Abstract
Objective
This study aimed to investigate the association between low plasma Platelet-derived growth factor-BB (PDGF-BB) levels and oestradiol in Postmenopausal osteoporosis (PMOP).
Methods
This prospective study measured plasma PDGF-BB and oestradiol levels in outpatients who were admitted to our hospital. Participants were screened and then allocated to three groups: normal young women, postmenopausal control, and PMOP. Additionally, Sprague–Dawley rats underwent either sham surgery or bilateral ovariectomy (OVX), and were divided into the following groups: sham, OVX, OVX + oestradiol, and OVX + PDGF-BB. Plasma oestradiol and PDGF-BB levels were measured using commercially available ELISA kits.
Results
A total of 121 participants, including 69 normal young women, 28 patients with primary PMOP, and 24 age-matched postmenopausal women were enrolled. Plasma oestradiol and PDGF-BB levels were lower in postmenopausal women, especially in PMOP (P < 0.01). Pearson correlations analysis showed that PDGF-BB levels were positively correlated with oestradiol levels and inversely correlated with age (P < 0.01). The OVX rat model showed that oestradiol replacement increased plasma PDGF-BB levels, while PDGF-BB systematic treatment had no effect on plasma oestradiol levels.
Conclusions
Plasma PDGF-BB levels are maintained by oestrogen in normal young women and play a major role in PMOP.
Keywords: PDGF-BB, oestradiol, postmenopausal osteoporosis
Introduction
PMOP, which is characterized by reduced bone mass, is associated with an imbalance between bone formation and bone resorption in older women.5 Consequently, there is an increase in bone fragility and susceptibility to fracture.6 Many studies have demonstrated that osteoporosis fractures are related to substantial morbidity and mortality in postmenopausal women.3,4 The aetiology of osteoporosis is still unclear, but osteoporosis is believed to be a multifactor disease, such as involvement of endocrine factors7 and genetic differences8,9. Many studies have shown that oestradiol deficiency plays a major role in the pathogenesis of PMOP.6
Platelet-derived growth factors (PDGFs) are growth factors that regulate different types of cell growth and division.14 PDGF-BB is one of the most recently studied isoforms and it plays an important role in skeletal development. Xie et al.20 found that Pdgfb−/− mice had less bone formation in trabecular and cortical bone compared with their normal littermates. Additional studies have demonstrated that PDGF-BB affects fracture healing, bone repair, osseous defects, and bone density loss.16–19
An in vitro study showed that PDGF-BB could be produced by endothelial cells and this depends on oestradiol levels.21 Finlay et al.22 found that oestradiol induced PDGF-BB production in ELT-3 cells, but not in vascular endothelial cells and vascular smooth muscle cells. However, no clinical studies have investigated plasma oestradiol and PDGF-BB levels in PMOP.
Materials and methods
Subjects
A total of 121 participants were enrolled in this study between June 2013 to September 2016. The participants included 69 normal young women aged 20–48 years, 28 patients with primary PMOP aged 45–76 years, and 24 age-matched women aged 46–73 years, from Xiangya Hospital of Central South University. Patients with PMOP had no history of taking medication to treat osteoporosis. Women with diseases, such as hyperparathyroidism, or treatment known to affect bone metabolism, were excluded from the young normal women group and control group. The spine and femoral neck T-scores were measured for each woman by a dual energy X-ray absorptiometry scanner. Osteoporosis was diagnosed by dual energy X-ray according to the WHO criteria.23 This study was approved by the Ethics Committee of Xiangya Hospital. Each participant who was enrolled in this study was informed of the project and had signed a written consent form.
Blood sample collection and biochemical analysis
For each participant, 5 ml of venous whole blood was drawn between 6 am to 8 am during the mid-menstrual cycle day. Whole blood samples were immediately centrifuged for 10 min at 3000 rpm to separate plasma and then stored at −80℃ until further analysis. Plasma oestradiol and PDGF-BB levels were measured using commercially available ELISA kits (KB11618 and KB10133, Laibio, Shanghai). The optical density of the samples was measured by using a microplate reader.
Animal model and treatment
Sprague–Dawley rats (female, 200–250 g) were purchased from the Department of Animal Experiments in Xiangya School of Medicine. All experimental protocols were approved by the Center for Laboratory Animal Medicine and Care at Xiangya Hospital. Each Sprague–Dawley rat underwent either sham surgery (sham group, the ovaries were exposed but not removed) or bilateral ovariectomy (OVX) under general anaesthesia with isoflurane. The rats were divided into the following groups (n = 10 in each group): (1) sham, (2) OVX, (3) OVX+ oestradiol (IH, 100 µg/kg/d), and (4) OVX+PDGF-BB (IV, 1 mg/3 d/wk). Oestradiol and PDGF-BB were administered after surgery for 4 weeks. Blood samples of the rats were obtained by cardiac puncture and were then immediately centrifuged for 5 min at 3000 rpm. Plasma oestradiol and PDGF-BB levels were measured using commercially available ELISA kits (KB12534 and KB12611; Laibio, Shanghai). All of the rats were housed in a SPF class room and allowed free access to water and a maintenance diet.
Statistical analysis
The collected data were entered and analysed in the GraphPad Prism 6 computer program (GraphPad Software, Inc.). The results are shown as mean ± SD. For data with a normal distribution, the independent sample t test was used to compare differences between two groups. Correlations between the measured parameters were determined with the Pearson rank correlation coefficient. Significant differences were established at P < 0.05
Results
The participants in this study ranged in age from 20 to 76 years. The mean WBC count, RBC count, PLT count, cholesterol levels, triacylglycerol (TG) levels, high-density lipoprotein cholesterol (HDLc) levels, blood pressure, blood glucose levels, haemoglobin levels, body mass index (BMI), and smoking status (normal young women, postmenopausal controls and postmenopausal osteoporosis) were not different among the three groups (Table 1). Furthermore, these variables were also not significantly different between the postmenopausal control and PMOP groups (data not shown).
Table 1.
General characteristics of the subjects.
| Normal young women | Postmenopausal controls | Postmenopausal osteoporosis | P value | |
|---|---|---|---|---|
| Number | 68 | 25 | 28 | >0.05 |
| Age (y) | 20–48 | 46–73 | 45–76 | >0.05 |
| WBC (109/L) | 6.2 ± 1.9 | 6.4 ± 2.5 | 6.0 ± 2.2 | >0.05 |
| RBC (1012/L) | 4.1 ± 1.3 | 4.2 ± 1.6 | 4.5 ± 2.1 | >0.05 |
| PLT (109/L) | 232 ± 35 | 241 ± 41 | 238 ± 39 | >0.05 |
| Cholesterol (mg/dL) | 200 ± 9.8 | 205 ± 10.5 | 206 ± 9.1 | >0.05 |
| TG (mg/dL) | 161.4 ± 15.7 | 158.4 ± 16.2 | 163.2 ± 17.1 | >0.05 |
| HDLc (mg/dL) | 50.8 ± 6.8 | 52.2 ± 7.4 | 52.1 ± 8.10 | >0.05 |
| Systolic blood pressure (mmHg) | 121 ± 8.1 | 123 ± 9.5 | 126 ± 10.1 | >0.05 |
| Diastolic blood pressure (mmHg) | 75 ± 3.6 | 76 ± 5.7 | 78 ± 7.8 | >0.05 |
| Glucose (mmol/L) | 5.5 ± 0.7 | 5.7 ± 0.8 | 5.8 ± 0.8 | >0.05 |
| Haemoglobin (g/L) | 122.8 ± 13.1 | 117.1 ± 15.3 | 115.9 ± 14.3 | >0.05 |
| BMI (kg/m2) | 24.6 ± 0.8 | 24.1 ± 0.9 | 23.9 ± 0.6 | >0.05 |
| Smoking | ||||
| Former | 2 | 1 | 2 | >0.05 |
| Current | 4 | 1 | 3 | >0.05 |
| Never | 62 | 23 | 23 | >0.05 |
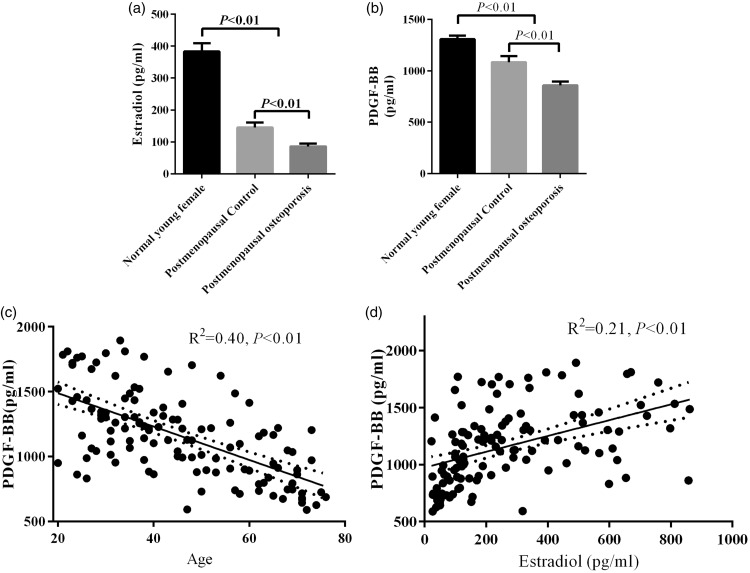
Plasma estradiol and PDGF-BB levels are low in PMOP
Plasma oestradiol levels were significantly higher in the normal young women group than in the postmenopausal control and PMOP groups (P < 0.05, Figure 1(a)). Additionally, plasma oestradiol levels in the postmenopausal control group were significantly higher than those in the PMOP group (P < 0.05, Figure 1(a)). Similar to oestradiol, plasma PDGF-BB levels were significantly lower in postmenopausal women, especially in patients with PMOP, compared with the normal young women group (Figure 1(b)). Pearson correlation analysis showed that PDGF-BB levels were inversely correlated with age (Figure 1(c)). Furthermore, plasma estradiol levels were positively correlated with PDGF-BB levels (Figure 1(d)).
Figure 1.
Plasma levels of estradiol (a) and PDGF-BB (b) in the normal young women, postmenopausal control, and PMOP groups. Pearson correlations analysis of PDGF-BB levels with age (c) and estradiol levels (d).
Plasma estradiol and PDGF-BB levels are reduced in OVX rats, while estradiol treatment can increase plasma PDGF-BB levels
To mimic conditions in PMOP, an OVX rat model of osteoporosis was established. Plasma oestradiol and PDGF-BB levels were significantly lower in OVX rats than SHAM group (Figure 2(a)–(b)). To further investigate the relationship between oestradiol and PDGF-BB, OVX rats were treated with either oestradiol or PDGF-BB. Oestradiol replacement increased plasma PDGF-BB levels, while PDGF-BB systematic treatment did not affect plasma estradiol levels (Figure 2(c)–(d)).
Figure 2.
Plasma levels of estradiol (a) and PDGF-BB (b) were significantly decreased in OVX. Estradiol replacement increased PDGF-BB levels (c). PDGF-BB treatment had no effect on plasma estradiol levels (d).
Discussion
Osteoporosis, which is the most common bone disorder, has become a serious health problem for women, especially postmenopausal women.4 Because of an increased risk of fracture, osteoporotic fracture is associated with morbidity and mortality in postmenopausal women.2,3 Several studies have suggested that PDGF-BB plays a major role in bone homeostasis.24,25 Clinical studies have shown that PDGF-BB is safe and effective in enhancing repair of the skeletal system.26 Therefore, PDGF-BB may be a useful drug for treating osteoporosis.15 However, little is known regarding plasma PDGF-BB levels in PMOP. This study showed that low plasma PDGF-BB levels were associated with oestradiol in PMOP. The OVX rat model demonstrated that normal PDGF-BB levels were maintained by the physiological function of oestradiol.
In the present study, we measured plasma estradiol and PDGF-BB levels in three populations, including normal young women, postmenopausal controls, and PMOP. The three groups of participants were similar regarding the WBC count, RBC count, PLT count, cholesterol levels, TG levels, HDLc levels, blood pressure, blood glucose levels, haemoglobin levels, BMI, and smoking status. Similar to oestradiol, plasma PDGF-BB levels were significantly lower in postmenopausal women, especially in patients with PMOP. These results suggest that PDGF-BB is likely to have an important role in maintaining normal bone density. Low PDGF-BB levels contribute to decreased bone mass and bone mineral density. To further examine the cause-effect between oestradiol and PDGF-BB, Pearson analysis was conducted in our study. We found that plasma PDGF-BB levels were positively correlated with estradiol, while inversely correlated with age. Therefore, as women age, plasma estradiol and PDGF-BB levels gradually decrease in the circulation.
Oestrogen deficiency causes osteoporosis. Glinskii et al.21 found that PDGF-BB levels were significantly decreased in ovariectomized female Yucatan miniature swine, while pulsed oestradiol treatment could prevent downregulation of PDGF-BB. They also showed that oestrogen simulates PDGF-BB production by endothelial cells in a dose-dependent manner. Another research team led by Finlay found that PDGF-BB production was regulated by oestrogen, which was associated with TSC2 gene expression.22 Furthermore, Xie et al. found that in the OVX mouse, bone marrow levels of PDGF-BB, which was partly produced by preosteoclasts, were significantly decreased. This occurred because an increase in mature bone resorption by osteoclasts reduced preosteoclasts and their secretion of PDGF-BB in OVX mice. Therefore, PDGF-BB is likely mediated by oestrogen in bone metabolism. Consequently, we hypothesized that decreased oestrogen results in a reduction in PDGF-BB, which eventually causes bone mass loss in postmenopausal women. We used an OVX rat model because of a lack of patients who had undergone oophorectomy. We found that oestradiol replacement increased plasma PDGF-BB levels, while PDGF-BB systematic treatment had no effect on plasma oestradiol levels. Taken together, these results suggest that plasma PDGF-BB levels are maintained by oestrogen in normal young women and play a major role in PMOP.
PDGF-BB was initially identified as a constituent of human plasma and was subsequently derived from human platelets.27 As a major mitogen, PDGF-BB can also be produced from other cell types, such as macrophages, endothelial cells, and osteoclasts.20,28,29 More importantly, the cells surrounding bone, such as myoblasts, vascular endothelial cells, smooth muscle cells, and even neurons, generate PDGF-BB.30 Therefore, decreased oestradiol in plasma reduces regulation of these cell types, causing low PDGF-BB levels in postmenopausal women and in PMOP.
There are several limitations in this study. Our study had a sample size of 121 subjects. There were only 25 and 28 participants in the postmenopausal control and PMOP groups, respectively. This small sample size should have been sufficient for our study, but whether the findings would be valid if more participants were included is unclear. Because of the lack of patients who had undergone OVX, we used an animal model to test the cause-effect relationship of oestradiol and PDGF-BB. The concentrations of estradiol and PDGF-BB in bone marrow are critical factors involved in bone metabolism. However, we were unable to measure estradiol and PDGF-BB levels in bone marrow. Therefore, this investigation is just a preliminary study. A study on the mechanism(s) of PDGF-BB in POMP needs to be performed in the future.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This study was supported by the Natural Science Foundation of Hunan Province (2016JJ6165).
References
- 1.Erlik Y, Meldrum DR, Judd HL. Estrogen levels in postmenopausal women with hot flashes. Obstet Gynecol 1982; 59: 403–407. [PubMed] [Google Scholar]
- 2.Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 2010; 17: 25–54; quiz 55-26. [DOI] [PubMed]
- 3.Teng GG, Curtis JR, Saag KG. Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin Exp Rheumatol 2008; 26(5 Suppl 51): S125–S137. [PMC free article] [PubMed] [Google Scholar]
- 4.Melton LJ., 3rd Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 2003; 18: 1139–1141. [DOI] [PubMed] [Google Scholar]
- 5.Mahdavi-Roshan M, Ebrahimi M, Ebrahimi A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin Cases Miner Bone Metab 2015; 12: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambacciani M, Vacca F. Postmenopausal osteoporosis and hormone replacement therapy. Minerva Med 2004; 95: 507–520. [in Italian, English Abstract]. [PubMed] [Google Scholar]
- 7.Stazi AV, Trinti B. Risk of osteoporosis in endocrine disorders and celiac disease. Ann Ist Super Sanita 2007; 43: 430–433. [PubMed] [Google Scholar]
- 8.Tranah GJ, Taylor BC, Lui LY, et al. Genetic variation in candidate osteoporosis genes, bone mineral density, and fracture risk: the study of osteoporotic fractures. Calcif Tissue Int 2008; 83: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei SF, Chen Y, Xiong DH, et al. Ethnic difference in osteoporosis-related phenotypes and its potential underlying genetic determination. J Musculoskelet Neuronal Interact 2006; 6: 36–46. [PubMed] [Google Scholar]
- 10.Jamal SA, Ridout R, Chase C, et al. Bone mineral density testing and osteoporosis education improve lifestyle behaviors in premenopausal women: a prospective study. J Bone Miner Res 1999; 14: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 11.Pearson JA, Burkhart E, Pifalo WB, et al. A lifestyle modification intervention for the treatment of osteoporosis. Am J Health Promot 2005; 20: 28–33. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest 2000; 106: 1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santi D, Madeo B, Carli F, et al. Serum total estradiol, but not testosterone is associated with reduced bone mineral density (BMD) in HIV-infected men: a cross-sectional, observational study. Osteoporos Int 2015; 27: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 14.Ostendorf T, Boor P, van Roeyen CR, et al. Platelet-derived growth factors (PDGFs) in glomerular and tubulointerstitial fibrosis. Kidney Int Suppl (2011) 2014; 4: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham S, Leonidou A, Lester M, et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs 2009; 18: 1633–1654. [DOI] [PubMed] [Google Scholar]
- 16.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol 2005; 76: 2205–2215. [DOI] [PubMed] [Google Scholar]
- 17.Mitlak BH, Finkelman RD, Hill EL, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res 1996; 11: 238–247. [DOI] [PubMed] [Google Scholar]
- 18.Nash TJ, Howlett CR, Martin C, et al. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone 1994; 15: 203–208. [DOI] [PubMed] [Google Scholar]
- 19.Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol 1997; 68: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 20.Xie H, Cui Z, Wang L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 2014; 20: 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glinskii OV, Huxley VH, Glinskii VV, et al. Pulsed estrogen therapy prevents post-OVX porcine dura mater microvascular network weakening via a PDGF-BB-dependent mechanism. PloS one 2013; 8: e82900–e82900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finlay GA, Hunter DS, Walker CL, et al. Regulation of PDGF production and ERK activation by estrogen is associated with TSC2 gene expression. Am J Physiol Cell Physiol 2003; 285: C409–C418. [DOI] [PubMed] [Google Scholar]
- 23.Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014; 25: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horner A, Bord S, Kemp P, et al. Distribution of platelet-derived growth factor (PDGF) A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone 1996; 19: 353–362. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Chen J, Jin D. Platelet-derived growth factor (PDGF)-BB stimulates osteoclastic bone resorption directly: the role of receptor beta. Biochem Biophys Res Commun 1998; 251: 190–194. [DOI] [PubMed] [Google Scholar]
- 26.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res 2011; 29: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 27.Bowen-Pope DF, Raines EW. History of discovery: platelet-derived growth factor. Arterioscler Thromb Vasc Biol 2011; 31: 2397–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaguin M, Fardel O, Lecureur V. AhR-dependent secretion of PDGF-BB by human classically activated macrophages exposed to DEP extracts stimulates lung fibroblast proliferation. Toxicol Appl Pharmacol 2015; 285: 170–178. [DOI] [PubMed] [Google Scholar]
- 29.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22: 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999; 79: 1283–1316. [DOI] [PubMed] [Google Scholar]