Abstract
Regulatory T cells (Tregs) are critical for self-tolerance. While adoptive transfer of expanded Tregs limits graft-versus-host disease (GVHD) after hematopoietic cell transplantation (HCT), ex vivo generation of large numbers of functional Tregs remains difficult. Here, we demonstrate that in vivo targeting of the TNF superfamily receptor TNFRSF25 using the TL1A-Ig fusion protein, along with IL-2, resulted in transient but massive Treg expansion in donor mice, which peaked within days and was nontoxic. Tregs increased in multiple compartments, including blood, lymph nodes, spleen and colon (a GVHD target tissue). Tregs did not expand in bone marrow, a critical site for graft-versus-malignancy (GVM) responses. Adoptive transfer of in vivo expanded Tregs in the setting of MHC-mismatched or MHC-matched allo-HSCT significantly ameliorated GVHD. Critically, transplant of Treg expanded donor cells facilitated transplant tolerance without GVHD, with complete sparing of GVM. This approach may prove valuable as a therapeutic strategy promoting transplantation tolerance.
Keywords: Hematopoietic stem cell transplantation (HSCT), Graft versus Host Disease (GVHD), T regulatory cells (Treg), Tumor Necrosis Factor Receptor Superfamily # 25 (TNFRSF25), Interleukin-2 (IL-2)
Introduction
CD4+FoxP3+ T regulatory cells (Tregs) are required for peripheral maintenance of self-tolerance1–4. The dependence of Tregs on IL-2 was demonstrated when its absence was linked to their loss concomitant with the induction of CD4-driven autoimmune disease5, 6. Subsequent investigations found that Treg-mediated suppression was not limited to autoimmune diseases, but was also critical for regulation of responses against cancers and transplantation antigens7–13. Trials of adoptive Treg therapy in clinical allogeneic hematopoietic stem cell transplantation (HSCT) demonstrated safety and potential efficacy, but also confirmed that the generation of adequate numbers of cells presented a potential barrier to their broader translational application14. Based in part on the practical difficulty of generating sufficient numbers of Tregs ex vivo for the optimal efficacy of adoptive therapy, several studies designed to expand Tregs have utilized a strategy of in vivo expansion by administration of low-dose IL-215–18.
Low-dose IL-2 selectively targets Tregs and recent studies indicate that IL-2–dependent STAT5 activation in Tregs occurs at a 10-fold lower concentration, relative to activated non-Tregs, including memory T cell populations19. Higher expression of IL-2Rα+γ chains by Tregs and endogenous protein serine/threonine phosphatase 1 and/or 2A activity may be responsible for the ability of Tregs to selectively respond to low-dose IL-2. Therefore, a number of completed and ongoing clinical trials are employing low-dose IL-2 treatment to favor expansion of high affinity IL-2R-expressing Tregs versus non-activated conventional T cells16, 18, 20, 21. Notably, we and others have administered IL-2 following experimental HCT to expand Tregs and block alloreactive T cells, an in vivo expansion approach that yields a ~2–3-fold increase in Treg frequency within the CD4 compartment several days following treatment22–24.
Binding of TL1A, the natural ligand of the tumor necrosis factor superfamily receptor 25 (TNFRSF25), provides a strong expansion signal to Tregs, which constitutively express TNFRSF25 at high levels, as well as activated conventional T cells (Tcon), which only express high levels upon activation. We previously found that in vivo stimulation of the TNFRSF25 pathway with an agonistic antibody (clone 4C12) led to a 3–4-fold selective expansion of Tregs (but not Tcon) within 4 days of administration in the absence of exogenous antigen. Based on the inability to expand Treg cells via TNFRSF25 in MHC class II deficient animals as well as in mice expressing a thymic targeted transgenic IL-2Rβ chain in IL-2Rβ−/− deficient mice, this effect was found dependent on the TCR, MHC-II and IL-2 signaling25. Importantly, Tregs expanded via TNFRSF25 provided protection against allergic lung inflammation in an experimental asthma model25 and delayed graft rejection in a heterotopic cardiac allograft model via increases in the numbers of local Tregs26. Recently, Kim et al.27 reported that 4C12 expanded Tregs in murine HSCT donors, resulted in a significant reduction of acute GVHD without impairing the GVL effect in a fully mismatched HSCT model. Despite the promise of TNFRSF25 demonstrated by this study, mAb have significant limitations, including long circulating half-lives and the potential for immunogenicity, limiting the ability to administer multiple doses.
To circumvent these inherent limitations of 4C12 antibody-mediated expansion of Tregs via TNFRSF25, we derived TL1A-Ig, a soluble fusion protein (FP) from the physiological ligand of TNFRSF2528. Here we report a new and more potent strategy to manipulate Tregs via 1) TNFRSF25 by infusion of the TL1A-Ig FP and 2) CD25 by using low dose IL-2, resulting in rapid and dramatic (5–7x) in vivo expansion of functional donor Tregs. Interestingly, differences between the Tregs present in the spleen and lymph node (LN) compartments were identified. Following their transient increase, Tregs returned to normal levels within 3 weeks and no long-term hematologic changes nor tissue pathology was detected. Transplant of splenocytes from donor mice after Treg expansion ameliorated GVHD severity in recipients following MHC-mismatched or MHC-matched transplants. Importantly, transplantation of Treg expanded donor cells preserved effective anti-tumor (GVT/L) responses, even when GVHD was effectively eliminated. Overall, these findings define a novel strategy to expand the Treg compartment to levels not previously reported and suggest a promising clinical strategy for the prevention and/or therapy of GVHD.
Materials and Methods
Mice
Wild-type BALB/c (H2d) mice were purchased from Taconic or Jackson Laboratory. B6-FoxP3rfp mice (provided by R. Flavell, Yale University, New Haven, Connecticut, USA)29, B6-CD45.1 and B10.D2 (H2d) mice were bred in our facility. Mice were used at 6–12 weeks of age and were maintained in pathogen-free conditions at the University of Miami animal facilities. All animal use procedures were approved by the UM IAUCUC.
Antibodies and reagents
Commercial antibodies for use in flow cytometry were purchased from BD Biosciences, Biolegend or eBioscience. Recombinant mouse IL-2 and α-IL-2 monoclonal antibody, clone JES6-5H4 (eBioscience). IL-2/α-IL-2 complex was generated by incubating 1.5 μg recombinant mouse IL-2 with 8 μg JES6-5H4 for 15 minutes at RT. TL1A-Ig was generated as described previously28. The A20luc/YFP cell line (derived from BALB/c mice) was a generous gift of Dr. Robert Negrin (Stanford University) and used for GVL experiments30. Luciferin was purchased from Perkin Elmer; G-CSF (Biolegend) and AMD3100 (Sigma Aldrich).
Flow cytometry and cell sorting
Single-cell suspensions were prepared from different organs (spleen, LN, BM, colon). Peripheral blood was collected in heparinized tubes. PBMCs were isolated by standard Ficoll density gradient centrifugation. 106 cells were pre-blocked with anti-mouse CD16/CD32 and stained with different antibody combinations. Intracellular staining was performed according to standard procedures. Flow cytometric analysis was performed on a BD LSR-Fortessa-HTS instrument. DIVA or FlowJo software was used for analysis. Cell sorting was done using a FACSAria II cell sorter (BD) after enrichment of T cells (sIG depletion of B cells.
In vitro Suppression Assay
FoxP3− splenocytes (105) were cultured in 96-well plates and activated with 1 μg soluble anti-CD3 (clone 2C11) antibody in the presence or absence of sorted CD4+FoxP3+ Tregs (different ratios). After 72 hours. proliferating cells were counted by Trypan blue exclusion using the Vi-cell XR cell counter (Beckman Coulter).
Functional assessment of spleen and LN cultures
Spleen and LN suspensions were activated in vitro with either 1 ug/ml soluble anti-CD3 (T cells) or 2 ug/ml LPS (B cells). After 72 hours, proliferating cells were counted as described above.
Methylation analyses
Tregs and conventional CD4 T cells from TL1A-Ig/IL-2 treated and un-expanded mice were flow sorted. Using the Applied Biosystems ‘Cells-to-CpG’ Bisulfite Conversion kit, genomic DNA(gDNA) from the T cell lysates was denatured and bisulfite converted. The samples were desalted and disulfonated. Bisulfite converted denatured gDNA was eluted and PCR amplification was performed. The FoxP3 CNS2 region was amplified by PCR using the following primers (Fw - ATGGAGGTTGTTTCTGGGACA and Rev- TTGAAGACTCAAGGGGGTCTC). The FoxP3 CNS2 amplicon was cloned using the Invitrogen TA Cloning Kit and vector pCR 2.1. Individual clones were sequenced. Sequence alignments were performed using Basic Local Alignment Search Tool (BLAST) from the NCBI comparing genomic mouse sequence NC_000086.7 as a reference to the cloned FoxP3 CNS2 sequences. Jmp Pro 13 software was used to assess distribution of methylated and unmethylated sites arranged by groups (Tcon, Non-expanded Treg and Expanded Treg). Confidence intervals determined at 95% alpha.
Histologic Analysis & Immunohistochemistry
Tissues from animals 6 months post-Treg expansion, and unexpanded controls or from recipients post HSCT were fixed in 10% formalin and embedded in paraffin. Sections were stained with Hematoxylin-Eosin (H&E) or Massons Trichrome for histologic examination.
HSCT and GVL experiments
For the HSCT in the major MHC-mismatch model (B6→BALB/c), female BALB/c mice (H2d) were ablatively conditioned with 8.5 Gy total body irradiation 1 day prior to transplant. Bone marrow (BM) cells were obtained from femurs, tibias, and vertebrae from sex-matched B6-CD45.1 (H2b; Thy1.2) donor animals. A single-cell suspension of marrow cells was prepared by flushing bones with a 21-gauge needle and the cells were filtered through a 100-μm nylon mesh. Donor marrow cells were depleted of T cells via complement-mediated lysis using anti-T-cell-specific antibody HO-13-4 (hybridoma supernatant, mouse anti-Thy1.2 IgM; ATCC) generously provided by Dr. Bruce Blazar (University of Minnesota), anti-CD4 mAb (clone 72.4), anti-CD8 mAb (clone H02.2) and rabbit complement (Cedarlane Laboratories). The marrow cells were incubated at 37°C for 45 minutes, washed twice in RPMI, and resuspended for hematopoietic cell transplant. Marrow T-cell depletion was routinely >99%. Donor T cells were prepared from spleens or LN obtained from B6-FoxP3rfp expanded or non-expanded animals. Donor cells were stained for T cells (anti-CD4, clone RM4–5; anti-CD8, clone 53-6-7) and adjusted to 1.2x106 T cells per mouse prior to mixing with BM. Recipient mice were transplanted (day 0) with TCD BM (5x106) and 1.2x106 T cells IV in a 0.2 ml volume via tail vein injection.
For the MHC-matched, minor antigen mismatch model (B10.D2→BALB/c), lethally irradiated (8.0 Gy) BALB/c mice were transplanted (Day 0) with 8x106 non-TCD BM cells and 25x106 splenocytes from B10.D2 mice.
GVHD was assessed by monitoring recipients for changes in total body weight, clinical signs and overall survival. The clinical signs of GVHD were recorded for individual mice. Recipients were scored on a scale from 0 to 2 for 5 clinical parameters31: (1) weight loss; (2) diarrhea; (3) fur texture; (4) posture; and (5) alopecia. To evaluate the GVL effect, 1x105 A20luc/YFP cells were injected IV at the time of BMT30.
Bioluminescence imaging
In vivo bioluminescence imaging (BLI) was performed as described previously32. Briefly, mice were injected intraperitoneally with luciferin (150 mg/kg of body weight), anesthetized and imaged using an IVIS Spectrum in vivo imaging system (Perkin Elmer) for up to 5 minutes. Imaging data were analyzed with Living Image Software (Perkin Elmer).
Statistical analysis
All graphing and statistical analysis were performed using GraphPad Prism. Values shown in graphs represent the mean of each group + SEM. Survival data were analyzed with the Mantel-Cox log-rank test. Nonparametric unpaired two-tailed t test was used for comparisons between 2 experimental groups, and multiple variable analysis was performed using ANOVA. A P-value less than 0.05 was considered significant.
Results
Synergistic effect of TL1A-Ig and IL-2 induces marked and transient expansion of CD4+FoxP3+ Tregs in vivo
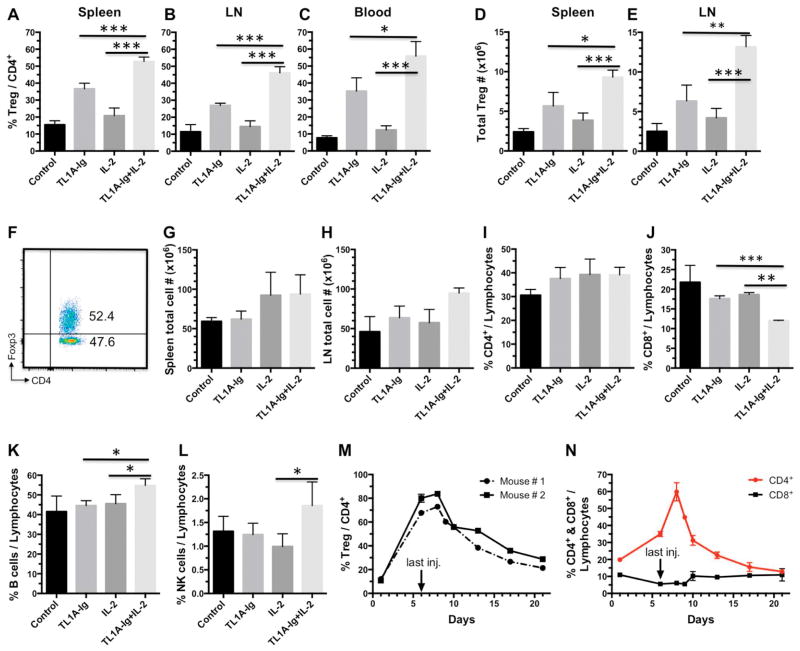
Our laboratory previously reported that infusion of an agonistic anti-TNFRSF25 (“DR3”) mAb (4C12), can systemically increase Treg levels ~3–4x within 4 days of administration25, 26. Since we and others have shown that infusion of rmIL-2 free or bound to anti-IL-2 mAb (JES6-5H4) expands the Treg compartment ~2–3x (Fig. 1A–C) 22, 23, we asked if targeting both receptors could dramatically elevate the level of Treg cells in the peripheral CD4+ T cell compartment. Since FP have shorter half-lives and lack immunogenicity relative to mAbs, we generated a TL1A-Ig FP28 capable of naturally activating TNFRSF25. Compared to all prior reported in vivo treatment regimens, assessment of Treg levels routinely demonstrated a dramatic and unprecedented in vivo expansion in the frequency (45–70% FoxP3+/CD4+) and absolute numbers (7x106–15x106 total vs. 2–3x106) of Tregs using the combination treatment (Fig. 1A–E). Consistent with increasing Treg levels, we observed slightly increased numbers of spleen and lymph node cells (Fig. 1 G,H) with elevated and diminished frequencies of CD4+ and CD8+ T cells, respectively (Fig. 1I,J). Treg expansion resulted in a significant shift from cTregs (central = CD62L+Ly6C+or −) to primarily eTregs (effector = CD62L−Ly6C−) in the LN and spleen (Supplemental Fig. 1)33, 34. The resultant CD62L−Ly6C− eTregs and CD62L+Ly6C− cTregs represent highly suppressive populations33. While Treg expansion was marked (5–7x over baseline, a level not previously reported), reaching maximal levels 6–8 days after protocol initiation (Fig. 1M), 2–3 weeks after the final injection there was a return to near normal frequencies and absolute numbers of Tregs (Fig. 1M). We observed no evidence of expansion of Tcon cells (which may upregulate TNFRSF25 upon activation), nor any systemic signs of inflammation suggesting nonspecific or bystander activation via other mechanisms. Furthermore, assessment of Treg and lymphocyte levels, CBC analyses and histopathological tissue examination demonstrated no abnormalities in recipients who underwent Treg expansion 6 months previously (Supplemental Fig. 2). These observations support the notion that after transient Treg expansion, the CD4+FoxP3+ compartment is phenotypically and functionally normal and therefore likely to be contributing to maintenance of peripheral self-tolerance in these animals.
Fig. 1. Synergistic effect of TL1A-Ig and IL-2 on Treg expansion in vivo.
A–E Combined administration of TL1A-Ig and rmIL-2 in vivo expands Tregs to much higher levels than single agents alone (n=6; p values are shown). TL1A-Ig (50 μg) was administered ip on days 1–4; rmIL-2 (1.5 μg) bound to α-IL-2 mAb (clone JES6-5H4; 8 μg) on days 4 and 6. Mice were sacrificed on day 7. Treg expansion is shown as frequency (%) of total CD4+ cells in spleen (A), LN (B) and PB (C) and total cell numbers in spleen (D) and LN (E). F Example dot plot of Treg expansion with TL1A-Ig+IL-2 in spleen on day 7. G–H Total cell numbers in spleen (G) and LN (H) post expansion on day 7 (n=6; p=ns if not shown). Consistent with an expansion of Tregs, increased numbers of spleen and lymph node cells (Fig. 1G,H) with elevated frequencies of CD4+ and diminished frequencies of CD8+ T cells, respectively were detected (Fig. 1I,J). I–L The frequency (%) of CD4 (I), CD8 (J), B (K) and NK1.1 cells (L) out of the lymphocyte compartment post Treg expansion in LN on day 7 is shown (n=6; p=ns if not shown). M Kinetics of Treg expansion indicate return to normal levels 2–3 weeks following final injection. N Kinetics of CD4, CD8 expression following final injection.
TL1A-Ig/IL-2 in vivo expanded Tregs are activated and functionally responsive
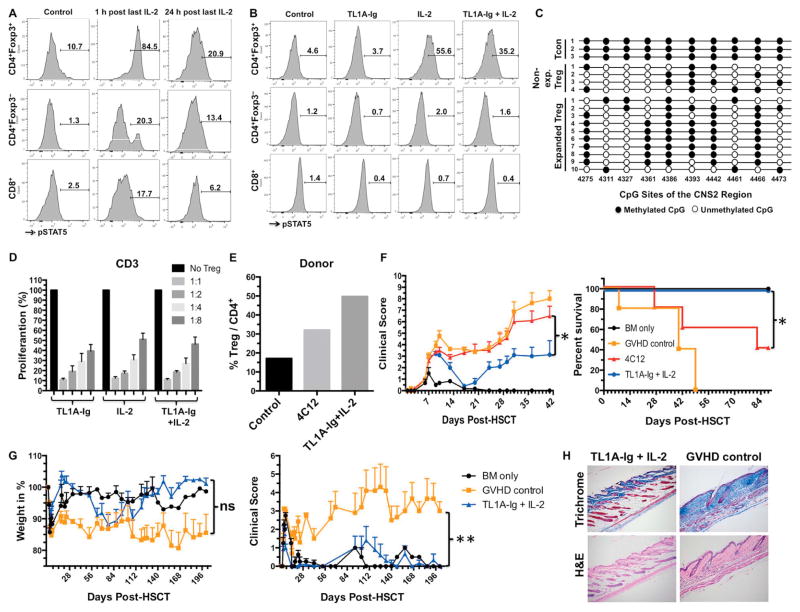
STAT5 signaling is critical to the function of Tregs and occurs downstream of the IL-2R5, 21, 35. Following the final IL-2 injection (d6), we directly assessed pSTAT5 levels in the expanded Tregs. pSTAT5 was sharply increased in the Treg fraction 1 hour post-injection and was still elevated 24 hours later (Fig. 2A). Analyses from animals treated with TL1A-Ig or IL-2 alone versus the combination demonstrated that one day following the final IL-2 injection: a) Tregs from mice receiving either IL-2 alone or the TL1A-Ig/IL-2 combination had elevated pSTAT5 expression; and b) there was little change in pSTAT5 levels in conventional (FoxP3-negative) T cells (Fig. 2B). Consistent with functional activity, the expanded Tregs maintained a high level of demethylation within the Treg-specific demethylation CNS2 (TSDR) region of the FoxP3 locus as reported previously for unexpanded Tregs (Fig. 2C)36, 37. To analyze their functional ability, we compared the suppressive capacity of sorted Tregs treated with TL1A-Ig or IL-2 alone and in combination (Fig. 2D). Tregs from expanded mice efficiently inhibited T conventional cell expansion in cultures stimulated with α-CD3 mAb regardless of whether individual or combined treatment was used to induce in vivo expansion (Fig. 2D). These results demonstrate that in vivo engagement of TNFRSF25 and IL-2R leads to rapid and marked expansion of functionally active Tregs.
Fig. 2. TL1A-Ig / IL-2 expanded Treg activation and function: Amelioration of GVHD following MHC mismatched and MHC-matched allogeneic hematopoietic stem cell transplantation.
Tregs were expanded with TL1A-Ig/IL-2 (single or combined) as in Fig. 1. Mice were sacrificed at different time points after the last IL-2 injection. A pSTAT5 staining (flow cytometry) showing the heightened activation status of splenic Tregs compared to Tcon CD4 and CD8 cells, 1 and 24 h after the last IL-2 injection (combined TL1A-Ig/IL-2) B Elevated pSTAT5 expression shown by flow cytometry in the spleens of IL-2 and TL1A-Ig/IL-2 but not TL1A-Ig only treated B6-FoxP3rfp animals one day following the last IL-2 injection. C Decreased methylation of FoxP3 CpG sites in the CNS2 (TSDR) region of TL1A-Ig/IL-2 expanded and unexpanded Tregs versus FoxP3− CD4 T (Tconv) cells. D Tregs expanded with single or combined treatment show comparable suppressive activity in a suppression assay. E, F Donor B6-FoxP3rfp mice were either left untreated or were treated with TL1A-Ig/IL-2 (TL1A: 50 μg on days -6 to -3; rmIL-2: 1.5 μg bound to α-IL-2 mAb [clone JES6-5H4] 8 μg on days -3 and -1) or 4C12 (0.5 mg/kg on day -4). Treg expansion was assessed on day 0 at the time of BMT (E). F A HSCT utilizing a B6 → BALB/c donor/recipient mouse model involving a complete MHC mismatch was performed on day 0. Lethally irradiated (8.5 Gy) BALB/c mice received 5x106 TCD B6-CD45.1 BM cells and spleen cells from expanded (TL1A-Ig/IL-2 or 4C12) or untreated B6-FoxP3rfp donor mice adjusted to contain 1.2x106 total T cells. Clinical scoring and survival are presented (F). HSCT with cells from TL1A-Ig/IL-2 expanded donors demonstrates more efficient amelioration of GVHD compared to 4C12 (n=5). G A HSCT utilizing a B10.D2 ; BALB/c donor/recipient mouse model across a MHC-matched, minor histocompatibility antigen mismatch was performed using 8x106 non-TCD BM cells + 25x106 spleen cells from either untreated or TL1A-Ig/IL-2 expanded B10.D2 donor mice. Percentage of initial weight and clinical score are presented (G). Amelioration of GVHD shown was readily detected using TL1A-Ig/IL-2 Treg expanded donor cells (n=7). p values are shown. H Massons Trichrome (collagen) and H&E staining from recipient skin 4–5 wks post-HSCT as described in G. In recipients of Treg expanded donor cells (TL1A-Ig + IL2) note the thin epidermis, scant collagen staining (blue) in the dermis and absence of inflammatory cells whereas recipients of non-expanded donor cells (GVHD control) exhibited a thickened epidermis, extensive collagen deposition in the dermis and a modest infiltration of chronic inflammatory cells. Magnification = 25x.
Transplant with TL1A-Ig/IL-2 in vivo expanded Treg donor cells efficiently ameliorates GVHD following MHC-mismatched and MHC-matched allogeneic HSCT
HSCT was performed using the B6 → BALB/c donor/recipient combination across a complete MHC-mismatch to assess GVHD after transplant using donor mice following transient Treg expansion. Since 4C12 mAb treated donor mice was previously found to ameliorate GVHD via Treg expansion, we compared efficacy of donor Treg expansion and subsequent GVHD amelioration achieved using combined TL1A-Ig/IL-2 therapy to the mAb strategy27. Expansion of Tregs was clearly superior following use of the combination strategy vs 4C12 mAb (Fig. 2E) as was the amelioration of GVHD following transplant of Treg expanded donor populations (Fig. 2F). Notably, using our TL1A-Ig/IL-2 protocol in an MHC-matched, minor transplantation antigen mismatched pre-clinical “MUD” model, GVHD was also dramatically diminished compared to recipients receiving cells from untreated donor mice and comparable to recipients receiving BM without spleen cells (Fig. 2G). Histological analysis of skin samples was performed comparing sections from recipients of donor cells treated with our TL1A-Ig/IL-2 protocol versus untreated donors (Fig. 2H). The epidermis was thin, the dermis showed scant collagen staining (blue) and inflammatory cells were absent in recipients of TL1A-Ig/IL-2 treated donors. In contrast, the epidermis was thickened, the dermis exhibited extensive collagen deposition and a modest infiltration of chronic inflammatory cells was apparent in recipients of untreated donors.
TL1A-Ig/IL-2 expansion of Tregs in vivo results in immune suppressive environments: comparison of spleen versus LN
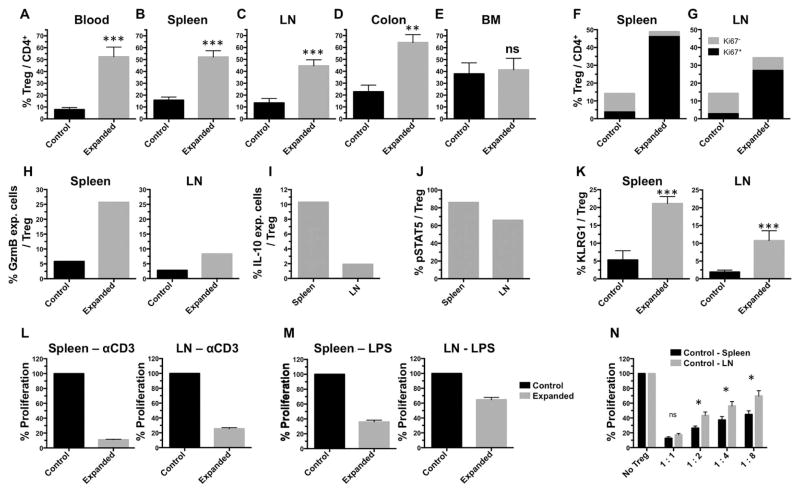
Compartmental assessment of in vivo Treg expansion in healthy donor mice demonstrated distinct differences. Whereas Tregs in blood, spleen, LN and colon were dramatically expanded (Figs. 1A–C, 3A–D), expansion of BM Tregs was modest (1.3–1.5x) (Fig. 3E). In all compartments, expanded Tregs were highly proliferative as evidenced by Ki67 (>80%+) staining (Fig. 3F,G). Within lymphoid organs, consistently higher Treg frequencies were observed in the spleen versus LN compartment (Fig. 3F,G). Interestingly, effector molecules including Granzyme B (GzmB) and IL-10 were expressed at a higher frequency of Tregs from the spleen compared to LN of TL1A-Ig/IL-2 treated animals (Fig. 3H,I) In addition to the increased IL-10 expression (Fig. 3I), both pSTAT5 (Fig. 3J) and KLRG1 (Fig. 3K) expression were higher in splenic versus LN Tregs of treated mice and this was observed in multiple independent experiments (Fig. 3). Furthermore, while the highly suppressive eTreg subset was elevated in both spleen and LN in Treg expanded animals, the strongly suppressive Ly6C− cTreg subset was significantly decreased (by 50%) in the LN but not in the spleen (Supplemental Fig. 1B). Consistent with these differences in functional (IL-10, pSTAT5) and differentiation (Ly6C, KLRG1) markers, following stimulation of spleen and LN cell suspensions obtained from TL1A-Ig/IL-2 treated mice with α-CD3 (Fig. 3L) and LPS (Fig. 3M), a suppressive environment was apparent in both compartments, however T and B cell suppression was more extensive in spleen cell cultures. The inability to respond to LPS is notable, as this implies constitutive suppression following TL1A-Ig/IL-2 treatment, since LPS-mediated activation does not require TCR engagement. Finally, we assessed the functionality of Tregs in the spleen versus LN of untreated B6-FoxP3rfp mice and found splenic Tregs were also more suppressive, suggesting an intrinsic property, rather than TL1A-Ig/IL-2 mediated functional changes (Fig. 3N).
Fig. 3. Expansion of Tregs in certain hematopoietic compartments is accompanied by a decrease in T and B cell immune responsiveness.
A–E Using standard protocol of treatment (TL1A-Ig/IL-2), the frequency of Tregs is increased in blood, spleen, LN and GI tract (colon) but barely bone marrow (n=13, except colon: n=3). F,G Expanded Tregs are highly proliferative indicated by expression of Ki67 in spleen and LN. One example is shown out of 3 independent experiments. In each experiment the elevation of Ki67 expression in expanded Tregs was ~10 fold greater than that in Tregs from control animals. H,I Increased cytokine production (Granzyme B, IL-10) by Tregs from expanded mice following PMA [1 ng/ml] + Ionomycin [1 μM] stimulation for 5 hours in the presence of Monensin indicate higher levels in splenic vs. LN derived Tregs. These increases were observed in each of 3 independent experiments performed ranging from 7x for GzmB between the control vs expanded Tregs in the spleen, ~4–5x for GzmB between the control vs expanded Tregs in the LN and 3–5x for IL-10 between the spleen vs. LN for expanded Tregs. J Higher pSTAT5 expression (assessed by flow cytometry) in spleen vs LN 1 h post final IL-2 injection. For H–J one example is shown out of 3 independent experiments. K Increased expression of KLRG1 on Tregs post expansion with higher expression on spleen compared to LN (n=5). L,M Treg expansion leads to a suppressive environment (T and B cells) in spleen and LN with increased suppression in the splenic compartment compared to LN despite similar Treg numbers (1.9x104 vs. 1.8x104 Tregs in spleen vs LN cultures respectively) (n=3). These cultures contained cell suspensions of spleen or lymph node cells obtained from mice which underwent TL1A-Ig+IL-2 induced expansion or normal, unexpanded mice. The cultures were then stimulated with either anti-CD3mAb or LPS for 72 hours (see Methods) N An in vitro suppression assay with sorted Tregs from control (normal) mice demonstrates more effective suppression by splenic vs LN derived CD4+FoxP3+ cells (n=3).
Spleens from TL1A-Ig/IL-2 expanded donors more effectively abrogate GVHD compared to LN cells
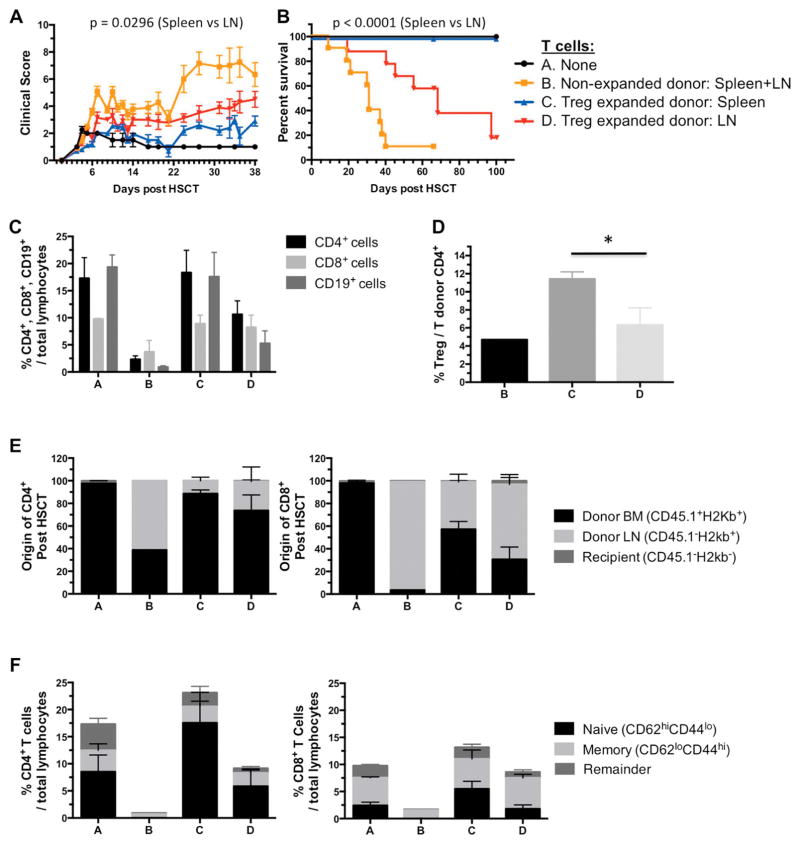
Based on the phenotypic differences and in vitro suppressive capacity of splenic versus LN Tregs post-TL1A-Ig/IL-2 expansion, we performed HSCT from both Treg sources to assess their ability to suppress GVHD. HSCT (B6→BALB/c) with either spleen or LN cells from TL1A-Ig/IL-2 expanded or untreated B6-FoxP3rfp mice were adjusted to contain identical numbers of total T cells. While GVHD was substantively reduced in recipients of either relative to recipients of donor cells from untreated mice, suppression of GVHD was significantly greater in recipients of donor spleen cells determined by weight loss (data not shown), clinical score (Fig. 4A) and survival (Fig. 4B) in two independent experiments. Recipients were bled one-month post-HSCT to assess engraftment and lymphoid immune reconstitution (Fig. 4C–F). Mice transplanted with spleen cells from Treg expanded donors exhibited higher CD4/CD8 ratios and greater B cell levels compared to recipients of LN cells from the same donors. However, recipients receiving either source of expanded donor cells exhibited a phenotype consistent with relatively preserved immune reconstitution, compared to mice receiving control donor grafts (Fig. 4E,F). In addition, recipients of spleen cells from expanded donors had more robust recovery of naïve T cells, compared to recipients of LN cells. Once again, recipients of Treg-expanded grafts demonstrated more healthy immune reconstitution versus mice transplanted with non-expanded grafts (Fig. 4E,F). RFP+ donor Treg analysis indicated the highest frequency was present in recipients of Treg expanded spleen cell donors (Fig. 4D). Finally, we assessed whether this approach could safely be accomplished in donors treated with filgrastim (G-CSF) and plerixafor (AMD3100), agents commonly used to mobilize stem cells for peripheral blood harvesting of human donors, and found that Treg expansion could also be achieved without apparent toxicity in such donors (Supplemental Fig 3).
Fig. 4. Hematopoietic stem cell transplant (HSCT) with cells from TL1A-Ig/IL-2 expanded donors indicates more efficient amelioration of GVHD by spleen cells compared to LN cells.
A B6 → BALB/c donor/recipient mouse model involving a complete MHC mismatch was utilized. Lethally irradiated mice received 5x106 TCD B6-CD45.1 BM cells and either spleen or LN cells from TL1A-Ig/IL-2 expanded or untreated B6-FoxP3rfp mice adjusted to contain 1.2x106 total T cells. A, B HSCT with cells from TL1A-Ig/IL-2 expanded donors indicates more efficient amelioration of GVHD by spleen cells compared to LN cells (n=10). A Clinical GVHD score. B Overall survival through 9–10 weeks post-HSCT. Median survival: A>100 days; B=31 days; C>100 days; D=68 days. p values are shown.
C–F Bleed day 31. C Frequency of CD4+, CD8+ and CD19+ cells out of total lymphocytes. D Frequency of Treg cells out of donor T CD4+ cells. Group A is not included in this graph since recipients did not receive donor T cells. E Cell origin/engraftment (BM donor, LN donor or Recipient) of CD4+ and CD8+ cells. F Naïve/Memory compartment of CD4+ and CD8+ cells.
TL1A-Ig/IL-2 Treg expanded and non-Treg expanded donor cells mediate equally effective GVL responses
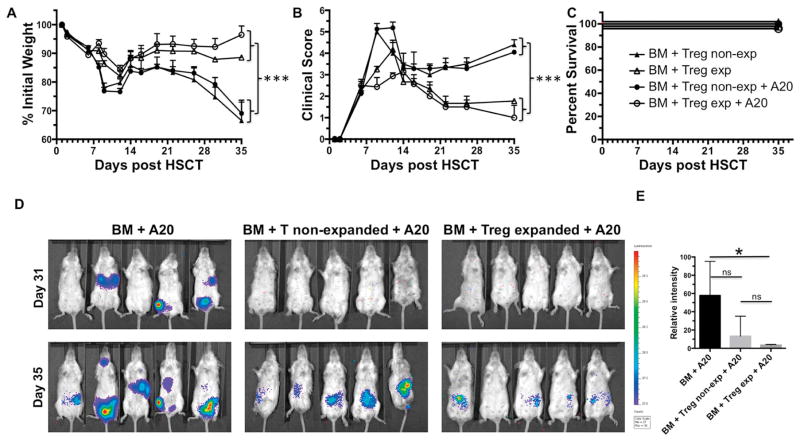
Given the importance of graft vs. malignancy responses in HSCT, it is critical that strategies that ameliorate recipient GVHD preserve graft vs. leukemia (GVT/L) responses essential for relapse-free survival. Therefore, A20luc/YFP tumor cells were injected on D0 with TCD-BM alone or together with TL1A-Ig/IL-2 Treg expanded or non-expanded donor spleen cells (Fig. 5). Recipients of Treg expanded donor spleen cells in the absence of A20luc/YFP tumor cells again exhibited reduced GVHD compared to recipients of non-expanded donor spleen cells (Fig. 5A, B). As expected, A20luc/YFP injected recipients receiving non-expanded B6-FoxP3rfp spleen cells had reduced tumor bulk by bioluminescence, indicative of GVL activity accompanying the presence of GVHD in these mice (Fig. 5D center). Critically, recipients of Treg expanded donor spleen cells had reduced GVHD but also comparable or slightly better GVL activity to that in recipients of non-expanded spleen cells (Fig. 5D right). Statistical analysis confirmed the IVIS imaging (Fig. 5E). These data indicate that transplant with TL1A-Ig/IL-2 expanded donors results in amelioration of GVHD that is selective, sparing GVL activity essential for the success of HSCT.
Fig. 5. Treg expanded donor cells mediate GVL responses as effective as normal donors.
HSCT with splenocytes from unexpanded or TL1A-Ig/IL-2 expanded B6-FoxP3rfp donors was performed as described in Fig. 4. Some groups also received 1x105 A20luc/YFP cells iv at the time of HSCT. Donor Treg expansion leads to an efficient amelioration of GVHD while retaining the GVL effect (n=8) shown by A % Initial weight, B Clinical GVHD score and C Survival. Statistical analyses (see Methods) was performed on Day 35. The GVL effect was identified by bioluminescence imaging D and quantified by measurement of the total photon flux from each recipient group E. Treg expanded donors (D, right panel) mediate GVL responses as effective as normal donors (D, middle panel). Positive (BM+A20) tumor growth control (D, left panel). Color bar represents signal intensity code over body surface area.
Discussion
Expansion of Tregs in vivo has the potential to be an important treatment advance in settings of transplantation tolerance and therapy for autoimmune diseases. Prior pre-clinical studies including our own found that Tregs could be consistently increased in vivo to achieve expansion to ~20–30% of the CD4 compartment by targeting CD25 or a number of cell surface receptors in the TNF family38–40. More recently, findings demonstrated that low-dose IL-2 usage not only expands Tregs in vivo, but selectively favors FoxP3+ Tregs providing a strategy to manipulate these cells in patients with chronic GVHD and type 1 diabetes16–18. Our laboratory and others have reported that administration of an agonistic antibody (4C12) or FP (TL1A-Ig) targeting TNFRSF25 rapidly expands Tregs in vivo25, 28. We hypothesized that the ‘limited’ 2–3x expansion resulting from targeting the TNFRSF25 receptor alone was in part a consequence of IL-2 levels available to sustain such increases. Consistent with this interpretation, our previous work found that CD25 expression was decreased following 4C12-mediated expansion compared with unstimulated Tregs25. Accordingly, we posited that addition of IL-2 could maintain and drive further Treg expansion together with TNFRSF25 stimulation. TL1A-Ig lacks immunogenicity and has a considerably shorter half-life than mAbs, making it an ideal reagent for both preclinical studies and also for potential translation to the human clinical setting. Based upon the theoretical reasons discussed, we assessed the effects of combined TL1A-Ig-based targeting of TNFRSF25 in combination with IL-2, hypothesizing that combined targeting would create therapeutic synergy. Our results strongly confirm the potential of this approach, demonstrating the highest levels of in vivo expansion of the FoxP3+CD4+ compartment reported to date.
Treg expansion resulted in FoxP3+ expressing cells comprising between 50–70% of the CD4 compartment, 5–7-fold higher levels than at baseline and typically achievable only with ex vivo expansion and/or selective enrichment of donor grafts using sorted Tregs. Expansion was observed in multiple sites critical for GVHD initiation and regulation, including peripheral lymphoid tissue, the lamina propria and intraepithelial compartments in the gastrointestinal tract. Interestingly, only Treg expansion in the BM was somewhat limited, with less than twofold expansion typically observed. This finding may reflect that the marrow compartment routinely contains a higher frequency of Tregs/CD4 T cells (15–25%) and/or a unique population vs spleen and LN. While these studies cannot establish whether the BM microenvironment and/or differences in trafficking may contribute to the lower increases observed, future adoptive transfer experiments involving peripheral versus marrow-derived Tregs may address these possibilities.
While quantitative Treg expansion was comparable in both the spleen and LN of TL1A-Ig/IL-2 treated mice, we detected differences in the phenotype, subsets and function of the Tregs from these tissues. Tregs from spleen expressed higher levels of IL-10 and GzmB and also demonstrated greater functional efficacy versus those from LN, as shown by experiments demonstrating an overall suppressive environment in the spleen that appeared to be Treg mediated. Although sorted ‘expanded’ and native ‘non-expanded’ Tregs may effectively suppress conventional T cells stimulated by anti-CD3mAb, we observed only the former suppressed LPS stimulated B cells in spleen cell cultures (containing extremely large numbers of TL1A-Ig+IL-2 expanded Treg cells) indicating that additional Treg TCR stimulation of this population was not required. The functionally active state of these expanded Tregs including their cytokine levels and pSTAT5 expression supports the notion that an amplification strategy using low-dose IL-2 to drive Treg expansion may selectively amplify Treg activity for therapeutic intervention in autoimmune diseases where native Treg numbers may be limiting.
Pre-clinical studies adding Tregs to donor inoculum pre-HSCT established that high ratios of Treg:Tconv were required to efficiently inhibit GVHD41. We reasoned that dramatic expansion of donor Tregs in vivo may be an effective strategy to improve the safety and efficacy of allogeneic HSCT, since these FoxP3+ CD4 T cells are capable of immediate suppression of alloreactive T cells that mediate potentially lethal GVHD. Consistent with this notion, we elevated donor Tregs to levels resulting in ~1 Treg:2–3 Tconv (CD4+CD8) and notably the expansion protocol significantly shifted the balance of Treg cells to more suppressive subsets (Figs. 1,3 and Supplemental Fig 1). We tested the efficacy of in vivo expansion of donor Tregs via combined TL1A-Ig/IL-2 treatment of donors, prior to HSCT across complete MHC disparities (B6→BALB/c). While both spleen or LN cells from TL1A-Ig/IL-2 treated mice clearly reduced GVHD, donor splenocytes were more effective, consistent with the phenotypic and functional observations noted above. In total we therefore conclude that while elevated numbers of expanded Tregs in the donor inoculum are crucial for the inhibition of GVHD observed in our studies, the stronger suppression observed following use of splenic vs LN Tregs is likely a result of their augmented effector activity. Additionally, we cannot formally exclude a contribution resulting from other (i.e. Tconv) donor populations. Based on CD44/CD62L expression – we can detect some phenotypic change in the CD4 T conv cells (data not shown). Experiments will need to be performed to determine if the level of these phenotypic changes translate into a difference in these cell’s ability to mediate development of GVHD. Regardless of these considerations, it is important to note that recipients of transplants using spleen cells from Treg expanded donors demonstrated preserved GVL activity using the A20 B cell lymphoma model - even when GVHD was suppressed, in contrast to recipients of normal donor spleen cells, wherein both GVHD and GVL occurred.
Additionally, transplants were also performed across MHC-identical minor antigen mismatched donor-recipient pairs and independent of the degree of mismatch, only marginal clinical GVHD signs were detected in recipients of expanded donor Tregs. Overall, the findings demonstrate that transplantation of donor cells following marked Treg expansion using TL1A-Ig/lL-2 treatment results in superior and dramatic reduction of GVHD which is selective, sparing efficient anti-tumor responses.
Fusion proteins as well as recombinant IL-2 are used clinically to treat inflammatory disorders and currently are under testing in a number of clinical trials42. Since clinical translation of this approach might involve in vivo expansion of Tregs in healthy human donors, it will be critical to perform additional studies to assess the consequences of transient Treg stimulation. However, detailed phenotypic, clinical and pathologic observations did not identify short- or long-term abnormalities following transient Treg expansion. Treg levels returned to normal within 3 weeks following elevation and animals which underwent expansion exhibited normal complete blood counts. Assessment of Treg function in mice long-term remained normal and consistent with these in vitro observations, histology of all tissues examined was unremarkable up to six months following TL1A-Ig/IL-2 administration. Critically, no clinical abnormalities were observed as long as nine months following Treg expansion. Based on the safety and efficacy of this approach in both donors and recipients, and the availability of reagents with the ideal characteristics and safety profile to consider translation to the human clinical setting, expansion of Tregs using fusion protein-based targeting of TNFRSF25 in combination to IL-2 has strong therapeutic potential in the setting of HSCT.
Supplementary Material
A Representative contour plot of Treg subset distribution (Ly-6C− cTregs, Ly-6C+ cTregs, and eTregs ) in control and TL1A-Ig/IL-2 expanded Tregs from (LN) and spleen on day 7. The distribution of Treg subsets was subdivided on the basis of Ly-6C and CD62-L expression. B Combined administration of TL1A-Ig/IL-2 in vivo increases the frequency (%) of eTreg population with a concomitant decrease on the Ly-6C− and Ly-6C+ cTregs from spleen and LN (left) (n=2; p values are shown * p<0.05; **p<0.01; ***p<0.001). Increased expression of KLRG1 on cTregs and eTregs post expansion on spleen and LN (right).
A, B Splenic Treg frequency and CD4/CD8 ratio are normal at 3 and 6 month post TL1A-Ig/IL-2 expansion (n=3). C Extended histopathological examination (H&E sections, 12x magnification) of tissues including pancreas, skin, uterus and lymphoid tissue were unremarkable 6 month post expansion. D Complete blood counts (CBC) from unexpanded B6-FoxP3rfp age- and sex-matched controls and Treg expanded B6-FoxP3rfp mice, 6 month post expansion are shown. E Global T and B cell as well as antigen specific MLR responses were unchanged in these mice >6 month post-expansion indicating Treg expansion has no long term effect on hematopoietic cell proliferation. Splenocytes from unexpanded controls as well as 3 and 6 month post expansion proliferate equally well after stimulation with α-CD3 (1 μg/ml), LPS (2 μg/ml) and irradiated BALB/c splenocytes (2x105 cells) (n=3). F Functional in vitro assessments demonstrated that Tregs from animals expanded at least 6 months earlier mediated comparable suppression to that by Tregs from ‘normal’, i.e. non-expanded mice.
A Combined expansion and mobilization protocol. TL1A-Ig (50 μg) was administered ip on days 1–4; recombinant mouse IL-2 (1.5 μg) bound to α-IL-2 mAb (clone JES6-5H4; 8 μg) on days 4 and 6 ip. G-CSF (2 μg/mouse) was given twice a day on day 3–6 s.c. and AMD3100 (5 mg/kg; sc) on day 7, 2–3 hrs before sacrificing the mice. B The combined protocol expands Tregs and Granulocytes. Frequencies of Treg out of total CD4+ and Granulocytes out of total live cells post expansion/mobilization in blood are shown. C Tregs expanded under mobilizing conditions are functional. LN cells were prepared from untreated control and expanded/mobilized mice and cultured for 72 h in the presence or absence of α-CD3 mAb. In LN cultures from Treg expanded and mobilized animals (right panel), T lymphocyte proliferation is impaired due to large numbers of Treg cells compared to control cultures from unexpanded mice (middle panel).
B6-FoxP3rfp mice were administered protocols used for optimal Treg expansion with either the combination protocol (TL1A-Ig+IL-2): TL1A-Ig (50 μg) was administered ip on days 1–4; rmIL-2 (1.5 μg) bound to α-IL-2 mAb (clone JES6-5H4; 8 μg) on days 4 and 6 (mice were sacrificed on day 7) or the mAb 4C12 (50ug / mouse) and sacrificed on Day 5. There were significantly greater levels of Treg cells using the combination protocol as assessed by the percentage of CD4+FoxP3+ / CD4+ cells.
Highlights.
TNFRSF25 receptor targeting with a fusion protein together with low dose IL-2 elevates levels of Tregs to >50 % of the CD4 compartment.
Treg levels are markedly increased in blood, spleen, lymph nodes, colon but not marrow
Donor Treg expansion results in ameliorated GVHD while preserving the GVL effect
Acknowledgments
We thank Dr. Oliver Umland and the SCC Flow Cytometry Core for their help with cell sorting and Dr. Zhibin Chen for use of his microscope. We are very grateful to Dr. Negrin (Stanford, CA) for generously providing us the A20luc/YFP cell line. We also thank Mr. Wasserlauf for his help with the IVIS system. This work was supported by funds from the Sylvester Comprehensive Cancer Center and NIH RO1 EY024484-01 to RBL; NCI 5PO1 CA109094-05; NIH 1 RO1 AI061807 to ERP and funds from the Kalish Family Foundation to KVK.
Footnotes
Disclosure of Conflicts of Interest
Dr. Podack was an inventor of patents used in the study and stands to gain royalties from future commercialization. Dr. Levy is a consultant for Capricor Therapeutics and Allergan. All other authors declared no conflict of interest.
Author contributions
DW - designed research studies/conducted experiments/analyzed data and wrote paper
HB, CSB, SC, COL and BJP - conducted experiments/acquired and analyzed data
NHA - acquired and analyzed data regarding histopathology
ERP - provided reagents
RBL & KVK – jointly supervised & supported the work and wrote the paper. RBL designed research studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 2.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 6.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shatry A, Chirinos J, Gorin MA, Jones M, Levy RB. Targeting Treg cells in situ: emerging expansion strategies for (CD4(+)CD25(+)) regulatory T cells. Biol Blood Marrow Transplant. 2009;15:1239–1243. doi: 10.1016/j.bbmt.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 12.Alpdogan O, van den Brink MR. Immune tolerance and transplantation. Semin Oncol. 2012;39:629–642. doi: 10.1053/j.seminoncol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9:243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 14.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartemann A, Bensimon G, Payan CA, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 18.Rosenzwajg M, Churlaud G, Mallone R, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48–58. doi: 10.1016/j.jaut.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 23.Shatry A, Levy RB. In situ activation and expansion of host tregs: a new approach to enhance donor chimerism and stable engraftment in major histocompatibility complex-matched allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:785–794. doi: 10.1016/j.bbmt.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinh TN, Kyaw TS, Kanellakis P, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber TH, Wolf D, Tsai MS, et al. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf D, Schreiber TH, Tryphonopoulos P, et al. Tregs expanded in vivo by TNFRSF25 agonists promote cardiac allograft survival. Transplantation. 2012;94:569–574. doi: 10.1097/TP.0b013e318264d3ef. [DOI] [PubMed] [Google Scholar]
- 27.Kim BS, Nishikii H, Baker J, et al. Treatment with agonistic DR3 antibody results in expansion of donor Tregs and reduced graft-versus-host disease. Blood. 2015;126:546–557. doi: 10.1182/blood-2015-04-637587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SQ, Tsai MS, Schreiber TH, Wolf D, Deyev VV, Podack ER. Cloning, expression, and functional characterization of TL1A-Ig. J Immunol. 2013;190:1540–1550. doi: 10.4049/jimmunol.1201908. [DOI] [PubMed] [Google Scholar]
- 29.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 31.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 32.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 33.Delpoux A, Yakonowsky P, Durand A, et al. TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J Immunol. 2014;193:5914–5923. doi: 10.4049/jimmunol.1400477. [DOI] [PubMed] [Google Scholar]
- 34.Toomer KH, Yuan X, Yang J, Dee MJ, Yu A, Malek TR. Developmental Progression and Interrelationship of Central and Effector Regulatory T Cell Subsets. J Immunol. 2016;196:3665–3676. doi: 10.4049/jimmunol.1500595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 36.Ohkura N, Hamaguchi M, Morikawa H, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Morikawa H, Ohkura N, Vandenbon A, et al. Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc Natl Acad Sci U S A. 2014;111:5289–5294. doi: 10.1073/pnas.1312717110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruby CE, Yates MA, Hirschhorn-Cymerman D, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao G, Nayak S, Regueiro JR, et al. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int Immunol. 2010;22:259–270. doi: 10.1093/intimm/dxq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannone F, Lopriore S, Bucci R, et al. Longterm Clinical Outcomes in 420 Patients with Psoriatic Arthritis Taking Anti-tumor Necrosis Factor Drugs in Real-world Settings. J Rheumatol. 2016 doi: 10.3899/jrheum.151042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Representative contour plot of Treg subset distribution (Ly-6C− cTregs, Ly-6C+ cTregs, and eTregs ) in control and TL1A-Ig/IL-2 expanded Tregs from (LN) and spleen on day 7. The distribution of Treg subsets was subdivided on the basis of Ly-6C and CD62-L expression. B Combined administration of TL1A-Ig/IL-2 in vivo increases the frequency (%) of eTreg population with a concomitant decrease on the Ly-6C− and Ly-6C+ cTregs from spleen and LN (left) (n=2; p values are shown * p<0.05; **p<0.01; ***p<0.001). Increased expression of KLRG1 on cTregs and eTregs post expansion on spleen and LN (right).
A, B Splenic Treg frequency and CD4/CD8 ratio are normal at 3 and 6 month post TL1A-Ig/IL-2 expansion (n=3). C Extended histopathological examination (H&E sections, 12x magnification) of tissues including pancreas, skin, uterus and lymphoid tissue were unremarkable 6 month post expansion. D Complete blood counts (CBC) from unexpanded B6-FoxP3rfp age- and sex-matched controls and Treg expanded B6-FoxP3rfp mice, 6 month post expansion are shown. E Global T and B cell as well as antigen specific MLR responses were unchanged in these mice >6 month post-expansion indicating Treg expansion has no long term effect on hematopoietic cell proliferation. Splenocytes from unexpanded controls as well as 3 and 6 month post expansion proliferate equally well after stimulation with α-CD3 (1 μg/ml), LPS (2 μg/ml) and irradiated BALB/c splenocytes (2x105 cells) (n=3). F Functional in vitro assessments demonstrated that Tregs from animals expanded at least 6 months earlier mediated comparable suppression to that by Tregs from ‘normal’, i.e. non-expanded mice.
A Combined expansion and mobilization protocol. TL1A-Ig (50 μg) was administered ip on days 1–4; recombinant mouse IL-2 (1.5 μg) bound to α-IL-2 mAb (clone JES6-5H4; 8 μg) on days 4 and 6 ip. G-CSF (2 μg/mouse) was given twice a day on day 3–6 s.c. and AMD3100 (5 mg/kg; sc) on day 7, 2–3 hrs before sacrificing the mice. B The combined protocol expands Tregs and Granulocytes. Frequencies of Treg out of total CD4+ and Granulocytes out of total live cells post expansion/mobilization in blood are shown. C Tregs expanded under mobilizing conditions are functional. LN cells were prepared from untreated control and expanded/mobilized mice and cultured for 72 h in the presence or absence of α-CD3 mAb. In LN cultures from Treg expanded and mobilized animals (right panel), T lymphocyte proliferation is impaired due to large numbers of Treg cells compared to control cultures from unexpanded mice (middle panel).
B6-FoxP3rfp mice were administered protocols used for optimal Treg expansion with either the combination protocol (TL1A-Ig+IL-2): TL1A-Ig (50 μg) was administered ip on days 1–4; rmIL-2 (1.5 μg) bound to α-IL-2 mAb (clone JES6-5H4; 8 μg) on days 4 and 6 (mice were sacrificed on day 7) or the mAb 4C12 (50ug / mouse) and sacrificed on Day 5. There were significantly greater levels of Treg cells using the combination protocol as assessed by the percentage of CD4+FoxP3+ / CD4+ cells.