Abstract
Background
Celiac disease (CD) often manifests with dyspeptic symptoms and chronic gastritis is a common finding.
Aim
To evaluate the frequency of lymphocytic gastritis (LG), chronic active gastritis (CAG), and chronic inactive gastritis (CIG) in patients with CD, before and after gluten-free diet (GFD).
Methods
A five-year prospective study including all consecutive patients with a new diagnosis of CD was conducted. Gastric and duodenal biopsy specimens taken both at the time of the CD diagnosis and at the first endoscopic control after 18–24 months on GFD were evaluated.
Results
213 patients with CD were enrolled. At the time of the diagnosis, 42 patients (19.7%) showed normal gastric mucosa, 34 (15.9%) LG, 67 (31.5%) CAG, and 70 (32.9%) CIG. Out of the 34 patients with LG, all were Helicobacter pylori negative and the majority of them showed an improvement both of gastritis (94.1%) and duodenal lesions (82.3%) after GFD. GFD did not show significant effects on CAG and CIG.
Conclusions
LG is present in 16% of CD patients, it is not associated with H. pylori infection, and it improves after GFD. Both CAG and CIG are also frequently associated with CD, but fail to respond to a GFD.
Keywords: Celiac disease, lymphocytic gastritis, chronic active gastritis, chronic inactive gastritis
Introduction
Celiac disease (CD) is a chronic, immune-mediated disorder of the small bowel triggered by gluten in genetically predisposed individuals.1 Untreated CD may cause intestinal and extraintestinal symptoms, malabsorption, iron deficiency, osteoporosis, reduced quality of life, and an increased risk of cancer, mainly lymphoma. CD patients may complain of mild dyspeptic symptoms, but the condition can often be asymptomatic. It has been estimated that CD affects approximately 1% of the whole world population, and is frequently associated with a number of autoimmune diseases – in particular diabetes mellitus type 1 and thyroiditis. Mucosal damage is not confined to the small bowel but may be found in the gastric mucosa.1 Indeed, lymphocytic gastritis (LG), chronic active gastritis (CAG), and chronic inactive gastritis (CIG) all appear to be more common in patients with CD.2 In particular, LG is strongly associated with CD, a strict correlation being found with severity of duodenal lesions, in particular villous atrophy.2
LG, first described by Haot et al. in 1986,3,4 is a very rare form of gastritis (0.8–1.6% of cases) with unclear pathogenesis.5 Its endoscopic appearance can vary from a normal mucosa to varioliform gastritis, nodularity, hypertrophic gastropathy, and aphthous erosions.1,6 Its etiopathogenesis is currently thought to be drug-induced, or a sprue-associated reaction, or an atypical inflammatory response to Helicobacter pylori (H.pylori) infection. LG was reported in almost 45% of cases of CD, with lymphocytic infiltration being more pronounced in the antrum and in patients with severe duodenal lesions.7 A correlation of LG with serologically and/or histologically confirmed H. pylori infection was found.5 The clinical significance of LG, in patients with or without CD, has not been evaluated, however, and there is no recognized treatment strategy for this histological condition.
The aim of the present study has been to evaluate the frequency of LG, CAG, and CIG in patients with CD and the histological improvements that a gluten-free diet (GFD) can determine in each case.
Methods
A prospective study was conducted at the Gastroenterology Unit of the University of L’Aquila enrolling all consecutive patients from January 2010 to June 2014. All patients underwent upper endoscopy with gastric and duodenal biopsies in response to one or more of the following signs and symptoms: dyspepsia, abdominal pain or discomfort, bloating, diarrhea, anemia, joint pain, dermatitis, autoimmune thyroiditis, type 1 diabetes, weight loss, family history of CD, positive screening for CD. All consecutive patients with a new diagnosis of CD were included.
The study was performed with the institutional review board approval and the written informed consent of all patients. All clinical investigations were conducted according to the principles laid down in the Declaration of Helsinki.
All enrolled patients underwent clinical, laboratory, endoscopic and histological evaluation. Laboratory examination included anti-tissue transglutaminase (anti-tTG) antibodies, anti-endomysial antibodies (anti-EmA), and anti-deamidated gliadin peptide antibodies (for all, both IgA and IgG), genetic testing for CD (HLA-DQ2 and HLA-DQ8 haplotypes), IgE antibodies to wheat, and skin prick test. Completed blood cell count, acute phase reactants, iron, ferritin, and immunoglobulins were also assessed. Gastric and duodenal biopsy specimens at the time of the diagnosis of CD and at the first endoscopic control after 18–24 months on GFD were assessed by hematoxylin and eosin (HE) and CD3 immunohistochemical staining.
The diagnosis of CD was made in the presence of anti-tTG antibodies and/or anti-EmA, associated with specific duodenal alterations at biopsy, such as increased intraepithelial lymphocytes (IELs), crypt hyperplasia, and villous atrophy (Figure 1), according to Marsh classification, as follows:
type 1: increased IELs;
type 2: increased IELs and crypt hyperplasia;
type 3: villous atrophy with increased IELs and crypt hyperplasia.8,9
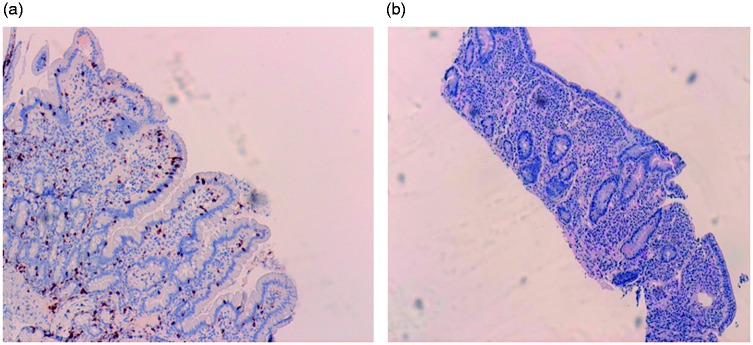
Figure 1.
Section from normal duodenal mucosa (a) and from mucosal biopsy with celiac disease (b). Panel (a) stained for CD3 (original magnification 10×) shows a normal architecture of the villi and crypts with only minimal numbers (<5/100 epithelial cells) of Intraepithelial lymphocytes (IELS). Panel (b) (original magnification 4×) shows a complete villous atrophy with marked increase of IELs and a dense inflammatory infiltrate, predominantly plasma cells, in the lamina propria (celiac disease type 3 according to the Marsh classification).
Patients without these findings were classified as having normal duodenal biopsies; those with active mucosal inflammation with or without erosions or foveolar cell metaplasia were classified as having duodenitis.
Patients with discordant results – i.e. positive antibodies and negative biopsies –were genetically tested for the presence of HLA DQ2 and/or DQ8 haplotypes; if this proved negative, CD was ruled out.
Gastric lesions were classified according to the updated Sydney system as follows:10
LG was defined as the presence of >20 lymphocytes/100 epithelial cells, with chronic inflammation in the lamina propria, in presence or absence of activity or H. pylori organisms (Figure 2).
CAG was defined as the presence of a mixed inflammatory infiltrate in the lamina propria, in presence or absence of H. pylori organisms.
CIG was defined as the presence of dense population of lymphocytes and plasma cells within the lamina propria, in presence or absence of activity or H. pylori organisms.
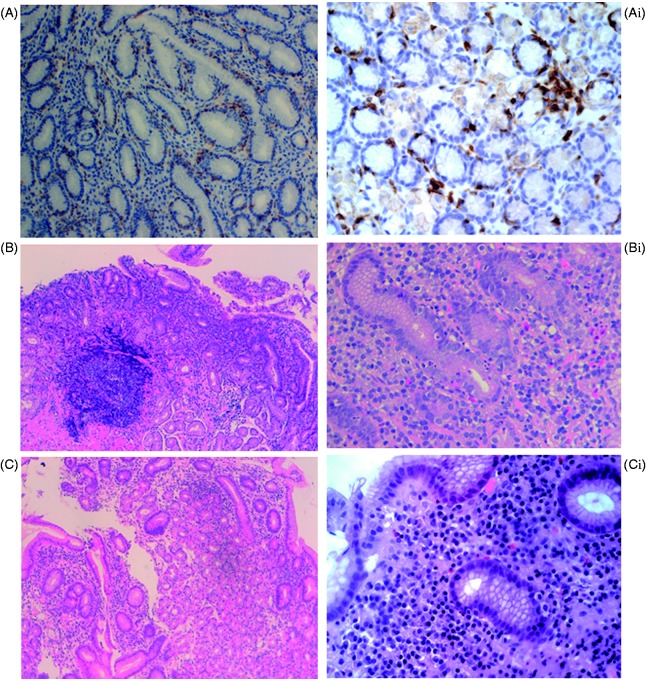
Figure 2.
Sections from gastric mucosa stained for CD3 (A–Ai) and with hematoxylin and eosin (B–Bi and C–Ci). Panel A (original magnification 10×), Ai (40×), shows a marked increase of lymphocytes (>20 lymphocytes/100 epithelial cells), with a chronic inflammatory infiltrate in the lamina propria (plasma cells, lymphocytes and rare neutrophils), typical findings of lymphocytic gastritis. Panel B (original magnification 4×), Bi (40×), shows erosion of the surface epithelium, activated lymphoid follicle with germinal center, glandular alteration with infiltrate of neutrophils and an increase in inflammatory cells in the lamina propria, findings consistent with the diagnosis of chronic active gastritis (CAG). The presence of H. pylori was also detected. Panel C (original magnification 4×), Ci (40×), shows a preserved glandular structure with a mild inflammatory infiltrate (plasma cells and lymphocytes) in the lamina propria, findings indicative of a chronic inactive gastritis. H. pylori was absent.
H. pylori infection was assessed by Giemsa staining of histological sections of biopsies taken in the antrum and fundus of the stomach. For confirmation, H. pylori negative CAG cases were also evaluated by the monoclonal enzyme immunoassay (EIA)-based stool antigen test (SAT). Autoimmune gastritis was diagnosed by enzyme-linked immunosorbent assay (ELISA) for anti-gastric parietal cell antibodies. Demographic and clinical data were collected. GFD was proposed to all CD patients enrolled in the study. Education to GFD and compliance with it were assessed throughout the study by monthly interviews of the patients by a trained nutritionist.
Statistical analysis
Comparisons among groups were assessed by the chi-square test and Fisher’s exact test, as appropriate, for categorical variables. A p-value of <0.05 was considered statistically significant. The prognostic value was expressed as risk ratio (RR) and corresponding 95% confidence interval (CI). McNemar test has been used to verify the efficacy of GFD on the gastric lesions. All statistical analyses were performed using SAS 9.4 Statistical software (SAS Institute Inc., Cary, NC, USA).
Results
During the 4 years study, 250 new cases of CD were diagnosed (191 F, 59 M, mean age 34 years at the diagnosis). Of these 250 patients, 22 were lost to the follow-up, 10 did not accept to undergo the control gastroscopy after 18–24 months of GFD, and five did not follow the GFD. Overall, 213 patients completed the study and were included in the statistical analysis.
No patients with CD had other concomitant causes of villous atrophy in the duodenum as tropical sprue, small-bowel bacterial overgrowth, autoimmune enteropathy, Whipple disease, collagenous sprue, Crohn’s disease, eosinophilic enteritis, intestinal lymphoma, infectious enteritis (e.g. giardiasis), graft versus host disease, acquired immune deficiency syndrome enteropathy, or drug-associated enteropathy (e.g. olmesartan).11,12 Small bowel bacterial overgrowth was evaluated by glucose H2 breath test. Drug-associated enteropathy was excluded through anamnestic data: no patient had taken olmesartan and no patients had taken nonsteroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs) during the three months before gastroscopy.
Gastric histological lesions at CD diagnosis
213 patients with CD were evaluated (174 F, 39 M, mean age 32 years at the diagnosis) showing the following duodenal lesions: Marsh-1 23 patients (10.8%); Marsh-2 18 (8.4%); Marsh-3 172 (80.8%). At the time of CD diagnosis, histological examination showed normal gastric mucosa in 42 patients (19.7%), gastritis in 171 patients (80.3 %), and 55 (32.1%) of them showed H. pylori infection. Frequencies of the subtypes of chronic gastritis are shown in Table 1.
Table 1.
Frequency of chronic gastritis subtypes in patients with celiac disease.
| LG | CAG | CIG | Normal mucosa | |
|---|---|---|---|---|
| Antrum, N | 30 | 27 | 38 | – |
| Fundus, N | 0 | 0 | 6 | – |
| Antrum + fundus, N | 4 | 40 | 26 | 42 |
| Total, N (%) | 34 (15.9%) | 67 (31.5%) | 70 (32.9%) | 42 42 (19.7%) |
LG = lymphocytic gastritis; CAG = chronic active gastritis; CIG = chronic inactive gastritis.
H. pylori infection was found in 77.6% of patients with CAG, in 4.3 % of patients with CIG, and in no patient with LG (Table 2).
Table 2.
Helicobacter pylori infection in the three subtypes of gastritis.
| H. pylori + | H. pylori − | |
|---|---|---|
| LG | ||
| Antrum, N | 0 | 30 |
| Fundus, N | 0 | 0 |
| Antrum + fundus, N | 0 | 4 |
| Total, N (%) | 0 | 34 (100%) |
| CAG | ||
| Antrum, N | 23 | 4 |
| Fundus, N | 0 | 0 |
| Antrum + fundus, N | 29 | 11 |
| Total, N (%) | 52 (77.6%) | 15 (22.4%) |
| CIG | ||
| Antrum, N | 2 | 36 |
| Fundus, N | 0 | 6 |
| Antrum + fundus, N | 1 | 25 |
| Total, N (%) | 3 (4.3%) | 67 (95.7%) |
LG = lymphocytic gastritis; CAG = chronic active gastritis; CIG = chronic inactive gastritis.
A significant correlation between LG and a more advanced intestinal villous atrophy (in according to Marsh classification) was found (p = 0.0001) (Figure 3).
Figure 3.
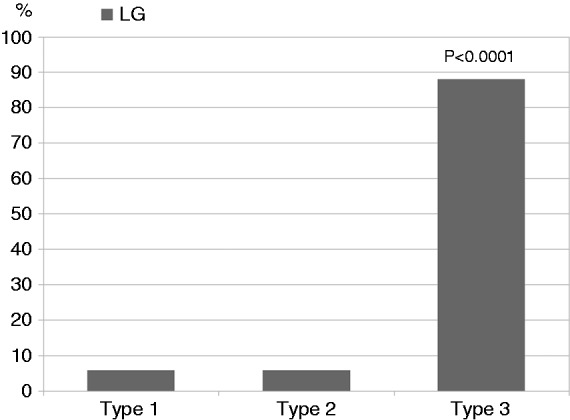
Lymphocytic gastritis (LG) significantly correlates with advanced intestinal villous atrophy (p < 0.0001).
Gastric histological changes after GFD
All the patients underwent control endoscopy with biopsies at a median follow up of 21 months (range 18–24 months) after the enrollment. Histological evaluations of gastric and duodenal biopsies were performed in order to evaluate the level of improvement of both gastric and duodenal lesions after GFD.
These findings with regard to those patients with H. pylori-negative gastritis at the time of the diagnosis were the following: out of the 34 patients with LG, all (100%) were H. pylori negative and the majority of them showed an improvement both of gastritis (94.1%) and duodenal histological lesions (82.3%) after GFD. Out of 67 patients with CAG, 15 (22.4%) patients were H. pylori negative and only 20% of them showed an improvement of gastritis after GFD. Out of 70 patients with CIG, 67 (95.7%) were H. pylori negative and none of them showed no change of gastritis after GFD. After 21 months of GFD, LG significantly improved compared to CAG (p = 0.0001) (Figure 4).
Figure 4.
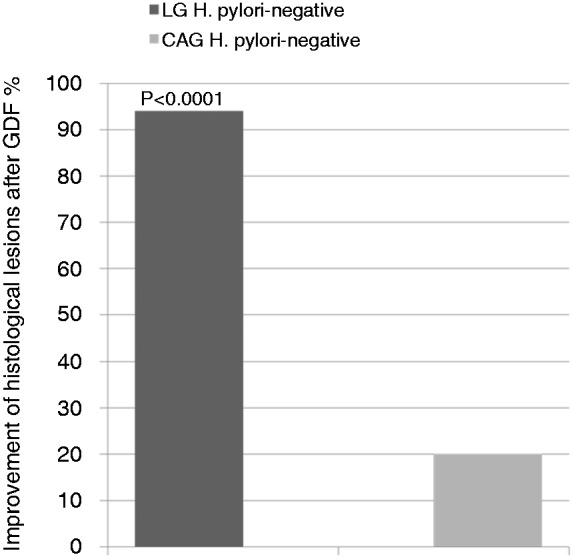
After 21 months of gluten-free diet (GFD), lymphocytic gastritis (LG) significantly improved compared to chronic active gastritis (CAG) (p < 0.0001).
Discussion
Chronic gastritis is frequently present in CD. We observed that about two thirds of CD patients have CIG or CAG (irrespective of H. pylori status), while LG is present in approximately 16% of the patients. None of patients with LG had H. pylori infection and almost all (94.1%) improved after GFD. GFD had lower influence on CAG and CIG. Patients with CAG not associated to H. pylori infection improved in only 20% of the cases, while those with CIG showed no improvement at all.
These data, therefore, confirm the association between CD and LG that has been described in both pediatric and adult patients.1,2,6,7,13–15 The prevalence observed in our cohort of patients was lower compared to that reported in the literature. Our data confirm the positive correlation with an advanced villous atrophy and the negative correlation with H. pylori infection.2,15–17
Concerning the natural history of LG, few data has been published. LG, when is associated with H. pylori infection, shows improvements after infection eradication.17 On the other hand, the effect of GFD on LG has yet to be identified. Evidence of clinical and biochemical improvement of LG after GFD has been reported in only two of four patients with LG and villous atrophy.18,19
In 1998, Feeley et al. described seven cases of LG, all occurring in the setting of untreated, symptomatic CD (both in patients with new CD diagnosis and in patients with poor adherence to the GFD), a finding which was associated with the small intestine villous atrophy.20
The real clinical significance of LG in CD patients is still unclear. When present in CD patients, LG seems to be responsible for dyspeptic symptoms and presumably related, at least in part, to the ingestion of gluten, as also confirmed by its clinical and histologic improvement with the GFD. The lack of improvement of LG after GFD should prompt to consider other possible causes. Indeed, dyspepsia is not-specific compliant due to various mechanisms and which can also improve spontaneously.
Lymphocytic infiltrate can also be present in the colonic mucosa of CD patients.21–23 Whether this association occurs only in a distinct subset of patients or is a more general phenomenon has yet to be determined.23
The examination of colonic biopsies in a subset of CD patients revealed that 31% of them also showed a striking lymphocytic infiltration of the superficial colonic epithelium.24 Out of four patients with celiac sprue and colonic lymphocytosis two of them also showed gastric lymphocytosis at gastric biopsies.24
Immune-mediated lymphocytic response linked to gluten occurs in the gastric epithelium, similar to that seen in the small intestine of patients with CD.17
It has been suggested that at least a subset of cases of CD may involve a diffuse T cell lymphocytic enteropathy occurring in response to gluten. Therefore, celiac associated LG might be the less florid gastric equivalent of the small intestinal response to gluten.20
In conclusion, gastric intraepithelial lymphocytosis may represent a concomitant manifestation of CD rather than a separate entity. In dyspeptic patients, histological finding of LG without H. pylori infection, suggests to test for the presence of CD. Adherence to GFD in CD patients with concomitant LG should be evaluated by histological assessment of both gastric and duodenal biopsies.
Other studies are needed to better understand the pathogenesis of LG, whether it is related to the duration of the CD or to a specific genetic susceptibility.
Declaration of conflicting interests
None declared.
Funding
This work was supported by the University of L’Aquila, L’Aquila, Italy.
References
- 1.Broide E, Sandbank J, Scapa E, et al. The immunohistochemistry profile of lymphocytic gastritis in celiac disease and Helicobacter pylori infection: interplay between infection and inflammation. Mediators Inflamm 2006; 2007: 81838–81838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebwohl B, Green PHR, Genta RM. The coeliac stomach: gastritis in patients with coeliac disease. Aliment Pharmacol Ther 2015; 42: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haot J, Jouret-Mourin A, Wallez Z, et al. Anatomoclinical study of a series of chronic gastritis characterized by intraepithelial lymphocytic infiltration. Acta Endosc 1986; 16: 69–74. [Google Scholar]
- 4.Haot J, Delos M, Wallez L, et al. Intraepithelial lymphocytes in inflammatory gastric pathology. Acta Endosc 1986; 16: 61–67. [Google Scholar]
- 5.Müller H, Rappel S, Volkholz H, et al. Lymphocytic gastritis-a rare disorder of the gastric mucosa. Pathologe 2001; 22: 56–61. [DOI] [PubMed] [Google Scholar]
- 6.Wolber R, Owen D, DelBuono L, et al. Lymphocytic gastritis in patients with celiac sprue or spruelike intestinal disease. Gastroenterology 1990; 98: 310–315. [DOI] [PubMed] [Google Scholar]
- 7.Bhatti TR, Jatla M, Verma R, et al. Lymphocytic gastritis in pediatric celiac disease. Pediatr Dev Pathol 2011; 14: 280–283. [DOI] [PubMed] [Google Scholar]
- 8.Marsh MN. Gluten, major histocompatibility complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102: 330–354. [PubMed] [Google Scholar]
- 9.Marsh MN, Johnson MW, Rostami K. Mucosal histopathology in coeliac disease: a rebuttal of Oberhuber’s sub-division of Marsh III. Gastroenterol Hepatol Bed Bench 2015; 8: 99–109. [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108: 656–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbure N, Lebwohl B, Arguelles-Grande C, et al. Olmesartan-associated sprue-like enteropathy: a systematic review with emphasis on histopathology. Hum Pathol 2016; 50: 127–134. [DOI] [PubMed] [Google Scholar]
- 13.De Giacomo C, Gianatti A, Negrini R, et al. Lymphocytic gastritis: a positive relationship with celiac disease. J Pediatr 1994; 124: 57–62. [DOI] [PubMed] [Google Scholar]
- 14.Prasad KK, Thapa BR, Lal S, et al. Lymphocytic gastritis and celiac disease in Indian children: evidence of a positive relation. J Pediatr Gastroenterol Nutr 2008; 47: 568–572. [DOI] [PubMed] [Google Scholar]
- 15.Brown I, Smith J, Rosty C. Gastrointestinal pathology in celiac disease. Am J Clin Pathol 2012; 138: 42–49. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen JA, Roberts CA, Lager DJ, et al. Lymphocytic gastritis is not associated with active Helicobacter pylori infection. Helicobacter 2014; 19: 349–355. [DOI] [PubMed] [Google Scholar]
- 17.Hayat M, Arora DS, Dixon MF, et al. Effects of Helicobacter pylori eradication on the natural history of lymphocytic gastritis. Gut 1999; 45: 495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsaigh N, Odze R, Goldman H, et al. Gastric and esophageal intraepithelial lymphocytes in pediatric celiac disease. Am J Surg Pathol 1996; 20: 865–870. [DOI] [PubMed] [Google Scholar]
- 19.Lynch DAF, Sobala GM, Dixon MF, et al. Lymphocytic gastritis and associated small bowel disease: a diffuse lymphocytic gastroenteropathy? J Clin Pathol 1995; 48: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeley KM, Heneghan MA, Stevens FM, et al. Lymphocytic gastritis and coeliac disease: evidence of a positive association. J Clin Pathol 1998; 51: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green PH, Yang J, Cheng J, et al. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol 2009; 7: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 22.Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: microscopic colitis. Aliment Pharmacol Ther 2006; 23: 1525–1534. [DOI] [PubMed] [Google Scholar]
- 23.Stewart M, Andrews CN, Urbanski S, et al. The association of coeliac disease and microscopic colitis: a large population-based study. Aliment Pharmacol Ther 2011; 33: 1340–1349. [DOI] [PubMed] [Google Scholar]
- 24.Wolber R, Owen D, Freeman H. Colonic lymphocytosis in patients with celiac sprue. Hum Pathol 1990; 21: 1092–1096. [DOI] [PubMed] [Google Scholar]