Abstract
Background
Meconium aspiration syndrome (MAS) is a leading cause of morbidity and mortality in term infants. Meconium-stained amniotic fluid (MSAF) occurs in approximately one of every seven pregnancies, but only 5% of neonates exposed to MSAF develop MAS. Why some infants exposed to meconium develop MAS while others do not is a fundamental question. Patients with MSAF have a higher frequency of intra-amniotic infection/inflammation than those with clear fluid. We propose that fetal systemic inflammation is a risk factor for the development of MAS in patients with MSAF.
Objective
To investigate whether intra-amniotic inflammation and funisitis, the histopathologic landmark of a fetal inflammatory response, predispose to MAS.
Study Design
A prospective cohort study was conducted from 1995 through 2009. Amniotic fluid (AF) samples (n=1,281) were collected at the time of cesarean delivery from women who delivered singleton newborns at term (gestational age ≥38 weeks). Intra-amniotic inflammation was diagnosed if the AF concentration of matrix metalloproteinase-8 (MMP-8) was >23 ng/ml. Funisitis was diagnosed by histologic examination if inflammation was present in the umbilical cord.
Results
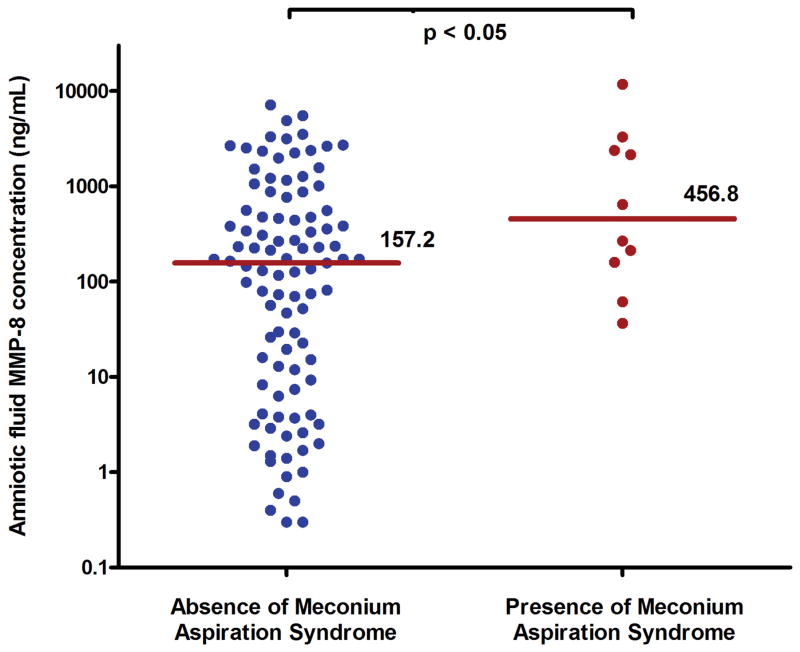
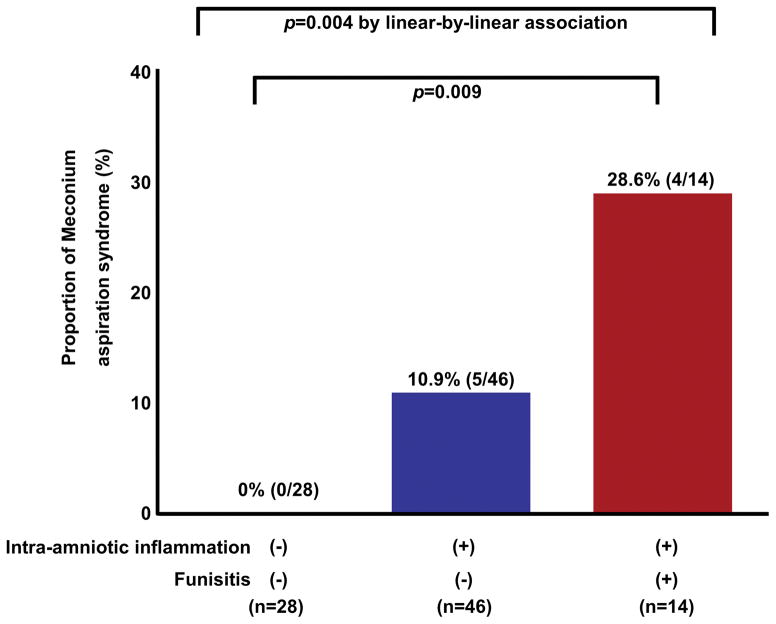
The prevalence of MSAF was 9.2% (118/1,281), and 10.2% (12/118) of neonates exposed to MSAF developed MAS. There were no significant differences in the median gestational age or umbilical cord arterial pH at birth between neonates who developed MAS and those who did not (each p > 0.1). Mothers whose newborns developed MAS had a higher median AF MMP-8 (456.8 ng/ml vs.157.2 ng/ml; p < 0.05). Newborns exposed to intra-amniotic inflammation had a higher rate of MAS than those who were not exposed to intra-amniotic inflammation [13.0% (10/77) vs. 0% (0/32), p = 0.03], as did those exposed to funisitis [31.3% (5/16) vs. 7.3% (6/82), relative risk, 4.3; 95% confidence interval, 1.5–12.3]. Among the 89 newborns for whom both AF and placental histology were available, MAS was more common in patients with both intra-amniotic inflammation and funisitis than in those without intra-amniotic inflammation and funisitis [28.6% (4/14) vs. 0% (0/28), p = 0.009], while the rate of MAS did not show a significant difference between patients with intra-amniotic inflammation alone (without funisitis) and those without intra-amniotic inflammation and funisitis [10.9% (5/46) vs. 0% (0/28)].
Conclusion
The combination of intra-amniotic inflammation with fetal systemic inflammation is an important antecedent of MAS. This concept has implications for the understanding of the mechanisms of disease responsible for MAS and for the development of prognostic models and therapeutic interventions for this disorder.
Keywords: amniocentesis, fetal inflammatory response syndrome, funisitis, intra-amniotic inflammation, matrix metalloproteinase-8 (MMP-8), meconium-stained amniotic fluid, pregnancy
Introduction
Meconium aspiration syndrome (MAS) is a major cause of neonatal morbidity and mortality, and is a leading cause of neonatal respiratory distress.1–12 An estimated 1,000 to 1,500 infants die in the U.S. each year as a result of meconium aspiration.1, 13, 14 Meconium-stained amniotic fluid (MSAF) is present in one of every seven pregnancies (range: 5%–20%; 400,000–600,000 deliveries in the U.S. per year),1, 15–20 but only approximately 5% (20,000–30,000) of neonates born to mothers with MSAF develop MAS.1, 15, 21–23 It is not known why only some, or which, neonates exposed to MSAF will develop MAS.1, 18, 24, 25 Attempts to prevent MAS have included oropharyngeal,26, 27 nasopharyngeal,26 and tracheal28–30 suctioning and amnioinfusion in women who have MSAF,31, 32 but none of these interventions has proven effective.33–36
Typically, MAS affects term newborns with low Apgar scores (< 7 at 5 minutes).1 Low Apgar scores are believed to be secondary to an intrauterine event that causes fetal hypoxia, which then causes meconium to be passed in utero,9, 11, 37–48 fetal gasping,49–54 and aspiration of the meconium before birth. However, MAS occurs in the absence of umbilical artery acidemia; therefore, other mechanisms must be involved.55–64
Clinical and experimental evidence suggests that lung inflammation induced by meconium plays a central role in the pathogenesis of MAS.4, 65–69 The pathophysiology has been attributed to: 1) the mechanical effect of meconium, which can obstruct the airways, and 2) the inflammatory effect of meconium.68, 70 We propose that intra-amniotic inflammation due to intra-amniotic infection or sterile intra-amniotic inflammation accompanied by a fetal inflammatory response predisposes fetuses exposed to MSAF to develop MAS. This concept is based on previous observations that MSAF is more likely to contain bacteria,71–73 endotoxin,73,74 and higher concentrations of inflammatory mediators, such as interleukin (IL)-1, tumor necrosis factor (TNF)-α,73,75,76 IL-8,67,76 and phospholipase-A2.77 Thus, meconium (with its pro-inflammatory properties), when aspirated before birth and combined with a fetal systemic inflammatory response involving the fetal lungs, could predispose to MAS. If this is correct, fetuses with MSAF and fetal inflammatory response syndrome (FIRS) should have a higher rate of MAS than those without FIRS. The purpose of this study was to determine whether the combination of intra-amniotic inflammation and a fetal systemic inflammatory response is associated with MAS in neonates who have been exposed to MSAF.
Material and methods
Study design
A prospective cohort study was conducted to establish a perinatal biobank to facilitate investigation of contributors to obstetric diseases. One of the features recorded was whether the Amniotic fluid (AF) was clear or meconium stained. AF samples were collected from consecutively enrolled women at term, undergoing cesarean deliveries at the Seoul National University Hospital from July 1995 through June 2009, who met the following inclusion criteria: 1) singleton pregnancy; 2) term gestation (gestational age ≥38 weeks); 3) AF obtained at the time of cesarean delivery; and 4) MSAF identified at delivery. Exclusion criteria were: 1) multiple gestation; 2) stillbirth or fetal death; and 3) presence of major congenital malformations. Written informed consent was obtained from all patients prior to the retrieval of AF.
The Institutional Review Board of Seoul National University Hospital approved the collection and use of these samples and information for research purposes. Seoul National University has a Federalwide Assurance with the Office for Human Research Protections of the U.S. Department of Health and Human Services.
Laboratory studies
AF was collected under direct ultrasound visualization at the time of hysterotomy during the course of a cesarean delivery, and an aliquot was cultured for aerobic and anaerobic bacteria and for genital mycoplasmas (Mycoplasma hominis and Ureaplasma species). The remaining fluid was centrifuged and stored in polypropylene tubes at −70°C. A concentration of matrix metalloproteinase-8 (MMP-8) was measured using a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Inc, Bucks, UK), following the instructions of the manufacturer.78 MMP-8 was assayed in duplicate per analytic run. The sensitivity of the test was 0.3 ng/mL, and the intra- and inter-assay coefficients of variation were <10%. Gram staining of amniotic fluid was not performed.
Histologic examination
Samples of the chorioamniotic membranes, chorionic plate, and umbilical cord were obtained from each placenta. Samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of tissue blocks were stained with Hematoxylin and Eosin. Histopathologic examinations were performed by a pathologist blinded to the clinical information.
Clinical definitions
The presence or absence of meconium was evaluated at the time of cesarean delivery under visual observation, consistent with standard practice in labor and delivery units. Intra-amniotic inflammation was defined as an elevated AF MMP-8 concentration (>23 ng/mL).78–84 A fetal systemic inflammatory response was defined by the presence of funisitis; i.e. the presence of neutrophil infiltration in the umbilical vessel walls or Wharton’s jelly, as previously described. 85–93 MAS was defined as respiratory distress in an infant, born to a mother with MSAF, requiring assisted mechanical ventilation or oxygen at a concentration of ≥40% for at least 48 hours, and by radiographic findings consistent with MAS and symptoms that could not otherwise be explained.1,31,94–96
Statistical analysis
Proportions were compared using the Fisher’s exact test. The Mann-Whitney U test was used for between-group comparisons of continuous variables. Poisson regression models with robust variance estimators were fitted to estimate relative risk (RR) and corresponding 95% confidence intervals (CI). These analyses were performed using SPSS, Version 19.0 (SPSS Inc., Chicago, IL, USA) and SAS, Version 9.3 (SAS Institute Inc., Cary, N.C., USA). A p-value of <0.05 was considered significant.
Results
Characteristics of the study population
AF was retrieved at the time of cesarean delivery from 1,281 patients with singleton pregnancies who delivered live-born term newborns (gestational age ≥38 weeks) without major congenital anomalies during the study. Altogether, 118 (9.2%) of the 1,281 women participating in this study had MSAF. Indications for cesarean delivery presented by the 118 women included the following: failure to progress during labor (n=76), non-reassuring fetal heart rate tracing (n=18), previous uterine surgery (n=15), fetal malpresentation (n=4), and other indicators (n=5); 12 (10.2%) of the 118 neonates developed MAS.
Table 1 describes the characteristics of the study population stratified according to the presence/absence of MAS; Table 2 shows the clinical characteristics of the 12 cases with MAS. The frequency of low Apgar scores (<7) at 5 minutes following birth was significantly higher in newborns who developed MAS than in those who did not [16.7% (2/12) vs. 0.9% (1/106), p<0.05]. There were no significant differences in the median of gestational age or umbilical cord arterial pH at birth between neonates who developed MAS and those who did not (40.4 vs. 40.4 weeks and 7.2 vs. 7.2, respectively, p >0.1 for each comparison). There was no difference in the rate of rupture of the membranes (ROM) at the time of cesarean delivery between mothers whose newborns developed MAS and those whose newborns did not [83.3% (10/12) vs. 65.7% (69/105), p>0.3]. However, patients whose newborns developed MAS had significantly higher rates of acute histologic chorioamnionitis [80% (8/10) vs. 32.6% (28/86)] and funisitis [45.5% (5/11) vs. 12.6% (11/87)] than those whose newborns did not develop MAS (p<0.05 for each).
Table 1.
Clinical characteristics of the study population according to the presence or absence of meconium aspiration syndrome in the context of meconium-stained amniotic fluid
| Meconium Aspiration Syndrome | P value | ||
|---|---|---|---|
| Absence N=106 |
Presence N=12 |
||
| Maternal age (years)* | 31 (25–44) | 32(27–38) | 0.655 |
| Nulliparity (%) | 78.3 (83/106) | 100.0 (12/12) | 0.120 |
| Indications for cesarean delivery (%) | 0.056 | ||
| Previous cesarean delivery | 14.2 (15/106) | 0.0 (0/12) | |
| Failure to progress | 66.0 (70/106) | 50.0 (6/12) | |
| Fetal malpresentation | 3.8 (4/106) | 0.0 (0/12) | |
| Non-reassuring FHR pattern | 12.3 (13/106) | 41.7 (5/12) | |
| Other | 3.8 (4/106) | 8.3 (1/12) | |
| Presence of labor at amniocentesis (%) | 76.4 (81/106) | 91.7 (11/12) | 0.461 |
| Gestational age at delivery (weeks)* | 40.4 (38.0–42.7) | 40.4 (39.4–42.0) | 0.742 |
| Birth weight (g)* | 3445 (2160–4520) | 3660 (2800–4850) | 0.134 |
| Infant male gender (%) | 56.6 (60/106) | 91.7 (11/12) | 0.026 |
| Apgar score <7 (%) | |||
| 1 minute | 13.2 (14/106) | 25.0 (3/12) | 0.377 |
| 5 minute | 0.9 (1/106) | 16.7 (2/12) | 0.027 |
| Umbilical arterial pH*† | 7.238(7.014–7.409) | 7.219(6.950–7.295) | 0.175 |
| <7.00 (%) | 0.0 (0/102) | 8.3 (1/12) | 0.105 |
| <7.10 (%) | 2.9 (3/102) | 16.7 (2/12) | 0.085 |
| <7.20 (%) | 24.5 (25/102) | 41.7 (5/12) | 0.296 |
| NICU admission (%) | 2.8 (3/106) | 100.0 (12/12) | <0.001 |
| Positive amniotic fluid culture (%) | 20.4 (21/103) | 40.0 (4/10) | 0.224 |
| MMP-8 > 23 ng/mL (%) | 67.7 (67/99) | 100.0 (10/10) | 0.032 |
| Acute histologic chorioamnionitis (%) | 32.6 (28/86) | 80.0 (8/10) | 0.005 |
| Funisitis (%) | 12.6 (11/87) | 45.5 (5/11) | 0.016 |
FHR, fetal heart rate; MMP, matrix metalloproteinase; NICU, neonatal intensive care unit
Values are presented as the median (range).
Four cases without a result of an umbilical arterial blood gas analysis were excluded from analysis.
Table 2.
Characteristics of 12 cases with neonatal meconium aspiration syndrome
| Case | GA (weeks) | Birth-weight (g) | Cord arterial pH | Apgar Score | Amniotic fluid | Placental pathology | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 min | 5min | MMP-8 (ng/mL) | Culture | Culture | Chorio-amnionitis | Funisitis | ||||
| 1 | 40.2 | 3660 | 6.950 | 5 | 6 | 2153.4 | (−) | NA | NA | |
| 2 | 40.3 | 4450 | 7.190 | 6 | 8 | 61.7 | (+) | Candida albicans, Streptococcus viridans | (+) | (+) |
| 3 | 39.3 | 3660 | 7.082 | 8 | 7 | 2383.0 | NA | (+) | (−) | |
| 4 | 41.2 | 3760 | 7.280 | 8 | 9 | 159.9 | (−) | (−) | (+) | |
| 5 | 39.6 | 3100 | 7.210 | 7 | 9 | 11,754.7 | NA | (+) | (+) | |
| 6 | 41.3 | 4850 | 7.238 | 9 | 9 | 212.8 | (−) | (+) | (−) | |
| 7 | 40.1 | 3270 | 7.244 | 8 | 9 | 266.9 | (+) | Streptococcus agalactiae | NA | (−) |
| 8 | 40.6 | 3940 | 7.295 | 7 | 8 | NA | (+) | Ureaplasma urealyticum | (+) | (+) |
| 9 | 40.1 | 3550 | 7.178 | 7 | 7 | 646.7 | (+) | Streptococcus mitis (Viridans Streptococcus) | (−) | (−) |
| 10 | 40.4 | 3320 | 7.146 | 8 | 8 | NA | (−) | (+) | (−) | |
| 11 | 41.0 | 2800 | 7.228 | 2 | 5 | 36.6 | (−) | (+) | (−) | |
| 12 | 39.6 | 4360 | 7.294 | 8 | 9 | 3292.6 | (−) | (+) | (+) | |
GA, gestational age; MMP, matrix metalloproteinase; NA, not available.
Microbial invasion of the amniotic cavity
The rate of positive AF cultures was two-fold higher in the MAS group than in the group without MAS. While this comparison did not reach statistical significance [40% (4/10) vs. 20.4% (21/103), p=0.2], the risk of a type II error is 67.5%. Microorganisms isolated from the AF using cultivation techniques included: Ureaplasma species (n=8), Escherichia coli (n=3), Enterococcus faecalis (n=3), Streptococcus anginosus (n=3), Staphylococcus epidermidis (n=3), and one isolate each of coagulase-negative staphylococci, Klebsiella pneumoniae, Streptococcus intermedius, Staphylococcus sciuri, Candida albicans, Streptococcus agalactiae, Lactobacillus jensenii, Lactobacillus species, and Pseudomonas. There were two cases with cultures positive for Gram-positive cocci and one case with Gram-negative rods; however, the precise organisms could not be identified at the genus level by the clinical laboratory.
Intra-amniotic inflammation
The median of AF MMP-8 concentrations was significantly higher in mothers whose newborns developed MAS than in those whose newborns did not [median 456.8 ng/mL (range 36.6–11,754.7 ng/mL) vs. median 157.2 ng/mL (range 0.3 –7163.4 ng/mL), p<0.05] (Figure 1). Similarly, intra-amniotic inflammation (defined as an MMP-8 concentration >23ng/mL) was significantly more frequent in patients whose newborns developed MAS than in those who did not [100% (10/10) vs. 67.7% (67/99), p<0.05]. MAS did not occur in the absence of intra-amniotic inflammation. There was no difference in the rate of intra-amniotic inflammation (AF MMP-8 concentration >23 ng/mL) according to the presence or absence of oligohydramnios [75.0% (6/8) vs. 68.8% (55/80), p>0.99]. In addition, the frequency of oligohydramnios before ROM did not differ between mothers whose newborns developed MAS and those whose newborns did not [9.1% (1/11) vs. 9.5% (8/84), p>0.99].
Figure 1.
Amniotic fluid (AF) matrix metalloproteinase-8 (MMP-8) in the context of neonatal development of meconium aspiration syndrome (MAS). The median AF MMP-8 was higher in newborns with MAS than in those without MAS (median 456.8 ng/mL; range, 36.6 to 11,754.7 ng/mL vs. median 157.2 ng/mL; range, 0.3 to 7163.4 ng/mL; p < 0.05).
Meconium aspiration syndrome in patients with and without funisitis
Newborns with funisitis were at a greater than four-fold greater risk of developing MAS than those without funisitis [31.3% (5/16) vs. 7.3% (6/82), RR, 4.3; 95% CI: 1.5–12.3]. Among the 89 newborns for whom both AF and placental histology were available, the rate of MAS in cases without intra-amniotic inflammation and funisitis (n=28), with intra-amniotic inflammation alone (n=46), and with both intra-amniotic inflammation and funisitis (n=14) was 0%, 10.9%, and 28.6%, respectively. Only 1 case had isolated funisitis without intraamniotic inflammation. MAS was more common in patients with both intraamniotic inflammation and funisitis than in those without intraamniotic inflammation and funisitis [28.6% (4/14) vs. 0% (0/28), P = .009], while the rate of MAS did not show a significant difference between patients with intraamniotic inflammation alone (without funisitis) and those without intraamniotic inflammation and funisitis [10.9% (5/46) vs. 0% (0/28), P = .15]. A Chi-squared test for trend showed that the frequency of MAS increased as the breadth of inflammation increased (from no inflammation to inflammation restricted to the amniotic cavity, or the combination of inflammation of the amniotic cavity and fetal inflammation; P = .004, from the analysis of linear-by-linear association, SPSS) (Figure 2).
Figure 2.
The frequency of neonatal development of meconium aspiration syndrome (MAS) in the context of intra-amniotic inflammation and funisitis. Neonates exposed to both intra-amniotic inflammation and funisitis were at significantly greater risk of MAS than newborns exposed to neither of these two conditions [28.6% (4/14) vs. 0% (0/28); p = 0.009]. In contrast, newborns exposed to only intra-amniotic inflammation, without funisitis, were not at greater risk of MAS than newborns exposed to neither of these two conditions [10.9% (5/46) vs. 0% (0/28)]. MAS did not occur in the absence of the intra-amniotic inflammation.
Comment
Principal finding of the study
The combination of intra-amniotic inflammation and fetal systemic inflammation (assessed by the presence of funisitis) predisposes to MAS.
Meconium-stained amniotic fluid is necessary but not sufficient to cause meconium aspiration syndrome
MSAF occurs in about one of every seven pregnancies1,15–20; Yet, only 5% of exposed infants develop MAS.1,15,21–23 The causes of MAS in newborns exposed to MSAF are unknown. Asphyxia (ante-partum and/or intra-partum) has been implicated in the pathogenesis of MAS,9,11,37–46,97 since fetal gasping can lead to aspiration of MSAF.49–54 Yet, a large fraction of neonates with MAS have no evidence of asphyxia at birth, which suggests the existence of alternative etiologies.55–64 Indeed, in the present study, we found that only two of 12 neonates with MAS had an Apgar score <7 at 5 minutes following birth, and only two of the 12 cases had an umbilical artery pH <7.1. Therefore, we focused on an alternative mechanism of disease: inflammation.
Inflammation predisposes to meconium aspiration syndrome
This focus is based on a considerable body of clinical and experimental evidence suggesting that inflammation plays a role in the pathogenesis of MAS,4,65–68,98–105 and that intra-amniotic inflammation occurs more commonly in MSAF than in clear AF.71,72,106–109 The key finding of our study is that newborns diagnosed with funisitis, a histopathologic hallmark of FIRS, were at more than four times the risk of MAS than those without funisitis. In contrast, intra-amniotic inflammation without funisitis was not significantly associated with MAS in this study. Together, these findings support the view that, in term newborns exposed to MSAF, a fetal inflammatory response predisposes to MAS.
A proposed pathophysiology for meconium aspiration syndrome
How could fetal systemic inflammation play a role in MAS? We propose that fetal swallowing of AF containing bacteria,110–112 endotoxin (or other microbial products),71,73,77 danger signals or alarmins,110,111,113–120 and other pro-inflammatory mediators67,73,75–77 can lead to increased bowel peristalsis and passage of meconium that can then be aspirated by the fetus. Meconium per se can block the airways and elicit a local inflammatory response in the lung (pneumonitis); yet, another component of the pathogenesis of MAS may be a systemic fetal inflammatory response. This may enhance the local effects of meconium, bacteria, and inflammatory mediators in the lungs, which may extend to the pulmonary circulation. Experimental evidence indicates that exposure to inflammatory mediators, such as endotoxin, induces vascular changes that predispose to persistent pulmonary hypertension.112,121–126 The combination of pneumonitis (caused by AF containing meconium and inflammatory mediators) and capillary damage/leakage developed during the course of fetal systemic inflammation could explain the association among MSAF, intra-amniotic inflammation, funisitis, and MAS. It has also been proposed that inflamed fetal vessels are more vulnerable to compression,127 and this may play a role in predisposing newborns with fetal systemic inflammation to MAS. Additionally, AF has physiologic antimicrobial properties impaired by the addition of meconium.71 Hence, it is also possible that the increased prevalence of microbial invasion of the amniotic cavity in women with MSAF is related to an alteration of the host-defense mechanisms due to the presence of meconium. Further studies are needed to establish time-order.
Clinical implications
The clinical course of MAS is unpredictable, and knowledge of the presence of systemic inflammation may be useful to identify newborns at the greatest risk. This is important because, among neonates born with MSAF, it is not easy to differentiate between those who will have transient respiratory distress from those who will develop full-blown MAS.
Therefore, the findings reported herein may have practical implications for the clinical management of newborns with meconium as it is now possible to assess the presence or absence of fetal systemic inflammation by examining the concentration of IL-6 and C-reactive protein in umbilical cord blood. Some investigators have proposed examination of frozen sections of the umbilical cord to assess the likelihood of systemic fetal inflammation.128–130 Determination of umbilical cord cytokines and acute phase reactants may be easier than examining frozen sections. The assessment of fetal systemic inflammation can be targeted to neonates born to mothers with MASF who have a clinical course suspicious for MAS. The rationale for this approach is that, among neonates who did not have funisitis (a hallmark of FIRS) only 7.3% (6/82) developed MAS, but the risk quadrupled to 31.3% (5/16) in neonates who had funisitis. Further studies are needed to determine whether the assessment of fetal systemic inflammation with biomarkers (cytokines, chemokines, acute phase reactants, or pathologic findings) can be used to predict MAS in neonates exposed to MSAF.
Strengths and limitations
This is the first study to report the relationship between MAS and the fetal, intra-amniotic, and placental inflammatory responses. Several markers of infection and/or inflammation were assessed, including AF culture, AF MMP-8 concentration, and placental pathology (funisitis). The observed prevalence of MSAF (9.2%, 118/1281) is consistent with that reported in the literature (5–20%).1,7,24 Yet, the prevalence of MAS (10.2%, 12/118) was somewhat higher than that previously reported.1,5,18,131 This is most likely attributable to our exclusive focus on term newborns with MSAF, whereas most prior studies included term and preterm neonates. In addition, all cases included in the current cohort study were delivered by cesarean section, a risk factor for the development of MAS among neonates with MSAF.13,132
During the study period from 1995 through 2009, the clinical management of neonates with MSAF changed at our hospital to follow the American Academy of Pediatrics-Neonatal Resuscitation Program (AAP-NRP) recommendations,5 and this might have influenced our findings.
In the present study, there was no difference in the rate of MAS according to the presence or absence of a positive AF culture, nor did we find a statistically significant association between intra-amniotic inflammation alone (without funisitis) and MAS; both findings might be attributed to type II error. A post-hoc power calculation indicated that there was 32% power (type II error: 68%) for detecting the association between a positive AF culture and MAS, and 42% power (type II error: 58%) for detecting the association between intra-amniotic inflammation alone (without funisitis) and MAS.
In our data, male neonates were at a higher risk of MAS than female neonates; we believe that this is an incidental finding. Moreover, male neonates did not show a higher rate of intraamniotic inflammation and funisitis than female neonates (for intraamniotic inflammation: 73.1% vs. 66.7%; for funisitis: 16.9% vs. 15.4%). Whether the male sex is a risk factor for MAS is controversial. Two previous studies have reported that male neonates were at higher risk of MAS than female neonates,133,134 while another study found no association between the male sex and MAS (OR 1.0; 95% CI, 0.92–1.2).1
Like all observational studies, causation cannot be inferred from the associations reported herein, and experimental studies would be required to explore this hypothesis.
Conclusion
We propose that the combination of intra-amniotic inflammation with fetal systemic inflammation is an important antecedent of MAS. This concept has implications for the understanding of the mechanisms of disease and development of therapeutic interventions.
Acknowledgments
Funding: Grant 03-2011-0200 of Seoul National University Hospital, Republic of Korea and grant HI12C0768 of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welafare, Republic of Korea. This work was also supported, in part, by the Perinatology Research Branch, Division of Intramural Research, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS, USA.
Footnotes
The authors report no conflicts of interest.
References
- 1.Dargaville PA, Copnell B. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics. 2006;117:1712–21. doi: 10.1542/peds.2005-2215. [DOI] [PubMed] [Google Scholar]
- 2.Wiswell TE. Delivery room management of the meconium-stained newborn. J Perinatol. 2008;28(Suppl 3):S19–26. doi: 10.1038/jp.2008.143. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan HB, Vidyasagar D. Meconium aspiration syndrome: current concepts and management. Compr Ther. 1999;25:82–9. doi: 10.1007/BF02889600. [DOI] [PubMed] [Google Scholar]
- 4.Vidyasagar D, Lukkarinen H, Kaapa P, Zagariya A. Inflammatory response and apoptosis in newborn lungs after meconium aspiration. Biotechnol Prog. 2005;21:192–7. doi: 10.1021/bp0497886. [DOI] [PubMed] [Google Scholar]
- 5.Bhat R, Vidyasagar D. Delivery room management of meconium-stained infant. Clin Perinatol. 2012;39:817–31. doi: 10.1016/j.clp.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Ross MG. Meconium aspiration syndrome--more than intrapartum meconium. N Engl J Med. 2005;353:946–8. doi: 10.1056/NEJMe058149. [DOI] [PubMed] [Google Scholar]
- 7.Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005;60:45–56. doi: 10.1097/01.ogx.0000149659.89530.c2. quiz 73–4. [DOI] [PubMed] [Google Scholar]
- 8.De Beaufort AJ. Early human development at the perinatal interface: meconium stained amniotic fluid (MSAF) and meconium aspiration syndrome (MAS) Early Hum Dev. 2009;85:605. doi: 10.1016/j.earlhumdev.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Sheiner E, Hadar A, Shoham-Vardi I, Hallak M, Katz M, Mazor M. The effect of meconium on perinatal outcome: a prospective analysis. J Matern Fetal Neonatal Med. 2002;11:54–9. doi: 10.1080/jmf.11.1.54.59. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell SC, Hallak M, Hotra JW, Refuerzo J, Sokol RJ, Sorokin Y. Prolonged in utero meconium exposure impairs spatial learning in the adult rat. Am J Obstet Gynecol. 2004;190:1551–5. doi: 10.1016/j.ajog.2004.03.048. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 11.Hayes BC, Mcgarvey C, Mulvany S, Kennedy J, Geary MP, Matthews TG, et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am J Obstet Gynecol. 2013;209:29e1–e19. doi: 10.1016/j.ajog.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Spain JE, Tuuli MG, Macones GA, Roehl KA, Odibo AO, Cahill AG. Risk factors for serious morbidity in term nonanomalous neonates. Am J Obstet Gynecol. 2015;212:799e1–7. doi: 10.1016/j.ajog.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Yoder BA, Kirsch EA, Barth WH, Gordon MC. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002;99(5 Pt 1):731–9. doi: 10.1016/s0029-7844(02)01942-7. [DOI] [PubMed] [Google Scholar]
- 14.Gelfand SL, Fanaroff JM, Walsh MC. Meconium stained fluid: approach to the mother and the baby. Pediatr Clin North Am. 2004;51:655–67. ix. doi: 10.1016/j.pcl.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Davis RO, Philips JB, 3rd, Harris BA, Jr, Wilson ER, Huddleston JF. Fatal meconium aspiration syndrome occurring despite airway management considered appropriate. Am J Obstet Gynecol. 1985;151:731–6. doi: 10.1016/0002-9378(85)90506-x. [DOI] [PubMed] [Google Scholar]
- 16.Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome. Unresolved issues Pediatr Clin North Am. 1993;40:955–81. doi: 10.1016/s0031-3955(16)38618-7. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez C, Little BB, Dax JS, Gilstrap LC, 3rd, Rosenfeld CR. Prediction of the severity of meconium aspiration syndrome. Am J Obstet Gynecol. 1993;169:61–70. doi: 10.1016/0002-9378(93)90132-3. [DOI] [PubMed] [Google Scholar]
- 18.Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome. An update Pediatr Clin North Am. 1998;45:511–29. doi: 10.1016/s0031-3955(05)70025-0. [DOI] [PubMed] [Google Scholar]
- 19.Connolly TP. Meconium-stained amniotic fluid (MSF) Am J Obstet Gynecol. 2004;191:2175–6. doi: 10.1016/j.ajog.2004.06.112. author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 20.Bhat RY, Rao A. Meconium-stained amniotic fluid and meconium aspiration syndrome: a prospective study. Ann Trop Paediatr. 2008;28:199–203. doi: 10.1179/146532808X335642. [DOI] [PubMed] [Google Scholar]
- 21.Greenough A. Meconium aspiration syndrome--prevention and treatment. Early Hum Dev. 1995;41:183–92. doi: 10.1016/0378-3782(95)01625-d. [DOI] [PubMed] [Google Scholar]
- 22.Van Ierland Y, De Boer M, De Beaufort AJ. Meconium-stained amniotic fluid: discharge vigorous newborns. Arch Dis Child Fetal Neonatal Ed. 2010;95:F69–71. doi: 10.1136/adc.2008.150425. [DOI] [PubMed] [Google Scholar]
- 23.Hutton EK, Thorpe J. Consequences of meconium stained amniotic fluid: what does the evidence tell us? Early Hum Dev. 2014;90:333–9. doi: 10.1016/j.earlhumdev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Van Ierland Y, De Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev. 2009;85:617–20. doi: 10.1016/j.earlhumdev.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Kaapa PO. Meconium aspiration syndrome (MAS) - Where do we go? Research perspectives. Early Hum Dev. 2009;85:627–9. doi: 10.1016/j.earlhumdev.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602. doi: 10.1016/S0140-6736(04)16852-9. [DOI] [PubMed] [Google Scholar]
- 27.Cuttini M. Intrapartum prevention of meconium aspiration syndrome. Lancet. 2004;364:560–1. doi: 10.1016/S0140-6736(04)16863-3. [DOI] [PubMed] [Google Scholar]
- 28.Wiswell TE, Gannon CM, Jacob J, Goldsmith L, Szyld E, Weiss K, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105(1 Pt 1):1–7. doi: 10.1542/peds.105.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Chettri S, Adhisivam B, Bhat BV. Endotracheal Suction for Nonvigorous Neonates Born through Meconium Stained Amniotic Fluid: A Randomized Controlled Trial. J Pediatr. 2015;166:1208–13. e1. doi: 10.1016/j.jpeds.2014.12.076. [DOI] [PubMed] [Google Scholar]
- 30.Vain NE, Musante GA, Mariani GL. Meconium Stained Newborns: Ethics for Evidence in Resuscitation. J Pediatr. 2015;166:1109–12. doi: 10.1016/j.jpeds.2015.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Fraser WD, Hofmeyr J, Lede R, Faron G, Alexander S, Goffinet F, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005;353:909–17. doi: 10.1056/NEJMoa050223. [DOI] [PubMed] [Google Scholar]
- 32.Hofmeyr GJ, Xu H, Eke AC. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev. 2014;1:CD000014. doi: 10.1002/14651858.CD000014.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmeyr GJ. What (not) to do before delivery? Prevention of fetal meconium release and its consequences. Early Hum Dev. 2009;85:611–5. doi: 10.1016/j.earlhumdev.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 34.SOGC Clinical Practice Guideline. Management of meconium at birth. No 224, April 2009. Int J Gynaecol Obstet. 2009;107:80–1. doi: 10.1016/j.ijgo.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Hahn S, Choi HJ, Soll R, Dargaville PA. Lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev. 2013;4:CD003486. doi: 10.1002/14651858.CD003486.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pop VJ, Kuppens SM. Management strategy in case of meconium stained amniotic fluid. Early Hum Dev. 2014;90:341–2. doi: 10.1016/j.earlhumdev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Low JA, Pancham SR, Worthington D, Boston RW. The incidence of fetal asphyxia in six hundred high-risk monitored pregnancies. Am J Obstet Gynecol. 1975;121:456–9. doi: 10.1016/0002-9378(75)90074-5. [DOI] [PubMed] [Google Scholar]
- 38.Urbaniak KJ, Mccowan LM, Townend KM. Risk factors for meconium-aspiration syndrome. Aust N Z J Obstet Gynaecol. 1996;36:401–6. doi: 10.1111/j.1479-828x.1996.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 39.Maymon E, Chaim W, Furman B, Ghezzi F, Shoham Vardi I, Mazor M. Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur J Obstet Gynecol Reprod Biol. 1998;80:169–73. doi: 10.1016/s0301-2115(98)00122-5. [DOI] [PubMed] [Google Scholar]
- 40.Oyelese Y, Culin A, Ananth CV, Kaminsky LM, Vintzileos A, Smulian JC. Meconium-stained amniotic fluid across gestation and neonatal acid-base status. Obstet Gynecol. 2006;108:345–9. doi: 10.1097/01.AOG.0000226853.85609.8d. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Mas-Calvet M, Wei SQ, Luo ZC, Fraser WD. Abnormal fetal heart rate tracing patterns in patients with thick meconium staining of the amniotic fluid: association with perinatal outcomes. Am J Obstet Gynecol. 2009;200:283, e1–7. doi: 10.1016/j.ajog.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 42.Cahill AG, Parks L, Harper L, Heitmann E, O’neill K. Abnormal fetal heart rate tracings in patients with thick meconium staining of the amniotic fluid: Xu et al. Am J Obstet Gynecol. 2009;200:342–3. doi: 10.1016/j.ajog.2008.12.054. discussion e1–4. [DOI] [PubMed] [Google Scholar]
- 43.Lee KA, Mi Lee S, Jin Yang H, Park CW, Mazaki-Tovi S, Hyun Yoon B, et al. The frequency of meconium-stained amniotic fluid increases as a function of the duration of labor. J Matern Fetal Neonatal Med. 2011;24:880–5. doi: 10.3109/14767058.2010.531329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey HA, Tuuli MG, Shanks AL, Macones GA, Cahill AG. Interpreting category II fetal heart rate tracings: does meconium matter? Am J Obstet Gynecol. 2014;211:644, e1–8. doi: 10.1016/j.ajog.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monen L, Hasaart TH, Kuppens SM. The aetiology of meconium-stained amniotic fluid: pathologic hypoxia or physiologic foetal ripening? (Review) Early Hum Dev. 2014;90:325–8. doi: 10.1016/j.earlhumdev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Pariente G, Peles C, Perri ZH, Baumfeld Y, Mastrolia SA, Koifman A, et al. Meconium-stained amniotic fluid - risk factors and immediate perinatal outcomes among SGA infants. J Matern Fetal Neonatal Med. 2015 Jun;28:1064–7. doi: 10.3109/14767058.2014.942634. [DOI] [PubMed] [Google Scholar]
- 47.Ensing S, Engelen M, Tamminga P, Abu-Hanna A, Mol BW, Ravelli A. Association between value of the individual components of the 5 minute apgar score and neonatal outcome (Abstract 707) Am J Obstet Gynecol. 2015;212:S10. [Google Scholar]
- 48.Caughey A. Neonatal acidemia in neonates with 5-minute apgar scores greater than 7 – What are the outcomes? Am J Obstet Gynecol (abstract 511) 2015;212:S256–S7. doi: 10.1016/j.ajog.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 49.Boddy K, Dawes GS. Fetal breathing. Br Med Bull. 1975;31:3–7. doi: 10.1093/oxfordjournals.bmb.a071237. [DOI] [PubMed] [Google Scholar]
- 50.Patrick JE, Dalton KJ, Dawes GS. Breathing patterns before death in fetal lambs. Am J Obstet Gynecol. 1976;125:73–8. doi: 10.1016/0002-9378(76)90895-4. [DOI] [PubMed] [Google Scholar]
- 51.Manning FA, Martin CB, Jr, Murata Y, Miyaki K, Danzler G. Breathing movements before death in the primate fetus (Macaca mulatta) Am J Obstet Gynecol. 1979;135:71–6. [PubMed] [Google Scholar]
- 52.Byrne DL, Gau G. In utero meconium aspiration: an unpreventable cause of neonatal death. Br J Obstet Gynaecol. 1987;94:813–4. doi: 10.1111/j.1471-0528.1987.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 53.Burgess AM, Hutchins GM. Inflammation of the lungs, umbilical cord and placenta associated with meconium passage in utero. Review of 123 autopsied cases. Pathol Res Pract. 1996;192:1121–8. doi: 10.1016/S0344-0338(96)80029-X. [DOI] [PubMed] [Google Scholar]
- 54.Kearney MS. Chronic intrauterine meconium aspiration causes fetal lung infarcts, lung rupture, and meconium embolism. Pediatr Dev Pathol. 1999;2:544–51. doi: 10.1007/s100249900160. [DOI] [PubMed] [Google Scholar]
- 55.Dijxhoorn MJ, Visser GH, Fidler VJ, Touwen BC, Huisjes HJ. Apgar score, meconium and acidaemia at birth in relation to neonatal neurological morbidity in term infants. Br J Obstet Gynaecol. 1986;93:217–22. doi: 10.1111/j.1471-0528.1986.tb07896.x. [DOI] [PubMed] [Google Scholar]
- 56.Yeomans ER, Gilstrap LC, 3rd, Leveno KJ, Burris JS. Meconium in the amniotic fluid and fetal acid-base status. Obstet Gynecol. 1989;73:175–8. [PubMed] [Google Scholar]
- 57.Trimmer KJ, Gilstrap LC., 3rd “Meconiumcrit” and birth asphyxia. Am J Obstet Gynecol. 1991;165(4 Pt 1):1010–3. doi: 10.1016/0002-9378(91)90460-9. [DOI] [PubMed] [Google Scholar]
- 58.Ramin SM, Gilstrap LC, 3rd, Leveno KJ, Dax JS, Little BB. Acid-base significance of meconium discovered prior to labor. Am J Perinatol. 1993;10:143–5. doi: 10.1055/s-2007-994647. [DOI] [PubMed] [Google Scholar]
- 59.Cornish JD, Dreyer GL, Snyder GE, Kuehl TJ, Gerstmann DR, Null DM, Jr, et al. Failure of acute perinatal asphyxia or meconium aspiration to produce persistent pulmonary hypertension in a neonatal baboon model. Am J Obstet Gynecol. 1994;171:43–9. doi: 10.1016/s0002-9378(94)70075-3. [DOI] [PubMed] [Google Scholar]
- 60.Andres RL, Saade G, Gilstrap LC, Wilkins I, Witlin A, Zlatnik F, et al. Association between umbilical blood gas parameters and neonatal morbidity and death in neonates with pathologic fetal acidemia. Am J Obstet Gynecol. 1999;181:867–71. doi: 10.1016/s0002-9378(99)70316-9. [DOI] [PubMed] [Google Scholar]
- 61.Blackwell SC, Moldenhauer J, Hassan SS, Redman ME, Refuerzo JS, Berry SM, et al. Meconium aspiration syndrome in term neonates with normal acid-base status at delivery: is it different? Am J Obstet Gynecol. 2001;184:1422–5. doi: 10.1067/mob.2001.115120. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 62.Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. Am J Obstet Gynecol. 2001;185:931–8. doi: 10.1067/mob.2001.116828. [DOI] [PubMed] [Google Scholar]
- 63.Westgate JA, Bennet L, Gunn AJ. Meconium and fetal hypoxia: some experimental observations and clinical relevance. BJOG. 2002;109:1171–4. doi: 10.1111/j.1471-0528.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 64.Zaki M, Greenwood C, Impey L. Meconium and fetal hypoxia : some experimental observations and clinical relevance. BJOG. 2003;110:713. doi: 10.1046/j.1471-0528.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- 65.De Beaufort AJ, Pelikan DM, Elferink JG, Berger HM. Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet. 1998;352:102–5. doi: 10.1016/S0140-6736(98)85013-7. [DOI] [PubMed] [Google Scholar]
- 66.Zagariya A, Bhat R, Navale S, Vidyasagar D. Cytokine expression in meconium-induced lungs. Indian J Pediatr. 2004;71:195–201. doi: 10.1007/BF02724267. [DOI] [PubMed] [Google Scholar]
- 67.Okazaki K, Kondo M, Kato M, Kakinuma R, Nishida A, Noda M, et al. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Pediatrics. 2008;121:e748–53. doi: 10.1542/peds.2007-1697. [DOI] [PubMed] [Google Scholar]
- 68.Vidyasagar D, Zagariya A. Studies of meconium-induced lung injury: inflammatory cytokine expression and apoptosis. J Perinatol. 2008;28(Suppl 3):S102–7. doi: 10.1038/jp.2008.153. [DOI] [PubMed] [Google Scholar]
- 69.Zagariya A, Sierzputovska M, Navale S, Vidyasagar D. Role of meconium and hypoxia in meconium aspiration-induced lung injury in neonatal rabbits. Mediators Inflamm. 2010;2010:204831. doi: 10.1155/2010/204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindenskov PH, Castellheim A, Saugstad OD, Mollnes TE. Meconium aspiration syndrome: possible pathophysiological mechanisms and future potential therapies. Neonatology. 2015;107:225–30. doi: 10.1159/000369373. [DOI] [PubMed] [Google Scholar]
- 71.Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991;164:859–62. doi: 10.1016/0002-9378(91)90529-z. [DOI] [PubMed] [Google Scholar]
- 72.Mazor M, Furman B, Wiznitzer A, Shoham-Vardi I, Cohen J, Ghezzi F. Maternal and perinatal outcome of patients with preterm labor and meconium-stained amniotic fluid. Obstet Gynecol. 1995;86:830–3. doi: 10.1016/0029-7844(95)00265-S. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Bacteria and endotoxin in meconium-stained amniotic fluid at term: could intra-amniotic infection cause meconium passage? J Matern Fetal Neonatal Med. 2014;27:775–88. doi: 10.3109/14767058.2013.844124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romero R, Kadar N, Lafreniere D, Durum S, Hobbins JC, Duff GW. Do blood and meconium affect the detection of endotoxin in amniotic fluid with the limulus amebocyte gel clot assay? Am J Perinatol. 1987;4:356–9. doi: 10.1055/s-2007-999807. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh TT, Hsieh CC, Hung TH, Chiang CH, Yang FP, Pao CC. Differential expression of interleukin-1 beta and interleukin-6 in human fetal serum and meconium-stained amniotic fluid. J Reprod Immunol. 1998;37:155–61. doi: 10.1016/s0165-0378(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 76.Yamada T, Minakami H, Matsubara S, Yatsuda T, Kohmura Y, Sato I. Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol. 2000;46:21–30. doi: 10.1016/s0165-0378(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 77.Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Secreted phospholipase A2 is increased in meconium-stained amniotic fluid of term gestations: potential implications for the genesis of meconium aspiration syndrome. J Matern Fetal Neonatal Med. 2014;27:975–83. doi: 10.3109/14767058.2013.847918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–61. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 79.Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med. 2009;22:917–23. doi: 10.1080/14767050902994705. [DOI] [PubMed] [Google Scholar]
- 80.Kim BJ, Romero R, Mi Lee S, Park CW, Shin Park J, Jun JK, et al. Clinical significance of oligohydramnios in patients with preterm labor and intact membranes. J Perinat Med. 2011;39:131–6. doi: 10.1515/JPM.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med. 2012;25:1212–21. doi: 10.3109/14767058.2011.629256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park CW, Yoon BH, Park JS, Jun JK. An elevated maternal serum C-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and AF inflammatory response are likely in patients with preterm labor: clinical implications. J Matern Fetal Neonatal Med. 2013;26:847–53. doi: 10.3109/14767058.2013.783806. [DOI] [PubMed] [Google Scholar]
- 83.Park CW, Yoon BH, Kim SM, Park JS, Jun JK. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet Gynecol Sci. 2013;56:167–75. doi: 10.5468/ogs.2013.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park CW, Kim SM, Park JS, Jun JK, Yoon BH. Fetal, amniotic and maternal inflammatory responses in early stage of ascending intrauterine infection, inflammation restricted to chorio-decidua, in preterm gestation. J Matern Fetal Neonatal Med. 2014;27:98–105. doi: 10.3109/14767058.2013.806898. [DOI] [PubMed] [Google Scholar]
- 85.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 86.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 87.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–7. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 89.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mi Lee S, Romero R, Lee KA, Jin Yang H, Joon Oh K, Park CW, et al. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. J Matern Fetal Neonatal Med. 2011;24:37–42. doi: 10.3109/14767058.2010.482622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim EN, Kim CJ, Park JW, Yoon BH. Acute funisitis is associated with distinct changes in fetal hematologic profile. J Matern Fetal Neonatal Med. 2015 Mar;28:588–93. doi: 10.3109/14767058.2014.927426. [DOI] [PubMed] [Google Scholar]
- 93.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute Chorioamnionitis and Funisitis: Definition, Pathologic Features, and Clinical Significance. Am J Obstet Gynecol. 2015;213(Suppl):S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rossi EM, Philipson EH, Williams TG, Kalhan SC. Meconium aspiration syndrome: intrapartum and neonatal attributes. Am J Obstet Gynecol. 1989;161:1106–10. doi: 10.1016/0002-9378(89)90643-1. [DOI] [PubMed] [Google Scholar]
- 95.Wiswell TE, Henley MA. Intratracheal suctioning, systemic infection, and the meconium aspiration syndrome. Pediatrics. 1992;89:203–6. [PubMed] [Google Scholar]
- 96.Wiedemann JR, Saugstad AM, Barnes-Powell L, Duran K. Meconium aspiration syndrome. Neonatal Netw. 2008;27:81–7. doi: 10.1891/0730-0832.27.2.81. [DOI] [PubMed] [Google Scholar]
- 97.Ensing S, Ravelli A, Mol BW, Abu-Hanna A. Prediction of birth asphyxia in term neonates. Am J Obstet Gynecol (abstract 584) 2014;210:S288. [Google Scholar]
- 98.Soukka HR, Ahotupa M, Ruutu M, Kaapa PO. Meconium stimulates neutrophil oxidative burst. Am J Perinatol. 2002;19:279–84. doi: 10.1055/s-2002-33089. [DOI] [PubMed] [Google Scholar]
- 99.De Beaufort AJ, Bakker AC, Van Tol MJ, Poorthuis BJ, Schrama AJ, Berger HM. Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured A549 epithelial cells. Pediatr Res. 2003;54:491–5. doi: 10.1203/01.PDR.0000082017.97479.39. [DOI] [PubMed] [Google Scholar]
- 100.Korhonen K, Soukka H, Halkola L, Peuravuori H, Aho H, Pulkki K, et al. Meconium induces only localized inflammatory lung injury in piglets. Pediatr Res. 2003;54:192–7. doi: 10.1203/01.PDR.0000072784.55140.1E. [DOI] [PubMed] [Google Scholar]
- 101.Wisniewski WM, Zagariya AM, Pavuluri N, Srinivasan H, Shankarao S, Vidyasagar D. Effects of meconium aspiration in isolated perfused rat lungs. Pediatr Pulmonol. 2005;39:368–73. doi: 10.1002/ppul.20123. [DOI] [PubMed] [Google Scholar]
- 102.Cayabyab RG, Kwong K, Jones C, Minoo P, Durand M. Lung inflammation and pulmonary function in infants with meconium aspiration syndrome. Pediatr Pulmonol. 2007;42:898–905. doi: 10.1002/ppul.20675. [DOI] [PubMed] [Google Scholar]
- 103.Salvesen B, Fung M, Saugstad OD, Mollnes TE. Role of complement and CD14 in meconium-induced cytokine formation. Pediatrics. 2008;121:e496–505. doi: 10.1542/peds.2007-0878. [DOI] [PubMed] [Google Scholar]
- 104.Kaapa P, Soukka H. Phospholipase A2 in meconium-induced lung injury. J Perinatol. 2008;28(Suppl 3):S120–2. doi: 10.1038/jp.2008.147. [DOI] [PubMed] [Google Scholar]
- 105.Martin GI, Vidyasagar D. Introduction: Proceedings of the First International Conference for Meconium Aspiration Syndrome and Meconium-induced Lung Injury. J Perinatol. 2008;28(Suppl 3):S1–2. doi: 10.1038/jp.2008.176. [DOI] [PubMed] [Google Scholar]
- 106.Halliday HL, Hirata T. Perinatal listeriosis--a review of twelve patients. Am J Obstet Gynecol. 1979;133:405–10. doi: 10.1016/0002-9378(79)90061-9. [DOI] [PubMed] [Google Scholar]
- 107.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10(4 Suppl):294–302. [PubMed] [Google Scholar]
- 108.Mazor M, Froimovich M, Lazer S, Maymon E, Glezerman M. Listeria monocytogenes. The role of transabdominal amniocentesis in febrile patients with preterm labor. Arch Gynecol Obstet. 1992;252:109–12. doi: 10.1007/BF02389637. [DOI] [PubMed] [Google Scholar]
- 109.Mazor M, Hershkovitz R, Bashiri A, Maymon E, Schreiber R, Dukler D, et al. Meconium stained amniotic fluid in preterm delivery is an independent risk factor for perinatal complications. Eur J Obstet Gynecol Reprod Biol. 1998;81:9–13. doi: 10.1016/s0301-2115(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014 Sep 24;:1–17. doi: 10.3109/14767058.2014.954243. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed AI, Chaemsaithong P, Chaiworapongsa T, Zhong D, Shaman M, Lannaman K, et al. A receptor for danger signals, advanced glycation end products (RAGE) in fetal systemic inflammation and clinical chorioamnionitis. Am J Obstet Gynecol. 2015;212:S298. [Google Scholar]
- 112.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–85. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 113.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–16. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015 Aug;28:1394–409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu JM, Yeh TF, Wang JY, Wang JN, Lin YJ, Hsieh WS, et al. The role of pulmonary inflammation in the development of pulmonary hypertension in newborn with meconium aspiration syndrome (MAS) Pediatr Pulmonol Suppl. 1999;18:205–8. [PubMed] [Google Scholar]
- 122.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–61. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, et al. Pulmonary and systemic inflammatory responses to intra-amniotic IL-1alpha in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L285–95. doi: 10.1152/ajplung.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ban R, Ogihara T, Mori Y, Oue S, Ogawa S, Tamai H. Meconium aspiration delays normal decline of pulmonary vascular resistance shortly after birth through lung parenchymal injury. Neonatology. 2011;99:272–9. doi: 10.1159/000318748. [DOI] [PubMed] [Google Scholar]
- 125.Jobe AH. Effects of chorioamnionitis on the fetal lung. Clin Perinatol. 2012;39:441–57. doi: 10.1016/j.clp.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kunzmann S, Collins JJ, Kuypers E, Kramer BW. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. Am J Obstet Gynecol. 2013;208:429–37. doi: 10.1016/j.ajog.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 127.Kovo M, Schreiber L, Ben-Haroush A, Klien H, Wand S, Golan A, et al. Association of non-reassuring fetal heart rate and fetal acidosis with placental histopathology. Placenta. 2011;32:450–3. doi: 10.1016/j.placenta.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Benirschke K, Clifford SH. Intrauterine bacterial infection of the newborn infant: frozen sections of the cord as an aid to early detection. J Pediatr. 1959;54:11–8. doi: 10.1016/s0022-3476(59)80031-7. [DOI] [PubMed] [Google Scholar]
- 129.Mendilcioglu I, Kilicarslan B, Gurkan Zorlu C, Karaveli S, Uner M, Trak B. Placental biopsy by frozen section: does it have a role in evaluation of fetal well-being? Aust N Z J Obstet Gynaecol. 2003;43:433–7. doi: 10.1046/j.0004-8666.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 130.Mahe E, Hamid J, Terry J, Jansen JW, Bourgeois J, Arredondo-Marin J. Frozen section of placental membranes and umbilical cord: an aid to early postpartum diagnosis of intra-amniotic infection. Am J Clin Pathol. 2014;142:202–8. doi: 10.1309/AJCPYN70DLUFFDVP. [DOI] [PubMed] [Google Scholar]
- 131.Sedaghatian MR, Othman L, Hossain MM, Vidyasagar D. Risk of meconium-stained amniotic fluid in different ethnic groups. J Perinatol. 2000;20:257–61. doi: 10.1038/sj.jp.7200367. [DOI] [PubMed] [Google Scholar]
- 132.Vivian-Taylor J, Sheng J, Hadfield RM, Morris JM, Bowen JR, Roberts CL. Trends in obstetric practices and meconium aspiration syndrome: a population-based study. BJOG. 2011;118:1601–7. doi: 10.1111/j.1471-0528.2011.03093.x. [DOI] [PubMed] [Google Scholar]
- 133.Wiswell TE, Tuggle JM, Turner BS. Meconium aspiration syndrome: have we made a difference? Pediatrics. 1990;85:715–21. [PubMed] [Google Scholar]
- 134.Wiswell TE. Handling the meconium-stained infant. Semin Neonatol. 2001;6:225–31. doi: 10.1053/siny.2001.0051. [DOI] [PubMed] [Google Scholar]