Abstract
Human papillomavirus (HPV) is the most commonly sexually transmitted infection in the world and the primary cause of cervical cancer. Canada introduced publicly funded HPV vaccination programs in 2006. The objectives of this study are twofold and aim to (1) determine the levels and (2) examine the various factors influencing vaccine uptake among the general Canadian population. A literature search was conducted on seven databases, followed by screening, methodological quality review (using modified Newcastle-Ottawa Scale), and data extraction. Pooled meta-analysis and a subgroup analysis were conducted stratifying by a number of variables (age, sex, type of program, and method of payment) determined apriori. A total of 718 peer-reviewed articles were initially identified with 12 remaining after screening and underwent methodological quality review. HPV vaccination uptake in Canada varied from 12.40% (95% confidence interval [CI] 6.77–20.26) to 88.20% (95% CI 85.72–90.39). The pooled random effects model showed the HPV vaccination uptake to be 55.92% (95% CI 44.87–66.65). The subgroup analysis showed that vaccination uptake was 66.95% (95% CI 55.00–77.89) in participants ≤ 18 years as compared to 13.58% (95% CI 10.93–16.46) in participants > 18 years. Uptake for females was higher 57.23% (95% CI: 45.40–68.66) when compared to that of 47.01% (95% CI: 0.82–97.75) in males. HPV vaccine uptake among school-based programs was 69.62% (95% CI 57.27–80.68) as compared to 18.66% (95% CI 6.66–34.92) for community-based programs. Vaccination uptake for publicly funded programs was significantly higher 66.95% (95% CI 55.00–77.89) when compared to 13.58% (95% CI 10.92–16.46) for programs where participants had to pay out of pocket. To prevent infections and reduce the burden of HPV-related diseases (including cervical cancer), communities should be made aware and encouraged to vaccinate their children. There is a documented need to direct effort and focus interventions toward improving HPV vaccination uptake in Canada.
Keywords: Canada, immunization, papillomavirus infections, papillomavirus vaccines, uterine cervical neoplasms, virus diseases
Introduction
Human papillomavirus (HPV) is the most commonly sexually transmitted infection in the world and the primary cause of cervical and other cancers.[1,2,3,4] Globally, cervical cancer is the second most common cancer and mainly affects women in the developing world.[4] However, even in developed countries such as Canada, cervical cancer remains a serious public health concern.[5] In 2016, it is estimated that 1500 Canadian women will be diagnosed with cervical cancer and 400 will die from it.[5] These statistics are unacceptably high for Canada, when one considers that it is a high-income country and cervical cancer is a highly preventable disease.
HPV infections are quite common and affect the majority of sexually active men and women.[6] Most HPV infections are asymptomatic and resolve spontaneously usually within 2 years.[6] However, longer lasting HPV types 16 and 18 infections are known to cause 70% of cervical cancers and precancerous cervical lesions, while HPV types 6 and 11 are associated with approximately 90% of all genital warts.[4] Most individuals do not even know that they have been infected with HPV and therefore may inadvertently transmit the HPV infection to their sex partners. In Canada, it is estimated that 550,000 people are infected with HPV each year and that approximately 80% of females of reproductive age will be infected at some point in their lifetime.[7]
Given the strong link between HPV infections (Types 16 and 18) and cervical cancer, a number of new interventions have been introduced in an effort to curtail the burden of the disease. Chief among them is the population-based use of HPV vaccines. In 2006, two HPV vaccines Cervarix (which covers HPV types 16 and 18) and Gardasil (which covers HPV types 6, 11, 16, and 18) were approved for use mainly among females but also for males aged 9–26 years in Canada.[8] Publically funded HPV immunization programs for females are available in all Canadian provinces and territories. In addition, four provinces (Alberta, British Columbia, Prince Edward Island, and Nova Scotia) have publically funded HPV vaccination programs for males and two others (Ontario and Quebec) are in the process of doing so in the near future.[9]
The HPV vaccine has been reported to be highly effective in preventing the targeted HPV types, as well as the diseases caused by them.[7,8] Across Canada, the HPV vaccine uptake is quite variable with initial vaccination rates (i.e. first dose) ranging from 47% in the Northwest Territories to 93.8% in Newfoundland and Labrador.[10,11] The rates are significantly lower when one considers the HPV vaccine completion rates (i.e. all three doses) with a number of provinces not even keeping records for these important statistics.[10,11] Even less is known about the factors that may influence HPV vaccine uptake in Canada. Public discussion regarding the new HPV vaccines is characterized by strong feelings and beliefs and significant financial interest, but more research is needed to help inform policy choices, public health interventions, and decision-makers.
To the best of our knowledge, there are no systematic reviews examining HPV vaccination uptake in Canada. Instead, previous studies have primarily focused on HPV vaccine knowledge, attitudes toward vaccination, acceptability, and intention to vaccinate.[12,13,14,15,16,17,18,19] However, to optimize the use of the HPV vaccination programs in Canada, it is critically important to determine the levels of HPV vaccine uptake. To this end, we conducted a systematic review and meta-analysis of the existing literature to address these key issues.
Methods
An extensive and systematic review of the literature was conducted on the following databases: Medline, PubMed, Cochrane Library, EMBASE, Global Health, ProQuest Public Health, and JSTOR. Searches were conducted using various combinations of keywords and Medical Subject Heading (MeSH) terms including “papillomavirus infections,” “virus diseases,” “uterine cervical neoplasms,” “papillomavirus vaccines,” “immunization,” and “Canada.”
Inclusion and exclusion criteria
Articles were included if they were in the English language, with a publication date of 2006 and later, were publically available, included human populations in Canada, involved an HPV vaccination intervention, and provided quantitative data regarding levels of HPV vaccination uptake. Articles involving case reports or case series studies were excluded.
Data extraction and quality assessment
Three steps were involved in the data extraction process. First, duplicates were removed and the remaining articles were screened by their titles and abstracts for relevance. Second, full-text articles were reviewed by two of the authors (OO and RM) to assess their conformity with the study inclusion criteria. Third, the selected articles underwent methodological quality review by using a modified Newcastle-Ottawa Scale (NOS).[20] Using the modified NOS, each study was assessed and scored under two domains: selection (representativeness of the vaccinated group, ascertainment of vaccination status, demonstration that outcome of interest was absent at start of study) and outcome (assessment of outcome, adequacy of follow-up of vaccinated group). Any disagreement between the two authors (OO and RM) was further discussed to reach a resolution, and if required, a third author (CN) provided the tie-breaking vote. Reference management and duplication were handled using the reference manager, Mendeley. Data extracted from the studies included vaccination rates, study design, participants’ size, participants’ demographic information, program location, period of vaccination, as well as key conclusions of the study. Data were collected into a common folder and shared between the researchers on Google Drive. Spreadsheets were constructed based on screening outcomes and data extraction from the final articles.
Statistical analysis
The meta-analysis was carried out using the MedCalc analytic software version 16.2.1 (MedCalc Software, Ostend, Belgium).[21] Weighted pooled vaccination rates were obtained with the aid of a random effects model using the Freeman-Tukey transformation.[22,23] Statistical analysis for heterogeneity was performed using Higgins I-squared (I2).[24,25] This allowed us to determine the proportion of observed variation in vaccination rates across studies that could be attributed to heterogeneity. A value of I2 >75% was considered a statistical indicator of the likely presence of heterogeneity.[24,25] Suspected heterogeneity was further explored using a subgroup analysis. The factors to be explored in the subgroup analysis were determined apriori and they included age (>18 vs. 18 years or younger), sex (male vs. female), type of program (community-based vs. school-based) and funding (publicly funded vs. out of pocket). The vaccination rates were pooled for the respective subgroups using a random effects model, with the subsequent computation of rate ratios and corresponding 95% confidence intervals (CIs), using the MedCalc analytic software version 16.2.1.[21] A funnel plot was used to assess the risk of publication bias for the included studies.
Results
Study selection
In the primary search, we found 718 peer-reviewed articles that were related to our topic. Of those, 205 were removed as duplicates. Of the remaining 513 articles, 366 were excluded after the title and abstract screening. Of the 147 articles that were assessed through full-text screening, 12 articles containing 624,604 participants remained. These articles underwent methodological quality review [Figure 1] and were included for analysis in our study.
Figure 1.
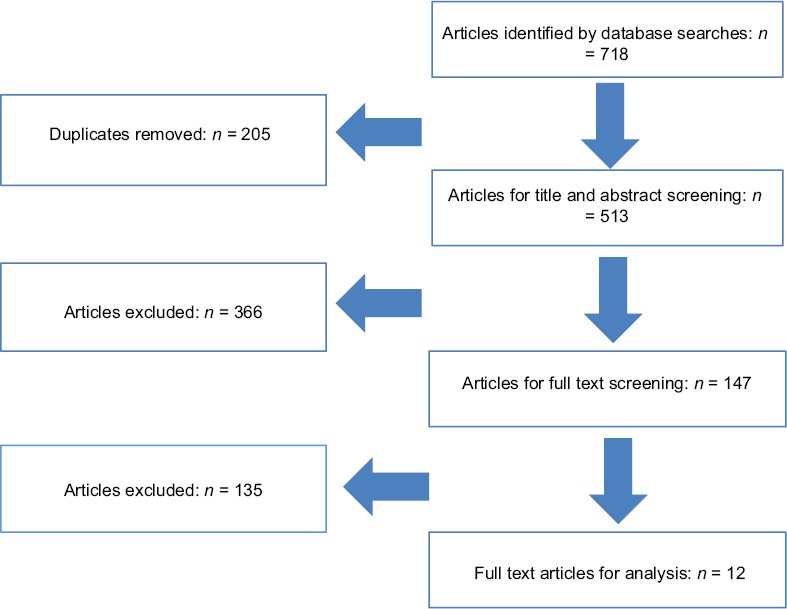
PRISMA flow diagram of the included studies
Study characteristics
Of the 12 studies,[26,27,28,29,30,31,32,33,34,35,36,37] eight were longitudinal and four were cross-sectional.[26,30,36,37] Sample size ranged from 105 to 223,051 participants.[26,27] Two studies[26,28] involved participants over 18 years old, who had to pay out of pocket to receive their HPV vaccination, whereas participants in the other ten studies were younger than or equal to 18 years old and their HPV vaccination was publicly funded. Two studies involved male and female participants,[29,32] while the remaining ten studies only used female participants. One study[21] was community based, six were school based,[27,28,29,35,36,37] and five were both community and school based. Overall, the risk of bias was found to be low across all studies. A summary table of the key characteristics of the included studies is shown in Table 1.
Table 1.
Summary of the key characteristics of eligible articles from the literature search
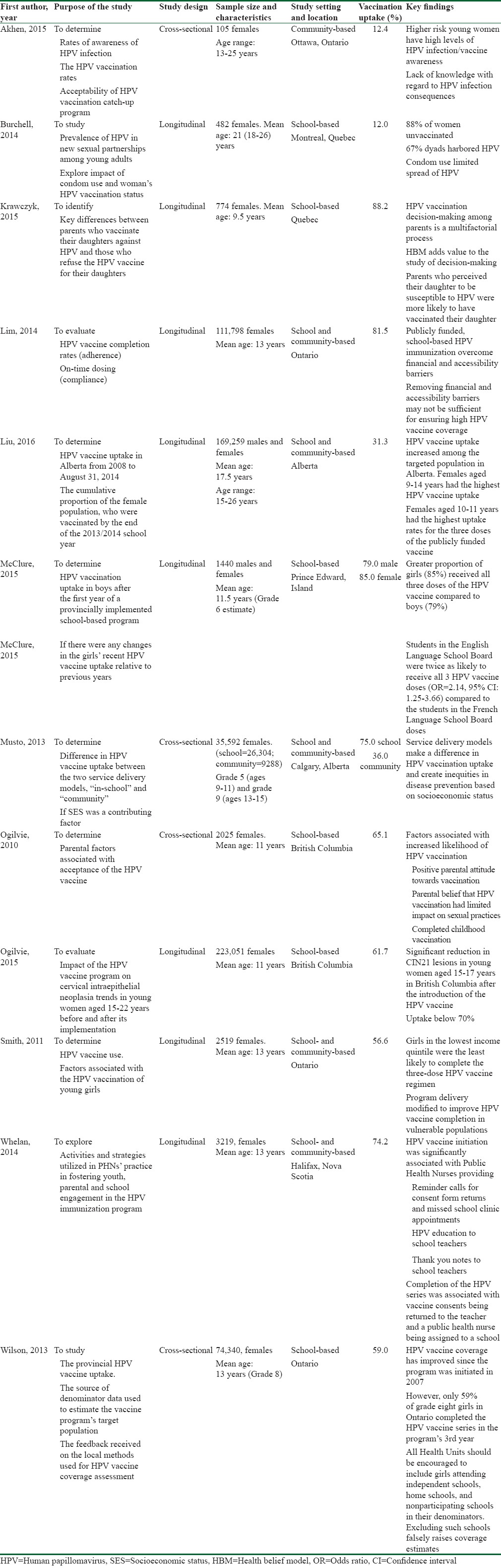
Vaccine uptake
Of the 12 studies, four were conducted in the province of Ontario, two in Quebec, two in Alberta, two in British Columbia, one in Prince Edward Island, and one in Nova Scotia. The reported vaccination uptake rates varied widely among the 12 studies, with the lowest reported rate at 12.40%[28] and the highest at 88.20%.[26] The pooled vaccination uptake using a random effects model was 55.91% (95% CI 44.87–66.65), with the test for heterogeneity; I2 = 99.98 (P < 0.0001). A summary of the pooled meta-analysis is shown in Table 2.
Table 2.
Pooled meta-analysis

Subgroup analysis
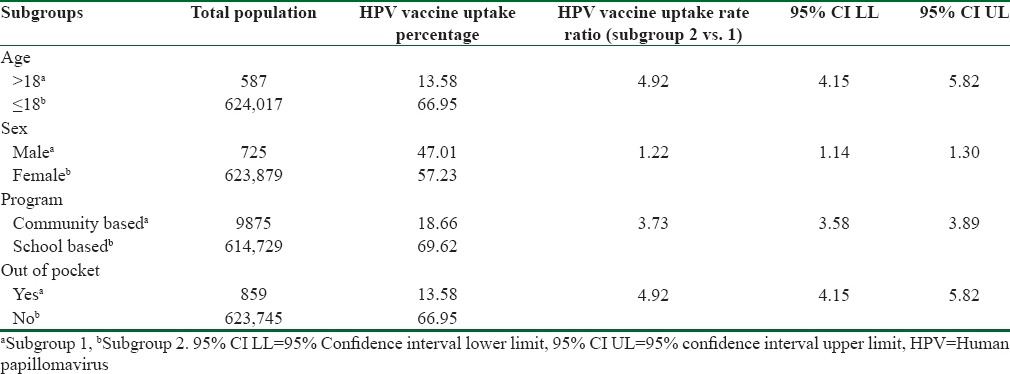
A subgroup analysis was conducted stratifying by a number of variables (age, sex, type of program, and method of payment) determined apriori. The pooled estimate for each subgroup was obtained using a random effects model after which rate ratios (with 95% CIs and P values) were calculated using the MedCalc analytic software to assess differences in vaccination rate between the predetermined variables.[21] The subgroup analysis by age found the HPV vaccination uptake for participants younger than or equal to 18 years old to be 66.95% (95% CI: 55.00–77.89). This rate was significantly higher than the one observed for participants older than 18 years, 13.58% (95% CI 10.93–16.46). Participants younger than or equal to 18 years were 4.92 times more likely to be vaccinated for HPV compared to those over the age of 18 years (P < 0.0001; 95% CI 4.15–5.82). Vaccination uptake for females was higher 57.23% (95% CI: 45.40–68.66) when compared to that of males 47.01% (95% CI: 0.82–97.75). Females were 1.22 times more likely to be vaccinated for HPV compared to males (P < 0.0001; 95% CI 1.14–1.30).
The subgroup analysis also showed that HPV vaccine uptake among school-based programs was significantly higher 69.62% (95% CI 57.27–80.68) than community-based programs 18.66% (95% CI 6.66–34.92). Participants in school-based programs were 3.73 times more likely to be vaccinated for HPV compared to those in community-based programs (P < 0.0001; 95% CI 3.58–3.89). Further, there were notable differences in the levels of HPV vaccination uptake when the source of funding was considered. Vaccination uptake for publicly funded programs was significantly higher 66.95% (95% CI 55.00–77.89) when compared to 13.58% (95% CI 10.93–16.46) for programs where participants had to pay out of pocket. Participants in publically funded programs were 4.92 times more likely to be vaccinated for HPV compared to those who had to pay out of pocket (P < 0.0001; 95% CI 4.15–5.82). A summary of the results for the subgroup analysis is shown in Table 3.
Table 3.
Subgroup analysis
Discussion
This systematic review was conducted to independently determine the HPV vaccine uptake in the Canadian population and to examine the various factors influencing vaccine uptake in different subpopulations that may require tailored interventions. Our pooled analysis showed the HPV vaccination uptake in Canada to be 55.91%, which is well below the >85% target set by the Canadian government.[10]
It has been well documented that receiving the HPV vaccine at younger ages (10–14 years) is more advantageous as it offers earlier protection against infection and better immune response to the vaccine when compared to older women and men.[38] Unsurprisingly, our study found that participants younger than or equal to 18 years old were 4.92 times more likely to be vaccinated for HPV compared to those over the age of 18. However, several clinical trials have shown that older girls and women, who are most at risk of infection (18–30 years), also have a strong immune response to the HPV vaccine, inducing high virus-neutralizing antibody titers.[39,40] Consequently, implementation of programs that improve the levels of HPV vaccine uptake among older girls and women could prove very beneficial to Canadian women and help prevent a significant burden of the HPV-related diseases (including cervical cancer) on the nation.
Our findings showed that females were 1.22 times more likely to be vaccinated against HPV compared to males. In Canada, HPV vaccination for females was introduced in 2006 and for males in 2013.[8] As of 2015, only four provinces (Alberta, Nova Scotia, British Columbia, and Prince Edward Island) offered free vaccination to males.[9] This might help explain the observed gender disparity in our study. While the National Advisory Committee on Immunization (NACI) recommends HPV vaccination be extended to males aged 9–26, they also advise that the benefit of expanding HPV immunization to include males be compared to improving uptake amongst females to 85% in areas where uptake is < 85%.[41] In addition, as many sectors are focusing on the direct association of HPV with cervical cancer, vaccination programs across the country are largely female oriented. These developments, alongside concerns regarding the financial cost,[42] have slowed progress toward achieving gender equity in HPV vaccination among Canadians.
Individuals participating in school-based programs were 3.73 times more likely to be vaccinated against HPV compared to community-based programs. This is similar to the findings in previous studies showing that school-based programs have higher rates of vaccination uptake in countries such as Spain, Scotland, Australia, and the USA.[43] It was reported that HPV vaccines delivered through schools in Australia and New Zealand had a high and relatively balanced uptake across socioeconomic groups, suggesting that school-based delivery can help reduce inequities in HPV vaccine delivery.[44,45] Moreover, school-based programs are known to provide an opportunity for children as well as their parents to be educated and make informed decisions about the importance of HPV vaccination.[44,45]
Participants in publicly funded programs were 4.92 times more likely to be vaccinated for HPV compared to those who had to pay out of pocket. This finding is not surprising as a systematic review conducted[13] among published articles in the USA found higher HPV vaccine uptake among individuals who had health insurance (private or public) as opposed to those who did not, suggesting that fee for service is negatively associated with vaccination uptake. Mathematical models of the clinical and economic impact of publically funded HPV vaccination programs have demonstrated significant clinical and cost benefits.[46,47] However, these studies assumed high levels of vaccine uptake (>70%), and therefore, the clinical and economic impact of the HPV vaccine may have been overestimated.[48,49,50]
The HPV vaccine uptake rates in Canada appear to be much lower than in many other developed countries, which have reported coverage rates of > 70%.[43] The reasons for this discrepancy are multifactorial. For instance, in 2013, the childhood National Immunization Coverage Survey (cNICS) found that approximately 75% of Canadian girls aged 12–14 years were immunized against HPV.[51] By comparison in 2014, the adult NICS found Canadian females aged 18–26 and 27–45 years to have HPV vaccination uptakes of 44.7% and 8.3%, respectively.[52] The dramatic fall in vaccination rates with increasing age among females may be attributed to the initial restriction of HPV vaccination programs to females in grades 4–8 (ages 10–14 years) in Canada.[8] By 2012, the NACI modified their HPV vaccination guidelines to include a larger age group (9–26 years).[41] However, despite these changes, our study results demonstrate that disparities in HPV vaccination uptake still persist by age group and setting as older cohorts, who are already out of school, are expected to pay out of pocket, potentially making the HPV vaccine unaffordable for them.
Limitations
Our study assessed the uptake of a relatively new vaccine, and as such, the amount of available data in the literature is scarce. Analysis of data showed significant heterogeneity that could be attributed to methodological and/or clinical variations in the characteristics of the included studies. There was little or no data available on the variation of vaccine uptake by ethnicity, especially with regards to the Aboriginal population in Canada. Furthermore, changing patterns of vaccine delivery, scheduling, and settings resulted in different uptake rates at different time periods. Finally, it is also possible that some of the findings may be due to factors unique to each study and could not be identified by means of a systematic review or meta-analysis.
Recommendations
Based on the findings of our systematic review and meta-analysis, we recommend expanding the HPV vaccination programs to include young males and older females, subsidizing the costs for the vaccination and developing a national immunization surveillance program based on provincial databases to better determine the levels of HPV vaccination uptake within the Canadian population. Better surveillance will help identify at-risk subpopulations and yield epidemiological data that guide effective use of resources and inform tailoring of vaccination interventions.
Conclusions
Due to the relatively low number of studies and lack of long-term results, no firm conclusions can be drawn. To prevent infections and reduce the burden of HPV-related disease (including cervical cancer), communities should be made aware and encouraged to vaccinate their children. This study found that HPV vaccination rates were higher for females aged 18 years or younger, who were part of school based, publically funded program. Better surveillance and additional research are needed in this area. The future success of the HPV immunization programs in Canada will depend on the concerted efforts and commitment of researchers, healthcare professionals, the public, and the provincial and federal government.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carter JR, Ding Z, Rose BR. HPV infection and cervical disease: A review. Aust N Z J Obstet Gynaecol. 2011;51:103–8. doi: 10.1111/j.1479-828X.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 3.Garland SM. Human papillomavirus update with a particular focus on cervical disease. Pathology. 2002;34:213–24. doi: 10.1080/00313020212469. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. HPV and Cervical Cancer: Key Facts. 2015. [Last accessed on 2016 Feb 28]. Available from: http://www.who.int/mediacentre/factsheets/fs380/en/
- 5.Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian Cancer Statistics. Toronto, ON: Canadian Cancer Society; 2016. [Last accessed on 2016 Jun 16]. Available from: http://www.cancer.ca/en/cancer.information/cancer.type/cervical/statistics/?region=on . [Google Scholar]
- 6.Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: Biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15:727–46. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 7.Crum CP, Abbott DW, Quade BJ. Cervical cancer screening: From the Papanicolaou smear to the vaccine era. J Clin Oncol. 2003;21(10 Suppl):224s–30s. doi: 10.1200/JCO.2003.01.116. [DOI] [PubMed] [Google Scholar]
- 8.Crum CP, Rivera MN. Vaccines for cervical cancer. Cancer J. 2003;9:368–76. doi: 10.1097/00130404-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 9.CTV News. Ontario Extending Free HPV Vaccines to Boys; 21 April. 2016. [Last accessed on 2016 Apr 23]. Available from: http://www.ctvnews.ca/health/ontario-extending-free-hpv-vaccines-to-boys-1.2869481 .
- 10.Cancer System Performance. Human Papillomavirus (HPV) Vaccination. 2016. [Last accessed on 2016 Apr 24]. Available from: http://www.systemperformance.ca/cancer-control-domain/prevention/hpv-vaccination/
- 11.Rogers C, Smith RJ. Examining Provincial HPV Vaccination Schemes in Canada: Should We Standardise the Grade of Vaccination or the Number of Doses? Int Sch Res Notices 2015. 2015 doi: 10.1155/2015/170236. 170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38:525–33. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–56. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Drolet M, Boily MC, Greenaway C, Deeks SL, Blanchette C, Laprise JF, et al. Sociodemographic inequalities in sexual activity and cervical cancer screening: Implications for the success of human papillomavirus vaccination. Cancer Epidemiol Biomarkers Prev. 2013;22:641–52. doi: 10.1158/1055-9965.EPI-12-1173. [DOI] [PubMed] [Google Scholar]
- 15.Cerigo H, Macdonald ME, Franco EL, Brassard P. Inuit women's attitudes and experiences towards cervical cancer and prevention strategies in Nunavik, Quebec. Int J Circumpolar Health. 2012;71:17996. doi: 10.3402/ijch.v71i0.17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gainforth HL, Cao W, Latimer-Cheung AE. Determinants of human papillomavirus (HPV) vaccination intent among three Canadian target groups. J Cancer Educ. 2012;27:717–24. doi: 10.1007/s13187-012-0389-1. [DOI] [PubMed] [Google Scholar]
- 17.Kiely M, Sauvageau C, Dubé E, Deceuninck G, De Wals P. Human papilloma virus: Knowledge, beliefs and behavior of Quebec women. Can J Public Health. 2011;102:303–7. doi: 10.1007/BF03404055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meghani H, Dubey V, Kadri O, Mathur A, Cameron J, Beckermann K. Factors contributing to uptake of the publicly-funded HPV vaccine in Toronto. Int J Infect Dis. 2010;14:E452. [Google Scholar]
- 19.Duval B, Gilca V, McNeil S, Dobson S, Money D, Gemmill IM, et al. Vaccination against human papillomavirus: A baseline survey of Canadian clinicians’ knowledge, attitudes and beliefs. Vaccine. 2007;25:7841–7. doi: 10.1016/j.vaccine.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. 2009. [Last cited on 2009 Oct 19]. Available from: http://www.ohrica/programs/clinical_epidemiology/oxfordhtm .
- 21.MedCalc. Easy-to-Use Statistical Software. [Last accessed on 2016 Apr 14]. Available from: https://www.medcalc.org/calc/
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11. [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahken S, Fleming N, Dumont T, Black A. HPV awareness in higher-risk young women: The need for a targeted HPV catch-up vaccination program. J Obstet Gynaecol Can. 2015;37:122–8. doi: 10.1016/S1701-2163(15)30333-9. [DOI] [PubMed] [Google Scholar]
- 27.Ogilvie GS, Naus M, Money DM, Dobson SR, Miller D, Krajden M, et al. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: An ecological analysis. Int J Cancer. 2015;137:1931–7. doi: 10.1002/ijc.29508. [DOI] [PubMed] [Google Scholar]
- 28.Burchell AN, Rodrigues A, Moravan V, Tellier PP, Hanley J, Coutlée F, et al. Determinants of prevalent human papillomavirus in recently formed heterosexual partnerships: A dyadic-level analysis. J Infect Dis. 2014;210:846–52. doi: 10.1093/infdis/jiu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure CA, MacSwain MA, Morrison H, Sanford CJ. Human papillomavirus vaccine uptake in boys and girls in a school-based vaccine delivery program in Prince Edward Island, Canada. Vaccine. 2015;33:1786–90. doi: 10.1016/j.vaccine.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Musto R, Siever JE, Johnston JC, Seidel J, Rose MS, McNeil DA. Social equity in Human Papillomavirus vaccination: A natural experiment in Calgary Canada. BMC Public Health. 2013;13:640. doi: 10.1186/1471-2458-13-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim WT, Sears K, Smith LM, Liu G, Lévesque LE. Evidence of effective delivery of the human papillomavirus (HPV) vaccine through a publicly funded, school-based program: The Ontario Grade 8 HPV Vaccine Cohort Study. BMC Public Health. 2014;14:1029. doi: 10.1186/1471-2458-14-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XC, Bell CA, Simmonds KA, Russell ML, Svenson LW. HPV Vaccine utilization, Alberta 2008/09-2013/14 School year. BMC Infect Dis. 2016;16:15. doi: 10.1186/s12879-016-1340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith L, Brassard P, Kwong J, Deeks S, Ellis A, Lévesque L. Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of grade 8 girls. BMC Public Health. 2011;11:645. doi: 10.1186/1471-2458-11-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelan NW, Steenbeek A, Martin-Misener R, Scott J, Smith B, D’Angelo-Scott H. Engaging parents and schools improves uptake of the human papillomavirus (HPV) vaccine: Examining the role of the public health nurse. Vaccine. 2014;32:4665–71. doi: 10.1016/j.vaccine.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk A, Knäuper B, Gilca V, Dubé E, Perez S, Joyal-Desmarais K, et al. Parents’ decision-making about the human papillomavirus vaccine for their daughters: I. Quantitative results. Hum Vaccin Immunother. 2015;11:322–9. doi: 10.1080/21645515.2014.1004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvie G, Anderson M, Marra F, McNeil S, Pielak K, Dawar M, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: Parental factors associated with HPV vaccine receipt. PLoS Med. 2010;7:e1000270. doi: 10.1371/journal.pmed.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson SE, Harris T, Sethi P, Fediurek J, Macdonald L, Deeks SL. Coverage from Ontario, Canada's school-based HPV vaccine program: The first three years. Vaccine. 2013;31:757–62. doi: 10.1016/j.vaccine.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;22;27:581–7. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 39.Muñoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: A randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 40.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 41.Eggertson L. Provinces weighing HPV vaccination of boys. CMAJ. 2012;184:E250–1. doi: 10.1503/cmaj.109-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25:5399–408. doi: 10.1016/j.vaccine.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 43.Hopkins TG, Wood N. Female human papillomavirus (HPV) vaccination: Global uptake and the impact of attitudes. Vaccine. 2013;31:1673–9. doi: 10.1016/j.vaccine.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 44.Brotherton JM, Deeks SL, Campbell-Lloyd S, Misrachi A, Passaris I, Peterson K, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. 2008;32:457–61. [PubMed] [Google Scholar]
- 45.Blakely T, Kvizhinadze G, Karvonen T, Pearson AL, Smith M, Wilson N. Cost-effectiveness and equity impacts of three HPV vaccination programmes for school-aged girls in New Zealand. Vaccine. 2014;32:2645–56. doi: 10.1016/j.vaccine.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 46.Kim JJ, Brisson M, Edmunds WJ, Goldie SJ. Modeling cervical cancer prevention in developed countries. Vaccine. 2008;26(Suppl 10):K76–86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev. 2006;28:88–100. doi: 10.1093/epirev/mxj006. [DOI] [PubMed] [Google Scholar]
- 48.Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96:604–15. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 49.Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology. 2002;13:631–9. doi: 10.1097/00001648-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Statistics Canada. Vaccine Coverage in Canadian Children: Highlights from the 2013 Childhood National Immunization Coverage Survey (cNICS) [Last updated on 2015 Jul 21; Last accessed on 2016 Apr 25]. Available from: http://www.healthycanadians.gc.ca/publications/healthy-living-vie-saine/immunization-coverage-children-2013-couverture- vaccinale-enfants/index-eng.php .
- 52.Statistics Canada. Vaccine uptake in Canadian adults: Results from the 2014 Adult National Immunization Coverage Survey (aNICS) [Last updated on 2016 Feb 02; Last accessed on 2016 Apr 25]. Available from: http://www.healthycanadians.gc.ca/publications/healthy-living-vie-saine/vaccine-coverage-adults-results-2014-resultats-couverture-vaccinale-adultes/index-eng.php .


