Abstract
Prescription drug monitoring programs are promising tools to use in addressing the prescription opioid epidemic, yet prescribers’ participation in these state-run programs remains low as of 2014. Statutory mandates for prescribers to register with their state’s program, use it, or both are believed to be effective tools to realize the programs’ full potential. Our analysis of aggregate Medicaid drug utilization data indicates that state mandates for prescriber registration or use adopted in 2011–14 were associated with a reduction of 9–10 percent in population-adjusted numbers of Schedule II opioid prescriptions received by Medicaid enrollees and amounts of Medicaid spending on these prescriptions. This effect was largely associated with mandates of registration, which were comprehensive in all adopting states, and not with mandates of use, which were largely limited in scope or strength before 2015. Our findings support the use of mandates of registration in prescription drug monitoring programs as an effective and relatively low-cost policy. Future research should further assess the value of strong mandates of use to ensure safer and more appropriate prescribing of opioids.
Between 1991 and 2010 the population-adjusted volume of opioid prescriptions in the United States more than doubled, increasing from 304 per 1,000 people to 680 per 1,000.1 The increase in prescription opioid use coincided with a rapid escalation in nonmedical use of prescription opioids2 and opioid overdose–related deaths.3,4 Prescribers of controlled substances are believed to be an important link in addressing the deadly drug overdose epidemic.5
Prescription drug monitoring programs are statewide databases that gather information from pharmacies on dispensed prescriptions of controlled substances. Prescribers are important intended users of these databases. A complete picture of each patient’s prescription history provided by the database can help prescribers identify patients at high risk of misusing controlled substances, while ensuring access to effective pain relief for patients who make legitimate use of the drugs. The surging prescription opioid epidemic and earmarked federal grant funding for prescription drug monitoring programs6 have spurred a wave of implementations or upgrades of these programs during the past decade. To date, forty-nine states and the District of Columbia have such programs in operation—Missouri is the only state without a program in place.
Recent evaluations have provided evidence of the impact of prescription drug monitoring programs. A study using data that analyzed pain-related visits to physicians’ offices in twenty-four states over a ten-year period found that implementation of monitoring programs was associated with reduced prescribing of Schedule II opioids7—the subclass of prescription opioids with the highest risk of abuse and dependence, according to the Drug Enforcement Agency. Another study, using mortality data from thirty-five states over a fifteen-year period, found substantial reduction in opioid overdose–related deaths associated with state implementation of a prescription drug monitoring program.8 Yet another study, using claims data for disability enrollees in Medicare for the period 2006–12, did not find an operating drug monitoring program to be associated with a decline in the rate of high-risk opioid use or treatment for prescription opioid overdose.9
Participation by prescribers in their state’s monitoring program remained low in the years covered by these studies. A report by the Prescription Drug Monitoring Program Center of Excellence at Brandeis University estimated a median program registration rate of 35 percent among licensed prescribers who prescribed at least one controlled substance in the period 2010–12.10 A national survey in 2014 found that 53 percent of primary care physicians used their state’s program at least once, but that many did not use it routinely.11 Given that two of the three studies described above found the monitoring programs to have taken effect immediately following implementation,7,8 the observed impact might be due to increased awareness by prescribers about the prescription opioid epidemic in response to the launching of the monitoring program in their states, instead of their use of the programs on a regular basis. As states deploy policies to increase prescribers’ use of prescription drug monitoring programs, the impact of the ongoing use of the programs needs to be evaluated.
Prominent policy strategies employed by states include mandates that prescribers register with the drug monitoring program (a prerequisite for using it) and mandates that prescribers use the system under certain clinical circumstances, such as upon initial prescribing and every three months thereafter.12 Prescriber mandates are believed to be more effective in inducing prescribers to use the monitoring programs consistently, compared to campaigns to recruit prescribers to participate in the program—which are resource intensive and have had lackluster outcomes.12 By the end of 2015, twenty-three states had adopted mandates for prescriber registration, and twenty-nine states had adopted some version of a mandate to use the monitoring program.12 Examinations of data from the drug monitoring programs of Kentucky13 and of New York, Ohio, and Tennessee12 indicated that after implementation of the mandates, there were rapid increases in prescribers’ participation in these programs and decreases in high-risk behaviors related to opioid prescriptions that suggested that drugs were being misused or diverted to people for whom they were not prescribed.
In this study we assessed the effects of prescriber mandates, of both registration and use, on the number of prescription opioids received by Medicaid enrollees and Medicaid spending on these drugs. Medicaid enrollees have excessive burdens of chronic pain14 and are at a much higher risk of substance use disorders,15,16 compared to populations with other types of insurance. Medicaid enrollees are thus at heightened risk for prescription opioid misuse15,17 and were five to six times as likely to die from opioid-related overdose compared to populations with other types of insurance.17,18 Reducing the number of unsafe prescriptions of opioids in the Medicaid population should be a priority for any state’s drug control policies.
We used state-level aggregate data on prescription opioids received by Medicaid enrollees and Medicaid program spending on prescription opioids to examine the effects of mandates, of both registration and use, adopted by twenty-five states in the period 2011–14. This comprehensive evaluation provides much-needed evidence that supports states’ policies designed to further improve the impact of prescription drug monitoring programs.
Study Data And Methods
Data
Our primary data source was the 2011–14 Medicaid State Drug Utilization Files from the Centers for Medicare and Medicaid Services (CMS). To be eligible for federal matching funds, all states are required to report to the CMS the numbers of prescriptions for Medicaid-covered outpatient drugs and Medicaid spending on these drugs through fee-for-service Medicaid and Medicaid managed care programs.19 This requirement resulted in nearly complete data on all Medicaid-covered prescription drug use nationwide.20
The Medicaid State Drug Utilization Files identify prescription drugs with their eleven-digit, three-segment National Drug Code numbers and provide information on the total number of prescriptions and pre-rebate Medicaid spending (that is, spending not accounting for rebates paid by drug manufacturers) associated with each National Drug Code in each calendar quarter. According to the data, in 2014, 165.5 million opioid prescriptions were dispensed to Medicaid enrollees nationwide, which accounted for 7.3 percent of the number of prescription drugs paid for by Medicaid programs in that year. Opioids containing hydrocodone (37 percent of all opioids) and opioids containing oxycodone (22 percent) were the top opioids dispensed. Per population opioid prescriptions dispensed varied greatly across states, with an interquartile range of 15–23 prescriptions per quarter per 100 enrollees in 2014 (see online Appendix A1).21 Opioids dispensed to patients in the emergency departments or inpatient settings or paid for with cash were not included in these data.
Study Population
We restricted our study period to 2011–14 because the pace of states’ adoption of mandates picked up only after 2012, and the most recent Medicaid State Drug Utilization Files available were for the fourth quarter of 2014. We excluded two states (Missouri and Pennsylvania) and the District of Columbia, none of which had implemented a monitoring program (based on user access date) before 2015. We also excluded two states (Alabama and Utah) with an effective date of mandates before 2011 but no changes to their mandates in our study period. Our study sample thus included 736 state-quarter pairs (forty-six states multiplied by sixteen calendar quarters).
Measures
The two outcome measures we examined were the number of filled prescriptions (including both new prescriptions and refills) and the amount of pre-rebate Medicaid spending on prescription opioids in each quarter per 100 Medicaid enrollees. The numbers of Medicaid enrollees for each state-quarter pair were included in public data from CMS.22 Prescription opioids were identified by linking the National Drug Code numbers with information in the Approved Drug Products with Therapeutic Equivalence Evaluations, known as the Orange Book, published by the Food and Drug Administration.23 We excluded buprenorphine, which is commonly used for medication-assisted treatment of opioid use disorder. Prescription opioids were further categorized as Schedule II or Schedule III opioids based on their classification by the Drug Enforcement Agency, which reflected a recent reclassification of all hydrocodone-containing combination opioids (such as Vicodin and Lortab) from Schedule III to Schedule II.24 Schedule II opioids are considered to have greater potential for abuse and dependence, compared to Schedule III opioids. We converted the nominal Medicaid spending values in the study period to December 2014 dollars, based on the national monthly Consumer Price Index.
Implementation of mandates for registration, use, or both related to prescription drug monitoring programs was defined based on the effective date of each statutory mandate. The Prescription Drug Monitoring Project of the National Alliance for Model State Drug Laws25 provided us with effective dates of state mandates. During our study period, seventeen states implemented mandates of registration, and twenty implemented some version of mandates of use. (Because many states implemented both types of mandates, twenty-five states implemented a mandate of some kind.) Appendix A221 provides a summary of mandate policies adopted by the forty-six states included in our study. For each state-quarter pair, we determined whether the state had any mandate (of registration or use), a mandate of registration only, a mandate of use only, or mandates of both registration and use. We set each of the policy indicators to 1 for each full quarter after the effective date of the mandate or mandates. Appendix A321 provides examples from four hypothetical states.
While mandates of registration typically apply to all licensed prescribers in a state, the comprehensiveness of mandates of use varies widely across states. They differ in terms of types of drugs and types of prescribers to which the mandate applies, the circumstances under which prescribers are mandated to use the system, and whether prescribers are to exercise their subjective judgment as to what constitutes inappropriate use in deciding whether to use the system12 (Appendix A2).21
In recent years, mandates of use have imposed increasingly broad and obligatory criteria and thus have become stronger and more comprehensive over time. In contrast, the mandates of use that states adopted in our study period were weak, with three exceptions: Kentucky (whose mandate became effective in 2012), New York (2013), and Tennessee (2013). We considered these three mandates to be strong for two reasons: They require all prescribers, regardless of practice settings, to query the monitoring programs when first prescribing an opioid or benzodiazepine and subsequently at least every twelve months should prescribing continue, and they do not allow prescriber discretion on whether to query the program based on subjective judgment about possible inappropriate use. Because of the limited number of state-quarter pairs in which there were strong mandates of use in our study period (21 of 736 state-quarter pairs), we chose not to differentiate between strong and weak mandates of use in our main analysis, but we examined them separately in an exploratory analysis.
Analysis
The staggered implementation of mandates across states created a natural experiment. Our analysis compared the numbers of opioid prescriptions and Medicaid spending on prescription opioids per 100 Medicaid enrollees in state-quarter pairs exposed to mandates and those not exposed to mandates. We estimated a series of linear models for both outcomes. In addition to the key independent variable of exposure to mandates, we included a set of dichotomous state indicators, one for each state (state fixed effects), to control for across-state differences in the population-adjusted volume of prescriptions and Medicaid spending on prescription opioids dispensed to enrollees. We also included a set of year fixed effects to control for nationwide trends in these outcomes.
In addition, our models controlled for state-level policies and general economic conditions that varied over time, including a dichotomous indicator of newly implemented prescription drug monitoring programs during the study period (for nineteen states);26 a dichotomous indicator of states’ implementation of Medicaid expansion under the Affordable Care Act in 201427 or the partial implementation of Medicaid expansion between 2011 and 2013;28 the Medicaid managed care penetration rate, measured as the percentage of all Medicaid enrollees in comprehensive managed care programs; the percentage of the noninstitutionalized population living in poverty (measured at the state-year level); and state-year level unemployment rate. Standard errors were derived by taking into account clustering at the state level.29
In our main analysis, we first estimated the effect of any mandate of registration or use. Then, in a separate analysis, we estimated the effects associated with mandates of registration only, mandates of use only, and mandates of both registration and use. Separate analyses were conducted for Schedule II, Schedule III, and all opioids. In an exploratory analysis, we further broke down mandates of both registration and use into mandates of registration and weak mandates of use versus mandates of registration and strong mandates of use, since all three states that adopted relatively strong mandates of use (Kentucky, New York, and Tennessee) also had mandates of registration when their mandates of use went into effect.
Limitations
Our study had several limitations. First, we could not tell whether the changes associated with states’ implementation of mandates that we found reflected a trend toward more appropriate and safer prescribing of opioids to Medicaid patients. Nor were we able to evaluate whether such mandates had any unintended effects that deterred appropriate opioid prescribing. To shed some light on this point, we conducted a separate analysis by focusing on fentanyl, morphine, and hydromorphone—opioids commonly used to treat cancer pain.13 Combined, these three drugs accounted for 7.6 percent of all prescription opioids dispensed to Medicaid enrollees.
Second, we did not have data on actual prescribers’ registration with and use of their state’s drug monitoring program. Because of their aggregate nature, the Medicaid State Drug Utilization Files did not allow us to derive patient-level measures such as daily morphine milligram-equivalent doses, multiple provider episodes (an indicator of possible misuse of controlled substances), or overlapping opioid prescriptions or total days of supply—measures that would capture high-risk patient behaviors related to opioid prescriptions. Thus, our study is unable to shed much light on the behavioral pathways by which state mandates had an effect on prescription opioid use.
Third, states that adopted mandates might have experienced more rapid growth in the opioid epidemic compared to other states, which would tend to bias our results in the direction of suggesting that the mandates lacked effects. Alternatively, implementation of mandates might have coincided with other reasons for changes in opioid prescriptions, such as Medicaid policies limiting coverage of high-dose opioid prescriptions or placing certain opioids on more restrictive formulary tiers,30 which would bias our estimates in the opposite direction. Thus, our results are associational rather than causal, but the net impact of the biases (after they cancel each other out) is unknown.
Study Results
Based on our data for 736 state-quarter pairs, the average number of opioid prescriptions per quarter per 100 Medicaid enrollees was 21.3 (95% confidence interval: 20.7, 21.8). Schedule II (average: 14.9; 95% CI: 14.5, 15.3) and Schedule III opioids (average: 6.4; 95% CI: 6.2, 6.6) accounted for 70 percent and 30 percent of all opioids, respectively. Average total Medicaid spending per quarter per 100 enrollees on all opioids was $874.0 (95% CI: 839.5, 908.4), of which $519.4 (95%CI: 500.1, 538.7) was spent on Schedule II and $354.5 (95% CI: 331.6, 377.5) on Schedule III opioids (dollar amounts do not sum to total because of rounding).
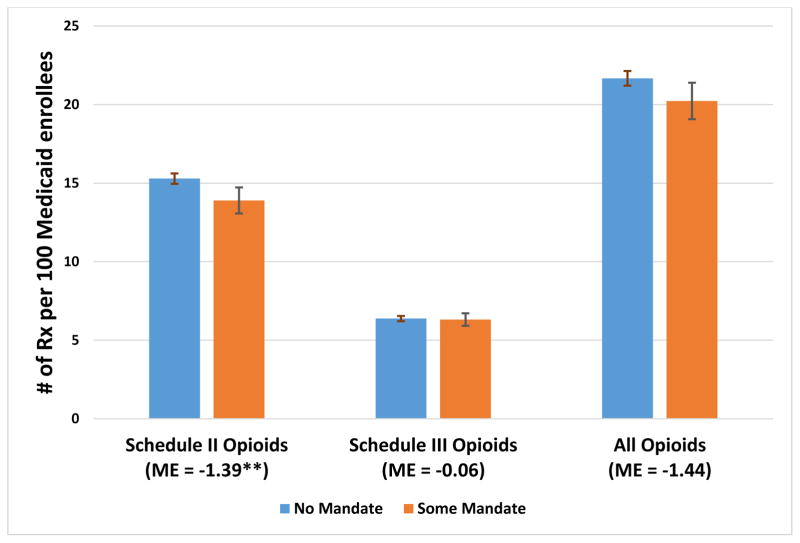
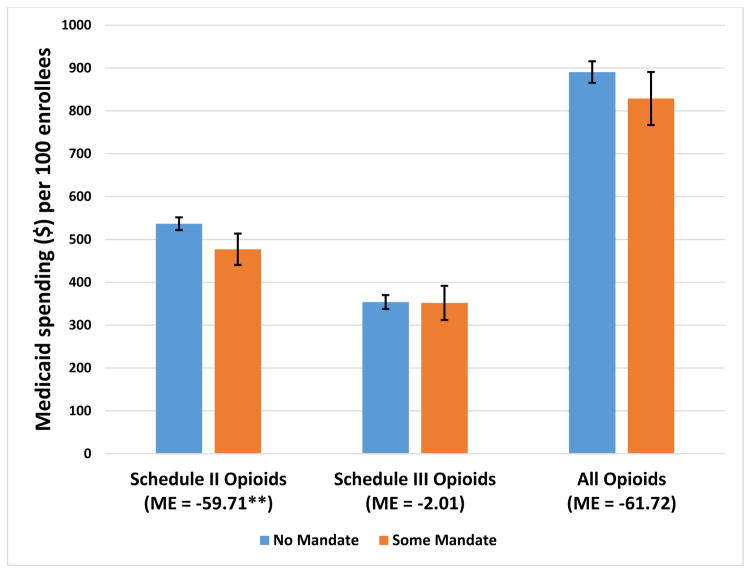
Our analysis indicated that mandates of any kind, of either registration or use, were associated with a 9–10 percent reduction in the use of Schedule II opioids by Medicaid enrollees over our study years, 2011–2014. The average numbers of Schedule II opioid prescriptions per quarter per 100 Medicaid enrollees were 15.3 without any mandate, compared to 13.9 with some kind of mandate (Exhibit 1). Average Medicaid spending on Schedule II opioids per quarter per 100 enrollees over the study years was $536.7 without any mandate, compared to $477.0 with a mandate (Exhibit 2). The difference in the numbers of Schedule III opioid prescriptions between states with and those without mandates was minimal and not significant (Exhibit 1). There was a difference of 6.6 percent in the numbers of all opioid prescriptions between states with and those without mandates, but that change was not significant.
Exhibit 1.
Average predicted numbers of opioid prescriptions per 100 Medicaid enrollees per quarter in states with and without mandates for prescribers to register with or use the prescription drug monitoring program, 2011–14
Source/Notes: SOURCE Authors’ analysis of data for 2011–14 from the Medicaid State Drug Utilization Files. NOTES Schedule II opioids are the subclass of prescription opioids with the highest risk of abuse and dependence. Schedule III opioids have a lower potential for abuse and dependence than Schedule II opioids. As explained in the text, Alabama, Missouri, Pennsylvania, Utah, and the District of Columbia were excluded from the analysis. The error bars indicate 95% confidence intervals. **p < 0.05
Exhibit 2.
Average predicted Medicaid spending on prescription opioids per 100 enrollees per quarter in states with and without mandates for prescribers to register with or use the prescription drug monitoring program, 2011–14
Source/Notes: SOURCE Authors’ analysis of data for 2011–14 from the Medicaid State Drug Utilization Files. NOTES Spending (in 2014 dollars) is before rebates paid by drug manufacturers. Schedule II and Schedule III opioids are explained in Exhibit 1 Notes. As explained in the text, Alabama, Missouri, Pennsylvania, Utah, and the District of Columbia were excluded from the analysis. The error bars indicate 95% confidence intervals. **p < 0.05
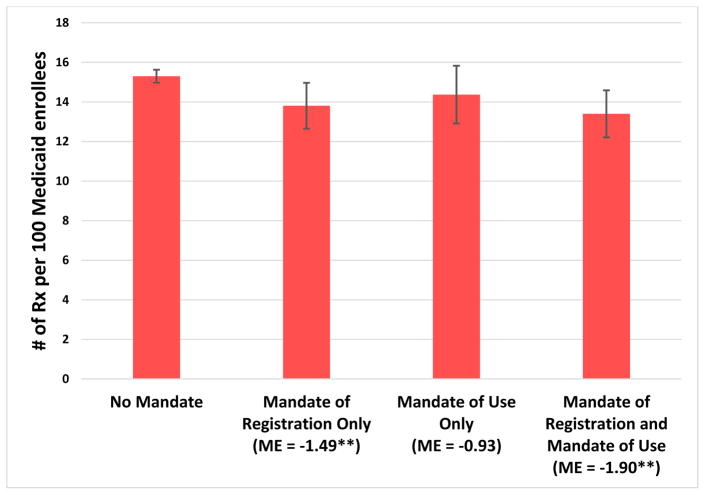
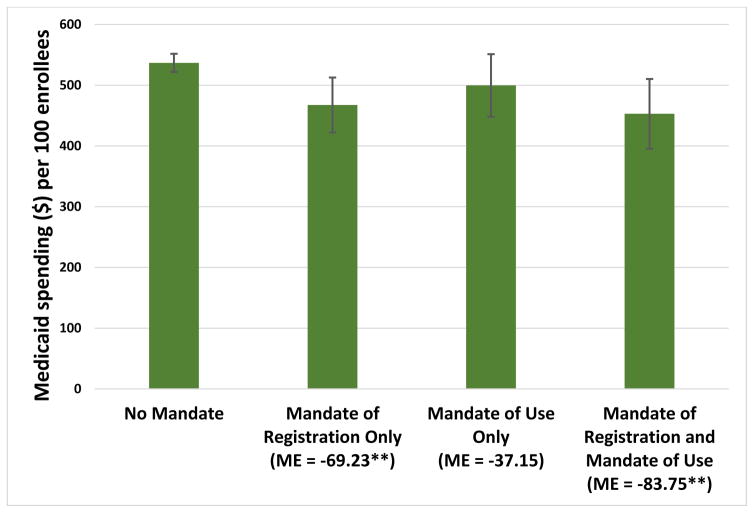
In our analysis that differentiated between mandates of registration and mandates of use, we found that both the numbers of prescriptions and the amounts of Medicaid spending were significantly lower (by approximately 10 percent) in states with a mandate of registration alone or a mandate of registration and use, compared to states with no mandate (Exhibits 3 and 4). In contrast, having a mandate of use alone was not associated with a significant decrease in Schedule II opioid prescriptions or spending. None of the mandate policy categories was associated with significant changes in the number of prescriptions of or spending on Schedule III opioids or all opioids (data not shown).
Exhibit 3.
Average predicted numbers of prescriptions for Schedule II prescription opioids per 100 Medicaid enrollees per quarter in states with and without mandates for prescribers to register with and/or to use the prescription drug monitoring program, 2011–14
Source/Notes: SOURCE Authors’ analysis of data for 2011–14 from the Medicaid State Drug Utilization Files. NOTES Schedule II opioids are explained in the Exhibit 1 Notes. Mandates of registration require prescribers to register with the state’s monitoring program. Mandates of use require that prescribers use the program under certain circumstances, such as for a new prescription. Significance denotes the difference between a mandate policy category and no mandate. As explained in the text, Alabama, Missouri, Pennsylvania, Utah, and the District of Columbia were excluded from the analysis. The error bars indicate 95% confidence intervals. **p < 0.05.
Exhibit 4.
Average predicted Medicaid spending on Schedule II prescription opioids per 100 enrollees per quarter in states with and without mandates for prescribers to register with and/or to use the prescription drug monitoring program, 2011–14
Source/Notes: SOURCE Authors’ analysis of data for 2011–2014 from the Medicaid State Drug Utilization Files. NOTES Spending (in 2014 dollars) is before rebates paid by drug manufacturers. Schedule II opioids are explained in the Exhibit 1 notes. Mandate policy categories are explained in the Exhibit 3 Notes. Significance denotes the difference between a mandate policy category and no mandate. As explained in the text, Alabama, Missouri, Pennsylvania, Utah, and the District of Columbia were excluded from the analysis. The error bars indicate 95% confidence intervals. **p < 0.05.
Full regression outputs of our main analyses are provided in Appendix A4.21
Our exploratory analysis further broke down mandates of use into strong versus weak mandates and examined changes in both outcomes associated with five categories of mandate policies (Appendix A5).21 Similar to the results in our main analysis, mandates of registration alone were associated with a reduction of more than 10 percent in both outcomes, compared to no mandates. Mandates of use alone (all of which were considered weak mandates) did not have an effect on either outcome. Changes associated with mandates of registration and weak mandates of use were similar to those associated with mandates of registration and strong mandates of use.
In the analysis that focused on drugs commonly used for cancer pain, we did not find any mandate policy to be associated with a significant change in the number of prescriptions of or Medicaid spending on these drugs (Appendix A6).21
Discussion
The past few years have seen an acceleration in states’ adoption of statutory mandates that prescribers register with their state’s prescription drug monitoring program, use the program, or both. Our analysis of data from the Medicaid State Drug Utilization Files indicated that such mandates implemented in the period 2011–14 were associated with a reduction of 9–10 percent in population-adjusted prescriptions of and Medicaid spending on Schedule II opioids for enrollees. No effect was seen for Schedule III opioids. Our results also suggest that reductions in numbers of Schedule II opioid prescriptions were largely associated with mandates of registration and not with mandates of use—which were generally weak during our study period. Our estimates suggest that if every state adopted a mandate of registration, Medicaid programs nationwide would save over $166 million (95% CI: 18 million, 314 million) from reduced spending on Schedule II opioids over 12 months (Appendix A7).21
An important policy implication is that mandates of registration alone could be effective in promoting safer and more contained prescribing of opioids with the highest potential for abuse and dependence. This is contrary to the common belief that mandating registration only might have limited effects, since mandates of registration alone do not guarantee that prescribers will actually use the monitoring programs.12,31 Since registration with the system was the prerequisite for querying it, mandates of registration might have substantially lowered the initial hurdle involved in using the system by making prescribers familiar with the process of using it. In addition, the fact that such mandates require all prescribers to register with the monitoring program might have further raised prescribers’ awareness of misuse and abuse of controlled substances among their patients, leading to subsequent changes in prescribing practices. If it is indeed the case that mandates of registration would encounter far less pushback from the provider community31 and are less costly to enforce (for example, registration can be enforced as a condition for license renewal), compared to mandates of use, states that do not yet have any mandate in place should consider adopting at least mandates of registration.
Our findings suggest that mandates of use alone implemented before 2015 had limited effects on the numbers of opioid prescriptions received by Medicaid enrollees or Medicaid spending on these drugs. Consistently, mandates of use had very limited incremental effects when combined with mandates of registration. Of note, in seventeen of the twenty states that implemented some mandate of use during our study period, the mandates were of limited scope (for example, applying only to prescribers in opioid addiction treatment programs), strength (for example, relying on prescribers’ judgment to determine the need to query the drug monitoring program), or both. Our findings suggest that weak mandates of use are unlikely to be an effective tool to induce population-wide changes in opioid prescribing.
Three states (Kentucky, New York, and Tennessee) adopted strong mandates of use during our study period. Our exploratory analysis did not find a greater reduction in opioid use by Medicaid patients associated with these strong mandates of use (all of which were in combination with mandates of registration), compared to weak mandates of use in combination with a mandate of registration. This is in contrast to findings of single-state evaluations that have reported rapid increases in prescribers’ use of the monitoring programs, reductions in multiple provider episodes, and reductions in total volume of prescribing of certain drugs after the programs were implemented.12 Although our study used a much stronger design than the single-state before-and-after approach, we had a limited number of states with strong mandates and limited follow-up time after their implementation (nine quarters for Kentucky, five for New York, and seven for Tennessee). Thus, our findings are at best preliminary and need to be revisited as more recent data become available. In addition, future studies using data that capture patient-level high-risk opioid prescription patterns will shed more definitive light on the effectiveness of mandates in changing prescribing behaviors.
Despite encouraging evidence that supports the use of mandates, enforcement remains a challenge—but more so for mandates of use than for mandates of registration. For example, Massachusetts adopted a policy according to which the renewal of a prescriber’s registration to prescribe controlled substances triggers the prescriber’s registration with the prescription drug monitoring program. Such approaches are believed to be more effective and efficient than campaigns to recruit prescribers to register voluntarily.
For mandates of use—especially strong mandates with comprehensive coverage of prescribers and clinical circumstances—no such ready regulatory mechanisms are available for enforcement. Extensive state monitoring of prescribers’ compliance may not be possible—or if it is possible, it may be costly. However, provider organizations may be able to regulate their members by integrating monitoring program reports with electronic medical records, health information exchange systems, or both32 and by enforcing queries of the drug monitoring program as a condition of prescribing under defined circumstances. Technical barriers to such regulation could be surmountable, but there might be a lack of incentives on the part of provider organizations to implement the regulation, especially if prescribers strongly resist complying with the mandate of use. In addition, data security and patient confidentiality remain serious concerns when patient data are integrated across different systems.32
Conclusion
Our analysis of aggregate Medicaid drug utilization data indicates that state mandates for prescribers to register with or use the prescription drug monitoring programs adopted in 2011–14 were associated with reductions of 9–10 percent in population-adjusted numbers of Schedule II opioid prescriptions received by Medicaid enrollees and amounts of Medicaid spending on these prescriptions. This reduction was largely associated with mandates for prescribers to register with their state’s monitoring program and not with the generally weak mandates to use the programs that we found in the study period. States’ adoption of mandates has accelerated in recent years. Future studies need to provide updated assessments of the role of strong mandates of use in ensuring safer and more appropriate use of prescription opioids.
Supplementary Material
Acknowledgments
An early version of this article was presented at the AcademyHealth Annual Research Meeting in Boston, Massachusetts, June 27, 2016. This work was funded by a pilot grant from the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV, a National Institute on Drug Abuse Center of Excellence (Grant No. P30DA040500). Bruce Schackman and Brandon Aden are funded by the National Institute on Drug Abuse (Grant No. P30DA040500). The authors thank the National Alliance for Model State Drug Laws for providing the effective dates of state mandates. The authors also thank Philip Jeng for his excellent assistance with the manuscript.
Biographies
Hefei Wen is an assistant professor of health management and policy at the University of Kentucky, in Lexington.
Bruce R. Schackman is a professor of healthcare policy and research at Weill Cornell Medical College, in New York City.
Brandon Aden is an assistant professor of medicine and of healthcare policy and research at Weill Cornell Medical College.
Yuhua Bao (yub2003@med.cornell.edu) is an associate professor of healthcare policy and research at Weill Cornell Medical College.
Notes
- 1.Volkow ND. Hearing before the Senate Caucus on International Narcotics Control [Internet] Washington (DC): US Congress; 2014. May 14, [cited 2016 Aug 16]. Available to download from: https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. [Google Scholar]
- 2.RTI International. Results from the 2014 National Survey on Drug Use and Health: detailed tables [Internet] Rockville (MD): Substance Abuse and Mental Health Services Administration; 2015. Sep 10, [cited 2017 Feb 10]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf. [Google Scholar]
- 3.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose–related deaths. JAMA. 2011;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–82. doi: 10.15585/mmwr.mm6450a3. (MMWR) [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services, Behavioral Health Coordinating Committee, Prescription Drug Abuse Subcommittee. Addressing prescription drug abuse in the United States: current activities and future opportunities [Internet] Washington (DC): HHS; [cited 2017 Feb 10]. Available from: http://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. [Google Scholar]
- 6.Catalog of Federal Domestic Assistance. Harold Rogers Prescription Drug Monitoring Program [Internet] Washington (DC): CFDA; [cited 2017 Feb 10]. Available from: http://www.cfda.gov/index?s=program&mode=form&tab=core&id=ce6f8849c6fd92bb108062ab1f9150bd. [Google Scholar]
- 7.Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff (Millwood) 2016;35(6):1045–51. doi: 10.1377/hlthaff.2015.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood) 2016;35(7):1324–32. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375(1):44–53. doi: 10.1056/NEJMsa1514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiner P, Nikitin R, Shields TP. Bureau of Justice Assistance prescription drug monitoring program performance measures report: January 2009 through June 2012 [Internet] Prescription Drug Monitoring Program Center of Excellence, Heller School for Social Policy and Management, Brandeis University; Waltham, MA: Washington (DC): Bureau of Justice Assistance; [last revised 2014 Apr 4; cited 2017 Feb 10]. Available from: http://www.pdmpexcellence.org/sites/all/pdfs/BJA%20PDMP%20Performance%20Measures%20Report%20Jan%202009%20to%20June%202012%20FInal_with%20feedback.pdf. [Google Scholar]
- 11.Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood) 2015;34(3):484–92. doi: 10.1377/hlthaff.2014.1085. [DOI] [PubMed] [Google Scholar]
- 12.Prescription Drug Monitoring Program Center of Excellence at Brandeis. PDMP prescriber use mandates: characteristics, current status, and outcomes in selected states. Waltham (MA): The Center; 2016. Revision 3, May 2016 [Internet] [cited 2017 Feb 10]. Available from: http://www.pdmpexcellence.org/sites/all/pdfs/COE%20briefing%20on%20mandates%203rd%20revision.pdf. [Google Scholar]
- 13.Freeman PR, Goodin A, Troske S, Talbert J. Kentucky House Bill 1 Impact Evaluation: executive summary prepared for the Kentucky Cabinet for Health and Family Services [Internet] Institute for Pharmaceutical Outcomes and Policy, Department of Pharmacy Practice and Science, College of Pharmacy, University of Kentucky; Lexington, KY: Frankfort (KY): The Cabinet; 2015. Mar, [cited 2017 Feb 10]. Available from: http://www.chfs.ky.gov/NR/rdonlyres/842D66B1-612C-4A26-9FE2-C526329D0BEE/0/KentuckyHB1ImpactStudyExecutiveSummary03262015.pdf. [Google Scholar]
- 14.Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009–10 National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken) 2016;6811:1688–94. doi: 10.1002/acr.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack KA, Zhang K, Paulozzi L, Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26(1):182–98. doi: 10.1353/hpu.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adelmann PK. Mental and substance use disorders among Medicaid recipients: prevalence estimates from two national surveys. Adm Policy Ment Health. 2003;31(2):111–29. doi: 10.1023/b:apih.0000003017.78877.56. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314(14):1468–78. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Overdose deaths involving prescription opioids among Medicaid enrollees—Washington, 2004–2007. MMWR Morb Mortal Wkly Rep. 2009;58(42):1171–5. (MMWR) [PubMed] [Google Scholar]
- 19.HealthData.gov. Medicaid drug programs data and resources [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; [cited 2017 Feb 10]. Available from: http://www.medicaid.gov/medicaid-chip-program-information/by-topics/benefits/prescription-drugs/medicaid-drug-programs-data-and-resources.html. [Google Scholar]
- 20.Department of Health and Human Services, Office of Inspector General. States’ collection of rebates for drugs paid through Medicaid managed care organizations. Washington (DC): HHS; 2012. Sep, [cited 2017 Feb 10]. Available from: https://oig.hhs.gov/oei/reports/oei-03-11-00480.pdf. [Google Scholar]
- 21.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 22.Medicaid.gov. Medicaid and CHIP Enrollment Data [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; 2016. Nov 21, Available from: https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/index.html. [Google Scholar]
- 23.Food and Drug Administration. Approved drug products with therapeutic equivalence evaluations (Orange Book) [Internet] Silver Spring (MD): FDA; [updated 2017 Jan 25; cited 2017 Feb 13]. Available for download from: http://www.fda.gov/Drugs/InformationOnDrugs/ucm129662.htm. [Google Scholar]
- 24.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: rescheduling of hydrocodone combination products from Schedule III to Schedule II. Fed Regist. 2014;79(163):49661–82. [PubMed] [Google Scholar]
- 25.National Alliance for Model State Drug Laws. Prescription drug monitoring programs [Internet] Manchester (IA): NAMSDL; c2017. [cited 2017 Feb 13]. Available from: http://www.namsdl.org/prescription-monitoring-programs.cfm. [Google Scholar]
- 26.National Alliance for Model State Drug Laws. PDMP dates of operation [Internet] Charlottesville (VA): NAMSDL; c2015. [cited 2017 Feb 13]. Available from: http://www.namsdl.org/library/580225E9-E469-AFA9-50E7579C1D738E71/ [Google Scholar]
- 27.Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision [Internet] Menlo Park (CA): KFF; c2017. [cited 2017 Feb 13]. Available from: http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/ [Google Scholar]
- 28.Sommers BD, Kenney GM, Epstein AM. New evidence on the Affordable Care Act: coverage impacts of early Medicaid expansions. Health Aff (Millwood) 2014;33(1):78–87. doi: 10.1377/hlthaff.2013.1087. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–75. [Google Scholar]
- 30.Keast SL, Nesser N, Farmer K. Strategies aimed at controlling misuse and abuse of opioid prescription medications in a state Medicaid program: a policymaker’s perspective. Am J Drug Alcohol Abuse. 2015;41(1):1–6. doi: 10.3109/00952990.2014.988339. [DOI] [PubMed] [Google Scholar]
- 31.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313(9):891–2. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark T, Eadie J, Kreiner P, Strickler G. Prescription drug monitoring programs: an assessment of the evidence for best practices [Internet] Prescription Drug Monitoring Program Center of Excellence, Heller School for Social Policy and Management, Brandeis University; Waltham, MA: Philadelphia (PA): Pew Charitable Trusts; 2012. Sep 20, [cited 2017 Feb 13]. Available from: http://www.pewtrusts.org/~/media/assets/0001/pdmp_update_1312013.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.