Abstract
Background
Duodenal intraepithelial lymphocytosis (DIL) is a histological finding characterized by the increase of intraepithelial CD3T-lymphocytes over the normal value without villous atrophy, mostly associated to coeliac disease (CD), Helicobacter pylori (Hp) gastritis and autoimmune diseases.
Objective
To assess the occurrence of DIL, CD and Hp gastritis in an endoscopic population over a 13 year period.
Methods
From 2003 to 2015 we included adult patients who consecutively underwent oesophago-gastro-duodenoscopy (OGD) with duodenal biopsies assessing the overall and annual occurrence of DIL and CD and the prevalence of Hp gastritis.
Results
160 (2.3%) patients with DIL and 275 (3.9%) with CD were detected among 7001 patients. CD occurrence was higher from 2003 to 2011, while since 2012 DIL occurrence gradually increased significantly compared to CD (p = 0.03). DIL patients were more frequently female (p = 0.0006) and underwent OGD more frequently for dyspepsia (p = 0.002) and for indications not related to gastrointestinal symptoms than CD patients (p = 0.0003). Hp gastritis occurred similarly in CD and DIL patients but the latter had higher frequency of atrophic body gastritis (p = 0.005).
Conclusions
DIL is a condition increasing in the general endoscopic population mainly diagnosed by chance. Concomitant gastric histological evaluation is able in one third of DIL patients to identify associated possible causes of DIL, such as Hp and atrophic gastritis.
Keywords: Coeliac disease, duodenal intraepithelial lymphocytosis, Helicobacter pylori, gastritis, atrophic gastritis
Introduction
Duodenal intraepithelial lymphocytosis (DIL) is a histological finding characterized by the increase of intraepithelial CD3 T-lymphocytes (IEL) over the normal value of 25–30/100 epithelial cells with preserved intestinal villous structure. Clinically, this condition may be associated with symptomatic malabsorption or more subtle micronutrient deficiencies related to specific or non-specific gastrointestinal (GI) symptoms, as recently stated in a Consensus Conference.1
DIL is commonly associated to a number of conditions involving the GI tract or due to systemic disease (non-GI diseases).2 Among GI diseases, DIL is mostly associated to gluten-related disorders, such as coeliac disease (CD) but also, more recently, non-coeliac gluten sensitivity.1–4 Clinical studies demonstrated that DIL is related to a final diagnosis of CD in a varying percentage (9–40%) of patients.3,5,6 Other non-gluten related conditions associated to DIL are Helicobacter pylori (Hp) gastritis,7,8 autoimmune disorders,9 drugs (non-steroidal anti-inflammatory agents, angiotensin II receptor antagonists and proton-pump inhibitors),10,11 bacterial overgrowth,4 inflammatory bowel diseases,12 parasitic infestations,13 food allergies, typically for cow’s milk and eggs,2 and immunodeficiency states (e.g. common variable immune deficiency).14
Reported prevalence of DIL is very heterogeneous, ranging from 1.3% in the general population up to 14.3% in a selected population.5,6,15,16 In the last decade, the frequency of this histological entity seems to have increased, reaching 7%, as reported in an endoscopic population.16
Hp infection is frequently associated to DIL, and its diagnosis and treatment may lead to normalization of duodenal IEL.7,8 Despite this strong relationship, accurate histological data on the frequency of Hp infection in patients with DIL are lacking. Most of the previous studies used a C13-urea breath test or non-standardized gastric sampling biopsies to detect Hp infection.4,8,10
The aim of this study was to assess the occurrence of DIL, CD and Hp-related conditions in an endoscopic population over a 13 year period.
Materials and methods
Study population
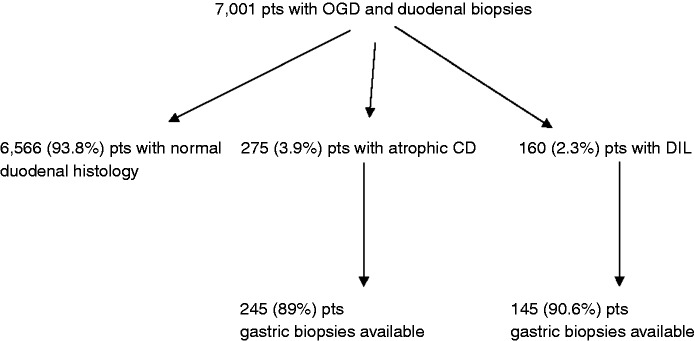
From January 2003 to December 2015 we evaluated in the Sant’Andrea Hospital pathology database for patients aged > 18 years who consecutively underwent oesophago-gastro-duodenoscopy (OGD) with duodenal biopsies. Among these patients, we considered those with a new diagnosis of DIL and CD. If some patients, during the studied period, did more than one gastroscopic procedure only the first one has been considered. We excluded from this population patients with previous intestinal resection (e.g. bariatric surgery) and/or bacterial overgrowth. The overall and annual occurrence of diagnosis of DIL and CD was assessed. Moreover in patients in whom besides duodenal biopsies also gastric biopsies were available, we assessed the presence of Hp infection and related gastric histological alterations both in DIL and CD patients (Figure 1).
Figure 1.
Flowchart of study population.
CD = celiac disease; DIL = duodenal intraepithelial lymphocytosis; OGD = oesophago-gastro-duodenoscopy.
All patients underwent an OGD using a flexible video-gastroscope, with at least four biopsies taken from the second part of the duodenum. Patients with gastric biopsies had at least five biopsies (3 from the antrum and 2 from the body) according to the updated Sydney system.17 We excluded 10 DIL patients and 20 CD patients with only duodenal and antral biopsies, 3 DIL patients and 7 CD patients with biopsies only from duodenum and body and 2 DIL patients and 3 CD patients with biopsies only from the duodenum.
Histological evaluation
The histological samples were evaluated by a GI-dedicated expert pathologist (EP). CD was diagnosed when duodenal biopsy samples showed intestinal villous atrophy (mild, moderate or severe) with crypt hyperplasia and intraepithelial lymphocytosis, according to the Marsh/Oberhuber classification,18,19 and previously described.20 Normal villous architecture and > 30 intraepithelial lymphocytes\100 enterocytes was defined as DIL, according to Veress et al.21
Intraepithelial lymphocytes T count was expressed as number of intraepithelial lymphocytes/100 epithelial cells and was carried out on immunohistochemical stained sections. Briefly, from a formalin-fixed, paraffin-embedded 3 µm tissue sections were stained with anti-CD3 mouse monoclonal antibody, clone F7.2.38 (Dako, Glostrup, Denmark), after antigen retrieval with EDTA at pH 9. Staining was visualized by Envision-Flex in automated stainer (Dako, Glostrup, Denmark).
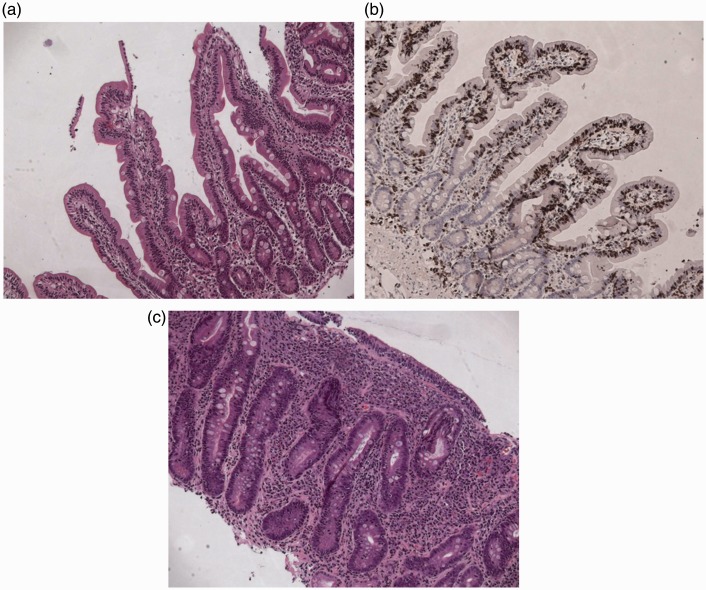
Figure 2 presents histological pictures in Haematoxylin & Eosin (H&E) and CD3 immunohistochemistry of DIL and in H&E of atrophic CD (Marsh 3C).
Figure 2.
Series of histological slides of typical mucosal biopsies in (a) DIL at H&E stain (×10), (b) DIL at CD3 immunohistochemistry (×10), (c) Marsh 3C lesion at H&E (×10).
Gastric histologic evaluation was performed according to the updated Sydney System classification.17 Gastric specimens were considered to be Hp negative if Hp was not detected with haematoxylin and eosin staining. Chronic active gastritis was defined as the presence of polymorphonuclear cells in the lamina propria in the absence of Hp organism. Chronic inactive gastritis was defined as the presence of lymphocytes and plasma cells within the lamina propria, in the absence of activity or Hp organism.22 Reactive gastritis includes foveolar or regenerative hyperplasia, oedema or hyperaemia of the lamina propria, erosions and smooth muscle proliferation.23 Lymphocytic gastritis was defined by the presence of at least 25 lymphocytes/100 epithelial cells on the surface and foveolar epithelium, with chronic inflammation also in the lamina propria.24 Atrophy of the corporal mucosa was defined as the focal or complete replacement of oxyntic glands by metaplastic pyloric or intestinal glands, atrophy of the antral mucosa was defined as focal or complete replacement of antral glands by intestinal metaplastic epithelium.25
Statistics
Data were expressed as values and percentages or medians and interquartile ranges. Comparisons between different groups were made using the Fisher’s exact test or chi-square test. A p value <0.05 was considered statistically significant. Dedicated software (Medcalc Belgium 12.0) was used for these purposes.
Results
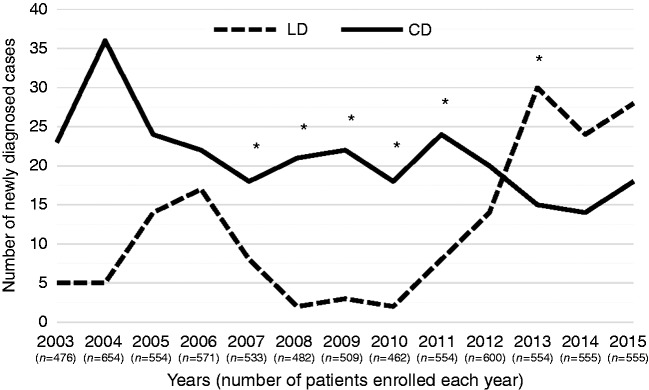
From January 2003 to December 2015, 7001 patients were identified over a 13 year period (Figure 1). DIL was diagnosed in 160 patients and CD in 275 patients with an overall occurrence of 2.3% and 3.9%, respectively. Figure 3 shows the yearly occurrence of the new diagnoses of DIL and CD during the study period. The number of patients enrolled each year remained almost steady during the entire time-period, with minimal fluctuations. Compared to DIL, the yearly occurrence of CD was higher in the years from 2003 to 2011. Since 2012 the number of patients diagnosed with DIL has increased, while the occurrence of new cases of CD has decreased. We observed a statistically significant higher occurrence (p = 0.001) of CD from 2003 to 2004 and from 2008 to 2011. From 2005 to 2007, the number of newly diagnosed cases of CD was higher than the number of DIL, but did not reach statistical significance (p = 0.5). Starting from 2012, the number of patients newly diagnosed with DIL was higher compared to those diagnosed with CD (p = 0.03).
Figure 3.
Yearly occurrence of duodenal intraepithelial lymphocytosis (DIL) and celiac disease (CD) over the 13-year study period.
Continuous line = celiac disease; dotted line = duodenal intraepithelial lymphocytosis.
*p < 0.05.
Concerning patients’ clinical features (Table 1), a significantly higher prevalence of females was observed both in DIL (87.5%) and CD (73.2%) compared with the endoscopic population with normal duodenal histology (66.5%) (p < 0.001 and p = 0.01, respectively). Moreover, female gender was more frequent in patients with DIL than in patients with CD (87.5% versus 73.2%) (p = 0.0006). The endoscopic population with normal duodenal histology was significantly older of age compared to both DIL and CD patients (p = 0.001). DIL patients were older of age (40.5 yrs, range 18–80) than CD patients (37 yrs, range 18–78; p = 0.007). As shown in Table 1, DIL patients underwent OGD more frequently for dyspepsia than CD patients (p = 0.002). DIL patients were more frequently diagnosed by indications to gastroscopy not related to GI symptoms than CD patients (p = 0.0003). As expected, signs of malabsorption, antibodies positivity or osteopenia/osteoporosis with a stronger suspicion for CD leaded more frequently to CD diagnosis (p < 0.0001).
Table 1.
Clinical features of patients with duodenal intraepithelial lymphocytosis (DIL) and celiac disease (CD).
| DIL n = 160 | CD n = 275 | p | |
|---|---|---|---|
| Female gender | 140 (87.5%) | 202 (73.2%) | <0.001 |
| Age, median (range) | 40.5 (18–80) | 37 (18–78) | 0.007 |
| Indications for oesophago-gastro-duodenoscopy | |||
| - Gastrointestinal symptoms (diarrhoea, abdominal pain) | 21 (13%) | 36 (13%) | Ns |
| - Iron deficiency anaemia | 64 (40%) | 87 (31.6%) | Ns |
| - Dyspepsia | 33 (20.6%) | 34 (12.3%) | 0.02 |
| - 1st degree family history for CD/ autoimmunity screening | 6 (3.7%) | 21 (7.6%) | Ns |
| - By chance (others indications not related to gastrointestinal symptoms) | 18 (11.5%) | 7 (2.5%) | <0.001 |
| - Suspected CD (antibodies positivity, osteopenia, signs of malabsorption) | 18 (11.2%) | 90 (33%) | <0.001 |
Data expressed as total number (percentage, %), when not otherwise indicated.
Ns = not significant.
Gastric histology in DIL and CD patients
As shown in Table 2, atrophic gastritis, both autoimmune and Hp-related, was found in 10 (6.9%) patients with DIL and 3 (1.2%) patients with CD (p = 0.005). Other gastric histological findings were not statistically different between DIL and CD, as normal gastric histology (42.7% vs 51%; p = 0.1173), Hp-positive gastritis (26.9% vs 22%, respectively; p = 0.325), Hp-negative gastritis (both chronic active and inactive gastritis) (17.2% vs 13.5%; p = 0.377), reactive gastritis (4.1% vs 9.4%; p = 0.071) and lymphocytic gastritis (2% vs 2.8%; p = 0.750).
Table 2.
Histological gastric mucosal findings in patients with duodenal intraepithelial lymphocytosis (DIL) and celiac disease (CD).
| DIL n = 145 | CD n = 245 | p | |
|---|---|---|---|
| Histologically normal gastric mucosa | 62 (42.7%) | 125 (51%) | Ns |
| Hp-related gastritis | 39 (26.9%) | 54 (22%) | Ns |
| – Antral gastritis | 6 | 12 | |
| – Pangastritis | 33 | 42 | |
| Negative Hp gastritis | 25 (17.2%) | 33 (13.5%) | Ns |
| – Chronic active gastritis | 3 (2%) | 7 (2.8%) | |
| – Chronic inactive gastritis | 22 (15.2%) | 26 (10.7%) | |
| Patients with reactive gastritis | 6 (4.1%) | 23 (9.4%) | Ns |
| Atrophic gastritis | 10 (6.9%) | 3 (1.2 %) | 0.005 |
| – Hp-related | 3 | 1 | |
| – Autoimmune | 7 | 2 | |
| Lymphocytic gastritis | 3 (2%) | 7 (2.8%) | Ns |
Data expressed as total number (percentage, %).
Hp = Helicobacter pylori.
Ns = not significant.
Discussion
This study assessed the occurrence of newly diagnosed cases of DIL and CD in an endoscopic population over a period of 13 years, showing an occurrence of 2.3% for DIL and 3.9% for CD. The respective occurrence changed over time: CD had the highest occurrence from 2003 to 2011, but from 2012 to 2015 DIL was diagnosed more frequently exceeding CD. Several studies evaluated the frequency of the DIL diagnosis leading to heterogeneous results. The frequency of newly diagnosed cases of DIL ranged from 1.34% to 14.3%.5,15 Such different results may arise from different inclusion criteria and time periods of the study population taken into account. However, it has been recently reported that the number of patients diagnosed with DIL is increasing.16,26 One of the reasons for such increase can be found in the expertise of the pathologists, who make use of the immunohistochemical stain for CD3 + lymphocytes. Another possible reason is the spread of the conditions which may lead to DIL, such as the chronic use of drugs or autoimmune diseases.4 But, the increase in newly diagnosed cases of DIL could be also explained by the reduction, compared to the past, in the number of IELs necessary for the diagnosis (25–30 IELs/100 enterocytes nowadays, while in the past the number of IELs to make diagnosis of DIL was 40).
In our study, we considered patients regardless of the indications for OGD. Although our unit is a tertiary academic centre, patients were not selected on the basis of indications for gastroscopy with high suspicion for CD. Furthermore, our cut-off to diagnose DIL (30 IELs/100 enterocytes) was maintained steady over the years thus avoiding the bias related to changed diagnostic criteria. Thus the findings of our study support the previously reported increase in DIL diagnosis.
Our study showed that patients with DIL and CD were mostly female and younger than patients with normal histological duodenum. The increased prevalence of female patients with DIL and CD has already been demonstrated by several studies,4,8,16,27 but there are still no data available in literature on the comparison between DIL and CD patients in terms of age and gender. In our study, we found that patients with DIL are more frequently female and older than CD patients. It has already been demonstrated in literature that female gender has a higher predisposition to autoimmune diseases, as autoimmune thyroid disease, rheumatoid arthritis or ankylosing spondylitis,28 conditions which are more frequently associated with DIL. Some of these conditions are linked to chronic pain requiring chronic use of analgesic drugs as NSAIDs,29 in turn related to DIL. Thus, it is possible that some factors related with DIL are more frequent in females. However, in general, the health-seeking behaviour and the attitude of seeking medical attention are more pronounced in females,30 possibly explaining the higher female prevalence amongst DIL and CD.
In this study, we also evaluated the indications to OGD for patients with DIL and CD. Table 1 shows that the indications are different between the two groups. On the one hand, patients with DIL were more often diagnosed when they underwent OGD for nonspecific symptoms, such as dyspepsia, or for other reasons not related to GI symptoms (i.e. family history of gastric cancer or thoracic pain). On the other hand, patients with CD were more often diagnosed by performing OGD for the common signs and symptoms suggestive of CD (i.e. malabsorption, diarrhoea, abdominal pain), as also shown by a very recent study reporting that patients with typical GI symptoms are more easily diagnosed as having CD disease.31 There are few studies on the evaluation of the indications to OGD and only two of them showed that dyspepsia is a common indication for OGD for those patients who are diagnosed with DIL.8,16 Moreover, a recent study showed that dyspepsia is more severe in patients with Hp infection who have also DIL.32 The same study also showed that dyspepsia may disappear or be significantly reduced after Hp eradication. This could suggest that duodenal biopsies should be taken, together with gastric biopsies, in patients who undergo OGD for gastric symptoms in order to exclude the presence of DIL.
With respect to the gastric histologic alterations related to DIL and CD, our study showed some peculiarities. First of all, the prevalence of Hp infection in patients with DIL and CD was not statistically different (26.9% versus 22%). Compared to the frequency of Hp infection in the Italian general population in an area where the infection has a medium to high prevalence,33 this figure is not different to that shown in the two groups of patients considered in our study. It has been described that Hp infection leads to DIL in a percentage of patients ranging from 6% to 14%, however the prevalence of such infection in DIL patients is still not clear.4,16,34 Only one study described a Hp prevalence of 19% in patients with DIL, a lower prevalence with respect to the local general population.35 This lack of data is most likely related to the fact that Hp infection is usually evaluated through C13 urea breath test or incomplete gastric biopsies (that is not taken from both gastric body and antrum, according to the updated Sydney System),4,8,10 which do not allow to assess the real prevalence of Hp infection. In our study, we enrolled only patients who had complete gastric biopsies in order to obtain an accurate evaluation of Hp infection prevalence in DIL patients, which was 26.9%. According to these results, eradication therapy for Hp should be administered in DIL patients, in order to tempt to obtain the regression of the histological alterations, both in the stomach and in duodenum.
With respect to the gastric histologic alterations not related to Hp infection, our study showed that 17.2% of DIL patients and 13.5% of CD patients had Hp negative gastritis (both chronic active and inactive gastritis). In particular, the percentage of patients with DIL who had chronic inactive gastritis was higher than the percentage showed by the study of Lebwohl et al.36 Such data may be related to geographical differences between the populations considered in the two studies. Moreover, so far, no study has been carried out to evaluate the possible role of this condition as a cause of DIL.
With regard to lymphocytic gastritis, there is only one study that evaluated the prevalence of such a condition in DIL.36 Consistent with that study, in our population the prevalence was low in patients with DIL (Table 2). We also observed that lymphocytic gastritis was lower among our CD patients compared to what has been observed before.36–38 This might be explained, again, by geographical and ethnic differences between the study populations.
Our study also showed that 6.9% of DIL patients had AG (atrophic gastritis of body and fundus), compared to 1.2% of CD patients. Our study is the first one to show a higher prevalence of AG in DIL patients compared to CD patients. This different AG occurrence in DIL patients may be explained by two pathogenic mechanisms, which are responsible for the gastric atrophy and which could lead to an increase in the number of IELs: autoimmunity and Hp infection.39 In our patients, AG seemed to arise from both the described pathogenic mechanisms (Table 2) and for such reason it is likely that the prevalence of AG is higher in DIL patients rather than CD patients. Therefore, a complete and accurate gastric mapping is useful to detect AG in DIL patients.
In conclusion, our study showed that DIL is increasing in the endoscopic population and that it is more frequently found in patients who undergo OGD for dyspeptic symptoms or for others indications not related to GI symptoms. Moreover, our study showed that the concomitant gastric histological evaluation is able, in one third of DIL patients, to identify associated possible causes of DIL, such as Hp and atrophic gastritis.
Declaration of conflicting interests
None declared.
Funding
This work was supported via grants from University Sapienza, Rome, in 2016.
References
- 1.Rostami K, Aldulami D, Holmes G, et al. Microscopic enteritis: Bucharest consensus. World J Gastroenterol 2015; 21: 2593–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown I, Mino-Kenudson M, Deshpande V, et al. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Pathol Lab Med 2006; 130: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 3.Wahab PJ, Crusius JB, Meijer JW, et al. Gluten challenge in borderline gluten-sensitive enteropathy. Am J Gastroenterol 2001; 96: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 4.Aziz I, Evans KE, Hopper AD, et al. A prospective study into the aetiology of lymphocytic duodenosis. Aliment Pharmacol Ther 2010; 32: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 5.Kakar S, Nehra V, Murray JA, et al. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol 2003; 98: 2027–2033. [DOI] [PubMed] [Google Scholar]
- 6.Mahadeva S, Wyatt JL, Howdle PD. Is a raised intraephitelial lymphocyte count with normal duodenal villous architecture clinically relevant? J Clin Pathol 2002; 55: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memeo L, Jhang J, Hibshoosh H, et al. Duodenal intraepithelial lymphocytosis with normal villous architecture: common occurrence in H. pylori gastritis. Mod Pathol 2005; 18: 1134–1144. [DOI] [PubMed] [Google Scholar]
- 8.Nahon S, Patey-Mariaud De Serre N, Lejeune O, et al. Duodenal intraepithelial lymphocytosis during Helicobacter pylori infection is reduced by antibiotic treatment. Histopathology 2006; 48: 417–423. [DOI] [PubMed] [Google Scholar]
- 9.Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep 2012; 14: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosinach M, Esteve M, Gonzalez C, et al. Lymphocytic duodenosis: aetiology and long-term response to specific treatment. Dig Liver Dis 2012; 44: 643–648. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe sprue-like enteropathy associated with olmesartan. Mayo Clin Proc 2012; 87: 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidali F, Di Sabatino A, Broglia F, et al. Increased CD8 + intraepithelial lymphocyte infiltration and reduced surface area to volume ratio in duodenum of patients with ulcerative colitis. Scand J Gastroenterol 2010; 45: 684–689. [DOI] [PubMed] [Google Scholar]
- 13.Hanevik K, Hausken T, Morken MH, et al. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect 2007; 55: 524–530. [DOI] [PubMed] [Google Scholar]
- 14.Washington K, Stenzel TT, Buckley RH, et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X linked agammaglobulinaemia. Am J Surg Pathol 1996; 20: 1240–1252. [DOI] [PubMed] [Google Scholar]
- 15.Biagi F, Bianchi I, Campanella J, et al. The prevalence and the causes of minimal intestinal lesions in patients complaining of symptoms suggestive of enteropathy: a follow-up study. J Clin Pathol 2008; 61: 1116–1118. [DOI] [PubMed] [Google Scholar]
- 16.Shmidt E, Smyrk TC, Boswell CL, et al. Increasing duodenal intraepithelial lymphocytosis found at upper endoscopy: time trends and associations. Gastrointest Endosc 2014; 80: 105–111. [DOI] [PubMed] [Google Scholar]
- 17.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 18.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102: 330–354. [PubMed] [Google Scholar]
- 19.Villanacci V, Ceppa P, Tavani E, et al. Coeliac disease: the histology report. Dig Liver Dis 2011; 43(Suppl 4): S385–S395. [DOI] [PubMed] [Google Scholar]
- 20.Galli G, Esposito G, Lahner E, et al. Histological recovery and gluten-free diet adherence: a prospective 1-year follow-up study of adult patients with coeliac disease. Aliment Pharmacol Ther 2014; 40: 639–647. [DOI] [PubMed] [Google Scholar]
- 21.Veress B, Franzèn L, Bodin L, et al. Duodenal intraepithelial lymphocyte-count revisited. Scand J Gastroenterol 2004; 39: 138–144. [DOI] [PubMed] [Google Scholar]
- 22.Genta RM, Sonnenberg A. Helicobacter-negative gastritis: a distinct entity unrelated to Helicobacter pylori infection. Aliment Pharmacol Ther 2015; 41: 218–226. [DOI] [PubMed] [Google Scholar]
- 23.Genta RM. Differential diagnosis of reactive gastropathy. Semin Diagn Pathol 2005; 22: 273–283. [DOI] [PubMed] [Google Scholar]
- 24.Carmack SW, Lash RH, Gulizia JM, et al. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol 2009; 16: 290–306. [DOI] [PubMed] [Google Scholar]
- 25.Annibale B, Aprile MR, D’Ambra G, et al. Cure of Helicobacter pylori infection in atrophic body gastritis patients does not improve mucosal atrophy but reduces hypergastrinemia and its related effects on body ECL-cell hyperplasia. Aliment Pharmacol Ther 2000; 14: 625–634. [DOI] [PubMed] [Google Scholar]
- 26.Lauwers GY, Fasano A, Brown I. Duodenal lymphocytosis with no or minimal enteropathy: much ado about nothing? Mod Pathol 2015; 28(Suppl 1): S22–S29. [DOI] [PubMed] [Google Scholar]
- 27.Zanini B, Lanzarotto F, Villanacci V, et al. Clinical expression of lymphocytic duodenosis in ‘mild enteropathy’ celiac disease and in functional gastrointestinal syndromes. Scand J Gastroenterol 2014; 49: 794–800. [DOI] [PubMed] [Google Scholar]
- 28.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 2008; 173: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atzeni F, Masala IF, Salaffi F, et al. Pain in systemic inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 2015; 29: 42–52. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen JT, Andersen JS, Tjønneland A, et al. Determinants related to gender differences in general practice utilization: Danish diet, cancer and health cohort. Scand J Prim Health Care 2016; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy A, Mehra S, Kelly CP, et al. The association between socioeconomic status and the symptoms at diagnosis of celiac disease: a retrospective cohort study. Ther Adv Gastroenterol 2016; 9: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirbagheri SA, Khajavirad N, Rakhshani N, et al. Impact of Helicobacter pylori infection and microscopic duodenal histopathological changes on clinical symptoms of patients with functional dyspepsia. Dig Dis Sci 2012; 57: 967–972. [DOI] [PubMed] [Google Scholar]
- 33.Lahner E, Zullo A, Hassan C, et al. Detection of gastric precancerous conditions in daily clinical practice: a nationwide survey. Helicobacter 2014; 19: 417–424. [DOI] [PubMed] [Google Scholar]
- 34.Hammer STG, Greenson JK. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch Pathol Lab Med 2013; 137: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 35.Simondi D, Ribaldone DG, Bonagura GA. Helicobacter pylori in celiac disease and in duodenal intraepithelial lymphocytosis: active protagonist or innocent bystander? Clin Res Hepatol Gastroenterol 2015; 39: 740–745. [DOI] [PubMed] [Google Scholar]
- 36.Lebwohl B, Green PH, Genta RM. The coeliac stomach: gastritis in patients with coeliac disease. Aliment Pharmacol Ther 2015; 42: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown IS, Smith J, Rosty C. Gastrointestinal pathology in celiac disease: a case series of 150 consecutive newly diagnosed patients. Am J Clin Pathol 2012; 138: 42–49. [DOI] [PubMed] [Google Scholar]
- 38.Villanacci V, Bassotti G, Liserre B, et al. Helicobacter pylori infection in patients with coeliac disease. Am J Gastroenterol 2006; 101: 1880–1885. [DOI] [PubMed] [Google Scholar]
- 39.Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol 2009; 15: 5121–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]