Abstract
Preventive medicine and food industry have shown an increased interest in the development of natural antioxidants, since those most commonly used synthetic antioxidants may have restricted use in food. This could explain why there is currently much research on the antioxidant properties from natural products such as mushrooms. Many mushrooms have been reported to possess antioxidant properties, which enable them to neutralize free radicals. The oxygen molecule is a free radical, which lead to the generation of the reactive oxygen species and can damage the cells. Cell damage caused by free radicals appears to be a major contributor to aging and degenerative diseases. Mushrooms antioxidant components are found in fruit bodies, mycelium and culture both, which include polysaccharides, tocopherols, phenolics, carotenoids, ergosterol and ascorbic acid among others. Fruit bodies or mycelium can be manipulated to produce active compounds in a relatively short period of time, which represent a significant advantage in antioxidant compounds extraction from mushrooms. Antioxidant compounds may be extracted to be used as functional additives or mushrooms can be incorporated into our food regime, representing an alternative source of food to prevent damage caused by oxidation in the human body.
Keywords: Antioxidant, Fruit body, Mushroom, Mycelium, Reactive oxygen species
Abbreviations: ROS, reactive oxygen species; DPPH•, 1,1-diphenyl-2-picrylhydrazyl; TEAC, Trolox equivalent antioxidant capacity; ABTS•+, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) radical cation; NBT, nitroblue tetrazolium
1. Introduction
1.1. Mushroom
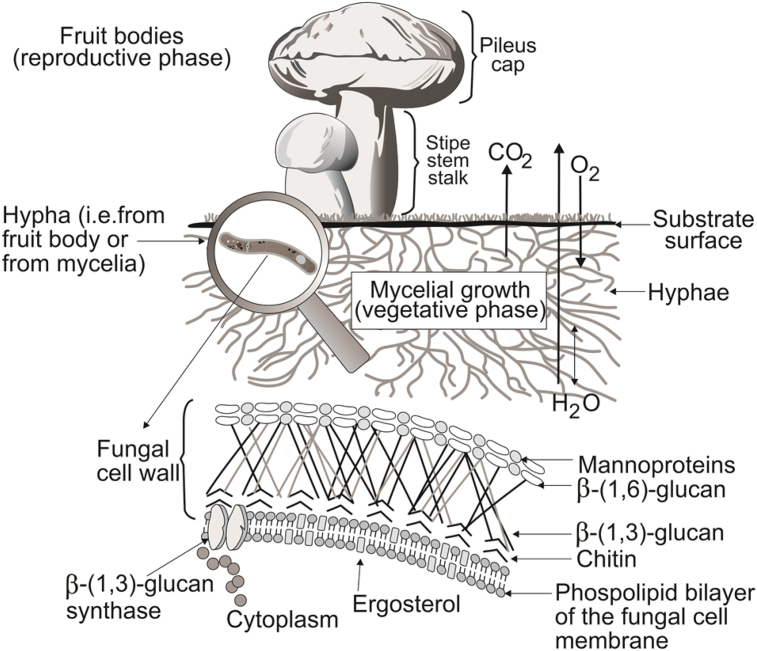
The term mushroom may come from the Latin word mucus (slime) [1]. According to Chang and Miles [2] “mushroom is a macro fungus with a distinctive fruit body, which can be either epigeous (grow above the earth) or hypogeous (grow underground; i.e. truffles) and large enough to be seen with naked eye to be picked by hand”. These organisms are a very large and diversified group of macrofungi (i.e. higher fungi) belonging to basidiomycetes and ascomycetes that can be edible or non-edible. The fungal spores for these two groups of macrofungi are located in a special structure called basidium (for basidiomycetes) or ascus (for ascomycetes). Mushrooms grow mostly above the earth and some of them have an umbrella-shaped fruiting body, where spores are produced (in lamellae, structures on the underside of the pileus or cap). Two phases of growth are distinguishable in these organisms; the reproductive phase (fruit bodies) and the vegetative phase (mycelia or mycelial growth). During substrate invasion, hyphae continually grow and branch to form a network of hyphae (mycelia) and a fruit body grows from underground mycelia by a process called fructification. Mycelial growth is generally coupled with increased enzyme production and respiration. Hyphae absorb digestive products, penetrating the substrate to some extent. The fungal cell wall can be formed by β-D-glucans, proteins, and chitin (Fig. 1). From the ecological point of view, mushroom can be saprotrophs, parasites and mycorrhiza. There are only few parasitic mushrooms. Most of the cultivated mushrooms are saprotrophs. Mycorrhizal mushrooms have a symbiotic relationship with some vegetation, mainly trees, having a relationship of mutual need. Saprotrophs are able to obtain nutrients from dead organic material and parasites obtain their food from living animals and plants, causing harm to the host [3]. Mushrooms have been eaten and appreciated for their exquisite flavor, economic and ecological values, and medicinal properties for many years. In general, mushrooms contain 90% water and 10% dry matter [4]. They have chemical composition which is attractive from the nutritional point of view [5]. Their nutritional value can be compared to those of eggs, milk, and meat [6]. Mushrooms contain vitamins (thiamine, riboflavin, ascorbic acid, ergosterol and niacin) as well as an abundance of essential amino acids. They also have proteins, fats, ash, and glycosides. Volatiles oils, tocopherols, phenolic compounds, flavonoids, carotenoids, folates, organic acids, etc [7], [8]. The total energetic value of mushroom caps is between 250 and 350 cal/kg of fresh mushrooms [4]. Mushrooms can be considered as functional food which provides health benefits in addition to nutritional value [9]. They have been collected in several countries for hundreds of years and technological improvements have made possible their cultivation world-wide.
Fig. 1.
Illustrative representation of fungal cell wall components and stages of mushroom growth.
1.2. Reactive oxygen species and antioxidant system
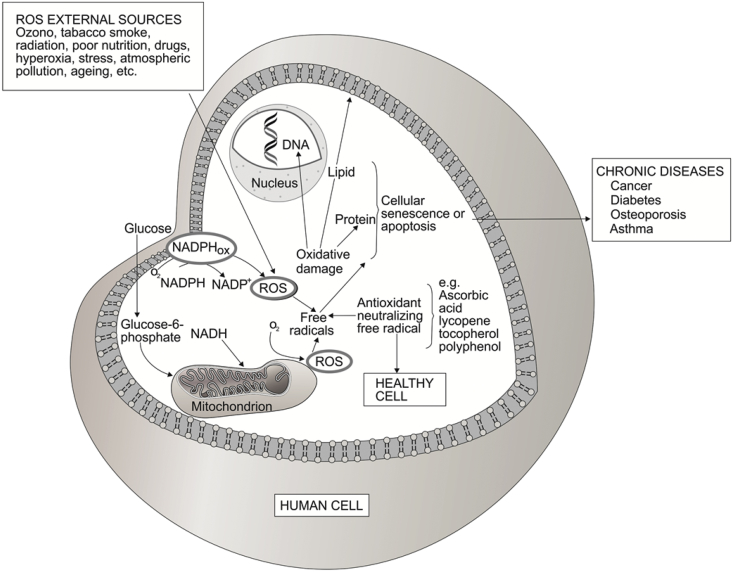
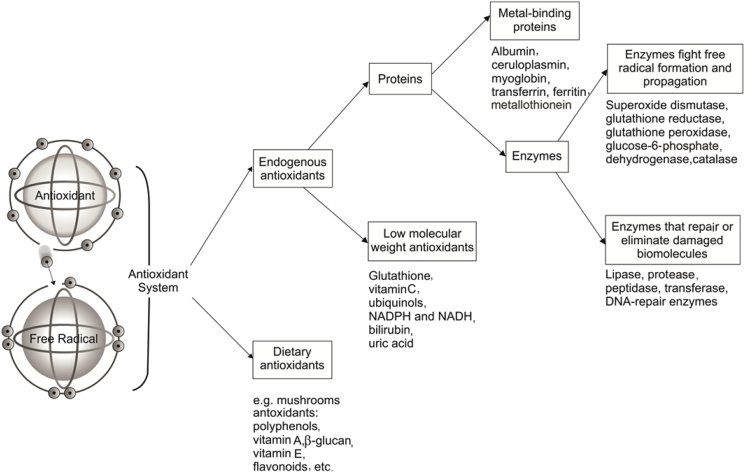
Around 2.45 billions of years ago molecular oxygen was introduced in our environment by the O2-evolving photosynthetic organisms and reactive oxygen species (ROS) has been present ever since in aerobic life [10]. The O2 molecule is a free radical (it has two impaired electrons), which lead to the generation of the ROS and can damage the cells of all organisms. A free radical is a chemical compound that contains one or more unpaired electrons in atomic or molecular orbitals [11]. Reactive molecules such as superoxide anion (O2•−), hydroxyl radical (OH•), hydroxyl ion (OH−), nitric oxide (NO•) and hydrogen peroxide (H2O2) are free radicals and non-radical molecular forms, respectively derived from molecular oxygen. In humans, oxidation is a process that the body uses for normal energy production and immune function. This is part of the process that enables the body to transform nutrients such as carbohydrates, fats, and proteins into energy. During oxidation, ROS are produced at low levels in normal physiological conditions, which are necessary for maintaining normal cell functions, and the endogenous antioxidant defense systems of the body have the capacity to avert any harmful effects. However, ROS are extremely harmful to organisms at high concentrations. When the level of ROS exceeds the defense mechanisms, they can affect many cellular functions by damaging nucleic acids, oxidizing proteins, and causing lipid peroxidation (Fig. 2). ROS can be produced either by external sources (e.g. tobacco smoke, ozone, stress, etc.) or as byproducts during the mitochondrial electron transport of aerobic respiration or by oxidoreductase enzymes and metal catalyzed oxidation [12]. Because they are reactive, radicals search out ways of pairing up their electron, so radicals often attack nearby chemical compounds. These chemical compounds may be involved in important enzyme reactions, may be components of cell walls or may be part of a DNA molecule. If their chemical structure is changed, their function in the cell may be lost and the result can be cellular senescence or apoptosis [12]. Cell damage caused by free radicals appears to be a major contributor to aging and degenerative diseases of aging such as cancer, cardiovascular disease, cataracts, immune system decline, liver diseases, diabetes mellitus, inflammation, renal failure, brain dysfunction and stress among others [11], [13] (Fig. 2). Neutralizing of free radicals or peroxide radicals by an antioxidant agent is important for cell protection against oxidative stress. Then, antioxidants are chemicals which inhibit the oxidation reaction of free radicals by exchanging one of their own electrons with the free radical molecules to stabilize them. These compounds can be endogenous and dietary antioxidants such as polyphenol, vitamin A (e. g. carotenoids), vitamin E (α-tocopherol), β-glucan, etc. Proteins and low molecular weight antioxidants such as ascorbic acid (vitamin C), glutathione, etc, are endogenous antioxidants. Glutathione may be the most important intra-cellular defense against the deleterious effects of ROS. It is a tripeptide (glutamyl-cysteinyl-glycine), which provides an exposed sulfhydryl group as target for attack. Metal-binding proteins and enzymes are antioxidant proteins. Enzymes that can fight free radical formation and propagation are superoxide dismutase, glutathione peroxidase, etc, and enzymes that repair or eliminate damage biomolecules include lipase, peptidase, transferase among others [14], [15] (Fig. 3).
Fig. 2.
Schematic representation of a human cell, which can be damaged by free radicals generated from internal and external sources. Neutralizing of free radicals by an antioxidant agent is important to maintain a healthy cell.
Fig. 3.
Schematic representation of antioxidant system and antioxidant molecules.
2. In vitro methods to assess antioxidant activity
Around 11 in vitro methods have been used for antioxidant evaluation activity in a biological material [16]. The most commonly used methods to measure mushrooms antioxidant activity are those involving chromogen compounds of radical nature that stimulate the reductive oxygen species (e.g. ABTS and DPPH methods). Examples of other methods that are also used are mentioned below.
2.1. DPPH• and ABTS•+ assays
DPPH• assay is based on scavenging of the purple chromogen radical 1,1-diphenyl-2-picrylhydrazyl (DPPH•) by the antioxidants, which produces a decrease in absorbance at 515 nm. When a solution of DPPH• is mixed with a substance that can donate a hydrogen atom, the reduced form of the radical is accompanied by loss of color. The activity is expressed as half inhibitory concentration IC50. It refers to the amount of antioxidant necessary to decrease by 50% the initial DPPH• concentration. Therefore, a lower IC50 means better radical scavenging activity or antioxidant activity [16], [17].
2,2′;-azinobis-(3-ethylbenzothiazoline-6-sulphonate) radical cation (ABTS•+) assay is also known as Trolox equivalent antioxidant capacity (TEAC) assay. The antioxidant reduces ABTS•+ to ABTS and decolorize it. The relative ability of hydrogen-donating antioxidants to scavenge ABTS•+ can be measured spectrophotometrically at 734 nm. Results are expressed by comparison with standard amounts of the synthetic antioxidant trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water-soluble analog of vitamin E that can be used as an antioxidant standard to give rise to the TEAC [16].
2.2. Superoxide anion radical scavenging activity assay
Superoxide anion (O2•−), resulting from the univalent reduction of O2, is considered as being the first step leading to oxidative stress. For measuring O2•− scavenging activity, a suitable system should be selected for generating these radicals. Two systems are used for producing O2•−; the xanthine/xanthine oxidase system and phenazine methosulphate system in the presence of nicotinamide adenine dinucleotide (NADH). The reaction of formation of O2•− is based on the catalysis of xanthine oxidase. In this case, nitroblue tetrazolium (NBT), a probe/target for measuring the O2•− scavenging capacities of samples is used. The color reaction of the O2•- with NBT is detected at 560 nm. Antioxidant activity of samples to inhibit the color to 50% is measured in terms of IC50 [16], [18].
2.3. Reducing power method
This method is based on the principle of increase in the absorbance of the reaction mixtures. Increase in the absorbance indicates the reducing power of the samples (antioxidant activity). In this method, antioxidant compound forms a colored complex with potassium ferricyanide, trichloro acetic acid and ferric chloride, which is measured at 700 nm [19].
2.4. Ferrous ion chelating assay
The interaction of ferrous ion (Fe2+) with hydrogen peroxide in biological systems can lead to formation of highly reactive hydroxyl radicals. Ferrozine is a ferroin compound that can form a complex with a red color by forming chelates with Fe2+. In the presence -of other chelating agents, the complex formation is disrupted, resulting in a decrease of the red color of the ferrozine-Fe2+ complexes. Measurement (spectrophotometrically at 562 nm) of the rate of color reduction therefore allows estimation of the chelating activity of the coexisting chelator. The chelation of ferrous ions is determined using the method of Dinis et al. [20]. EDTA is used as a reference standard for this assay. A lower absorbance value indicates a better ferrous ion-chelating ability of the test sample.
3. Antioxidant compounds from mushrooms
A wide range of mushrooms have been reported to possess antioxidant properties. Extracts from mushrooms contain many components, each of which is unique of a specific mushroom. Antioxidant compounds are found in fruit bodies, mycelium and culture both, which can be phenolics, polysaccharides, tocopherols, flavonoids, carotenoids, glycosides, ergothioneine and ascorbic acid (Table 1).
Table 1.
Antioxidant compounds in mushrooms.
| Mushroom scientific name | Mushroom common names | Phyllum/Edibility | Antioxidant compounds | Biomaterial source | Reference |
|---|---|---|---|---|---|
| Agaricus arvensis | Horse mushroom | B/E | β-Carotene, ascorbic acid, lycopene, phenolic compounds | Fruit bodies extracts | [42], [43], [44] |
| Agaricus bisporus | Common mushroom, button mushroom, white mushroom, champignon mushroom | B/E | Pyrogallol l-ergothioneine, α- and β-glucans Catechin, gallic acid, rutin, caffeic acid |
Fruit bodies and mycelia Fruit bodies hot water extracts |
[17], [33], [35], [44], [45], [46], [47], [48], [49] |
| Agaricus blazei | Almond mushroom, mushroom of the sun | B/E | Benzoic acid, myricetin, quercetin, pyrogallol α- and β-Glucans |
Fruit bodies hot water extract Mycelia extracts |
[17], [35], [50] |
| Agaricus romagnesii | NA | B/NE | Phenolic compounds, β-carotene | Fruit bodies | [43], [44] |
| Agaricus silvaticus | Scaly wood mushroom | B/E | Phenolic compounds, β-carotene | Fruit bodies | [43], [44] |
| Agaricus silvicola | Wood mushroom | B/E | β-Carotene, ascorbic acid, lycopene, phenolic compounds | Fruit bodies extracts | [42], [44], [45] |
| Agrocybe cylindracea | Black poplar mushroom | B/E | α-Tocopherol, β-tocopherol | Fruit bodies | [32], [51] |
| Amanita rubescens | blusher | B/NE | Phenolics compounds, flavonoids | Methanolic extract | [33], [52] |
| Armillaria mellea | Honey mushroom | B/NE | Antioxidant components, ascorbic acid, flavonoids and phenolic compounds | Dried mycelia and mycelia-free broth extracts | [53] |
| Armillaria ostoyae | Humongous fungus | B/NE | Phenolic compounds | Fruit bodies extracts | [33] |
| Auricularia auricula-judae | Jelly mushroom; judas's ear fungus | B/E | Polysaccharides, phenolic compounds | Fruit bodies | [40], [54] |
| Auriculariapolytricha | Cloud ear, jelly ear | B/E | Phenolic compounds | Fruit bodies extracts | [34], [55] |
| Boletus badius | Bay bolete | B/E | β-Carotene, α-tocopherol, phenolic compounds, flavonoids |
Fruit bodies methanolic extracts | [36], [49], [56], [57] |
| Boletus edulis | Porcini, penny bun | B/E | β-Carotene, ascorbic acid, flavonoids, tocopherols | Fruit bodies extracts | [34], [47], [49], [56], [58], [59] |
| Calocybe gambosa | St. George's mushroom | B/E | Phenolic compounds, flavonoids | Fruit bodies methanolic extracts | [47] |
| Cantharellus cibarius | Chanterelle | B/E | Phenolic compounds, flavonoids | Fruit bodies extracts | [34], [45], [47], [49], [52] |
| Cantharellus clavatus | Pig's ears, violet chanterelle | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Chlorophyllum rhacodes | Shaggy parasol | B/NE | Phenolic compounds | Fruit bodies extracts | [33] |
| Clavaria vermicularis | Fairy fingers, white worm coral | B/E | Flavonoids, ascorbic acid | Fruit bodies methanolic extracts | [25] |
| Clitocybe alexandri | Alexander's Funnel | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36], [60] |
| Clitocybe geotropa | Trooping funnel | B/E | Phenolic compounds | Fruit bodies extracts | [61] |
| Coprinopsis atramentaria | Common ink cap, inky cap | B/NE | β-Glucans | Fruit bodies extract | [48] |
| Coprinus comatus | Shaggy ink cap | B/E | β-Carotene, ascorbic acid, lycopene, phenolic compounds | mycelium ethanolic extract | [62] |
| Coriolus versicolor | Polypore mushroom | B/NE | Gallic, p-coumaric, protocatechin, caffeic and vanillc acids | Methanolic extracts | [63] |
| Cortinarius glaucopus | Blue-foot webcap | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Craterellus cornucopioides | Horn of plenty | B/E | Phenolic compounds, flavonoids | Fruit bodies methanolic extracts | [47] |
| Fistulina hepatica | Beefsteak fungus, beefsteak polypore | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Flammulina velutipes | Golden needle mushroom; enokitake (Japanese name) | B/E | Gallic acid, pyrogallol, homogentisic acid, 5-sulfosalicylic acid, protocatechuic acid, quercetin, caffeic acid | Fruit bodies methanolic extracts Mycelium extracts |
[35], [64], [65] |
| Ganoderma applanatum | Artist's bracket, artist's conk, bear bread | B/NE | Gallic, p-coumaric, protocatechin, caffeic and vanillc acids | Methanolic extracts | [63] |
| Ganoderma lucidum | Lingzhi mushroom, Reishi (Japanese name) | B/E | Quercetin, kaempferol, Triterpenoids, polysaccharides |
Fruit bodies Mycelium |
[17], [55], [66] |
| Ganoderma tsugae | Hemlock varnish shelf | B/NE | Polysaccharides | Fruit bodies, mycelia and extracts | [67] |
| Gomphus clavatus | Pig's ears, violet chanterelle | B/E | Ergosterol, phenolic compounds | Fruit bodies extracts | [68] |
| Grifola frondosa | Hen-of-the-woods, ram's head and sheep's head. | B/E | Phenolic compounds, β-1,6 and β-1,3-glucan |
Fruit bodies extract | [69], [70] |
| Helvella crispa | White saddle, elfin saddle, common helvel | A/NE | Phenolic compounds | Fruit bodies extracts | [34] |
| Hericium erinaceus | Lion's mane mushroom, | B/E | Phenolic compounds | Fruit bodies and mycelium extract | [71] |
| Hydnum repandum | Sweet tooth, wood hedgehog, hedgehog mushroom | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [33], [34], [36], [72] |
| Hygrophoropsis aurantiaca | False chanterelle | B/NE | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Hygrophorus marzuolus | March mushroom | B/E | Phenolic compounds, flavonoids | Fruit bodies methanolic extracts | [47] |
| Hypholoma capnoides | NA | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Hypholoma fasciculare | Sulphur tuft, sulfur tuft, | B/NE | Tocopherols, phenolics, flavonoids, ascorbic acid, β-carotene | Fruit bodies extracts | [42] |
| Hypsizygus marmoreus | Brown Beech Mushroom | B/E | Ascorbic acid, β-carotene, tocopherols | Fruit bodies methanolic extracts | [73] |
| Inonotus obliquus | Chaga mushroom | B/E | p-Hydroxybenzoic acid, quercetin, kaempferol | Mycelium; methanolic and water extracts | [35], [55] |
| Laccaria amethystine | Amethyst deceiver | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Laccaria laccata | The deceiver, waxy laccaria | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Lactarius aurantiacus | NA | B/NE | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Lactarius citriolens | NA | B/NE | Free sugars, fatty acids, tocopherols and phenolic acids | Fruit bodies methanolic extracts | [74] |
| Lactarius deliciosus | Saffron milk cap and red pine mushroom | B/E | Phenolic compounds, flavonoids | Fruit bodies extracts | [33], [34], [43], [47], [75] |
| Lactarius piperatus | Peppery milk-cap | B/E | Phenolic compounds, flavonoids | Methanolic extract | [33], [52], [76] |
| Lactarius salmonicolor | Milky agaric | B/E | Phenolic compounds | Methanolic extract | [33], [77] |
| Lactarius sanguifluus | Bloody milk cap | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Lactarius turpis | Ugly milk-cap | B/NE | Free sugars, fatty acids, tocopherols, phenolic acids | Fruit bodies methanolic extracts | [74] |
| Lactarius volemus | The weeping milk cap | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Laetiporus sulphureus | Crab-of-the-woods, sulphur polypore | B/E | Gallic, p-coumaric, protocatechin, caffeic and vanillc acids | Methanolic extracts | [63] |
| Leccinum scabrum | Rough-stemmed bolete | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Leccinum spp | NA | B/NE | Phenolic compounds, β-carotene, lycopene | Aqueous and methanolic extracts of dried fruiting bodies | [49] |
| Lentinula edodes | Forest mushroom, shiitake | B/E | Gallic acid, protocatechuic acid, catechin, tocopherols | Fruit bodies Fruit bodies extracts |
[35], [78], [59], [55], [64] |
| Lentinus squarrolosus | NA | B/E | β-Carotene, lycopene, flavonoids | Fruit bodies extracts | [79] |
| Lenzites betulina | Gilled polypore | B/E | Betulinan A, B Benzoquinone | Fruit bodies extracts | [79] |
| Lepista inversa | NA | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36], [60] |
| Lepista nuda | Wood blewit, blue stalk mushroom | B/E | β-Carotene, α-tocopherol | Fruit bodies methanolic extracts | [56], [75] |
| Lepista sordida | Fairy rings | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Leucopaxillus giganteus | Giant leucopax | B/E | β-carotene, ascorbic acid, lycopene, phenolic compounds | Fruit bodies extracts | [75] |
| Lycoperdon molle | The smooth puffball | B/E | Phospoethanolamida, lysophosphatidyl choline | Fruit bodies extracts | [26], [80] |
| Lycoperdon perlatum | Common puffball | B/E | Flavonoids, ascorbic acid | Fruit bodies methanolic extracts | [45], [25] |
| Macrolepiota mastoidea | NA | B/E | Tocopherols, phenolics, flavonoids, ascorbic acid, β-carotene | Fruit bodies extracts | [75] |
| Macrolepiota procera | The parasol mushroom | B/E | Phenolic compounds | Fruit bodies extracts | [75], [33], [34], [40], [49] |
| Marasmius oreades | Scotch bonnet, fairy ring mushroom | B/E | Flavonoids, ascorbic acid | Fruit bodies methanolic extracts | [25] |
| Meripilus giganteus | Giant polypore, black-staining polypore, | B/E | Gallic, p-coumaric, protocatechin, caffeic and vanillc acids | Methanolic extracts | [63] |
| Mycena rosea | Rosy bonnet | B/NE | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Panus conchatus | Lilac oysterling | B/NE | Phenolic compounds, flavonoids | Fruit bodies | [40] |
| Panus tigrinus | NA | B/NE | Gallic, p-coumaric, protocatechin, caffeic and vanillc acids | Methanolic extracts | [63] |
| Phellinus igniarius | Willow bracket, fire sponge | B/NE | Hispidin | Dried mushrooms | [81] |
| Phellinus linteus | Black hoof mushroom, meshimakobu (Japanese name) | B/NE | β-Tocopherol, protocatechuic acid, gallic acid; pyrogallol; homogentisic acid, α- and β-glucans |
Fruit bodies methanolic and ethanolic extracts Fruit bodies hot water extract |
[35], [17], [82] |
| Pleurotus albidus | NA | B/E | Phenolic compounds | Mycelium extracts | [83] |
| Pleurotus cornucopiae | NA | B/E | Phenolic compounds | Fruit bodies extracts | [61] |
| Pleurotus cystidiosus | Abalone oyster; summer oyster mushroom | B/E | Tocopherols | Fruit bodies methanolic extracts | [64] |
| Pleurotus djamor | Pink oyster mushroom | B/E | Phenolic compounds | Fruit bodies extracts | [34], [84] |
| Pleurotus dryinus | NA | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Pleurotus eous | NA | B/E | Flavonoids | Fruit bodies water extracts | [85] |
| Pleurotus eryngii | King oyster, king trumpet mushroom; | B/E | Gallic acid, protocatechuic acid, naringin, kaempferol, rutin, resveratrol, catechin | Fruit bodies methanolic extracts | [45], [86] |
| Pleurotus ostreatus | Oyster mushroom | B/E | β-Glucans, gallic acid, homogentisic acid, naringin, myricetin, tocopherols, glycoproteins, β-D-Glucan (pleuran) Lectin |
Fruit bodies methanolic extracts | [35], [49], [61], [62], [64], [81], [87] |
| Pleurotus pulmonarius | Indian oyster, italian oyster, phoenix mushroom | B/E | Flavonoids, ascorbic acid | Fruit bodies methanolic extracts | [25], [81] |
| Pleurotus sajor-caju | Grey oyster mushroom | B/E | Phenolic compounds | Fruit bodies water and methanolic extracts | [34], [88] |
| Polyporus squamosus | Dryad's saddle and pheasant's back mushroom | B/NE | β-Carotene, α-tocopherol | Fruit bodies methanolic extracts | [56] |
| Polyporus tenuiculus | NA | B/E | Phenolic compounds | Fruit bodies | [40] |
| Pycnoporus sanguineus | NA | B/NE | Phenolic compounds | Mycelium extracts | [80] |
| Ramaria botrytis | Clustered coral, the pink-tipped coral mushroom | B/NE | Tocopherols, phenolic compounds, ascorbic acid, β-carotene | Fruit bodies extracts | [26] |
| Ramaria formosa | Beautiful clavaria, pink coral fungus | B/NE | Ascorbic acid, flavonoids | Fruit bodies extracts | [25] |
| Russula brevipes | Short-stemmed russula, the stubby brittlegill | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Russula cyanoxantha | Charcoal burner | B/E | Phenolic compounds | Methanolic extract | [52] |
| Russula delica | Milk-white brittlegill | B/E | β-carotene, α-tocopherol phenolic compounds |
Fruit bodies methanolic extracts | [33], [56] |
| Russula integra | The entire russula | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Russula nigricans | Blackening brittlegill | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Russula vesca | Bare-toothed russula | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Russula vinosa | Darkening brittlegill | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Sarcodon imbricatus | Shingled hedgehog, scaly hedgehog | B/E | β-Carotene, ascorbic acid, lycopene, phenolic compounds | Fruit bodies extracts | [42], [75] |
| Schizophyllum commune | Split-gill fungus | B/NE | α- and β-Glucans, phenolic compounds | Fruit bodies extracts | [89] |
| Sparassis crispa | Cauliflower fungus | B/E | Protocatechuic acid, benzoic acid, p-hydroxibenzoic acid | Fruit bodies | [35], [72] |
| Suillus bovinus | Jersey cow mushroom, bovine bolete | B/E | Phenolic compounds, β-carotene | Aqueous and methanolic extracts of dried fruiting bodies | [49] |
| Suillus collinitus | NA | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Suillus luteus | Slippery jack or sticky bun | B/E | Phenolic compounds | Fruit bodies extracts | [33] |
| Suillus mediterraneensis | NA | B/E | Tocopherols, phenolic compounds | Fruit bodies extracts | [36] |
| Suillus variegatus | Velvet bolete | B/E | Phenolic compounds, β-carotene | Aqueous and methanolic extracts of dried fruiting bodies | [49] |
| Termitomyces heimii | Termite nest fungus | B/E | Phenolic compounds | Fruit bodies extracts | [34], [66] |
| Termitomyces microcarpus | NA | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Termitomyces mummiformis | NA | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Termitomyces schimperi | NA | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Termitomyces tylerance | N/A | B/E | Phenolic compounds | Fruit bodies extracts | [34] |
| Tremella fuciformis | White jelly fungus, white wood ear, snow mushroom | B/E | 3,4-dihydroxybenzaldehyde, vanillic acid, caffeic acid, syringic acid and 3,4-dihydroxybenzlacetone | Ethanolic and water extracts | [55] |
| Tricholoma acerbum | Bitter knight | B/E | Tocopherols, phenolic compounds, ascorbic acid, β-carotene | Fruit bodies extracts | [26] |
| Tricholoma equestre | Man on horseback, Yellow knight | B/NE | Phenolic compounds, β-carotene | Aqueous and methanolic extracts of dried fruiting bodies | [49] |
| Verpa conica | Bell morel, the thimble fungus | A/NE | β-Carotene, α-tocopherol | Fruit bodies methanolic extracts | [56] |
| Volvariella volvaceae | Paddy straw mushroom | B/E | Phenolic compounds | Fruit bodies extracts | [66] |
| Xerocomus subtomentosus | Suede bolete, boring brown bolete | B/E | Phenolic compounds, β-carotene | Aqueous and methanolic extracts of dried fruiting bodies | [49] |
B: Basidiomycota, A: Ascomycota, E: Edible, NE: Non-edible, NA: not available.
3.1. Phenolic compounds
Phenolic compounds are aromatic hydroxylated compounds with one or more aromatic rings and one or more hydroxyl groups. They include phenolic acids, flavonoids, hydroxybenzoic acids, hydroxycinnamic acids, lignans, tannins, stilbenes and oxidized polyphenols. Furthermore, some of them stimulate synthesis of endogenous antioxidant molecules in the cell [21], [22]. It has been reported that phenolic compounds exhibit antioxidant activity in biological systems, acting as free radical inhibitors, peroxide decomposers, metal inactivators or oxygen scavengers [23], [24]. Phenolic compounds are present in all the mushrooms. These compounds can be pyrogallol, myricetin, caffeic acid, quercetin and catechin among others (Table 1). Mushrooms contain large amounts of polyphenols at concentrations in the range of 6.25–3.62 mg/mL [25], [26]. It has been reported that grapes and wine contain between 1.0 and 1.8 μg/mL of these compounds [27].
3.2. Polysaccharides
A glucan is a d-glucose homopolysaccharide linked by glycosidic bonds. Glucans are classified as α-or β-glucans according to types of glycosidic bonds. α-Glucans are mainly present for storage of glucose such as starch, glycogen, and dextran. β-Glucan is a non-starch polysaccharide comprised of β-linked d-glucose molecules linked to one another by 1–3 glycosidic chain with 1–6 glycosidic branches. The physicochemical properties of β-glucans differ depending on characteristics of their primary structure, including linkage type, degree of branching, molecular weight, and conformation (e.g., triple helix, single helix, and random coil structures) [28], [29]. β-Glucan is one of the key components of the fungal cell wall (Fig. 1). Hence, antioxidant properties of mushrooms are mainly attributed to β-glucans [13]. Trznadel et al. [30] reported an increase of superoxide dismutase when the commercial yeast β-glucan (Zymosan®) was administrated to chronic uremic patients. Superoxide dismutase is one of the basic antioxidant enzymes we have that fight free radicals (Fig. 3). The length and branches of fungal β-glucan from various mushrooms is widely different [31].
3.3. Ascorbic acid
l-ascorbic acid (vitamin C), being water-soluble, can work inside and outside the cell to combat free radical damage. Vitamin C has been detected in several mushrooms. Among them Boletus edulis [32], B. pseudosulphureus) [33], Lactarius deliciosus [34], Pleurotus ostreatus [35], Suillus luteus [36] among others. Ramesh and Pattar [25] reported that mushrooms contain vitamic C at concentrations in the range of 0.15–0.06 mg/mL. Orange juice contains around 0.37 mg of vitamic C/mL [37].
3.4. Tocopherols
Vitamin E is a common term for eight different compounds; four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols. Among them α-tocopherol is the most biologically active. This fat-soluble compound is embedded within the cell membrane, which has a protective fatty layer of lipids [13]. In this way, α-tocopherol can disable free radicals. Tocopherols have been detected in most mushrooms (Table 1).
3.5. β-carotene and lycopene
β-carotene and lycopene are carotenoids, which are natural pigments present in food (e. g. vegetables, fruits and mushrooms) but are not synthetized by animals. β-carotene is the precursor for the synthesis of vitamin A. Lycopene is an acyclic isomer of β-carotene, and has no vitamin A activity. It is a highly unsaturated, straight chain hydrocarbon containing conjugated and two non-conjugated double bonds, which makes it a potent antioxidant [38], [39]. Hussein et al. [40] reported that the amount of β-carotene and lycopene detected in Lentinus squarrolosus, were in abundance compared to the concentration reported in some vegetables (e.g. carrot, persimmon and tomato) (Table 1).
3.6. Ergosterol
Some mushrooms possess ergosterol (Table 1), which is the precursor of vitamin D. In mushrooms, ergosterol is converted to vitamin D2 (ergocalciferol) when exposed to UV radiation. Vitamin D2 serves as the only available dietary source of vitamin D for those who eat no animal products. Vitamin D is crucial for bone health [41].
4. Concluding remarks
Mushrooms are a natural source of food and antioxidant are becoming important in human health. Interestingly, antioxidant potential in mushrooms is higher than in most vegetables and fruits. The consumption of dietary antioxidant will protect against free radical damage for the prevention of various diseases and aging. Technological developments in cultivation technologies make it possible to produce a wide variety of mushrooms. In addition, research into devising method for cultivation of wild mushrooms is being carried out, so these organisms can be cultivated through artificial methods. A significant advantage in antioxidant compounds extraction from mushrooms is that fruit bodies or mycelium can be manipulated to produce active compounds in a relatively short period of time. Antioxidant compounds may be extracted to be used as functional ingredients or mushrooms can be incorporated to our diet to help the human body to reduce oxidative damage. Health benefits and delicious taste of mushrooms make them a good choice of food to include in our food regime.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Baker T. Origin of the word 'mushroom'. Mycol. 1989;3:88–90. [Google Scholar]
- 2.Chang S.T., Miles P.G. Mushrooms biology - a new discipline. Mycol. 1992;6:64–65. [Google Scholar]
- 3.Cheung P.C.K. Wiley; NJ, USA: 2008. Mushrooms as functional food; p. 280. [Google Scholar]
- 4.Sánchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol. 2010;85(5):1321–1337. doi: 10.1007/s00253-009-2343-7. [DOI] [PubMed] [Google Scholar]
- 5.Dundar A., Acy H., Yildiz A. Yield performance and nutritional contents of three oyster mushroom species cultivated on wheat stalk. Afr J Biotechnol. 2008;7:3497–3501. [Google Scholar]
- 6.Oei P. TOOL Publications; Amsterdam: 2003. Manual on mushroom cultivation: techniques species and opportunities for commercial application in developing countries. [Google Scholar]
- 7.Sánchez C. Modern aspects of mushroom culture technology. Appl Microbiol Biotechnol. 2004;64(6):756–762. doi: 10.1007/s00253-004-1569-7. [DOI] [PubMed] [Google Scholar]
- 8.Patel S., Goyal A. Recent developments in mushrooms as anti–cancer therapeutics: a review. 3 Biotech. 2012;2(1):1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathee S., Rathee D., Rathee D., Kumar V., Rathee P. Mushrooms as therapeutic agents. Braz J Pharmacog. 2012;22(2):459–474. [Google Scholar]
- 10.Kump L.R. The rise of atmospheric oxygen. Nature. 2008;451:277–278. doi: 10.1038/nature06587. [DOI] [PubMed] [Google Scholar]
- 11.Alliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 12.Cederbaum A.I., Lu Y., Wu D. Role of oxidative stress in alcohol–induced liver injury. Arch Toxicol. 2009;83(6):519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 13.Kozarski M., Klaus A., Jakovljevic D., Todorovic N., Vunduk J., Petrović P. Antioxidants of edible mushrooms. Molecules. 2015;20:19489–19525. doi: 10.3390/molecules201019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatua S., Roy T., Acharya K. Antioxidant and free radical scavenging capacity of phenolic extract from Russula laurocerasi. Asian J Pharm Clin Res. 2013;6(4):156–160. [Google Scholar]
- 15.Held P. 2015. An introduction to reactive oxygen species: measurement of ROS in cells.http://www.biotek.com/resources/articles/reactive–oxygen–species.html [Accessed 5 April 2006] [Google Scholar]
- 16.Boligon A.A., Machado M.M., Athayde M.L. Technical evaluation of antioxidant activity. Med Chem. 2014;4:517–522. [Google Scholar]
- 17.Kozarski M., Klaus A., Niksic M., Jakovljevic D., Helsper J.P.F.G. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushroom Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phelinus linteus. Food Chem. 2011;129:1667–1675. [Google Scholar]
- 18.Patel R.M. Comparative antioxidant activity evaluation and HPTLC phyto–chemical fingerprinting of some Indian medicinal plant extracts. CIBTech J Bio Protoc. 2014;3(1):19–34. [Google Scholar]
- 19.Oyaizu M. Studies on products of browning reactions:antioxidant activities of products of browning reaction prepared from glucosamine. J Nutr. 1986;44:307–315. [Google Scholar]
- 20.Dinis T.C.P., Madeira V.M.C., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyradical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 21.Cote J., Caillet S., Doyon G. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr. 2010;50(7):666–679. doi: 10.1080/10408390903044107. [DOI] [PubMed] [Google Scholar]
- 22.D'Archivio M., Filesi C., Vari R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziezak J.D. Antioxidants-The ultimate answer to oxidation. Food Technol. 1986;40(9):94–102. [Google Scholar]
- 24.Yagi K. A rapid method for evaluation of oxidation and antioxidants. Agric Biol Chem. 1970;34(1):142–145. [Google Scholar]
- 25.Ramesh C., Pattar M.G. Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of Western Ghats of Karnataka, India. Phcog Res. 2010;2:107–112. doi: 10.4103/0974-8490.62953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barros L., Venturini B.A., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. J Agric Food Chem. 2008;56:3856–3862. doi: 10.1021/jf8003114. [DOI] [PubMed] [Google Scholar]
- 27.Macheix J.J., Fleuriet A., Billot J. CRC Press, Taylor and Francis Group; FL, USA: 1990. Fruit phenolics. [Google Scholar]
- 28.Tada R., Tanioka A., Iwasawa H., Hatashima K., Shoji Y., Ishibashi K. Structural characterisation and biological activities of a unique type β-D-glucan obtained from Aureobasidium pullulans. Glycoconj J. 2008;25(9):851–861. doi: 10.1007/s10719-008-9147-3. [DOI] [PubMed] [Google Scholar]
- 29.Vetvicka V., Vetvickova J. Physiological effects of different types of β-glucan. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):225–231. doi: 10.5507/bp.2007.038. [DOI] [PubMed] [Google Scholar]
- 30.Trznadel K., Luciak M., Pawlicki L., Kedziora J., Blaszczyk J., Buczyinski A. Superoxide anion generation and lipid peroxidation processes during hemodialysis with reused cuprophan dialyzers. Free Radic Biol Med. 1990;8:429–432. doi: 10.1016/0891-5849(90)90055-n. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Herrera J. second ed. CRC Press, Taylor and Francis Group; Boca Raton, FL, USA: 2012. Fungal cell wall: structure, synthesis, and assembly. [Google Scholar]
- 32.Tsai S.Y., Tsai H.L., Mau J.L. Antioxidant properties of Agaricus blazei, Agrocybe cylindracea, and Boletus edulis. LWT Food Sci Technol. 2007;40:1392–1402. [Google Scholar]
- 33.Keleş A., Koca I., Genҫcelep H. Antioxidant properties of wild edible mushrooms. J Food Process Technol. 2011;2:3–6. [Google Scholar]
- 34.Puttaraju N.G., Venkateshaiah S.U., Dharmesh S.M., Urs S.M.N., Somasundaram R. Antioxidant activity of indigenous mushrooms. J Agric Food Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.Y., Seguin P., Ahn J.K., Kim J.J., Chun S.C., Kim E.H. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 2008;56:7265–7270. doi: 10.1021/jf8008553. [DOI] [PubMed] [Google Scholar]
- 36.Heleno S.A., Barros L., Sousa M.J., Martins A., Ferreira I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010;119:1443–1450. [Google Scholar]
- 37.Chem Fax Comparing vitamin C content in fruit juices. Flinn Scientific, Inc; 2009. http://www.flinnsci.com/Documents/newsPDFs/CF030300.pdf/ [Accessed 30 April 2016] [Google Scholar]
- 38.Rao A.V., Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res. 1999;19:305–323. [Google Scholar]
- 39.Mueller L., Boehm V. Antioxidant activity of β–carotene compounds in different in vitro assays. Molecules. 2011;16:1055–1069. doi: 10.3390/molecules16021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussein J.M., Tibuhwa D.D., Mshandete A.M., Kivaisi A.K. Antioxidant properties of seven wild edible mushrooms from Tanzania. Afr J Food Sci. 2015;9(9):471–479. [Google Scholar]
- 41.Thacher T.D., Clarke B.L. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barros L., Baptista P., Correia D.M., Casal S., Oliveira B., Ferreira I.C.F.R. Fatty acid and sugar compositions and nutritional value of five wild edible mushrooms from northeast Portugal. Food Chem. 2007;105:140–145. [Google Scholar]
- 43.Barros L., Correia D.M., Ferreira I.C.F.R., Baptista P., Santos-Buelga C. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem. 2008;110:1046–1050. doi: 10.1016/j.foodchem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Barros L., Falcāo S., Baptista P., Freire C., Vilas-Boas M., Ferreira I.C.F.R. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–66. [Google Scholar]
- 45.Barros L., Cruz T., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Wild and comercial mushrooms as source of nutrients and nutraceuticals. Food Chem Toxicol. 2008;46:2742–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Ghahremani-Majad H., Dashti F. Chemical composition and antioxidant properties of cultivated button mushroom (Agaricus bisporus) Hort Environ Biotechnol. 2015;56(3):376–382. [Google Scholar]
- 47.Palacios I., Lozano M., Moro C., D'Arrigo M., Rostagno M.A., Martínez J.A. Antioxidant properties of phenolic compound occurring in edible mushrooms. Food Chem. 2011;128:674–678. [Google Scholar]
- 48.Khan A.A., Adilgani Famasoodi, Kousar S., Ahmad M. vol. 1. ICMBMP8; 2014. Antioxidant and functional properties of β-glucan extracted from edible mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus atramentarius; pp. 210–214. (Proceedings of the 8th international conference on mushroom biology and mushroom products). [Google Scholar]
- 49.Robaszkiewicz A., Bartosz G., Lawrynowicz M., Soszyński M. The role of polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J Nutr Metab. 2010:1–9. doi: 10.1155/2010/173274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvajal A.E.S.S., Koehnlein E.A., Soares A.A., Eler G., Nakashima A.T.A., Bracht A. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT Food Sci Technol. 2012;46:493–499. [Google Scholar]
- 51.Lo KM, Cheung PC. Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem89(4):533–539.
- 52.Kosanic M., Rankovic B., Dasic M. Antioxidant and antimicrobial properties of mushrooms. Bulg J Agric Sci. 2013;19(5):1040–1046. [Google Scholar]
- 53.Lung M.Y., Chang Y.C. Antioxidant properties of the edible Basidiomycete Armillaria mellea in submerged cultures. Int J Mol Sci. 2011;12:6367–6384. doi: 10.3390/ijms12106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Wang Z.Y., Zhang Z., Wang X. Purified Auricularia auricular–judae polysaccharide (AAP–a) prevents oxidative stress in an ageing mouse model. Carbohydr Polym. 2011;84:638–648. [Google Scholar]
- 55.Zhang N., Chen H., Zhang Y., Xing L., Li S., Wang X. Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. Int J Food Sci Technol. 2015;50:465–471. [Google Scholar]
- 56.Elmastas M., Isildak O., Turkekul I., Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal. 2007;20:337–345. [Google Scholar]
- 57.Jaworska G., Pogoń K., Skrzypezak A., Bernaś E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J Food Sci Technol. 2015;52(12):7944–7953. doi: 10.1007/s13197-015-1933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vamanu E., Nita S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed Res Int. 2013:1–11. doi: 10.1155/2013/313905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heleno S.A., Ferreira R.C., Antonio A.L., Queiroz M.J.R.P., Barros L., Ferreira I.C.F.R. Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci. 2015;10:26–41. [Google Scholar]
- 60.Vaz J.A., Heleno S.A., Martins A., Aimeida G.M., Vaconcelos M.H., Ferreira I.C.F.R. Wild mushrooms Citocybe alexandii and Lepista inversa: in vitro antioxidant activity and growth inhibition of human tumor cell lines. Food Chem Toxicol. 2010;48:2881–2884. doi: 10.1016/j.fct.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Kolayli S., Sahin H., Aliyazicioglu R., Sesli E. Phenolic components and antioxidant activity of three edible wild mushrooms from Trabzon, Turkey. Chem Nat Comp. 2012;48(1):123–126. [Google Scholar]
- 62.Vamanu E. In vitro antioxidant and antimicrobial activities of two edible mushroom mycelia obtained in the presence of different nitrogen sources. J Med Food. 2013;16(2):155–156. doi: 10.1089/jmf.2012.0030. [DOI] [PubMed] [Google Scholar]
- 63.Karaman M., Jovin E., Malbaša Matavuly M., Popović M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother Res. 2010;24:1473–1481. doi: 10.1002/ptr.2969. [DOI] [PubMed] [Google Scholar]
- 64.Yang J.H., Lin H.C., Mau J.L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. [Google Scholar]
- 65.Zhao C., Zhao K., Liu X., Huang Y.F., Liu B. In vitro antioxidant and antitumor activities of polysaccharides extracted from the mycelia of liquid-cultured Flammulina velutipes. Food Sci Technol Res. 2013;19(4):661–667. [Google Scholar]
- 66.Abdullah N., Ismail S.M., Aminudin N., Shuib A.S., Lau B.F. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. J Evid Based Complement Altern Med. 2012:1–12. doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tseng Y.H., Yang J.H., Mau J.L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008;107:732–738. [Google Scholar]
- 68.Makropoulou M., Aligiannis N., Gonou-Zagou Z., Pratsinis H., Skaltsounis A.L., Fokialakis N. Antioxidant and cytotoxic activity of the wild edible mushroom Gomphus clavatus. J Med Food. 2012;15(2):216–221. doi: 10.1089/jmf.2011.0107. [DOI] [PubMed] [Google Scholar]
- 69.Yeh J.Y., Hsieh L.H., Wu K.T., Tsai C.F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake) Molecules. 2011;16:3197–3201. doi: 10.3390/molecules16043197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin Y.J., Lee S.C. Antioxidant activity and β-glucan contents of hydrothermal extracts from maitake (Griofola frondosa) Food Sci Biotechnol. 2014;23(1):277–282. [Google Scholar]
- 71.Wong K.H., Sabaratnam V., Abdullah N., Kuppusamy U.R., Naidu M. Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.:Fr.) Pers. extracts. Food Technol Biotechnol. 2009;47(1):47–55. [Google Scholar]
- 72.Sulkowska-Ziaja K., Muszyńska B., Szewczyk A. Antioxidant components of selected indigenous edible mushrooms of the obsolete order Aphyllophorales. Rev Iberoam Micol. 2015;32(2):99–102. doi: 10.1016/j.riam.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Lee Y.L., Yen M.T., Mau J.L. Antioxidant properties of various extracts from Hypsizigus marmoreus. Food Chem. 2007;104:1–9. [Google Scholar]
- 74.Vieira V., Barros L., Martins A., Ferreira I.C.F.R. Expanding current knowledge on the chemical composition and antioxidant activity of the genus Lactarius. Molecules. 2014;19:20650–20663. doi: 10.3390/molecules191220650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barros L., Baptista P., Correia D.M., Morais J.S., Ferreira I.C.F.R. Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms. J Agri Food Chem. 2007;55:4781–4788. doi: 10.1021/jf070407o. [DOI] [PubMed] [Google Scholar]
- 76.Barros L., Baptista P., Ferreira I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem Toxicol. 2007;45:1731–1737. doi: 10.1016/j.fct.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Athanasakis G., Aligiannis N., Gonou-Zagou Z., Skaltsounis A.L. Antioxidant properties of the wild edible mushroom Lactarius salmonicolor. J Med Food. 2013;16(8):760–764. doi: 10.1089/jmf.2012.0297. [DOI] [PubMed] [Google Scholar]
- 78.Cheung L.M., Cheung P.C.K. Mushrooms extracts with antioxidant activity against lipid peroxidation. Food Chem. 2005;89:403–409. [Google Scholar]
- 79.Lee I.K., Yun B.S., Cho S.M., Kim W.G., Kim J.P., Ryoo I.J. Betulinans A and B, two benzoquinone compounds from Lenzites betulina. J Nat Prod. 1996;59:1090–1092. doi: 10.1021/np960253z. [DOI] [PubMed] [Google Scholar]
- 80.Singh P., Singh A., D'Souza L.M., Roy U., Singh S.M. Chemical constituents and antioxidant activity of the Arctic mushroom Lycoperdon molle Pers. Polar Res. 2012;31:17329. [Google Scholar]
- 81.Li N., Zhao L., Ng T.B., Wong J.H., Yan Y., Shi Z. Separation and purification of the antioxidant compound hispidin from mushrooms by molecularly imprinted polymer. Appl Microbiol Biotechnol. 2015;99:7569–7577. doi: 10.1007/s00253-015-6499-z. [DOI] [PubMed] [Google Scholar]
- 82.Reis F.S., Barreira J.C.M., Calhelha R.C., van Griensven L.J.I.D., Ćiric A., Glamoclija J. Chemical characterization of the medicinal mushroom Phellinus linteus (Berkeley & Curtis) Teng and contribution of different fractions to its bioactivity. LWT Food Sci Technol. 2014:1–8. [Google Scholar]
- 83.Gambato G., Todescato K., Pav Pavão E.M., Scortegagna A., Fontana R.C., Salvador M. Evaluation of productivity and antioxidant profile of solid-state cultivated macrofungi Pleurotus albidus and Pycnoporus sanguineus. Biores Technol. 2016;207:46–51. doi: 10.1016/j.biortech.2016.01.121. [DOI] [PubMed] [Google Scholar]
- 84.Sánchez J.E., Jimenez–Pérez G., Liedo P. Can consumption of antioxidant rich mushrooms extend longevity?: antioxidant activity of Pleurotus spp. and its effects on Mexican fruit flies' (Anastrepha ludens) longevity. AGE. 2015;37:107. doi: 10.1007/s11357-015-9847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sudha G., Vadivukkasrasi S., Shree R.B.I., Lakshmanan P. Antioxidant activity of various extracts from an edible mushroom Pleurotus eous. Food Sci Biotechnol. 2012;21(3):661–668. [Google Scholar]
- 86.Yaldirim N.C., Turkoglu S., Yaldirim N., Ince O.K. Antioxidant properties of wild edible mushroom Pleurotus eryngii colected from Tunceli province of Turkey. Dig J Nanomater Bios. 2012;7(4):1647–1654. [Google Scholar]
- 87.Mitra P., Khatua S., Acharya K. Free radical scavenging and NOS activation properties of water soluble crude polysaccharides from Pleurotus ostreatus. Asian J Pharm Clin. 2013;6(3):67–70. [Google Scholar]
- 88.Gogakevar S.S., Rokade S.A., Ranveer R.C., Ghosh J.S., Kalyani D.C., Sahoo A.K. Important nutritional constituents, flavour components, antioxidant and antibacterial properties of Pleurotus sajor-caju. J Food Sci Technol. 2014;51(8):1483–1491. doi: 10.1007/s13197-012-0656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klaus A., Kosarski M., Niksic M., Jakovljevic D., Todorovic N. Antioxidative activities and chemical characterization of polysaccharides extracted from the basidiomycete Schizophyllum commune. LWT Food Sci Technol. 2012;44:2005–2011. [Google Scholar]