Abstract
We recently reported that increased NADPH oxidase 4 (NOX4) expression and activity during aging results in enhanced cellular and mitochondrial oxidative stress, vascular inflammation, dysfunction, and atherosclerosis. The goal of the present study was to elucidate the molecular mechanism(s) for these effects and determine the importance of NOX4 modulation of proinflammatory gene expression in mouse vascular smooth muscle cells (VSMCs). A novel peptide-mediated siRNA transfection approach was used to inhibit Nox4 expression with minimal cellular toxicity. Using melittin-derived peptide p5RHH, we achieved significantly higher transfection efficiency (92% vs. 85% with Lipofectamine) and decreased toxicity (p < 0.001 vs. Lipofectamine in MTT and p < 0.0001 vs. Lipofectamine in LDH assays) in VSMCs. TGFβ1 significantly upregulates Nox4 mRNA (p < 0.01) and protein (p < 0.01) expression in VSMCs. p5RHH-mediated Nox4 siRNA transfection greatly attenuated TGFβ1-induced upregulation of Nox4 mRNA (p < 0.01) and protein (p < 0.0001) levels and decreased hydrogen peroxide production (p < 0.0001). Expression of pro-inflammatory genes Ccl2, Ccl5, Il6, and Vcam1 was significantly upregulated in VSMCs in several settings cells isolated from aged vs. young wild-type mice, in atherosclerotic arteries of Apoe−/− mice, and atherosclerotic human carotid arteries and correlated with NOX4 expression. p5RHH-mediated Nox4 siRNA transfection significantly attenuated the expression of these pro-inflammatory genes in TGFβ1-treated mouse VSMCs, with the highest degree of inhibition in the expression of Il6. p5RHH peptide-mediated knockdown of TGFβ-activated kinase 1 (TAK1, also known as Map3k7), Jun, and Rela, but not Nfkb2, downregulated TGFβ1-induced Nox4 expression in VSMCs. Together, these data demonstrate that increased expression and activation of NOX4, which might result from increased TGFβ1 levels seen during aging, induces a proinflammatory phenotype in VSMCs, enhancing atherosclerosis.
Keywords: Inflammation, Atherosclerosis, Cytokines, Nanoparticles, Reactive oxygen species
1. Introduction
Oxidative stress and inflammation are interrelated and work in a vicious feed-forward cycle during atherogenesis: Reactive oxygen species (ROS) activate transcription factors that regulate inflammatory cytokines, chemokines and soluble mediators including metabolites of arachidonic acid. Cytokines, and chemokines secreted by inflammatory cells recruit more inflammatory cells to the sites of inflammation resulting in the production of more ROS, thus exacerbating this adverse cycle [1,2]. Accumulating evidence points to inflammation/immunity as a central hub for integrating multiple pathways that drive atherogenesis and its sequelae [3].
Broad experimental evidence supports the key role of oxidative stress in all stages of atherosclerosis pathophysiology, including the formation and progression of atheroma and plaque stability [4,5]. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) are an important and widely expressed enzyme family with ROS generation as its primary function. The NOX family consists of 7 catalytic homo-logues. Of these, NOX1, NOX2, NOX4, and NOX5 are expressed in the vasculature [6,7]. All NOX catalytic subunits, except NOX5, associate with p22phox to make the membrane-bound catalytic core. NOX1 and NOX2 induce atherogenesis by promoting inflammation in cells both intrinsic and extrinsic to the vessel wall [7]. Unlike NOX1/2-dependent oxidases, the activity of NOX4-dependent NOX does not require cytosolic regulatory subunits and is regulated at the expression level, generating predominantly H2O2 [8,9]. NOX4 is ubiquitously expressed, including in vascular smooth muscle cells (VSMCs), a dominant constituent of the vessel wall whose functions are critical determinants of vascular homeostasis and disease [10,11]. Uniquely to the NOX family, NOX4 localizes to mitochondria, contributing to mitochondrial ROS levels [12–14].
NOX4 expression and activity is significantly increased in vascular wall cells by proinflammatory mediators and cytokines, including transforming growth factor β1 (TGFβ1) [15–18]. We have shown recently that NOX4 is a mediator of cardiovascular disease (CVD) in aging hyperlipidemic mice, and NOX4 expression levels in the arterial wall in humans correlate with age and atherosclerotic severity [14]. Xu et al. also observed an age-dependent increase in ROS levels in aortas of hypercholesterolemic mice, with induction of NOX4 expression in the later stages of atherosclerosis [19]. VSMCs from atherosclerotic lesions as well as normal aortas with overexpression of NOX4 showed increased ROS levels, senescence, and susceptibility to apoptosis. NOX4 expression progressively increased from stage I to stage IV atherosclerosis in human coronary arteries [18]. The precise role of NOX4 in atherosclerosis has been questioned, however, based on modulation of NOX4 expression in endothelial cells in Apoe−/− mice [20,21].
siRNAs in conjunction with a safe and effective nanoparticle delivery system enable effective targeted gene silencing both in cell culture and in vivo [22]. p5RHH, a transfection agent with the ability to form nanocomplexes with siRNA, is an N-terminal peptide of melittin, a bee venom component, which has arginine and histidine residues at the C-terminus [23]. p5RHH-siRNA complexes are stable in serum and are internalized into cells by micropinocytosis, and siRNA is released in the cell following endosomal acidification and lysis of the endosomal membrane [24]. The studies reported here, using VSMCs from young and aged wild-type and young Nox4−/− mice, young and aged Apoe−/− mice, young and aged human carotid arteries, a NOX1/NOX4 inhibitor, and anti-NOX4 peptide-siRNA self-assembling nanocomplexes, yielded consistent results showing that NOX4 regulates inflammatory gene expression in VSMCs in response to TGFβ1 treatment, in aging and aging-associated atherosclerosis.
2. Materials and methods
2.1. VSMC isolation and culture
Mouse aortic VSMCs were isolated from 4-month (young) and 16-month old (aged) male wild-type and 4-month old Nox4−/− (C57BL/ 6J strain) mice. Briefly, aortas were removed under sterile conditions, rinsed, and incubated in collagenase type II (Worthington) at 37 °C. Adventitia and endothelium were removed and the aortas were digested in collagenase type II and elastase at 37 °C for 1 h. Cells were cultured in DMEM supplemented with 10% FBS and an antibiotic, antimycotic solution (Gibco). Cells were maintained under standard cell culture conditions at 37 °C and 5% CO2 in a humidified incubator. All experiments were performed using VSMCs between passages 3 and 11. DMEM supplemented with 0.1% FBS was used for growth arrest (48 h). Quiescent VSMCs were incubated for another 24 h in serum-free DMEM after adding freshly thawed TGFβ1 (1 ng/mL). Protein and RNA were extracted from lysed samples and analyzed by Western Blot, ELISA, and realtime PCR.
2.2. VSMC transfection and RNA interference
Nox4 (M-058509-01), Map3K7 (M-040718-01), and Jun (M-043776-01) expressions were silenced in VSMCs using appropriate commercial siRNAs (GE Healthcare). Custom Rela (GGAGUACCCUGAAGCUAUA) and Nfkb2 (GAAAGAAGACAGAGCCUAU) siRNAs were purchased from Sigma-Aldrich. A scrambled siRNA (Sigma-Aldrich) was used as a negative control.
Peptide-siRNA nanocomplexes were prepared by dilution of p5RHH (10 mM stock in DNAse-, RNAse-, and protease-free water (Sigma-Al-drich)) and siRNA (100 µM stock in siRNA buffer (Thermo Scientific)) 1:200 in Opti-MEM medium, followed by incubation for 40 min at 37 °C. VSMCs were seeded in 6-well plates, allowed to reach 70% confluence and quiesced for 48 h in DMEM containing 0.1%FBS. After in cubation with transfection complexes for 4 h at 37 °C, cells were washed with PBS and treated with TGFβ1 as described above.
Lipofectamine RNAiMAX reagent(Invitrogen)and siRNA were diluted as described in the manufacturer’s protocol. VSMCs were grown in 6-well plates to reach 70% confluence and quiesced for 48 h. After quiescence, cells were incubated with transfection complexes (7.5 µL of Lipofectamine and 50 pmol siRNA per well) for 24 h, washed in PBS and treated with TGFβ1 (1 ng/mL).
2.3. Measurement of cellular hydrogen peroxide production and mitochondrial hydrogen peroxide levels
VSMCs were incubated in 6-well plates for 24 h in serum- and Phenol Red-free DMEM (Gibco). Media were collected and H2O2 concentrations were analyzed using the Amplex Red Hydrogen Peroxide Assay Kit (Invitrogen) according to the manufacturer’s protocol. Fluorescence was measured at 530 nm excitation and 590 nm emission, using the Wallac 1420 VICTOR2 Multilabel plate reader (PerkinElmer).
Mitochondrial-derived H2O2 was detected in living cells using Mitochondria Peroxy Yellow 1 (MitoPY1; Sigma-Aldrich) as described previously [25]. Briefly, VSMCs were plated on 8-well chamber slides (Thermo Scientific) and grown to 70% confluence. After quiescing for 48 h, VSMCs were transfected and/or treated with TGFβ1 as described above. Cells were incubated with 10 µM MitoPY1 for 1 h, then washed and incubated in DPBS for 1 h. After washing, cells were mounted in mounting medium with DAPI (Vector Laboratories) and imaged on a fluorescence microscope (Nikon Eclipse TE-2000E) using 460 nm (DAPI) and 530 nm (MitoPY1) emission filters. Mean fluorescence levels were analyzed using ImageJ software.
2.4. RNA extraction, real-time reverse transcription PCR
Total RNA from VSMCs was isolated using RNeasy Micro Kit (Qiagen) according to manufacturer’s recommendations. Total RNA was used for cDNA synthesis by reverse transcription using iScript Reverse Transcription Supermix (Biorad). Real-time reverse transcription-polymerase chain reaction was performed on the Applied Biosystems 7900 HT Sequence Detection System using TaqMan PCR Master Mix and TaqMan Gene Expression Assays for Nox4 (Mm00479246_m1), Ccl2 (Mm00441242_m1), Ccl5 (Mm01302427_m1), Il6 (Mm00446190_m1), Vcam1 (Mm01320970_m1), Map3k7 (Mm00554514_m1), Jun (Mm00495062_s1), Rela (Mm00501346_m1), and Nfkb2 (Mm00479807_m1). Relative expression levels were determined by normalization to 18S rRNA using REST 2009 software (Qiagen).
2.5. Protein extraction, Western Blot, and ELISA analysis
To extract protein for Western Blot analysis, VSMCs were lysed in 100 µL M-PER Mammalian Protein Extraction Reagent supplemented with Halt Protease Inhibitor Cocktail (Thermo Scientific) and incubated on ice for 30 min with subsequent centrifugation at 16,000g for 10 min at 4 °C. Protein concentration was determined using Bio-Rad Protein Assay Reagent.
Equal amounts of protein were resolvedon 10% sodium dodecyl sulfate-polyacrylamide gels by electrophoresis and transferred to a nitrocellulose membrane (GE Healthcare). Membranes were blocked with 5% non-fat milk, incubated with primary antibody, and then with a species appropriate secondary anti-rabbit or anti-mouse HRP-conjugated antibody (GE Healthcare). Primary antibodies used in these studies include anti-NOX4 (Abcam), anti-IkB (Cell Signaling Technologies), and anti-β-actin (Sigma-Aldrich). Blots were developed using SuperSignal substrate (Thermo Scientific) and visualized on X-ray film. The intensity of the bands was analyzed using ImageJ software.
Concentrations of IL6, CCL2, and CCL5 were measured in conditioned media using Mouse IL6 DuoSet ELISA, Mouse CCL2/JE/MCP1 DuoSet ELISA, and Mouse CCL5/RANTES DuoSet ELISA kits (R&D Systems), respectively. Concentration of VCAM1 was measured in cell lysates using Mouse VCAM1 ELISA Kit (Sigma-Aldrich). Measurements were made according to the manufacturer’s instructions. Absorbance was measured at 450 nm on SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices).
2.6. Cell viability and cytotoxicity analysis
VSMCs were seeded in 96-well plates, grown to 70% confluence and then quiesced for 24 h. Cells were transfected with scrambled or Nox4 siRNA and then treated with or without TGFβ1, and cell viability and cytotoxicity were measured. To determine cell viability, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) Cell Proliferation Assay Kit (Cayman Chemical) was used following manufacturer’s protocol. Absorbance was measured at 570 nm. Cytotoxicity was analyzed with LDH Cytotoxicity Assay Kit (Thermo Scientific) according to manufacturer’s protocol. Absorbance was measured at 490 nm on SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices).
2.7. Mouse aortic root samples
Male Apoe−/− C57Bl/6 J mice were purchased from Jackson Laboratory and bred in-house. Mice were housed at 22 °C and in a 12-h light/ dark cycle with free access to food and water. Mice were fed standard rodent chow until 1 month (young) and 13 months (aged) and then Western diet (Harlan Teklad #88137) for 3 months. Cohorts of 13-month old mice received a selective NOX1/NOX4 NADPH oxidase inhibitor AK120765 (Ark Pharm) at a concentration of 60 mg/kg/day by oral gavage once daily for 12 weeks or vehicle as described previously [14]. At 16 months of age, AK120765 and vehicle-treated mice were euthanized with inhaled Isoflurane and aortas were perfused with phosphate-buffered saline. Aortic root segments were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek) and snap-frozen in liquid nitrogen. Transverse serial sections were collected through the aortic root segments.
2.8. Human carotid artery samples
Carotid artery samples collected during autopsy 12–24 h postmortem were acquired from National Disease Research Interchange. All specimens were deidentified and their use was approved by University of Michigan Medical School Institutional Review Board.
2.9. Immunofluorescence staining
Mouse aortic root frozen sections (n = 6) were fixed in acetone and immunostained with rabbit anti-CCL2, and NOX4 (Abcam), CCL5, IL6, and VCAM1 (Bioss), AlexaFluor 594-conjugated goat anti-rabbit IgG (Thermo Scientific), and mouse FITC-conjugated anti-α-smooth muscle actin (Sigma-Aldrich). Human carotid artery frozen sections (n = 8) were fixed in acetone and immunostained with rabbit anti-TGFβ1 (Cell Signaling Technology), anti-NOX4 (Abcam), anti-CCL2, and anti-IL6 (Bioss), AlexaFluor 594-conjugated goat anti-rabbit IgG (Thermo Scientific), and mouse FITC-conjugated anti-α-smooth muscle actin (Sigma-Aldrich). Sections were mounted in Vectashield mounting medium with DAPI for fluorescence studies (Vector Laboratories). All images were acquired with Nikon Microphot-FX microscope at the same photomultiplier tube voltage, exposure, gain and offset. Single-channel images were merged using Adobe Photoshop CC 2014.
2.10. Statistical analysis
In this study, all statistical analyses were performed using Prism 6 GraphPad software and the results are given as the mean values ± SEM. The statistical significance of differences within parameters was assessed using Student’s t-test or one–way analysis of variance (ANOVA) using Newman-Keuls post hoc test. A value of p < 0.05 was considered significant.
3. Results
3.1. p5RHH mediated efficient Nox4 siRNA transfection of mouse primary VSMCs
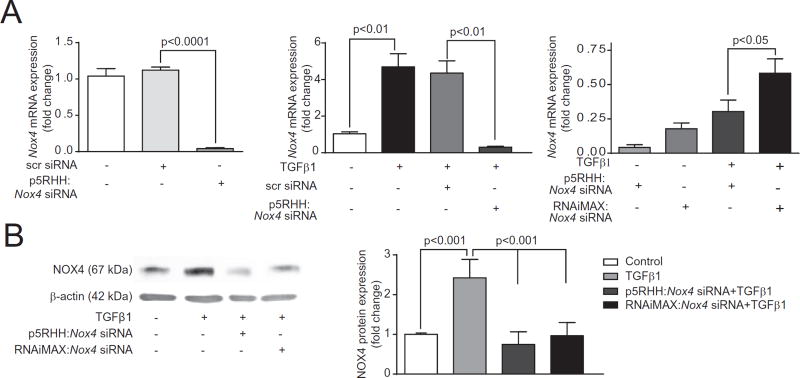
RT-PCR analysis showed maximal knockdown of Nox4 expression in mouse VSMCs at a p5RHH:Nox4 siRNA ratio of 200:1 (not shown), which was used for the characterization of TGFβ1-regulated intracellular signaling pathways. p5RHH:Nox4 siRNA transfection significantly inhibited basal Nox4 mRNA levels in VSMCs (p < 0.0001 vs. control), whereas scrambled siRNA had no effect on Nox4 expression (Fig. 1A, left panel). TGFβ1 significantly (452%) increased NOX4 expression in VSMCs and p5RHH:Nox4 siRNA transfection inhibited TGFβ1-induced Nox4 mRNA expression as well (p < 0.01 vs. TGFβ1) (Fig. 1A, middle panel). In direct comparison, Nox4 mRNA expression was significantly lower in cells transfected with p5RHH:Nox4 siRNA than with Lipofectamine RNAiMax:Nox4 siRNA (Fig. 1A, right panel). Concordant with the knock down in mRNA expression, p5RHH:Nox4 siRNA transfection had greater effect suppressing TGFβ1-induced NOX4 protein expression compared to Lipofectamine RNAiMax:Nox4 siRNA transfection (92% vs. 85%) (Fig. 1B).
Fig. 1.
p5RHH:Nox4 siRNA nanocomplex transfection downregulates Nox4 mRNA and protein expression in VSMC from 4-month old wild-type mice. (A) p5RHH:Nox4 siRNA nanocomplex transfection efficiency compared to RNAiMAX Nox4 siRNA using quantitative RT-PCR (mean ± SEM, n = 4). (B) Representative Western Blot and quantification of NOX4 protein expression in VSMC treated with TGFβ1 (mean ± SEM, n = 3). [2 columns].
3.2. p5RHH:Nox4 siRNA transfection attenuates basal and TGFβ1-induced H2O2 generation in mouse VSMCs, without impacting cell viability
To determine the functional effect of Nox4 knockdown using p5RHH:siRNA delivery system, we measured H2O2 release into the culture medium by the Amplex Red assay. p5RHH:Nox4 siRNA nanoparticles decreased H2O2 release by 37% in untreated VSMCs and ~43% in TGFβ1-treated cells (Fig. 2A). Because NOX4 localizes to mitochondria, contributing to mitochondrial ROS levels [1,2,14], we measured the effect of p5RHH:Nox4 siRNA transfection on mitochondrial H2O2 levels imaging using Mitochondria peroxy yellow 1 (MitoPY1), a small molecule fluorescent probe [26]. MitoPY1 contains a triphenylphosphonium group to localize to mitochondria and a boronate-based molecular switch to selectively respond to H2O2 vs. other ROS within the mitochondria. p5RHH:Nox4 siRNA transfection significantly inhibited the increase in mitochondrial H2O2 levels in TGFβ1-treated VSMC (Fig. 2B). As extensive cytotoxicity was observed with the use of traditional transfection agents [24], we compared the cytotoxic profiles of p5RHH:siRNA and RNAiMax:siRNA transfection methods by determining cell viability using MTT and lactate dehydrogenase (LDH) leakage assays. MTT assay showed that p5RHH is significantly superior to Lipofectamine RNAiMax in decreasing cytotoxicity (p < 0.001), exhibiting a minimal decrease (10%) in cell viability (Fig. 2C). LDH assay confirmed that p5RHH has no measurable effect on cell viability whereas cells treated with Lipofectamine RNAiMax exhibited 9% cytotoxicity (Fig. 2D).
Fig. 2.
p5RHH:Nox4 siRNA nanocomplex transfection downregulates H2O2 production and shows low cytotoxicity in VSMCs from 4-month old wild-type mice. (A) H2O2 production assayed with Amplex Red in media after 24 h incubation of VSMCs with TGFβ1 (mean ± SEM, n = 5). (B) Representative fluorescent images of VSMCs stained with MitoPY1 for mitochondrial H2O2 production. (C) Cell viability tested in MTT assay (mean ± SEM, n = 6). (D) Cytotoxicity assessed with the LDH assay (mean ± SEM, n = 6). [1.5 columns].
3.3. Nox4 knockdown with p5RHH:Nox4 siRNA nanoparticles attenuates TGFβ1-induced pro-inflammatory gene expression
TGFβ1 is a pleiotropic cytokine produced by several cardiovascular cell types, including SMCs [26]. Conflicting data exist with regard to atherogenic and anti-atherogenic effects of this cytokine [27–29]. However, TGFβ1 production is increased in aging, obesity, and other inflammatory conditions [30–32]. To assess the regulatory role of NOX4 in TGFβ1-induced proinflammatory gene expression, we measured cytokine and adhesion molecule expression levels in mouse VSMCs transfected with p5RHH:Nox4 siRNA nanoparticles. Increased levels of chemokines such as CCL2 and CCL5 and the adhesion molecules such as VCAM1 enhance atherogenesis by inducing immune cell migration from the circulation into the arterial wall [33]. Ccl2 mRNA expression which was increased 320%in young mouse VSMCs in response to TGFβ1 treatment was inhibited by p5RHH:Nox4 siRNA nanoparticles (Fig. 3A). Similarly, p5RHH:Nox4 siRNA nanoparticle transfection inhibited Ccl5 expression, which was increased by 422% in TGFβ1-treated VSMCs (Fig. 3B). Circulating IL6 is a well-established biomarker for cardiovascular disease. Cell types that secrete the cytokine include vascular wall cells [34–35]. TGFβ1 increased Il6 expression 24 fold in young mouse VSMCs and p5RHH:Nox4 siRNA nanoparticle transfection significantly attenuated increase in Il6 expression induced by TGFβ1 (Fig. 3C). Vcam1 expression increased 257% in cells treated with TGFβ1 and p5RHH:Nox4 siRNA nanoparticle transfection significantly attenuated TGFβ1-induced adhesion molecule expression (Fig. 3C). p5RHH:scrambled siRNA nanoparticles had no effect on either the basal or the TGFβ1-induced inflammatory gene expression in VSMCs.
Fig. 3.
p5RHH:Nox4 siRNA nanocomplex transfection of VSMCs attenuates TGFβ1-stimulated expression of pro-inflammatory genes from 4-month old wild-type mice. Relative mRNA expression was assessed by quantitative RT-PCR (mean ± SEM, n = 4); (A) Ccl2, (B) Ccl5, (C) Il6, and (D) Vcam1. Secretion of cytokines into conditioned media and VCAM1 protein expression were determined by ELISA (mean ± SEM, n = 4); (E) CCL2, (F) CCL5, (G) IL6, and (H) VCAM1. [1.5 columns].
In concert with the changes in mRNA levels, secretion of CCL2 (Fig. 3E), CCL5 (Fig. 3F), and IL6 (Fig. 3G) was significantly higher in the conditioned media from VSMCs treated with TGFβ1 compared to the controls (by 200, 180, and 600%, respectively) and transfection with p5RHH:Nox4 siRNA nanoparticles significantly attenuated TGFβ1-induced cytokine secretion. Similarly, increase in VCAM1 protein expression in response to TGFβ1 treatment (50% vs. control) was suppressed in cells transfected with Nox4 siRNA nanoparticles (Fig. 3H). These results indicate that TGFβ1 exerts a pro-inflammatory effect on aortic VSMCs by regulating NOX4 expression.
3.4. Nox4 deficiency inhibits TGFβ1-induced pro-inflammatory gene expression
To confirm the role of NOX4 in TGFβ1 stimulated inflammation, we assessed the effect of Nox4 deletion on the expression of the above mentioned genes in wild-type and Nox4−/− VSMCs with and without TGFβ1 treatment. TGFβ1 significantly increased expression of Ccl2 and Ccl5 in wild-type VSMCs. Both basal and TGFβ1-induced Ccl2 and Ccl5 levels were significantly inhibited in Nox4−/− VSMCs compared to the wild-type (p < 0.05 for Ccl2 in Nox4−/− vs. wild-type + TGFβ1; p < 0.001 for Ccl5 in Nox4−/− vs. wild-type + TGFβ1; Fig. 4A and B). Nox4 deficiency had no effect on basal Il6 and Vcam1 expression, whereas it inhibited TGFβ1-induced increase in Il6 (p < 0.001 vs. TGFβ1-treated wild-type, Fig. 4C) and Vcam1 expression (p < 0.05 vs. TGFβ1-treated wild-type, Fig. 4D).
Fig. 4.
TGFβ1-stimulated expression of pro-inflammatory genes is inhibited in 4-month old Nox4−/− VSMC. Relative mRNA expression was assessed by quantitative RT-PCR (mean±SEM, n =4); (A) Ccl2 (B) Ccl5 (C) Il6, and (D) Vcam1. Secretion of cytokines into conditioned media and VCAM1 protein expression was determined by ELISA (mean ± SEM, n = 4); (E) CCL2, (F) CCL5, (G) IL6, and (H) VCAM1. [1 column].
Similarly, secretion of CCL2 (Fig. 4E), CCL5 (Fig. 4F), and IL6 (Fig. 4G) proteins into the conditioned media and VCAM1 protein (Fig. 4H) from VSMCs was significantly increased in wild-type cells treated with TGFβ1. The TGFβ1-induced expression of the proteins was attenuated in Nox4−/− VSMCs (p < 0.0001 for CCL2, CCL5, and IL6 in Nox4−/− vs. wild-type + TGFβ1; p < 0.001 VCAM-1 in Nox4−/− vs. wild-type + TGFβ1). These data confirm the regulatory role of NOX4-induced signaling pathways in TGFβ1-induced inflammation in VSMCs, indicated by the data from Nox4 knockdown with p5RHH:Nox4 siRNA nanoparticles.
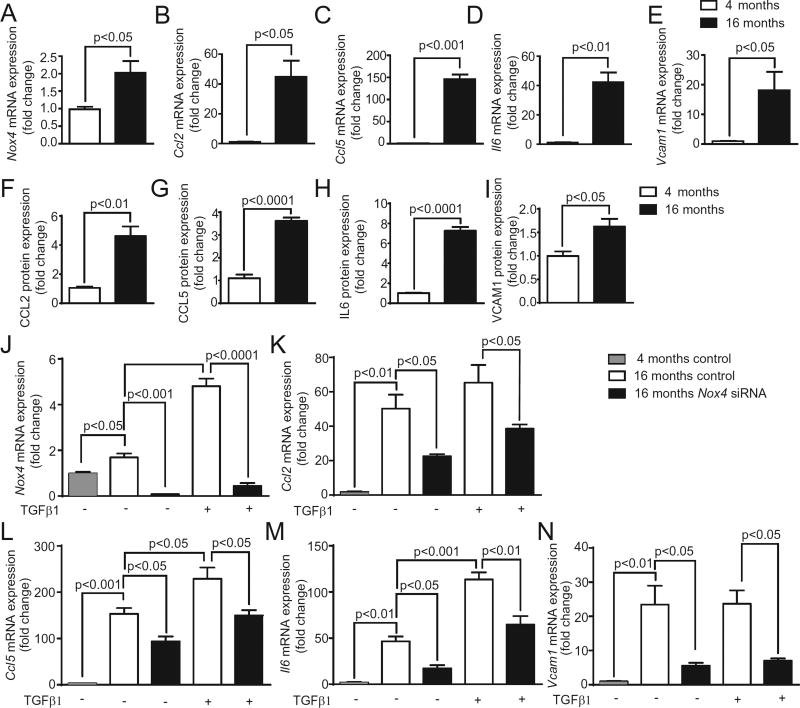
3.5. Aging increases proinflammatory gene expression in aortic VSMCs
Pro-inflammatory phenotypes of VSMCs and increased cytokine levels have also been implicated in enhanced atherosclerosis with aging [35]. To further evaluate the importance of a regulatory role of NOX4 in inflammation, we measured Nox4 and pro-inflammatory gene expression in VSMCs isolated from young (4 months) and aged (16 months) mice under basal and TGFβ1-stimulated conditions. As reported by us recently [14], Nox4 expression was significantly increased in aged VSMCs compared to the young (p< 0.05, Fig. 5A). Interestingly, a sharp increase in proinflammatory gene expression was also observed in aged VSMCs. VSMC Ccl2 expression was increased 45 fold with age (Fig. 5B). Basal Ccl5 mRNA expression increased 146 fold in aged VSMCs compared to the young (Fig. 5C). Il6 expression increased 42 fold (p < 0.01, Fig. 5D), and Vcam1 mRNA levels were increased 18 fold (p < 0.05, Fig. 5E) with age. Similarly, the CCL2, CCL5, and IL6 secretion into the conditioned media by the VSMCs from the aged mice and cellular VCAM1 protein levels in the cells from the aged mice were significantly higher when compared to the cells from young mice (Fig. 5F – I).
Fig. 5.
VSMCs isolated from 16 months-old mice show elevated pro-inflammatory gene expression vs. VSMCs from 4-month old mice. Relative mRNA expression was assessed by quantitative RT-PCR (mean ± SEM, n = 4); (A) Nox4, (B) Ccl2, (C) Ccl5, (D) Il6, and (E) Vcam1. Secretion of cytokines into conditioned media and VCAM1 protein expression in VSMCs from 4 and 16 months-old mice treated with or without TGFβ1(mean ± SEM, n = 4); (F) CCL2, (G) CCL5, (H) IL6, and (I) VCAM1. p5RHH:Nox4 siRNA nanocomplex transfection of VSMCs from 16 months-old mice attenuates TGFβ1-stimulated expression of pro-inflammatory genes. Relative mRNA expression (mean ± SEM, n = 3); (J) Ccl2, (K) Ccl5, (L) Il6, and (I) Vcam1. [2 columns].
Transfection with p5RHH:Nox4 siRNA decreased basal as well as TGFβ1-induced Nox4 mRNA expression in VSMCs from aged mice (Fig. 5J). While TGFβ1 increased the expression of Ccl5 (Fig. 5L) and Il6 (Fig. 5M), it had no discernable effect on Ccl2 (Fig. 5K) and Vcam1 (Fig. 5N) expression inVSMCs from the aged mice. Nox4 siRNA transfection significantly reduced Ccl2, Ccl5, Il6, and Vcam1 expression in untreated and TGFβ1-treated VSMCs from the aged mice. These data support the central role of NOX4 in aging-associated increase in inflammation and suggest that VSMC-derived chronic inflammation is a contributing factor to age-associated atherosclerosis.
3.6. Cytokine and VCMA1 protein expression increases in atherosclerotic lesions with age, and attenuation of NOX4 activity inhibits the aging-induced chemokine increases in the lesions of aged Apoe−/− mice
To assess whether Nox4 regulated inflammatory gene expression in VSMC cultures is of significance to aging-associated increases in atherosclerosis, we measured CCL2, CCL5, IL6, and VCAM1 expression by immunofluorescence in atherosclerotic lesions of young (4 month) and aged Apoe−/− (16 month) mice that were fed high-fat diet for the last three months. Consistent with our recent report [14], a marked increase in immunoreactive NOX4 expression in medial VSMCs in aortic root sections was observed in Apoe−/− mice with age (Fig. 6A). CCL2, CCL5, IL6, and VCAM1 expression in aortic medial VSMCs was increased in the atherosclerotic lesions in the aged vs. young Apoe−/− mice (Fig. 6B – E). More importantly, a selective NOX1/NOX4 NADPH oxidase inhibitor AK120765 (GKT136901), significantly attenuated CCL2, CCL5, IL6, and VCAM1 expression in the aortic medial VSMCs of atherosclerotic lesions in the aged Apoe−/− mice. These data support the notion that the age-associated increase in NOX4 activity increases atherosclerosis, in part, by stimulating inflammatory gene expression, while inhibition of NOX4 activity exerts a salutary effect on age-associated atherosclerosis under hyperlipidemic conditions [14]. Although Nox1 expression is either not significantly affected [14] or decreased with age in aortic SMCs in hypercholesterolemic mice [19], we can’t rule out a role for NOX1 in vascular inflammation and in atherosclerosis during aging as NOX1 activity is also regulated by NADPH oxidase complex formation, post-translational modification, and localization [36].
Fig. 6.
Cytokines and adhesion molecules expression in atherosclerotic lesions of Apoe−/− mice correlates with NOX4 expression. Representative immunofluorescent images of fresh frozen aortic root sections stained for NOX4 (A), CCL2 (B), CCL5 (C), IL6 (D), and VCAM1 (E) (red fluorescence) costained with smooth muscle α-actin (green), and DAPI (blue). [1.5 columns].
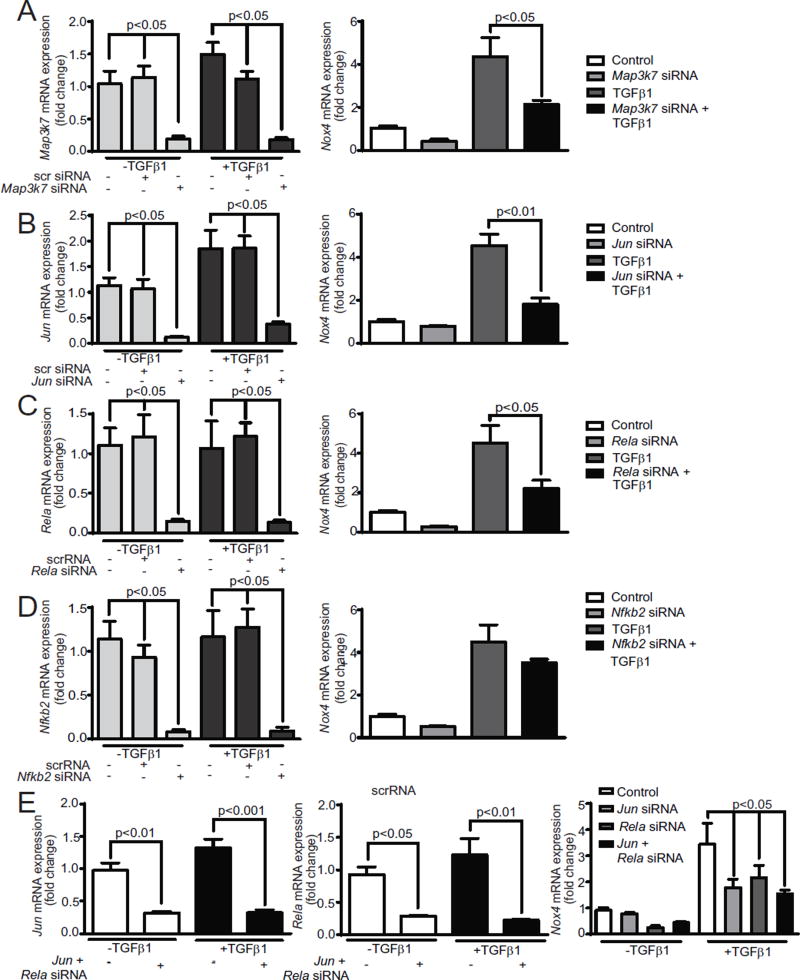
3.7. Transforming growth factor-β-activated kinase 1 (TAK1), NFκB, and AP1 mediated signaling regulate TGFβ1-induced Nox4 expression in VSMCs
TAK1 (MAP3K7) is a mitogen-activated protein kinase kinase kinase family member, which is activated by TGFβ1 and is known to be a key regulator of immune and pro-inflammatory signaling pathways [37– 39]. Song et al. [40] reported that a small molecule inhibition of TAK1 significantly inhibited NOX activity and pro-inflammatory gene expression induced by CD40L/CD40 stimulation. To determine whether MAP3K7 regulates Nox4 expression, we measured Map3k7 and Nox4 mRNA expression in VSMCs treated with and without TGFβ1 and transfected with p5RHH:Map3k7 siRNA nanoparticles. Map3k7 mRNA expression increased 37% in TGFβ1 treated VSMCs (Fig. 7A). p5RHH:Map3k7 siRNA nanoparticle transfection significantly inhibited Map3k7 mRNA expression in unstimulated (p < 0.05) as well as TGFβ1 treated VSMCs. Nox4 mRNA expression was also significantly inhibited in the control and treated VSMCs upon transfection with p5RHH:Map3k7 siRNA nanoparticles, indicating that TAK1 mediates the transcriptional regulation of Nox4 by TGFβ1.
Fig. 7.
TAK1, AP1, and canonical NFκB pathways regulate Nox4 expression in VSMCs from 4-month old mice. Relative Nox4 mRNA expression was assessed by quantitative RT-PCR (mean ± SEM, n = 4) in cells transfected with p5RHH:siRNA nanoparticles; (A) Map3k7, (B) Jun, (C) Rela, (D) Nfkb2, and (E) Jun + Rela. [1.5 columns].
Thannickal and colleagues [41] reported that an AP1/Smad binding box regulates TGFβ1-induced Nox4 gene expression in human lung fibroblasts. To ascertain whether AP1 regulates Nox4 expression in mouse VSMCs, we transfected cells with p5RHH :Jun siRNA and p5RHH:scrambled siRNA nanoparticles in the presence and absence of TGFβ1. Jun expression increased with TGFβ1 treatment and Jun siRNA transfection inhibited basal as well as TGFβ1-stimulated Jun mRNA expression (p < 0.05; Fig. 7B).Jun siRNA transfection significantly inhibited Nox4 mRNA expression in untreated and TGFβ1 treated cells (p < 0.05), indicating that AP-1 indeed plays a role in Nox4 gene regulation in response to cytokine exposure.
NFκB is a redox-sensitive transcription factor whose activation causes a sustained expression of inflammatory cytokines, affecting plaque development [42]. Activation of NFκB upregulated Nox4 expression in human aortic endothelial cells exposed to hyperglycemia [43]. TAK1 stimulation activates CD40L/CD40-induced NFκB activation by increasing the phosphorylation of IKKα/β, IkBα, and NFκB p65 (RelA) [40]. To assess the role of NFκB in TGFβ1 induced Nox4 expression, VSMCs were transfected with p5RHH:Rela siRNA and p5RHH:scrambled siRNA nanoparticles. Rela siRNA transfection significantly inhibited Rela mRNA expression in unstimulated as well as TGFβ1 treated VSMCs (p < 0.05 vs. scrambled siRNA) (Fig. 7C). Suppressing NFκB p65 expression inhibited both basal and TGFβ1-induced Nox4 mRNA expression in VSMC (p < 0.05), supporting a role for NFκB activation in the regulation of Nox4 expression. Because TGFβ1 can stimulate several noncanonical signaling pathways [44], we examined whether noncanonical NFκB activation contributes to Nox4 expression by transfecting VSMCs with p5RHH:Nfkb2 siRNA and p5RHH:scrambled siRNA nanoparticles. Basal and TGFβ1-stimulated Nfkb2 mRNA expression was significantly inhibited in VSMCs transfected with Nfkb2 siRNA (Fig. 7D). However, suppressing Nfkb2 mRNA expression had no significant effect on Nox4 gene expression in VSMCs treated with and without TGFβ1, indicating that a noncanonical NFκB pathway is not involved in TGFβ1-induced Nox4 expression and subsequent inflammation.
We also tested the possibility of independent regulation of Nox4 expression by Jun- and Rela-dependent pathways. Both basal and TGFβ1-stimulated levels of Jun and Rela mRNA were significantly decreased in VSMCs transfected with either Jun siRNA or Rela siRNA nanoparticle complexes or the combination of Jun and Rela p5RHH:siRNA nanoparticle complexes (Fig. 7E). The TGFβ1-stimulated expression of Nox4 was significantly decreased (p < 0.05; Fig. 7E) in these transfected cells without an additive effect on inhibition, suggesting that Jun and Rela act in the same pathway in Nox4 gene regulation. Furthermore, to determine whether TAK1 is upstream of NFκB or AP1, we measured Jun, Rela, and Nfkb2 mRNA expression in VSMCs transfected with p5RHH:Map3k7 siRNA and p5RHH:Scr siRNA and then treated VSMCs with and without TGFβ1. p5RHH:Map3k7 siRNA transfection had no significant effect on the expression of either mRNA, which indicates that TAK1 is not upstream of NFκB and AP1 (data not shown).
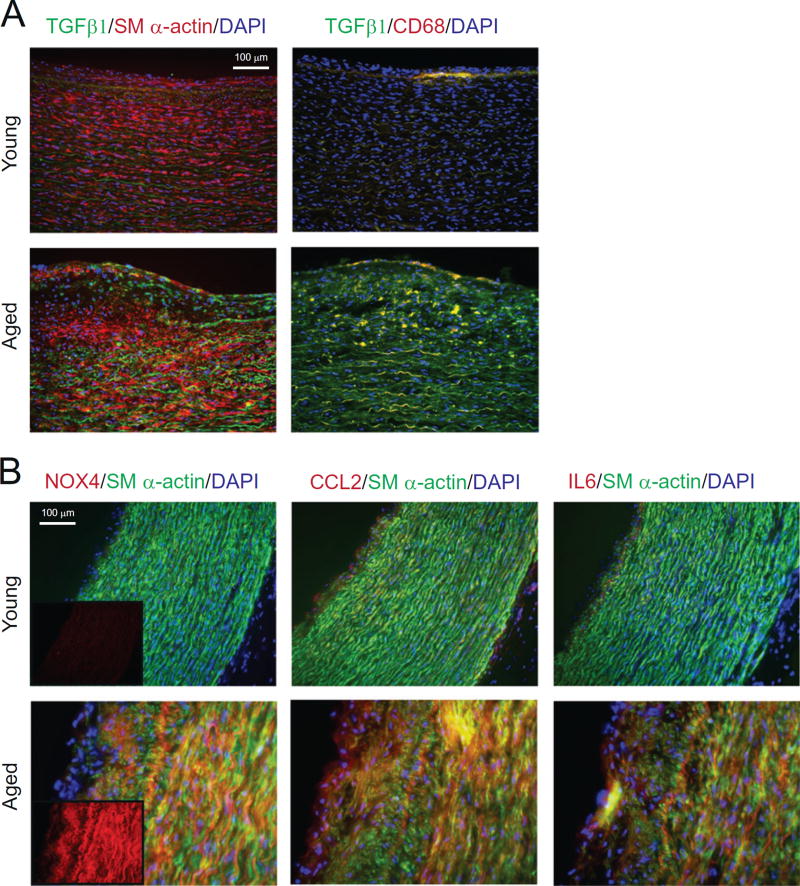
3.8. Increased NOX4 expression with aging is associated with enhanced cytokine levels in human carotid arteries
We previously reported an increased NOX4 expression with aging and a positive correlation between increased NOX4 expression in human carotid artery medial SMCs and atherosclerotic lesion severity [14]. Because VSMC migration is a crucial event in the pathogenesis of atherosclerosis and restenosis and further, because TGFβ1-stimulated formation of focal adhesions and migration of VSMC is mediated by increased NOX4 expression [17], we investigated whether increased NOX4 expression is associated with higher levels of TGFβ1 in human atherosclerotic carotid artery lesions. Immunofluorescence staining showed markedly enhanced immunoreactive TGFβ1 staining in the medial layers of carotid arteries from aged patients compared to young patients, as indicated by co-localization with smooth muscle α-actin (Fig. 8A). In addition, TGFβ1 in macrophage-positive (CD68+ staining) subendothelial areas of carotid arteries increased with age.
Fig. 8.
NOX4 expression in SMCs in human atherosclerotic carotid arteries from aged patients is correlated with TGFβ1, CCL2, and IL6 protein levels. (A) Representative immunofluorescence images of human carotid artery sections stained for TGFβ1 (green) and costained with smooth muscle α-actin (left panels) and CD68 (right panels) (red) and DAPI (blue). (B) Representative immunofluorescence images of human carotid artery sections stained for NOX4, CCL2, and IL6 (red) and co-stained with smooth muscle α-actin (green), and DAPI (blue) (inset: NOX4 staining). [1.5 columns].
Consistent with our previous findings [14], and corresponding to the increase in TGFβ1 levels, expression of immunoreactive NOX4 was markedly increased in the intimal and medial carotid artery SMCs in the aged vs. young patients (Fig. 8B). In correlation with NOX4 levels, expression of immunoreactive CCL2 and IL6 significantly increased in the intimal and medial SMCs in the carotid arteries of the aged (Fig. 8B), supporting our data from in vitro studies and mouse models indicating a central role for NOX4 expression in vascular inflammation in aging.
4. Discussion
In this study, we demonstrated that NOX4 is a key regulator of inflammatory gene expression in aortic VSMCs in response to TGFβ1 treatment, in aging, aortic atherosclerotic lesions in Apoe−/− mice, and in carotid arteries from aged patients. Consistent with these findings, VSMCs from Nox4−/− mice exhibit dramatically reduced cytokine and adhesion molecule expression. TGFβ1-induced NOX4 expression in VSMCs is regulated by TAK1 and AP1 and canonical NFκB pathways. Collectively, these data indicate that NOX4 is a nodal regulator of inflammation in aging, pathophysiological conditions, and atherosclerosis.
Accumulating evidence has demonstrated that atherosclerosis is a chronic inflammatory disease of the arterial wall, with inflammation driving all stages of pathogenesis, including the formation, progression and rupture of atherosclerotic plaque [45,46]. While the function of chemokines and adhesion molecules produced by the inflammatory cells and endothelium in atherosclerosis is well documented, the pro-inflammatory role of VSMCs in atherosclerosis is only now emerging [11,35,47]. Erren et al. [29] reported that an “inflammatory signature” of advanced atherosclerosis includes, among other markers, increased plasma levels of TGFβ1. Corroborating its role in aging-associated CVD, TGFβ1 levels were higher in atherosclerotic lesions than in non-atherosclerotic aortic tissue [48] and active TGFβ1, its receptor, and receptor-mediated signaling all increase within the aortic wall and in VSMCs with aging [32]. While TGFβ1 induces NOX4 expression and increases ROS levels in various cell types, ROS, in turn, induce/activate TGFβ1, resulting in a self-perpetuating cycle that amplifies oxidative stress [49]. ROS can activate latent TGFβ1 directly by oxidizing latency-associated protein bound to TGFβ1 in the inactive complex and indirectly by activating matrix metaloproteinases that cleave latency-associated protein to release active TGFβ1. Consistent with the TGFβ1-NOX4 vicious cycle hypothesis, Subramanian et al. [50] reported ROS-mediated TGFβ1 production in VSMCs treated with Angiotensin II.
The literature strongly supports the notion that TGFβ1 increases mitochondrial ROS production in various cell types via several different mechanisms [49]. The mechanisms implicated in the redox imbalance induced by TGFβ1 comprise suppression of antioxidant enzymes, including mitochondrial superoxide dismutase 2 [51]. Interestingly, it was reported that inhibition of mitochondrial ROS by mitochondriatargeted antioxidant suppressed TGFβ1-induced NOX4 expression [48], indicating that TGFβ1-NOX4 vicious cycle includes mitochondrial oxidative stress. Taken together, these data suggest that increased mitochondrial oxidative stress during aging may increase TGFβ1 activity/expression, inducing NOX4 expression and contributing to aging-associated CVD, consistent with our prior studies [52,53].
Regulatory mechanisms that control Nox4 gene transcription are critical to understanding its role in vascular homeostasis as NOX4 expression is primarily regulated at the level of gene transcription [44]. Our current results showing that suppression of Map3k7 mRNA expression inhibits Nox4 expression, together with the reports that TGFβ1 levels increase in injured vessels [54,55] and Map3k7 inhibition attenuates NOX4 activity and vascular oxidative stress in response to arterial injury and the pro-inflammatory phenotype of VSMCs [40] lend support to the notion that TAK1 is an important regulatory kinase affecting NOX4 expression in pathophysiological conditions. Moreover, inhibition of pro-inflammatory genes in VSMCs by CD40 ligand with blockage of TAK1 was mediated, in part, by suppressing phosphorylation of IKKα/β, IκBα, and NFκB and attenuating p65 nuclear translocation [40]. These data, together with our observation that Rela levels regulate Nox4 expression reinforce the notion that NOX4 is a nodal regulator of inflammation under pathophysiologic conditions. Our data are also consistent with the report of Manea et al. [56] that NFκB is an essential regulator of NOX4 in SMCs. AP1-dependent regulation of Nox4 expression observed in the present study is in agreement with similar reports observed in human aortic SMCs treated with Ang II and TNFα [57] and in lung fibroblasts with TGFβ1 [44]. It is possible that the redox imbalance induced by TGFβ1 activates redox-regulated transcription factors such as Jun and NFκB, which in turn stimulate Nox4 expression, resulting in the promotion and amplification of inflammation.
Inflammation is not merely a biological manifestation of increased ROS, but a major contributor to the development of CVD. By binding to G protein-coupled cell surface receptors, chemokines activate target cells and also induce leukocyte migration to inflammatory sites [58]. CCL2 is an important contributor to atherosclerosis as ablation of the gene in low density lipoprotein receptor-deficient (Ldlr−/−) mice reduced atherosclerosis [59], and plasma CCL2 levels are associated with atherosclerosis risk factors in the general population and adverse clinical events in acute coronary syndrome patients [60,61]. Veillard et al. [58] demonstrated that CCL5 is highly expressed within atheroma and antagonism of RANTES receptors reduces atherosclerotic plaque formation in Ldlr−/− mice. The analysis of Atherosclerosis Risk in Communities (ARIC) carotid MRI study revealed that CCL5 levels are positively associated with the severity of carotid atherosclerosis and high risk plaques [62]. Increased CCL2 and CCL5 levels observed in aged VSMCs in the current study are similar to the findings of Goldstein and colleagues [35], who attributed the aging-associated VSMC pro-inflammatory phenotype, in part, to increased Toll-like receptor (TLR) 4 and MyD88 signaling. Interestingly, a direct interaction of NOX4 with TLR4 was shown essential for lipopolysaccharide-induced activation of NFκB and ROS production [63]. TLRs are activated by both microbial ligands and endogenous ligands, including hyaluronan and oxidized LDL [35]. We previously reported NADPH oxidase-dependent increase in ROS production by hyaluronan in VSMCs as well as an NADPH oxidase-mediated synthesis of hyaluronan and its receptor, CD44, in atherosclerotic lesions [52], augmenting the evidence for a role of NOX4 in aging-associated CVD. A positive correlation between NOX4 and IL6 levels in VSMCs in mice in aging and in human atherosclerotic lesions observed here, together with the published reports that recombinant IL6 enhances atherogenesis in mice [64], and that elevated levels of IL6 are associated with increased risk of future myocardial infarction in apparently healthy men [65], may further affirm the modulatory role of NOX4 in inflammation and atherosclerosis in aging. Similar to our data showing an increase in cellular VCAM1 levels in aged VSMCs, age-dependent increases in circulating VCAM1 levels were observed in apparently healthy subjects [66]. Increased VCAM1 levels may contribute to atherosclerosis as it plays an important role in atherosclerosis in mouse models [14,67] and is markedly increased in patients with coronary artery disease [68]. Together, our in vitro and in vivo data showing Nox4-dependent/associated upregulation of inflammatory gene expression provide further mechanistic insight into the role of NOX4 in aging associated CVD [14].
It is important to note that studies investigating the role of NOX4 in atherosclerosis have yielded seemingly contradictory data. Apoe−/− mice with Nox4 overexpression in endothelial cells had less atherosclerosis compared to the Apoe−/− mice [21], while tamoxifen-inducible Nox4-Apoe double knockout mice had accelerated atherosclerosis [20]. Varying effects of Nox4 deletion on diabetic atherosclerosis were reported, including no effect [69] and increased atherosclerosis [70]. Because transcriptional responses to changes in redox status, including the ones mediated by NOX4, are cell-type specific [71], we propose that increased expression of NOX4 has different pathophysiologic effects in VSMCs vs. endothelial cells. In support of this hypothesis and our findings, Tong et al. [72] recently showed that SMC NOX4 plays a critical role in atherogenesis.
Although showing enhanced overall specificity for NOXs, existing NOX inhibitors, including AK120765 exhibit limited isoform specificity [73], a significant limitation for characterization of molecular pathways. Gene silencing is a viable alternative to achieve specificity lacking in small molecule inhibitors. Coupled with safe and effective delivery nanovehicles, such as p5RHH and cell-specific markers, gene silencing may enable targeted NOX4 suppression in VSMCs.
In summary, we have shown that NOX4 is a nodal regulator of inflammation in arterial VSMC in response to changes in redox status and pathophysiological conditions. Correcting the redox imbalance by inhibiting arterial VSMC NOX4 expression/activity may offer a new therapeutic approach to treat aging-associated inflammation and CVD.
Acknowledgments
This work was supported in part by NIH grants AG024282 and HL111664. We thank Dr. Judith Connett for careful reading of the manuscript.
Footnotes
Disclosures
Dr. Samuel Wickline has equity in Trasir Therapeutics, LLC.
References
- 1.Martinon F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 2.Reuter S, Gupta S, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ. Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 5.Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Radic. Biol. Med. 2013;61:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lassègue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, et al. Kruppel-like factor 15 is critical for vascular inflammation. J. Clin. Invest. 2013;123:4232–4241. doi: 10.1172/JCI68552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, et al. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid. Redox Signal. 2015;23:1389–1409. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, et al. A role for NADPH ox-idase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic. Biol. Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peshavariya HM, Chan EC, Liu GS, Jiang F, Dusting GJ. Transforming growth factor-β1 requires NADPH oxidase 4 for angiogenesis in vitro and in vivo. J. Cell. Mol. Med. 2014;18:1172–1183. doi: 10.1111/jcmm.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez I, Martin-Garrido A, Zhou DW, Clempus RE, Seidel-Rogol B, Valdivia A, et al. Hic-5 mediates TGFβ-induced adhesion in vascular smooth muscle cells by a Nox4-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2015;35:1198–1206. doi: 10.1161/ATVBAHA.114.305185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 19.Xu S, Chamseddine AH, Carrell S, Miller FJ., Jr Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–650. doi: 10.1016/j.redox.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schürmann C, Rezende F, Kruse C, Yasar Y, Löwe O, Fork C, et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, et al. Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free Radic. Biol. Med. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HF, Yan H, Pan H, Hou KK, Akk A, Springer LE, et al. Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis. J. Clin. Invest. 2014;124:4363–4374. doi: 10.1172/JCI75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou KK, Pan H, Lanza GM, Wickline SA. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34:3110–3119. doi: 10.1016/j.biomaterials.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano. 2013;7:8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson BC, Lin VS, Chang CJ. Preparation and use of MitoPY1 for imaging hydrogen peroxide in mitochondria of live cells. Nat. Protoc. 2013;8:1249–1259. doi: 10.1038/nprot.2013.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 27.Toma I, McCaffrey TA. Transforming growth factor-β and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347:155–175. doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XL, Liu SX, Wilcken DEL. Circulating transforming growth factor-beta1 and coronary artery disease. Cardiovasc. Res. 1997;34:404–410. doi: 10.1016/s0008-6363(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 29.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler. Thromb. Vasc. Biol. 1999;19:2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Zhang H, Yin Y, Li J, Tang Y, Purkayastha S, et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat. Med. 2014;20:1001–1008. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler. Thromb. Vasc. Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 33.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 34.Rodondi N, Marques-Vidal P, Butler J, Sutton-Tyrrell K, Cornuz J, Satterfield S, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am. J. Epidemiol. 2010;171:540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gimenez M, Schickling BM, Lopes LR, Miller FJ., Jr Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin. Sci. (Lond.) 2016;130:151–165. doi: 10.1042/CS20150404. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 38.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 40.Song Z, Zhu X, Jin R, Wang C, Yan J, Zheng Q, et al. Roles of the kinase TAK1 in CD40-mediated effects on vascular oxidative stress and neointima formation after vascular injury. PLoS One. 2014;9:e101671. doi: 10.1371/journal.pone.0101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai G, Hock TD, Logsdon N, Zhou Y, Thannickal VJ. A far-upstream AP-1/Smad binding box regulates human NOX4 promoter activation by transforming growth factor-β. Gene. 2014;540:62–67. doi: 10.1016/j.gene.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 43.Williams CR, Lu X, Sutliff RL, Hart CM. Rosiglitazone attenuates NF-KB-mediated Nox4 upregulation in hyperglycemia-activated endothelial cells. Am. J. Phys. Cell Phys. 2012;303:C213–C223. doi: 10.1152/ajpcell.00227.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang YE. Non-Smad pathways in TGF-β. signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ES, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7. doi: 10.1093/rheumatology/kem202. [DOI] [PubMed] [Google Scholar]
- 46.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 47.Gardner SE, Humphry M, Bennett MR, Clarke MC. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler. Thromb. Vasc. Biol. 2015;35:1963–1974. doi: 10.1161/ATVBAHA.115.305896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piao M, Tokunaga O. Significant expression of endoglin (CD105), TGFbeta-1 and TGFbeta R-2 in the atherosclerotic aorta: an immunohistological study. J. Atheroscler. Thromb. 2006;13:82–89. doi: 10.5551/jat.13.82. [DOI] [PubMed] [Google Scholar]
- 49.Liu RM, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian V, Golledge J, Hey wood EB, Bruemmer D, Daugherty A. Regulation of peroxisome proliferator-activated receptor-γ by angiotensin II via transforming growth factor-β.1-activated p38 mitogen-activated protein kinase in aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2012;32:397–405. doi: 10.1161/ATVBAHA.111.239897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-β regulates Nox4 MnSOD catalase expression, and IL-6 release in airway smooth muscle cells. Am J. Phys. Lung Cell. Mol. Phys. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, et al. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J. Biol. Chem. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, et al. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler. Thromb. Vasc. Biol. 2012;32:745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward MR, Agrotis A, Kanellakis P, Dilley R, Jennings G, Bobik A. Inhibition of protein tyrosine kinases attenuates increases in expression of transforming growth factor-beta isoforms and their receptors following arterial injury. Arterioscler. Thromb. Vasc. Biol. 1997;17:2461–2470. doi: 10.1161/01.atv.17.11.2461. [DOI] [PubMed] [Google Scholar]
- 55.Sinha S, Heagerty AM, Shuttleworth CA, Kielty CM. Expression of latent TGF-beta binding proteins and association with TGF-beta 1 and fibrillin-1 following arterial injury. Cardiovasc. Res. 2002;53:971–983. doi: 10.1016/s0008-6363(01)00512-0. [DOI] [PubMed] [Google Scholar]
- 56.Manea A, Tanase LI, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010;396:901–907. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler. Thromb. Vasc. Biol. 2008;28:878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 58.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ. Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 59.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 60.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 61.de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, et al. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J. Am. Coll. Cardiol. 2007;50:2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 62.Virani SS, Nambi V, Hoogeveen R, Wasserman BA, Coresh J, Gonzalez F, 2, et al. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur. Heart J. 2011;32:459–468. doi: 10.1093/eurheartj/ehq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 64.Huber S, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 65.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of inter-leukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 66.Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T, et al. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology. 2003;49:293–300. doi: 10.1159/000071710. [DOI] [PubMed] [Google Scholar]
- 67.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semaan HB, Gurbel PA, Anderson JL, Muhlestein JB, Carlquist JF, Horne BD, et al. Soluble VCAM-1 and E-selectin, but not ICAM-1 discriminate endothelial injury in patients with documented coronary artery disease. Cardiology. 2000;93:7–10. doi: 10.1159/000006995. [DOI] [PubMed] [Google Scholar]
- 69.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–02. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 70.Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016;36:295–07. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 71.Murray TV, Smyrnias I, Shah AM, Brewer AC. NADPH oxidase 4 regulates cardiomyocyte differentiation via redox activation of c-Jun protein and the cis-regulation of GATA-4 gene transcription. J. Biol. Chem. 2013;288:15745–15759. doi: 10.1074/jbc.M112.439844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, et al. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J. Mol. Cell. Cardiol. 2016;92:30–40. doi: 10.1016/j.yjmcc.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]