Abstract
Objective
Mid-trimester amniocentesis continues to be used for the prenatal diagnosis of chromosomal anomalies and other genetic disorders. Analysis of amniotic fluid obtained at the time of mid-trimester genetic amniocentesis identifies those patients who are at risk for early spontaneous preterm delivery. This is based on a solid body of evidence that found subclinical intra-amniotic inflammation/infection to be causally linked to early spontaneous preterm birth. Although several biomarkers have been proposed to identify intra-amniotic inflammation, the accumulated data suggest that the determination of amniotic fluid matrix metalloproteinase-8 (MMP-8), or neutrophil collagenase, is a powerful predictor of spontaneous preterm delivery. MMP-8 is released by inflammatory cells in response to microbial products or “danger signals.” A rapid point-of-care test has been developed to determine MMP-8 at the bedside within 20 minutes, and without the requirement of laboratory equipment. The objective of this study was to determine whether an elevation of MMP-8 in the amniotic fluid, measured by a rapid point-of-care test, can identify those patients at risk for spontaneous preterm delivery after a mid-trimester genetic amniocentesis.
Study Design
A case-control study was designed to obtain amniotic fluid from asymptomatic singleton pregnant women who underwent mid-trimester genetic amniocentesis. An MMP-8 bedside test was performed to analyze the amniotic fluid of 64 patients with early spontaneous preterm delivery (<30 weeks) and 128 matched controls with normal pregnancy outcomes.
Results
1) The MMP-8 bedside test (Yoon’s MMP-8 Check™) was positive in 42.2% (27/64) of patients with spontaneous preterm delivery but in none (0/128) of the control cases (p<0.001); 2) the MMP-8 bedside test had a sensitivity of 42.2%, and a specificity of 100% in the prediction of spontaneous preterm delivery (<30 weeks) following a mid-trimester genetic amniocentesis; and 3) among the patients with spontaneous preterm delivery, those with a positive MMP-8 bedside test had a significantly higher rate of spontaneous delivery within 2 weeks and 4 weeks of an amniocentesis [40.7% (11/27) vs. 5.4% (2/37); 63.0% (17/27) vs. 24.3% (9/37)] and a shorter interval-to-delivery period than those with a negative test [interval-to-delivery: median (range), 16 days (0–95 days) vs. 42 days (2–91 days); p<0.05 for each].
Conclusion
We conclude that 42% of patients with an early spontaneous preterm delivery (<30 weeks) could be identified by a rapid MMP-8 bedside test at the time of their mid-trimester genetic amniocentesis. The MMP-8 bedside test is a powerful predictor of early spontaneous preterm birth in asymptomatic pregnant women.
Keywords: amniotic fluid, intra-amniotic inflammation/infection, point-of-care (POC) test, preterm parturition
Introduction
Mid-trimester genetic amniocentesis has been widely used for the prenatal diagnosis of fetal chromosomal disorders [1, 2]. With the introduction of and interest in non-invasive prenatal testing (NIPT) [3–13], amniocentesis continues to be used as a final diagnostic test after aneuploidy has been detected by means of NIPT [8, 14–25]. Indeed, Papp has recently proposed that an amniocentesis is preferable to chorionic villous sampling (CVS) in this setting in order to avoid the diagnostic problems related to confined placental mosaicism [26, 27].
A solid body of evidence indicates that subclinical intra-amniotic inflammation or infection is causally linked with spontaneous preterm delivery [28–52] and adverse pregnancy outcome [38, 40, 46, 53–68]. Such pathologic processes can be detected by analyzing amniotic fluid for microorganisms (bacteria or viruses) [69–92] or inflammatory markers [38, 50, 53, 57, 74, 76, 90, 93–160]. We previously reported that amniotic fluid matrix metalloproteinase-8 (MMP-8) [121, 123, 124, 127, 128, 132–134, 136, 161] and interleukin (IL)-6 [53, 74, 76, 95, 103, 105] are powerful biomarkers of intra-amniotic inflammation/infection. These studies were largely conducted using enzyme-linked immunoassays with research reagents. Translation of the findings to clinical practice requires that results be available quickly and easily. Therefore, we have developed a qualitative immunochromatographic kit that detects the presence of MMP-8 in amniotic fluid at the patient’s bedside. In previous studies, we documented the value of the amniotic fluid MMP-8 bedside test for patients with preterm labor [127] as well as those with prelabor rupture of the membranes (PROM) [128]. Moreover, we found that a positive amniotic fluid MMP-8 bedside test is a predictor of funisitis (a marker of fetal systemic inflammation) [129]. The objective of this study was to evaluate the performance of this MMP-8 rapid test in the identification of patients at risk for spontaneous preterm delivery after a mid-trimester genetic amniocentesis.
Materials and Methods
Study design
A case-control study was designed using amniotic fluid from asymptomatic pregnant women who underwent a genetic amniocentesis during the mid-trimester. The study population included patients with singleton gestations who underwent spontaneous preterm delivery before 30 weeks. These patients were matched for maternal age (within 5 years), parity (nulliparous vs. multiparous), gestational age at amniocentesis (within 2 weeks), and year of amniocentesis (within 3 years) with 128 controls who underwent genetic amniocentesis and delivered singleton newborns after 37 weeks of gestation (1:2 matching). Patients with abnormal fetal karyotypes, major fetal anomalies, or any symptom or sign of preterm delivery at the time of genetic amniocentesis were excluded (e.g. suspicion of ruptured or dilated membranes). Written innformed consent was obtained from each patient prior to the procedure. The Institutional Review Board of Seoul National University Hospital approved the collection and use of amniotic fluid samples and clinical information for research purposes.
MMP-8 Rapid Test
Amniotic fluid was processed for fetal karyotyping and the unused fluid was centrifuged at 2,000 rpm for 10 minutes at 4 °C, aliquoted and pipetted, and then stored at −70 °C until assay. After thawing the stored amniotic fluid, the MMP-8 rapid test (Yoon’s MMP-8 Check™; OBMed Co., Ltd., Seoul, Republic of Korea) was performed by personnel blinded to the clinical information. Yoon’s MMP-8 Check™ is a qualitative immunochromatographic test that detects the presence of MMP-8 in human amniotic fluid with a threshold of 10 ng/ml. The manufacturer recommends the addition of 25 μl of amniotic fluid and 75 μl (3 drops) of buffer to the test window (Figure 1). The results were scored after 20 minutes. When the results were equivocal (very weak bands), the test was repeated using 12.5 μl of amniotic fluid and 75 μl (3 drops) of the buffer.
Figure 1.
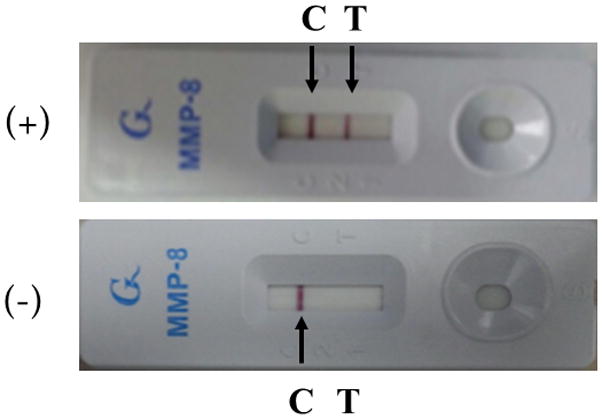
The MMP-8 rapid test (Yoon’s MMP-8 Check™) was considered positive when two bands were visible, corresponding to the control (C) and testing (T) channels of the kit (arrows).
Statistical analysis
The Mann-Whitney U test was used to compare continuous variables, and the Fisher’s exact test was used for the comparison of proportions.
Results
The clinical characteristics and pregnancy outcomes of the study population are shown in Table 1. There were no significant differences in the maternal age, frequency of nulliparity, and gestational age at amniocentesis between the two groups.
Table 1.
Clinical characteristics and pregnancy outcome of the study population
| Characteristics | Spontaneous early preterm delivery < 30 weeks (n=64) | Term delivery (n=128) | P-value |
|---|---|---|---|
| Mean maternal age, years (±SD) | 34.9 ± 4.5 | 35.0 ± 4.0 | NS |
| Nulliparity | 15 (23.4%) | 30 (23.4%) | NS |
| Previous spontaneous preterm delivery | 6 (9.4%) | 5 (3.9%) | NS |
| Median gestational age at amniocentesis, weeks (range) | 17.7 (15.6–22.3) | 17.6 (15.4–23.0) | NS |
| Median gestational age at delivery, weeks (range) | 23.0 (16.6–29.7) | 39.0 (37.0–41.4) | <0.001 |
| Positive MMP-8 rapid kit result | 42.2% (27/64) | 0.0% (0/128) | <0.001 |
SD = Standard deviation; NS = Not significant; MMP = Matrix metalloproteinase
Data are presented as median (range) or n (%).
The MMP-8 bedside test was positive in 42.2% (27/64) of patients with early spontaneous preterm delivery (<30 weeks) but in none (0/128) of the control group (p<0.001). The MMP-8 bedside test had a sensitivity of 42.2%, and a specificity of 100% in the prediction of spontaneous preterm delivery (<30 weeks) after mid-trimester genetic amniocentesis in asymptomatic singleton pregnant women.
Table 2 demonstrates the comparison of clinical characteristics and pregnancy outcomes according to the results of the MMP-8 bedside test among the patients with spontaneous preterm delivery. There were no significant differences in the maternal age, frequency of nulliparity, and gestational age at amniocentesis between the two groups. However, the median interval-to-delivery period after amniocentesis was significantly shorter for those with a positive MMP-8 bedside test than for those with a negative test [median (range), 16 days (0–95 days) vs. 42 days (2–91 days); p=0.011]. Patients with a positive MMP-8 bedside test had a significantly higher rate of delivery within 2 to 4 weeks after an amniocentesis than those with a negative test [40.7% (11/27) vs 5.4% (2/37), p=0.001; 63.0% (17/27) vs 24.3% (9/37), p=0.004].
Table 2.
Clinical characteristics and pregnancy outcomes of 64 cases with spontaneous early preterm delivery (<30 weeks of gestation) according to results of the MMP-8 bedside test
| Characteristic | MMP-8 bedside test Positive (n=27) | MMP-8 bedside test Negative (n=37) | P-value |
|---|---|---|---|
| Mean maternal age, years (±SD) | 33.8 ± 4.5 | 35.6 ± 4.4 | NS |
| Nulliparity | 7 (25.9%) | 8 (21.6%) | NS |
| History of spontaneous preterm delivery | 1 (3.7%) | 5 (13.5%) | NS |
| Median gestational age at amniocentesis, weeks (range) | 18.0 (15.6–22.3) | 17.6 (15.6–21.7) | NS |
| Median gestational age at delivery, weeks (range) | 21.0 (16.6–29.6) | 24.4 (17.2–29.7) | NS |
| Indication for delivery | NS | ||
| • Preterm labor | 10 (37.0%) | 17 (45.9%) | |
| • PROM | 15 (55.6%) | 17 (45.9%) | |
| • Cervical insufficiency | 0 (0.0%) | 3 (8.1%) | |
| • Spontaneous abortion | 2 (7.4%) | 0 (0.0%) | |
| Median amniocentesis-to-delivery interval, day (range) | 16 (0–95) | 42 (2–91) | 0.011 |
| • Time from amniocentesis to delivery ≤1week | 6 (22.2%) | 2 (5.4%) | NS |
| • Time from amniocentesis to delivery ≤2weeks | 11 (40.7%) | 2 (5.4%) | 0.001 |
| • Time from amniocentesis to delivery ≤4weeks | 17 (63.0%) | 9 (24.3%) | 0.004 |
MMP = Matrix metalloproteinase; SD = Standard deviation; NS = Not significant; PROM = Premature rupture of the membranes
Data are presented as median (range) or n (%).
DISCUSSION
Principal finding of the study
A positive MMP-8 bedside test identified about one-half of the patients who underwent a mid-trimester genetic amniocentesis and subsequently had a spontaneous abortion or an early spontaneous preterm delivery (<30 weeks of gestation).
Clinical implications
The current practice of mid-trimester genetic amniocentesis is specifically focused on the prenatal diagnosis of chromosomal abnormalities; however, amniotic fluid analysis provides a powerful tool to identify patients at risk for spontaneous preterm delivery by determining the presence of intra-amniotic inflammation. An obstacle to the assessment of inflammation has been the availability of a rapid test that can be informative, reliable, and inexpensive.
The MMP-8 bedside test is a simple point-of-care method for the rapid identification of intra-amniotic inflammation without laboratory equipment [127–130, 135, 136]. The test has performed well in the identification of intra-amniotic inflammation/infection in patients with preterm labor [127, 136] and preterm PROM [128, 135]. Moreover, a positive MMP-8 bedside test is a marker for fetal systemic inflammation, presumably because neutrophils found in the amniotic fluid in cases of inflammation are of fetal origin [162]. Indeed, an elevated concentration of MMP-8 in the amniotic fluid is associated with funisitis [129], the hallmark of the fetal inflammatory response syndrome.
We previously reported that the odds ratio of an elevated concentration of MMP-8 (>23 ng/mL) in the amniotic fluid is approximately 68 [122]. In the current study, the odds ratio could not be calculated due to the lack of false-positive results. Once patients are identified as “at risk,” the next step is to investigate the etiology of the inflammatory process. A rapid test allows identification of the samples that need to be sent to the microbiologic laboratory for both cultivation and molecular microbiologic studies. A positive culture for microorganisms has been the gold standard for the diagnosis of intra-amniotic infection. However, recent data suggest that the combined use of cultivation and molecular microbiologic methods results in the increased detection of microorganisms: some bacteria are fastidious or cannot be cultured with traditional techniques available in hospital clinical laboratories [75, 78, 81, 82, 85, 86, 163–166]. The performance of an amniotic fluid rapid MMP-8 test is of great value because it allows immediate identification of the samples that need to be worked up for the presence of bacteria or viruses. The immediate results of the amniotic fluid MMP-8 rapid test will prevent the need to retrieve the sample if the laboratory informs the clinician that there is evidence of intra-amniotic inflammation based on conventional methods such as an amniotic fluid white blood cell count, glucose, or other methods [71, 73, 74, 76, 96, 98, 167–175]. The identification of intra-amniotic infection is crucial because antibiotics would be helpful only in patients with infection. Sterile intra-amniotic inflammation has been recently recognized as a major factor in patients with preterm labor [47, 48], preterm PROM [46], and a sonographic short cervix [176]. The identification of danger signals responsible for such inflammatory processes has not been determined [156, 177–186]. However, it is possible that patients with sterile intra-amniotic inflammation may benefit from anti-inflammatory agents rather than antibiotics [187–192]. Sterile intra-amniotic inflammation could be detected when a patient has a positive amniotic fluid MMP-8 rapid test but is negative for bacteria and virus when a combination of cultivation and molecular microbiologic techniques is used. Randomized clinical trials are required to address the optimal treatment in patients with intra-amniotic inflammation and microorganisms and of those with sterile intra-amniotic inflammation. An important observation in this study is that the interval between amniocentesis and delivery was approximately 16 days in patients with intra-amniotic inflammation. Therefore, there is a window of time during which treatment may address the pathologic process responsible for the intra-amniotic inflammatory response.
Strengths and Limitations
The major strengths of this study are the large number of early spontaneous preterm deliveries and that personnel were blinded to the clinical outcome. The limitations are related to its case-control nature. A cohort study would be an ideal way to estimate the predictive values of the MMP-8 rapid test.
Conclusion
A positive MMP-8 rapid test of amniotic fluid obtained at the time of mid-trimester genetic amniocentesis identified about one-half of those patients who were at risk of an early spontaneous preterm delivery (<30 weeks). This information has prognostic value and could be the basis for the design of intervention trials to determine whether anti-inflammatory agents and/or antibiotics can reduce the rate of preterm delivery in patients with intra-amniotic infection or sterile intra-amniotic inflammation.
Acknowledgments
This research was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C0768). This research was also supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS).
Footnotes
Presented as a poster presentation in 35th Society of Maternal-Fetal Medicine Annual Meeting, February 2–7, 2015, San Diego, CA.
Declaration: The test described in this article is the subject of a patent by the Seoul National University in Seoul, Republic of Korea. Dr. B.H. Yoon is listed as an inventor and has a financial interest in OBMed Co., Ltd., Seoul, Republic of Korea, the manufacturer of this test. A financial interest is defined as a potential gain or a potential loss derived from the activity of this company.
References
- 1.Eisenberg B, Wapner RJ. Clinical proceduress in prenatal diagnosis. Best Pract Res Clin Obstet Gynaecol. 2002;16:611–627. doi: 10.1053/beog.2002.0328. [DOI] [PubMed] [Google Scholar]
- 2.Simpson JL. Invasive procedures for prenatal diagnosis: any future left? Best Pract Res Clin Obstet Gynaecol. 2012;26:625–638. doi: 10.1016/j.bpobgyn.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Lo YM. Fetal nucleic acids in maternal blood: the promises. Clin Chem Lab Med. 2012;50:995–998. doi: 10.1515/CCLM.2011.765. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314:162–169. doi: 10.1001/jama.2015.7120. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Mahoney MJ. Noninvasive prenatal testing and detection of maternal cancer. JAMA. 2015;314:131–133. doi: 10.1001/jama.2015.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canadian Agency for Drugs and Technologies in Health. Non-invasive prenatal testing: a review of the cost effectiveness and guidelines. Ottawa, ON: 2014. [cited 23 Oct 2015]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK274056/ [PubMed] [Google Scholar]
- 7.Daley R, Hill M, Chitty LS. Non-invasive prenatal diagnosis: progress and potential. Arch Dis Child Fetal Neonatal Ed. 2014;99:F426–30. doi: 10.1136/archdischild-2013-304828. [DOI] [PubMed] [Google Scholar]
- 8.Platt LD, Janicki MB, Prosen T, et al. Impact of noninvasive prenatal testing in regionally dispersed medical centers in the United States. Am J Obstet Gynecol. 2014;211:368e1–e7. doi: 10.1016/j.ajog.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 9.Porreco RP, Garite TJ, Maurel K, et al. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol. 2014;211:365e1–e12. doi: 10.1016/j.ajog.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Salomon LJ, Alfirevic Z, Audibert F, et al. ISUOG consensus statement on the impact of non-invasive prenatal testing (NIPT) on prenatal ultrasound practice. Ultrasound Obstet Gynecol. 2014;44:122–123. doi: 10.1002/uog.13393. [DOI] [PubMed] [Google Scholar]
- 11.Allyse M, Minear MA, Berson E, et al. Non-invasive prenatal testing: a review of international implementation and challenges. Int J Womens Health. 2015;7:113–126. doi: 10.2147/IJWH.S67124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137e1–e8. doi: 10.1016/j.ajog.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Oepkes D, Tabor A, Yaron Y. Prenatal aneuploidy screening using cell free DNA. Am J Obstet Gynecol. 2015;21:596–597. doi: 10.1016/j.ajog.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi DW. Pregnancy: Prepare for unexpected prenatal test results. Nature. 2015;522:29–30. doi: 10.1038/522029a. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll DA, Gross SJ. Screening for fetal aneuploidy and neural tube defects. Genet Med. 2009;11:818–821. doi: 10.1097/GIM.0b013e3181bb267b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaney SA, Palomaki GE, Scott JA, et al. Noninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysis. JAMA. 2011;306:627–636. doi: 10.1001/jama.2011.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norwitz ER, Levy B. Noninvasive prenatal testing: the future is now. Rev Obstet Gynecol. 2013;6:48–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Yu SC, Jiang P, Choy KW, et al. Noninvasive prenatal molecular karyotyping from maternal plasma. PLoS One. 2013;8:e60968. doi: 10.1371/journal.pone.0060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner AJ, Mitchell ME, Tomita-Mitchell A. Use of cell-free fetal DNA in maternal plasma for noninvasive prenatal screening. Clin Perinatol. 2014;41:957–966. doi: 10.1016/j.clp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Ferres MA, Hui L, Bianchi DW. Antenatal noninvasive DNA testing: clinical experience and impact. Am J Perinatol. 2014;31:577–582. doi: 10.1055/s-0034-1371706. [DOI] [PubMed] [Google Scholar]
- 21.Robinson C, van den Boom D, Bombard AT. Noninvasive prenatal detection of aneuploidy. Clin Obstet Gynecol. 2014;57:210–225. doi: 10.1097/GRF.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 22.Society for Maternal-Fetal Medicine (SMFM) Publications Committee. #36: Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212:711–716. doi: 10.1016/j.ajog.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Thompson AE. JAMA PATIENT PAGE. Noninvasive prenatal testing. JAMA. 2015;314:198. doi: 10.1001/jama.2015.7655. [DOI] [PubMed] [Google Scholar]
- 24.Mennuti MT, Cherry AM, Morrissette JJ, et al. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy? Am J Obstet Gynecol. 2013;209:415–419. doi: 10.1016/j.ajog.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Williams J, 3rd, Rad S, Beauchamp S, et al. Utilization of noninvasive prenatal testing: impact on referrals for diagnostic testing. Am J Obstet Gynecol. 2015;213:102e1–e6. doi: 10.1016/j.ajog.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Papp C, Beke A, Mezei G, et al. Chorionic villus sampling: a 15-year experience. Fetal Diagn Ther. 2002;17:218–227. doi: 10.1159/000059373. [DOI] [PubMed] [Google Scholar]
- 27.Papp C, Papp Z. Chorionic villus sampling and amniocentesis: what are the risks in current practice? Curr Opin Obstet Gynecol. 2003;15:159–165. doi: 10.1097/00001703-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 30.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 33.Fidel PL, Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 34.Gravett MG, Witkin SS, Haluska GJ, et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 35.Gomez R, Ghezzi F, Romero R, et al. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 36.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 37.Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–176. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 38.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 39.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 40.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17:12–19. doi: 10.1016/j.siny.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combs CA, Gravett M, Garite T, et al. Abstract No. 73: Intramniotic inflammation may be more important than the presence of microbes as a determinant of perinatal outcome in preterm labor. Am J Obstet Gynecol. 2013;208:S44. [Google Scholar]
- 46.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–358. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;211:708. doi: 10.1016/j.ajog.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 50.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125e1–e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Combs CA, Gravett M, Garite TJ. Reply: To PMID 24274987. Am J Obstet Gynecol. 2014;211:708–709. doi: 10.1016/j.ajog.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 52.Bujold E, Tétu A, Laforest G, et al. Abstract No. 22: Midtrimester microbial invasion of the amniotic cavity and the risk of very preterm birth. Am J Obstet Gynecol. 2014;210:S15. [Google Scholar]
- 53.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 54.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 55.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 56.Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 57.Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 58.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 59.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newnham JP, Moss TJ, Kramer BW, et al. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186:1062–1068. doi: 10.1067/mob.2002.122293. [DOI] [PubMed] [Google Scholar]
- 61.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–F135. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Been JV, Rours IG, Kornelisse RF, et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol. 2009;201:587e1–e8. doi: 10.1016/j.ajog.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 63.Kramer BW, Kallapur S, Newnham J, et al. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jobe AH, Kallapur SG. Chorioamnionitis, surfactant, and lung disease in very low birth weight infants. J Pediatr. 2010;156:3–4. doi: 10.1016/j.jpeds.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Iliodromiti Z, Zygouris D, Sifakis S, et al. Acute lung injury in preterm fetuses and neonates: mechanisms and molecular pathways. J Matern Fetal Neonatal Med. 2013;26:1696–1704. doi: 10.3109/14767058.2013.798284. [DOI] [PubMed] [Google Scholar]
- 66.Kim SM, Romero R, Park JW, et al. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med. 2015;28:1500–1509. doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016;29:1525–1529. doi: 10.3109/14767058.2015.1053862. [DOI] [PubMed] [Google Scholar]
- 68.Miyazaki K, Furuhashi M, Ishikawa K, et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the Neonatal Research Network Japan. J Matern Fetal Neonatal Med. 2016;29:331–337. doi: 10.3109/14767058.2014.1000852. [DOI] [PubMed] [Google Scholar]
- 69.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–952. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 70.Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63:38–43. [PubMed] [Google Scholar]
- 71.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 73.Asrat T, Nageotte MP, Garite TJ, et al. Gram stain results from amniocentesis in patients with preterm premature rupture of membranes--comparison of maternal and fetal characteristics. Am J Obstet Gynecol. 1990;163:887–889. doi: 10.1016/0002-9378(90)91089-u. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 75.Blanchard A, Hentschel J, Duffy L, et al. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 76.Gomez R, Romero R, Galasso M, et al. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 77.Markenson GR, Martin RK, Tillotson-Criss M, et al. The use of the polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1997;177:1471–1477. doi: 10.1016/s0002-9378(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 78.Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 79.Oyarzun E, Yamamoto M, Kato S, et al. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179:1115–1119. doi: 10.1016/s0002-9378(98)70115-2. [DOI] [PubMed] [Google Scholar]
- 80.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol. 1999;11:541–547. doi: 10.1097/00001703-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 82.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 83.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 84.Jacobsson B, Mattsby–Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 85.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holst RM, Hagberg H, Wennerholm UB, et al. Prediction of microbial invasion of the amniotic cavity in women with preterm labour: analysis of multiple proteins in amniotic and cervical fluids. BJOG. 2011;118:240–249. doi: 10.1111/j.1471-0528.2010.02765.x. [DOI] [PubMed] [Google Scholar]
- 89.Kacerovsky M, Pliskova L, Bolehovska R, et al. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol. 2011;205:213e1–e7. doi: 10.1016/j.ajog.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 90.Marconi C, de Andrade Ramos BR, Peracoli JC, et al. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65:549–556. doi: 10.1111/j.1600-0897.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 91.Gervasi MT, Romero R, Bracalente G, et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:2002–2013. doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen-Daniels MJ, Serrano MG, Pflugner LP, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol. 2015;212:779e1–e13. doi: 10.1016/j.ajog.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. discussion 20–3. [DOI] [PubMed] [Google Scholar]
- 95.Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–183. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 96.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 97.Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 98.Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901–911. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 99.Yoon BH, Romero R, Jun JK, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 100.Ghidini A, Jenkins CB, Spong CY, et al. Elevated amniotic fluid interleukin-6 levels during the early second trimester are associated with greater risk of subsequent preterm delivery. Am J Reprod Immunol. 1997;37:227–231. doi: 10.1111/j.1600-0897.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 101.Wenstrom KD, Andrews WW, Hauth JC, et al. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 102.Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–178. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 103.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 104.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–343. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med. 2014;27:757–769. doi: 10.3109/14767058.2013.844123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2015 Sep;28:1510–1519. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29:349–359. doi: 10.3109/14767058.2015.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360–367. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 111.Romero R, Sepulveda W, Mazor M, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863–872. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell MD, Edwin SS, Silver RM, et al. Potential agonist action of the interleukin-1 receptor antagonist protein: implications for treatment of women. J Clin Endocrinol Metab. 1993;76:1386–1388. doi: 10.1210/jcem.76.5.8496334. [DOI] [PubMed] [Google Scholar]
- 114.Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 115.Athayde N, Romero R, Maymon E, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 116.Pacora P, Romero R, Maymon E, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 117.Greig PC, Herbert WN, Robinette BL, et al. Amniotic fluid interleukin-10 concentrations increase through pregnancy and are elevated in patients with preterm labor associated with intrauterine infection. Am J Obstet Gynecol. 1995;173:1223–1227. doi: 10.1016/0002-9378(95)91358-0. [DOI] [PubMed] [Google Scholar]
- 118.Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 120.Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 121.Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 122.Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 123.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–1155. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 124.Maymon E, Romero R, Chaiworapongsa T, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–1148. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 125.Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–306. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 126.Biggio JR, Jr, Ramsey PS, Cliver SP, et al. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:109–113. doi: 10.1016/j.ajog.2004.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–1030. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 128.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292e1–e5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 129.Park CW, Lee SM, Park JS, et al. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee SJ, Won HS, Kim MN, et al. Diagnostic value of the matrix metalloproteinase-8 rapid test for detecting microbial invasion of the amniotic cavity. Eur J Clin Microbiol Infect Dis. 2008;27:1257–1260. doi: 10.1007/s10096-008-0566-7. [DOI] [PubMed] [Google Scholar]
- 131.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, et al. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118:1042–1054. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 132.Park CW, Yoon BH, Kim SM, et al. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet Gynecol Sci. 2013;56:167–175. doi: 10.5468/ogs.2013.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaemsaithong P, Romero R, Docheva N, et al. A rapid point-of-care test (MMP-8) for the identification of intra-amniotic inflammation and impending preterm delivery. Abstract presented at the 12th World Congress of Perinatal Medicine; 3–6 November 2015; Madrid, Spain. [Google Scholar]
- 134.Chaemsaithong P, Romero R, Chaiyasit N, et al. Rapid MMP-8 as a point-of-care test in the identification of intra-amniotic inflammation in patients with preterm PROM. Abstract presented at the 12th World Congress of Perinatal Medicine; 3–6 November 2015; Madrid, Spain. [Google Scholar]
- 135.Park HS, Kim SA. Abstract No. 322: The value of the genedia MMP-8 rapid test for diagnosing intraamniotic infection/inflammation and predicting adverse pregnancy outcomes in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2015;212:S174. [Google Scholar]
- 136.Kim SM, Lee JH, Park CW, et al. Abstract No. 556: One third of early spontaneous preterm delivery can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. Am J Obstet Gynecol. 2015;212:S277. [Google Scholar]
- 137.Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 138.Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 139.Park KH, Chaiworapongsa T, Kim YM, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 140.Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545–1553. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 141.Athayde N, Romero R, Gomez R, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8:213–219. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 142.Locksmith GJ, Clark P, Duff P, et al. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol. 1999;94:1–6. doi: 10.1016/s0029-7844(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 143.Maymon E, Romero R, Pacora P, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 144.Locksmith GJ, Clark P, Duff P, et al. Amniotic fluid concentrations of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during pregnancy and labor. Am J Obstet Gynecol. 2001;184:159–164. doi: 10.1067/mob.2001.108860. [DOI] [PubMed] [Google Scholar]
- 145.Harirah H, Donia SE, Hsu CD. Amniotic fluid matrix metalloproteinase-9 and interleukin-6 in predicting intra-amniotic infection. Obstet Gynecol. 2002;99:80–84. doi: 10.1016/s0029-7844(01)01632-5. [DOI] [PubMed] [Google Scholar]
- 146.Esplin MS, Romero R, Chaiworapongsa T, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 147.Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 148.Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–257. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nhan-Chang CL, Romero R, Kusanovic JP, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–775. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keelan JA, Wang K, Chaiworapongsa T, et al. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod. 2003;9:535–540. doi: 10.1093/molehr/gag068. [DOI] [PubMed] [Google Scholar]
- 151.Keelan JA, Yang J, Romero RJ, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod. 2004;70:253–259. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 152.Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell MD, Chang MC, Chaiworapongsa T, et al. Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J Clin Endocrinol Metab. 2005;90:4244–4248. doi: 10.1210/jc.2004-2496. [DOI] [PubMed] [Google Scholar]
- 154.Pacora P, Romero R, Chaiworapongsa T, et al. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med. 2009;37:503–511. doi: 10.1515/JPM.2009.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kusanovic JP, Romero R, Chaiworapongsa T, et al. Amniotic fluid sTREM-1 in normal pregnancy, spontaneous parturition at term and preterm, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:34–47. doi: 10.3109/14767050903009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero R, Espinoza J, Hassan S, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–398. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016 Jan;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015 Dec;213:836.e1–e18. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park CW, Yoon BH, Jun JK, et al. Abstract No. 469: Approximately one-fifth of patients with preterm labor and intact membranes and low amniotic fluid white blood cell counts have evidence of intra-amniotic inflammation and are at risk for impending preterm delivery when MMP-8 is used. American Journal of Obstetrics and Gynecology. 2012;206:S214. [Google Scholar]
- 162.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 163.Kim M, Kim G, Romero R, et al. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–152. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 164.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 165.Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211.e1–e8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rodriguez N, Fernandez C, Zamora Y, et al. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol. 1988;5:64–69. doi: 10.1055/s-2007-999657. [DOI] [PubMed] [Google Scholar]
- 168.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 169.Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991;165:1105–1110. doi: 10.1016/0002-9378(91)90480-f. [DOI] [PubMed] [Google Scholar]
- 170.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167:1092–1095. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 171.Greig PC, Ernest JM, Teot L. Low amniotic fluid glucose levels are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. Am J Obstet Gynecol. 1994;171:365–370. doi: 10.1016/s0002-9378(94)70036-2. discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 172.Hazan Y, Mazor M, Horowitz S, et al. The diagnostic value of amniotic fluid Gram stain examination and limulus amebocyte lysate assay in patients with preterm birth. Acta Obstet Gynecol Scand. 1995;74:275–280. doi: 10.3109/00016349509024449. [DOI] [PubMed] [Google Scholar]
- 173.Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87:231–237. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 174.Carroll SG, Philpott-Howard J, Nicolaides KH. Amniotic fluid gram stain and leukocyte count in the prediction of intrauterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther. 1996;11:1–5. doi: 10.1159/000264270. [DOI] [PubMed] [Google Scholar]
- 175.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8:155–158. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<155::AID-MFM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 176.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014 Sep 24;:1–17. doi: 10.3109/14767058.2014.954243. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 178.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–778. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oppenheim JJ, Tewary P, de la Rosa G, et al. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 180.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 181.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323:1683–1684. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 183.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dubicke A, Andersson P, Fransson E, et al. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010;84:86–94. doi: 10.1016/j.jri.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 185.Nunez G. Intracellular sensors of microbes and danger. Immunol Rev. 2011;243:5–8. doi: 10.1111/j.1600-065X.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 186.Ahmed AI, Chaemsaithong P, Chaiwaropongsa TZD, et al. 599: A receptor for danger signals, advanced glycation end products (RAGE) in fetal systemic inflammation and clinical chorioamnionitis. American Journal of Obstetrics and Gynecology. 2015;212:S298. [Google Scholar]
- 187.Keelan JA, Khan S, Yosaatmadja F, et al. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-kappaB inhibitor. J Immunol. 2009;183:5270–5278. doi: 10.4049/jimmunol.0802660. [DOI] [PubMed] [Google Scholar]
- 188.Pirianov G, Waddington SN, Lindstrom TM, et al. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 189.Nath CA, Ananth CV, Smulian JC, et al. Can sulfasalazine prevent infection-mediated pre-term birth in a murine model? Am J Reprod Immunol. 2010;63:144–149. doi: 10.1111/j.1600-0897.2009.00773.x. [DOI] [PubMed] [Google Scholar]
- 190.Stinson LF, Ireland DJ, Kemp MW, et al. Effects of cytokine-suppressive anti-inflammatory drugs on inflammatory activation in ex vivo human and ovine fetal membranes. Reproduction. 2014;147:313–320. doi: 10.1530/REP-13-0576. [DOI] [PubMed] [Google Scholar]
- 191.Ireland DJ, Kemp MW, Miura Y, et al. Intra-amniotic pharmacological blockade of inflammatory signalling pathways in an ovine chorioamnionitis model. Mol Hum Reprod. 2015;21:479–489. doi: 10.1093/molehr/gav005. [DOI] [PubMed] [Google Scholar]
- 192.Ng PY, Ireland DJ, Keelan JA. Drugs to block cytokine signaling for the prevention and treatment of inflammation-induced preterm birth. Front Immunol. 2015;6:166. doi: 10.3389/fimmu.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]


