Abstract
Background
Effective management of irritable bowel syndrome (IBS), a common functional gastrointestinal disorder, can be challenging for physicians because of the lack of simple diagnostic tests and the wide variety of treatment approaches available.
Objective
The objective of this article is to outline a simple algorithm for day-to-day clinical practice to help physicians navigate key stages to reaching a positive IBS diagnosis and guidance on how to prioritise the use of specific management strategies.
Methods
This algorithm was based on the opinion of an expert panel evaluating current evidence.
Results
The key principles forming the foundation of this evidence-supported algorithm are: confidently naming and explaining an IBS diagnosis for the patient, followed by assessment of key patient characteristics likely to influence the choice of therapy, such as predominant symptoms, and exploring the patient agenda and preferences. Consultation should always include education and reassurance with an explanatory model of IBS tailored to the patient. Individualised lifestyle changes, dietary modifications, pharmacological therapies, psychological strategies or a combination of interventions may be used to optimise treatment for each patient.
Conclusion
The simple visual tools developed here navigate the key stages to reaching a positive diagnosis of IBS, and provide a stepwise approach to patient-centred management targeted towards the most bothersome symptoms. Establishing a strong patient-physician relationship is central to all stages of the patient journey from diagnosis to effective management.
Keywords: Irritable bowel syndrome, diagnosis, treatment, management
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID), with prevalence rates ranging between 5% and 20%, depending on the geographical region and the criteria used for assessment.1 The defining features of IBS are the presence of recurrent abdominal pain in association with altered bowel habits (diarrhoea, constipation or both). The spectrum, duration and severity of symptoms can range from inconvenient to incapacitating,2 and can prevent individuals from participating in everyday activities.3
Despite the prevalence of IBS, its diagnosis and management remain as challenges for global healthcare systems.
Diagnosis
FGIDs most likely exist on a continuum rather than in isolation as separate and discrete disorders, with significant symptom overlap among these conditions.4 Furthermore, the symptoms of IBS can mimic those associated with organic diseases, posing a challenge for diagnosis.5 The recently updated Rome IV criteria were designed to facilitate making a positive diagnosis of IBS, based on the presence of characteristic symptoms and the absence of objective findings from a limited number of standard diagnostic tests and investigations.4 While the majority of patients with IBS are diagnosed and treated in primary care, only a minority of general practitioners use the Rome criteria to make a diagnosis.6 Even experts have difficulty in consistently making a positive diagnosis.7 Differences in national guidelines and practice approaches as well as the availability and costs of diagnostic tests may also influence the diagnostic approach used.7
Management
While a variety of different therapy options are available to target the symptoms of IBS, many have not been evaluated in high-quality, randomised controlled trials. Several recent review articles,8–10 along with multiple systematic reviews and meta-analyses,11–14 have provided detailed evaluation of the clinical evidence for different treatment approaches for the management of IBS. However, guidance in terms of how to prioritise the use of different agents is lacking.
Our objective was to develop a simplified algorithm to be used in day-to-day clinical practice to support practitioners to reach a positive diagnosis of IBS, prioritise the use of specific therapies to target predominant symptoms, and provide guidance on how to incorporate key patient characteristics into a tailored management approach.
Methods
A panel of 13 international experts in the field of FGIDs met twice to discuss and agree on the development of simplified algorithms for the diagnosis and management of IBS. The algorithms developed during this process were based on the expert opinion of the panel, taking into consideration professional guidelines, the quality of the clinical evidence for specific management strategies and the group’s experience from clinical practice.
During the first meeting, as a group, the panel participated in a series of workshop activities to: (1) define the key stages in reaching a positive diagnosis of IBS; (2) determine what patient factors physicians need to consider before starting treatment; (3) provide guidance on the sequence in which to use different therapy options, based on key patient characteristics and the quality of evidence supporting the use of specific agents in each context; (4) discuss how to assess treatment success and what this may mean to each individual patient; and (5) discuss the long-term management of IBS. The workshop format allowed all participants to provide their individual opinions equally, allowing sufficient time for group discussion and agreement. Based on these initial outputs, draft algorithms were developed. The panel then met a second time to discuss further refinement and finalisation of the algorithms. The final algorithms presented here were agreed upon by all members of the panel.
Reaching a positive diagnosis of IBS
Patient presenting with symptoms suggestive of IBS
Typically, the first point at which patients with suspected IBS will consult a physician is when their symptoms are bothersome enough to affect their daily life and warrant them seeking medical attention (Figure 1). Factors that may drive this consultation are symptom severity, number of symptoms, concomitant psychological disorders and concerns that symptoms might indicate an underlying severe disease.9,15
Figure 1.
Simplified algorithm for irritable bowel syndrome (IBS) diagnosis.
As there is no specific biomarker or test to confirm or rule out a diagnosis of IBS, the Rome IV guidelines outline how the diagnosis of IBS requires a thoughtful approach, limited diagnostic tests, and careful follow-up.4 The presence of recurrent abdominal pain in association with abnormal bowel habits are the defining features of IBS (Table 1).4 Abdominal bloating and distention are also commonly present but not required to make the diagnosis of IBS. While meeting these criteria serves as a firm basis for reaching a positive diagnosis of IBS, they do not ‘confirm’ IBS or ‘rule out’ other conditions, nor do they capture all dimensions of the patient’s clinical condition in order to optimise treatment.16
Table 1.
| Recurrent abdominal pain, on average, at least one day per week in the last three months, associated with ≥ 2 of the following criteria: |
| 1. Related to defecation. |
| 2. Associated with a change in frequency of stool. |
| 3. Associated with a change in form (appearance) of stool. |
Criteria fulfilled for the last three months with symptom onset at least six months before diagnosis.
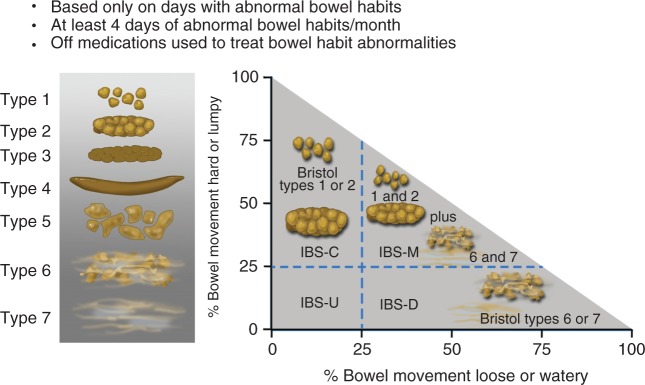
Determining the predominant disorder in bowel habit will be part of the initial clinical evaluation of the patient. The Bristol Stool Form Scale (BSFS; Figure 2)17 should be used to record stool consistency on days when patients have abnormal bowel habits (BSFS type 1–2 or 6–7); for accurate assessment this should be performed when patients are not taking medications that alter their bowel habits (e.g. laxatives or anti-diarrhoeal agents). These assessments can be used to identify the IBS subtype (Figure 2):4
IBS with predominant constipation (IBS-C): Patient reports that abnormal bowel movements are usually constipation (BSFS type 1 or 2).
IBS with predominant diarrhoea (IBS-D): Patient reports that abnormal bowel movements are usually diarrhoea (BSFS type 6 or 7).
IBS with mixed bowel habits (IBS-M): Patient reports that abnormal bowel movements are usually both constipation and diarrhoea (more than one-quarter constipation and more than one-quarter diarrhoea).
Figure 2.
Irritable bowel syndrome subtypes.4
Reproduced with permission (Lacy et al., 2016).
IBS: irritable bowel syndrome; IBS-C: IBS with constipation; IBS-D: IBS with diarrhoea; IBS-M: IBS with constipation/diarrhoea; IBS-U: IBS unclassifiable.
Assessment and investigation (history/physical exam)
Performing a battery of tests in all patients suspected of having IBS is not warranted as most patients <50 years old have a very low probability of harbouring organic disease.18 Limited diagnostic testing can play an important role in distinguishing IBS from other gastrointestinal (GI) conditions associated with similar symptoms (e.g. coeliac disease, inflammatory bowel disease (IBD), lactose intolerance, and microscopic colitis).4 For the majority of patients with a clinical history compatible with IBS, the tests or investigations required will vary according to patient demographics, clinical situation and reported symptoms.9 Tests that may be performed at this stage include complete blood count (because anaemia or an elevated white blood cell count should warrant further investigation), and C-reactive protein and faecal calprotectin in those with diarrhoea to exclude IBD.4 Routine thyroid tests are not indicated in all patients, but can be checked if clinical suspicion of thyroid disorder is high.4 Diagnostic testing for coeliac disease may be warranted in patients from areas with a high prevalence of the disease. A colonoscopy is indicated for all patients ≥50 years, with biopsies indicated for patients with diarrhoea or mixed bowel habits.4
Exploring personal disease triggers is an important starting point for medical intervention in IBS.19 A detailed patient history (Table 2) can be used to assess the impact of symptoms on daily life, to explore the patient’s agenda in terms of what they want to achieve with therapy, and to identify any precipitating factors that may be associated with symptoms.4,20 These assessments should focus on gaining an understanding of the patient’s dietary habits, and on determining whether patients are consuming foods or drinks that can mimic or exacerbate the symptoms of IBS. The history should also identify lifestyle factors that may be contributing to symptoms and to gain an understanding of additional comorbidities (e.g. psychological, gynaecological, urological, rheumatological) that could affect management. Understanding how specific symptoms affect a patient’s quality of life can also help develop a more targeted management approach beyond treating the dominant disordered bowel habit.
Table 2.
Initial patient assessments.
| • Identify symptom triggers (e.g. diet, stress). |
| • Assess impact on daily life. |
| • Assess for psychological comorbidities. |
| • Assess for other physical comorbidities (e.g. gynaecological, urological). |
| • Explore patient’s values and preferences. |
Factors supporting IBS diagnosis
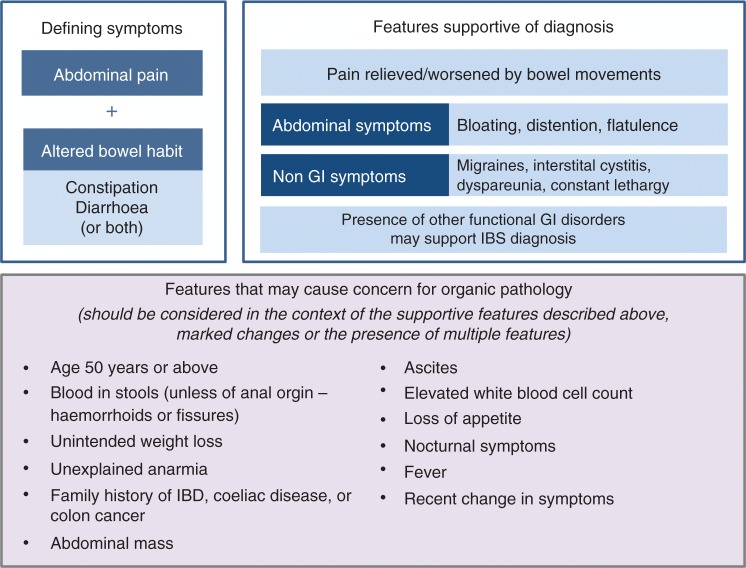
What further questions need to be addressed to confirm a positive diagnosis of IBS? The pattern of abdominal pain or discomfort should be considered in terms of the duration, type, location, time of occurrence and its relation to defecation, e.g. whether pain is relieved with bowel movements.4,20 Other abdominal symptoms that are consistent with a diagnosis of IBS (but not present in all patients) include bloating, distention and flatulence. The presence of other FGIDs may also support a diagnosis of IBS. Non-GI symptoms that are supportive of an IBS diagnosis also include migraine headaches, interstitial cystitis and dyspareunia (Figure 3). Constant lethargy is also commonly experienced by patients21 with IBS and it can be reassuring for patients to be informed that this is a well-recognised symptom.
Figure 3.
Patient features supportive of an IBS diagnosis or raising concern for organic pathology.
IBS: irritable bowel syndrome; IBD: inflammatory bowel disease; GI: gastrointestinal.
The nature and onset of symptoms is also important; for example, onset after gastroenteritis would suggest post-infectious IBS. The onset of IBS-like symptoms after an acute episode of diverticulitis has also been observed.22 Stressful events in a patient’s history such as domestic abuse23 or serving in the military24 may also be linked to the risk of developing IBS.
Ruling out organic pathology
Specific factors that could raise concern for underlying organic pathology and warrant further testing to exclude other GI diseases are summarised in Figure 3. These factors should be considered in the context of each individual patient and the presence of additional features that would raise or lower the level of concern. In general, the risk of organic pathology is very low in young patients with symptoms suggestive of IBS, so invasive investigation is not warranted even in the presence of features outlined in Figure 3 unless there is a combination of features or an individual feature that is markedly abnormal (e.g. significant weight loss). Specific investigations that may be required for some patients are outlined in Table 3. To put the use of some of these investigations into context, an example patient vignette is provided in Table 4. As this patient is female, nearing 50 years of age, has a co-existing autoimmune disease and is taking nonsteroidal anti-inflammatory drugs, further investigations to rule out microscopic colitis (MC) would be warranted, as these factors are all associated with an increased likelihood of MC.25
Table 3.
Further investigations that may be required in some patients.
| Features that may raise concerna | Investigation |
|---|---|
| All patients |
Limited laboratory studies • Complete blood count • C-reactive protein and faecal calprotectin to exclude IBD or other inflammatory conditions |
| Persistent diarrhoea Areas with high coeliac disease prevalence Onset after gastroenteritis/recent visit to (sub)tropics Not responsive to traditional therapy |
• Serological tests followed by (if positive) upper GI endoscopy with duodenal biopsy • Stool tests (culture, parasites (relevant to areas recently visited)) • Breath test (to rule out carbohydrate malabsorption) |
| Family history of colon cancer Unintended weight loss Rectal bleeding (not of anal origin) Abdominal mass |
• Colonoscopy with colonic biopsy |
| Age >50 years | • Colonoscopy |
Should be considered in the context of the supportive features of IBS, marked changes or the presence of multiple features.
IBS: irritable bowel syndrome; IBD: inflammatory bowel disease; GI: gastrointestinal.
Table 4.
Patient vignette – example of a case of suspected irritable bowel syndrome requiring further investigation.
| A 45-year-old woman presents with a six-month history of watery diarrhoea (Bristol Stool scale 6) occurring 10 times per day with mild abdominal pain relieved by opening her bowels. There is no weight loss and no other associated symptoms. Patient takes non-steroidal anti-inflammatory drugs (NSAIDs) for osteo-arthritis but is on no other medication. There is no relevant family history and clinical examination is unremarkable. It is important to check thyroid function and coeliac antibodies. Colonoscopy (or flexible sigmoidoscopy) with colonic biopsies are warranted to exclude microscopic colitis. |
Management algorithm
1. Consider key patient characteristics
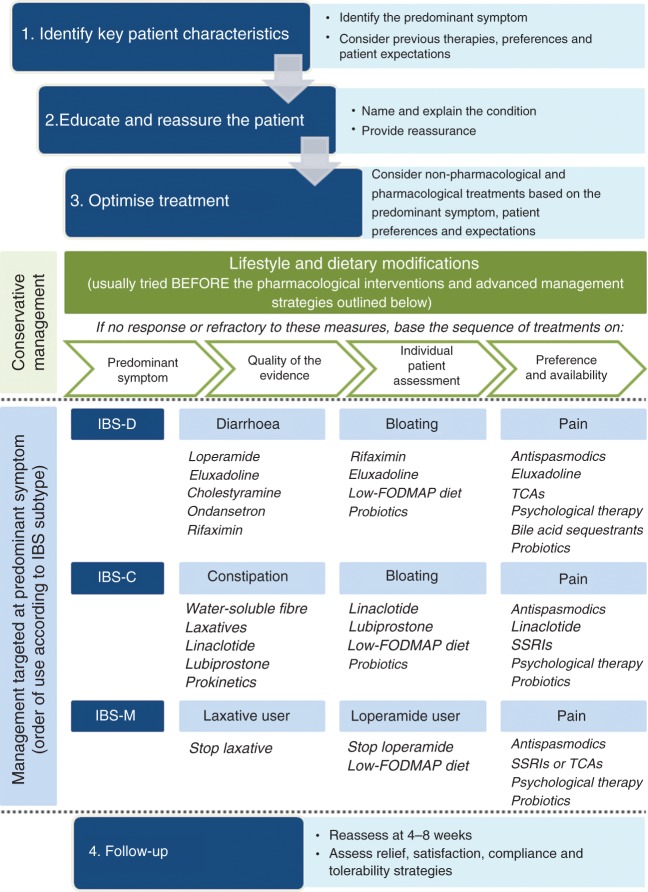
When deciding on an appropriate treatment strategy, it is important to understand the clinical profile of the patient, particularly in terms of the most predominant symptom. The pattern and severity of the GI symptoms experienced, along with the impact of these symptoms on daily activities and quality of life, will be the key determinants of the choice of management strategy, in addition to patient preferences (e.g. preference for non-pharmacological therapies) and treatment history (of both over-the-counter and prescription medications). Psychological comorbidities that could be contributing to the presence or worsening of IBS symptoms should also be considered (Figure 4).
Figure 4.
Management algorithm for irritable bowel syndrome.
FODMAP: fermentable oligosaccharides, disaccharides, monosaccharides and polyols; SSRIs: selective serotonin re-uptake inhibitors; TCAs: tricyclic antidepressants.
It is additionally important to explore the patient’s goals to elicit their personal perspective of their condition, and to understand what their expectations are in terms of treatment success. Knowing what patients with IBS want or expect from their medical care is critical in helping them to manage their symptoms, as failure to do so can lead to patient dissatisfaction with care, lack of compliance with prescribed treatments, and the inappropriate use of medical resources.26
2. Educating, supporting and sharing information with the patient
Exploring the patient’s goals also helps the physician understand the level of knowledge the patient has about their condition, and in turn allows information to be shared in an appropriate patient-specific way. Many physicians may feel uncomfortable giving a diagnosis of IBS until other possible explanations (i.e. organic disease) for a patient’s symptoms have been completely ruled out.11,27 However, continued unnecessary investigations can have a negative effect on patient management by undermining the ultimate diagnosis of IBS and the patient’s confidence in their treating physician.27 Effective management of IBS is therefore reliant on physicians being able to provide information and instruction for the patient by naming the condition through a confident diagnosis, with a clear explanation of what they believe is causing a patient’s symptoms and how they intend to target these factors with specific management strategies.28 Providing patients with a written diagnosis of IBS rather than just verbal confirmation can also encourage them to understand and appreciate their new diagnosis.
Simple explanatory models can provide a basis from which clinicians can explain IBS to their patients in ‘lay’ terms (Table 5).27 Important components of these models include providing an explanation of the underlying disease mechanisms and multifactorial nature of disease, the relationship between the brain and the gut, and the link between symptoms (patterns and severity) and stress, along with providing confidence about the benign nature of IBS.29 The use of simple visual tools can also help to simplify the complex pathophysiology of IBS using patient-friendly terminology (Figure 5). Ultimately, these models can help increase patient knowledge of IBS pathophysiology, helping to clarify any misconceptions around what IBS is and is not.29 They may also serve as a useful guide for future avenues to explore for IBS interventions, counselling and caregiving.27
Table 5.
Examples of ‘lay’ language for communicating with patients27.
| • The brain sends signals in such a way that they are over-interpreted by the bowel. |
| • The bowel is processing signals over-sensitively and this affects function. |
| • The function of the bowel is affected by the nervous system. |
| • The bowel sends signals in such a way that they are over-interpreted by the brain. |
| • The brain is receiving or processing signals too sensitively. |
| • The brain is misinterpreting normal signals from the body as signs of disease. |
| • Food, bacteria, or substances found in the gut can sometimes cause the gut to malfunction and trigger symptoms. |
Figure 5.
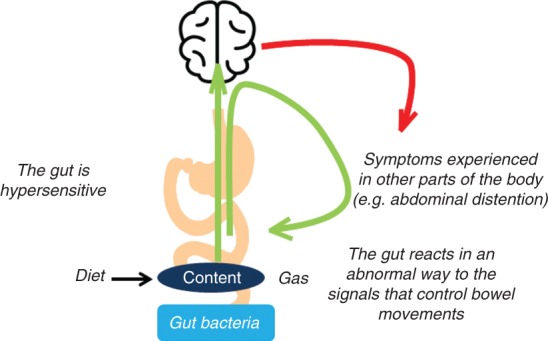
Explaining irritable bowel syndrome pathophysiology to the patient.
Fernando Azpiroz©. Published with permission. All rights reserved.
The more time that is spent at this important early stage in establishing a strong patient-physician relationship – getting to the root of the patient’s concerns and explaining their condition – the greater the chance of finding an effective management strategy. Key factors that can help build this relationship include active listening, not interrupting, using empathy, setting realistic patient expectations, and using nonverbal techniques such as eye contact and open body posture.11 Patient education and reassurance can also help teach patients simple self-management strategies related to diet and stress management, and assuage symptom-related fears and anxiety.29 Continuity of care is also important, with the development of a good patient-doctor relationship in follow-up appointments.
3. Create the optimal management strategy for each individual patient
Ideally, therapies that have been evaluated in high-quality randomised controlled trials would be the therapy of choice for patients with IBS. The reality is that many readily available treatments for IBS are either not specifically approved for the symptomatic treatment of IBS, or the evidence supporting their use is poor. However, physicians are familiar with their use and they are often inexpensive. Newly approved therapies have been developed specifically for the treatment of multiple IBS symptoms and have been assessed in high-quality clinical trials, but there is no direct evidence suggesting that newer agents are superior to traditional therapies, and it is very unlikely that comparative trials will ever be conducted to assess this. First-line usage of newly developed agents is therefore highly unlikely in the short term.
Lifestyle/dietary interventions
Regardless of subtypes or predominant symptoms, for many patients, the first-line approach of lifestyle and dietary modifications may provide relief from IBS without the need for further interventions.28 These include promoting increased physical activity30 and encouraging healthy eating habits such as modifying the intake of alcohol, caffeine, fat, spicy food, and gas-producing foods.31 Investigating the possibility of carbohydrate malabsorption, restricting milk and dairy products, and modifying dietary fibre may also be considered at this stage.31 Patients who do not respond or are refractory to the above measures may require symptom-modifying drugs or psychological treatments,28 along with more advanced dietary interventions.31 If suspected, functional outlet obstruction should be investigated and treated.
Pharmacological therapies for IBS management should be targeted towards the patient’s predominant symptom, which could be their dominant abnormal bowel habit, abdominal pain or bloating. With our increased understanding of the pathophysiology of IBS, pharmacological agents targeting the underlying disease mechanisms and thereby multiple symptoms of IBS associated with specific subtypes have also been developed.
A number of recent review articles,8–10 along with multiple systematic reviews and meta-analyses11–14 have provided a detailed overview of the quality of the clinical evidence for different management strategies for IBS. Taking this evidence into account, we summarise below the therapy options available for the management of different IBS subtypes according to their suitability for targeting specific symptoms and the order in which to prioritise their use (Figure 4).
Management of IBS-C
Initial management
Water-soluble fibre (e.g. psyllium) has been shown to provide overall symptom relief in IBS,32 while the osmotic laxative, polyethylene glycol, has been found to improve stool frequency and consistency, but has not shown a significant effect on abdominal pain or bloating.33 As the evidence base for soluble fibre for management of IBS-C is as strong as that for most pharmacological therapies, it is reasonable to use these agents as a first-line strategy because of their low cost, over-the-counter availability and favourable tolerability profile.9,32,33
Pharmacological treatments
By definition, all patients with IBS-C will experience abdominal pain and constipation, potentially along with other characteristic symptoms. The pharmacological agents available for the management of IBS-C vary from those targeting specific symptoms to those with a more global effect on multiple symptoms. The optimal therapy choice will ultimately depend on the severity of the symptoms that are most bothersome to the patient, and on local availability of treatments and patient preferences.
Agents that target multiple symptoms in patients with IBS-C include linaclotide and lubiprostone. Linaclotide is a guanylate cyclase C agonist that increases the production of cyclic guanosine monophosphate, which is proposed to reduce constipation by increasing fluid secretion and accelerating intestinal transit, and to target abdominal pain by reducing visceral hypersensitivity.34 Although linaclotide has highest-quality evidence to support its use for the management of IBS-C, it is unlikely to be used before more traditional therapy approaches such as laxatives and soluble fibre. In phase 3 clinical trials, linaclotide was shown to significantly improve abdominal pain and discomfort, and to provide significant relief from the symptoms of IBS-C, including abdominal bloating, stool consistency and severity of straining, as well as increase the mean number of spontaneous and complete spontaneous bowel movements per week. Linaclotide is approved by the European Medicines Agency (EMA) for the treatment of IBS-C.35,36
Another agent targeting fluid secretion in the GI tract is the chloride channel activator, lubiprostone, which has been shown to provide significant relief from IBS-C symptoms, including bloating, bowel movement frequency, abdominal pain, straining, constipation severity and stool consistency.37 Although lubiprostone has been evaluated in patients with IBS-C in phase 3 clinical trials with moderate evidence quality, it is not currently approved for this indication by the EMA.
For patients with IBS with pain predominance, antispasmodics (which include anticholinergic or calcium channel-blocking agents) are an established therapy option, as they relax gut smooth muscle.38 Pain in IBS is thought to be in part the result of smooth muscle spasms.9 While the quality of most studies assessing the efficacy of antispasmodics in IBS is suboptimal, the results of meta-analyses suggest benefits of these agents as a class over placebo for abdominal pain,39 and they may provide symptomatic short-term relief.12 However, not all antispasmodics have been shown to be effective, and the availability of specific agents varies significantly from country to country. Moreover, it is not clear whether they act differently in different IBS subgroups.
Antidepressants are also widely used for the treatment of IBS pain because of their observed effects on pain perception, mood and motility,11 and were first introduced into the management of IBS based on the observation that depression and anxiety were frequent comorbidities among patients seen in secondary and tertiary care.12 In a meta-analysis of randomised controlled trials of patients with IBS treated with antidepressants, these agents as a class were shown to improve abdominal pain along with global IBS symptoms.14 The symptomatic benefit of antidepressants in IBS seems to be unrelated to the presence or improvement of coexistent depression. Patient preferences may come into play when considering the suitability of these agents, as some patients may be averse to the idea of taking antidepressants.12 Of this class, selective serotonin re-uptake inhibitors (SSRIs) may be an appropriate choice for patients with IBS-C due to the prokinetic effects of these drugs.11 SSRIs tend to be prescribed at dosages standard for treating mental health disorders, so may also be more suitable for patients with psychological comorbidities.40 However, tricyclic antidepressants (TCAs) could also be used, as they are effective for abdominal pain and do not induce constipation when administered at low dosage.
Prokinetic agents, such as prucalopride, may also be considered as a treatment option specifically targeting constipation. Although prucalopride has demonstrated efficacy in the treatment of chronic idiopathic constipation, there are currently no data on the efficacy or safety for this agent in the treatment of IBS-C.12
Management of IBS-D
Initial management
Water-soluble fibre (e.g. psyllium) has a high water-holding/gel-forming capacity that is preserved throughout the large bowel, and can act as a bulking agent to firm loose/liquid stools in patients with diarrhoea.41 A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may also decrease symptoms in patients with IBS, particularly those with IBS-D.10,42 However, rigorous trials of dietary manipulations in patients with IBS are currently lacking, making it difficult to make strong recommendations on the optimal use of this approach.
Pharmacological treatments
As all patients with IBS-D experience pain and diarrhoea, the optimal therapy choice will be determined based on the severity of the symptoms that are most bothersome to the patient, local availability of treatments, and on patient preferences. The pharmacological agents available for the management of IBS-D vary from those specifically targeting diarrhoea, such as loperamide, to those targeting multiple symptoms.
Loperamide is a μ-opioid receptor agonist which improves diarrhoea by decreasing peristalsis, prolonging GI transit time, and through reduction of fluid secretion in the intestinal lumen.9 Although loperamide is established as an effective anti-diarrhoeal agent, the evidence base supporting its use for the treatment of IBS-D is not as strong as that of other pharmacological therapies as it has not been shown to improve overall IBS symptoms.12 Adverse effects of loperamide include abdominal cramps, constipation, bloating and nausea. However, because of its availability over the counter, established effect on diarrhoea and relatively low cost, loperamide is frequently used as a first-line therapy for IBS-D. Loperamide may also be used prophylactically when a patient anticipates episodes of diarrhoea.11
Eluxadoline is a mixed μ-opioid receptor agonist, δ-opioid receptor antagonist and κ-opioid receptor agonist peripherally acting in the gut with minimal oral bioavailability.43 Based on preclinical evidence, eluxadoline has been shown to reduce visceral hypersensitivity without completely disrupting intestinal motility, suggesting that peripheral δ-opioid receptor antagonism may reduce μ-opioid receptor-mediated constipation and, similar to its documented effects on central analgesia, enhance μ-opioid receptor-mediated peripheral analgesia.43 In phase 3 clinical trials, eluxadoline was shown to provide sustained relief from the global symptoms of IBS-D over the six-month trial period,44 and it has recently received regulatory approval for the treatment of IBS-D from the EMA, the United States Food and Drug Administration (FDA) and Health Canada.
There is increasing evidence to support a role for bile acids in the pathophysiology of IBS-D, with a subset of patients proposed to have bile acid malabsorption.45 Bile salt sequestrants, such as cholestyramine or colesevelam, may therefore be effective against diarrhoeal symptoms in some patients with IBS-D. The efficacy of cholestyramine is linked to the degree of bile acid malabsorption with patients affected by severe malabsorption responding better than those with milder forms.46 It is suggested that bile acid sequestrants should be considered after other therapies targeting diarrhoea have been unsuccessful.4,47
Serotonin (5-hydroxytryptamine (5-HT)) is one of the most important neurotransmitters in the gut, influencing gut motility and secretion.48 The 5-HT3 receptor antagonist, ondansetron, has been shown to slow colonic transit. In a randomised controlled trial, ondansetron improved the characteristic symptoms of IBS-D: loose stools, frequency and urgency. However, a significant reduction in abdominal pain was not seen.49 Patients with severe diarrhoea did not respond as well, suggesting that ondansetron treatment is most suited to patients with mild-to-moderate symptoms.
Recent evidence suggests a role for gut bacteria and antibiotics in the pathophysiology and treatment of IBS, respectively.50 Small intestinal bacterial overgrowth has also been suggested to be associated with IBS in general, and with IBS-D in particular, although this connection is still a topic of considerable debate.51 Rifaximin is an oral, minimally absorbed, broad-spectrum antimicrobial agent that targets the GI tract and is associated with a low risk of clinically relevant bacterial antibiotic resistance.52,53 In randomised controlled trials in patients with non-constipated IBS, two weeks of rifaximin treatment provided adequate relief of global IBS symptoms and individual symptoms of loose stools, abdominal pain, and bloating.52 However, this effect gradually disappears and re-treatment is necessary in a large proportion of patients to retain symptom improvement, so the optimal use of this agent for long-term management of IBS symptoms is yet to be defined.
Antispasmodics and antidepressants are widely used to target pain-predominance in all subtypes of IBS; however, specific agents within these classes may be more suited to patients with IBS-D. As TCAs increase intestinal transit time, they may be preferable to SSRIs for patients with IBS-D.11 However, data on the efficacy of these agents specifically for IBS-D are limited. Because of the potential for adverse effects, TCAs are generally prescribed for patients with IBS at dosages much lower than those used for depression or anxiety.40
Management of IBS-M
As patients with IBS-M essentially experience both IBS-C and IBS-D, the management strategy needs to be adaptable. Physicians should target the treatment approach towards the most bothersome symptoms experienced at a particular time, and then be prepared to re-evaluate this approach based on the response to therapy. There are limited studies evaluating the efficacy of specific management strategies for IBS-M and the evidence base for the use of most treatment options in this context is low. Usually the approach is selected from those outlined in the IBS-C and IBS-D sections above, depending on the bowel habit symptom that bothers the patient the most.
Individual patient characteristics are likely to be the key determinant of how to proceed with management. Patients who are habitual users of anti-diarrhoeal or laxative agents may need to discontinue these therapies in order to normalise their bowel habits. Some patients may also benefit from further investigations in order to determine the most appropriate treatment modality. Dietary management strategies (e.g. the low-FODMAP diet) that target bloating and diarrhoea (if present), and do not precipitate constipation may also be considered.
It is also important to note that some patients may have ‘pseudo’-IBS-M due to the intermittent use of laxatives and anti-diarrhoeal agents making them appear to have the mixed subtype. For these patients, simple strategies such as reducing the dose of laxatives may be enough to improve management. Patients with IBS-C may also experience overflow diarrhoea due to severe constipation causing a blockage in the bowel. As a consequence, watery stools may pass around the blockage and leak from the bowel, causing watery diarrhoea or faecal incontinence.
Targeting bloating
While there are currently no management strategies that specifically target bloating in IBS, some of the newer therapy options designed to target specific IBS subtypes have demonstrated efficacy against bloating in addition to other key symptoms. These include linaclotide and lubiprostone for IBS-C, and rifaximin and eluxadoline for IBS-D (Figure 4).
In recent years, data have also emerged supporting the use of the low-FODMAP diet for the management of IBS symptoms.54 The low-FODMAP diet has been shown to alleviate overall IBS symptoms as well as individual symptoms such as abdominal pain, bloating, constipation, diarrhoea, abdominal distention and flatulence in clinical trials.54,55 There is also evidence to suggest that the low-FODMAP diet may be more beneficial for patients with bloating predominance.56 The diet appears safe for short-term use; however, further research is needed to determine the long-term health effects of dietary restriction and the potential detrimental impact on the gut microbiota, making it difficult to provide evidence-based recommendations on optimal use.56 Recent studies have also reported comparable symptomatic benefit with the less stringent National Institute of Health and Care Excellence (NICE) diet.57,58 Again, data are limited to short-term efficacy studies.
There is a growing body of evidence to suggest that perturbations in the intestinal microbiota are linked to the pathophysiology of IBS, and that the composition of the gut microbiota of patients with IBS differs from that seen in ‘healthy’ individuals.59 A number of meta-analyses and systematic reviews suggest that probiotics may provide relief from overall or individual (e.g. bloating and abdominal pain) IBS symptoms, and these agents have been widely used for decades.9,59 However, the conclusions of these studies vary owing to inadequate sample size, poor study design and use of various probiotic strains in the reviewed studies,59 making it difficult to make definitive recommendations on their use or conclusions as to whether specific strains or preparations are effective against specific symptoms.9,11,12
Psychological therapies
Thoughts, emotions, and behaviours are proposed to be bi-directionally related to gut physiology and symptom manifestations in IBS.60 Psychological and behavioural treatments can help patients with IBS control and reduce their pain and discomfort and are seen as ancillary to or augmenting medical treatments.4 A variety of psychological interventions have demonstrated efficacy in improving IBS symptoms, including cognitive behavioural therapy (CBT), hypnotherapy, multicomponent psychotherapy and dynamic psychotherapy.11 CBT is the most studied form of psychological therapy and is associated with overall improvement in IBS symptoms, with good short-term and long-term efficacy.61 In clinical practice, the limited availability of therapists skilled in applying psychological therapy to GI problems, the high costs of delivering the treatment, and the practical difficulties for patients of scheduling weekly visits at a clinic may limit widespread use and suggest that these strategies are reserved for later lines of therapy.11,62
4. Follow-up
Once a patient has been started on a specific management strategy, they should be reassessed after four to eight weeks for their response to treatment. The key factors that should be assessed during this follow-up consultation are summarised in Table 6. Understanding what the most bothersome aspect of the condition is to the patient is fundamental to this approach, and this should be an ongoing dialogue between the patient and physician, starting from their initial consultations and continuing through to follow-up and long-term management.
Table 6.
What are the key factors to consider during follow-up in order to optimise management?
| • Understanding what treatment success means to the patient and what level of improvement is acceptable to them (overall and individual symptoms). |
| – Is the patient achieving satisfactory relief from their most bothersome symptoms? |
| • Considering strategies to optimise treatment tolerability. |
| – Encourage patient to report any adverse events experienced with therapy as soon as they occur. |
| ▪ Being contactable to the patient (e.g. phone, email) can facilitate this. |
| – Determining the minimum effective dose to minimise the potential for adverse effects. |
| – Assessing the need to continue or to interrupt treatment. |
| • Assessing patient compliance (frequency, timing, etc.). |
| • As most of the pharmacological therapies used for the management of irritable bowel syndrome target specific symptoms, the use of a combination of therapies may be a valid approach to target multiple symptoms. |
| • Considering the need for further investigations if there is no response to therapy. |
Firstly, it is important to define patient expectations in terms of treatment success and if they are achieving satisfactory relief from overall or individual symptoms. The severity of symptoms can play into setting realistic expectations for therapy. For example, in patients with more severe symptoms, achieving improved management and daily functioning may be a more achievable goal and acceptable to the patient, than anticipating complete resolution of symptoms.63
If the efficacy of a particular treatment is suboptimal there are a number of factors that could play a role. Before stopping a treatment, the physician should evaluate treatment adherence, changes in lifestyle and dietary habits, and concomitant therapies that might potentially interact with the treatment. Patients should be encouraged to contact their physician should they experience any adverse events so they can be advised on simple management strategies to optimise treatment tolerability. This does not have to take the form of a formal consultation: Being contactable by other means such as phone or email may be sufficient or even preferable for some patients. Dose adjustments to determine the minimum effective dose to minimise the potential for adverse effects can also be considered. As many of the symptom-modifying treatments for IBS target a specific mechanism, using a combination of management strategies may also be a valid approach for some patients to achieve a more global effect on symptoms.
Assessing the need to continue or interrupt treatment
The minimum time required to assess whether a treatment approach has been successful will vary according to the specific management strategy being applied (Table 7). If any ‘alarm features’ appear during management and follow-up, patients should be referred for further investigation as appropriate.
Table 7.
Guidance on optimising the use of specific therapies.
| Which patients may benefit? | Time to achieve efficacy on key symptoms | Common adverse effects and management | |
|---|---|---|---|
| Advanced dietary strategies | |||
| Low-FODMAP diet | • After conservative dietary management strategies have failed • Used alongside pharmacological therapies | • Reduction in severity of overall GI symptoms within seven days55 • May take up to eight weeks for symptom response to appear if dietary-mediated changes to gut microbiota are the cause of the improvement64 | • Further research needed to determine if there are potential adverse effects on the gut microbiota associated with long-term use54,55 • Potential for inadequate nutrient intake with stringent dietary restriction54 |
| Therapies targeting constipation | |||
| Linaclotidea,34–36 | • Constipation, pain or bloating as the predominant symptom | • Improvement in bowel frequency seen as early as week 1 • Maximal effect on abdominal pain and bloating may take longer (8–10 weeks) | • Diarrhoea – usually resolves within seven days or with temporary cessation of treatment |
| Lubiprostone37 | • Constipation as the predominant symptom | • Improvements in bowel movement frequency, straining, constipation severity and stool consistency seen at month 1 • Improvements in abdominal pain and bloating seen at month 2 | • Diarrhoea and nausea • To limit dose-dependent nausea, should be taken with meals |
| Therapies targeting diarrhoea | |||
| Loperamideb | • Diarrhoea as the dominant symptom (for acute episodes) | • Can be used on an as-needed basis, but patients may take a fixed dose to avoid diarrhoea episodes | • Constipation (treatment should be stopped in severe cases) |
| Eluxadolinec,44 | • Diarrhoea, abdominal pain or bloating as the predominant symptom | • Significant improvement in abdominal pain and stool symptoms from week 1 onwards • Maximum effect on pain may take four to six weeks | • Constipation – can be minimised by avoiding concomitant use with other medicines that may cause constipation |
| Cholestyramine | • IBS-D with increased colonic bile acid • After other pharmacological therapies targeting diarrhoea have been tried | • Within one to three weeks • Stop after one to three months if the therapeutic effect is not adequate | • Should be started at a low dose and gradually increased to reduce the incidence and intensity of adverse effects such as nausea and upper GI symptoms47 • Can reduce the bioavailability of other drugs so should be taken at a different time of day |
| Ondansetron49 | • Mild to moderate symptoms of diarrhoea (not severe cases) | • Onset of effect within one week in most cases • Improves loose stools, frequency, and urgency | • Constipation (can be managed with dose reduction) |
| Rifaximin52,53 | • Bloating as the predominant symptom | • Significant relief of IBS symptoms, bloating, abdominal pain, and loose or watery stools after two weeks | • Antibiotic resistance of GI flora a concern if use widespread12 • Long-term efficacy uncertain – effect gradually disappears and re-treatment is necessary in a large proportion of patients to retain symptom improvement53 |
| Therapies targeting pain | |||
| Antispasmodics | • Pain as the predominant symptom (to provide symptomatic short-term relief) | • Effect on pain is usually immediate (within an hour) | • Use may be limited by anticholinergic adverse events12 |
| TCAs | • Patients with IBS-D • Patients with insomnia, anorexia, or weight loss | • Patients usually started at low doses to minimise the potential for side effects • If an effect is not seen within a month, the dose may be increased | • Constipation • Drowsiness, dry mouth • Side effects frequently develop as the dose is increased |
| SSRIs | • Patients with IBS-C • Patients with anxiety or depression | • Onset of therapeutic benefit seems to occur within the first three to four weeks (but may take up to eight weeks) | • Diarrhoea • Sleep disturbances • Nervousness |
EMA-approved for the symptomatic treatment of moderate to severe IBS-C.
EMA-approved for the symptomatic treatment of acute episodes of diarrhoea associated with IBS-D.
EMA-approved for the treatment of IBS-D.
All other treatments included in the table are not currently approved by the EMA for the management of IBS.
EMA: European Medicines Agency; FODMAP: fermentable oligosaccharides, disaccharides, monosaccharides and polyols; GI: gastrointestinal; IBS-C: irritable bowel syndrome with constipation predominance; IBS-D: irritable bowel syndrome with diarrhoea predominance; SSRIs: selective serotonin re-uptake inhibitors; TCAs: tricyclic antidepressants.
Conclusions
The main challenges faced by physicians managing individuals with IBS today are the lack of simple diagnostic tests and the complex nuanced approach required for successful management. In order to simplify this process for day-to-day clinical practice, here we have developed simple visual tools to help navigate the key stages to reaching a positive diagnosis of IBS and a stepwise approach to patient-centred management targeted towards the most bothersome symptoms.
The basic tenets of IBS diagnosis and management highlighted by this simplified algorithm are very much aligned with the guiding principles proposed by recent reviews by Simrén and colleagues,8 Enck and colleagues,9 and Lacy and colleagues,10 along with the Rome IV guidelines.4 Successful management starts with the ability to make a confident positive diagnosis of IBS, along with being able to explain the underlying causes in relatable terms for the patient. These measures can help establish a strong patient-physician relationship and instil patient confidence in their treating physician. A thorough patient history can help to identify potential dietary and lifestyle triggers that can be modified as the first stage of IBS management. If symptoms are not effectively managed by these measures, pharmacological treatments can be considered, along with psychological therapies. The optimal choice of management strategy will ultimately depend on the predominant symptoms, patient preferences and a thorough understanding of the patient agenda in terms of their treatment expectations.
Acknowledgements
Editorial support for this manuscript was provided by Farah Dalwai from inVentiv Medical Communications, and was funded by Allergan, along with support for the organisation of the workshop meetings. The final content was developed independently by the authors without input from Allergan.
Declaration of conflicting interests
PM has received speaker’s fees from Allergan and Abbvie, and research support from Allergan and Takeda, and has served on advisory boards for Allergan, Shire and Lupin.
FM has received honoraria from Allergan.
FA has received grants from Danone, Clasado and Noventure, and served on advisory boards for Danone, Clasado and Allergan.
VA has received speaker and/or consulting fees from: Allergan, AstraZeneca, Boehringer Ingelheim, Falk, Ferring, KyowaKirin, Nordmark and Shionogi.
GB has received speaker bureau and/or advisory and/or research support from: Alfa Wassermann, Allergan, Cadigroup, Commonwealth, Danone, Falk Pharma, Ironwood, Italchimici, Lorenzatto, Malesci, Menarini, Noos, Parmalat, Shire, Synergy, Sofar, Yakult and Zespri.
MC has acted as a consultant for Allergan and Kiowa Kirin, and as a speaker for Shire and Menarini.
AE has served on advisory boards for Allergan, Almirall, Shire and Takeda.
APSH is the chair of the Rome IV Primary Care Committee. He has received fees from Allergan for advisory boards and research funding from Danone.
PL has received speaker’s fees for Abbott, Allergan, Falk, Nordmark and Shire, and served on advisory boards for Abbott and Allergan.
VS has received speaker’s fees from Alfa Wassermann, Allergan, Angelini and Valeas, and research support from Alfa Wassermann, Allergan and Shire, and has also served on advisory boards for Alfa Wassermann, Allergan, Angelini Farmaderma, Shire and Takeda.
PW has acted as a consultant for, or received research grant support from, the following pharmaceutical companies in the past five years: Almirall Pharma, Chr. Hansen, Danone Research, Ironwood Pharmaceuticals, Salix, Shire UK, Sucampo Pharmaceuticals and Allergan.
FZ has received speaker fees from Allergan, Reckitt-Benckiser, Takeda, Coloplast, Vifor pharma and Mayoli Spindler, and research support from Medtronic and Sandhill Scientific, and has served on advisory boards for Allergan and Reckitt-Benckiser.
JT has provided scientific advice to Abide Therapeutics, Alfa Wassermann, Allergan, Chr. Hansen, Danone, Genfit, Ironwood, Janssen, Kyowa Kirin, Menarini, Mylan, Novartis, Nutricia, Ono Pharma, Rhythm, Shionogi, Shire, SK Life Science, Takeda, Theravance Biopharma, Tsumura, Yuhan, Zealand and Zeria, has received research grants or support from Abide Therapeutics, Shire and Zeria, and has served on speakers’ bureaus for Abbott, Allergan, AstraZeneca, Janssen, Kyowa Kirin, Menarini, Mylan, Novartis, Shire, Takeda and Zeria.
Funding
Funding for editorial support and organisation of the workshop meetings was provided by Allergan.
References
- 1.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2016; 66: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: A Rome Foundation Working Team report. Am J Gastroenterol 2011; 106: 1749–1759. quiz 1760. [DOI] [PubMed] [Google Scholar]
- 3.Farndale R, Roberts L. Long-term impact of irritable bowel syndrome: A qualitative study. Prim Health Care Res Dev 2011; 12: 52–67. [DOI] [PubMed] [Google Scholar]
- 4.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407.e1395. [DOI] [PubMed] [Google Scholar]
- 5.Sood R, Camilleri M, Gracie DJ, et al. Enhancing diagnostic performance of symptom-based criteria for irritable bowel syndrome by additional history and limited diagnostic evaluation. Am J Gastroenterol 2016; 111: 1446–1454. [DOI] [PubMed] [Google Scholar]
- 6.Mujagic Z, Jonkers DMAE, Hungin AP, et al. Use of Rome criteria for the diagnosis of irritable bowel syndrome in primary care: A survey among European countries. Eur J Gastroenterol Hepatol 2017; 29: 651–656. [DOI] [PubMed] [Google Scholar]
- 7.Andresen V, Whorwell P, Fortea J, et al. An exploration of the barriers to the confident diagnosis of irritable bowel syndrome: A survey among general practitioners, gastroenterologists and experts in five European countries. United European Gastroenterol J 2015; 3: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simrén M, Törnblom H, Palsson OS, et al. Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2017; 2: 112–122. [DOI] [PubMed] [Google Scholar]
- 9.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016; 2: 16014–16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 2016; 9: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014; 109(Suppl 1): S2–S26. quiz S27. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–1561. quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: Systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1350–1365. quiz 1366. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Sanchez MI, Ford AC, Avila CA, et al. Anxiety and depression increase in a stepwise manner in parallel with multiple FGIDs and symptom severity and frequency. Am J Gastroenterol 2015; 110: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology. Epub ahead of print 19 February 2016. DOI: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed]
- 17.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 18.Ford AC, Talley NJ, Veldhuyzen van Zanten SJ, et al. Will the history and physical examination help establish that irritable bowel syndrome is causing this patient’s lower gastrointestinal tract symptoms? JAMA 2008; 300: 1793–1805. [DOI] [PubMed] [Google Scholar]
- 19.Casiday RE, Hungin APS, Cornford CS, et al. Patients’ explanatory models for irritable bowel syndrome: Symptoms and treatment more important than explaining aetiology. Fam Pract 2009; 26: 40–47. [DOI] [PubMed] [Google Scholar]
- 20.Quigley EM, Fried M, Gwee K, et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A global perspective. J Clin Gastroenterol 2016; 50: 704–713. [DOI] [PubMed] [Google Scholar]
- 21.Corsetti M, Whorwell PJ. Managing irritable bowel syndrome in primary care. Practitioner 2015; 259: 21–24. [PubMed] [Google Scholar]
- 22.Spiller R. Editorial: New thoughts on the association between diverticulosis and irritable bowel syndrome. Am J Gastroenterol 2014; 109: 1906–1908. [DOI] [PubMed] [Google Scholar]
- 23.Perona M, Benasayag R, Perello A, et al. Prevalence of functional gastrointestinal disorders in women who report domestic violence to the police. Clin Gastroenterol Hepatol 2005; 3: 436–441. [DOI] [PubMed] [Google Scholar]
- 24.Riddle MS, Welsh M, Porter CK, et al. The epidemiology of irritable bowel syndrome in the US military: Findings from the Millennium Cohort Study. Am J Gastroenterol 2016; 111: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macaigne G, Lahmek P, Locher C, et al. Microscopic colitis or functional bowel disease with diarrhea: A French prospective multicenter study. Am J Gastroenterol 2014; 109: 1461–1470. [DOI] [PubMed] [Google Scholar]
- 26.Halpert A. Irritable bowel syndrome: What do patients really want? Curr Gastroenterol Rep 2011; 13: 331–335. [DOI] [PubMed] [Google Scholar]
- 27.Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: An integrated explanatory model for clinical practice. Neurogastroenterol Motil 2015; 27: 750–763. [DOI] [PubMed] [Google Scholar]
- 28.Almquist E, Törnblom H, Simrén M. Practical management of irritable bowel syndrome: A clinical review. Minerva Gastroenterol Dietol 2016; 62: 30–48. [PubMed] [Google Scholar]
- 29.Labus J, Gupta A, Gill HK, et al. Randomised clinical trial: Symptoms of the irritable bowel syndrome are improved by a psycho-education group intervention. Aliment Pharmacol Ther 2013; 37: 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannesson E, Ringström G, Abrahamsson H, et al. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol 2015; 21: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie YA, Bowyer RK, Leach H, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet 2016; 29: 549–575. [DOI] [PubMed] [Google Scholar]
- 32.Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: A systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 33.Chapman RW, Stanghellini V, Geraint M, et al. Randomized clinical trial: Macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol 2013; 108: 1508–1515. [DOI] [PubMed] [Google Scholar]
- 34.Quigley EM, Tack J, Chey WD, et al. Randomised clinical trials: Linaclotide phase 3 studies in IBS-C – a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 2013; 37: 49–61. [DOI] [PubMed] [Google Scholar]
- 35.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- 36.Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1724; quiz 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome – results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther 2009; 29: 329–341. [DOI] [PubMed] [Google Scholar]
- 38.Annaházi A, Róka R, Rosztóczy A, et al. Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol 2014; 20: 6031–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011, pp. CD003460–CD003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacy BE, Weiser K, De Lee R. The treatment of irritable bowel syndrome. Therap Adv Gastroenterol 2009; 2: 221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol 2013; 108: 718–727. [DOI] [PubMed] [Google Scholar]
- 42.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014; 146: 67–75.e65. [DOI] [PubMed] [Google Scholar]
- 43.Wade PR, Palmer JM, McKenney S, et al. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol 2012; 167: 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016; 374: 242–253. [DOI] [PubMed] [Google Scholar]
- 45.Slattery SA, Niaz O, Aziz Q, et al. Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2015; 42: 3–11. [DOI] [PubMed] [Google Scholar]
- 46.Wedlake L, A’Hern R, Russell D, et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009; 30: 707–717. [DOI] [PubMed] [Google Scholar]
- 47.Mottacki N, Simrén M, Bajor A. Review article: Bile acid diarrhoea – pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2016; 43: 884–898. [DOI] [PubMed] [Google Scholar]
- 48.Gershon MD. Review article: Serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004; 20(Suppl 7): 3–14. [DOI] [PubMed] [Google Scholar]
- 49.Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014; 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saadi M, McCallum RW. Rifaximin in irritable bowel syndrome: Rationale, evidence and clinical use. Ther Adv Chronic Dis 2013; 4: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghoshal UC, Gwee KA. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: The missing link. Nat Rev Gastroenterol Hepatol 2017; 14: 435–441. [DOI] [PubMed] [Google Scholar]
- 52.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 53.Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 54.Nanayakkara WS, Skidmore PM, O’Brien L, et al. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin Exp Gastroenterol 2016; 9: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr 2016; 55: 897–906. [DOI] [PubMed] [Google Scholar]
- 56.Lacy BE. The science, evidence, and practice of dietary interventions in irritable bowel syndrome. Clin Gastroenterol Hepatol 2015; 13: 1899–1906. [DOI] [PubMed] [Google Scholar]
- 57.Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015; 149: 1399–1407.e1392. [DOI] [PubMed] [Google Scholar]
- 58.Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol 2016; 111: 1824–1832. [DOI] [PubMed] [Google Scholar]
- 59.Distrutti E, Monaldi L, Ricci P, et al. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol 2016; 22: 2219–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laird KT, Tanner-Smith EE, Russell AC, et al. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: A systematic review and meta-analysis. Clin Psychol Rev 2017; 51: 142–152. [DOI] [PubMed] [Google Scholar]
- 61.Laird KT, Tanner-Smith EE, Russell AC, et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 937–947.e934. [DOI] [PubMed] [Google Scholar]
- 62.Sinagra E, Romano C, Cottone M. Psychopharmacological treatment and psychological interventions in irritable bowel syndrome. Gastroenterol Res Pract 2012; 2012: 486067–486067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucak S, Chang L, Halpert A, et al. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Therap Adv Gastroenterol 2017; 10: 253–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012; 142: 1510–1518. [DOI] [PubMed] [Google Scholar]