Abstract
Objective:
To determine the optimal environmental conditions for chondrogenic differentiation of human adipose tissue–derived mesenchymal stromal/stem cells (AMSCs). In this investigation we specifically investigate the role of oxygen tension and 3-dimensional (3D) culture systems.
Design:
Both AMSCs and primary human chondrocytes were cultured for 21 days in chondrogenic media under normoxic (21% oxygen) or hypoxic (2% oxygen) conditions using 2 distinct 3D culture methods (high-density pellets and poly-ε-caprolactone [PCL] scaffolds). Histologic analysis of chondro-pellets and the expression of chondrocyte-related genes as measured by reverse transcriptase quantitative polymerase chain reaction were used to evaluate the efficiency of differentiation.
Results:
AMSCs are capable of expressing established cartilage markers including COL2A1, ACAN, and DCN when grown in chondrogenic differentiation media as determined by gene expression and histologic analysis of cartilage markers. Expression of several cartilage-related genes was enhanced by low oxygen tension, including ACAN and HAPLN1. The pellet culture environment also promoted the expression of hypoxia-inducible cartilage markers compared with cells grown on 3D scaffolds.
Conclusions:
Cell type–specific effects of low oxygen and 3D environments indicate that mesenchymal cell fate and differentiation potential is remarkably sensitive to oxygen. Genetic programming of AMSCs to a chondrocytic phenotype is effective under hypoxic conditions as evidenced by increased expression of cartilage-related biomarkers and biosynthesis of a glycosaminoglycan-positive matrix. Lower local oxygen levels within cartilage pellets may be a significant driver of chondrogenic differentiation.
Keywords: mesenchymal stem cells, chondrogenesis, chondrocytes, biomaterials
Introduction
Articular cartilage is a well-organized tissue that is adapted for gliding during joint movements. Focal cartilage defects in articular cartilage caused by trauma or osteochondritis dissecans are leading causes of osteoarthritis in young patients.1 When these focal lesions are loaded, increased stress on the surrounding cartilage leads to expansion of the defect size and further cartilage breakdown. These cartilage defects have poor healing capacity that has been attributed to avascularity, reduced cellularity, and low cell turnover.2 In particular, the poor vascularity may impair normal tissue repair by humoral factors and stem/progenitor cells.3 Due to its limited healing capacity, the regeneration of articular cartilage is a major clinical challenge for preventing future progression of degenerative joint disease.
Popular treatments that have been used for cartilage repair with variable success include stimulation procedures such as microfracture, subchondral drilling, abrasion, arthroplasty, and osteochondral allograft transplantation.4 Cell-based therapies such as autologous chondrocyte implantation (ACI) have become increasingly popular for cartilage repair and regeneration. ACI is widely used for functional restoration of focal injuries (2-4 cm2)5-7 and has demonstrated promising clinical results in younger patients.8-10 This procedure has a number of limitations,11,12 including donor site morbidity, limited availability of donor cells, and the production of fibrocartilage rather than physiologic articular cartilage. In addition, a major drawback of ACI is the dedifferentiation of chondrocytes resulting from the expansion of the cells for multiple passages in monolayer, which is performed in order to acquire a workable amount of cells for downstream clinical applications.13-15 As dedifferentiation progresses, molecular markers associated with chondrocyte phenotype and cartilage formation, such as aggrecan (ACAN), type II collagen (COL2A1), decorin (DCN), cartilage oligomeric matrix protein (COMP), and SRY (sex determining region Y)-box 9 (SOX9), start to decrease, whereas the expression of fibroblastic and osteogenic markers increase.16 Moreover, spontaneous articular cartilage healing typically occurs via the development of fibrocartilaginous scar tissue that exhibits inferior biomechanical properties to hyaline articular cartilage and which may interfere with currently available clinical strategies for cartilage repair.17-20 Fibrocartilaginous healing also fails to restore the smooth gliding motion of the articular surface and is unable to withstand normal loads and compression forces, thus placing the joint at risk for progressive degeneration.21 Promoting effective articular cartilage regeneration remains a clinical challenge.
Use of stem cells and in particular mesenchymal stem cells (MSCs) from adult sources has emerged as a viable solution to overcome limitations of current cartilage restoration procedures. Multipotent adult MSCs reside in several tissues including bone marrow, skeletal muscle, neural tissue, adipose tissue, and synovium. MSCs are currently being used in various clinical trials with promising clinical results.22,23 However, promoting chondrogenic differentiation of MSCs can be challenging. Currently, most studies for cartilage repair have focused on bone marrow–derived MSCs. As an alternative, human adipose tissue–derived mesenchymal stem cells (AMSCs) are an attractive cellular therapeutic due to minimally invasive tissue harvest, high abundance of cells, and rapid expansion ex vivo.24 Moreover, these cells are multipotent and can produce musculoskeletal extracellular matrix proteins once confluent and are able to differentiate into osteogenic and chondrogenic lineages.24,25 While there are many studies on chondrogenic differentiation of bone marrow–derived MSCs, our studies address a major gap in our knowledge by investigating the chondrogenic potential of AMSCs under multiple culture conditions relevant to cartilage tissue engineering, including hypoxia and propagation on a 3-dimensional (3D) culture environment.
Materials and Methods
Cell Harvest and Expansion
AMSCs were derived from lipoaspirates obtained from consenting healthy donors. This study used the same 3 representative donors that were previously validated for standard stem cell markers, normal cell growth characteristics, and tri-lineage differentiation potential.24,25 Human primary chondrocytes were obtained from healthy donors undergoing amputation procedures for congenital limb deformity. All tissues/cell lines were collected under protocols approved by the Mayo Clinic Institutional Review Board. Maintenance media for AMSCs was composed of Advanced MEM (Gibco/Thermo Fisher Scientific, Waltham, MA) supplemented with 5% human platelet lysate (PL-Max, MillCreek Life Sciences, Rochester, MN), 1% penicillin/streptomycin, 1% Glutamax (Gibco/Thermo Fisher Scientific), and 0.2% heparin (Baxter, Deerfield, IL). Human primary chondrocytes were cultured in maintenance medium that contained Advanced MEM (Gibco/Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA) and 1% penicillin/streptomycin (Gibco/Thermo Fisher Scientific). Cells were cultured using standard technique in T-175 cm2 flasks at 37°C, 95% humidity, and 5% CO2 until they reached 80% confluence. Prior to each experiment, cells were detached from T175 flasks by trypsinization using TrypLE Express (Gibco/Thermo Fisher Scientific).
2D Monolayer Cell Culture
For monolayer culture, AMSCs were plated in 6-well plates at a density of 3,000 cells/cm2 and maintained for 21 days in chondrogenic media, which consisted of culture media supplemented with 40 mg/mL of L-proline (Sigma-Aldrich, Saint-Louis, MO), 50 mg/mL Insulin Transferrin Selenium-premix (Gibco/Thermo Fisher Scientific), 50 mg/mL of ascorbic acid (Sigma-Aldrich), 10 ng/mL of TGF-β1 and 0.1 µM dexamethasone (Sigma-Aldrich). Cell culture medium was replaced every 3 days. Chondrocytes were cultured in monolayer only for proliferative expansion.
3D Cell Culture in High-Density Pellets or on Polycaprolactone (PCL) Scaffolds
Induction of chondrocyte differentiation in high-density cellular aggregates was performed using pellet cultures in accordance with a protocol published previously.26 To induce chondrogenic differentiation, 250,000 cells (AMSCs or human chondrocytes) were seeded in 96 round-bottom well plates. Pellets were formed by centrifugation and maintained for 24 hours at 37°C, 95% humidity, and 5% CO2 until cells coalesced. Following pellet formation, maintenance media was exchanged with chondrogenic differentiation media, which consisted of culture media supplemented with 40 mg/mL of L-proline, 50 mg/mL Insulin Transferrin Selenium-premix, 50 mg/mL of ascorbic acid, 10 ng/mL of TGF-β1, and 0.1 µM dexamethasone and was replaced every 3 days.
PCL scaffolds were fabricated using an electrospinning technique described previously.27 Scaffolds were composed of 9.5 wt% homogeneous solution of poly-ϵ-caprolactone. This solution was prepared by dissolving 1.05 g of 80,000 Mn poly-ϵ-caprolactone (Sigma-Aldrich) in 0.8 g N,N-dimethylformamide (Thermo Fisher Scientific), 6.0 g chloroform (Thermo Fisher Scientific), and 3.2 g acetone (Thermo Fisher Scientific). The solution was stirred for 4 hours. The polymer solution was subsequently placed in a 20 mL glass syringe with a 20-G needle. The solution was dispensed at a rate of 3.3 mL/h while applying a 30 kV electrical field. All procedures were performed in a chemical hood. A glass plate mounted on an aluminum block placed 15 cm away from the needle collected the fibers. This procedure was followed until the sheets reached a thickness of 1 mm. Discs of 10 mm in diameter were cut from the sheets using a dermal punch. The final dimensions of the scaffolds were approximately 10 mm diameter and 1 mm thickness. Both sides of the scaffold were sterilized by ultraviolet irradiation in a laminar flow hood for 30 minutes. To provide a hydrophilic surface for efficient cell attachment, scaffolds were prewetted by immersion in different ethanol concentrations for 30 minutes each (95%, 50%, and 25% ethanol), 30 minutes in distilled water, and finally overnight in HBSS buffer in the 37°C incubator. Induction of differentiation on PCL scaffolds was achieved as follows. AMSCs and human primary chondrocytes grown in T175 cell culture flasks were trypsinized, counted, and plated at a density of 400,000 cells/scaffold. Scaffolds were placed in 24-well culture plates precoated with 0.3% poly-(hydroxyethyl-methacrylate) to prevent cell attachment to the tissue-culture plate surface. Scaffolds were incubated at 37°C for 4 hours to allow correct diffusion of the cells trough the nanofibers. During the first 4 hours, 30 µL of complete media was added every 30 minutes to each scaffold to prevent desiccation of the scaffold fibers. After 4 hours of incubation 2 mL of chondrogenic differentiation media was added, which consisted of culture media supplemented with 40 mg/mL of L-proline, 50 mg/mL Insulin Transferrin Selenium-premix, 50 mg/mL of ascorbic acid, 10 ng/mL of TGF-β1, and 0.1 µM dexamethasone. Cell viability was monitored using a Live/Dead staining kit (Life Technologies, Carlsbad, CA) at Days 1, 3, and 7 in culture to visualize the live cells attached to the nanofibrous PCL scaffold. Fluorescence observations were performed using a semiautomated ZEISS Axio inverted light microscope with appropriate filters (Zeiss, Oberkochen, Germany). Cell culture medium was replaced every 3 days.
Cell Culture under Normoxia and Hypoxia Conditions
To evaluate the response to oxygen tension, both AMSCs and human primary chondrocytes were allowed to differentiate for up to 21 days in chondrogenic differentiation media under either normoxic (21% oxygen) or hypoxic (2% oxygen) conditions using an integrated system with a cell culture hood and incubator accessible through a gas-lock (I-Glove, BioSpherix, New York, NY).
Gene Expression Analysis
Total RNA was isolated using the miRNeasy Micro Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. For pellet cultures, at least 5 pellets were pooled from each condition to obtain sufficient RNA. Pooled pellets from each time point were lysed using an 18-gauge needle and Qiazol lysis buffer (Qiagen, Hilden, Germany) to yield an RNA sample for one biological replicate. Cells from scaffold cultures were lysed using the same syringe-needle homogenization method, and one scaffold yields sufficient RNA for one biological replicate. Each time course was repeated 3 times to yield 3 biological replicates per time point.
For baseline gene expression values, lysates were obtained from expanded cells in monolayer immediately prior to plating cells on scaffolds or in pellet culture on day 0 (D0: plating day). Subsequent samples were harvested at D1, D7, and D14 after culture under various conditions (e.g., pellets or scaffolds). Three different biological replicates for each experimental condition (e.g., 3 samples for normoxia on D0, 3 samples for hypoxia on D0, etc.) were used concurrently for RNA isolation and subsequent expression analysis to ensure consistency in RNA quality and expression values.
RNA concentrations and purity levels were measured using a NanoDrop (Thermo Fisher Scientific) and isolated RNA was reverse transcribed into cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Gene expression was quantified using quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) with primers for representative chondrogenic genes (Supplementary Table S1). Real-time qPCR reactions were performed with 10 ng cDNA per 10 µL with QuantiTect SYB R Green PCR Kit (Qiagen) and the CFX384 Real-Time System (BioRad, Hercules, CA). Gene expression levels were quantified using the 2−ΔΔCt method described previously.28 As illustrated in Supplementary Figure S1, several housekeeping genes were tested for low variation across our samples among the different conditions, cell types (AMSCs and chondrocytes), and over time. Housekeeping genes GAPDH, ACTB, and AKT1 were evaluated according to the stability of the Ct values and the variation from the mean Ct. Because the housekeeping gene AKT1 was found to be more stably expressed than GAPDH and ACTB, we normalized all data relative to AKT1 (mean ± standard error of the mean, n = 3).
Histological Analysis
Cell pellets and scaffolds were fixed overnight in 10% neutral buffered formalin. Samples were then washed and dehydrated in graded series of ethanol (70% to 100%) and processed with xylene (50% to 100%) prior to paraffin embedding. Paraffin blocks were cut into consecutive sections of 5 µm thickness using a microtome and placed onto charged microscope glass slides for histological staining. Following deparaffinization, sections were stained for glycosaminoglycan content using 0.5% (wt/vol) Alcian Blue 8GX dye (Sigma-Aldrich) and counterstained with Nuclear Fast Red (Sigma-Aldrich).
The presence of type II collagen was localized by immunohistochemistry (IHC). In brief, after removal of paraffin and rehydration, sections were washed with distilled water. Pepsin antigen retrieval was performed using 10 mg/mL pepsin for 10 minutes at 37°C in a humidified chamber. The Mouse and Rabbit Specific HRP (ABC) Detection IHC Kit (Abcam, Cambridge, UK) was used according to the manufacturer’s instructions. Briefly, protein block was applied after which slides were incubated overnight with 1 µg/mL of anti-Collagen Type II Antibody (Millipore, Darmstadt, Germany) or mouse IgG1 Isotype Control (Biosciences, Osage, IA) at 4°C in a humidified chamber. Slides were sequentially incubated with biotinylated polyvalent secondary antibody and streptavidin peroxidase plus (supplied in the kit) and were then treated with 3,3′-diaminobenzidine (DAB) enhanced liquid substrate system for IHC (Sigma-Aldrich) diluted 1:1 with TBS (Tris-buffered saline) for 10 minutes until color development. Stained sections were analyzed using a ZEISS Axio inverted light microscope as described above.
Statistical Analysis
Quantitative data for gene expression profiles have been presented as mean ± standard deviation from 3 independent donors for AMSCs and one donor for human primary chondrocytes for each experimental condition and time point. The experimental factors analyzed for their association with gene expression values included oxygen level (2 levels: normoxia, hypoxia), culture condition (3 levels: 2D monolayer, 3D high-density pellet, 3D PCL scaffold), time (5 levels: baseline and days 1, 3, 7, and 14), and cell type (2 levels: AMSCs, chondrocytes). Each combination of the experimental conditions was performed in triplicate. Separate analyses were performed for each of the 12 targeted genes. The analyses were performed using generalized linear models utilizing generalized estimating equations (GEE) to account for the within-donor correlation among the subsamples from each donor. Significant main effects with more than 2 levels were analyzed further by generating pairwise contrasts to identify levels that are significantly different from each other. In order to protect against an increased type I error rate associated with multiple comparisons, the reported P values were adjusted using the Benjamini-Hochberg method to control the false discovery rate. All analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC). A summary of the statistical analysis can be found in Supplementary Tables S2 and S3.
Results
Chondrogenic Differentiation of AMSCs
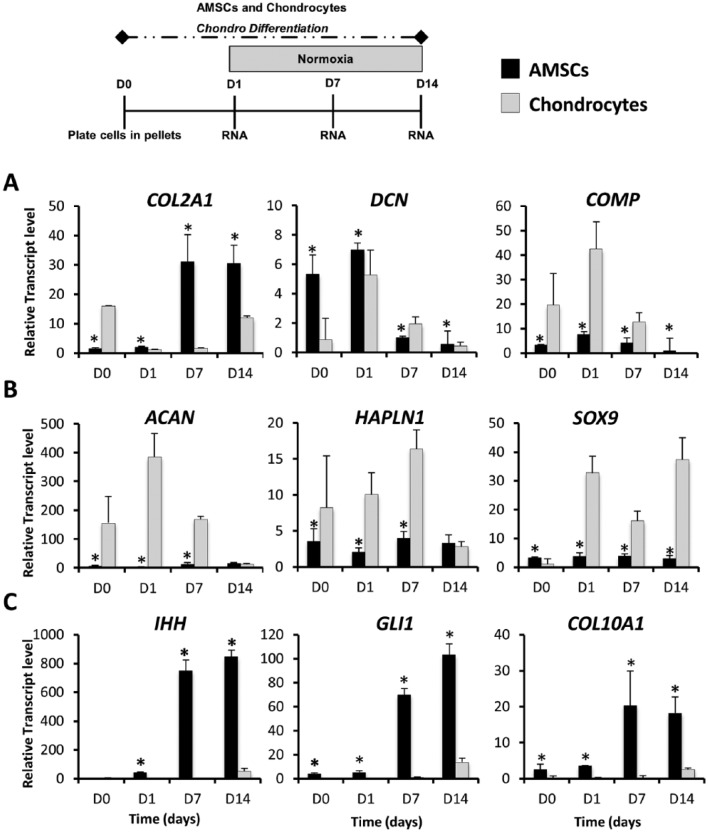
To evaluate the ability of AMSCs to undergo chondrogenic differentiation, we compared the expression of chondrogenic markers in AMSCs to those of human chondrocytes using traditional high-density pellet cultures under normoxic conditions ( Fig. 1 ). On differentiation induction, AMSCs express significantly higher levels of classic cartilage markers including COL2A1 (2.3-fold increase at day 14), DCN (12-fold increase at day 14), and COMP (276-fold change at day 14) ( Fig. 1A ). AMSCs also showed expression of ACAN, HAPLN1, and SOX9. However, these genes are expressed at significantly lower levels compared to chondrocytes on days 1 and 7 ( Fig. 1B ). AMSCs also have significantly higher expression of hypertrophic markers, including IHH (4-fold increase at day 7), GLI1 (3.7-fold increase at day 14), and COL10A1 (6.6-fold increase at day 14). These results show that AMSCs have chondrogenic potential and that their molecular response to induction of differentiation is distinct from that observed for chondrocytes. This observation is expected because AMSCs have tri-lineage potential while primary human chondrocytes are precommitted to a cartilage cell fate. The enhanced expression of hypertrophic cartilage markers in AMSCs suggests that AMSCs may have undergone accelerated end-stage differentiation into cells characteristic of calcifying cartilage.
Figure 1.
Gene expression profiles of AMSCs (human adipose tissue–derived mesenchymal stem cells) and human primary chondrocytes in high-density pellet cultures during chondrogenic differentiation. Expression of chondrocyte-specific markers was analyzed at days 0, 1, 7, and 14. All values graphed for pellet cultures were derived from at least 5 pellets per time point, and each differentiation time course was repeated 3 times to obtain 3 independent biological replicates. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to plating as a pellet (3D) culture. (A) AMSCs showed increased expression of COL2A1 and upregulated mRNA levels of ECM proteins DCN and COMP when compared to human primary chondrocytes. (B) Earlier and robust expression of ECM proteins ACAN and HAPLN1, and transcription factor SOX9, was observed in human primary chondrocytes over the chondrogenic time course. (C) Hypertrophic phenotype is induced in AMSCS over time, which is evidenced by the upregulation of IHH, GLI1, COL10A1 over time in AMSCs. Data have been presented as mean ± standard. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to chondrocytes.
Chondrogenic Differentiation of AMSCs Is Enhanced in Pellet Cultures Compared to Monolayer Culture
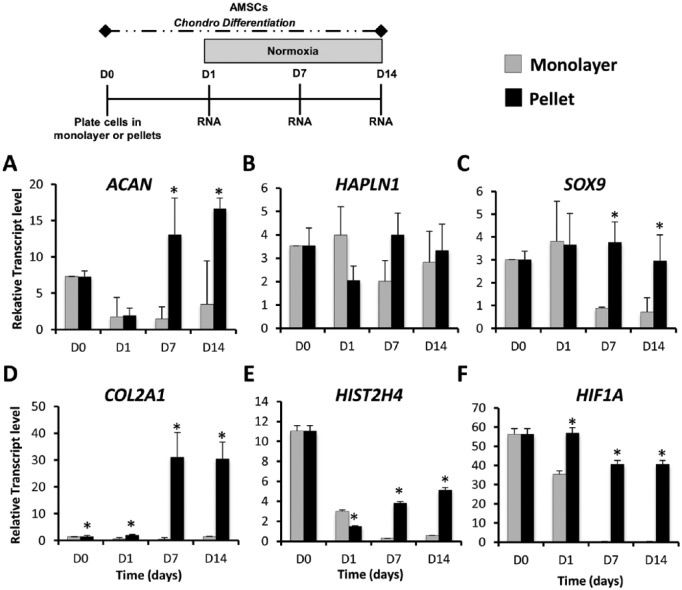
Because clinical delivery of chondro-pellets is not always desirable or feasible, we sought to investigate if AMSCs retain their chondrogenic potential in monolayer culture, a scenario that could potentially arise if AMSCs are directly seeded into a cartilage defect. To evaluate this we compared gene expression during chondrogenic differentiation of AMSCs grown as monolayers and as pellet cultures under normoxic conditions. High-density pellet cultures expressed considerably higher levels of chondrogenic markers including extracellular matrix (ECM) proteins ACAN (8.8-fold increase at day 7), HAPLN1 (2.0-fold increase at day 7), COL2A1 (62.0-fold increase at day 7), and the chondrogenic transcription factor SOX9 (4.2-fold increase at day 7). Statistical differences were observed in ACAN and SOX9 at days 7 and 14 ( Fig. 2A , B , C , and D ). Significant upregulation of the proliferation marker HIST2H4 was also observed in pellet cultures at days 1, 7, and 14 ( Fig. 2E ). Because articular cartilage is normally an avascular tissue with low oxygen tension, we also examined whether the differences between monolayer and pellet culture were due to differences in the oxygen availability within both culture systems. The expression of hypoxia inducible factor 1 (HIF1A) was used as a surrogate indicator of oxygen levels. This gene had significantly greater expression in AMSCs cultured in pellets compared to monolayer at days 1, 7, and 14 ( Fig. 2F ). These findings indicate that the low oxygen microenvironment within a cell pellet may act to modulate chondrogenic differentiation of AMSCs.
Figure 2.
Gene expression profiles of AMSCs (human adipose tissue–derived mesenchymal stem cells) in monolayer and high-density pellets cultures during chondrogenic differentiation. Cultures were subjected to analysis of chondrocyte specific markers by gene expression analysis using reverse transcriptase quantitative polymerase chain reaction at the indicated times: days 0, 1, 7, and 14. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to plating as a pellet (3D) culture. (A, B, C, and D) AMSCs cultured in pellets significantly upregulated expression of chondrogenic markers ACAN, HAPLN1, SOX9, and COL2A1 when compared to monolayer culture. (E) Increased expression of the proliferation marker HIST2H4A was also observed in pellet cultures. (F) HIF1A expression was greater in AMSCs cultured in pellets over monolayer. Data have been presented as mean ± standard. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to monolayer culture.
Culture on PCL Scaffolds Supports Cartilage-Specific Extracellular Matrix Formation of AMSCs
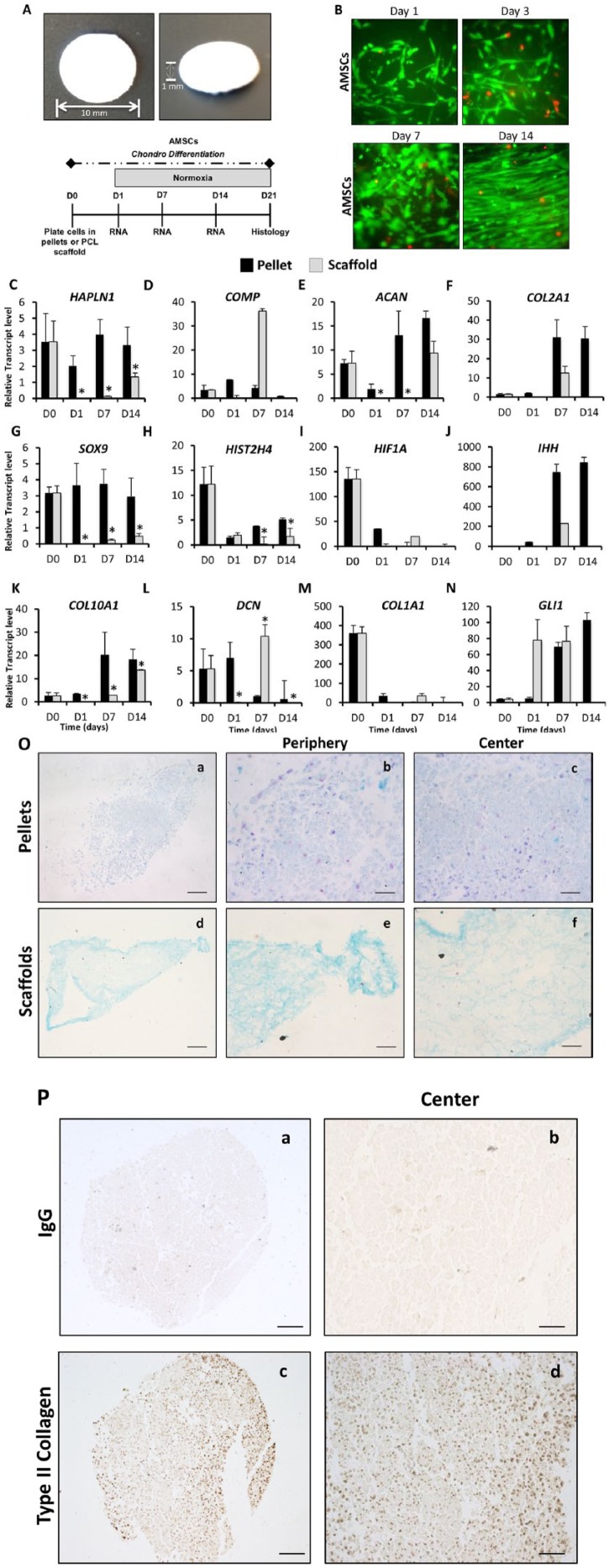
To aid in the clinical translation of AMSCs for the treatment of cartilage defects, we investigated whether 3D nanofibrous PCL scaffolds could support chondrogenic differentiation of AMSCs. After fabrication, scaffolds were visualized by light microscopy and were observed to be homogenous in size and shape ( Fig. 3A ). AMSCs were seeded onto the scaffold and live/dead staining revealed that the cells attached and proliferated within the scaffold after 1, 3, 7, and 14 days. At all time points, the majority of cells retained their viability as evidenced by live/dead assays (green staining). Moreover, fluorescence microscopy at day 14 revealed cells were tightly packed indicating cells reached a confluence state ( Fig. 3B ).
Figure 3.
Gene expression and histological analysis differences between high-density pellets and polycaprolactone (PCL) scaffold cultures using AMSCs (human adipose tissue–derived mesenchymal stem cells). The AMSC phenotype was evaluated during chondrogenic induction in either high-density pellets and PCL scaffolds. (A) Visualization of PCL scaffold after fabrication. (B) Live (green)/dead (red) analysis showing AMSCs attached and proliferated on the scaffold after 1, 3, 7, and 14 days of culture. (C-N) Gene expression analysis of chondrocyte-specific markers, HIF1A and hypertrophic markers using reverse transcriptase quantitative polymerase chain reaction was performed at days 0, 1, 7, and 14. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to culturing cells as pellets or on scaffolds in 3D culture. (C, D, E, F, and G) Pellet culture promotes the expression of several chondrogenic markers including HAPLN1, COMP, ACAN, COL2A1, and SOX9. (H and I) An enhanced expression of HIST2H4 and HIF1A was also achieved in pellet culture over the time course. (J and K) Hypertrophic genes IHH and COL10A1 were also increased when cultured in pellets. (L) DCN protein was the only chondrogenic marker to show similar expression in both pellet and PCL scaffold cultures. (M and N) Osteogenic marker COL1A1 and hypertrophic marker GLI1 were increased in scaffolds over pellets. Data have been presented as mean ± standard. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to PCL scaffolds. (O) Histological analysis of AMSCs in either high-density pellets or PCL scaffold cultures at day 21 of chondrogenic differentiation. Formation of cartilaginous extracellular matrix was analyzed using Alcian Blue and Nuclear Fast Red counterstaining for each culture condition (Scale bar = 100 µm). (O a and d) Formation of a positive but low glycosaminoglycan matrix staining was observed for AMSCs cultured in both pellet and PCL scaffolds. (O b, c, e, and f) High-magnification pictures were taken at the center and periphery of the pellets or scaffolds to visualize the cells phenotype. (P) Presence of type II collagen was localized by immunohistochemistry in pellets at day 21 of culture, shown at 10× and 40× magnification. (P a and b) IgG antibody was used as a control and showed no staining. (P c and d) Type II collagen positive staining was shown as the brown staining area at low and high magnifications.
Gene expression analyses revealed differences between the pellet culture and scaffold system for AMSCs. Pellet culture favored the expression of several chondrogenic ECM markers including HAPLN1 (2.4-fold increase at day 14), COMP (276-fold increase at day 14), ACAN (13-fold increase at day 7), COL2A1 (23.2-fold increase at day 14), and the transcription factor SOX9 (4.3-fold increase at day 14), compared to scaffolds. Statistical differences were observed in HAPLN1 at days 1, 7, and 14, ACAN days 1 and 7, and SOX9 days 1, 7, and 14 ( Fig. 3C , D , E , F , and G ). Moreover, as we observed in monolayer culture, there was enhanced expression of HIST2H4 (significant fold increase at days 7 and 14) and HIF1A in pellet cultures compared with 3D scaffolds ( Fig. 3H and I ). The upregulation of HIF1A suggests a hypoxic microenvironment within the pellet even when cultured under normoxic conditions. Hypertrophic genes IHH and COL10A1 (significant fold increase at days 1, 7, and 14) were also increased in pellets after 1 week in culture ( Fig. 3J and K ). DCN protein showed a significant difference between both pellet and scaffold at days 1, 7, and 14 ( Fig. 3L ). In contrast, the osteogenic/fibroblastic marker COL1A1 and hypertrophic marker GLI1 were increased in scaffolds over pellets by 34-fold and 1.1-fold, respectively, after 1 week ( Fig. 3M and N ). Histological analysis of AMSCs cultured in pellets and scaffolds at day 21 of chondrogenic differentiation indicated that both pellet and scaffold cultures permit development of an Alcian Blue–positive matrix ( Fig. 3O , a and d ). However, the matrix was denser at the center of pellets ( Fig. 3O , b and c ), in the region expected to have the lowest oxygen tension. In scaffolds, cells and corresponding matrix were located primarily along the periphery ( Fig. 3O , e and f ). To validate the expression of COL2A1, type II collagen was localized by IHC in pellets at day 21 of culture ( Fig. 3P ). In summary, AMSCs grown on 3D scaffolds are capable of producing a cartilaginous matrix with potential clinical utility. However, chondrogenic differentiation of AMSCs in pellet culture is superior to that observed on a basic 3D scaffold, possibly due to lower oxygen concentrations within the pellets.
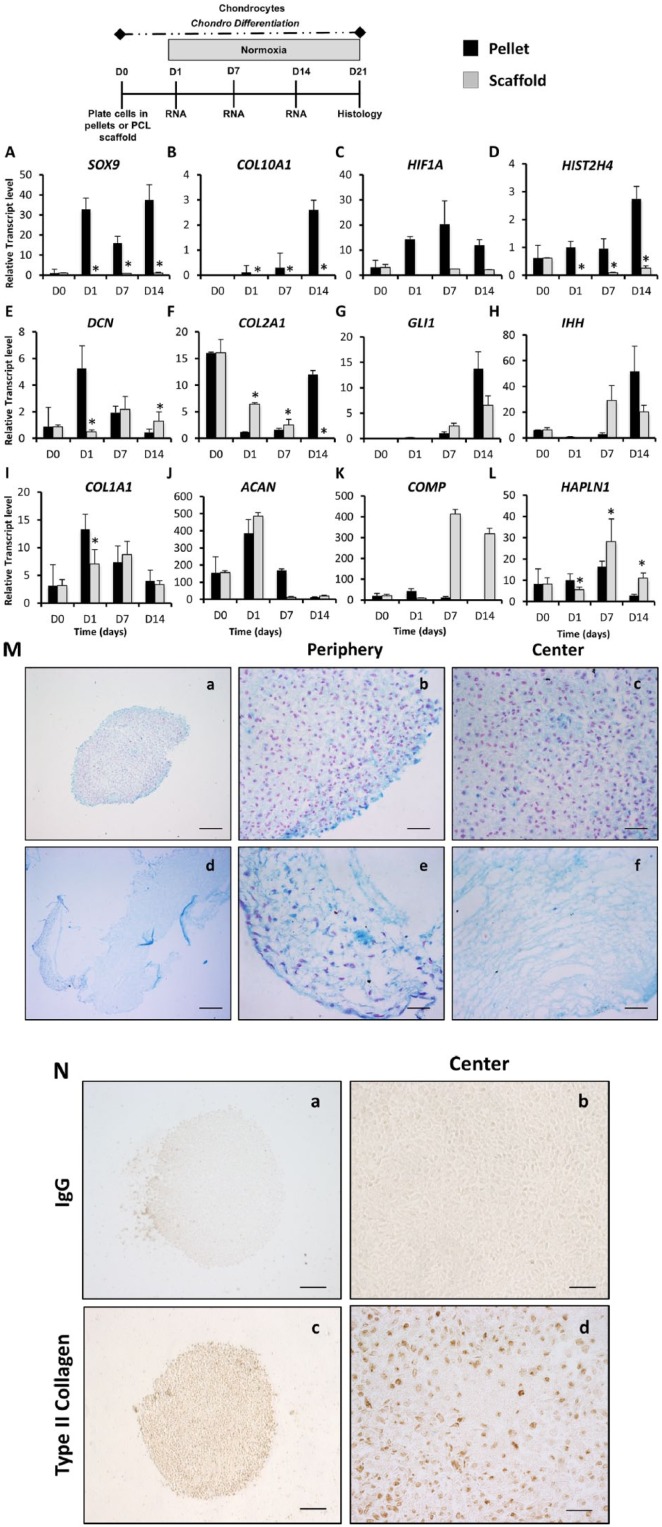
To verify the efficiency of AMSC chondrogenic differentiation on 3D scaffolds, we also compared them with primary human chondrocytes grown under the same conditions. Gene expression analysis revealed similarities between AMSCs and chondrocytes under both 3D culture conditions. Pellet culture again seemed to show preferential expression of the chondrogenic transcription factor SOX9 (significant increase at days 1, 7, and 14), hypertrophic marker COL10A1 (significant increase at days 1, 7, and 14), hypoxia inducible factor HIF1A and HIST2H4 (significant increase at days 1, 7, and 14) in both AMSCs and chondrocytes ( Fig. 4A , B , C , and D ). The mRNA levels of the ECM protein DCN were significantly increased in pellets when compared to scaffolds at days 1 and 14 in both AMSCs and chondrocytes ( Fig. 4E ). Expression of the chondrogenic marker COL2A1was significantly increased in scaffolds at days 1 and 7, whereas at day 14 was significantly higher in pellets ( Fig. 4F ). Chondrogenic markers ACAN and hypertrophic markers IHH and GLI1 mRNA levels were similar for chondrocytes grown in both culture systems ( Fig. 4G , H , and J ). The nonchondrogenic marker COL1A1 gene was significantly higher in pellets at day 1 but similar between pellets and scaffolds at days 7 and 14 ( Fig. 4I ).
Figure 4.
Analysis of human primary chondrocytes differentiated in high-density pellets and 3D-scaffold cultures. The effect of 3D cultures (high-density pellets and 3D scaffolds) on the phenotype, gene expression, and proliferation of human primary chondrocytes was evaluated by reverse transcriptase quantitative polymerase chain reaction at days 0, 1, 7, and 14. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to culturing cells as pellets or on scaffolds in 3D culture. (A-E) Pellet culture enhances expression of chondrogenic transcription factor SOX9, hypertrophic marker COL10A1, as well as HIF1A, HIST2H4, and DCN. (F-J) Chondrogenic markers COL2A1 and ACAN, hypertrophic markers IHH and GLI1, and osteogenic marker COL1A1 gene expression was similar in both 3D culture conditions. (K and L) Chondrocytes extracellular matrix (ECM) proteins HAPLN1 and COMP were enhanced in 3D scaffolds when compared to pellets. Data have been presented as mean ± standard deviation. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to PCL scaffolds. (M) Histological analysis of primary chondrocytes cultured in high-density pellets and 3D-scaffolds cultures at day 21 of chondrogenic differentiation. Formation of cartilaginous ECM matrix was analyzed using Alcian Blue and Nuclear Fast Red counterstaining for each culture condition (scale bar = 100 µm). (M a and d) Alcian Blue staining was evident in chondrocytes when culture in both 3D conditions at day 21 of culture. (M b, c, e, and f) High-magnification pictures were taken at the center and periphery of the pellets or scaffolds to visualize the cells phenotype. (N) Presence of type II collagen was localized by IHC in pellets at day 21 of culture, shown at 10× and 40× magnification. (N a and b) IgG antibody was used as a control and showed no staining. (N c and d) Type II collagen positive staining was shown as the brown staining area at low and high magnifications.
In contrast, mRNA levels of ECM proteins COMP and HAPLN1 (significant increase at days 7 and 14) were enhanced in scaffolds compared to pellet cultures ( Fig. 4J , K , and L ). Histological analysis showed intense Alcian Blue staining in chondrocytes when cultured in both 3D conditions at day 21 ( Fig. 4M ). Within the primary chondrocyte pellets, a dense chondrogenic matrix was observed throughout the pellet ( Fig. 4M , b and c ). In scaffolds, poor migration of the cells within the scaffold was also observed with AMSCs ( Fig. 4M e and f ). Chondrocytes did not show any specific orientation or arrangement within the pellet or scaffold. Expression of type II collagen was detected by IHC in pellets at day 21 of culture ( Fig. 4N ), indicating a chondrogenic matrix. Together, these results suggest that both high-density pellets and PCL scaffold cultures are conducive to the production of a glycosaminoglycan positive matrix, and the cartilage-like gene expression profile indicates that the chondrocytic phenotype is preserved.
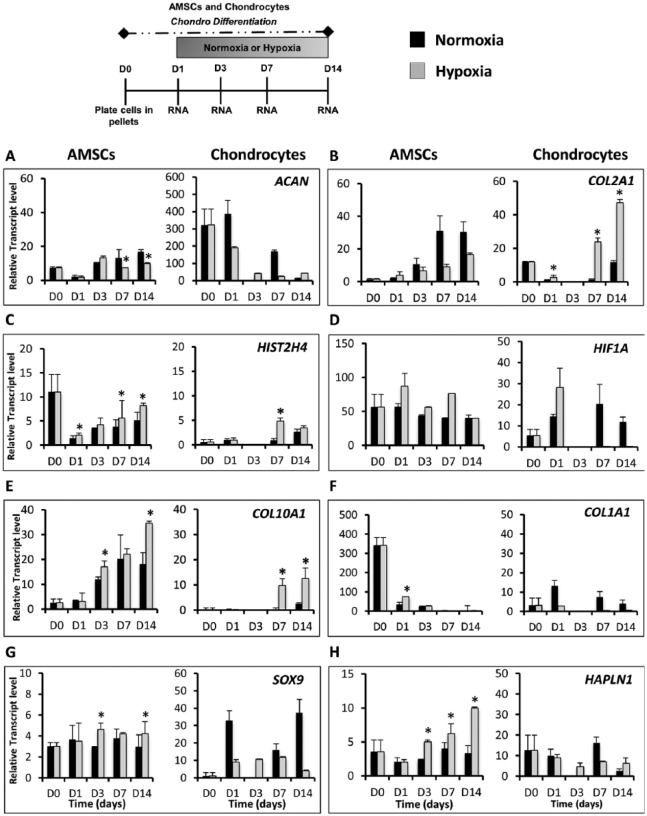
Hypoxia Promotes Cartilage-Specific Gene Expression in AMSCs in Both Pellets and PCL Scaffolds
Hypoxia has been previously shown to enhance chondrogenic differentiation of MSCs. To determine if low oxygen tension also enhances chondrogenic differentiation of AMSCs, we compared pellet cultures of both AMSCs and chondrocytes in normoxia (21% O2) and hypoxia (2% O2). Gene expression analyses revealed that while the expression of a number of cartilage-related genes were independent of oxygen levels, several cell type–specific responses to hypoxia were observed. In AMSCs, hypoxia stimulated the expression of several chondrogenic genes, including the transcription factor SOX9 (1.6-fold, day 3), HAPLN1 (2.1-fold, day 3) ( Fig. 5G and H ) and COMP (1.8-fold, day 1) (data not shown). Both SOX9 and HAPLN1 markers significantly increased in hypoxia at days 3, 7, and 14. Hypoxia also stimulated the preferential expression of the proliferation marker HIST2H4 (significant increase at days 7 and 14) as well as HIF1A, which is the master regulator of the cellular response to hypoxia29-31 ( Fig. 5C and D ). Low oxygen conditions also significantly increased levels of hypertrophic mRNA markers COL10A1 at days 3 and 14 ( Fig. 5E ), as well as GLI1 and IHH (data not shown). A reduced oxygen environment also accelerated downregulation of COL1A1, thus decreasing the osteogenic and/or fibroblastic potential of the cells ( Fig. 5F ). In AMSCs, normoxia stimulated the expression of ECM proteins ACAN (significantly increase at days 7 and 14), COL2A1 ( Fig. 5A and B ), and DCN (data not shown) after 1 week of culture.
Figure 5.
The effect of oxygen tension on differentiation potential of AMSCs (reverse transcriptase quantitative polymerase chain reaction) and chondrocytes cultured in high-density pellets. AMSCs and chondrocytes were placed in pellet cultures and were exposed to normoxic (27% O2) or hypoxic conditions (2.1% O2) for 14 days. Gene expression analysis of chondrocyte-specific markers, hypoxia-related factor protein HIF1A, and hypertrophic markers was performed at days 0, 1, 3, 7, and 14. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to culturing cells as pellets. (A and B) Normoxia stimulated the expression of ACAN and COL2A1 in AMSCs. Hypoxia increased COL2A1 expression in chondrocytes. (C) Expression level of proliferation marker HIST2H4 was significantly enhanced during the chondrogenic time course in low oxygen conditions. (D) Hypoxia inducible factor HIF1A was also upregulated in response to low oxygen conditions. (E) Other genes enhanced by hypoxia included COL10A1 whose activity is restrictive to later stages of chondrocyte maturation. (F) COL1A1 was downregulated earlier in low oxygen conditions, suggesting a reduced osteogenic potential of both AMSCs and chondrocytes. (G and H) Hypoxia stimulates upregulation of gene transcripts toward cartilage like phenotype. HAPLN1 and SOX9 were upregulated in AMSCs when cultured under low oxygen, whereas in chondrocytes both HAPLN1 and SOX9 mRNA levels decreased in hypoxia. Data have been presented as mean ± standard deviation. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to normoxia.
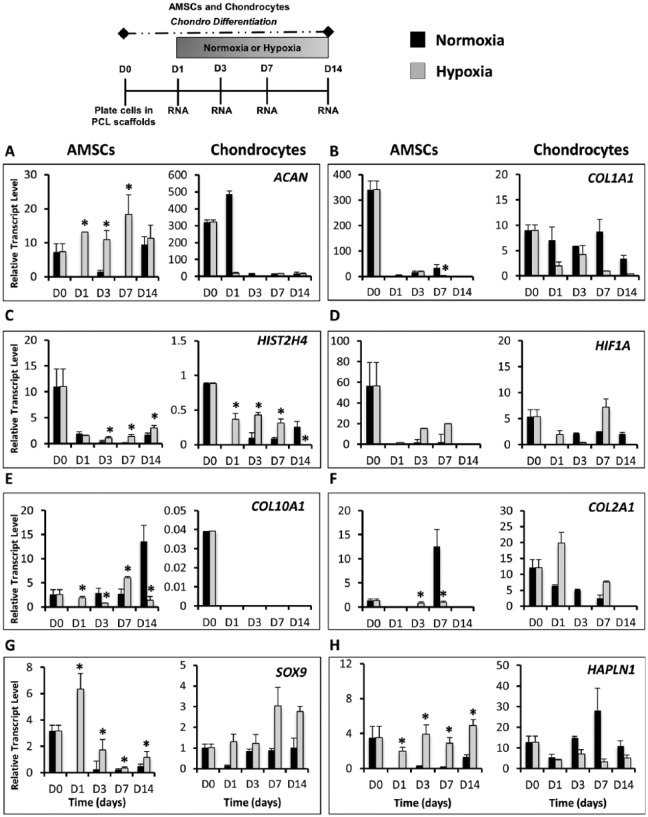
We also evaluated whether oxygen tension modulated chondrogenic differentiation of AMSCs and chondrocytes cultured on scaffolds. Gene expression analysis revealed similarities between AMSCs and chondrocytes in both low and high oxygen cultures, although cell type–specific differences were observed. In AMSCs differentiated on scaffolds, hypoxia significantly increased the expression of chondrogenic markers ACAN (5-fold increase at day 3) ( Fig. 6A ), SOX9 (5.6-fold increase at day 3) ( Fig. 6G ), and HAPLN1 (4-fold increase at day 3) ( Fig. 6H ), whereas normoxia upregulated osteogenic/fibroblastic marker COL1A1 ( Fig. 6B ) and hypertrophic markers COL10A1 (3-fold increase at day 3) ( Fig. 6E ), GLI1 and IHH (data not shown). Moreover, normoxia also significantly increased mRNA levels of COL2A1 at day 7 ( Fig. 6F ). In chondrocytes, hypoxia upregulated the expression of transcription factor SOX9 ( Fig. 6G ), HIST2H4 (significant increase at days 1, 3, and 7), and HIF1A ( Fig. 6C and D ), while normoxia increased COL1A1 ( Fig. 6B ). Interestingly, low oxygen tension enhanced expression of chondrogenic genes in chondrocytes that were repressed in AMSCs, including COL2A1 ( Fig. 6F ), DCN and GLI1 and IHH (data not shown). Conversely, chondrocytes on scaffolds cultured under normoxic conditions upregulated several chondrogenic genes including ACAN ( Fig. 6A ) and HAPLN1 ( Fig. 6H ). The hypertrophic marker COL10A1 was the only gene that was not expressed by chondrocytes grown on scaffolds ( Fig. 6E ). These data show that many of the chondrogenic genes that are upregulated in AMSCs and chondrocytes in pellet culture are also many of the same genes that show upregulation under hypoxic conditions, including SOX9, HAPLN1, and COMP, thus indicating that the hypoxic microenvironment within cell pellets is partially responsible for promoting chondrogenic differentiation of AMSCs.
Figure 6.
The effect of oxygen tension on differentiation potential of AMSCs (reverse transcriptase quantitative polymerase chain reaction) and primary human chondrocytes cultured in polycaprolactone (PCL) scaffolds. PCL scaffolds were seeded with either AMSCs or chondrocytes and placed under hypoxic or normoxic conditions in the presence of chondrogenic differentiation medium for 14 days. Gene expression analysis was performed at days 0, 1, 3, 7, and 14. D0 data represent baseline gene expression values obtained for cells expanded cells in monolayer (2D) immediately prior to culturing cells on scaffolds in 3D culture. (A) Hypoxia stimulates upregulation of ACAN in AMSCs while decreasing levels were observed in chondrocytes. (B) COL1A1 was downregulated earlier in low oxygen conditions, indicating a reduction in osteogenic potential of both AMSCs and chondrocytes. (C) Expression levels of proliferation marker HIST2H4 were significantly enhanced during the chondrogenic time course in low oxygen conditions. (D) HIF1A was also upregulated in response to low oxygen conditions. (E) COL10A1 was enhanced by normoxia in AMSCs but was not expressed by chondrocytes. (F) COL2A1 was upregulated by normoxia in AMSCs but increased by hypoxia in chondrocytes. (G) SOX9 was upregulated in both AMSCs and chondrocytes when cultured under low oxygen. (H) Hypoxia increased the expression of extracellular matrix protein HAPLN1 in AMSCs while decreasing the expression in chondrocytes. Data have been presented as mean ± standard deviation. Statistical difference was set to a P < 0.05 and has been indicated with an asterisk. *P < 0.05 compared to normoxia.
Discussion
Tissue engineering offers possibilities for optimization of cartilage repair by combining different cell types, biomaterials, and growth factors for the support of cartilage regeneration.2 Although several procedures have been developed and studied, no standardized clinical protocol has yet been established.32
Currently, studies on tissue engineering approaches using stem cells and particularly bone marrow–derived MSCs have emerged as promising solutions for cartilage repair/regeneration strategies. However, current protocols for chondrogenic differentiation efficiently create hypertrophic cartilage, which on implantation undergoes endochondral ossification and mineralization.33 Several studies have attempted to understand the mechanisms by which MSCs can be programmed for chondrogenic differentiation while avoiding further progression into hypertrophic cartilage.34,35 Mimicking the natural hypoxic environment of cartilage development under in vitro conditions is beneficial because it mediates metabolic programming of the chondrogenic fate of MSCs into different subtypes of hyaline cartilage. The results presented in this study validate the biological properties of self-renewing capacity and chondrogenic potential of AMSCs for cartilage regeneration applications. Moreover, in our studies we investigated the chondrogenic potential of AMSCs under different microenvironments (3D cultures and variations in oxygen conditions.
The comparison between normoxia and hypoxia of AMSCs and chondrocytes cultured in pellets revealed an enhanced expression of chondrogenic markers that were expressed in both oxygen conditions. Previous studies have described neo-hyaline cartilage tissue formation as gene expression of COL2A1 and higher glycosaminoglycan content.18,36 Furthermore, extracellular matrix proteins (e.g., ACAN, DCN, COMP, and HAPLN1) are highly expressed in human cartilage and human articular chondrocytes.24,26,37 In our studies, both cell types respond to hypoxia by enhancing the expression of several chondrogenic markers including SOX9, HAPLN1, and COMP, but also HIST2H4 and HIF1A. These findings indicate that a low oxygen microenvironment may act to modulate cell proliferation and chondrogenic differentiation of both AMSCs and chondrocytes. The avascularity of the cartilage tissue provides a low oxygen environment for the chondrocytes, whereas oxygen measurements of tissues known to harbor stem cells revealed lower oxygen tension to maintain the stemness of the niche.38-40 In our experiments, we observed an increased expression of HIF1A mRNA levels even in normoxic conditions at early time points in AMSCs. The main regulation of HIF1A occurs at the level of protein stability, and its protein levels are strongly stimulated by low oxygen conditions.30 We believe that robust expression of HIF1A mRNA in normoxia in AMSCs thus supports a high sentry level of labile proteins that can rapidly accumulate upon oxygen deprivation. As a consequence of continuous hypoxia under chondrogenic induction, stem cells start to produce hyaline cartilage that is resistant to hypertrophic differentiation, whereas incubation under normoxia conditions results in hypertrophic cartilage that resembles epiphyseal cartilage.26,41 Our data indicate that under chondrogenic induction AMSCs develop a chondrocyte-like phenotype, but may have a propensity to exhibit a hypertrophic response unless exposed to a hypoxic environment. Furthermore, our study suggests that molecular pathways utilized by AMSCs undergoing chondrogenic differentiation are different from those used by primary human chondrocytes. We note that the use of human primary chondrocytes from a single donor and a single anatomical location for examination of gene expression is a limitation of the present work that must be addressed in future studies in which chondrogenically differentiated AMSCs are compared with articular cartilage chondrocytes from multiple donors to define the ideal conditions for cartilage engineering strategies.
Oxygen responsiveness and chondrogenic potential also appear to be affected by the choice of cell culture model. In the current investigation, we found that changing the cell environment from a 2D culture to a 3D culture model (i.e., high-density pellets or PCL scaffold) improved the chondrogenic potential of AMSCs and primary chondrocytes by increasing cell-cell interactions and generating a low oxygen environment within cellular aggregates. We observed that conventional monolayer culture did not support the chondrogenic differentiation of AMSCs, whereas both high-density pellets and scaffold cultures are conducive to the production of a glycosaminoglycan positive matrix, while a cartilage-like gene expression profile indicates that AMSCs acquire a chondrocytic phenotype. Moreover, pellet cultures promote proliferation and a hypoxic microenvironment, whereas PCL scaffolds increase hypertrophic marker expression and the maintenance of a fibroblastic phenotype of the AMSCs. Our results using nanofibrous PCL scaffolds corroborate previous studies and supports the chondrogenic differentiation potential of MSCs when cultured under appropriate inductive conditions.42
The potential use of scaffolds for implantation relies on their flexible designs, excellent biomechanical properties, and slow degradation rate. There is evidence that MSCs favor nanotopographical geometry and porous materials over plastic for growth and differentiation.2 Scaffolds have been largely used to provide cells with structural support for attachment, proliferation, and differentiation in a 3D environment. Moreover, scaffolds facilitate optimal filling of the entire defect and provide mechanical support for the healing tissue, potentially reducing the rehabilitation time for the patient.43-46 A combination of a biodegradable scaffold with stem cells would better resemble the native tissue composition and mechanical properties, and could potentially regenerate hyaline cartilage tissue with the distinct molecular and biological properties of natural human cartilage.47-48 For articular cartilage surface regeneration, several scaffolds have been used including nanomaterials, functional mechanocompatible scaffolds, multilayered scaffolds, and ECM scaffolds.14 Some of these scaffolds can cover the entire chondral defect but their use is typically accompanied by production of a type I collagen rich ECM, thus producing a biomechanical inferior and nondurable matrix that does not resemble native cartilage. In our studies, low oxygen cultures repressed the expression of fibroblastic/osteogenic marker COL1A1 in both AMSCs and chondrocytes on both 3D cultures. Taken together, the results presented here support the important role of oxygen regulation in chondrogenic differentiation of AMSCs for clinical applications.
The cell type–specific role of low oxygen in differentiation of AMSCs indicates that mesenchymal cell fate and differentiation potential is remarkably sensitive to oxygen. The oxygen status is a dominant environmental parameter for directing chondrogenic differentiation of AMSCs and reduces their tendency to generate a fibroblastic ECM, while helping chondrocytes to maintain their phenotype by enhancing type II collagen expression and inhibiting additional matrix production. Hence, hypoxia is more effective in promoting a chondrocytic phenotype at earlier stages of differentiation. Our findings indicate that genetic programming of AMSCs to a chondrocytic phenotype is effective in cellular aggregates (pellet culture) under hypoxic conditions as reflected by increased expression of cartilage-related biomarkers and biosynthesis of a glycosaminoglycan-positive matrix. Although expression of cartilage-related genes is more robust in pellets than scaffolds, hypoxia enhances the low levels observed in scaffold cultures. Our results provide a new starting point for optimization of platelet lysate expanded AMSCs in cartilage engineering strategies that leverage the chondrogenic effects of hypoxia in the 3D environment of an implantable scaffold for treatment of focal cartilage defects.
Supplementary Material
Footnotes
Supplementary material is available for this article online.
Acknowledgments and Funding: We thank the members of our research group, including Dakota Jones, Chris Paradise, and Martina Gluscevic, for stimulating discussions, as well as Dr. Michael Yaszemski and his team for the use of the electro-spinner for the manufacturing of PCL scaffolds. This work was supported, in whole or in part, by National Institutes of Health Grants R01 AR049069 (AJvW) and F32 AR066508 (AD). We also appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their names.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Aaron Krych is a paid consultant for Arthrex, Inc. Dr. Allan Dietz is affiliated with MillCreek Life Sciences. Dr. Karperien is founder and shareholder of Hy2Care B.V.
Ethical Approval: Ethical approval for this study was obtained from the Institutional Review Board of Mayo Clinic (approval number 13-005619).
Informed Consent: Written informed consent was obtained from all subjects or legally authorized representatives before the study.
References
- 1. Falah M, Nierenberg G, Soudry M, Hayden M, Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34:621-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152-67. [DOI] [PubMed] [Google Scholar]
- 3. Murtaugh LC, Chyung JH, Lassar AB. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13:225-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pintan GF, de Oliveira AS, Jr, Lenza M, Antonioli E, Ferretti M. Update on biological therapies for knee injuries: osteoarthritis. Curr Rev Musculoskel Med. 2014;7:263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Claerbout MT, Matricali GA. Technique tip: modification of a long-leg cast for use in patients with functional impairment or muscle weakness of the upper extremities. Foot Ankle Int. 2004;25:176. [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 7. Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955-63. [DOI] [PubMed] [Google Scholar]
- 8. Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagninin S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35:2014-21. [DOI] [PubMed] [Google Scholar]
- 9. Marsano A, Millward-Sadler SJ, Salter DM, Adesida A, Hardingham T, Tognana E, et al. Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthritis Cartilage. 2007;15:48-58. [DOI] [PubMed] [Google Scholar]
- 10. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23:381-7. [DOI] [PubMed] [Google Scholar]
- 11. Brittberg M. Autologous chondrocyte transplantation. Clin Orthop Relat Res. 1999;367:147-55. [DOI] [PubMed] [Google Scholar]
- 12. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 13. Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313-21. [DOI] [PubMed] [Google Scholar]
- 14. Mayne R, Vail MS, Miller EJ. The effect of embryo extract on the types of collagen synthesized by cultured chick chondrocytes. Dev Biol. 1976;54:230-40. [DOI] [PubMed] [Google Scholar]
- 15. von der Mark K, von der Mark H. The role of three genetically distinct collagen types in endochondral ossification and calcification of cartilage. J Bone Joint Surg Br. 1977;59:458-64. [DOI] [PubMed] [Google Scholar]
- 16. Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2007;26:1230-7. [DOI] [PubMed] [Google Scholar]
- 17. Chang NJ, Lin CC, Shie MY, Yeh ML, Li CF, Liang PI, et al. Positive effects of cell-free porous PLGA implants and early loading exercise on hyaline cartilage regeneration in rabbits. Acta Biomater. 2015;28:128-37. [DOI] [PubMed] [Google Scholar]
- 18. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmoski MJ, Colyer RA, Brandt KD. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheumatol. 1980;23:325-34. [DOI] [PubMed] [Google Scholar]
- 20. Chang NJ, Lin CC, Li CF, Su K, Yeh ML. The effect of osteochondral regeneration using polymer constructs and continuous passive motion therapy in the lower weight-bearing zone of femoral trocheal groove in rabbits. Ann Biomed Eng. 2013;41:385-97. [DOI] [PubMed] [Google Scholar]
- 21. Song JQ, Dong F, Li X, Xu CP, Cui Z, Jiang N, et al. Effect of treadmill exercise timing on repair of full-thickness defects of articular cartilage by bone-derived mesenchymal stem cells: an experimental investigation in rats. PLoS One. 2014;9:e90858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129:1277-90. [DOI] [PubMed] [Google Scholar]
- 23. Veriter S, Andre W, Aouassar N, Poitel HA, Lafosse A, Docquier PL, et al. Human adipose-derived mesenchymal stem cells in cell therapy: safety and feasibility in different “hospital exemption” clinical applications. PLoS One. 2015;10:e0139566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115:1816-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797-811. [DOI] [PubMed] [Google Scholar]
- 26. Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111:13954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schagemann JC, Paul S, Casper ME, Rohwedel J, Kramer J, Kaps C, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stromal cells via biomimetic and bioactive poly-ϵ-caprolactone scaffolds. J Biomed Mater Res A. 2013;101:1620-8. [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402-408. [DOI] [PubMed] [Google Scholar]
- 29. Wang GL, Jiang BH, Semenza GL. Effect of protein kinase and phosphatase inhibitors on expression of hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1995;216:669-75. [DOI] [PubMed] [Google Scholar]
- 30. Lyer NV, Leing SW, Semenza GL. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159-65. [DOI] [PubMed] [Google Scholar]
- 31. Lyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan CM, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457-65. [DOI] [PubMed] [Google Scholar]
- 33. van Gool SA, Emons JA, Leijten JC, Decker E, Sticht C, van Houwelingen JC, et al. Fetal mesenchymal stromal cells differentiating towards chondrocytes acquire a gene expression profile resembling human growth plate cartilage. PLoS One. 2012;7:e44561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, et al. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage. 2013;21:599-603. [DOI] [PubMed] [Google Scholar]
- 35. Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17:1425-36. [DOI] [PubMed] [Google Scholar]
- 36. Chang NJ, Lin YT, Lin CC, Wang HC, Hsu HC, Yeh ML. The repair of full-thickness articular cartilage defect using intra-articular administration of N-acetyl-D-glucosamine in the rabbit knee: randomized controlled trial. Biomed Eng Online. 2015;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A. Epub Oct 2015. doi: 10.1002/jbm.a.35603. [DOI] [PubMed] [Google Scholar]
- 38. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-61. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, et al. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaewsuwan S, Song SY, Kim JH, Sung JH. Mimicking the functional niche of adipose-derives stem cells for regenerative medicine. Expert Opin Biol Ther. 2012;12:1575-88. [DOI] [PubMed] [Google Scholar]
- 41. Georgi N, Cillero-Pastor B, Eijkel GB, Periyasamy PC, Kiss A, van Blitterswijk C, et al. Differentiation of mesenchymal stem cells under hypoxia and normoxia: lipid profiles revealed by time-of-flight secondary ion mass spectrometry and multivariate analysis. Anal Chem. 2015;87:3981-8. [DOI] [PubMed] [Google Scholar]
- 42. Casper ME, Fitzsimmons JS, Stone JJ, Meza AO, Huang Y, Ruesink TJ, et al. Tissue engineering of cartilage using poly-epsilon-caprolactone nanofiber scaffolds seeded in vivo with periosteal cells. Osteoarthritis Cartilage. 2010;18:981-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Georgi N, van Blitterswijk C, Karperien M. Mesenchymal stromal/stem cell or chondrocyte-seeded microcarriers as building blocks for cartilage tissue engineering. Tissue Eng Part A. 2014;20:2513-23. [DOI] [PubMed] [Google Scholar]
- 44. Ng YC, Berry JM, Butler M. Optimization of physical parameters for cell attachment and growth on macroporous microcarriers. Biotechnol Bioeng. 2006;50:627. [DOI] [PubMed] [Google Scholar]
- 45. Malda J, Frondoza CG. Microcarriers in the engineering of cartilage and bone. Trends Biotechnol. 2006;24:299-304. [DOI] [PubMed] [Google Scholar]
- 46. Mastbergen SC, Saris DB, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9:277-90. [DOI] [PubMed] [Google Scholar]
- 47. Lin Y, Lewallen EA, Camilleri ET, Bonin CA, Jones DL, Dudakovic A, et al. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J Orthop Res. 2016;34:1950-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lewallen EA, Bonin CA, Li X, Smith J, Karperien M, Larson AN, et al. The synovial microenvironment of osteoarthritic joints alters RNA-seq expression profiles of human primary articular chondrocytes. Gene. 2016. 2016;591:456-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.