Abstract
Pneumonia remains the single leading cause of childhood death worldwide. Despite the commercial availability of multiple pneumococcal conjugate vaccines (PCVs), high dosage cost and supply shortages prevent PCV delivery to much of the developing world. The current work presents high-yield pneumococcal conjugates that are immunogenic in animals and suitable for use in human vaccine development. The 13-valent pneumococcal conjugate vaccine (PCV-13) investigated in this research incorporated serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. Pneumococcal polysaccharides (PnPSs) and CRM197 carrier protein were produced and purified in-house, and used to prepare PnPS-CRM conjugates using unique, cyanide-free, in vacuo glycation conjugation methods. In vitro characterization confirmed the generation of higher molecular weight PnPS-CRM conjugates low in free protein. In vivo animal studies were performed to compare PnuVax's PCV-13 to the commercially available PCV-13, Prevnar®13 (Pfizer, USA). A boost dose was provided to all groups post-dose 1 at t = 14 days. Post-dose 2 results at t = 28 days showed that all 13 serotypes in PnuVax's PCV-13 were boostable. Per serotype IgG GMCs demonstrated that PnuVax's PCV-13 is immunogenic for all 13 serotypes, with 10 of the 13 serotypes statistically the same or higher than Prevnar®13 post-dose 2. As a result, the novel polysaccharide-protein conjugates developed in this work are highly promising for use in human PCV development. The in vacuo conjugation technique applied in this work could also be readily adapted to develop many other conjugate vaccines.
Keywords: Pneumonia, Vaccine development, PCV, Pneumococcal conjugate vaccine, Polysaccharide, Carrier protein, In vacuo glycation
1. Introduction
Streptococcus pneumoniae is a major cause of pneumonia, meningitis, sepsis, and otitis media, amongst other diseases [1], [2]. There are approximately 98 serotypes of S. pneumoniae that each produce immunologically distinct polysaccharide (PS) capsules [3]. In the current approach to the manufacture of pneumococcal polysaccharide vaccines (PPVs), capsular pneumococcal PSs (PnPSs) produced by S. pneumoniae during fermentations are purified as antigenic material for use in vaccine formulation. However, although antigenic when introduced to a developed human immune system, PSs induce a T-cell independent response [4] and are poor antigens in newborns and young children [5], [6].
Infants are particularly susceptible to S. pneumoniae infections because they do not mount a useful immune response to capsular PnPSs [4]. Approximately 23% of all childhood deaths in the developing world in 2015 were attributed to S. pneumoniae [7] with pneumonia killing nearly 1 million children globally [8]. To date, pneumonia remains the single leading cause of childhood death under age 5 worldwide [1], [8].
Significant efforts have therefore been made to develop semi-synthetic antigens that are immunogenic in infants, and are prepared by chemically conjugating pathogen-produced PSs to immunogenic but otherwise irrelevant carrier proteins [9]. This conjugated PS approach allows for the immunogenicity of the carrier protein to be extended to the covalently coupled PSs, and ultimately elicit immune responses in infants and young children otherwise not observed [4]. Avery and Godel (1931) demonstrated enhanced antigenicity to S. pneumoniae by chemically conjugating PnPS to horse globulin via a diazotization reaction [10]. The resulting semi-synthetic antigen produced a strong antibody response in rabbits, which normally do not mount a significant immune response to purified PS [5], [10], [11]. However, it was not until the approval of the first pneumococcal conjugate vaccine (PCV) Prevnar® (Wyeth, USA) in 1999, that a semi-synthetic PS-protein antigen was approved for widespread use to prevent S. pneumoniae infection in humans [12]. Following the introduction of 7-valent Prevnar® to the USA, substantial declines in invasive pneumococcal disease (IPD) in children and adults were reported, as compared to pre-Prevnar® years [12], [13], [14].
Serotypic coverage of this vaccine was more recently expanded to include the additional 6 serotypes resulting in the currently licensed 13-valent PCV, Prevnar®13 (Pfizer, USA) [15]. The second currently licensed PCV is Synflorix® (GlaxoSmithKlein, UK), which is 10-valent and uses a different conjugation chemistry, and different quantities and combinations of PnPSs and carrier proteins to generate PnPS-protein conjugates [16].
Prevnar®13 uses reductive amination conjugation chemistry [17], [18], while Synflorix® uses 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) conjugation chemistry [19], [20]. Prevnar®13 incorporates CRM197 as a carrier protein, which is a non-toxic diphtheria toxin mutant resulting from a single amino acid substitution at residue 52 (glycine to glutamine) in the diphtheria toxin Fragment A that causes loss of the enzymatic activity responsible for toxicity [21]. Synflorix® incorporates protein D, tetanus toxoid, or diphtheria toxoid as a carrier protein depending on the serotype [16]. Prevnar®13 and Synflorix® report different ranges of total carrier protein content in their formulated products. However, both products elicit effective immunogenicity in infants indicating that protective semi-synthetic antigens can be formed under a variety of conjugation and preparation conditions.
To date, reductive amination has been the most broadly used method to successfully conjugate PSs to proteins for the purpose of manufacturing conjugate vaccines. PS-protein conjugates for H. influenzae type b [22], N. meningitidis serotypes A, C, W, Y136 [23], [24], and 13 serotypes of S. pneumoniae [25], have all been approved for worldwide use to date [20]. With respect to PCV development, though many other PnPS conjugation chemistries have been used experimentally, only reductive amination (Prevnar®13) and CDAP (Synflorix®) processes have resulted in conjugated antigens that are effectively immunogenic and sufficiently stable to achieve regulatory approval to date, as well as adequately scalable to enable the high volume manufacturing that is required to significantly impact global health. Conjugation by reductive amination typically has lower PS-protein conjugate yields compared to CDAP [20], and requires the addition of sodium cyanoborohydride in excess [26] that must later be removed [27], resulting in vaccines that are relatively expensive to manufacture.
Moreover, despite the commercial availability of multiple safe and highly effective pneumococcal conjugate vaccines, high dosage costs and limitations in supply prevent the delivery of PCV to much of the developing world [28]. The development, approval, and launch of a safe and effective childhood PCV available at high volumes and reduced costs would have wide-reaching impacts on reducing childhood death and improving global health.
Therefore, the long-term goal of this work is to create scalable manufacturing processes to produce effective, low-cost PCVs and other needed conjugate vaccines. The current study presents the development and preliminary animal testing of a set of semi-synthetic antigens manufactured from 13 different polysaccharides of S. pneumoniae, each coupled to a protein by applying the in vacuo conjugation method originally established by Kaplan et al. (2005) [29], [30], [31]. Batches of purified PnPSs and CRM197 produced in-house during this work were explored as compendial-grade starting materials through comprehensive in vitro characterization. PnPS-CRM conjugates for serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F were prepared via in vacuo glycation [29], [30], [31] and used to formulate investigational 13-valent pneumococcal conjugate vaccine (PCV-13). Preliminary in vivo studies were performed using a rabbit model to assess the per serotype immunogenicity of PnuVax's PCV-13 as compared to unconjugated PnPSs and commercially available PCV-13 (Prevnar®13).
2. Material and methods
All materials used in this work were certified as free of animal-derived components, and were United States Pharmacopoeia (USP) grade, or equivalent. Reagents were sourced from Sigma-Aldrich (Canada) unless otherwise specified.
2.1. Pneumococcal seed bank generation
S. pneumoniae isolates for serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F were procured from the US Centers for Disease Control and Prevention (CDC). Isolates were selectively passaged and expanded to yield Master and Working pneumococcal seed banks. Each seed bank was tested for identity and purity by the Quellung (or Neufeld) reaction with type- and factor-specific antisera (SSI, Denmark), supplemented with standard S. pneumoniae taxonomic tests [32].
2.2. Pneumococcal polysaccharides
Pilot-scale fermentations were performed for the 13 serotypes using customized soy peptone/yeast medium recipes. Following culture inactivation, each monovalent PnPS was purified using precipitation and membrane filtration techniques before terminal sterile-filtration (0.22 μm pore size) and refrigeration. PnPS identity was evaluated using Size Exclusion Chromatography - Multi-Angle Laser Light Scattering (SEC-MALLS) and proton Nuclear Magnetic Resonance (1H NMR) spectroscopy (600 Mhz) using commercially available purified PnPSs (SSI, Denmark) as standards. Purified PnPS identity and specificity were immunologically evaluated using Ouchterlony double immunodiffusion with type- and factor-specific pneumococcal antisera (SSI, Denmark). PnPS nucleic acid content was determined by UV absorbance at 280 nm. PnPS total protein content was determined by the Bradford protein assay.
2.3. Carrier protein
Investigational CRM197 carrier protein used to prepare pneumococcal conjugates in this work was prepared in-house by fermentation of the commercially certified non-toxigenic C. diphtheriae strain (ATCC). In-process cultures were tested for purity by Gram staining. Fermentation broth was purified using standard precipitation, ion exchange chromatography, and membrane separation techniques [33] before terminal sterile-filtration (0.22 μm pore size), lyophilization, and storage at −80 °C. All upstream and downstream CRM197 manufacturing steps were performed free of animal-derived components. Purified CRM197 was evaluated for identity and purity by antisera-based (Abcam, USA) Ouchterlony double immunodiffusion testing and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analyses, together with SEC-MALLS analyses.
2.4. Pneumococcal conjugate preparation
For each serotype, PnuVax polysaccharide samples were sonicated and evaluated for molecular weight by SEC-MALLS, before sterile-filtration (0.22 μm pore size) and refrigeration until use in conjugation experiments. In preparation for conjugation, PnPS samples (1–9 mg input scale) were activated using sodium periodate (S398, Fisher Scientific, USA), combined with PnuVax CRM197 carrier protein, dry-glycated in vacuo at high temperature [29], [30], [31], and capped using sodium borohydride (480886, Sigma Aldrich, Canada) to yield PnPS-CRM conjugates. PnuVax PnPS-CRM conjugates were suspended in WFI (Water for Injection), dialyzed (100 kDa), and terminally sterile-filtered (0.22 μm pore size) in preparation for in vitro characterization, investigational vaccine formulation, and in vivo preliminary animal studies.
2.5. Pneumococcal conjugate in vitro characterization
PnuVax PnPS-CRM conjugates were characterized by SEC-MALLS, SDS-PAGE, the Bradford protein assay, and the Anthrone saccharide assay. PnPS-CRM conjugates were then refrigerated until use in formulating investigational PCV-13.
2.6. Investigational pneumococcal conjugate vaccine preparation
PnuVax PnPS-CRM conjugates for serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F were combined in QS to yield final antigen concentrations ranging from 4 to 8 μg PS/mL. NaCl solution was used as diluent, and aluminum phosphate (Adju-Phos®, Brenntag, USA) was used as an investigational adjuvant at concentrations of up to 0.25 mg aluminum/mL. PCV-13 was aseptically hand-filled into 2 mL sterile vials (Wheaton, USA). Unconjugated PnPS vaccine was also prepared using comparable PS antigen concentrations. All vials, seals, and vial contents were visually inspected directly before sterility testing, refrigeration, shipment, and immediate use in animal studies thereafter.
2.7. Assessment in vivo using New Zealand White rabbits
A New Zealand White (NZW) rabbit model was selected in this work to compare the immunogenicity of PnPS-CRM conjugates prepared using in vacuo glycation, to commercially available Prevnar®13 and unconjugated PnPS vaccine (Table 1). All work was performed in accordance with the Canadian Council on Animal Care (CCAC) guidelines for the care and use of laboratory animals, and all animal study protocols for the current work were reviewed and approved by the University Animal Care Committee (UACC) of Queen's University (Kingston, Canada). Immunization experiments were performed using NZW rabbits (Charles River). Rabbits from all groups were examined for clinical signs before and during the 28-day immunization periods. Group sizes ranged across the studies from 3 to 10 rabbits (n = 3–10). For all groups, pre-immunization (t = 0) bleeds were performed before using intramuscular (IM) injection to provide a 0.5 mL dose of test product at t = 0 (dose 1), and a 0.5 mL boost dose at t = 14 days (dose 2). Terminal blood collection was performed by cardiac puncture at t = 28 days. The serum fraction of the unpooled samples from all timepoints was aliquoted and stored at −80 °C until use during immunogenicity characterization.
Table 1.
Animal study design for New Zealand White rabbits.
| Day | 0 | 14 | 28 |
|---|---|---|---|
| Dosing | Dose 1 | Dose 2 (Boost) | None |
| Sample | Pre-Immunization | Post-Dose 1 | Post-Dose 2 |
| Bleed | First Bleed | Second Bleed | Terminal Bleed |
2.8. Multiplexed magnetic microsphere immunoassay
Per serotype IgG antibody responses in NZW rabbits were measured using a multiplexed magnetic microsphere-based immunoassay. Methods were adapted from the literature and the manufacturer's instructions to prepare MagPlex® carboxylated polystyrene microspheres (Luminex, USA) coupled to pneumococcal polysaccharide-poly-l-lysine (PnPS-PLL) conjugates for each of the required 13 serotypes [34], [35], [36], [37], [38]. StabliGuard (SG01, SurModics, USA) was included in the microsphere storage buffer to prevent non-specific binding [39].
In preparation for analyses, WHO human reference serum (NIBSC, UK) and rabbit test serum samples were diluted and pre-absorbed for cross-reacting antibodies by treatment with pneumococcal common cell wall PSs (CWPSs) and 22F PnPS [40] obtained from SSI (Denmark). The human reference serum was serially diluted 3-fold from a 1/15 starting dilution for use in standard curve generation, and study rabbit serum samples were diluted as needed depending on the timepoint and test product under evaluation. To each well of a 96-well microplate, 50 μL of 13-valent microsphere master mix and 50 μL of pre-absorbed diluted serum were added, incubated, and washed as per the manufacturer's instructions [38]. 50 μL of phycoerythrin (PE)-labeled anti-human and PE-labeled anti-rabbit IgG reporter antibodies (Southern Biotech, USA) were added to the human serum and rabbit serum wells, respectively [34], [38]. Finally, the plate was re-incubated, washed, and read using the Bio-Plex® MAGPIX™ Multiplex Reader (Bio-Rad, USA), as per the manufacturer's instructions. Per serotype fluorescence intensity (FI) values were collected and converted to IgG concentrations using Bio-Plex Data Pro™ Manager MP v.1.0 software (Bio-Rad, USA) and the reference serum assigned IgG antibody concentrations as the assay standard [41].
2.9. Statistical analysis
Immunogenicity data were expressed as the log-transformed geometric mean IgG antibody concentrations (GMCs) ± their standard deviations (SDs). GMCs and their SDs were calculated individually for each animal study group, and IgG GMCs were evaluated on a per serotype basis. Statistical comparisons of GMCs between different test products and between different study timepoints were performed by one-way ANOVA with a Tukey's post-hoc comparison of the means in Origin®2016 software (OriginLab, USA). Differences between GMCs were considered statistically significant at p < 0.05.
3. Results
3.1. Pneumococcal seed banks
All S. pneumoniae seed banks were successfully identified as pure stocks. Type- and factor-specific antisera applied to live culture samples for each bank resulted in readily apparent capsular swelling indicating a positive Quellung reaction for serotypic identity. No reaction was observed when treated with all other type- and factor-specific antisera included in the vaccine. Supplemental taxonomic tests confirmed all pneumococcal seed bank cultures were gram-positive in liquid culture, alpha-hemolytic with round mucoidal colonies on agar, catalase-negative, and soluble in bile and/or optochin-sensitive.
3.2. Pneumococcal polysaccharides
Physicochemical characterization results for the 13 PnPSs produced during this work are shown in Table 2. All purified PnPSs met the World Health Organization (WHO) recommendations and European Pharmacopoeia (EP) specifications for identity and purity [27]. Spectral correlation analyses performed to compare the 1H NMR spectra obtained for PnuVax PnPS batches to reference spectra in the literature (references listed in Table 2) confirmed the identity of all 13 PnPSs with demonstrated acceptably low C—PS levels, ranging from 0.9 to 7.5% depending on the serotype. All 1H NMR spectral identity results for the PnPSs produced in-house were independently reviewed and confirmed by Dr. J. Richards (NRC, Ottawa, Canada). Immunological test results by Ouchterlony double immunodiffusion confirmed PnPS immunological identity and specificity for all 13 serotypes, with a positive precipitin band formed only between each PnPS and its specific type- and factor-specific antisera. No reaction was observed for each PnPS when tested against all other type- and factor-specific antisera included in the vaccine. For all 13 serotypes, nucleic acid and protein levels (dry weight basis) met the WHO recommendations of <2% and <3%, respectively [27].
Table 2.
Purified pneumococcal polysaccharide characterization data across multiple batches (n = 3).
| Serotype | NMR correlation | C—PS (%) | Ouchterlony | Nucleic acid (%) | Protein (%) |
|---|---|---|---|---|---|
| 1 | + [42], [43] | <5 | + | <2 | <1 |
| 3 | + [44] | <2 | + | <1 | <1 |
| 4 | + [45] | <7.5 | + | <1 | <1 |
| 5 | + [46] | <7.5 | + | <1 | <1 |
| 6A | + [47], [48] | <5 | + | <1 | <1 |
| 6B | + [48], [49] | <2 | + | <2 | <1 |
| 7F | + [50] | <2 | + | <1 | <1 |
| 9V | + [51], [52] | <2 | + | <1 | <1 |
| 14 | + [53], [54] | <5 | + | <1 | <1 |
| 18C | + [55], [56] | <5 | + | <1 | <1 |
| 19A | + [57] | <5 | + | <1 | <1 |
| 19F | + [58], [59] | <2 | + | <1 | <1 |
| 23F | + [60] | <5 | + | <1 | <1 |
3.3. CRM197 carrier protein
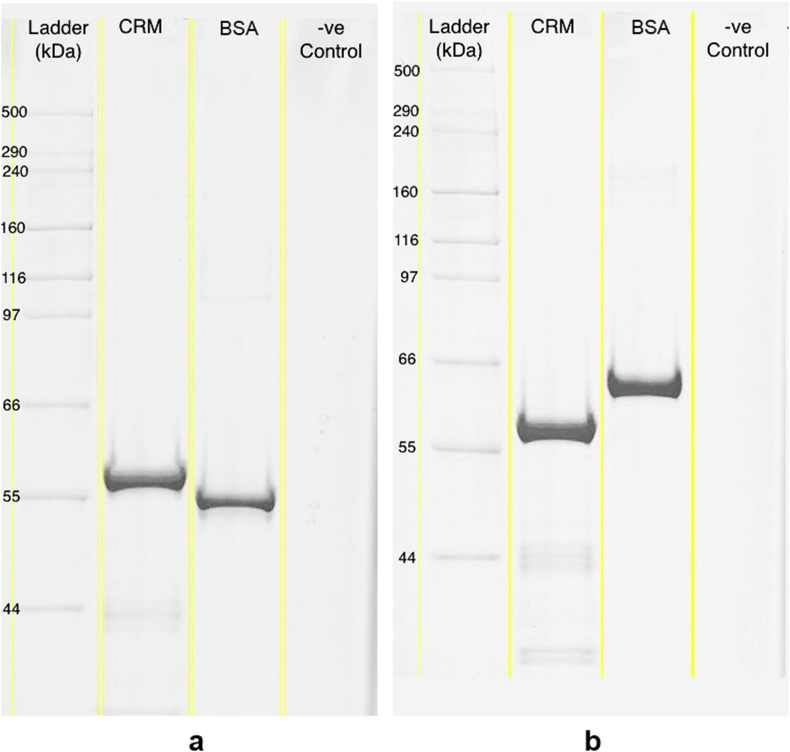
Characterization results for CRM197 carrier protein produced during this work met the WHO recommendations for carrier protein identity and purity [27]. The identity of all characterized batches (n = 3) of CRM197 was confirmed by positive precipitin band formation when loaded against CRM197 immunological antisera. Non-reduced and reduced SDS-PAGE gels are shown in Fig. 1. Gel analyses confirmed an average CRM197 molecular weight of 58.3 ± 1.3 kDa, with the major band well within the EP specification of ±10% of the 58.35 kDa reference value reported in the literature [61]. Results also demonstrated adequate purity across all batches, with CRM197 comprising 92.6± 0.7% of the total protein content. This exceeds the 90% purity specification set forth by the EP. Together, the CRM197 characterization results confirmed carrier protein identity and adequate purity. Therefore, CRM197 produced in-house during this work was released for use in subsequent PnPS conjugation experiments.
Fig. 1.
Representative SDS-PAGE Tris-Acetate pre-cast gel (7% acrylamide, Coomassie R-350 staining) of purified CRM197 under (a) non-reduced conditions and b) reduced conditions. 1.5 μg of total protein were loaded into each respective lane.
3.4. In vitro characterization of pneumococcal conjugates
In vacuo glycation was successfully used to prepare PnPS-CRM conjugates for serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. Characterization results pointed to the generation of wide, higher molecular weight PnPS-CRM conjugate bands on SDS-PAGE gels, with approximated low free protein content across all serotypes (Table 3). Fig. 2 shows a representative SDS-PAGE demonstrating the ladder (Fig. 2a) next to the initial mixture of activated free PS and free protein before conjugation (Fig. 2b) as compared to the lower molecular weight portion of the broad PnPS-CRM conjugate band (Fig. 2c). The SDS-PAGE method for detecting free CRM197 in the PnPS-CRM conjugates did not adequately resolve higher molecular weights, and so the free protein results shown in Table 3 could be an underestimation.
Table 3.
Preliminary physicochemical characterization data for activated pneumococcal polysaccharides and monovalent pneumococcal conjugates prepared using in vacuo glycation.
| Serotype | Activated PS MW (kDa) | Conjugate MW (kDa) | PS:Protein ratio | Free protein (%) | Appearance | Soluble |
|---|---|---|---|---|---|---|
| 1 | >100 | 690 | <10 | <1 | Clear & colorless | + |
| 3 | >100 | 650 | <10 | <1 | Clear & colorless | + |
| 4 | >100 | 320 | <10 | <1 | Clear & colorless | + |
| 5 | <100 | 250 | <10 | <1 | Clear & colorless | + |
| 6A | <100 | 320 | <10 | <1 | Clear & colorless | + |
| 6B | >100 | 200 | <10 | <1 | Clear & colorless | + |
| 7F | <100 | 340 | <10 | <4 | Clear & colorless | + |
| 9V | >100 | 240 | <10 | <1 | Clear & colorless | + |
| 14 | >100 | 300 | <10 | <1 | Clear & colorless | + |
| 18C | >100 | 440 | <10 | <1 | Clear & colorless | + |
| 19A | <100 | 640 | <10 | <1 | Clear & colorless | + |
| 19F | <100 | 700 | <10 | <1 | Clear & colorless | + |
| 23F | <100 | 270 | <10 | <9 | Clear & colorless | + |
Fig. 2.
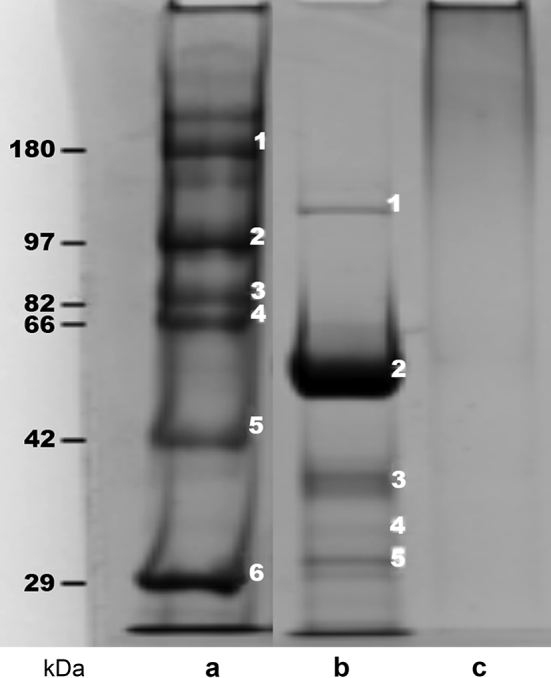
Representative SDS-PAGE gel Tris-Acetate pre-cast gel (7% acrylamide, Coomassie R-350 staining) demonstrating the use of CRM197 to form higher molecular weight PnPS-CRM conjugate using in vacuo glycation; (a) reference ladder in kDa, (b) activated PS and free protein mixture before conjugation with evident free CRM197 band near 58 kDa, and (c) PnPS-CRM197 conjugate, with low to no free protein as evidenced by the absence of bands below the 66 kDa ladder line. 1.5 μg samples were loaded into each respective lane.
Based on the higher molecular weight of the conjugate formed, only the lower molecular weight portion of the conjugate entered the gel shown in Fig. 2. Therefore, SEC-MALLS analyses of the resulting PnPS-CRM conjugates were used to quantify the increase in molecular weight across in vacuo glycation (Table 3). The calculated high preliminary conjugation yields ranged in value from approximately 40 to 85% (PS input basis) across the 13 different serotypes. PnPS-CRM conjugates selected for in vivo evaluation were clear, colorless, and soluble. Conjugate PS:protein ratios in purified conjugates were less than 10.0 for all serotypes. The total protein content of the formulated investigated PCV-13 in this work was comparable to or lower than the total carrier protein content ranges reported for licensed PCVs (Prevnar®13 and Synflorix®). Following formulation, all investigational test products passed visual inspection and sterility tests.
3.5. In vivo safety and tolerability
Findings from daily and post-immunization animal examinations showed that all rabbits gained weight, and no vaccine-related adverse events or other abnormal clinical signs were observed for any of the rabbits included in the study. No animals died before their sacrifice by terminal bleeding. Injections with PnuVax's PCV-13 were reported as comparatively well tolerated relative to Prevnar®13 (A. Winterborn, personal communication, May 27, 2016).
3.6. In vivo immunogenicity
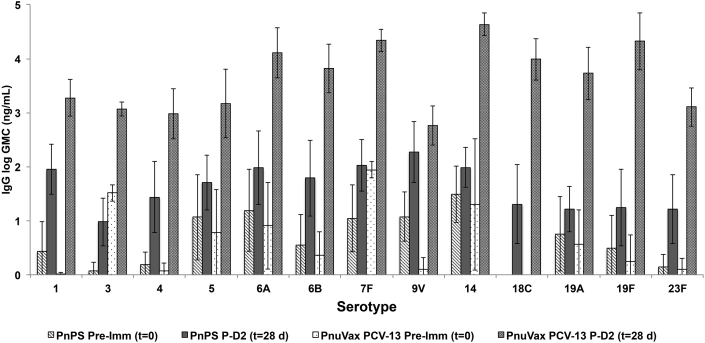
Rabbits that received unconjugated PnPSs as a test product did not demonstrate a boost response. No increase in geometric mean IgG concentration (GMC) was observed at t = 28 days as compared to t = 14 days, despite the boost dose (dose 2) provided at t = 14 days (data not shown). Post-dose 2 (t = 28 days) GMC values for the PnPS vaccine group were the same or lower than the post-dose 1 (t = 14 days) GMC values, with no statistical difference in GMCs observed between t = 14 and t = 28 days.
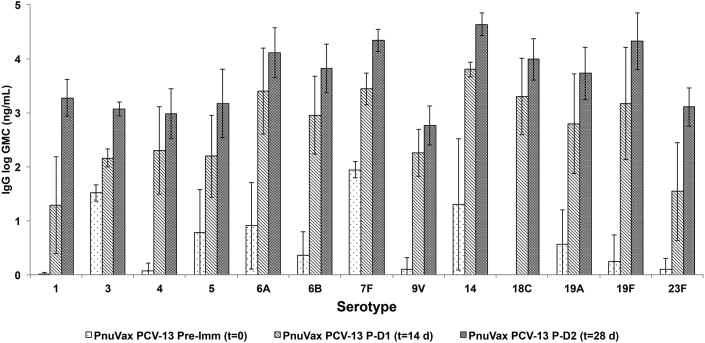
In contrast, a boost response was evident for all rabbits injected with PnuVax's PCV-13 (Fig. 3). Post-dose 2 (t = 28 days) GMC values were greater than the post-dose 1 (t = 14 days) GMC values for all 13 serotypes in PnuVax's PCV-13. All PnuVax PCV-13 per serotype GMCs were statistically higher at post-dose 2 (t = 28 days) than at post-dose 1 (t = 14 days), with the exception of serotype 9V. As compared to the PnPS group, post-dose 2 (t = 28 days) GMC values for PnuVax's PCV-13 were higher for all 13 serotypes, with only serotype 9V not statistically different (Fig. 4). The presence of this boost response across all 13 serotypes confirms the generation of covalently linked PnPS-CRM conjugates using in vacuo glycation for all investigated serotypes.
Fig. 3.
Pre-immunization (t = 0), post-dose 1 (t = 14 days), and post-dose 2 (t = 28 days) serotype-specific IgG concentrations for New Zealand White rabbits immunized with PnuVax PCV-13. Data are expressed as logarithmic GMCs ± SD, with error bars representing one standard deviation from the geometric mean concentration (n = 3–10 NZW rabbits/group).
Fig. 4.
Pre-immunization (t = 0) and post-dose 2 (t = 28 days) serotype-specific IgG concentrations of New Zealand White rabbits immunized with PnPS vaccine and PnuVax's PCV-13. Data are expressed as logarithmic GMCs ± SD, with error bars representing one standard deviation from the geometric mean concentration (n = 3–10 NZW rabbits/group).
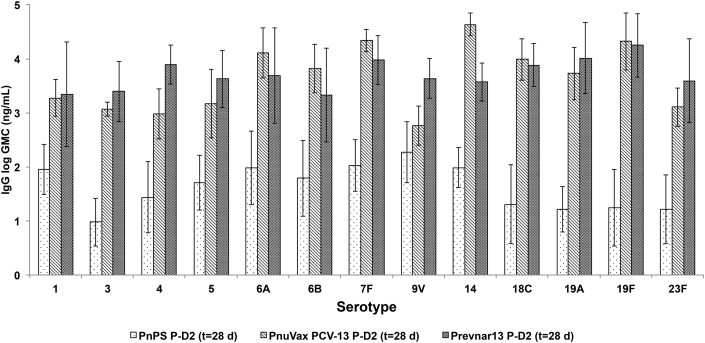
As compared to Prevnar®13, the post-dose 2 (28-day) per serotype GMCs for PnuVax's PnPS-CRM conjugates were statistically comparable or higher, with the exception of serotypes 4, 5, and 9V (Fig. 5). PnuVax's PnPS-CRM conjugate for serotype 14 demonstrated a statistically higher GMC than Prevnar®13 at t = 28 days.
Fig. 5.
Post-dose 2 (t = 28 days) serotype-specific IgG concentrations of New Zealand White rabbits immunized with PnuVax PCV-13, Prevnar®13, and unconjugated PnPS vaccine. Data are expressed as logarithmic GMCs ± SD, with error bars representing one standard deviation from the geometric mean concentration (n = 3–10 NZW rabbits/group).
Overall, immunogenicity results clearly demonstrated that PnuVax's PCV-13 was highly immunogenic as well as boostable in New Zealand White (NZW) rabbits.
4. Discussion
Vaccines have a profound and lifelong effect on the immune system. A human conjugate vaccine dose is typically 2–10 μg/person [20], with 3 doses providing life-long immunity to a given pathogen. In the past century, the nearly universal use of purified natural antigens extracted from pathogenic organisms to protect from such lethal or debilitating diseases as tetanus, diphtheria, pertussis, haemophilus b, meningococcal meningitis, and pneumococcal pneumonia, decreased the deaths due to these diseases from high to negligible levels in most of the world. More recently, conjugate vaccines for S. pneumoniae, as well as Haemophilus influenzae b, Neisseria meningitidis, and Salmonella typhi, have all been successfully developed and commercially licensed, further reducing childhood deaths, and in some cases also indicated for use in adults [62].
Despite this significant progress in preventive medicine, the discrepancy between vaccine availability and realized delivery to the developing world remains highly problematic for global health, and a stigma to the vaccine manufacturing industry. This issue could be greatly alleviated by successfully establishing large-scale manufacturing processes that yield high volumes of safe and effective vaccines available at a fraction of the current costs.
As pneumonia is the single leading cause of childhood death globally, using in vacuo conjugation to firstly prepare immunogenic PnPS-protein conjugates was a logical starting point from a vaccine development perspective. The few steps and high yields of the in vacuo approach, combined with sourcing starting materials in-house where possible and consciously avoiding exorbitant profit margins, could together greatly reduce the final cost per dose of this PCV-13 for the developing world.
Based on the planned evaluation of in vacuo PnPS-protein conjugates in human clinical trials, significant efforts were made to produce and characterize the PnPSs and CRM197 according to current Good Manufacturing Practices (cGMPs) appropriate to this pre-clinical stage of development. The adequately low C—PS (teichoic acid) levels achieved for all 13 monovalent PnPSs used in this work were particularly important, as antibodies to teichoic acid are not protective for S. pneumoniae [63]. NMR spectra used to confirm PnPS identification and composition, were comparable for all 13 serotypes to the 1H NMR reference spectra established in the literature (J. Richards, personal communication, January 14, 2016). By incorporating a robust immunological PnPS identity assay that employed both type- and factor-specific antisera, purified PnPs from both different types and sub-types could be successfully identified from one another (for example, serotypes 19A and 19F). CRM197 characterization testing was also included in this work to confirm identity and purity. All batches of monovalent PnPSs and CRM197 used in this work met the required PnPS and CRM197 standards [27], rendering them suitable for use in pre-clinical PCV research and development.
Animal-derived components at all process stages in this work were strictly avoided. Soy peptone-based media recipes, which are now well-established, modern, growth substrate alternatives [64], [65], [66], [67], were adapted to perform the fermentations. The careful selection of materials at this early stage was critical, as it will later allow our resulting vaccine to meet all quality standards as well as international specifications for Halal, Kosher, vegetarian, or vegan certification(s). This may increase acceptability of the vaccine in strongly religious or belief-based groups worldwide, and help achieve the high immunization rates needed to effectively reduce global disease incidence.
Process-wise, all PnPS and CRM production protocols developed during this work are directly scalable for large-scale manufacturing, as they use standard, commercially available unit operations. Conjugation experiments adopted the same long-term mindset of attention to material sourcing and process scalability, to ensure the developed protocols can be readily transferred to large-scale vaccine production under cGMPs. Successful technology transfer is a critical aspect of vaccine development [68], [69]. While many different experimental conjugation approaches have been investigated at the laboratory-scale, the direct scalability of in vacuo conjugation, together with our consideration of cGMP needs from an early stage of development, render the overall approach directly suitable for transfer to large-scale manufacturing.
Many of the advantages of in vacuo glycation are due to its inherent simplicity. The in vacuo conditions serve to thermodynamically drive the glycation reaction forward. This is in direct contrast to reductive amination, which requires the use of toxic reducing reagents such as sodium cyanoborohydride to achieve the same type of covalent PS-protein bonds achieved by in vacuo glycation. Because no toxic reagents are required, purification of conjugates prepared using in vacuo glycation is straightforward. Further, there is no linker molecule required to couple the activated PnPSs to the carrier protein using our method. The result is a few-step process that occurs readily. Incomplete glycation is avoided under in vacuo conditions, meaning that theoretically 100% of the costly carrier protein is utilized and industrially advantageous yields can be achieved. This was exemplified in the current study by the >40% yields (PS input basis) estimated by SEC-MALLS for our purified PnPS-CRM conjugates.
During formulation, the decision to include the same 13 serotypes as those in Prevnar®13 was made to allow for a direct comparator group in the animal studies. However, based on the varying epidemiological serotypic prevalence and strain antibiotic resistance in different global regions [70], [71], [72], [73], [74], it is possible that serotype substitutions and/or additions may hereafter be performed to maximize coverage of S. pneumoniae protection.
In vitro, it is possible to use high levels of PS oxidation to achieve high molecular weight conjugates, yet this often destroys the epitopes needed to elicit an immune response once in vivo. As a result, immunogenicity is not tied to any one physicochemical attribute, and the animal studies performed in this work were critical to evaluate conjugate immunogenicity. Several animal models were considered to mimic the infant human immune system, which does not respond to T-cell independent antigens such as unconjugated PSs [75]. Rabbits were ultimately selected, as mice are known to respond to T-cell independent antigens [5] and so are unsuitable for PCV evaluation.
In vivo results demonstrated that the serotype-specific epitopes were well preserved in our PnPS-CRM conjugates, based on the elevated per serotype GMCs measured for all serotypes at post-dose 2 (28 days) as compared to the unconjugated PnPS group. Further, the boostability demonstrated by our conjugates for all 13 serotypes was considered an important immunological confirmation of the in vacuo conjugation technique, given that no GMC increase was observed at t = 28 days for any of the 13 serotypes in the PnPS group.
The ability for the in vacuo conjugation protocols used in this work to yield PnPS-CRM conjugates for all 13 serotypes that were highly immunogenic, 10 of which were statistically the same as or higher than Prevnar®13, confirmed the success of both the covalent linkage of the PnPSs to the CRM197, as well as the preservation of the required serotype-specific epitopes.
Assay-wise, the magnetic multiplexed microsphere immunoassay in this work drastically reduced overall serum use and labour. The WHO human reference serum is a highly limited, finite resource. Minimizing its use will help extend the WHO reference serum stockpile's lifetime until a new human reference serum must be developed [41]. Using WHO reference serum in this work allowed for quantitative IgG antibody concentrations to be calculated, as opposed to simple titers.
Opsonophagocytic activity (OPA) assays are also often performed to confirm the protective functionality of the generated antibodies [76], [77], [78], [79], [80]. This is particularly relevant in cases where the PnPSs may have elevated levels of C—PS, as C—PS does not confer immunological protection for S. pneumoniae [63]. Based on the low C—PS levels in our PnPSs, together with measuring serotype-specific IgG concentrations, protective functionality is anticipated for all serotypes. Promisingly, precursory PnPS-CRM conjugates prepared by in vacuo glycation during earlier work generated serotype-specific protective antibodies in NZW rabbits, using a multiplexed microsphere OPA assay [81], [82].
Overall, the in vitro and in vivo results from this work support the use of in vacuo glycation to produce lower-cost, highly immunogenic, polysaccharide-protein conjugates suitable for use in human vaccine development. It is possible that a one-pot conjugation approach could later be developed to even further lower the cost of this conjugation process. Together with OPA assaying and additional animal studies, ongoing and upcoming research will focus on increasing the process scale and reproducibility, and performing further assay development and validation, before initiating our planned PCV human clinical trials.
5. Conclusions
In the current study, the application of in vacuo glycation to prepare immunogenic pneumo-conjugates suitable for use in PCV development was investigated for 13 different pneumococcal serotypes. Results from this work clearly show that we have developed a new 13-valent pneumococcal conjugate vaccine that is highly immunogenic in NZW rabbits. Quantification of per serotype GMCs confirmed that the PnuVax investigational PnPS-CRM conjugates prepared during this work induced high per serotype IgG antibody responses for all 13 serotypes included in PnuVax's PCV-13. At 28 days (post-dose 2), PnuVax PnPS-CRM conjugate GMCs were higher than the PnPS vaccine GMCs for all 13 serotypes. Efficacy of the in vacuo conjugation technique was further supported by the boost response demonstrated by our PnPS-CRM conjugates, but not observed for any serotypes with the PnPS vaccine group. Based on these promising immunogenicity results, together with the industrially relevant yields and few process steps required to perform in vacuo glycation, this work presents significant progress toward developing an effective pneumococcal conjugate vaccine. Following additional characterization and optimization, plans are in preparation to evaluate PnuVax's PCV-13 in human clinical trials. Once licensed, this vaccine is expected to measurably reduce global childhood death, through increasing the supply of reduced cost PCV on a globally relevant scale. Moreover, the in vacuo conjugation approach demonstrated in this work is wide reaching, as it could be readily adapted to develop reduced cost versions of other conjugate vaccines currently needed worldwide.
Role of the funding source
The Bill & Melinda Gates Foundation was in no way involved in the study design, in the collection, analysis, or interpretation of the data, or in the writing of this paper.
Potential conflicts of interest
Financial Relationships
-
•
All authors are employees of PnuVax SL Biopharmaceuticals Inc.
-
•
Jonas E Gerson and Donald F Gerson are shareholders of PnuVax Inc.
-
•
This work is funded by the Bill & Melinda Gates Foundation.
Personal Relationships
-
•
Donald F Gerson is the father of Jonas E Gerson and father-in-law of Allison EB Turner, and Allison EB Turner is the spouse of Jonas E Gerson.
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation (Seattle, WA) [grants OPP1103687 & OPP1132049]. The authors are grateful to Dr. S. Berkley for his encouragement to research and develop a low-cost PCV. The authors acknowledge Dr. H. Kaplan et al. for establishing the in vacuo conjugation that was explored in this work, and Dr. M. G. Meadows for overseeing all data review and quality aspects of this work. The authors thank Dr. M. Kole for his technical assistance, Dr. J. Richards of the National Research Council of Canada (NRC) for kindly reviewing the PnPS NMR spectra, Dr. D. W. Cameron of the Ottawa Hospital Research Institute (OHRI) for providing doses of Prevnar®13, and Dr. A. Winterborn, Ms. D. Harrington, and their team at the Queen's University Animal Care Services for their patience and skill in performing the animal studies and serum collections. NMR samples were run by Dr. F. Ni (NRC). The authors also acknowledge the many PnuVax contributors for their sample preparation and characterization performed during this work; B. Badger, A. Bernèche-d’Amours, K. Bishop, H. Bransch, A. Chhoeu, M. Coville, J. Evans, E. Grohmann, T. Hachie, W. Han, E. Henderson, C. Irick, J. Ko, G. Kwak, J. Mersereau, R. Murre, Y. Pan, T. Paradis, P. Parrott, R. Pollowy, G. Promnitz, C. Romanin, T. Seelert, S. Sequeira, E. Shepherdson, E. Skwara, and A. Smith.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.O'Brien K.L., Wolfson L.J., Watt J.P., Henkle E., Deloria-Knoll M., McCall N. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.AlonsoDeVelasco E., Verheul A., Verhoef J., Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austrian R. The pneumococcus at the millennium: not down, not out. J Infect Dis. 1999;179 doi: 10.1086/513841. S338–S41. [DOI] [PubMed] [Google Scholar]
- 4.Stein K.E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165:S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 5.Bishop C., Jennings H. Immunology of polysaccharides. Polysacch. 1982;1:291–330. [Google Scholar]
- 6.Balmer P., Borrow R., Arkwright P.D. The 23-valent pneumococcal polysaccharide vaccine does not provide additional serotype antibody protection in children who have been primed with two doses of heptavalent pneumococcal conjugate vaccine. Vaccine. 2007;25:6321–6325. doi: 10.1016/j.vaccine.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Liu L., Hill K., Oza S., Hogan D., Chu Y., Cousens S. vol. 71. Reproductive, Maternal, Newborn, and Child Health; 2016. (Levels and causes of mortality under age five years). [PubMed] [Google Scholar]
- 8.Kallander K., Burgess D.H., Qazi S.A. Early identification and treatment of pneumonia: a call to action. Lancet Glob Health. 2016;4 doi: 10.1016/S2214-109X(15)00272-7. e12–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantino P., Rappuoli R., Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 10.Goebel W., Avery O. Chemo-immunological studies on conjugated carbohydrate proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of Type III Pneumococcus with foreign proteins. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eby R. Springer; 1995. Pneumococcal conjugate vaccines. Vaccine Design; pp. 695–718. [DOI] [PubMed] [Google Scholar]
- 12.DeStefano F., Pfeifer D., Nohynek H. Safety profile of pneumococcal conjugate vaccines: systematic review of pre-and post-licensure data. Bull World Health Organ. 2008;86 doi: 10.2471/BLT.07.048025. 373–80A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poehling K.A., Talbot T.R., Griffin M.R., Craig A.S., Whitney C.G., Zell E. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. Jama. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 14.Isaacman D.J., Fletcher M.A., Fritzell B., Ciuryla V., Schranz J. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar) Vaccine. 2007;25:2420–2427. doi: 10.1016/j.vaccine.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Sucher A.J., Chahine E.B., Nelson M., Sucher B.J. Prevnar 13, the new 13-valent pneumococcal conjugate vaccine. Ann Pharmacother. 2011;45:1516–1524. doi: 10.1345/aph.1Q347. [DOI] [PubMed] [Google Scholar]
- 16.Croxtall J.D., Keating G.M. Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix™; PhiD-CV) Pediatr Drugs. 2009;11:349–357. doi: 10.2165/11202760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh C. Characterization of saccharide-CRM197 conjugate vaccines. Dev Biol. 1999;103:93–104. [PubMed] [Google Scholar]
- 18.Reinert R.R., Paradiso P., Fritzell B. Advances in pneumococcal vaccines: the 13-valent pneumococcal conjugate vaccine received market authorization in Europe. Expert Rev Vaccines. 2010;9:229–236. doi: 10.1586/erv.10.6. [DOI] [PubMed] [Google Scholar]
- 19.Lees A. Activation of polysaccharides via the cyanylating agent, 1-cyano-4-pyrrolidinopyridinium tetrafluoroborate (CPPT), in the preparation of polysaccharide/protein conjugate vaccines. Google Patents; 2015.
- 20.Frasch C.E. Preparation of bacterial polysaccharide–protein conjugates: analytical and manufacturing challenges. Vaccine. 2009;27:6468–6470. doi: 10.1016/j.vaccine.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Uchida T., Pappenheimer A.M., Greany R. Diphtheria toxin and related proteins I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973;248:3838–3844. [PubMed] [Google Scholar]
- 22.Adams W.G., Deaver K.A., Cochi S.L., Plikaytis B.D., Zell E.R., Broome C.V. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. Jama. 1993;269:221–226. [PubMed] [Google Scholar]
- 23.Pichichero M., Casey J., Blatter M., Rothstein E., Ryall R., Bybel M. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two-to ten-year-old children. Pediatr Infect Dis J. 2005;24:57–62. doi: 10.1097/01.inf.0000148928.10057.86. [DOI] [PubMed] [Google Scholar]
- 24.Girard M.P., Preziosi M.-P., Aguado M.-T., Kieny M.P. A review of vaccine research and development: meningococcal disease. Vaccine. 2006;24:4692–4700. doi: 10.1016/j.vaccine.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Gruber W.C., Scott D.A., Emini E.A. Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann N Y Acad Sci. 2012;1263:15–26. doi: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz B.A., Gray G.R. Proteins containing reductively aminated disaccharides: synthesis and chemical characterization. Arch Biochem Biophys. 1977;181:542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- 27.WHO Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. WHO Tech Rep Ser. 2013:91–151. (No. 977, Annex 3). http://www.who.int/biologicals/vaccines/TRS_977_Annex_3.pdf. [Google Scholar]
- 28.Moffitt K.L., Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011;23:407–413. doi: 10.1016/j.coi.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart N, Kaplan H, King M. In vacuo glycation of proteins. United States Patent and Trademark Office; 2005.
- 30.Kaplan H. Non-aqueous synthesis of polysaccharide-protein conjugates for vaccines. US Patent 9,120,848; 2015.
- 31.Kaplan H, Hefford MA, Simons BL. Zero length cross-linking of proteins and related compounds. US Patent 20,040,260,019; 2004.
- 32.CDC . Centers for Disease Control and Prevention; 2011. Identification and characterization of Streptococcus pneumoniae laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. [Google Scholar]
- 33.Rappuoli R., Perugini M., Marsili I., Fabbiani S. Rapid purification of diphtheria toxin by phenyl sepharose and DEAE-cellulose chromatography. J Chromatogr A. 1983;268:543–548. [Google Scholar]
- 34.Pickering J.W., Hill H.R. Measurement of antibodies to pneumococcal polysaccharides with Luminex xMAP microsphere-based liquid arrays. Carbohydr Microarrays Methods Protoc. 2012:361–375. doi: 10.1007/978-1-61779-373-8_24. [DOI] [PubMed] [Google Scholar]
- 35.Pickering J.W., Martins T.B., Greer R.W., Schroder M.C., Astill M.E., Litwin C.M. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am J Clin Pathol. 2002;117:589–596. doi: 10.1309/lmch-c4q2-vfl9-3t1a. [DOI] [PubMed] [Google Scholar]
- 36.Whaley M.J., Rose C., Martinez J., Laher G., Sammons D.L., Smith J.P. Interlaboratory comparison of three multiplexed bead-based immunoassays for measuring serum antibodies to pneumococcal polysaccharides. Clin Vaccine Immunol. 2010;17:862–869. doi: 10.1128/CVI.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elberse K.E., Tcherniaeva I., Berbers G.A., Schouls L.M. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol. 2010;17:674–682. doi: 10.1128/CVI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angeloni S., Cordes R., Dunbar S., Garcia C., Gibson G., Martin C. vol. 2. 2014. (xMAP cookbook. A collection of methods and protocols for developing multiplex assays with xMAP technology). [Google Scholar]
- 39.Pickering J.W., Larson M.T., Martins T.B., Copple S.S., Hill H.R. Elimination of false-positive results in a luminex assay for pneumococcal antibodies. Clin Vaccine Immunol. 2010;17:185–189. doi: 10.1128/CVI.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Concepcion N.F., Frasch C.E. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–272. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldblatt D., Plikaytis B., Akkoyunlu M., Antonello J., Ashton L., Blake M. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2011;18:1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindberg B., Lindqvist B., Lönngren J., Powell D.A. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr Res. 1980;78:111–117. doi: 10.1016/s0008-6215(00)83664-2. [DOI] [PubMed] [Google Scholar]
- 43.Stroop C.J., Xu Q., Retzlaff M., Abeygunawardana C., Bush C.A. Structural analysis and chemical depolymerization of the capsular polysaccharide of Streptococcus pneumoniae type 1. Carbohydr Res. 2002;337:335–344. doi: 10.1016/s0008-6215(01)00318-4. [DOI] [PubMed] [Google Scholar]
- 44.Lefeber D.J., Gallego R.G., Grün C.H., Proietti D., D'Ascenzi S., Costantino P. Isolation of oligosaccharides from a partial-acid hydrolysate of pneumococcal type 3 polysaccharide for use in conjugate vaccines. Carbohydr Res. 2002;337:819–825. doi: 10.1016/s0008-6215(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 45.Jones C., Currie F., Forster M.J. Nmr and conformational analysis of the capsular polysaccharide from Streptococcus pneumoniae type 4. Carbohydr Res. 1991;221:95–121. doi: 10.1016/0008-6215(91)80051-n. [DOI] [PubMed] [Google Scholar]
- 46.Jansson P.-E., Lindberg B., Lindquist U. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 5. Carbohydr Res. 1985;140:101–110. doi: 10.1016/0008-6215(85)85053-9. [DOI] [PubMed] [Google Scholar]
- 47.Cai P., Moran J., Pavliak V., Deng C., Khoury N., Marcq O. NMR structural analysis of the capsular polysaccharide from Streptococcuspneumoniae serotype 6C. Carbohydr Res. 2012;351:98–107. doi: 10.1016/j.carres.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Kenne L., Lindberg B., Madden J.K. Structural studies of the capsular antigen from Streptococcus pneumoniae type 26. Carbohydr Res. 1979;73:175–182. doi: 10.1016/s0008-6215(00)85487-7. [DOI] [PubMed] [Google Scholar]
- 49.Bubb W.A. NMR spectroscopy in the study of carbohydrates: characterizing the structural complexity. Concepts Magn Reson Part A. 2003;19:1–19. [Google Scholar]
- 50.Moreau M., Richards J.C., Perry M.B., Kniskern P.J. Application of high-resolution NMR spectroscopy to the elucidation of the structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 7F. Carbohydr Res. 1988;182:79–99. doi: 10.1016/0008-6215(88)84093-x. [DOI] [PubMed] [Google Scholar]
- 51.Perry M.B., Daoust V., Carlo D.J. The specific capsular polysaccharide of Streptococcus pneumoniae type 9V. Can J Biochem. 1981;59:524–533. doi: 10.1139/o81-073. [DOI] [PubMed] [Google Scholar]
- 52.Rutherford T.J., Jones C., Davies D.B., Elliott A.C. Location and quantitation of the sites of O-acetylation on the capsular polysaccharide from Streptococcus pneumoniae type 9V by 1 Hn. mr spectroscopy: comparison with type 9A. Carbohydr Res. 1991;218:175–184. doi: 10.1016/0008-6215(91)84096-w. [DOI] [PubMed] [Google Scholar]
- 53.Joosten J.A., Kamerling J.P., Vliegenthart J.F. Chemo-enzymatic synthesis of a tetra-and octasaccharide fragment of the capsular polysaccharide of Streptococcus pneumoniae type 14. Carbohydr Res. 2003;338:2611–2627. doi: 10.1016/s0008-6215(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 54.Lindberg B., Lonngren J., Powell D.A. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr Res. 1977;58:177–186. doi: 10.1016/s0008-6215(00)83413-8. [DOI] [PubMed] [Google Scholar]
- 55.Chang J., Serrano Y., Garrido R., Rodríguez L.M., Pedroso J., Cardoso F. Relevance of O-acetyl and phosphoglycerol groups for the antigenicity of Streptococcus pneumoniae serotype 18C capsular polysaccharide. Vaccine. 2012;30:7090–7096. doi: 10.1016/j.vaccine.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 56.Lugowski C., Jennings H.J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 18C (56) Carbohydr Res. 1984;131:119–129. doi: 10.1016/0008-6215(84)85409-9. [DOI] [PubMed] [Google Scholar]
- 57.Katzenellenbogen E., Jennings H.J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 19A (57) Carbohydr Res. 1983;124:235–245. doi: 10.1016/0008-6215(83)88459-6. [DOI] [PubMed] [Google Scholar]
- 58.Jennings H.J., Rosell K.-G., Carlo D.J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type-19 (19F) Can J Chem. 1980;58:1069–1074. [Google Scholar]
- 59.Sheng S., Cherniak R. Identification of phosphorylation sites in polysaccharides by 1-D1H-31P HMQC experiments. J Carbohydr Chem. 1998;17:317–321. [Google Scholar]
- 60.Richards J.C., Perry M.B. Structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 23F (American type 23) Biochem Cell Biol. 1988;66:758–771. doi: 10.1139/o88-087. [DOI] [PubMed] [Google Scholar]
- 61.Bröker M., Costantino P., DeTora L., McIntosh E.D., Rappuoli R. Biochemical and biological characteristics of cross-reacting material 197 (CRM 197), a non-toxic mutant of diphtheria toxin: use as a conjugation protein in vaccines and other potential clinical applications. Biologicals. 2011;39:195–204. doi: 10.1016/j.biologicals.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Control CfD, Prevention . vol. 61. MMWR Morbidity and Mortality Weekly Report; 2012. Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older; p. 394. [PubMed] [Google Scholar]
- 63.Szu S.C., Schneerson R., Robbins J.B. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986;54:448–455. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demain A.L., Gerson D.F., Fang A. Effective levels of tetanus toxin can be made in a production medium totally lacking both animal (eg, brain heart infusion) and dairy proteins or digests (eg, casein hydrolysates) Vaccine. 2005;23:5420–5423. doi: 10.1016/j.vaccine.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Fang A., Gerson D., Demain A. Menstrum for culture preservation and medium for seed preparation in a tetanus toxin production process containing no animal or dairy products. Lett Appl Microbiol. 2006;43:360–363. doi: 10.1111/j.1472-765X.2006.01984.x. [DOI] [PubMed] [Google Scholar]
- 66.Demain A.L., Gerson D.F., Kole M., Fang A. The role of reduced iron powder in the fermentative production of tetanus toxin. Appl Microbiol Biotechnol. 2006;73:55–59. doi: 10.1007/s00253-006-0450-2. [DOI] [PubMed] [Google Scholar]
- 67.Fang A., Gerson D.F., Demain A.L. Production of Clostridium difficile toxin in a medium totally free of both animal and dairy proteins or digests. Proc Natl Acad Sci. 2009;106:13225–13229. doi: 10.1073/pnas.0906425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerson D.F., Mukherjee B. Manufacturing process development for high-volume, low-cost vaccines. BioProcess Int. 2005;3 [Google Scholar]
- 69.Gerson D.F., Himes V., Hopfer R., Khandke L., Kohn F., Komotar A. Transfer of processes from development to manufacturing. Drug Inf J. 1998;32:19–26. [Google Scholar]
- 70.Kim L., McGee L., Tomczyk S., Beall B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre-and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev. 2016;29:525–552. doi: 10.1128/CMR.00058-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaur R., Casey J.R., Pichichero M.E. Emerging streptococcus pneumoniae Strains colonizing the nasopharynx in children after 13-Valent (PCV13) pneumococcal conjugate vaccination in comparison to the 7-Valent (PCV7) Era, 2006–2015. Pediatr Infect Dis J. 2016;35(8):901–906. doi: 10.1097/INF.0000000000001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porat N., Benisty R., Givon-Lavi N., Trefler R., Dagan R. The impact of pneumococcal conjugate vaccines on carriage of and disease caused by streptococcus pneumoniae serotypes 6C and 6D in southern Israel. Vaccine. 2016;34:2806–2812. doi: 10.1016/j.vaccine.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 73.Earnshaw S.R., McDade C.L., Zanotti G., Farkouh R.A., Strutton D. Cost-effectiveness of 2+ 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect Dis. 2012;12:1. doi: 10.1186/1471-2334-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hausdorff W.P. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. Eur J Pediatr. 2002;161 doi: 10.1007/s00431-002-1066-x. S135–S9. [DOI] [PubMed] [Google Scholar]
- 75.Kuberan B., Linhardt R. Carbohydrate based vaccines. Curr Org Chem. 2000;4:653–677. [Google Scholar]
- 76.Winkelstein J.A. The role of complement in the host's defense against Streptococcus pneumoniae. Rev Infect Dis. 1981;3:289–298. doi: 10.1093/clinids/3.2.289. [DOI] [PubMed] [Google Scholar]
- 77.Song J.Y., Moseley M.A., Burton R.L., Nahm M.H. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013;19:412–425. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose C.E., Romero-Steiner S., Burton R.L., Carlone G.M., Goldblatt D., Nahm M.H. Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera. Clin Vaccine Immunol. 2011;18:135–142. doi: 10.1128/CVI.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleck R., Romero-Steiner S., Nahm M. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol. 2005;12:19–27. doi: 10.1128/CDLI.12.1.19-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee L.H., Frasch C.E., Falk L.A., Klein D.L., Deal C.D. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine. 2003;21:2190–2196. doi: 10.1016/s0264-410x(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 81.Martinez J.E., Clutterbuck E.A., Li H., Romero-Steiner S., Carlone G.M. Evaluation of multiplex flow cytometric opsonophagocytic assays for determination of functional anticapsular antibodies to Streptococcus pneumoniae. Clin Vaccine Immunol. 2006;13:459–466. doi: 10.1128/CVI.13.4.459-466.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romero-Steiner S., Frasch C.E., Carlone G., Fleck R.A., Goldblatt D., Nahm M.H. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]