Abstract
Objective
To analyze the postoperative recurrence of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 tRCC).
Methods
This retrospective study was approved by the institutional review board and performed in accordance with the ethical standards established by the institution. Demographic, clinical, pathological, and follow-up data were compiled for the study cohort.
Results
During a mean follow-up of 41.3 months (range, 3–104 months), 8 of 34 patients with Xp11.2 tRCC were confirmed to have recurrence. Three of these patients died with poor outcomes due to a vena cava tumor embolus, and one died of distant metastasis 48 months after the initial nephrectomy during which lymph node metastasis was found. Three patients survived after cytoreduction surgery. One patient was diagnosed with lung metastasis 11 months postoperatively.
Conclusions
The TNM classification provides significant prognostic information for Xp11.2 tRCC. A relatively active surveillance algorithm is recommended, and cytoreduction surgery is an effective approach for recurrent Xp11.2 tRCC. Larger studies are required to more extensively investigate the recurrence of these potentially aggressive tumors.
Keywords: RCC, Xp11.2, TFE3, recurrence
Introduction
Renal cell carcinoma (RCC) associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 tRCC) was recognized as a new entity in the 2004 World Health Organization classification of renal tumors.1 The classification of RCC has been updated along with improvements in genetic profiling and technology. Xp11.2 tRCC and t(6;11) RCC were recently included as microphthalmia transcription factor-associated tRCC, a new subgroup of RCC in the International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia.2 Xp11.2 tRCC is characterized by various fusions of the TFE3 gene and predominantly occurs in children and young adults.3 The incidence of Xp11.2 tRCC is considered to be relatively low.4–7 Most reports of Xp11.2 tRCC have been published by pathologists and geneticists. Obtaining clinically relevant data of recurrent Xp11.2 tRCC useful for daily urological practice is difficult and time-consuming. We herein present several recurrent cases of adult Xp11.2 tRCC after surgical therapy with a focus on data that are important for practicing urologists.
Methods
This retrospective study was approved by the institutional review board and performed in accordance with the ethical standards established by the institution. Of 1239 patients with RCC treated from January 2007 to January 2016, 82 patients showed a positive reaction to TFE3 immunohistochemistry (TFE3-IHC), and 34 patients were eventually pathologically confirmed to have Xp11.2 tRCC by TFE3-IHC staining and fluorescence in situ hybridization (FISH) assay8 (Figure 1). IHC staining was performed on formalin-fixed, paraffin-embedded tissue sections with a TFE3 antibody, and a positive result was defined as 2 + to 3 + nuclear TFE3 immunoreactivity in > 10% of cells. Polyclonal break-apart probes for TFE3 gene rearrangement in the Xp11.2 region were utilized on samples from patients who had a positive TFE3-IHC result on formalin-fixed, paraffin-embedded tissue microarray slides.
Figure 1.
Typical histopathology of Xp11.2 tRCC, including abundant eosinophilic cytoplasm, distinct cell borders, and papillary architecture. (a) × 100. (b) × 200. (c) Strong nuclear positivity for TFE3 (×100). (d) TFE3 break-apart FISH assay characterized by separate red and green signals (red and green arrowheads) (×1000).
All patients with Xp11.2 tRCC underwent surgical therapy and systemic adjuvant therapy only in our institution, and none of them received chemotherapy before. All patients were followed up after discharge with computed tomography (CT) or contrasted-enhanced ultrasonography every 3 months until the time of death or loss to follow-up.9 At each follow-up, thoracic, abdominal, and pelvic CT was required for all patients with adequate renal function. For patients with poor renal function, abdominal and pelvic magnetic resonance imaging and thoracic CT were routinely performed. Recurrence was defined as the occurrence of local or distant metastatic disease after attempted curative surgery.
During a mean follow-up of 41.3 months, 8 of 34 patients (23.5%) were diagnosed with recurrence. The demographic, clinical, pathological, and follow-up data were analyzed for the study cohort. The TNM stage was determined based on the 7th American Joint Committee on Cancer (AJCC) staging criteria, 2010.10 The ISUP grading system was used to determine the nuclear grade. In this study, categorical data are presented as proportions and continuous variables are presented as mean (range). GraphPad Prism software version 5.0 (GraphPad Software, La Jolla, CA) was used to generate the survival curve.
Results
Of 1239 patients with RCC, 34 (2.74%) were confirmed to have Xp11.2 tRCC. The overall survival curve is shown in Figure 2 and Figure 3. The estimated 5-year overall survival rate was 86.6%, and the progression-free survival rate was 70.3%.
Figure 2.
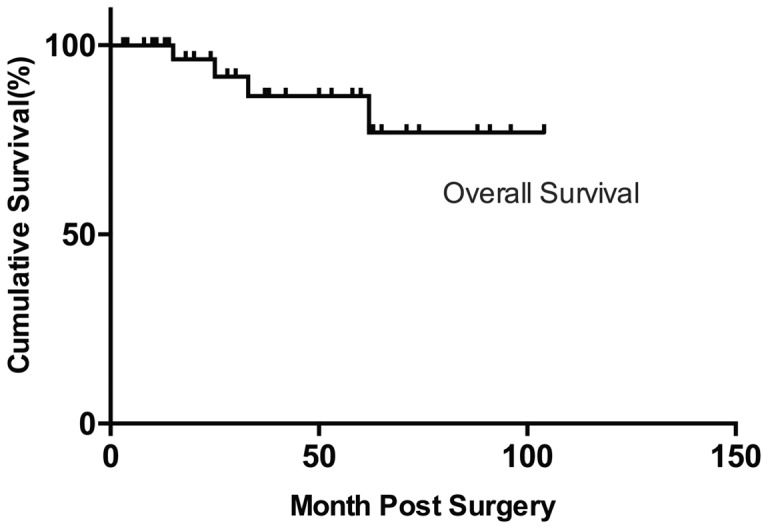
Overall survival of patients with Xp11.2 tRCC.
Figure 3.

Progression-free survival of patients with Xp11.2 tRCC.
The clinical data of all 34 patients with Xp11.2 tRCC are shown in Table 1. Eight of the 34 patients were confirmed to have recurrence during follow-up. These patients included six women and two men with a mean age of 35 years (range, 22–46 years). None of the patients had a history of malignant tumors or chemotherapy. The tumors were located in the right (5/8, 62.5%) and left (3/8, 37.5%) kidneys. No bilateral or multifocal disease was observed. The mean diameter of the tumors was 8.4 ± 3.1 cm (range, 3.9–13.0 cm). All patients with recurrence received surgical treatment, including radical nephrectomy (5/8, 62.5%) and open radical nephrectomy with vena cava tumor embolus resection (3/8, 37.5%).
Table 1.
Clinical data of 34 patients with Xp 11.2 tRCC.
| Case | Age (years)/ Sex/Laterality | Tumor size (cm) | Operation | ACJJ stage | ISUP | Adjuvant therapy | Recurrence time, (months) | Follow-up (months) | Disease status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36/F/R | 8.6 | ORN + VCTER | pT3cN1M0, III | 3 | TMT | 2 | 33 | Dead |
| 2 | 39/F/R | 13 | ORN + VCTER | pT3bN1M0, III | 3 | TMT | 12 | 25 | Dead |
| 3 | 46/F/R | 5.8 | ORN + VCTER | pT3cN0M0, III | 3 | TMT | 7 | 15 | Dead |
| 4 | 22/F/R | 3.9 | LRN | pT1aN1M0, III | 4 | TMT | 48 | 62 | Dead |
| 5 | 27/M/L | 8.5 | LRN | pT3aN0M0, III | 1 | TMT | 16 | 24 | Alive |
| 6 | 45/F/L | 12 | ORN | pT3aN0M0, III | 3 | TMT | 12 | 30 | Alive |
| 7 | 30/F/L | 9.5 | LRN | pT3aN0M0, III | 3 | TMT | 14 | 20 | Alive |
| 8 | 35/M/R | 6 | LRN | pT1bN0M0, I | 2 | TMT | 11 | 50 | Alive |
| 9 | 21/F/R | 4 | LRN | pT1aN0M0, I | 2 | None | – | 60 | Alive |
| 10 | 25/M/R | 7.1 | LRN | pT2aN0M0, II | 3 | IT | – | 18 | Alive |
| 11 | 26/M/L | 3.7 | ORN | pT1aN0M0, I | 2 | IT | – | 74 | Alive |
| 12 | 26/F/R | 5 | LRN | pT1bN0M0, I | 3 | None | – | 96 | Alive |
| 13 | 7/M/L | 3 | ORN | pT1aN0M0, I | 2 | None | – | 104 | Alive |
| 14 | 30/F/R | 3.2 | RA + LNSS | pT1aNxM0, I | 3 | IT | – | 63 | Alive |
| 15 | 7/M/L | 10 | ORN | pT4N1M0, IV | 3 | None | – | 65 | Alive |
| 16 | 25/F/L | 3.8 | LRN | pT1aN0M0, I | 3 | IT | – | 58 | Alive |
| 17 | 24/F/R | 3.9 | LRN | pT1aN0M0, I | 3 | IT | – | 42 | Alive |
| 18 | 51/F/R | 5 | LNSS | pT1bNxM0, I | 2 | IT | – | 53 | Alive |
| 19 | 27/F/R | 6 | LRN | pT1bN0M0, I | 3 | IT | – | 53 | Alive |
| 20 | 26/M/L | 3.7 | LNSS | pT1aN0M0, I | 3 | IT | – | 18 | Alive |
| 21 | 3/F/R | 4 | ORN | pT1aN1M0, III | 3 | None | – | 71 | Alive |
| 22 | 11/F/R | 5.6 | ORN | pT1bN0M0, I | 3 | None | – | 88 | Alive |
| 23 | 40/M/L | 3.9 | LRN | pT1aN0M0, I | 2 | None | – | 37 | Alive |
| 24 | 19/F/L | 5 | LRN | pT1bN0M0, I | 3 | None | – | 24 | Alive |
| 25 | 38/M/R | 3 | LRN | pT1aN0M0, I | 3 | IT | – | 28 | Alive |
| 26 | 29/M/L | 3.5 | LNSS | pT1aN0M0, I | 1 | IT | – | 10 | Alive |
| 27 | 25/F/R | 8.1 | LRN | pT2aN0M0, II | 2 | None | – | 91 | Alive |
| 28 | 22/F/R | 5 | LRN | pT1bN0M0, I | 3 | IT | – | 38 | Alive |
| 29 | 45/M/R | 5.5 | LNSS | pT1bNxM0, I | 3 | IT | – | 14 | Alive |
| 30 | 25/F/R | 3.5 | LRN | pT1aNxM0, I | 3 | IT | – | 13 | Alive |
| 31 | 39/F/R | 4.5 | LNSS | pT1bNxM0, I | 3 | IT | – | 11 | Alive |
| 32 | 64/M/L | 3 | LNSS | pT1aN0M0, I | 3 | IT | – | 3 | Alive |
| 33 | 55/F/R | 3 | LNSS | pT1aN0M0, I | 3 | IT | – | 8 | Alive |
| 34 | 42/M/L | 3.5 | LNSS | pT1aN0M0, I | 4 | IT | – | 4 | Alive |
Abbreviations: M = male; F = female; R = right; L = left; AJCC = American Joint Committee on Cancer; VCTER: vena cava tumor embolus resection; LRN = laparoscopic radical nephrectomy; ORN = open radical nephrectomy; RA = radiofrequency ablation; LNSS = laparoscopic nephron-sparing surgery.
The patients were diagnosed with ISUP nuclear grade 1 (1/8, 12.5%), grade 2 (1/8, 12.5%), grade 3 (5/8, 62.5%), and grade 4 RCC (1/8, 12.5%). Among patients with recurrence, the postoperative AJCC stages were stage III (7/8, 87.5%) and II (1/8, 12.5%). Histopathological examination showed T1 and T3 RCC in two and six patients, respectively. The receptor tyrosine-kinase inhibitor sunitinib or sorafenib was applied as adjuvant molecular-targeted therapy after surgery in all patients with recurrence. Sunitinib was administrated at 50 mg every day on a 4/2 schedule (4 weeks of treatment and 2 weeks of rest) with or without food, and sorafenib was administrated at 400 mg twice a day without food.
The mean time of recurrence was 15.3 months (range, 2–48 months), and the mean overall survival of the patients with recurrence was 32.4 months (range, 15–62 months). Three patients with a vena cava tumor embolus found during the initial nephrectomy were diagnosed with multi-site distant metastasis, including the lungs, bone, liver, and brain, with a mean time of recurrence of 7 months (range, 2–12 months). Additionally, three patients underwent second surgeries to excise recurrent lesions at different metastatic sites, including the abdominal wall, descending colon, and abdominal cavity (Figure 4), with a mean time of recurrence of 14 months (range, 12–16 months). All three of these patients underwent a period of molecular-targeted therapy suspension before and after the surgery, and they were still alive at a mean follow-up time of 24.7 months (range, 20–30 months). During the mean follow-up time of 32.4 months, three patients died with poor outcomes due to a vena cava tumor embolus, one died of distant metastasis 48 months after surgery with lymph node metastasis found during the initial nephrectomy, three were alive after cytoreduction surgeries, and one was alive after receiving molecular-targeted therapy for a diagnosis of lung metastasis 11 months after surgery.
Figure 4.
A 45-year-old woman was diagnosed with recurrent Xp11.2 tRCC 12 months after open radical nephrectomy (red and green crossed lines). (a) Transaxial view of a lesion in the left abdominal cavity (maximum diameter = 5.0 cm, depicted by plain computed tomography). (b) Increased fluorodeoxyglucose uptake in the lesion (maximum standardized uptake value = 3.3, depicted by a fusion positron emission tomography/computed tomography image).
Discussion
TFE3 has been widely used for the diagnosis of Xp11.2 tRCC in daily clinical practice; however, the positive predictive value of TFE3 immunostaining is low. In this study, although 82 patients showed a positive reaction to TFE3-IHC, only 34 patients were eventually pathologically confirmed to have Xp11.2 tRCC by FISH assay. This suggests that TFE3-IHC can be performed proactively as a screening test and that FISH can be performed as a verification test. The sensitivity and specificity can be improved by combination of TFE3-IHC and FISH.
Because of the relatively low prevalence of Xp11.2 tRCC, treatment guidelines for this relatively newly classified tumor are not available. Among relevant articles on tRCC, reports of Xp11.2 tRCC recurrence are uncommon except for a number of studies reporting the poorer prognosis of Xp11.2 tRCC compared with non-Xp11.2 tRCC. Part of the reason for this lack of reports on recurrence is that Xp11.2 tRCC is predominantly diagnosed among children and is rare in adults (very low incidence of 0.2%–5.0%),11–14 and the disease seems to be more advanced and aggressive in adults than in children.15–17 Thus, the details of recurrence and treatment for recurrent Xp11.2 tRCC remain largely unknown. The data presented in this study are inadequate for a full understanding of recurrent Xp11.2 tRCC.
In this study, eight patients developed postoperative recurrence of Xp11.2 tRCC, including three patients with a vena cava tumor embolus, one with node-positive metastasis who also had a relatively long survival time, three who were still alive after the second surgery during which the recurrent mass was excised, and one who was receiving molecular-targeted therapy for a diagnosis of lung metastasis. The postoperative AJCC stage was stage III (identified as locally advanced RCC) in seven patients. This finding is consistent with those of previous studies,18,19 suggesting that the TNM classification provides significant prognostic information for RCC.
Patients 1 to 3 underwent open radical nephrectomy and vena cava tumor embolus resection for removal of a vena cava tumor embolus. An RCC tumor thrombus in the inferior vena cava is a significant adverse prognostic factor,20 indicting a poor outcome and shorter overall survival. Although the lymph node in Patient 4 was diagnosed as positive on postoperative pathological examination, this patient had a relatively favorable outcome with a 62-month survival time. Notably, the diameter of the tumor was 3.9 cm and had an integrated pseudocapsule, and a tumor-negative surgical margin was achieved without extra-pseudocapsule extension. It has been found that extra-pseudocapsule extension is associated with clinical and pathologic tumor dimensions in small RCCs (≤4.0 cm) based on studies of four subcategories of RCC: clear cell RCC, papillary RCC, chromophobe RCC, and oncocytoma.21,22 Cheng et al.23 also reported a relatively high integrity rate of small Xp11.2 RCC. Aoyagi et al.17 reported an adult patient with node-positive Xp11.2 tRCC who had not developed recurrence 4.5 years after two surgical resections for recurrent nodal disease. This patient underwent radical nephrectomy at the first treatment, and the overall survival time is unknown. Small Xp11.2 tRCC may have a favorable survival time with regular physical examinations and timely treatment for recurrent masses. Patients 5 to 7 underwent timely treatment for their recurrent masses. Resection of recurrent masses has been shown to extend overall survival of patients with RCC.24,25 Because of the limited follow-up duration, the survival times of these three patients are unknown, and we will continue to encourage them to participate in regular examinations to detect signs of recurrence.
Because of the lack of consistent guidelines regarding systemic adjuvant therapy for Xp11.2 tRCC, systemic adjuvant therapies for RCC are included in this report for reference. Systemic treatments mainly include chemotherapy, immunotherapy, and targeted molecular therapy.26 Chemotherapy plays a limited role in the treatment of RCC because of its poor sensitivity and significant toxicity.27 The ability of renal cancer to evoke an immune response to immunotherapy has been explored,28 but several randomized trials have failed to show any survival advantages.29–31 The only exception is that low-dose interleukin-2 plus interferon-α has shown efficacy in treating patients with low-grade tumors and an age of < 60 years with only mild toxicity.32 Targeted molecular therapy has shown advantages in treating metastatic RCC with higher sensitivity, with longer progression-free survival and overall survival than achieved with immunotherapy.33 Three classes of drugs with antiangiogenic activity are available: circulating vascular endothelial growth factor inhibitors, multitargeted receptor tyrosine-kinase inhibitors of vascular endothelial growth factor receptor, and inhibitors of mammalian target of rapamycin.26 In the current study, Patients 1, 2, 3, 5, 6, and 7 were diagnosed with an ACJJ stage of higher than T3N0M0 at the time of the first treatment, and Patient 4 was found to have one positive lymph node. Patient 8 was eventually found to have lung metastasis 11 months after the first surgery, although the initial diagnosis was localized RCC. Although no standard adjuvant therapy regimens have been established for advanced RCC, targeted molecular therapy was used for these patients with recurrence considering the potential aggressive nature of this disease. Some reports34,35 have suggested that targeted molecular therapy achieves objective responses and prolonged progression-free survival compared with immune therapy in the treatment of locally advanced and metastatic Xp11.2 tRCC. Although Patients 15 and 21 also had locally advanced RCC, the use of targeted therapy in young patients is usually limited because of its toxicity to growth and the better prognosis of juvenile than adult Xp11.2 tRCC. With technological improvements and further exploration, the signaling pathways involved in Xp11.2 RCC have become more completely understood. Studies have suggested that phosphorylated 4EBP1 may be a critical factor for improved outcomes of Xp11.2 RCC by effectively inhibiting upstream proliferative oncogenic signals.36
The aggressive and often insidious nature of Xp11.2 tRCC underscores the importance of postsurgical surveillance. However, no consensus has been reached regarding a surveillance algorithm for postoperative follow-up of Xp11.2 tRCC. Traditional surveillance algorithms uniformly follow patients without tailored time points, reflecting the likelihood of recurrence.37 The UCLA Integrated Staging System (UISS), which incorporates the 1997 TNM classification with the Eastern Cooperative Oncology Group, combines the performance status and Fuhrman grade into a single prognostic algorithm and categorizes patients into low-, intermediate-, and high-risk groups.38 Klaassen et al.9 proposed classifying Xp11.2 tRCC in adults as high-risk based on its depiction in the UISS, illustrating the potential aggressive and unpredictable nature of the disease. This system encourages history-taking and physical examination, laboratory tests, and chest CT every 6 months for the first 3 years, then yearly for 10 years of follow-up. Additionally, an abdominal CT scan is recommended every 6 months for the first 2 years, yearly thereafter for 2 to 5 years, and every 2 years thereafter for 10 years of follow-up. Because of the further aggressive nature of Xp11.2 tRCC with a vena cava tumor embolus, relatively active surveillance is recommended for early detection of recurrence or metastasis. Notably, an individual surveillance algorithm must take patient comorbidities, patient compliance and mindset, and willingness into account for additional treatment.
This study has some limitations. First, because of the low incidence of this rare disease, we were able to include only a small number of recurrent cases. Second, the follow-up time was relatively short, and the outcomes of Patients 5 to 8 remain unknown. Third, statistical analysis with other subtypes of RCC was lacking because of the small sample size. Further studies of recurrent Xp11.2 tRCC are currently ongoing within our center.
In conclusion, patients with Xp11.2 tRCC with tumor thrombus have a poorer prognosis than patients with node-positive but small tumors. The TNM classification provides significant prognostic information for Xp11.2 tRCC. A relatively active surveillance algorithm is recommended and cytoreduction surgery is effective for the treatment of recurrent Xp11.2 tRCC.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study received financial support from the National Natural Science Foundation of China (81572512, 21377052) and the Natural Science Foundation of Jiangsu Province of China (BK20131281).
References
- 1.Lopez-Beltran A, Scarpelli M, Montironi R, et al. 2004 WHO classification of the renal tumors of the adults. Eur Urol 2006; 49: 798–805. [DOI] [PubMed] [Google Scholar]
- 2.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal Neoplasia. Am. J. Surg. Pathol 2013; 37: 1469–1489. [DOI] [PubMed] [Google Scholar]
- 3.Kmetec A, Jeruc J. Xp 11.2 translocation renal carcinoma in young adults; recently classified distinct subtype. Radiol Oncol 2014; 48: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Yang R, Gan W, et al. Xp11.2 translocation renal cell carcinomas in young adults. BMC Urol 2015; 15: 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan BC, Mackinnon AC, Al-Ahmadie HA. Recent developments in the pathology of renal tumors: morphology and molecular characteristics of select entities. Arch Pathol Lab Med 2009; 133: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Aulmann S, Karanjawala Z, et al. Melanotic Xp11 translocation renal cancers: a distinctive neoplasm with overlapping features of PEComa, carcinoma, and melanoma. Am. J. Surg. Pathol 2009; 33: 609–619. [DOI] [PubMed] [Google Scholar]
- 7.Camparo P, Vasiliu V, Molinie V, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am. J. Surg. Pathol 2008; 32: 656–670. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Yang Y, Gan W, et al. Newly designed break-apart and ASPL-TFE3 dual-fusion FISH Assay are useful in diagnosing Xp11.2 translocation renal cell carcinoma and ASPL-TFE3 renal cell carcinoma: a STARD-compliant article. Medicine (Baltimore) 2015; 94: e873–e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaassen Z, Tatem A, Burnette JO, et al. Adult Xp11 translocation associated renal cell carcinoma: time to recognize. Urology 2012; 80: 965–968. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 11.Sukov WR, Hodge JC, Lohse CM, et al. TFE3 rearrangements in adult renal cell carcinoma: clinical and pathologic features with outcome in a large series of consecutively treated patients. Am. J. Surg. Pathol 2012; 36: 663–670. [DOI] [PubMed] [Google Scholar]
- 12.Komai Y, Fujiwara M, Fujii Y, et al. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry. Clin Cancer Res 2009; 15: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 13.Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am. J. Surg. Pathol 2007; 31: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 14.Geller JI, Argani P, Adeniran A, et al. Translocation renal cell carcinoma: lack of negative impact due to lymph node spread. Cancer 2008; 112: 1607–1616. [DOI] [PubMed] [Google Scholar]
- 15.Asaki HE, Moshero G, Stanton ML, et al. Xp11.2 translocation tumor: a rare cause of gross hematuria. JAAPA 2014; 27: 24–27. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda N, Mikami S, Pan CC, et al. Review of renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions with focus on pathobiological aspect. Histol Histopathol 2012; 27: 133–140. [DOI] [PubMed] [Google Scholar]
- 17.Aoyagi T, Shinohara N, Kubota-Chikai K, et al. Long-term survival in a patient with node-positive adult-onset Xp11.2 translocation renal cell carcinoma. Urol Int 2011; 86: 487–490. [DOI] [PubMed] [Google Scholar]
- 18.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015; 67: 913–924. [DOI] [PubMed] [Google Scholar]
- 19.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 2007; 25: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Salamanca JI, Huang WC, Millan I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011; 59: 120–127. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Feng J, Alvarez H, et al. Critical histologic appraisal of the pseudocapsule of small renal tumors. Virchows Arch 2015; 467: 311–317. [DOI] [PubMed] [Google Scholar]
- 22.Minervini A, di Cristofano C, Lapini A, et al. Histopathologic analysis of peritumoral pseudocapsule and surgical margin status after tumor enucleation for renal cell carcinoma. Eur Urol 2009; 55: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X, He J, Gan W, et al. Pseudocapsule of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion: a clue for tumor enucleation? Int J Clin Exp Pathol 2015; 8: 5403–5410. [PMC free article] [PubMed] [Google Scholar]
- 24.Itano NB, Blute ML, Spotts B, et al. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol 2000; 164: 322–325. [PubMed] [Google Scholar]
- 25.Tanguay S, Pisters LL, Lawrence DD, et al. Therapy of locally recurrent renal cell carcinoma after nephrectomy. J Urol 1996; 155: 26–29. [PubMed] [Google Scholar]
- 26.Buti S, Bersanelli M, Donini M, et al. Systemic adjuvant therapies in renal cell carcinoma. Oncol Rev 2012; 6: e18–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asakura T, Imai A, Ohkubo-Uraoka N, et al. Relationship between expression of drug-resistance factors and drug sensitivity in normal human renal proximal tubular epithelial cells in comparison with renal cell carcinoma. Oncol Rep 2005; 14: 601–607. [PubMed] [Google Scholar]
- 28.Bleumer I, Oosterwijk E, De Mulder P, et al. Immunotherapy for renal cell carcinoma. Eur Urol 2003; 44: 65–75. [DOI] [PubMed] [Google Scholar]
- 29.Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol 2001; 19: 425–431. [DOI] [PubMed] [Google Scholar]
- 30.Messing EM, Manola J, Wilding G, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol 2003; 21: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 31.Hinotsu S, Kawai K, Ozono S, et al. Randomized controlled study of natural interferon alpha as adjuvant treatment for stage II or III renal cell carcinoma. Int J Clin Oncol 2013; 18: 68–74. [DOI] [PubMed] [Google Scholar]
- 32.Passalacqua R, Caminiti C, Buti S, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): A phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother 2014; 37: 440–447. [DOI] [PubMed]
- 33.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 34.Malouf GG, Camparo P, Oudard S, et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): A report from the Juvenile RCC Network. Ann Oncol 2010; 21: 1834–1838. [DOI] [PubMed]
- 35.Kakoki K, Miyata Y, Mochizuki Y, et al. Long-term treatment with sequential molecular targeted therapy for Xp11.2 translocation renal cell carcinoma: a case report and review of the literature. Clin Genitourin Cancer 2016. [DOI] [PubMed] [Google Scholar]
- 36.Qu Y, Zhao R, Wang H, et al. Phosphorylated 4EBP1 is associated with tumor progression and poor prognosis in Xp11.2 translocation renal cell carcinoma. Sci Rep 2016; 6: 23594–23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin AI, Lam JS, Figlin RA, et al. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol 2006; 8: 1–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol 2004; 22: 3316–3322. [DOI] [PubMed] [Google Scholar]




