Abstract
Aim
To evaluate the association between cervical strain assessed with quasi-static elastography and spontaneous preterm delivery.
Methods
Quasi-static elastography was used to estimate cervical strain in 545 pregnant women with singleton pregnancies from 11 weeks to 28 weeks of gestation. Cervical strain was evaluated in one sagittal plane and in the cross-sectional planes of the internal cervical os and external cervical os. The distribution of strain values was categorized into quartiles for each studied region and their association with spontaneous preterm delivery at ≤34 weeks and at <37 weeks of gestation was evaluated using logistic regression.
Results
The prevalence of spontaneous preterm delivery at <37 weeks of gestation was 8.2% (n=45), and that at ≤34 weeks of gestation was 3.8% (n=21). Strain in the internal cervical os was the only elastography value associated with spontaneous preterm delivery. Women with strain values in the third and fourth quartiles had a significantly higher risk of spontaneous preterm delivery at ≤34 weeks and at <37 weeks of gestation when compared to women with strain values in the lowest quartile. When adjusting for a short cervix (<25 mm) and gestational age at examination, women with strain values in the third quartile maintained a significant association with spontaneous preterm delivery at ≤34 weeks (OR 9.0; 95% CI, 1.1–74.0; p=0.02), whereas women with strain values in the highest quartile were marginally more likely than women with lowest quartile strain values to deliver spontaneously at ˂37 weeks of gestation (OR 2.8; 95% CI: 0.9–9.0; p=0.08).
Conclusion
Increased strain in the internal cervical os is associated with higher risk of spontaneous preterm delivery both at ≤34 and <37 weeks of gestation.
Keywords: Cervical elasticity, cervical stiffness, prematurity, preterm labor, short cervix
Introduction
Throughout gestation, the cervix undergoes dynamic changes in tissue composition characterized by a dynamic remodeling of the collagen network and an increased concentration of glycosaminoglycans and water content in the extracellular matrix.1–11 These changes provide the basis for the process of ripening before the onset of labor and may reflect the elastic properties of the cervix.6,12–23
Different methods have been applied to evaluate the elastic properties of the cervix such as: aspiration,24–26 cervical consistency index,27,28 and elastography.29,30 Elastography was first proposed by Ophir et al.31 as an ultrasound technique able to estimate tissue displacement or deformation when an oscillatory compression is applied. Tissue displacement or strain can be tracked using Doppler techniques or cross-correlation analysis and converted to an elastic modulus as an indirect estimation of tissue stiffness.32–40 Modalities of ultrasound elastography can be classified as: 1) quasi-static, whereby an external compression is applied to create tissue displacement; and 2) continuous, in which an acoustic impulse is produced by the ultrasound system, and the propagation of the shear-wave in the tissues is tracked by ultrafast ultrasound.41
Elastography was first applied to the cervix to differentiate between normal and tumoral cervical tissue;42,43 during pregnancy, it has been used to identify women with higher probabilities for a successful induction of labor.44,45 Our group reported regional differences in cervical strain and strain changes throughout pregnancy using quasi-static elastography.46 We also reported a reduced risk for preterm delivery at <37 weeks of gestation in women with low strain in the internal cervical os.47 The association between cervical strain and preterm delivery at <37 weeks has been recently confirmed by other authors.48–50 Currently, identification of a short cervix by transvaginal ultrasound is the most powerful predictor for spontaneous preterm delivery.51–62 The use of elastography to evaluate cervical strain has shown promising results in its association with preterm delivery; however, there is still the need for data to support the value of this technique in women who will present with moderately/early preterm delivery, and with adjustment for a short cervix. The aim of this study was to estimate whether strain or deformation obtained in different regions of the cervix is associated with spontaneous preterm delivery at <37 weeks of gestation, and with spontaneous preterm delivery at ≤34 weeks of gestation,63–66 and whether this association is altered by the presence of a short cervix.
Methods
Study design and participants
This cross-sectional study was conducted at the Center for Advanced Obstetrical Care and Research [Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, and the Wayne State University School of Medicine, Detroit Medical Center/Hutzel Women’s Hospital, Detroit, MI]. All patients provided written informed consent and were enrolled in research protocols approved by the Human Investigation Committee of Wayne State University and the Institutional Review Board of the NICHD. For this analysis, women evaluated at 11 weeks to 28 weeks of gestation with singleton pregnancies and without structural or chromosomal abnormalities were included. This range of gestational age was defined to include different gestational periods when identification of a short cervix has been associated with preterm delivery.67–71 One cervical examination per patient was analyzed. The elastography recordings of women with a short cervix obtained before treatment with vaginal progesterone were evaluated. Patients with a cervical cerclage were not included. Spontaneous preterm delivery was considered as having resulted from the spontaneous onset of labor or spontaneous rupture of membranes at ≤34 weeks and at <37 weeks of gestation.
Ultrasound and elastography recordings
Gestational age was confirmed in all patients at or before 11 weeks of gestation by the crown-rump length measurement. Cervical length was measured using transvaginal ultrasound (Hitachi 8–4 MHz, HI Vision 900, Hitachi Medical Corporation, Tokyo, Japan) in a sagittal plane, providing clear images of the internal cervical os and external cervical os and complete visualization of the endocervical canal while maintaining equal sizes of the anterior and posterior cervical lips.72,73 The elastography evaluation was performed in a sagittal view of the cervix at the same anatomical plane as that used to measure cervical length, and in cross-sectional planes of the internal and external os. The elastography color box was adjusted to cover the anatomical plane of the cervix, and continuous oscillatory pressure was gently applied by the operator using the transducer without creating additional discomfort to that experienced during the routine vaginal ultrasound examination. The elastography equipment provides a press indicator that displays the average displacement of all structures localized within the color box elastogram; values range from 0 (none) to 7 (maximum). The press indicator can be considered an estimator of the oscillatory compression applied to that specific region. All measurements in this study were performed while maintaining the press indicator at a value of 3; additionally, the ultrasound probe in each image was located in the middle of the cervix and was kept at approximately the same distance from each of its lateral parts. Strain was calculated in two regions of interest for each anatomical plane. For the cross-sectional images, a circular region of interest was applied to cover the endocervical canal, and another included most of the internal or external cervical os; and for the sagittal plane, the regions of interest were adjusted according to the boundaries of the endocervix and the entire cervix (Figure 1). The measured strain within the region of interest represents the percentage of tissue displacement or deformation that resulted during the manual application of oscillatory pressure. Studies were performed by operators with more than two years of experience in cervical elastography and with a previously reported inter-observer correlation coefficient of 0.73, an exact agreement of 65%, and a weighted kappa of 0.46.46
Figure 1.
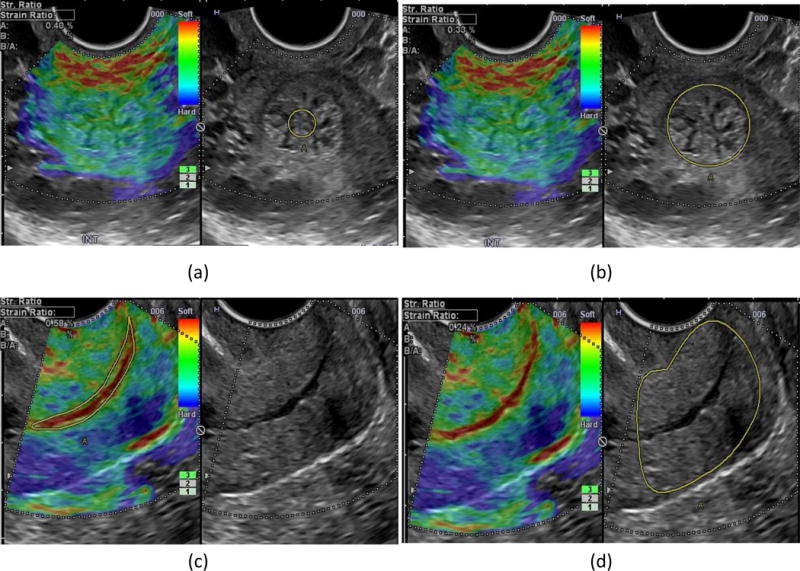
Cervical elastography; upper panel, cross-sectional plane of the internal cervical os: a) strain in the endocervical canal, b) strain in the entire cervix; lower panel, sagittal plane of the cervix: c) strain in the endocervical canal, d) strain in the entire cervix.
Statistical analysis
The distribution of strain values was categorized into quartiles for each studied region, and their association with spontaneous preterm delivery at ≤34 and at <37 weeks of gestation was evaluated using logistic regression. Covariables considered as potential confounders included gestational age and the presence of a short cervix at examination. Chi-square or Fisher’s exact tests were used to examine differences in proportions. Statistical significance was defined as a p value <0.05. Analyses were performed using SPSS® 19 (IBM Corp., Armonk, New York, USA), SAS version 9.4 (Cary, NC, USA), and Med Calc 9.0.1.0 (Ostend, Belgium) statistical software.
Results
Characteristics of the study population
Forty-five [8.2%] women delivered preterm (<37 weeks), and 21 [3.8%] women delivered at ≤34 weeks of gestation. Clinical characteristics of the study population grouped by gestational age at delivery are presented in Table 1. Patients who delivered preterm had a shorter cervix at the time of the ultrasound scan, a higher prevalence of a short cervix (<25 mm) and previous preterm delivery, and they were more frequently identified as smokers than women who delivered at term.
Table 1.
Descriptive characteristics of patients presenting with spontaneous preterm delivery (sPTD) and patients delivering at term
| sPTD ≤34 weeks (n=21) |
sPTD <37 weeks (n=45) |
Delivery ≥37 weeks (n=500) |
|
|---|---|---|---|
| Maternal Age, years (median, range) | 24 (18–27) | 25 (18–38) | 24 (18–38) |
| African-American (n, %) | 20 (95%) | 44 (97%) | 460 (92%) |
| Smoker (n, %) | 5 (23%) | 14 (31%) | 90 (18%) |
| Nulliparity (n, %) | 8 (38%) | 13 (28%) | 197 (29%) |
| Body Mass Index (median, range) | 27 (16–42) | 28 (16–48) | 24 (16–41) |
| Cervical Length | 30.5 (9.6%) | 31.5 (9.2) | 37 (6.3%)* |
| Prevalence of a short cervix (<25 mm) | 7 (33%)* | 12 (26%)* | 19 (3.85) |
| Prior preterm delivery (n, %) | 8 (38%)* | 22 (48%)* | 81 (16%) |
| Gestational weeks at scan (median, range) | 19 (12–27) | 20 (12–28) | 17 (11–28) |
| Gestational weeks at delivery (median, range) | 32 (22–34)* | 35 (22–36)* | 39 (37–41)* |
P<0.05.
Strain in different cervical areas and spontaneous preterm delivery
Logistic regression analysis showed that,among elastography parameters, only strain from the internal cervical os was associated with spontaneous preterm delivery. The rates of preterm delivery at ≤34 weeks and at <37 weeks of gestation by internal os endocervical strain quartiles (A and B), and by internal os complete cervix strain quartiles (C and D), are shown in Figure 2. The lowest prevalence of spontaneous preterm delivery at ≤34 weeks and at <37 weeks of gestation was observed among patients with the lowest quartile strain values, whereas the highest prevalence was observed in patients with strain values in the third quartile of the distribution. There were no appreciable differences in these associations for measurements performed for the entire cervix as opposed to the endocervical canal. Strain values obtained from the internal os endocervical canal were used for all subsequent analyses.
Figure 2.
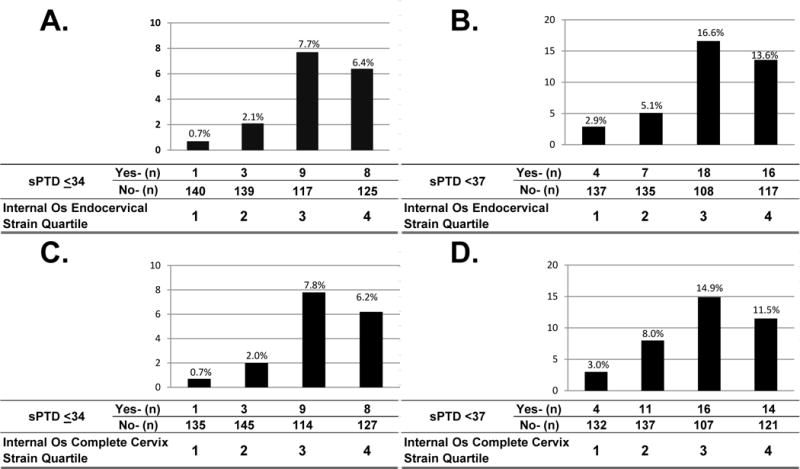
Prevalence of spontaneous preterm delivery at ≤34 and at <37 weeks of gestation by internal os endocervical strain quartile (A and B), and by internal os entire cervix strain quartile (C and D). Endocervical strain quartiles were defined as: 1st quartile ≤0.16; 2nd quartile >0.16–0.27; 3rd quartile >0.27–0.41; and 4th quartile >0.41. Entire cervix strain quartiles were defined as: 1st quartile ≤0.29; 2nd quartile <0.29–0.4; 3rd quartile >0.4–0.52: and 4th quartile >0.52.
Internal os cervical strain and spontaneous preterm delivery at ≤34 weeks of gestation
The magnitude of association between spontaneous preterm delivery at ≤34 weeks and the internal os endocervical strain quartile classifications, with and without multivariable adjustment for gestational age at examination and a short cervix (<25 mm), are presented in Table 2. Women with either third or fourth quartile strain values were significantly more likely to deliver spontaneously at ≤34 weeks than those with first quartile strain values, with and without multivariable adjustment for gestational age at the examination. After adjusting for both gestational age and a short cervix (<25 mm), women with third quartile strain values were at a significantly higher risk of spontaneous preterm delivery at ≤34 weeks when compared to women with first quartile strain values.
Table 2.
Magnitude of association between internal os endocervical strain quartiles and spontaneous preterm delivery at ≤34 weeks of gestation
| Model I | Model II | Model III | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Strain Classification | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Higher vs. lowest quartiles | |||||||||
| 4 | 9.0 | 1.1–72.5 | 0.016 | 8.2 | 1.0- 67.3 | 0.024 | 5.7 | 0.7–49.3 | 0.12 |
| 3 | 10.8 | 1.3–86.1 | 0.006 | 10.1 | 1.3–81.6 | 0.004 | 9.0 | 1.1–74.0 | 0.02 |
| 2 | 3.0 | 0.3–29.4 | 0.42 | 3.0 | 0.3–29.0 | 0.44 | 2.6 | 0.3–26.0 | 0.95 |
| 1 | Reference | ||||||||
|
| |||||||||
| Extreme vs. middle quartiles | |||||||||
| 4 | 1.4 | 0.5–3.4 | 0.44 | 1.3 | 0.5–3.3 | 0.48 | 1.0 | 0.4–2.7 | 0.82 |
| 2 and 3 | Reference | ||||||||
| 1 | 0.15 | 0.02–1.2 | 0.09 | 0.16 | 0.02–1.2 | 0.11 | 0.18 | 0.02–1.4 | 0.19 |
|
| |||||||||
| Highest Quartiles | |||||||||
| Yes | 1.9 | 0.8–4.8 | 0.14 | 1.8 | 0.7–4.5 | 0.21 | 1.3 | 0.5–3.4 | 0.60 |
| No | Reference | ||||||||
|
| |||||||||
| Lowest Quartiles | |||||||||
| Yes | 0.14 | 0.02–1.02 | 0.06 | 0.15 | 0.02–1.10 | 0.08 | 0.18 | 0.02–1.4 | 0.12 |
| No | Reference | ||||||||
Model I, unadjusted; Model II, adjusted for gestational age at examination; Model III, adjusted for gestational age at examination and a short cervix (<25mm). Endocervical strain cut-offs (quartiles): 1=<0.16; 2=0.16–0.27; 3=0.28–0.41; and 4=>0.41. OR, odds ratio; CI, confidence interval.
Internal os cervical strain and spontaneous preterm delivery at <37 weeks of gestation
The magnitude of association between preterm delivery at <37 weeks and the internal os endocervical strain quartile classifications, with and without multivariable adjustment for potentially confounding factors, are presented in Table 3. The overall pattern of association was similar to that observed for spontaneous preterm delivery at ≤34 weeks, yet the odds for spontaneous preterm delivery at <37 weeks were significantly lower among women with first quartile strain values compared either to those with higher (2nd, 3rd, 4th) or middle (2nd, 3rd) quartile strain values, with and without adjustment for gestational age. Yet, adjusting for both gestational age and a short cervix at examination, women with third quartile strain values were at a significantly higher risk of spontaneous preterm delivery at <37 weeks, whereas women with highest quartile strain values were still at marginally higher risk (p=0.08) when each was compared to women with first quartile strain values.
Table 3.
Magnitude of association between internal os endocervical strain quartiles and spontaneous preterm delivery at <37 weeks of gestation
| Model I | Model II | Model III | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Strain Classification | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Higher vs. lowest quartiles | |||||||||
| 4 | 4.7 | 1.5–14.4 | 0.002 | 3.9 | 1.3–12.1 | 0.004 | 2.8 | 0.9–9.0 | 0.08 |
| 3 | 5.7 | 1.9–17.4 | 0.004 | 5.1 | 1.7–15.6 | 0.001 | 4.5 | 1.4–13.8 | 0.004 |
| 2 | 1.8 | 0.5–6.3 | 0.53 | 1.7 | 0.5–6.1 | 0.56 | 1.5 | 0.4–5.4 | 0.88 |
| 1 | Reference | ||||||||
|
| |||||||||
| Extreme vs. middle quartiles | |||||||||
| 4 | 1.3 | 0.7–2.6 | 0.33 | 1.2 | 0.6–2.3 | 0.53 | 1.0 | 0.5–2.0 | 0.93 |
| 2 and 3 | Reference | ||||||||
| 1 | 0.28 | 0.10–0.8 | 0.02 | 0.31 | 0.10–0.9 | 0.04 | 0.35 | 0.12–1.04 | 0.08 |
|
| |||||||||
| Highest Quartile | |||||||||
| Yes | 1.8 | 0.9–3.4 | 0.08 | 1.5 | 0.8–3.0 | 0.19 | 1.2 | 0.6–2.4 | 0.59 |
| No | Reference | ||||||||
|
| |||||||||
| Lowest Quartile | |||||||||
| Yes | 0.26 | 0.09–0.73 | 0.02 | 0.29 | 0.10–0.82 | 0.03 | 0.35 | 0.12–1.03 | 0.08 |
| No | Reference | ||||||||
Model I, unadjusted; Model II, adjusted for gestational age at examination; Model III, adjusted for gestational age at examination and a short cervix (<25mm). Endocervical strain cutoffs (quartiles): 1=<0.16; 2=0.16–0.27; 3=0.28–0.41; and 4=>0.41. OR, odds ratio; CI, confidence interval.
There was no difference in the association between strain quartile and spontaneous preterm delivery at ≤34 and at <37 weeks when comparing patients who were examined at <18 weeks, 18 to <24 weeks, or at 24+ weeks (p= 0.93 and 0.99, respectively).
Discussion
Principal findings of the study
1) Women with strain values in the third or fourth quartiles in the internal cervical os had an increased risk of spontaneous preterm delivery at ≤34 weeks and at <37 weeks of gestation compared to women with lowest quartile strain values; 2) after adjusting for gestational age and a short cervix, women with strain values in the third quartile maintained significantly elevated risk for spontaneous preterm delivery, whereas those with highest quartile strain values had marginally increased risk, relative to women with lowest quartile strain values; and 3) strain evaluated in the external os or in the sagittal plane of the cervix did not show a significant association with spontaneous preterm delivery. This is the first study describing the association between cervical strain and preterm delivery at ≤34 weeks; and it is also the first report on the magnitudes of association between cervical strain and preterm delivery adjusted for both gestational age at examination and the presence of a short cervix.
Association between cervical strain evaluated by elastography and preterm delivery
The association of cervical strain and the risk of spontaneous preterm delivery was first reported by our group, showing that women with low strain values in the internal cervical os had a reduced risk for spontaneous preterm delivery at <37 weeks of gestation.47 Several authors recently reported an association between cervical elastography and preterm delivery. Wozniak et al.,50 using a semi-quantitative index based on the color of the elastogram, demonstrated a significant association between a ‘soft’ cervix and an increased risk of preterm delivery at <37 weeks. Using a similar approach, Swiatkowska-Freund et al.48 also reported an association between a color-based cervical elastography index and the risk of preterm delivery at <37 weeks; and Köbbing et al.49 showed a significant association between cervical strain and preterm delivery at <37 weeks of gestation. Despite differences in the evaluation of tissue displacement, the available data seemed to support an association between cervical strain evaluated by elastography and the risk of preterm delivery at <37 weeks.61,74 The association of elastography with preterm delivery at ≤34 weeks of gestation had not previously been reported. The present study shows that strain in the internal cervical os evaluated between 11 weeks and 28 weeks of gestation is associated with increased risk of spontaneous preterm delivery at ≤34 and at <37 weeks of gestation; this association was independent of gestational age at examination and the presence of a short cervix in women with third quartile strain values. Women with highest quartile strain values were at marginally higher risk (p=0.08) for spontaneous preterm delivery ˂37 weeks after adjustment for gestational age and presence of a short cervix.
Previously, the association of the internal cervical os with spontaneous preterm delivery was noted when it was found to be open during clinical examination.75,76 The internal os is usually visualized at the time of cervical length estimation and constitutes one of the required landmarks for a correct measurement.51,77 Recently, other investigators have also applied elastography techniques to study cervical areas located close to the internal os. Molina et al.29 reported that the internal, inferior parts of the cervix were significantly stiffer than the external, superior parts. Carlson et al.78 reported an increased shear wave propagation in the proximal part (internal os) compared to the distal part (external os) in cervical samples obtained after hysterectomy. Using shear wave elastography, our group also reported a faster propagation of the shear wave in the internal cervical os, suggesting that this region is denser and stiffer than the external cervical os.79 Changes in tissue composition of the internal os might be early manifestations of the process of cervical ripening before preterm or term labor.
Cervical regions may have distinctive tissue compositions resulting in differences in strain or deformation which may explain the lack of association between strain obtained from the external cervical os and spontaneous preterm delivery.15,80–83 The lack of association between strain in the sagittal plane and spontaneous preterm delivery can be related to the mixture of strain values from different areas of the cervix. Applying other methods, such as ultrasound attenuation,84–86 gray-level histogram characteristics,87,88 or shear wave elastography,78,79,89 or using reference materials for strain comparisons,90 may allow for the reliable evaluation of cervical regions other than the internal os.
Technical factors affecting ultrasound elastography evaluation
Quasi-static elastography estimates cervical strain produced by the natural movement of the tissue or after external oscillatory compression; however, researchers use different methods for quantification, either based on the color of the elastogram45,50 or as a ratio of tissue displacement.91 A uniform method to express the elastic properties of the cervix is still needed for external validation of this technique. The potential factors affecting quasi-static elastography are: 1) standardization of the oscillatory compression, 2) reproducibility among operators, and 3) regional differences in strain. In order to overcome these technical challenges, we applied a similar oscillatory stimulus to all patients, recordings were obtained by trained operators, and the selected anatomical plane was aligned with the ultrasound probe for a homogenous distribution of pressure. The cross-sectional plane of the internal os allowed for the uniform propagation of the oscillatory compression, thereby reducing the variation in strain calculation. We have previously reported a better agreement in strain estimation assessed in the internal cervical os than in other cervical areas.46 Good reproducibility of cervical elastography evaluations has also been reported by Swiatkowska-Freund et al.92
Clinical implications of cervical elastography
Elastography is an emerging field in ultrasound imaging; several systems already include elastography in their abdominal and vaginal probes. Elastography can be performed at the same time when cervical length is measured, and the combination of both might improve the identification of women at risk of spontaneous preterm delivery. Elastography does not increase the scanning time and does not create more discomfort than the routine transvaginal scan. It does not require a sophisticated set-up, and can be applied by trained operators. However, a clear definition of the targeted cervical areas and a uniformed quantification of strain are still needed to propose elastography for clinical use.
Strengths and limitations
The association between cervical strain and preterm delivery was adjusted for the presence of a short cervix; a standard protocol for applying oscillatory compression was followed; well-defined regions of interest in the cervix were analyzed; strain values were reported numerically, providing more robust estimations than qualitative evaluation of color elastograms; and magnitudes of association were adjusted for gestational age as well as short cervix. The main limitation is that small variations in the technique for applying oscillatory compression might affect the results.
Conclusion
Elevated strain in the internal cervical os is associated with increased risk of spontaneous preterm delivery at ≤34 and at <37 weeks of gestation, and these associations were independent of gestational age at examination and the presence of a short cervix in women with strain values in the third quartile of the distribution. The clinical benefit of introducing elastography in combination with cervical length measurement for identification of women at risk of spontaneous preterm delivery should be further evaluated.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. The ultrasound experience and technical support of senior Registered Diagnostic Medical Sonographers (RDMS) Catherine Ducharme and Denise Haggerty are gratefully acknowledged.
References
- 1.Uldbjerg N, Malmstrom A, Ekman G, Sheehan J, Ulmsten U, Wingerup L. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J. 1983;209:497–503. doi: 10.1042/bj2090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minamoto T, Arai K, Hirakawa S, Nagai Y. Immunohistochemical studies on collagen types in the uterine cervix in pregnant and nonpregnant states. Am J Obstet Gynecol. 1987;156:138–144. doi: 10.1016/0002-9378(87)90225-0. [DOI] [PubMed] [Google Scholar]
- 3.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J., Jr The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, Khalek N, Camacho N, Mittal P, Yoon BH, Espinoza J, Kim CJ, Sorokin Y, Malone J., Jr Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197:250 e251–257. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104–116. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008;27:487–497. doi: 10.1016/j.matbio.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S82–89. doi: 10.1016/j.ejogrb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol. 2009;33:300–307. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, Gonzalez JM, Chaiworapongsa T, Ali-Fehmi R, Dong Z, Than NG, Kim CJ. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472 e1–472.e14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 11.Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod. 2011;84:1053–1062. doi: 10.1095/biolreprod.110.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleissl HP, van der Rest M, Naftolin F, Glorieux FH, de Leon A. Collagen changes in the human uterine cervix at parturition. Am J Obstet Gynecol. 1978;130:748–753. doi: 10.1016/0002-9378(78)90003-0. [DOI] [PubMed] [Google Scholar]
- 13.El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, Kobayashi T, Terao T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod. 1997;12:1080–1088. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Changes in cervical resistance and collagen fluorescence during gestation in rats. J Perinat Med. 1999;27:188–194. doi: 10.1515/JPM.1999.026. [DOI] [PubMed] [Google Scholar]
- 15.Winkler M, Rath W. Changes in the cervical extracellular matrix during pregnancy and parturition. J Perinat Med. 1999;27:45–60. doi: 10.1515/JPM.1999.006. [DOI] [PubMed] [Google Scholar]
- 16.Fittkow CT, Shi SQ, Bytautiene E, Olson G, Saade GR, Garfield RE. Changes in light-induced fluorescence of cervical collagen in guinea pigs during gestation and after sodium nitroprusside treatment. J Perinat Med. 2001;29:535–543. doi: 10.1515/JPM.2001.074. [DOI] [PubMed] [Google Scholar]
- 17.House M, Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet Gynecol. 2006;28:745–749. doi: 10.1002/uog.3850. [DOI] [PubMed] [Google Scholar]
- 18.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–340. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6:e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179:838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–438. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol. 2013;97:112–119. doi: 10.1016/j.jri.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poellmann MJ, Chien EK, McFarlin BL, Wagoner Johnson AJ. Mechanical and structural changes of the rat cervix in late-stage pregnancy. J Mech Behav Biomed Mater. 2013;17:66–75. doi: 10.1016/j.jmbbm.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazza E, Nava A, Bauer M, Winter R, Bajka M, Holzapfel GA. Mechanical properties of the human uterine cervix: an in vivo study. Med Image Anal. 2006;10:125–136. doi: 10.1016/j.media.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Bauer M, Mazza E, Jabareen M, Sultan L, Bajka M, Lang U, Zimmermann R, Holzapfel GA. Assessment of the in vivo biomechanical properties of the human uterine cervix in pregnancy using the aspiration test: a feasibility study. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S77–81. doi: 10.1016/j.ejogrb.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn. 2013;33:737–741. doi: 10.1002/pd.4116. [DOI] [PubMed] [Google Scholar]
- 27.Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol. 2011;38:44–51. doi: 10.1002/uog.9010. [DOI] [PubMed] [Google Scholar]
- 28.Al Naimi A, Fittschen M, Bahlmann F. Measuring cervical strain with tissue Doppler imaging depending on the shape and placement of the region of interest and its correlation with cervical consistency index. Eur J Obstet Gynecol Reprod Biol. 2014;179:246–250. doi: 10.1016/j.ejogrb.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Molina F, Gomez L, Florido J, Padilla M, Nicolaides K. Quantification of cervical elastography. A reproducibility study. Ultrasound Obstet Gynecol. 2012;39:685–689. doi: 10.1002/uog.11067. [DOI] [PubMed] [Google Scholar]
- 30.Fruscalzo A, Steinhard J, Londero AP, Frohlich C, Bijnens B, Klockenbusch W, Schmitz R. Reliability of quantitative elastography of the uterine cervix in at-term pregnancies. J Perinat Med. 2013;41:421–427. doi: 10.1515/jpm-2012-0180. [DOI] [PubMed] [Google Scholar]
- 31.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 32.Cespedes I, Ophir J, Ponnekanti H, Maklad N. Elastography: elasticity imaging using ultrasound with application to muscle and breast in vivo. Ultrason Imaging. 1993;15:73–88. doi: 10.1177/016173469301500201. [DOI] [PubMed] [Google Scholar]
- 33.Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, Varghese T. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213:203–233. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 34.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 35.Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003;23:1657–1671. doi: 10.1148/rg.236035163. [DOI] [PubMed] [Google Scholar]
- 36.Fleury Ede F, Roveda Junior D, Fleury JC, do Carmo Queiroz M, Piato S. Elastography: theory into clinical practice. Breast J. 2009;15:564–566. doi: 10.1111/j.1524-4741.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 37.Manickam K, Machireddy RR, Seshadri S. Characterization of biomechanical properties of agar based tissue mimicking phantoms for ultrasound stiffness imaging techniques. J Mech Behav Biomed Mater. 2014;35:132–143. doi: 10.1016/j.jmbbm.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Gilman G, Khandheria BK, Hagen ME, Abraham TP, Seward JB, Belohlavek M. Strain rate and strain: a step-by-step approach to image and data acquisition. J Am Soc Echocardiogr. 2004;17:1011–1020. doi: 10.1016/j.echo.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Neves LP, Jiang J, Hall TJ, Carneiro AA. Acoustic elastography under dynamic compression using one-dimensional track motion. Conf Proc IEEE Eng Med Biol Soc Conference. 2007;2007:83–86. doi: 10.1109/IEMBS.2007.4352228. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Aglyamov SR, Park S, O’Donnell M, Emelianov SY. An autocorrelation-based method for improvement of sub-pixel displacement estimation in ultrasound strain imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:838–843. doi: 10.1109/TUFFC.2011.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–184. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 42.Thomas A. Imaging of the cervix using sonoelastography. Ultrasound Obstet Gynecol. 2006;28:356–357. doi: 10.1002/uog.3813. [DOI] [PubMed] [Google Scholar]
- 43.Thomas A, Kummel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14:193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Preis K, Swiatkowska-Freund M, Pankrac Z. Elastography in the examination of the uterine cervix before labor induction. Ginekol Pol. 2010;81:757–761. [PubMed] [Google Scholar]
- 45.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez-Andrade E, Hassan SS, Ahn H, Korzeniewski SJ, Yeo L, Chaiworapongsa T, Romero R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol. 2013;41:152–161. doi: 10.1002/uog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Andrade E, Romero R, Korzeniewski SJ, Ahn H, Aurioles-Garibay A, Garcia M, Schwartz AG, Yeo L, Chaiworapongsa T, Hassan SS. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J Perinat Med. 2014;42:159–169. doi: 10.1515/jpm-2013-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swiatkowska-Freund M, Traczyk-Los A, Preis K, Lukaszuk M, Zielinska K. Prognostic value of elastography in predicting premature delivery. Ginekol Pol. 2014;85:204–207. doi: 10.17772/gp/1714. [DOI] [PubMed] [Google Scholar]
- 49.Köbbing K, Fruscalzo A, Hammer K, Mollers M, Falkenberg M, Kwiecien R, Klockenbusch W, Schmitz R. Quantitative elastography of the uterine cervix as a predictor of preterm delivery. J Perinatol. 2014;34:774–780. doi: 10.1038/jp.2014.87. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak S, Czuczwar P, Szkodziak P, Milart P, Wozniakowska E, Paszkowski T. Elastography in predicting preterm delivery in asymptomatic, low-risk women: a prospective observational study. BMC Pregnancy Childbirth. 2014;14:238. doi: 10.1186/1471-2393-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 52.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 53.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 54.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 55.Welsh A, Nicolaides K. Cervical screening for preterm delivery. Curr Opin Obstet Gynecol. 2002;14:195–202. doi: 10.1097/00001703-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 58.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 59.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, Romero V, Ward C, Chaiworapongsa T, Mittal P, Sorokin Y, Hassan SS. Clinical significance of early (< 20 weeks) vs. late (20–24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–481. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433 e431–438. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sananes N, Langer B, Gaudineau A, Kutnahorsky R, Aissi G, Fritz G, Boudier E, Viville B, Nisand I, Favre R. Prediction of spontaneous preterm delivery in singleton pregnancies: where are we and where are we going? A review of literature. J Obstet Gynaecol. 2014;34:457–461. doi: 10.3109/01443615.2014.896325. [DOI] [PubMed] [Google Scholar]
- 62.Barros-Silva J, Pedrosa AC, Matias A. Sonographic measurement of cervical length as a predictor of preterm delivery: a systematic review. J Perinat Med. 2014;42:281–293. doi: 10.1515/jpm-2013-0115. [DOI] [PubMed] [Google Scholar]
- 63.Engle WA. A recommendation for the definition of “late preterm” (near-term) and the birth weight-gestational age classification system. Semin Perinatol. 2006;30:2–7. doi: 10.1053/j.semperi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40:739–751. doi: 10.1016/j.clp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Mahoney AD, Jain L. Respiratory disorders in moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40:665–678. doi: 10.1016/j.clp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Ananth CV, Friedman AM, Gyamfi-Bannerman C. Epidemiology of moderate preterm, late preterm and early term delivery. Clin Perinatol. 2013;40:601–610. doi: 10.1016/j.clp.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Souka AP, Papastefanou I, Michalitsi V, Salambasis K, Chrelias C, Salamalekis G, Kassanos D. Cervical length changes from the first to second trimester of pregnancy, and prediction of preterm birth by first-trimester sonographic cervical measurement. J Ultrasound Med. 2011;30:997–1002. doi: 10.7863/jum.2011.30.7.997. [DOI] [PubMed] [Google Scholar]
- 68.Greco E, Gupta R, Syngelaki A, Poon LC, Nicolaides KH. First-trimester screening for spontaneous preterm delivery with maternal characteristics and cervical length. Fetal Diagn Ther. 2012;31:154–161. doi: 10.1159/000335686. [DOI] [PubMed] [Google Scholar]
- 69.Benshalom-Tirosh N, Tirosh D, Aricha-Tamir B, Weintraub AY, Erez O, Mazor M, Hershkovitz R. The clinical utility of sonographic cervical length in the management of preterm parturition at 28–32 weeks of gestation. J Matern Fetal Neonatal Med. 2015;28:1929–1933. doi: 10.3109/14767058.2014.972929. [DOI] [PubMed] [Google Scholar]
- 70.Hiersch L, Yogev Y, Domniz N, Meizner I, Bardin R, Melamed N. The role of cervical length in women with threatened preterm labor: is it a valid predictor at any gestational age? Am J Obstet Gynecol. 2014;211:532 e1–9. doi: 10.1016/j.ajog.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Souka AP, Papastefanou I, Papadopoulos G, Chrelias C, Kassanos D. Cervical length in late second and third trimesters: a mixture model for predicting delivery. Ultrasound Obstet Gynecol. 2015;45:308–312. doi: 10.1002/uog.13407. [DOI] [PubMed] [Google Scholar]
- 72.Burger M, Weber-Rossler T, Willmann M. Measurement of the pregnant cervix by transvaginal sonography: an interobserver study and new standards to improve the interobserver variability. Ultrasound Obstet Gynecol. 1997;9:188–193. doi: 10.1046/j.1469-0705.1997.09030188.x. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalil MR, Thorsen P, Uldbjerg N. Cervical ultrasound elastography may hold potential to predict risk of preterm birth. Dan Med J. 2013;60:A4570. [PubMed] [Google Scholar]
- 75.Leitich H, Brunbauer M, Kaider A, Egarter C, Husslein P. Cervical length and dilatation of the internal cervical os detected by vaginal ultrasonography as markers for preterm delivery: A systematic review. Am J Obstet Gynecol. 1999;181:1465–1472. doi: 10.1016/s0002-9378(99)70407-2. [DOI] [PubMed] [Google Scholar]
- 76.Benham BN, Balducci J, Atlas RO, Rust OA. Risk factors for preterm delivery in patients demonstrating sonographic evidence of premature dilation of the internal os, prolapse of the membranes in the endocervical canal and shortening of the distal cervical segment by second trimester ultrasound. Aust N Z J Obstet Gynaecol. 2002;42:46–50. doi: 10.1111/j.0004-8666.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 77.Vayssiere C, Moriniere C, Camus E, Le Strat Y, Poty L, Fermanian J, Ville Y. Measuring cervical length with ultrasound: evaluation of the procedures and duration of a learning method. Ultrasound Obstet Gynecol. 2002;20:575–579. doi: 10.1046/j.1469-0705.2002.00854.x. [DOI] [PubMed] [Google Scholar]
- 78.Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Munoz Del Rio A, Hall TJ. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet Gynecol. 2014;43:452–458. doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hernandez-Andrade E, Aurioles-Garibay A, Garcia M, Korzeniewski SJ, Schwartz AG, Ahn H, Martinez-Varea A, Yeo L, Chaiworapongsa T, Hassan SS, Romero R. Effect of depth on shear-wave elastography estimated in the internal and external cervical os during pregnancy. J Perinat Med. 2014;42:549–557. doi: 10.1515/jpm-2014-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- 81.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol. 1993;81:88–92. [PubMed] [Google Scholar]
- 82.Ross M, Wojciech P. Female Reproductive System. In: Pawlina RMW, editor. Editor Histology: A Text and Atlas. 1st. Lippincott Williams & Wilkins; China: 2010. pp. 830–892. [Google Scholar]
- 83.Kling E, Kitahara S, Posligua L, Malpica A, Silva EG. The 2 stromal compartments of the normal cervix with distinct immunophenotypic and histomorphologic features. Ann Diagn Pathol. 2012;16:315–322. doi: 10.1016/j.anndiagpath.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Bigelow TA, McFarlin BL, O’Brien WD, Oelze ML. In vivo ultrasonic attenuation slope estimates for detecting cervical ripening in rats: Preliminary results. J Acoust Soc Am. 2008;123:1794–1800. doi: 10.1121/1.2832317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McFarlin BL, Bigelow TA, Laybed Y, O’Brien WD, Oelze ML, Abramowicz JS. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol. 2010;36:218–225. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Labyed Y, Bigelow TA, McFarlin BL. Estimate of the attenuation coefficient using a clinical array transducer for the detection of cervical ripening in human pregnancy. Ultrasonics. 2011;51:34–39. doi: 10.1016/j.ultras.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tekesin I, Wallwiener D, Schmidt S. The value of quantitative ultrasound tissue characterization of the cervix and rapid fetal fibronectin in predicting preterm delivery. J Perinat Med. 2005;33:383–391. doi: 10.1515/JPM.2005.070. [DOI] [PubMed] [Google Scholar]
- 88.Kuwata T, Matsubara S, Taniguchi N, Ohkuchi A, Ohkusa T, Suzuki M. A novel method for evaluating uterine cervical consistency using vaginal ultrasound gray-level histogram. J Perinat Med. 2010;38:491–494. doi: 10.1515/jpm.2010.079. [DOI] [PubMed] [Google Scholar]
- 89.Carlson LC, Feltovich H, Palmeri ML, del Rio AM, Hall TJ. Statistical analysis of shear wave speed in the uterine cervix. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:1651–1660. doi: 10.1109/tuffc.2014.006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hee L, Sandager P, Petersen O, Uldbjerg N. Quantitative sonoelastography of the uterine cervix by interposition of a synthetic reference material. Acta Obstet Gynecol Scand. 2013;92:1244–9. doi: 10.1111/aogs.12246. [DOI] [PubMed] [Google Scholar]
- 91.Fuchs T, Pomorski M, Zimmer M. Quantitative cervical elastography in pregnancy. Ultrasound Obstet Gynecol. 2013;41:712. doi: 10.1002/uog.12474. [DOI] [PubMed] [Google Scholar]
- 92.Swiatkowska-Freund M, Pankrac Z, Preis K. Intra- and inter-observer variability of evaluation of uterine cervix elastography images during pregnancy. Ginekol Pol. 2014;85:360–364. doi: 10.17772/gp/1740. [DOI] [PubMed] [Google Scholar]


