Abstract
Background
Fecal microbiota transfer (FMT) is an effective treatment for recurrent Clostridium difficile infection (CDI), but data on procedure-related complications and long-term outcome are scarce.
Methods
All patients treated with FMT for recurrent CDI at the Academic Medical Center between July 2010 and January 2016 were included. FMT was performed according to the FECAL trial protocol: administration of fresh donor feces (related or unrelated donor) through a duodenal tube after pre-treatment with vancomycin and bowel lavage. We collected information on FMT-related complications, recurrent CDI, and short- and long-term adverse events by telephone interviews using a structured questionnaire at three months after FMT, and at the time of data collection of this study.
Results
In total, 39 patients were treated with FMT. The primary cure rate (no recurrence ≤8 weeks after one infusion with donor feces) was 82% (32 of 39 patients). Of the seven patients with recurrent CDI after FMT, four were cured by antibiotic therapy alone (fidaxomicin in three patients, metronidazole in one patient) and three by repeat FMT. Peri-procedural complications occurred in five patients, comprising fecal regurgitation or vomiting. One patient died one week post-FMT due to pneumonia; a causal relation with FMT could not be excluded. The follow-up period ranged between 3 and 68 months. No long-term side effects were reported.
Conclusions
Our data underline the efficacy of FMT as treatment for recurrent CDI. Importantly, it is possible to cure post-FMT recurrences with antibiotic therapy alone. Peri-procedural complications do occur and should be closely monitored to help identify high-risk patients. To minimize the risk of complications, all FMT candidates should be evaluated to assess the most ideal delivery method.
Keywords: Clostridium difficile, infection (CDI), fecal microbiota transplantation (FMT), long-term follow-up, complications, nasoduodenal tube
Introduction
Clostridium difficile is the most common cause of nosocomial diarrhea and healthcare-associated infection.1,2 Symptoms of C. difficile infection (CDI) range from self-limiting disease to fulminant colitis. The estimated recurrence rate after treatment of an initial healthcare-associated CDI is 20–25%, with increased risk of recurrence (up to 80%) after each subsequent CDI episode.3,4 Persistent disruption of the intestinal microbiota, mostly caused by the use of antibiotics, is the most important cause of recurrent CDI.5 Even antibiotics used to treat CDI, such as oral vancomycin, influence the balance of the microbiota.6 Use of fecal microbiota transfer (FMT) has gained momentum across the globe, since it was established as a highly effective method to recurrent CDI, with cure rates around 85%.7,8 FMT leads to the restoration of the gut microbiota, resulting in colonization resistance preventing germination of C. difficile spores. However, worldwide implementation of FMT is currently limited by a lack of uniform guidelines, concerns about safety, and remaining uncertainty of long-term side effects. A recent review of the literature about adverse events in FMT reported three deaths potentially attributable to FMT for CDI,9 including septic shock with decompensated toxic megacolon,10 aspiration during sedation for colonoscopy,11 and fatal aspiration pneumonia.12 One case of microperforation after biopsy and one case of cecal perforation necessitating colectomy have been described.13,14 Norovirus transmission possibly associated with FMT has been reported in two cases.15 Mild short-term side effects include diarrhea, flatulence, transient abdominal discomfort and bloating.7,9,13,16–18 More follow-up data are needed to capture the efficacy and safety profile of FMT. Here we report the results of follow-up of patients who were treated with FMT at the Academic Medical Center (AMC), Amsterdam.
Methods
Participants
We included all patients treated with FMT for recurrent CDI between July 2010 and January 2016, at the AMC in Amsterdam, The Netherlands (after termination of the FECAL trial).7 Patients (≥18 years) were referred from across The Netherlands, and were evaluated by an infectious disease specialist (authors AG and EN) to determine eligibility before they received FMT. Pregnancy and antibiotic usage other than for C. difficile at the day of the expected infusion were absolute exclusion criteria for FMT treatment. All patients had documented recurrent CDI after at least one course of adequate CDI antibiotic therapy. Recurrent CDI was defined as the reappearance of diarrhea (≥3 unformed stools per 24 hours) in combination with a positive C. difficile toxin ELISA (after June 2015 toxin PCR). The potential risks, benefits, logistics, and procedural details were discussed during an outpatient clinic visit. If the patient had any acute medical conditions other than CDI on the day of donor feces infusion, FMT was rescheduled.
Patients gave oral consent for anonymous use of their data for scientific purposes.
Fecal microbiota transfer
The collection and infusion of donor feces was performed according to the protocol used in the FECAL trial.7 In summary, fresh donor feces was obtained from an adult family member (e.g. spouse, brother or sister, son or daughter) or an anonymous young healthy donor, after screening for infectious diseases (the anonymous donor was screened every three months).7 Donor feces was collected on the day of infusion and diluted with 500 mL of sterile saline (0.9%). This solution was poured through an unfolded gauze in a sterile bottle. On the day of FMT a nasoduodenal tube was placed under direct imaging, using Cortrak® electromagnetic imaging system. Within six hours after collection of feces by the donor, the donor feces solution was slowly administered to the patient through a nasoduodenal tube (two minutes per 50 mL syringe, with a break of at least half an hour after 250 mL).
Prior to FMT, until one day before the procedure, patients received vancomycin (250 mg q.i.d.) for a minimum of four days, followed by bowel lavage one day prior to FMT.
Pre- and post-FMT data
Pre- and post-FMT data were collected from all patients. Pre-FMT data included patient demographics, health status, and number and type of previous failed treatment courses. Short-term (<6 months) and long-term (≥6 months) post-FMT data were obtained by telephone interviews by their treating physician. Shortly after the procedure, and at three months after the procedure, patients were questioned about the occurrence of diarrhea, recurrent CDI, the use of antibiotics, and short-term adverse events (e.g. diarrhea, abdominal cramps, nausea, vomiting) in the context of standard patient care. At the time of data collection of this study patients were questioned about the occurrence of late recurrences, the use of antibiotics, and long-term adverse events (e.g. infectious complications, development of auto-immune diseases, metabolic disorders, obesity, hospital admissions) using a structured questionnaire (see Appendix A). Also, medical charts were reviewed for additional data about recurrences, hospital visits, and hospital admissions.
Primary cure was defined on clinical grounds as absence of diarrhea after initial FMT with no recurrence in the subsequent 8 weeks. Because patients were referred from all parts of the country, it was not feasible to obtain post-FMT stool samples to confirm resolution of CDI with a repeat toxin test. Secondary cure was defined as the resolution of symptoms subsequent to repeat treatment with antibiotics and/or second FMT, in the case of recurrent CDI within 8 weeks following initial FMT. The treating physician was interviewed, and medical charts were reviewed for data on the procedure, and procedure-related complications. A serious adverse event (SAE) was defined as any death, unplanned hospitalization or extension of the admission, or important medical event within 12 weeks after FMT. Following the World Health Organization–Uppsala Monitoring Centre (WHO-UMC) causality assessment system (Appendix B), SAEs were unlikely, possibly, probably, or certainly related to FMT.
Results
Pre-FMT data
Between July 2010 and January 2016, 43 patients were treated with FMT for recurrent CDI. Four patients (9%) were lost to follow-up; therefore 39 patients were included in this study. Patient characteristics and pre-FMT data are shown in Table 1. The median age of the treated patients was 73 years (range 17–97 years). Data on pre-FMT antibiotic treatment for CDI were missing in 12 of 39 patients (31%). Antibiotic treatment for CDI had failed in all patients. Regimens included metronidazole (21/27 patients), oral vancomycin in standard (25/27) or pulse-tapered regimens (15/27), and/or fidaxomicin (15/27). The time between the first episode of CDI and FMT ranged from 3 to 25 months.
Table 1.
Patient characteristics and pre-FMT data (N = 39).
| Patient characteristics | ||
|---|---|---|
| Age (yr) – median (range) | 73 | (17–97) |
| Male sex – no. (%) | 16 | (41) |
| Charlson comorbidity index19 (mean ± SD) | 3.0 | (±2.9) |
| Recurrences of CDI – median (range)* | 4 | (3–10) |
| Antibiotic courses – mean (range)** | 4.2 | (2–9) |
| Metronidazole – no. (%) | 21/27 | (84) |
| Vancomycin – no. (%) | 25/27 | (93) |
| Tapered vancomycin – no. (%) | 15/27 | (56) |
| Fidaxomicin – no. (%) | 15/27 | (56) |
| Time between first episode of CDI and FMT in months – median (range)* | 6 | (3–25) |
Data are missing for 4 patients (10%).
Data are missing for 12 (31%) patients.
Follow-up data
Follow-up was assessed in 39 patients (Table 2). For 37 patients the follow-up was ≥6 months. Of this cohort, 32 patients were successfully cured with FMT, yielding a primary cure rate of 82%. Seven patients experienced an early (≤8 weeks post-FMT) CDI recurrence. In two of these, CDI recurrence was associated with use of antibiotics within 8 weeks after FMT. In the other five patients, no antibiotics had been prescribed post-FMT.
Table 2.
Post-FMT data (N = 39).
| N | % | |
|---|---|---|
| Primary cure of FMT* | 32 | 82 |
| Recurrent CDI after FMT* | 7 | 18 |
| Early recurrence (≤8 weeks) | 7 | 100 |
| Late recurrence (>8 weeks) | 0 | 0 |
| Secondary cure | 6 | 97 |
| Serious adverse event within 12 weeks after FMT | 9 | 23 |
| Procedure related (all probably related) | 4 | 13 |
| Medical event (vomiting/regurgitation donor feces) | 4 | 13 |
| Death | 1 | 3 |
| Material related | 1 | 3 |
| Unplanned hospitalization (possibly related) | 1 | 3 |
| Not FMT related | 4 | 13 |
| Short follow-up (<6 months) | 2 | 5 |
| Long follow-up (≥6 months) | 37 | 95 |
| Follow-up period in months – median (range) | 21 | 3–68 |
| Long-term side effects | 0 | 0 |
Data are missing for 1 (4%) patient.
Recurrent CDI post-FMT was successfully treated with antibiotics alone in four of seven patients, without subsequent need of a repeat FMT. Of these, one patient was treated with metronidazole and three with fidaxomicin. The other three patients were successfully treated with a repeat FMT, of which one had first been treated with fidaxomicin but had relapsed a few weeks post treatment.
For one patient the indication of FMT had been dubious; previous CDI episodes were diagnosed based on ongoing diarrhea in combination with an inconclusive stool toxin test result for C. difficile. In addition, the patient had not responded at all to earlier treatments with either metronidazole, vancomycin, or fidaxomicin, during which diarrhea had persisted. This patient did also not respond to FMT, after which his symptoms remained unchanged. An alternative diagnosis was considered, for which he was referred to a gastroenterologist.
Fifteen patients (38%) received donor feces from a family member, 24 (62%) from an anonymous donor. There was no statistically significant difference in the development of recurrent CDI between these groups (3/15 in the family donor group versus 4/24 in the anonymous donor group, p = 0.79).
Seven patients died after the 12-week post-FMT period (range 4–28 months). None of these were related to CDI or FMT, all were associated with pre-existing chronic progressive illnesses (progressive cholangiocarcinoma, metastatic stomach cancer, Alzheimer’s disease, pulmonary embolus, acute myocardial infarct, heart failure, renal insufficiency).
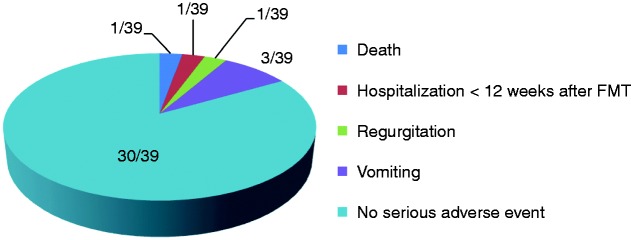
Serious adverse events
SAEs were observed in nine patients within 12 weeks post-FMT of which five (12%) were (probably) related to the FMT procedure: one patient died, and four patients experienced a medical event (Figure 1). One patient (3%) was hospitalized after FMT which was possibly related to the FMT material.
Figure 1.
Serious adverse events within 12 weeks after FMT (possibly or probably related).
One patient died 15 days after FMT due to pneumonia; a causal relation with FMT could not be excluded. This patient was fed through a PEG tube because of a swallowing disorder following oropharyngeal radiation after surgical removal of a maxillary carcinoma two years earlier. The donor feces was administered through nasoduodenal tube, which was placed (without sedation) besides the PEG tube. In the three-hour observation period after FMT, the patient had experienced some regurgitation, which at that moment did not appear to be severe. However, within one week after FMT, the patient developed pneumonia and died despite antibiotic treatment. Although no causative organism was identified, possible aspiration of donor feces could have been the cause of this pneumonia.
Procedural complications were observed in four other patients. The first patient, following uncomplicated FMT delivery, consumed a meal within one hour after FMT, after which the patient developed nausea and subsequent vomiting. The second patient had a medical history of continuous ambulatory peritoneal dialysis. During administration of the first half of donor feces suspension the patient experienced some abdominal cramps. Therefore, administration of the second half of donor feces suspension (250 mL) was postponed. After an observation period of one hour, during which the abdominal cramps had resolved, the second half of donor feces suspension was administered, and FMT was completed. However, shortly after the FMT procedure, the patient developed acute diarrhea and nausea, with subsequent vomiting. The third patient had a history of a congenital syndrome, which included mental retardation and a swallowing disorder. The patient was fed by PEG tube. This patient developed nausea and some regurgitation during FMT. Despite immediate discontinuation of the procedure, removal of the nasoduodenal tube and a symptom free post-FMT observation period of three hours, the patient vomited on the way home after discharge from the hospital. In the fourth patient, FMT was immediately halted and the nasoduodenal tube removed, when nausea and mild regurgitation occurred, after which symptoms resolved. None of these four patients developed further complications. One of these patients developed a post-FMT recurrence, which was successfully cured by a 10-day course of fidaxomicin.
Three patients were hospitalized within 12 weeks after FMT, with problems unrelated to FMT or recurrent CDI. One patient was admitted one month post-FMT with symptoms of vomiting and diarrhea, which could have been related to the FMT material. The stool sample of this patient was positive for both Yersinia enterocolitica and C. difficile. At the time of screening, the donor feces had been screened negative for these pathogens. Although the donor may have acquired Y. enterocolitica after the screening, the incubation time makes it very unlikely that the Y. enterocolitica infection was caused by FMT. The diarrhea resolved after treatment of the Y. enterocolitica infection only, without treatment for CDI. However, two months later the patient developed diarrhea again, with a positive stool toxin test for C. difficile (Y. enterocolitica negative). The patient was then successfully treated with a 10-day course of fidaxomicin and did not experience a relapse since (follow-up 36 months).
Discussion
Further implementation of FMT is hampered by the lack of uniform guidelines, concerns about safety, and remaining uncertainty about long-term side effects. With a primary cure rate of 82% in 39 patients, this study supports the currently available evidence that FMT is a very effective treatment for recurrent CDI. Importantly, four of seven patients who experienced a post-FMT recurrence were successfully cured by antibiotic therapy alone, without the need of repeat FMT. This suggests that patients treated with FMT for recurrent CDI may have a partially restored microbiota reflected by increased efficacy of antibiotic treatment compared to the pre-FMT state. We now treat a first recurrence of CDI after FMT always with antibiotics, with a preference for fidaxomicin, because of the narrow antibiotic spectrum associated with the lowest risk of recurrence in this high-risk patient group.20 In two patients, recurrent CDI was associated with use of antibiotics within 12 weeks after FMT. This finding suggests that for the restoration of the gut microbiota by FMT, the early period is crucial. Therefore we recommend to avoid antibiotics during the first month after FMT unless strictly necessary.
A classic feature of recurrent CDI is the resolution of symptoms during antibiotic treatment, but rapid recurrence of symptoms after cessation of antibiotic therapy. When patients do not respond to antibiotic treatment at all, as illustrated by one patient in this study, it should be questioned whether CDI is the actual cause of symptoms before proceeding to treatment with FMT.
In general, FMT is considered to be safe. In our cohort, with a follow-up period of more than five years in some patients, no long-term side effects (e.g. infectious complications, auto-immune disease, obesity, diabetes) were reported. However, five of 39 patients (13%) experienced regurgitation or vomiting after FMT by nasoduodenal tube. One of these patients died due to pneumonia two weeks after FMT. The possibility of aspiration due to FMT could not be excluded. Regurgitation or vomiting after FMT has been described earlier in a case series,11,21 and one case report,12 and seems the main concern of FMT through a duodenal tube compared to FMT via colonoscopy or enema. In our cohort the amount of donor feces suspension (500 mL) could have contributed to this complication. We have adjusted our protocol to prevent this complication by reducing the load of the macrogol solution prior to the procedure, and by reducing the volume of the donor feces suspension to a maximum of 200 mL, if the patient develops abdominal discomfort or nausea. It is suggested that the required amount of donor feces is 50 g.22 Therefore, it might be well possible to further decrease this volume. With the advent of lower volume solutions or oral capsules derived from stored frozen samples,23,24 the problem of regurgitation/vomiting may become less prominent.
Other preventive measures to avoid regurgitation or vomiting are:
^ Reduce stress/anxiety.
^ Avoid ingestion of food or fluids shortly (<1 hour) after FMT.
^ Use caution with pre-existing abdominal conditions (such as a calcified gut secondary to chronic peritoneal dialysis in one of our patients).
^ In case of patients who are fed across a PEG tube, consider consultation of a gastroenterologist, who can pass a jejunal extension through the PEG tube.
^ Assess aspiration risk in each patient, and if increased consider administration of FMT via colonoscopy.
^ Patients with a swallowing disorder should be excluded from FMT via a nasoduodenal tube.
^ During FMT, perform continuous monitoring of symptoms of abdominal discomfort or nausea, and discontinue FMT immediately when symptoms develop.
^ If nausea develops, consider administration of metoclopramide.
^ After FMT, patients should be observed in the hospital for at least three hours.
Donor feces is usually administered through a nasogastric or duodenal tube, colonoscopy, or enema. All methods have advantages and disadvantages, and in every patient the ideal delivery route should be assessed. In general, we prefer FMT delivery through a duodenal tube, because it is generally well tolerated by patients and less invasive compared to colonoscopy.11,25 In particular, use of the Cortrak® electromagnetic imaging system, enables safe, accurate and deep intra-duodenal positioning of the tube at patient’s bedside without assistance of endoscopy (and sedation). In our cohort, patients received bowel lavage before FMT in attempt to remove the pre-existent flora, and C. difficile spores prior to FMT.7 This may, however, not be necessary, since there are reports on FMT via upper gastrointestinal delivery (upper FMT) without bowel lavage, with similar effectiveness.21,26,27 To date, we have performed upper-FMT without bowel lavage in three patients (for various reasons), all of whom responded well. Because bowel lavage is considered to be the most inconvenient part of FMT (Van Nood et al.; submitted for publication), a study comparing upper-FMT with and without bowel lavage would be welcome. If effectiveness would be equal, bowel lavage could be discarded, greatly increasing patient comfort. Furthermore it would support the use of the upper gastrointestinal route. Administration through colonoscopy has the advantage of visibility of relevant pathology, and the capacity to infuse larger volume suspension without the risk of aspiration. However, colonoscopy carries the risk of perforation.13,14 Currently, no randomized controlled trials have been performed comparing duodenal vs. colonic delivery of donor feces. Furuya-Kanamori et al. compared upper with lower gastrointestinal delivery routes of FMT pooling data of 14 studies.28 They showed that FMT via lower gastrointestinal delivery seems to be the most effective route. However, the authors did not address the fact that more than half of the patients who received donor feces via upper delivery, received less than the recommended 50 g of donor feces.22,29 Although a recent randomized, open-label, controlled pilot study (n = 20) showed that nasogastric administration of donor feces appears to be as effective as colonoscopic administration,30 a randomized controlled trial comparing duodenal vs. colonic delivery of donor feces is required to determine the optimal route for FMT delivery. Delivery via enema has advantages in its accessibility, since it does not require endoscopy or anesthesia. Although administration via enema is well tolerated by patients, usually multiple infusions are required to reach clinical cure. In addition, some patients have problems with fecal incontinence.25 Nasogastric administration of donor feces has been less favored, presumably due to the location of insertion of the donor feces.31
A limitation of our study is its retrospective design. Four out of 43 patients were lost to follow-up, and pre-FMT data were incomplete in a subset of patients. Information was obtained through telephone calls, and information had to be recalled from memory by the patients. Another limitation of this study is that we did not collect stool samples before and after FMT. Therefore, data about dynamics of the gut microbiota over time are lacking.
In conclusion, FMT is a very effective treatment for CDI recurrence, with long-term benefits over antibiotic use. Importantly, a first post-FMT recurrence of CDI can be successfully treated with antibiotics, with a theoretical preference for fidaxomicin. FMT remains an invasive procedure and complications do occur. To avoid the risk of regurgitation or vomiting after FMT, volume reduction of donor feces suspension should be performed without hesitation in case of abdominal symptoms or nausea. In patients with risk factors for aspiration, delivery of the donor feces suspension through colonoscopy should be considered. In the first month after FMT, antibiotics should be avoided unless strictly necessary to minimize the risk of recurrent CDI after FMT.
Appendix A
Questionnaire follow-up FMT for recurrent Clostridium difficile infection






Appendix B
WHO-UMC causality categories
| Causality term | Assessment criteria |
|---|---|
| Certain | • Event or laboratory test abnormality, with plausible time relationship to drug intake • Cannot be explained by disease or other drugs • Response to withdrawal plausible (pharmacologically, pathologically) • Event definitive pharmacologically or phenomenologically (i.e. an objective and specific medical disorder or a recognized pharmacological phenomenon) • Rechallenge satisfactory, if necessary |
| Probable/likely | • Event or laboratory test abnormality, with reasonable time relationship to drug intake • Unlikely to be attributed to disease or other drugs • Response to withdrawal clinically reasonable • Rechallenge not required |
| Possible | • Event or laboratory test abnormality, with reasonable time relationship to drug intake • Could also be explained by disease or other drugs • Information on drug withdrawal may be lacking or unclear |
| Unlikely | • Event or laboratory test abnormality, with a time to drug intake that makes a relationship improbable (but not impossible) • Disease or other drugs provide plausible explanations |
| Conditional/unclassified | • Event or laboratory test abnormality • More data for proper assessment needed, or • Additional data under examination |
| Unassessable/unclassifiable | • Report suggesting an adverse reaction • Cannot be judged because information is insufficient or contradictory • Data cannot be supplemented or verified |
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ghose C. Clostridium difficile infection in the twenty-first century. Emerging Microbes Infect 2013; 2: e62–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 2009; 58: 403–410. [DOI] [PubMed] [Google Scholar]
- 4.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 2008; 197: 435–438. [DOI] [PubMed] [Google Scholar]
- 6.Louie TJ, Emery J, Krulicki W, et al. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother 2009; 53: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 8.Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection: a systematic review. Ann Internal Med 2015; 162: 630–638. [DOI] [PubMed] [Google Scholar]
- 9.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect 2016; 92: 117–127. [DOI] [PubMed] [Google Scholar]
- 10.Solari PR, Fairchild PG, Noa LJ, et al. Tempered enthusiasm for fecal transplant. Clin Infect Dis 2014; 59: 319–319. [DOI] [PubMed] [Google Scholar]
- 11.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014; 109: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter M, Ahmad T, Colville A, et al. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis 2015; 61: 136–137. [DOI] [PubMed] [Google Scholar]
- 13.Patel NC, Griesbach CL, DiBaise JK, et al. Fecal microbiota transplant for recurrent Clostridium difficile infection: Mayo Clinic in Arizona experience. Mayo Clinic Proc 2013; 88: 799–805. [DOI] [PubMed] [Google Scholar]
- 14.Potakamuri L, Turnbough L, Maheshwari A, et al. Effectiveness of fecal microbiota transplantation for the treatment of recurrent clostridium difficile infection: community hospital experience. Am J Gastroenterol 2013; 108: S175–S175. [Google Scholar]
- 15.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013; 108: 1367–1367. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis 2014; 33: 1425–1428. [DOI] [PubMed] [Google Scholar]
- 17.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46: 145–149. [DOI] [PubMed] [Google Scholar]
- 18.Aroniadis OC, Brandt LJ, Greenberg A, et al. Long-term follow-up study of fecal microbiota transplantation for severe and/or complicated Clostridium difficile infection: a multicenter experience. J Clin Gastroenterol 2016; 50: 398–402. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 20.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–431. [DOI] [PubMed] [Google Scholar]
- 21.Gweon TG, Kim J, Lim CH, et al. Fecal microbiota transplantation using upper gastrointestinal tract for the treatment of refractory or severe complicated Clostridium difficile infection in elderly patients in poor medical condition: the first study in an Asian country. Gastroenterol Res Pract 2016; 2016: 2687605–2687605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53: 994–1002. [DOI] [PubMed] [Google Scholar]
- 23.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 312: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016; 315: 142–149. [DOI] [PubMed] [Google Scholar]
- 25.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 2014; 48: 693–702. [DOI] [PubMed] [Google Scholar]
- 26.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36: 580–585. [DOI] [PubMed] [Google Scholar]
- 27.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection 2012; 40: 643–648. [DOI] [PubMed] [Google Scholar]
- 28.Furuya-Kanamori L, Doi SA, Paterson DL, et al. Upper versus lower gastrointestinal delivery for transplantation of fecal microbiota in recurrent or refractory Clostridium difficile infection: a collaborative analysis of individual patient data from 14 studies. J Clin Gastroenterol. Epub ahead of print 31 May 2016. DOI: 10.1097/MCG.0000000000000511. [DOI] [PubMed]
- 29.Terveer EM, van Beurden YH, van Dorp S, et al. Is the lower gastrointestinal route really preferred over the upper gastrointestinal route for fecal microbiota transfer? J Clin Gastroenterol 2016; 50: 895–895. [DOI] [PubMed] [Google Scholar]
- 30.Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014; 58: 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zipursky JS, Sidorsky TI, Freedman CA, et al. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis 2012; 55: 1652–1658. [DOI] [PubMed] [Google Scholar]