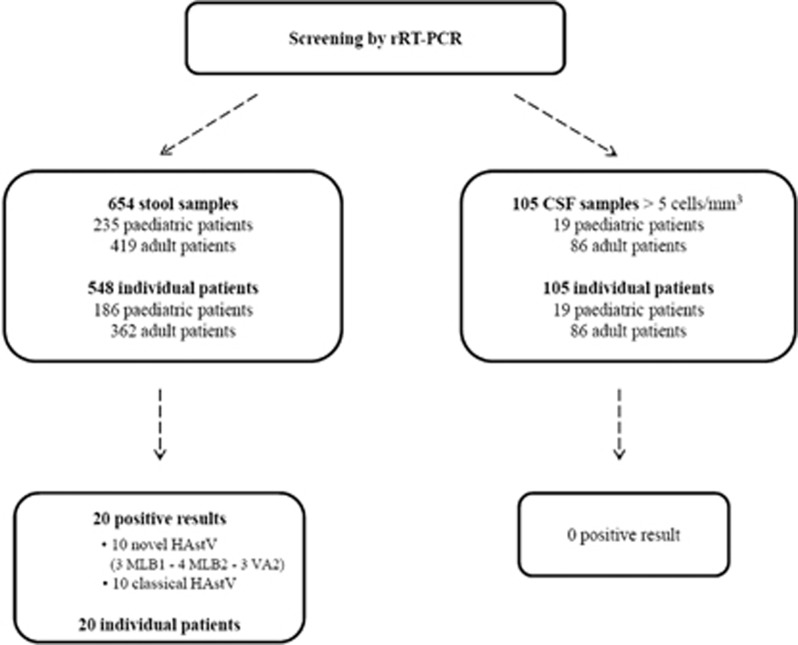
A total of 654 stool specimens collected from 548 individual patients (186 pediatric and 362 adult cases) were tested by rRT-PCR (Figure 1 and Supplementary Figure S1). The overall median age of the 548 screened patients was 52 years old (range, 0–100). The median age of the 186 pediatric patients was 1 year old (range, 0–15). Twenty of the 654 analyzed stool samples were positive for HAstV (3.1% overall prevalence) with 10 classical HAstV and 10 novel HAstV (Figure 2 and Table 1). Fifteen of the 235 pediatric samples (6.4%) and five of the 419 adult samples (1.2%) were positive for HAstV. A comparative analysis of positive stool samples in the pediatric and adult populations showed a significantly higher prevalence of all HAstV among pediatric patients (P=0.0002) with a risk ratio of 5.4 (95% CI (1.9, 14.5)). Similarly, when analyzing novel and classical HAstV separately, the prevalence of either a novel or a classical HAstV was also significantly higher among pediatric patients (P=0.006 and P=0.04, respectively) with a risk ratio of 7.1 (95% CI (1.5, 33.3)) and 4.2 (95% CI (1.1, 15.9), respectively). Classical HAstV were detected in 1.5% of all stool samples (pediatric, 3% adult, 0.7%). Novel HAstV were also detected in 1.5% of all stool samples (pediatric, 3.4% adult, 0.5%). Regarding pediatric HAstV-positive subjects, novel HAstV were detected in neonates (one case), infants (that is, 1 month–2 years; three cases) and children (four cases), whereas classical HAstV were restricted to infants (four cases) and children (three cases) (Table 1).
Figure 1.
Study flowchart using eight rRT-PCRs to detect human astroviruses in stool and cerebrospinal (CSF) samples sent for viral screening in the laboratory of virology, University Hospitals of Geneva, 2015–2016.
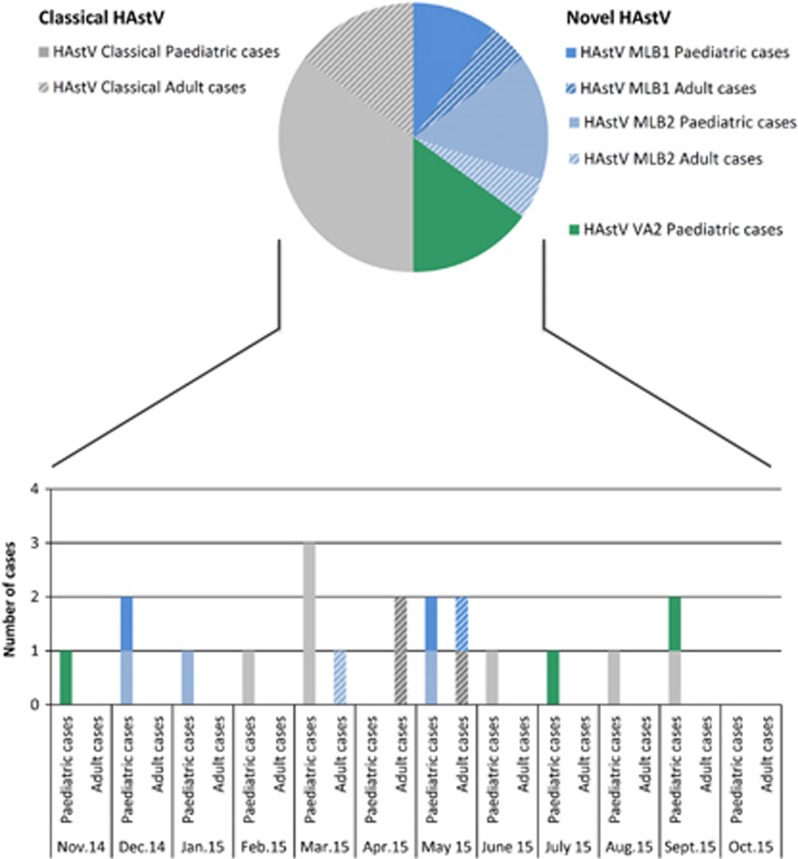
Figure 2.
Positive human astroviruses in stool samples. Top: Pie chart showing the prevalence and composition of classical and novel astroviruses among HAstV-positive patients. Bottom: histogram of the temporal distribution of cases. human astrovirus, HAstV.
Table 1. Clinical cases of classical and novel HAstV detection in stool samples.
|
Patient characteristics |
HAstV characteristics |
Enteric virus stool screening |
Clinical manifestations |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age | Immunodeficiency, etiology | Time between transplantation and HAstV detection | HAstV detected | Viral load (RNA copies/mL of resuspended stool) | Virus specie | Fever | Digestive symptoms, time between symptoms and HAstV detection | Type of digestive symptoms | Respiratory symptoms, time between symptoms and HAstV detection | Type of respiratory symptoms |
| 1 | M | 3.1 years | No | HAstV-MLB1 | 5.5 × E4 | Neg. | No | Yes, 2 days | Vomiting, diarrhea | Yes, 15 days | Cough | |
| 2 | F | 3.4 years | No | HAstV-MLB1 | 6.1 × E3 | Neg. | Yes | Yes, 4 days | Diarrhea, abdominal pain | No | ||
| 3 | F | 51.0 years | Yes, liver transplant recipient | 26.4 months | HAstV-MLB1 | 3.3 × E2 | Rotavirus | No | Yes, 1 day | Vomiting, diarrhea, abdominal pain | Yes, 1 day | Cough |
| 4 | M | 4.2 years | Yes, liver transplant recipient | 37.5 months | HAstV-MLB2 | 7.1 × E2 | Neg. | No | Yes, 1 day | Diarrhea | Yes, 13 days | Cough, rhinorrhea |
| 5 | F | 9.8 months | No | HAstV-MLB2 | 2.9 × E6 | Neg. | No | Yes, 14 days | Diarrhea | Yes | Cough | |
| 6 | M | 2.4 years | Yes, liver transplant recipient | 15.4 months | HAstV-MLB2 | 7.2 × E3 | Neg. | No | Yes, 21 days | Diarrhea | Yes, 10 days | Odynophagia |
| 7 | M | 38.2 years | Yes, allogeneic stem cell transplant recipient | 4.7 months | HAstV-MLB2 | 3.3 × E9 | Neg. | No | Yes, 3 days | Vomiting | No | |
| 8 | F | 1.4 months | No | HAstV-VA2 | 4.8 × E2 | Neg. | Yes | Yes, 4 days | Diarrhea | Yes, 10 days | Rhinorrhea | |
| 9 | F | 23 days | No | HAstV-VA2 | 8.4 × E2 | Enterovirus | Yes | Yes, 2 days | Diarrhea | No | ||
| 10 | M | 5.1 months | No | HAstV-VA2 | 3.4 × E2 | Neg. | No | Yes, 1 day | Diarrhea | Yes, 16 days | Cough, rhinorrhea | |
| 11 | F | 9.6 months | Yes, liver transplant recipient | 6 days | Classical HAstV undet. | 2.3 × E7 | Neg. | Yes | Yes, 1 day | Diarrhea | No | |
| 12 | M | 4.2 years | No | Classical HAstV-8 | 2.0 × E10 | Enterovirus | Yes | Yes, 2 days | Diarrhea | No | ||
| 13 | M | 4.8 years | Yes, kidney transplant recipient | 37.9 months | Classical HAstV undet. | 2.1 × E5 | Neg. | No | Yes, 4 days | Diarrhea | Yes, 7 days | Cough |
| 14 | M | 1.4 years | No | Classical HAstV-1 | 2.4 × E10 | Neg. | No | Yes, >30 days | Diarrhea | No | ||
| 15 | F | 9.9 months | No | Classical HAstV-2 | 1.0 × E11 | Neg. | No | Yes, 1 day | Vomiting, diarrhea | No | ||
| 16 | F | 6.4 months | No | Classical HAstV-1 | 5.3 × E6 | Neg. | Yes | Yes, 14 days | Vomiting, diarrhea | No | ||
| 17 | F | 3.4 years | Yes, liver transplant recipient | 20.6 months | Classical HAstV-4 | 1.6 × E9 | Neg. | Yes | Yes, 2 days | Vomiting, diarrohea | No | |
| 18 | M | 40.2 years | Yes, allogeneic stem cell transplant recipient | 25.4 months | Classical HAstV-1 | 7.5 × E9 | Neg. | No | Yes, 5 days | Diarrhea | No | |
| 19 | F | 30.4 years | No | Classical HAstV-3 | 2.0 × E10 | Neg. | No | Yes, 1 day | Vomiting, diarrhea | No | ||
| 20 | F | 53.0 years | Yes, allogeneic stem cell transplant recipient | 11.3 months | Classical HAstV-5 | 6.0 × E11 | Neg. | NA | NA | NA | NA | NA |
Abbreviations: human astrovirus, HAstV; not available, NA; negative, neg; undetermined, undet.
Screening revealed the presence of 3 MLB1, 4 MLB2, 3 VA2 and 10 classical HAstV (Table 1 and Figure 2), resulting in a prevalence of 0.46%, 0.61%, 0.46% and 1.53%, respectively. No cross-reaction was observed between the different rRT-PCR assays, demonstrating their excellent respective specificities. HAstV-1 was the most frequent serotype detected among classical HAstV (Table 1). These results show that novel HAstV were detected with the same frequency as classical HAstV in stool samples sent for routine screening.
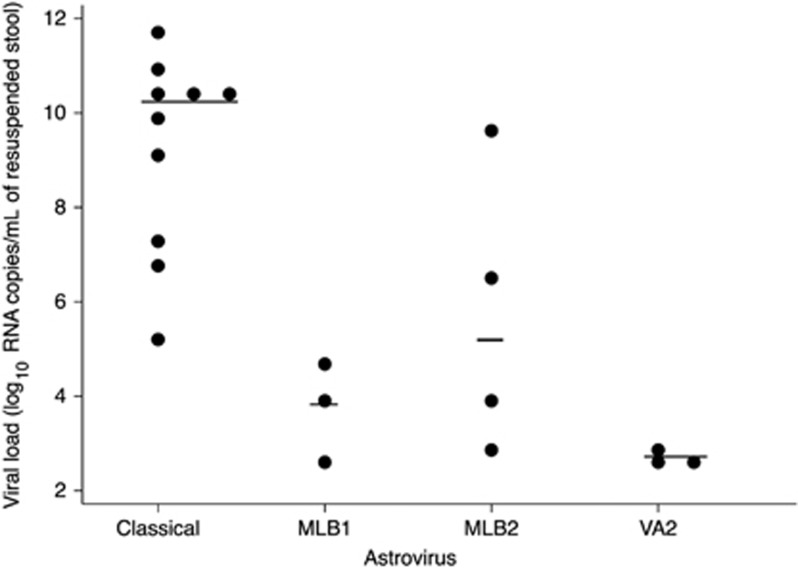
The viral load was estimated for each positive case (Table 1 and Figure 3) and ranged from 7.1 × E2 to 3.3 × E9 for MLB2, 3.3 × E2 to 5.5 × E4 for MLB1, 3.4 × E2 to 8.4 × E2 for VA2 and 2.1 × E5 to 6.0 × E11 for classical HAstV. Overall, the viral load was significantly lower (P=0.0007) in novel HAstV-positive (MLB1, MLB2 and VA2) than in classical HAstV-positive samples (Table 2). This latter observation did not result from different time intervals between the symptom onsets and the collection of samples (P=0.95). Due to the small number of positive samples, a statistical analysis could not be performed to compare viral loads between the different groups of novel HAstV. The mean viral load of all HAstV-positive samples was similar between adults and children (P=0.137) and when novel and classical HAstV were considered separately (P=0.433 and P=0.253, respectively). The distribution of novel and classical HAstV-positive cases was uniform throughout the year, irrespective of the studied population (Figure 2).
Figure 3.
Classical and novel human astrovirus viral load in stool samples. The horizontal lines denote median log values. ***P<0.001. human astrovirus, HAstV.
Table 2. HAstV-positive stool samples: overall and stratified (classical and novel) HAstV patient characteristics.
| All HAstV n=20 | Classical HAstV n=10 | Novel HAstV n=10 | P valuea | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male sex – no. (%) | 9 (45) | 4 (40) | 5 (50) | |
| Age of pediatric and adult patients (yo) | 0.3258 | |||
| Median (range) | 3.3 (0.06–53.0) | 3.8 (0.5–53.0) | 2.8 (0.06–51.0) | |
| Mean±sd | 12.2±18.6 | 14.0±19.6 | 10.4±18.4 | |
| Pediatric patients, no. (%) | 15 (75) | 7 (70) | 8 (80) | 1.000 |
| Age of pediatric patients, months | 0.3545 | |||
| Median (range) | 16.7 (0.8–57.7) | 16.7 (6.4–57.7) | 19.2 (0.8–50.0) | |
| Mean±sd | 24.4±20.1 | 27.3±21.6 | 21.8±19.8 | |
| Immunocompromised – no. (%) | 9 (45) | 5 (50) | 4 (40) | 1.000 |
| Fever – no. (%) | 7 (36.8)b | 4 (44.4)c | 3 (30) | |
| With respiratory symptoms – no. (%) | 8 (42.1)b | 1 (11.1)c | 7 (70) | 0.020 |
| Time interval between respiratory symptoms and HAstV detection (days) | ||||
| Median (range) | 11.5 (1–20) | 7 | 13 (1–20) | |
| Mean±sd | 11.5±5.9 | 7 | 12.1±6.0 | |
| Time interval between transplantation and HAstV detection (days) | ||||
| Median (range) | 617 (6–1139) | 617 (6–1139) | 626.5 (142–1126) | |
| Mean±sd | 598.1±399.9 | 572.4±428.3 | 630.3±423.6 | |
| HAstV | ||||
| Viral load (RNA copies/mL of resuspended stool) | 0.0007 | |||
| Median (range) | 4.1 × E6 (3.4 × E2–6.0 × E11) | 1.4 × E10 (2.1 × E5–6.0 × E11) | 3.5 × E3 (3.4 × E2–3.3 × E9) | |
| Mean±sd | 3.9 × E10±1.3E × 11 | 7.7 × E10±1.9 × E11 | 3.3 × E8±1.0 × E9 | |
Abbreviations: human astrovirus, HAstV; standard deviation, sd; years old, yo.
P-values are for the comparison between the classical and novel HAstV-positive stool sample groups.
Percentage calculated based on 19 patients (1 missing data, Table 1).
Percentage calculated based on 9 patients (1 missing data, Table 1).
A total of 105 CSF samples collected from 105 individual patients (19 pediatric; 86 adult) were tested. Among these, none were positive (Figure 1).
Clinical features in HAstV-positive cases
Among the 20 patients whose samples were positive, the median age was 3.3 years old (range, 23 days–53 years old). The median age of pediatric patients was 1 year old (range, 23 days–4 years old) with a male-to-female ratio of 0.88:1. The median age of adult patients was 40 years old (range, 30–53 years old) with a male-to-female ratio of 0.7:1. Nine (45%) of the 20 patients were immunocompromised (Table 1). Six patients were solid organ transplant recipients and three were allogeneic stem cell transplant recipients. The median time between transplantation and HAstV detection was 617 days (range, 6–1139). Of the 20 positive patient samples, 13 were collected from outpatients. Seven patients were hospitalized at the time of stool sample collection.
Fever was reported in seven of the 20 HAstV-positive patients (Table 1). Upper respiratory manifestations were reported in 8 of 20 patients, with significantly more patients in the novel HAstV group (n=7, 70%) compared to the classical HAstV group (n=1, 10%) (P=0.02). Upper respiratory manifestations were reported in all patients (n=3) with HAstV-VA2-positive stool samples (Tables 1 and 2). The mean time between upper respiratory manifestations and HAstV detection was 11.5±5.9 days.
Routine diagnostic rRT-PCR was performed to detect enteric viruses (norovirus,29 rotavirus,30 enterovirus31 and adenovirus (adapted from ref. 32)) in the 20 HAstV-positive cases. This screening was performed on the same stool samples used in HAstV analysis, and enteric viruses were detected in three patients. One was a liver transplant recipient who tested positive for rotavirus (patient no. 3; Table 1) while the remaining two were immunocompetent patients with enterovirus (patient nos. 9 and 12; Table 1).
DISCUSSION
This study describes a unique survey of classical and novel HAstV among pediatric and adult patients in a tertiary care hospital using a panel of specifically designed rRT-PCR assays.
Our investigation showed that novel and classical HAstV were detected with the same frequency in stool samples obtained from routine viral screening in a tertiary care hospital. Similar to other studies, HAstV-1 was the most frequent serotype detected among classical HAstV.18 Our systematic approach over a 1-year period revealed that, of the novel HAstV, only MLB1, MLB2 and VA2 were consistently detected. This finding is in agreement with previous observations in which MLB1, MLB2, MLB3 and VA2 were the novel HAstV most frequently detected in studies among patients with diarrhea.14 However, in our study, screening did not reveal the presence of MLB3, VA1, VA3 or VA4; this observation cannot be attributed to a lower sensitivity of rRT-PCR assays (Supplementary Table S1). While these observations might be expected for VA1, VA3 and VA4, the absence of MLB3 may result from differences in prevalence, depending on factors such as geographical location, climate or year of the study.18 An analysis of the monthly distribution of HAstV-positive cases revealed a uniform distribution of both the number of stool samples tested and the positivity rate throughout the study period (Supplementary Figure S2). Given the small number of cases, it is not possible to form conclusions on seasonality trends.
Investigating the patient profile of HAstV-positive patients revealed a clearly susceptible population. A significantly higher prevalence of novel and classical HAstV-positive samples was observed among pediatric patients compared to adults. All novel and classical HAstV-positive pediatric subjects were ≤four years old. Almost half of all HAstV-positive cases were immunocompromised patients. Children and immunocompromised patients have been previously described as key susceptible populations for classical HAstV. Our results corroborate this finding for classical HAstV and further show this trend to be mirrored in novel HAstV (Table 1). As viremia and dissemination to other organs have been described for HAstV,10, 11, 33 all available additional samples collected for routine care before and/or after the positive samples (respiratory, stool, serum, plasma, CSF and/or biopsy samples) were tested retrospectively for the 20 HAstV-positive cases. However, none of these additional samples returned a positive result (data not shown), thus suggesting that HAstV in these 20 patients were present as a transient infection, not a chronic one. The absence of positive CSF samples for novel and classical HAstV is consistent with other studies. Indeed, only three cases of novel HAstV-associated encephalitis with HAstV-positive CSF have been described.19, 20, 24 The remaining published cases of novel HAstV-associated encephalitis were detected in brain biopsies.14 Of note, of the 105 patients screened in this study, 89 were immunocompetent; this could explain the absence of novel HAstV detection since most of the novel HAstV-associated meningoencephalitis cases were reported in immunocompromised patients. Taken together, HAstV-associated meningoencephalitis cases are rare events, and CSF screening should be considered in specific patients.
A review of medical records revealed that up to 70% of novel HAstV-positive subjects presented upper respiratory manifestations a few days before stool sampling vs only 10% of classical HAstV-positive cases. Although this observation needs to be confirmed with larger and specific prospective studies, it reflects the observation of a recently published case in which HAstV VA1 was detected in a nasopharyngeal specimen of a 13-month-old Tanzanian child with acute respiratory symptoms.25 Furthermore, the mean viral load of novel HAstV was significantly lower than that seen in classical HAstV-positive cases even though the sensitivity of the Hast rRT-PCR assay appears to be the lowest (Supplementary Table S1). However, this observation should be considered with caution as the difference is probably biased by the very low viral loads detected in the three VA2-positive subjects (Table 1). Taken together, and similar to observations for the different adenovirus and enterovirus genotypes, our study offers potential evidence for a divergence in the pathogenic pathways of classical and novel HAstV. Although all novel HAstV-positive cases presented gastroenteritis symptoms (Table 1), it could be postulated that some novel HAstV have a tropism for upper respiratory epithelia, eventually leading to upper respiratory manifestations and followed by subsequent gastroenteritis-like symptoms. However, it remains unknown whether the presence of gastroenteritis-like symptoms reflects a possible viral replication in enteric cells (as suggested by the high MLB2 viral loads detected in patient nos. 5 and 7; Table 1) or if these symptoms are indirect or confounding phenomena. Indeed, recent data showed that the TAstV-2 capsid protein could act as an enterotoxin and that its oral administration was sufficient to induce diarrhea in turkey poults.34 Comprehensive HAstV screening in routine respiratory samples is needed to better investigate the potentially divergent pathogenic pathways of novel and classical HAstV.
Similar to previous findings, the present study revealed the co-detection of novel or classical HAstV with other pathogens (Table 1).16, 35 Despite the well-known association between classical HAstV and gastroenteritis, it remains to be determined whether novel HAstV have the potential to act as unique pathogenic agents or if they are simply bystanders of other enteric agents.
To our knowledge, this study represents the first epidemiologic description of novel HAstV in a tertiary care hospital based on pediatric and adult sample screening. Since this study included all stool samples collected from pediatric and adult patients (with or without diarrheal disorders) for which a viral screening was requested by physicians, the observed prevalence of novel and classical HAstV is likely to be underestimated. Nevertheless, although our results are in line with previously published prevalence reports in patients <5 years old with moderate-severe diarrhea in Kenya and The Gambia,17 the observed prevalence of novel HAstV in the pediatric population (3.4%) was far higher than what has been previously reported in most studies. This result is true either in symptomatically selected patients (that is, those with diarrhea or acute flaccid paralysis) or specifically among hospitalized children.14 Given the retrospective nature of this study, patient characteristics were also based on a retrospective analysis of medical records. This analysis method is particularly important for clinical characteristics, such as respiratory manifestations. Finally, the medical records of HAstV-negative patients were not reviewed in this study. However, further investigations comparing the clinical features between HAstV-positive and -negative patients would be valuable.
In conclusion, this study confirms the clinical and epidemiological relevance of novel HAstV and may help advance the future implementation of novel HAstV molecular assays, such as rRT-PCR. We also identify the target population, namely, immunocompromised and pediatric patients, in which routine screening may yield clinically valuable information.
Acknowledgments
The authors thank Antoine Poncet (AP), Christine Deffert (CD), Rosemary Sudan (RS) (University Hospitals of Geneva) and Mary-Anne Hartley (MAH) (University Hospital Center of Lausanne) for statistical (AP), technical (CD) and editorial assistance (RS and MAH). This study was supported by the Swiss National Science Foundation (Grant NO 310030_165873 to Laurent Kaiser).
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Finkbeiner SR, Holtz LR, Jiang Y et al. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 2009; 6: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Hargest V, Cortez V et al. Astrovirus pathogenesis. Viruses 2017; 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix S, Caballero S, Villena C et al. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J Clin Microbiol 2002; 40: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser LA, Schultz-Cherry S. Pathogenesis of astrovirus infection. Viral Immunol 2005; 18: 4–10. [DOI] [PubMed] [Google Scholar]
- Borrows CL, Turner PC. Seasonal screening for viral gastroenteritis in young children and elderly hospitalized patients: is it worthwhile? J Hosp Infect 2014; 87: 98–102. [DOI] [PubMed] [Google Scholar]
- Gray JJ, Wreghitt TG, Cubitt WD et al. An outbreak of gastroenteritis in a home for the elderly associated with astrovirus type 1 and human calicivirus. J Med Virol 1987; 23: 377–381. [DOI] [PubMed] [Google Scholar]
- Jarchow-Macdonald AA, Halley S, Chandler D et al. First report of an astrovirus type 5 gastroenteritis outbreak in a residential elderly care home identified by sequencing. J Clin Virol 2015; 73: 115–119. [DOI] [PubMed] [Google Scholar]
- Coppo P, Scieux C, Ferchal F et al. Astrovirus enteritis in a chronic lymphocytic leukemia patient treated with fludarabine monophosphate. Ann Hematol 2000; 79: 43–45. [DOI] [PubMed] [Google Scholar]
- Wood DJ, David TJ, Chrystie IL et al. Chronic enteric virus infection in two T-cell immunodeficient children. J Med Virol 1988; 24: 435–444. [DOI] [PubMed] [Google Scholar]
- Wunderli W, Meerbach A, Gungor T et al. Astrovirus infection in hospitalized infants with severe combined immunodeficiency after allogeneic hematopoietic stem cell transplantation. PLoS ONE 2011; 6: e27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A, Pinto RM, Guix S. Human astroviruses. Clin Microbiol Rev 2014; 27: 1048–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans MP, Bijen MH, Monroe SS et al. Age-stratified seroprevalence of neutralizing antibodies to astrovirus types 1 to 7 in humans in the Netherlands. Clin Diagn Lab Immunol 1998; 5: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston S, Willcocks MM, Carter MJ et al. Seroprevalence of astrovirus types 1 and 6 in London, determined using recombinant virus antigen. Epidemiol Infect 1996; 117: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu DL, Cordey S, Brito F et al. Novel human astroviruses: novel human diseases? J Clin Virol 2016; 82: 56–63. [DOI] [PubMed] [Google Scholar]
- Holtz LR, Bauer IK, Rajendran P et al. Astrovirus MLB1 is not associated with diarrhea in a cohort of Indian children. PLoS ONE 2011; 6: e28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamrin P, Thongprachum A, Okitsu S et al. Multiple astrovirus MLB1, MLB2, VA2 clades, and classic human astrovirus in children with acute gastroenteritis in Japan. J Med Virol 2016; 88: 356–360. [DOI] [PubMed] [Google Scholar]
- Meyer CT, Bauer IK, Antonio M et al. Prevalence of classic, MLB-clade and VA-clade astroviruses in Kenya and The Gambia. Virol J 2015; 12: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier Mda P, Carvalho Costa FA, Rocha MS et al. Surveillance of human astrovirus infection in Brazil: the first report of MLB1 astrovirus. PLoS One 2015; 10: e0135687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Morfopoulou S, Hubb J et al. Astrovirus VA1/HMO-C: an increasingly recognized neurotropic pathogen in immunocompromised patients. Clin Infect Dis 2015; 60: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordey S, Vu DL, Schibler M et al. Astrovirus MLB2, a new gastroenteric virus associated with meningitis and disseminated infection. Emerg Infect Dis 2016; 22: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond ML, Perot P, Muth E et al. Next-generation sequencing for diagnosis and tailored therapy: a case report of astrovirus-associated progressive encephalitis. J Pediatric Infect Dis Soc 2015; 4: e53–e57. [DOI] [PubMed] [Google Scholar]
- Naccache SN, Peggs KS, Mattes FM et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin Infect Dis 2015; 60: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan PL, Wagner TA, Briese T et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis 2010; 16: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kuroda M, Kasai M et al. Acute encephalopathy in an immunocompromised boy with astrovirus-MLB1 infection detected by next generation sequencing. J Clin Virol 2016; 78: 66–70. [DOI] [PubMed] [Google Scholar]
- Cordey S, Brito F, Vu DL et al. Astrovirus VA1 identified by next-generation sequencing in a nasopharyngeal specimen of a febrile Tanzanian child with acute respiratory disease of unknown etiology. Emerg Microbes Infect 2016; 5: e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler M, Yerly S, Vieille G et al. Critical analysis of rhinovirus RNA load quantification by real-time reverse transcription-PCR. J Clin Microbiol 2012; 50: 2868–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C, O’Leary JJ, O’Sullivan N. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J Virol Methods 2007; 146: 36–44. [DOI] [PubMed] [Google Scholar]
- Noel JS, Lee TW, Kurtz JB et al. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol 1995; 33: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehne M, Schreier E. Detection of Norovirus genogroup I and II by multiplex real-time RT- PCR using a 3'-minor groove binder-DNA probe. BMC Infect Dis 2006; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MM, Kerin T, Hull J et al. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J Med Virol 2008; 80: 1489–1496. [DOI] [PubMed] [Google Scholar]
- Tapparel C, Cordey S, Van Belle S et al. New molecular detection tools adapted to emerging rhinoviruses and enteroviruses. J Clin Microbiol 2009; 47: 1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen J, Timmen-Wego M, Laudien R et al. Detection of adenoviruses and rotaviruses in drinking water sources used in rural areas of Benin, West Africa. Appl Environ Microbiol 2009; 75: 2798–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz LR, Wylie KM, Sodergren E et al. Astrovirus MLB2 viremia in febrile child. Emerg Infect Dis 2011; 17: 2050–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliopoulos VA, Marvin SA, Freiden P et al. Oral administration of astrovirus capsid protein is sufficient to induce acute diarrhea in vivo. mBio 2016; 7: e01494–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pativada M, Nataraju SM, Ganesh B et al. Emerging trends in the epidemiology of human astrovirus infection among infants, children and adults hospitalized with acute watery diarrhea in Kolkata, India. Infect Genet Evol 2012; 12: 1685–1693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.