Abstract
Background
Recent studies have highlighted the relationship between gut microbiota and bowel movements.
Objective
We aimed to evaluate transglucosidase treatment efficacy for bowel movements in patients with type 2 diabetes mellitus and to clarify the relationship between bowel movements, dietary habits, gut microbiota and fecal short-chain fatty acids.
Methods
In this randomized double-blind, placebo-controlled study, 66 patients received placebo or transglucosidase (300 or 900 mg/day) orally, for 12 weeks. Fecal bacterial communities and short-chain fatty acids were analyzed before and after the treatment.
Results
Transglucosidase treatment significantly (p < 0.05) affected fecal microbiota (Prevotella spp., Bacteroides spp., Bifidobacterium spp., and Clostridium subcluster XIVa) and fecal short-chain fatty acid (acetate, valerate, succinate and lactate) content. Clostridium cluster IV, Clostridium subcluster XIVa, Clostridium cluster XVIII and fecal pH increased significantly and order Lactobacillales decreased in patients with bowel movement disorder compared with controls. Transglucosidase treatment significantly improved bowel movements compared with placebo treatment (46.2%, 95% confidence interval: 19.2–74.9% vs. 0%, 95% confidence interval: 0–33.6%, p < 0.05). This effect was not observed in patients without bowel movement disorder.
Conclusion
Patients with bowel movement disorder suffer from gut dysbiosis. Transglucosidase treatment alleviates bowel movement disorder symptoms in type 2 diabetes mellitus patients by increasing fecal acetate level.
Keywords: Transglucosidase, type 2 diabetes mellitus, bowel movements, intestinal microbiota, constipation, short-chain fatty acids
Introduction
We have recently developed a novel strategy employing Aspergillus niger transglucosidase (TGD) to produce oligosaccharides from starch in the human digestive tract. This strategy aimed to decrease postprandial blood glucose levels in individuals with impaired glucose tolerance and at high risk of developing type 2 diabetes mellitus (T2DM).1 Furthermore, we demonstrated that TGD administration decreases glycosylated hemoglobin (HbA1c) and insulin levels in T2DM patients.2 We subsequently proposed that the TGD treatment decreases blood glucose levels and prevents body weight gain in T2DM patients by inducing the production of oligosaccharides in the alimentary tract and modulating the composition of gut microbiota.3 We reported that TGD treatment is safe because of a lack of serious adverse events in the treatments.4–6
Intestinal enteropathy, which can cause diarrhea and/or constipation, is one of the major gastrointestinal complications in T2DM patients. Chronic bowel movement disorder (BMD) is a common gastrointestinal symptom in T2DM, and it decreases the patient’s quality of life.4–6 Increasing evidence points to a relationship between BMD and gut microbiota.7,8 However, whether the changes of gut microbiota reflected the effects of BMD or the confounding effects of altered fiber or fat intake was unclear. To the best of our knowledge, no data on gut dysbiosis in T2DM patients suffering from BMD have been reported.
This study aimed to compare the gut microbiota of T2DM patients with and without BMD. Furthermore, we aimed to clarify the relationship between gut microbiota and patients’ dietary habits, and also to clarify the effect of TGD on bowel movements in T2DM patients.
Materials and methods
Trial design and study subjects
This study was conducted according to the World Medical Association Declaration of Helsinki. It was designed as a randomized, double-blind, placebo-controlled trial and was conducted in Japan. The ethics review committee of the Nagoya City Graduate University School of Medical Sciences granted approval of this study (approval number 45-07-0009), and written informed consent was obtained from all the subjects.
The patient enrollment criteria and study design were as described previously.1 The 74 patients recruited during our previous trial were enrolled for this study as well.1
Based on previous experiments, we used TGD dosages of 300 and 900 mg/day.2,9 TGD (3,000,000 U/g) was purchased from Amano Enzyme Inc. (Nagoya, Japan). Capsules containing TGD (50 and 150 mg) and placebo were prepared by Adaptogen Pharmaceutical Co. Ltd (Tajimi, Japan). Patients took two capsules after every meal for 12 weeks, and fecal and blood sampling was performed prior to and at the end of the study.
Using simple randomization technique, the patients were randomized into three groups (1:1:1 proportion) according to treatment. The allocation of capsules and placebo was blinded until the end of the study. A statistician generated the randomization list managed by a clinical research coordinator.
The primary outcome was the change in HbA1c levels (previously reported).1 Important secondary outcomes included the change in fecal microbiota and bowel movements. Patients whose fecal samples were collected before and after the treatment in our previous study1 were included in the evaluation. The patients received stable dosages of medication throughout the study. The sample size calculation was based on a previous study,2,9 and based on an alpha of 0.05 with a power of 80%. Taking into account a 10% drop-out, total sample size of 66 patients was randomized.
Assessment of patient nutrient intake
To evaluate nutrient intake, a data-based short food frequency questionnaire10 was used.
Assessment of bowel movements
Bowel movement type was categorized as follows: (1) BMs-C: mostly constipation, <1 bowel movement per two days, need for laxatives because of constipation; (2) BMs-D: mostly diarrhea, loose or watery stools, or urgency; (3) BMs-M: mixed type, constipation and diarrhea; and (4) control: normal bowel movements. BMD includes BMs-C, BMs-D and BMs-M.
Before the treatment, the study participants answered the following: ‘Do you think you have a constipation: yes, no?’ After the 12-week treatment, the changes in bowel movement were subjectively assessed by the patients (patient-reported outcome). The participants answered the following: ‘Which of the following best describes your bowel movements compared to before treatment: improved, not changed, worsened?’
Extraction of fecal DNA
The fecal samples (20 mg per 200 µl of sterile distilled water) were suspended in 800 µl of a solution containing 4 M guanidinium thiocyanate, 100 mM Tris-HCl (pH 9.0) and 40 mM EDTA. The samples were then beaten with glass beads, using a mini bead beater (BioSpec Products, Bartlesville, OK, USA). Thereafter, DNA was extracted from the bead-treated suspension using benzyl chloride, as described by Zhu et al.11 The DNA extract was then purified using a GFX polymerase chain reaction DNA and Gel Band Purification Kit (Amersham Biosciences, Piscataway, NJ, USA). The final concentration of each DNA sample was adjusted to 10 ng/µl.
Terminal restriction fragment length polymorphism (T-RFLP)
The amplification of 16S rDNA, restriction enzyme digestion, size fractionation and analysis of T-RFLP data were performed according to the protocol described by Nagashima et al.12 Terminal restriction fraction (T-RF) length was determined using an ABI PRISM 3130 × 1 genetic analyzer (Applied Biosystems, CA, USA) in GeneScan mode. Standard size markers were used (MapMarker X-Rhodamine Labeled 50–1000 bp, BioVentures, TN, USA). The fragment sizes were estimated using the local southern method of the GeneMapper software (Applied Biosystems, CA, USA). T-RFs were divided into 30 operational taxonomic units (OTUs), according to the methods described by Nagashima et al.12 The OTUs were quantified as percentages of an individual OTU per total OTU area, expressed as peak percent area under the curve (%AUC). Cluster analyses were performed using the GeneMaths software (Applied Maths, Kortrijk, Belgium), based on the BslI T-RFLP patterns.
Fecal short-chain fatty acid (SCFA) analysis
For SCFA determinations, 0.1 g of feces was transferred to a 2.0 ml tube containing 1.1 g of zirconia beads and suspended in 900 µl of 0.1 mM perchloric acid solution with 3% phenol. Samples were heated at 80℃ for 15 min, vortex-mixed for 45 s using a FastPrep 24 homogenizer (MP Biomedicals, Santa Ana, CA, USA) at 5 m/s, and centrifuged at 15,350 × g for 10 min. The supernatant was passed through a 0.45-µm filter (DISMIC13HP; ADVANTEC, Tokyo, Japan). SCFAs (acetic, propionic, butyric, iso-butyric, succinic, lactic, formic, valeric and iso-valeric acids) were determined by high performance liquid chromatography (Prominence, SHIMADZU, Kyoto, Japan) using a post-column reaction with a detector (CDD-10A, SHIMADZU), two tandemly arranged columns (Shim-pack SCR-102(H), 300 mm × 8 mm i.d.; SHIMADZU), and a guard column (Shim-pack SCR-102(H), 50 mm × 6 mm i.d.; SHIMADZU). A mobile phase of 5 mM p-toluenesulfonic acid and a reaction solution of 5 mM p-toluenesulfonic acid, 100 × M EDTA, and 20 mM Bis-Tris were used. The flow rate was 0.8 ml/min and oven temperature was 45℃. The detector cell temperature was maintained at 48℃.
Statistical analysis
All statistical analyses were performed using the JMP statistical software package, version 15.0.4849.1003 for Windows (SAS Institute Inc., Cary, NC, USA). The results were expressed as mean ± standard deviation (SD) or median (range). Patients’ characteristics and nutrient intake profiles were compared in the placebo and the two TGD groups, and between control, BMs-C, BMs-M and BMs-D groups using the Kruskal–Wallis and Steel tests. The Mann–Whitney U test was used to compare the control with BMD patients. The relative abundances of specific bacterial groups were reflected through their T-RF peak areas, and the percentage values and amount of SCFAs were compared between placebo, TGD300 and TGD900 groups using two-way analysis of variance and Bonferroni post hoc test before and after treatment. Microbiota and SCFA contents were compared in the control, BMs-C, BMs-M and BMs-D groups using the Kruskal–Wallis and Steel tests. The Mann–Whitney U test was used to compare the control and BMD groups. The improvement ratio of bowel movements was calculated as (number of BM improved patients)/(number of BM improved, unchanged, and worsened patients)×100 and was compared in placebo and the two TGD groups, or the control, BMs-C, BMs-M and BMs-D groups, using χ2 test. A p value < 0.05 was considered statistically significant.
Results
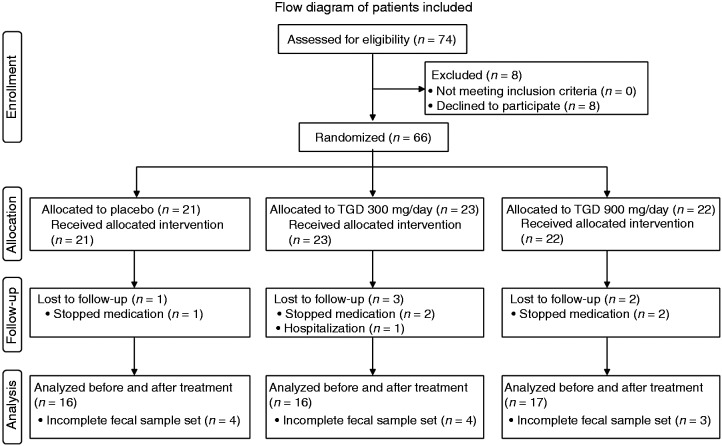
Flow diagram of patients included in the study
In a previous study,1 between December 2007 and March 2009, 74 T2DM patients from our outpatient clinic (Nagoya City University Hospital) were recruited and 66 (38 men, 28 women) were randomized into the following groups: placebo (n = 21), TGD300 (n = 23) receiving 300 mg of TGD a day, and TGD900 (n = 22) receiving 900 mg of TGD a day. Six patients did not complete the study, with five discontinuing the treatment; one patient was hospitalized because of recurrence of cerebral infarction and left the study. Because fecal samples of 11 patients, before or after treatment, were lost, 49 patients (16 in the placebo group, 16 in the TGD300 group and 17 in the TGD900 group) were included in the evaluation in this study (Figure 1).
Figure 1.
Flow diagram of patients included.
TGD: transglucosidase
Patient characteristics
The distribution of baseline clinical characteristics (age, sex, body mass index (BMI) and HbA1c) of patients in the three groups was similar. No significant differences were observed between the three groups concerning bowel movements, exercise frequency and nutrient intake profiles (Table 1).
Table 1.
Characteristics and nutrients intake in patients.
| Placebo | TGD300 | TGD900 | p value | |
|---|---|---|---|---|
| (n = 16) | (n = 16) | (n = 17) | ||
| Characteristics | ||||
| Sex (male/female) | 7/9 | 10/6 | 10/7 | 0.53a |
| Age (year) | 64.5 ± 6.1 | 63.5 ± 10.3 | 64.2 ± 6.4 | 0.94b |
| Body mass index (kg/m2) | 23.1 ± 4.7 | 25.5 ± 5.0 | 23.4 ± 3.0 | 0.29b |
| HbA1c (%) | 6.8 ± 0.5 | 6.7 ± 0.5 | 6.6 ± 0.5 | 0.53b |
| Bowel movements | ||||
| Control | 7 | 9 | 11 | 0.53a |
| BMs-C | 3 | 2 | 3 | |
| BMs-M | 2 | 4 | 1 | |
| BMs-D | 4 | 1 | 2 | |
| Exercise (n) | 8 | 10 | 12 | 0.47a |
| Nutrients | ||||
| Energy (kcal/day) | 1570 ± 349 | 1724 ± 432 | 1637 ± 304 | 0.61b |
| Protein (g/day) | 51.8 ± 11.9 | 55.9 ± 11.2 | 53.2 ± 8.6 | 0.77b |
| Fat (g) | 39.3 ± 7.5 | 43.2 ± 7.3 | 37.9 ± 6.2 | 0.22b |
| Carbohydrate (g/day) | 222.6 ± 61.5 | 250.5 ± 72.5 | 238.6 ± 56.7 | 0.31b |
| Fiber (g/day) | 11.6 ± 2.9 | 10.7 ± 2.3 | 11.3 ± 4.2 | 0.70b |
Data are mean ± standard deviation (SD).
Analyzed by χ2 test.
Analyzed by Kruskal-Wallis and Steel tests.
TGD300: transglucosidase, 300 mg/day; TGD900: transglucosidase, 900 mg/day; HbA1c: glycolated hemoglobin; BMs-C: constipation; BMs-M: constipation and diarrhea; BMs-D: diarrhea
The effect of TGD treatment on fecal microbiota and fecal SCFAs
To investigate the effect of TGD on fecal bacterial communities, the bacteria from the placebo, TGD300, and TGD900 groups were analyzed by T- RFLP (Table 2).
Table 2.
The effect of transglucosidase treatment on the patient fecal microbiota and fecal short-chain fatty acid content.
| Placebo (n = 16) |
TGD300 (n = 16) |
TGD900 (n = 17) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p value | Before | After | p value | Before | After | p value | |
| Fecal bacteria predicted by T-RF length | |||||||||
| Bifidobacterium spp. (%) | 23.0 ± 14.6 | 21.4 ± 13.9 | 0.36 | 20.7 ± 14.9 | 24.7 ± 17.0 | p < 0.05 | 28.0 ± 13.6 | 18.2 ± 12.5 | p < 0.05 |
| Lactobacillales spp. (%) | 16.3 ± 14.8 | 15.2 ± 13.5 | 0.60 | 16.9 ± 14.7 | 16.6 ± 17.4 | 0.87 | 18.2 ± 15.1 | 21.1 ± 19.4 | 0.15 |
| Bacteroides spp. (%) | 12.1 ± 10.2 | 17.9 ± 7.8 | p < 0.05 | 14.7 ± 11.5 | 13.9 ± 11.8 | 0.49 | 14.9 ± 12.7 | 19.8 ± 13.1 | p < 0.05 |
| Prevotella spp. (%) | 0.8 ± 2.9 | 0.8 ± 2.9 | 0.87 | 0.4 ± 1.0 | 0.2 ± 1.0 | 0.68 | 0.4 ± 1.1 | 1.6 ± 3.5 | p < 0.05 |
| Clostridium cluster IV (%) | 7.8 ± 8.4 | 9.1 ± 7.4 | 0.23 | 6.5 ± 7.1 | 6.2 ± 5.3 | 0.82 | 8.3 ± 8.7 | 8.4 ± 7.9 | 0.91 |
| Clostridium subcluster XIVa (%) | 23.3 ± 14.8 | 21.4 ± 11.0 | 0.31 | 22.7 ± 13.3 | 20.8 ± 12.2 | 0.30 | 14.8 ± 11.2 | 18.5 ± 13.0 | p < 0.05 |
| Clostridium cluster XI (%) | 2.3 ± 4.8 | 1.0 ± 1.4 | p < 0.05 | 0.6 ± 1.3 | 1.4 ± 2.1 | 0.06 | 1.4 ± 2.1 | 1.0 ± 1.8 | 0.33 |
| Clostridium cluster XVIII (%) | 5.8 ± 6.0 | 1.0 ± 1.7 | p < 0.05 | 1.2 ± 2.1* | 3.0 ± 6.4 | p < 0.05 | 2.1 ± 3.3* | 1.0 ± 2.2 | 0.10 |
| Others | 8.8 ± 4.0 | 12.0 ± 8.7 | p < 0.05 | 15.7 ± 12.6 | 14.3 ± 12.3 | 0.29 | 11.9 ± 10.1 | 10.4 ± 6.1 | 0.21 |
| Fecal SCFA and pH | |||||||||
| Total SCFA (mg/g) | 8.0 ± 4.1 | 7.5 ± 4.4 | 0.36 | 6.7 ± 3.3 | 6.7 ± 2.8 | 0.99 | 6.7 ± 3.4 | 7.5 ± 3.2 | 0.09 |
| Acetate (mg/g) | 4.3 ± 2.6 | 4.1 ± 2.9 | 0.58 | 3.9 ± 1.9 | 3.6 ± 1.6 | 0.28 | 3.6 ± 2.1 | 4.2 ± 1.6 | p < 0.05 |
| Propionate (mg/g) | 1.7 ± 1.0 | 1.4 ± 0.8 | p < 0.05 | 1.6 ± 0.9 | 1.5 ± 0.9 | 0.78 | 1.2 ± 0.9 | 1.5 ± 0.7 | 0.10 |
| Butyrate (mg/g) | 1.2 ± 0.9 | 1.3 ± 0.9 | 0.43 | 0.9 ± 0.9 | 1.0 ± 0.8 | 0.17 | 1.1 ± 1.0 | 1.3 ± 1.1 | 0.40 |
| Valerate (mg/g) | 0.8 ± 0.7 | 0.8 ± 0.6 | 0.74 | 0.4 ± 0.4* | 0.6 ± 0.6 | p < 0.05 | 0.3 ± 0.3* | 0.6 ± 0.6 | p < 0.05 |
| Succinate (mg/g) | 0.06 ± 0.05 | 0.05 ± 0.05 | 0.72 | 0.15 ± 0.37 | 0.08 ± 0.05 | 0.11 | 0.22 ± 0.37 | 0.12 ± 0.21 | p < 0.05 |
| Formate (mg/g) | 0.03 ± 0.04 | 0.06 ± 0.04 | p < 0.05 | 0.02 ± 0.03 | 0.06 ± 0.07 | p < 0.05 | 0.05 ± 0.10 | 0.04 ± 0.04 | 0.59 |
| Lactate (mg/g) | 0.02 ± 0.07 | 0.01 ± 0.02 | 0.89 | 0.23 ± 0.91 | 0.36 ± 1.40 | 0.19 | 0.35 ± 1.12 | 0.05 ± 0.13 | p < 0.05 |
| pH | 6.8 ± 0.8 | 6.7 ± 0.8 | 0.62 | 6.6 ± 0.7 | 6.8 ± 0.8 | 0.14 | 6.6 ± 0.9 | 6.7 ± 0.7 | 0.68 |
Data are mean ± standard deviation (SD). Total SCFA = acetate + propionate + butyrate + valerate. Analyzed by two-way ANOVA and Bonferroni post hoc test.
p < 0.05 vs. placebo.
T-RF: terminal restriction fragment; SCFA: short-chain fatty acid; TGD300: transglucosidase, 300 mg/day; TGD900: transglucosidase, 900 mg/day
Some changes in the fecal flora were shared between the groups, and some were group-specific. For example, before TGD treatment, bacteria from Clostridium subcluster XVIII were significantly less abundant in TGD300 and TGD900 groups than in placebo group (p < 0.05). No such differences were noted for other bacterial groups. After TGD therapy, the relative abundance of Bifidobacterium spp. was significantly decreased in TGD900 group (p < 0.05) while that of Prevotella spp. and Clostridium subcluster XIVa was significantly increased (p < 0.05). The relative abundance of Bacteroides spp. in TGD900 and placebo groups, and that of Clostridium cluster XVIII in TGD300 group was significantly increased after treatment (p < 0.05).
We also analyzed fecal SCFA levels and fecal pH to evaluate the effect of TGD treatment on the intestinal environment (Table 2). Before the treatment, valerate levels in TGD300 and TGD900 groups were significantly lower than in placebo group (p < 0.05). Following TGD900 therapy, fecal acetate and valerate levels were significantly increased, while succinate and lactate were significantly reduced (p < 0.05). Total SCFAs were marginally increased after this treatment (p = 0.09). Formate and valerate levels were significantly increased after TGD300 treatment; however, significant increase of the former was also observed in the placebo group (p < 0.05).
Comparison of characteristics of patients with and without BMD
The distribution of clinical characteristics of patients with and without BMD (age, sex, BMI and HbA1c) was similar. No significant differences were observed in the control, BMs-C, BMs-M and BMs-D groups or the control and BMD groups with respect to exercise, the treatment with TGD, and nutrient intake profiles (Table 3).
Table 3.
Characteristics and nutrient intake of patients, grouped by bowel movement disorder (BMD).
| Control | BMs-C | BMs-M | BMs-D | p value | BMD | p valuea | |
|---|---|---|---|---|---|---|---|
| (n = 27) | (n = 8) | (n = 7) | (n = 7) | (n = 22) | |||
| Characteristics | |||||||
| Sex (male/female) | 16/11 | 3/5 | 5/2 | 3/4 | 0.49b | 11/11 | 0.57b |
| Age (years) | 65.3 ± 6.1 | 67.8 ± 2.7 | 60.1 ± 14.0 | 52.3 ± 5.7 | 0.10c | 62.6 ± 9.1 | 0.51d |
| Body mass index (kg/m2) | 23.7 ± 3.3 | 21.7 ± 3.4 | 27.1 ± 6.5 | 24.6 ± 5.1 | 0.24c | 24.3 ± 5.4 | 0.88d |
| HbA1c (%) | 6.8 ± 0.4 | 6.6 ± 0.4 | 6.8 ± 0.4 | 6.7 ± 0.6 | 0.72c | 6.7 ± 0.5 | 0.66d |
| Nutrients | |||||||
| Energy (kcal/day) | 1592 ± 326 | 1606 ± 295 | 1643 ± 387 | 1887 ± 505 | 0.66c | 1707 ± 400 | 0.56d |
| Protein (g/day) | 52.2 ± 9.9 | 57.4 ± 5.9 | 52.0 ± 11.5 | 56.4 ± 15.7 | 0.56c | 55.4 ± 8.6 | 0.38d |
| Fat (g) | 39.5 ± 5.9 | 44.6 ± 7.7 | 39.1 ± 9.5 | 38.0 ± 8.2 | 0.34c | 40.8 ± 8.6 | 0.75d |
| Carbohydrate (g/day) | 231.3 ± 56.8 | 222.3 ± 57.0 | 230.9 ± 36.7 | 283.6 ± 100.5 | 0.73c | 244.5 ± 71.5 | 0.86d |
| Fiber (g/day) | 11.2 ± 3.1 | 12.6 ± 4.4 | 11.4 ± 2.5 | 9.3 ± 1.7 | 0.39c | 11.2 ± 3.3 | 0.76d |
| Yogurt (n) | 14 | 4 | 4 | 2 | 0.69b | 10 | 0.78b |
| Exercise (n) | 17 | 6 | 4 | 3 | 0.63b | 13 | 0.51b |
| TGD | 7/9/11 | 3/2/3 | 2/4/1 | 4/1/2 | 0.78b | 9/7/6 | 0.54b |
| (Placebo/300 mg/day/ 900 mg/day) |
Data are mean ± standard deviation (SD).
BMs-C,: constipation; BMs-M: constipation and diarrhea; BMs-D: diarrhea; BMD: BMs-C + BMs-M + BMs-D
Compared with control.
Analyzed by χ2 test.
Analyzed by Kruskal–Wallis and Steel tests.
Analyzed by Mann–Whitney U test.
Fecal microbiota and fecal SCFA levels in patients with and without BMD
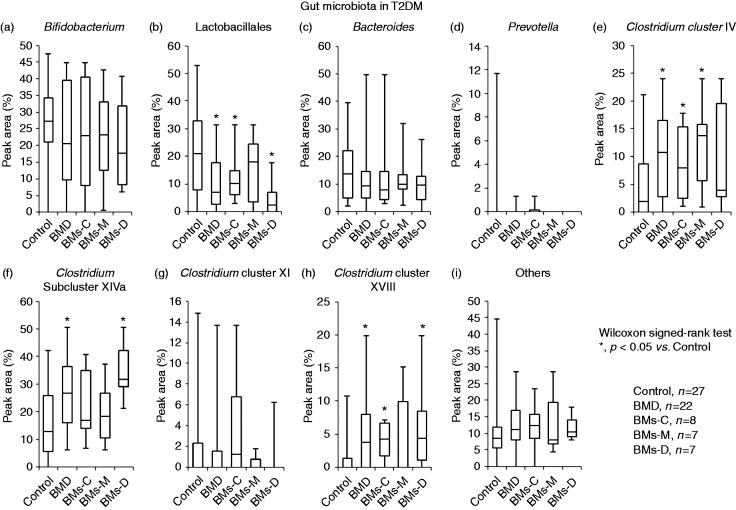
To compare the intestinal microenvironments of patients with and without BMD, the relative abundances of fecal bacteria, SCFA content and pH were analyzed (Figure 2).
Figure 2.
Gut microbiota in type 2 diabetes mellitus (T2DM) patients with and without bowel movement disorder (BMD). The occurrence of gut microbes is given as percentage of peak area. The data are presented as a box-and-whisker plot and were analyzed by Kruskal–Wallis and Steel tests or the Mann–Whitney U test. Asterisks denote p < 0.05 vs. control.
BMD: BMs-C + BMs-M + BMs-D; BMs-C: constipation; BMs-M: constipation and diarrhea; BMs-D: diarrhea
No difference between the relative abundances of Bifidobacterium spp. was observed between the groups (Figure 2(a)). In contrast, the abundance of bacteria from the order Lactobacillales was significantly lower in patients with BMD than in control patients (p < 0.05, Figure 2(b)). Bacteria from Clostridium cluster IV, Clostridium subcluster XIVa and Clostridium cluster XVIII were significantly more abundant in samples from patients with BMD than in control patients (p < 0.05, Figure 2(e), (f) and (h)). Compared with control, bacteria from the order Lactobacillales were significantly less abundant in BMs-C and BMs-D group samples, and Clostridium cluster IV bacteria were significantly more abundant in BMs-C and BMs-M group samples (p < 0.05, Figure 2(b) and (e)). Furthermore, compared with the control group samples, Clostridium subcluster XIVa bacteria were significantly more abundant in BMs-D group samples, while Clostridium cluster XVIII bacteria were significantly more abundant in BMs-C and BMs-D groups (p < 0.05, Figure 2(f) and (h)). Compared with the status quo before the treatment, no differences in fecal microbiota were observed after treatment within each bowel movement group (data not presented).
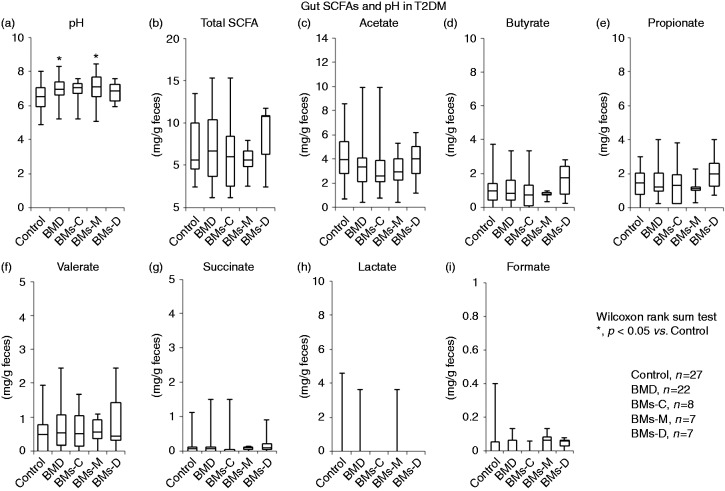
The fecal pH was significantly higher in samples from BMD patients than in control samples (p < 0.05, Figure 3(a)).
Figure 3.
Fecal short-chain fatty acids (SCFAs) in type 2 diabetes mellitus (T2DM) patients with and without bowel movement disorder (BMD). SCFA levels were quantified as mg/g feces. The data are presented as a box-and-whisker plot and were analyzed using Kruskal–Wallis and Steel tests or the Mann–Whitney U test. Asterisks denote p < 0.05 vs. control.
BMD: BMs-C + BMs-M + BMs-D; BMs-C: constipation; BMs-M: constipation and diarrhea; BMs-D: diarrhea
Furthermore, the fecal pH was significantly higher in the BMs-M group, but not in BMs-C and BMs-D groups, than in the control groups (p < 0.05, Figure 3(a)). No significant differences in individual SCFA content were observed in no BMD versus BMD comparisons (Figure 3(b) to (i)). After treatment, fecal SCFAs and pH were unchanged in each group (data not presented).
The effect of TGD treatment on bowel movements
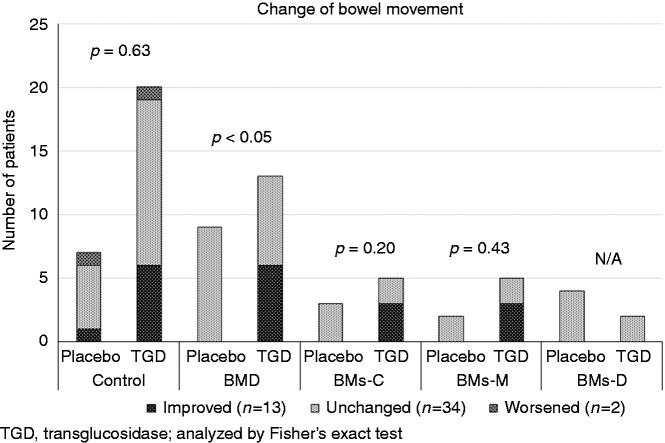
To investigate the effect of TGD treatment on bowel movements, bowel movements before and after treatment were analyzed. The bowel movement improvement ratio was 6.3% (1/16; 95% confidence interval (CI): 0.2–30.2%), 43.8% (7/16; 95% CI: 19.8–70.1%) and 29.4% (5/17, 95% CI: 10.3–56.0%) in the placebo, TGD300 and TGD900 groups, respectively, and there was a marginal difference in the bowel movement improvement ratio in the TGD-treated group compared with the placebo group (p = 0.053). Considering the bowel movements before TGD treatment, bowel movement was significantly improved in the BMD group after treatment compared with placebo treatment (46.2% (95% CI: 14.7–94.7%) vs. 0% (95% CI: 0–33.3%), p < 0.05, Figure 4). One patient in the placebo group had constipation and one patient in the TGD900 group had diarrhea. Bowel movements in BMs-C and BMs-M groups were improved by TGD but not by placebo treatment. Some patients in the control group had improved bowel movement, probably because the assessment relied on a patient-reported outcome. In the control group, there was no difference in the bowel movement improvement ratio between placebo and TGD treated group (Figure 4).
Figure 4.
The effect of transglucosidase (TGD) treatment on bowel movement in type 2 diabetes mellitus patients. The improved ratios of bowel movements for placebo and TGD treatments, were, respectively: 16.7% (95% CI: 0.4–64.1%) and 31.6% (95% CI: 12.6–56.69%) in the control group; 0% (95% CI: 0–33.3%) and 46.2% (95% CI: 19.2–74.9%) in the BMD group; 0% (95% CI: 0–100%) and 60.0% (95% CI: 14.7–94.7%) in the BMs-C group; 0% (95% CI: 0–100%) and 60.0% (95% CI: 14.7–94.7%) in the BMs-M group; and 0% (95% CI: 0–75.0%) and 0% (95% CI: 0–100%) in the BMs-D group. The data are presented as the number of patients and were analyzed by χ2 test.
CI: confidence interval; BMD: BMs-C + BMs-M + BMs-D; BMs-C: constipation; BMs-M: constipation and diarrhea; BMs-D: diarrhea
Discussion
In the current study, we investigated the gut microbiota and fecal SCFA levels of patients with and without BMD before and after TGD treatment. To the best of our knowledge, this is the first report of this kind.
T2DM is a metabolic disease primarily caused by obesity-linked insulin resistance, and recent studies have demonstrated a relationship between the composition of intestinal microbiota and metabolic diseases, such as obesity and diabetes.13,14 We had previously shown that T2DM in human is associated with compositional changes in intestinal microbiota, and these compositional changes were involved in lipid and glucose metabolism. TGD treatment improves metabolic condition of T2DM and affects fecal microbiota, that is, increases the proportion of Bacteroidetes.3,15 Gut dysbiosis in irritable bowel syndrome (IBS) has been well documented and the improvement of symptoms, including altered bowel habit and stool formation, by the manipulation of gut microbiota using probiotics, prebiotics or antibiotics suggests that dysbiosis may play a potential role in the pathogenesis of IBS.16,17 Gut dysbiosis is also involved in the pathogenesis of functional constipation, which is supported by the accumulating evidence on the efficacy of probiotics, prebiotics and symbiotics in treating this condition.7,8 In the present study, significant differences were observed in gut microbiota of T2DM patients with and without BMD. TGD treatment (TGD300 + TGD900 group) improved bowel movement compared with the placebo group (p < 0.05, data not shown) via improvement of intestinal microbiota. Regarding the reason for the higher improvement ratio of bowel movement in the TGD300 group than in the TGD900 group, it is possible that the individuals in the TGD300 group exercised more, but the actual reason is unclear. Lower level of Bifidobacterium spp. and Bacteroides were reported in stool in IBS patients both with constipation and diarrhea,18 but no difference in their relative fecal abundances was observed in the present study. Furthermore, Lactobacillus spp. levels were similar in IBS patients and healthy controls;18 however, in the present study, we noted a lower abundance of the order Lactobacillales in BMD than in the control in T2DM patients. Different microbiome might be involved in the pathogenesis of BMD in patients with IBS and T2DM.
As one of the major metabolites endogenously produced in the colon, SCFAs serve as an energy source for colonocytes and regulate colonic motility and secretion.19–21 Based on their physiological effects, excess of certain SCFAs, for example iso-butyrate, might contribute to the pathophysiology of constipation.22 However, Soret and colleagues23 reported that butyrate increases the proportion of enteric neurons that express acetyltransferase, which can promote colonic motility by the cholinergic pathway. Therefore, the role of butyrate in constipation is unclear. Shi and colleagues24 demonstrated that acetate, propionate and butyrate levels in patients with mixed refractory constipation are all significantly lower than in control individuals who had no history of constipation. Considering the previous reports and present results, gut dysbiosis may be closely associated with the pathogenesis of BMD in T2DM patients.
Using rat gastrointestinal and gastric ligation models, we previously demonstrated that TGD can convert carbohydrates to oligosaccharides, such as panose and isomaltooligosaccharide,9 which were fermented to SCFAs by gut bacteria. In the present study, TGD treatment altered the gut microbiota profile and fecal SCFA levels, including acetate, which may explain the improvement of bowel movements in subjects. SCFAs stimulate active sodium and chloride absorption in the colon, and may thus aggravate the symptoms of constipation;20 however, the effect of SCFAs on colonic contractility, motility and transit time remains unclear.25 Further studies are required to clarify the roles of SCFAs in the pathophysiology of human constipation.
One limitation of this study is a small number of samples and subjective definition of BMD. Rome IV criteria,26 which utilize a subjective symptom-based classification system, are commonly used to assess chronic functional constipation. In the present study, the definition of BMD was similar to Rome IV, but bowel movement improvement was subjectively assessed by patients. However, the double-blind placebo control treatment improved bowel movements in T2DM patients with BMD, suggesting that TGD treatment indeed improves the quality of life of T2DM patients when bowel symptoms are concerned. Further larger scale study using an objective bowel movement evaluation in T2DM patients is required to verify the findings of the current trial.
In summary, we provided evidence that gut microbiota differ in patients with and without BMD, and fecal SCFA levels accompany different frequency of bowel movement in human subjects with T2DM. The TGD treatment improved bowel movements of T2DM patients. Based on the results of the current trial, we suggest that the TGD treatment alleviates BMD in T2DM patients by inducing the production of oligosaccharides in the alimentary tract and modulating gut SCFA levels.
Acknowledgements
Clinical trial registration: UMIN-CTR UMIN000010318.
Declaration of conflicting interests
K Kasugai received a research grant from AstraZeneca K.K., Daiich Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd. and lecture fees from AstraZeneca K.K.; Daiich Sankyo Co., Ltd.; and Takeda Pharmaceutical Co., Ltd. Other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by Nagoya City University (Grant-in-Aid for Research); the Japan Science and Technology Agency (Grant-in-Aid for Research for Promoting Technology Seeds of Comprehensive Support Programs for Creation of Regional Innovation); and the Japanese Research Foundation for Clinical Pharmacology (Grant-in-Aid for Research).
References
- 1.Sasaki M, Imaeda K, Okayama N, et al. Effects of transglucosidase on diabetes, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2012; 14: 379–382. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki M, Joh T, Koikeda S, et al. A novel strategy in production of oligosaccharides in digestive tract: prevention of postprandial hyperglycemia and hyperinsulinemia. J Clin Biochem Nutr 2007; 41: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki M, Ogasawara N, Funaki Y, et al. Transglucosidase improves the gut microbiota profile of type 2 diabetes mellitus patients: A randomized double-blind, placebo-controlled study. BMC Gastroenterol 2013; 13: 81–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maleki D, Locke GR, 3rd, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med 2000; 160: 2808–2816. [DOI] [PubMed] [Google Scholar]
- 5.Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149. [DOI] [PubMed] [Google Scholar]
- 6.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med 1983; 98: 378–384. [DOI] [PubMed] [Google Scholar]
- 7.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–1561. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulides S, Dimidi E, Fragkos KC, et al. Systematic review with meta-analysis: Effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment Pharmacol Ther 2016; 44: 103–116. [DOI] [PubMed] [Google Scholar]
- 9.Kariya K, Ogawa T, Joh T. Effect of gut flora of oligosaccharide synthesizing enzymes. Digestion & Absorption 2000; 23: 107–109. [Google Scholar]
- 10.Tokudome S, Goto C, Imaeda N, et al. Development of a data-based short food frequency questionnaire for assessing nutrient intake by middle-aged Japanese. Asian Pac J Cancer Prev 2004; 5: 40–43. [PubMed] [Google Scholar]
- 11.Zhu H, Qu F, Zhu LH. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res 1993; 21: 5279–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashima K, Hisada T, Sato M, et al. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 2003; 69: 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 14.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010; 5: e9085–e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Adachi K, Sugiyama T, et al. Association of intestinal microbiota with metabolic markers and dietary habits in patients with type 2 diabetes. Digestion 2016; 94: 66–72. [DOI] [PubMed] [Google Scholar]
- 16.Parkes GC, Brostoff J, Whelan K, et al. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am J Gastroenterol 2008; 103: 1557–1567. [DOI] [PubMed] [Google Scholar]
- 17.Silk DB, Davis A, Vulevic J, et al. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 2009; 29: 508–518. [DOI] [PubMed] [Google Scholar]
- 18.Shukla R, Ghoshal U, Dhole TN, et al. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: An evidence of dysbiosis. Dig Dis Sci 2015; 60: 2953–2962. [DOI] [PubMed] [Google Scholar]
- 19.Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol 1985; 368: 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 1989; 96: 989–996. [DOI] [PubMed] [Google Scholar]
- 21.Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care 2014; 17: 139–144. [DOI] [PubMed] [Google Scholar]
- 22.Kang DW, DiBaise JK, Ilhan ZE, et al. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe 2015; 33: 33–41. [DOI] [PubMed] [Google Scholar]
- 23.Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010; 138: 1772–1782. [DOI] [PubMed]
- 24.Shi Y, Chen Q, Huang Y, et al. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Colorectal Dis 2016; 18: 803–810. [DOI] [PubMed] [Google Scholar]
- 25.Kassam Z, Collins SM, Moayyedi P. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2013; 368: 577–578. [DOI] [PubMed] [Google Scholar]
- 26.Stanghellini V, Talley NJ, Chan F, et al. Rome IV – gastroduodenal disorders. Gastroenterology 2016; 150: 1380–1392. [DOI] [PubMed] [Google Scholar]