Significance
Climate change is altering the seasonal timing of biological events, effectively rescheduling the potential interactions among species. We know specialist consumers suffer when they fail to synchronize with their prey; however, little is known about how generalist consumers respond to phenological shifts across multiple food resources. This reshuffling may create novel temporal overlap between foods that were once separated in time. We examined how a generalist consumer, the Kodiak brown bear, responded when two key foods, red elderberry and sockeye salmon, became synchronized. Bears switched from eating salmon to elderberries, disrupting an ecological link that typically fertilizes terrestrial ecosystems and generates high mortality rates for salmon. These results demonstrate an underappreciated mechanism by which climate-altered phenologies can alter food webs.
Keywords: climate change, mismatch, grizzly bear, phenology, prey switch
Abstract
Climate change is altering the seasonal timing of life cycle events in organisms across the planet, but the magnitude of change often varies among taxa [Thackeray SJ, et al. (2016) Nature 535:241–245]. This can cause the temporal relationships among species to change, altering the strength of interaction. A large body of work has explored what happens when coevolved species shift out of sync, but virtually no studies have documented the effects of climate-induced synchronization, which could remove temporal barriers between species and create novel interactions. We explored how a predator, the Kodiak brown bear (Ursus arctos middendorffi), responded to asymmetric phenological shifts between its primary trophic resources, sockeye salmon (Oncorhynchus nerka) and red elderberry (Sambucus racemosa). In years with anomalously high spring air temperatures, elderberry fruited several weeks earlier and became available during the period when salmon spawned in tributary streams. Bears departed salmon spawning streams, where they typically kill 25–75% of the salmon [Quinn TP, Cunningham CJ, Wirsing AJ (2016) Oecologia 183:415–429], to forage on berries on adjacent hillsides. This prey switching behavior attenuated an iconic predator–prey interaction and likely altered the many ecological functions that result from bears foraging on salmon [Helfield JM, Naiman RJ (2006) Ecosystems 9:167–180]. We document how climate-induced shifts in resource phenology can alter food webs through a mechanism other than trophic mismatch. The current emphasis on singular consumer-resource interactions fails to capture how climate-altered phenologies reschedule resource availability and alter how energy flows through ecosystems.
Many trophic resources are ephemerally available and do not provide foraging opportunities year-round. One way consumers are able to persist on ephemeral resources is by integrating across multiple resource taxa that exhibit complementary dynamics and occur sequentially through time (1). As climate change differentially shifts the phenology of resource taxa (2–4), foraging opportunities that once occurred sequentially may begin to occur simultaneously. Many studies have explored how phenological shifts in single resources affect specialist consumers (5–10). In contrast, beyond wide agreement that generalists likely will fare better than specialists (7–11), there is poor understanding of how generalist consumers respond to heterogeneous phenological shifts across multiple resources. Past work has shown that changes in the relative abundance of alternative prey taxa can cause predators to switch between them (12), but it remains largely unknown whether changes in the relative phenology of prey can have similar effects. Prey switching can affect the stability of food webs (12), and may have profound ecological effects when apex predators are involved (13). Here we document how variation in the phenological responses of multiple resources affects a typically strong predator–prey interaction.
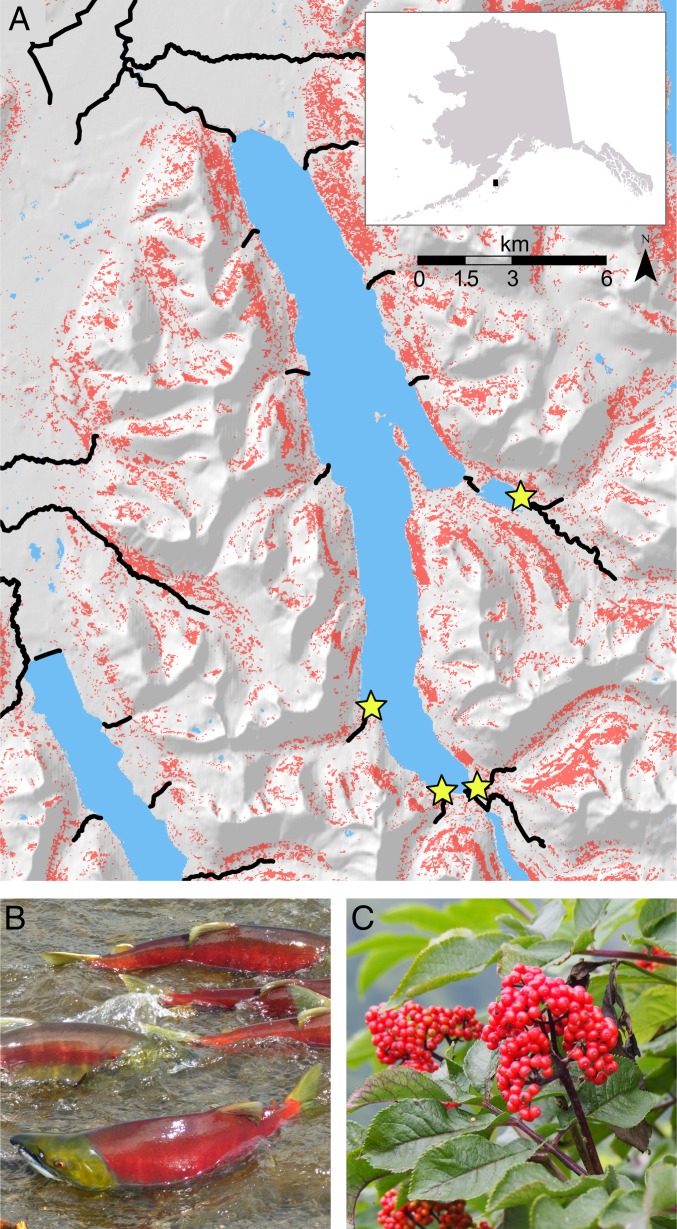
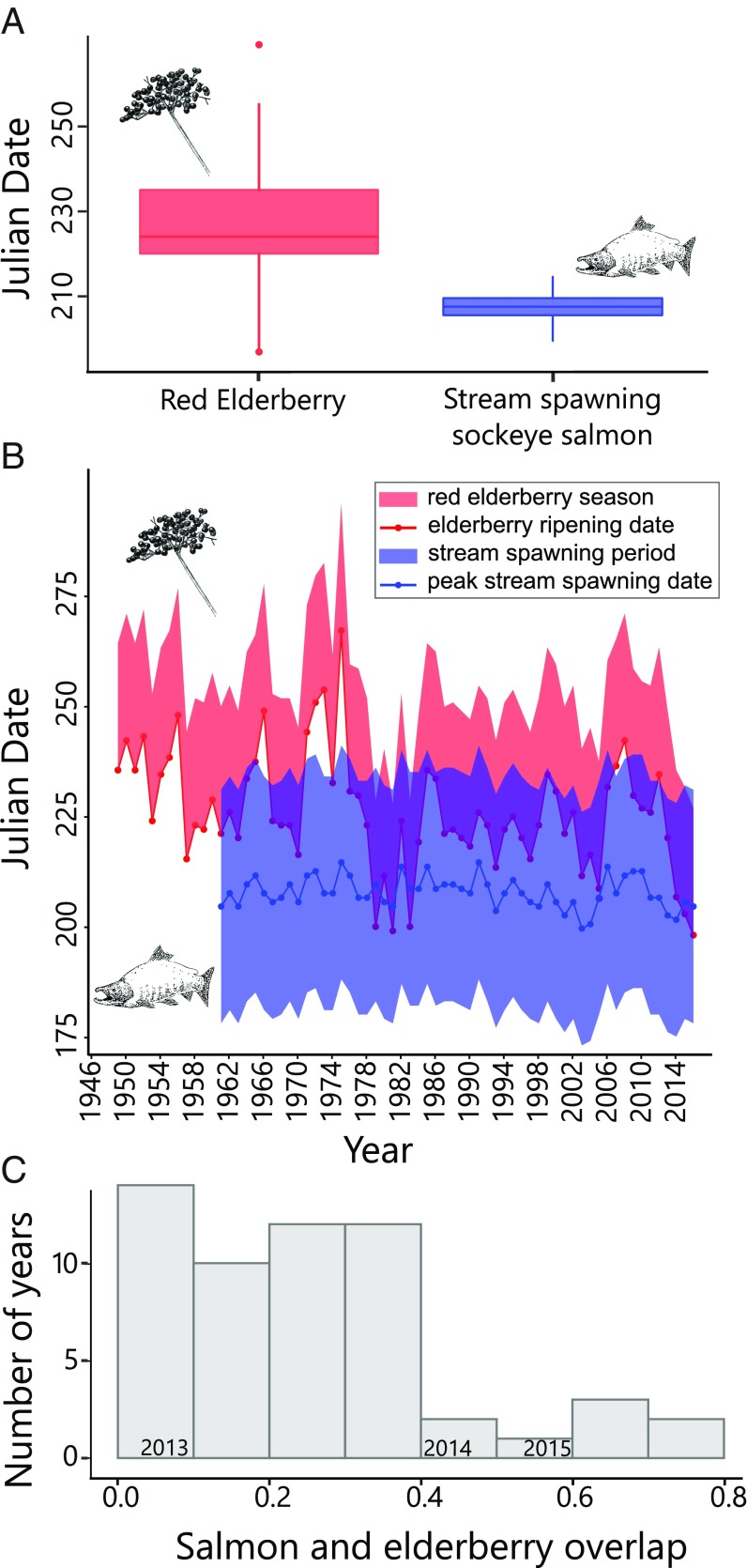
Brown bears (Ursus arctos) are apex predators that prey intensively on spawning salmon (Oncorhynchus spp.) in coastal ecosystems of the North Pacific. This interaction has far-reaching effects, influencing not only the energy budgets and demographic rates of bears (14, 15) but also the evolution of salmon populations (16), the coevolution of plant pollinators (17), and the dispersal of marine-derived nutrients into terrestrial food webs (18, 19). As generalists, bears consume a wide array of temporally complementary resources, including salmon, neonate ungulates, herbaceous vegetation, and fruit. For brown bears on Kodiak Island, Alaska (Fig. 1A), sockeye salmon (Oncorhynchus nerka, Fig. 1B) and red elderberry (Sambucus racemosa, Fig. 1C) are key foods with complementary temporal dynamics. Across all Kodiak’s spawning habitats, sockeye salmon spawn for more than 4 mo (20), but are most vulnerable to predation while spawning in shallow streams (21, 22) from early July through August. In shallow streams, bears can kill prespawn salmon that are up to three times more energy dense than senescent fish (22, 23). Red elderberries typically ripen at the end of the salmon predation window and are available to bears from late August into early September. We estimated the phenology of salmon spawning and elderberry ripening over the course of 55 y, based on observed data and a calibrated growing degree day model (for salmon and elderberries, respectively; Methods). Estimated elderberry fruiting phenology varied substantially among years, ranging from July 14 to September 28 (mean, August 14; SD = 13.9 d), whereas estimated salmon spawning phenology was relatively consistent, ranging from July 19 to August 2 (mean, July 27; SD = 3.5 d; Fig. 2 A and B). The different phenological sensitivities of berries and salmon generated strong interannual variation in their temporal overlap, with a trend of increasing overlap over the time-series (due to increasing spring air temperatures; Fig. S1A; Fig. 2B). We studied bear foraging behavior under different scenarios of phenological overlap that correspond to current and projected average conditions under climate change.
Fig. 1.
Key Kodiak brown bear trophic resources and their distribution. (A) Map of the study system showing areas with 10–40% red elderberry land cover (red), and the streams/rivers where sockeye salmon (Oncorhynchus nerka) spawn (black lines). Yellow stars indicate the four focal tributaries where we quantified salmon abundance and monitored bear activity. (B) Spawning sockeye salmon entering a tributary stream within the study area. (C) Red elderberry shrub with ripe berries.
Fig. 2.
Phenological variation in brown bear trophic resources. (A) Estimated onset of red elderberry ripening (1949–2015, n = 67 y) and estimated dates of peak sockeye salmon spawning in the Karluk basin, Alaska streams (1961–2016, n = 56 y). (B) Time series of estimated availability of ripe red elderberry (red) and stream spawning sockeye salmon (blue). (C) Histogram of annual proportion of salmon run in streams temporally overlapped by red elderberry availability.
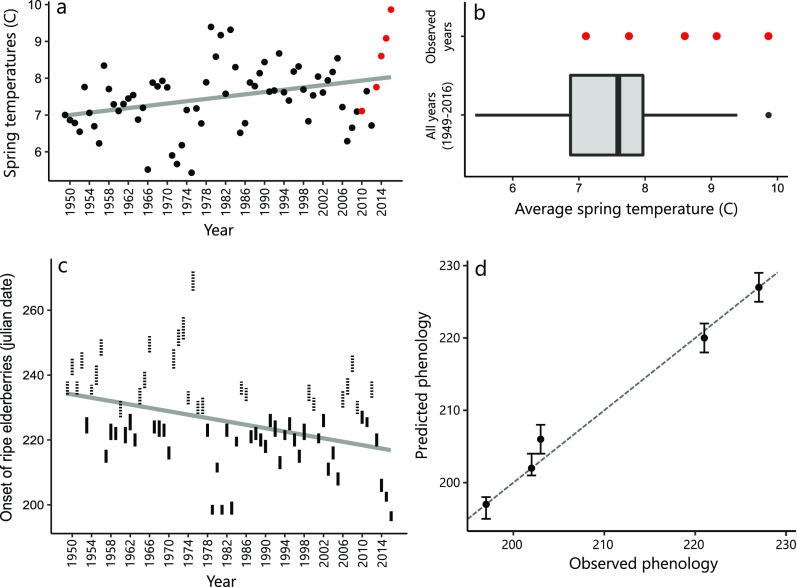
Fig. S1.
Observed air temperatures and red elderberry phenology model performance. (A) Mean spring/summer (March–August) temperatures from 1949 to 2016 at Karluk Lake, Alaska. The gray line indicates the least squares regression line with equation y = 0.016× − 23.3 (P < 0.01). Red points indicate the 5 y where red elderberry phenology was observed. (B) Tukey boxplot of mean spring/summer temperature (March–August), calculated for 1949–2016 (bold vertical line shows median, box represents 25th and 75th quartiles, lines extend to 1.5× the interquartile range, beyond which outliers are shown as points). The red points indicate mean growing season temperature in years where elderberry phenology was observed (spanning 62% of GDD conditions expressed from 1949 to 2016). (C) Hindcast of onset of ripe elderberries from 1949 to 2016. Length of vertical bars shows uncertainty in estimated berry phenology calculated as the 95% confidence intervals of the mean growing degree days required to produce ripe berries. The dashed lines indicate extrapolated predictions and the gray line shows the least squares regression line with equation y = −0.2508× + 723.4 (t = −3.11; P < 0.01). (D) Observed red elderberry phenology vs. predicted phenology using leave one out cross-validation. The gray dashed line indicates the 1:1 line, and the error bars indicate 95% confidence intervals of predicted phenology. The mean absolute error of predicted phenology was 1.4 d. Observed and predicted phenology were highly positively correlated (Pearson’s r = 0.99).
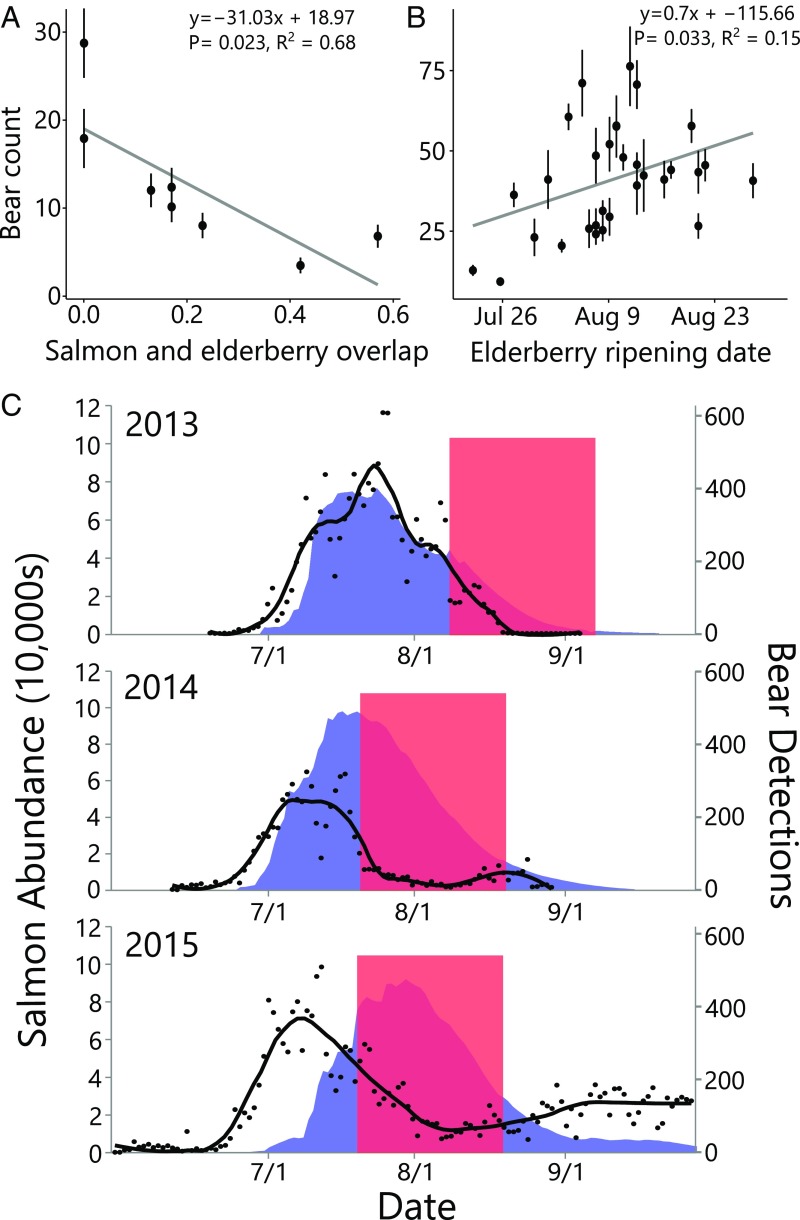
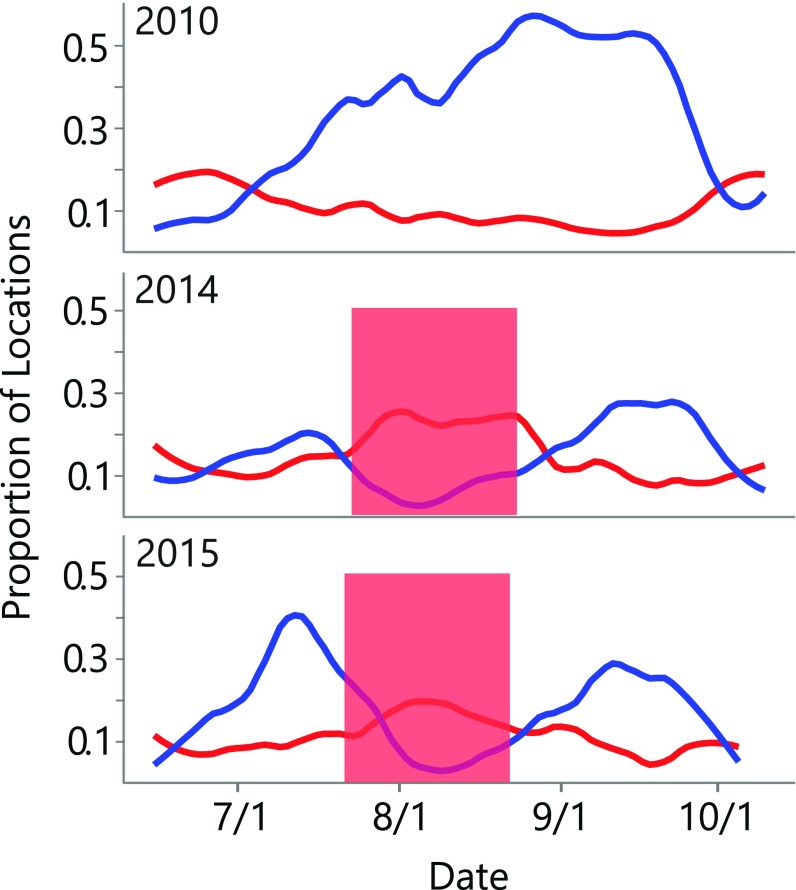
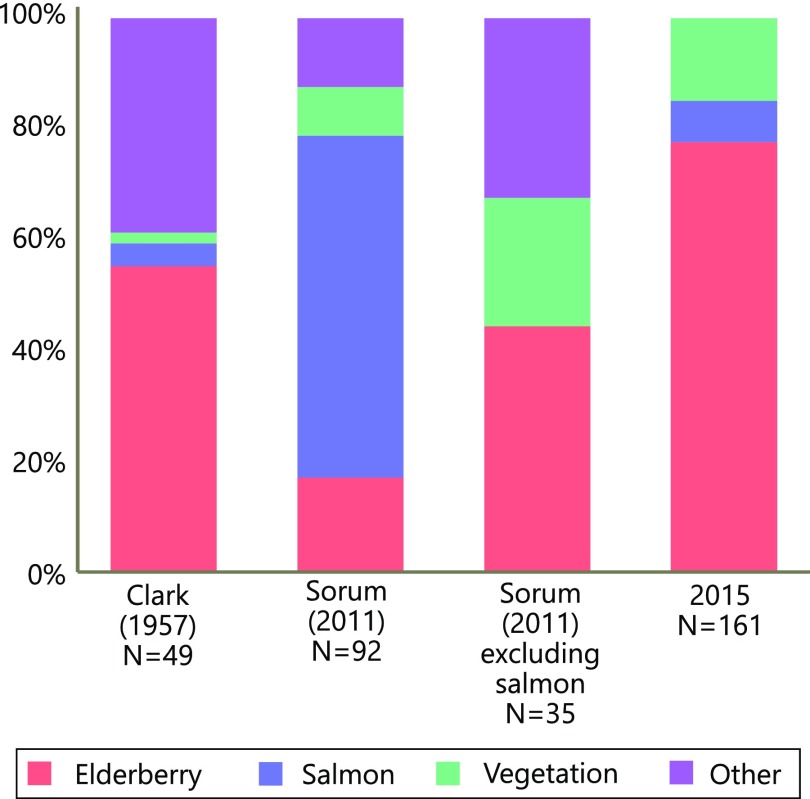
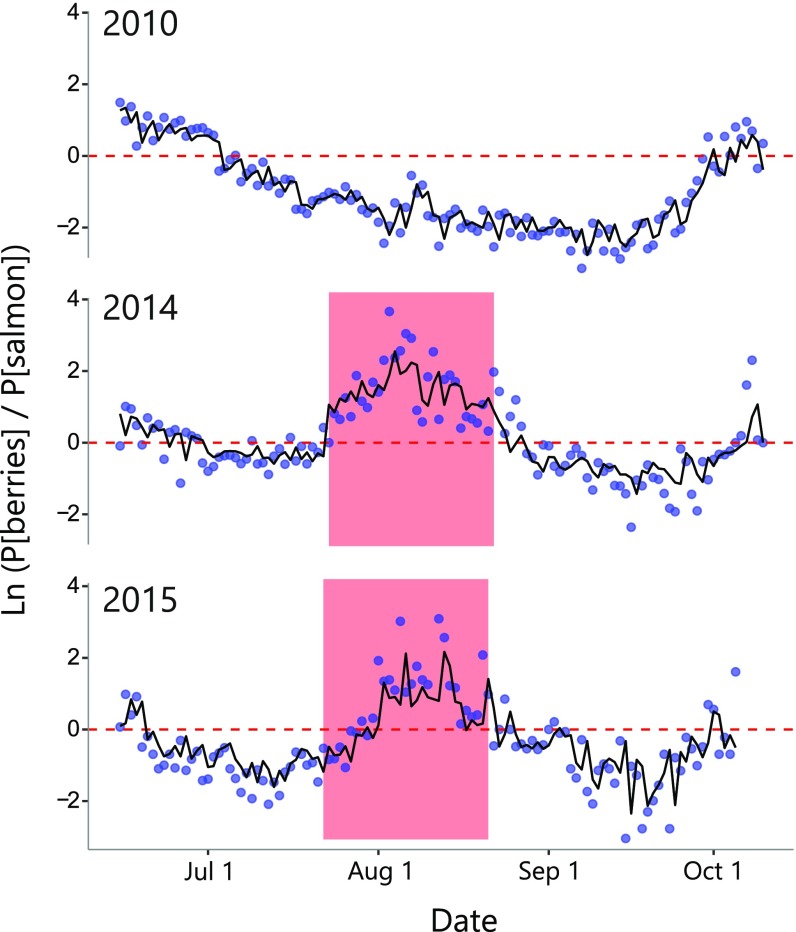
We used multiple independent methods to quantify the foraging behavior of bears during years with varying levels of resource overlap (Fig. 2 B and C). All lines of evidence suggested bears switch from salmon to berries when the two resources co-occur in time. For example, 8 y of aerial surveys in the Karluk River catchment showed that higher phenological synchrony between salmon and berries was associated with lower bear counts at stream spawning sites (i.e., the number of individuals feeding on salmon) (Fig. 3A). Similarly, 31 y of aerial surveys outside the Karluk River catchment showed that earlier elderberry ripening dates (which generate overlap with salmon) were associated with lower bear counts at streams where salmon spawn (Fig. 3B). Within years, bear counts at prime salmon foraging sites declined when elderberries became available during the salmon run (Fig. 3C). In the anomalously warm years of 2014 and 2015, GPS-collared bears moved from salmon foraging habitats to berry foraging habitats during periods of phenological overlap (Fig. 4). Analysis of scat contents in the high-overlap year of 2015 provided direct evidence that bears were indeed consuming red elderberries rather than some other nonsalmon food: 125 of 151 scats encountered near salmon streams contained mostly red elderberries (Fig. S2). In 2010, when we observed no ripe red elderberries, GPS-collared bears did not exhibit a shift between foraging habitats as they continued to feed primarily on salmon (Fig. 4).
Fig. 3.
Effect of resource phenology on bear counts at salmon streams. (A) Mean bear counts across seven salmon spawning streams in the Karluk watershed vs. the temporal overlap between red elderberry and salmon. Data from 113 aerial surveys spanning 2008–2015. Points show annual mean bear count (n = 8), error bars = 1 SEM. (B) Mean bear counts vs. elderberry ripening date (a proxy for resource overlap). Data from 317 aerial surveys (1982–2015, n = 31 due to missing years) of six streams and rivers outside of the Karluk watershed. Error bars = 1 SEM. (C) Daily bear detections (loess smoothed black line and points) and estimated abundance of sockeye salmon in four Karluk Lake, Alaska, tributaries (blue shaded region). The red squares indicate the approximate periods of ripe red elderberry availability. To explain variability in bear numbers, we fit a negative binomial generalized linear model with predictors including year, berry presence, salmon abundance, and the interaction between berry presence and salmon abundance (Fig. S5). The positive relationship between salmon abundance and bear detections was significantly dampened in the presence of berries [salmon, z = 11.18 (P < 0.0001); berries, z = −2.46 (P = 0.014); berries*salmon, z = −2.70 (P = 0.0070); n = 78, 80, 135 for years 2013, 2014, 2015, respectively].
Fig. 4.
Habitat use of GPS collared female bears. The proportion of hourly female brown bear locations recorded in either salmon (blue) or red elderberry (red) habitat patches. The red boxes indicate the periods during which ripe red elderberries were available to bears. No berry phenology box is shown for 2010 because no ripe berries were observed that year. To explain variability in habitat use, we fit a dynamic linear model to these data. When ripe berries were available, bears used berry habitat more than salmon habitat (P < 0.0001; Fig. S5). After September 1, salmon primarily spawn in lake and river habitats (20), where salmon are much less vulnerable to bears (21, 22).
Fig. S2.
Brown bear fecal composition data from Karluk basin, Alaska. Each survey was conducted using a different protocol, but all show the proportion of scats consisting entirely, or mostly, of a single food. The 1957 survey (52) was a visual estimate of 140 scats, 49 of which consisted entirely of one food. The 2011 scats (38) were collected from the bed sites of eight collared female bears in 2011. The contents were examined microscopically, and values were corrected for differential disappearance (60). The 2015 data are from a survey of Karluk area streams during a period with both salmon and elderberries (August 21, 2016; Methods). Although some scats had mixed food residues, each was classified by its predominant food.
Fig. S5.
Dynamic linear model of bear habitat use in relation to berry availability. The blue points show daily natural log transformed ratios of total brown bear locations recorded in berry habitat patches to total locations recorded in salmon habitat patches (see Methods for habitat classification details). The red boxes indicate the periods during which ripe red elderberries were available to bears. No berry phenology box is shown for 2010 because no ripe berries were observed that year. To explain variability in habitat use, we fit a dynamic linear model to these data (black line). A dynamic linear model can be thought of as an extension of a linear regression model, with either the intercept or coefficients varying through time as a random walk. One of the advantages in using a dynamic linear model is that the flexibility of the models may capture underlying processes better than models with constant parameters (regression, generalized additive models) and reduce temporal autocorrelation (61, 62). We implemented dynamic linear models with time-varying intercepts and constant fixed effects of year, Julian date (and Julian date squared), and an indicator using the presence/absence of a berry window. In both 2014 and 2015, when ripe berries were available, bears use of berry habitat exceeded use of salmon habitat (P < 0.0001).
By triggering food switching, shifts in elderberry fruiting phenology disrupted a normally strong predator–prey interaction between brown bears and salmon. The inferred food switching was surprising because elderberries are only ∼50% as energy dense as spawning salmon (11.4 kJ/g dry mass vs. 21.7 kJ/g dry mass) (23), and bears preying on salmon can selectively consume portions of the carcass that are even more energy dense (24). If bears are constrained by assimilation and limited by prey energy density, as suggested by their selective consumption of salmon (24), why would they switch to a less energy dense food? Diets that maximize energy intake may fail to maximize growth if the macronutrient composition of food is suboptimal and reduces food conversion efficiency (25). Bears in feeding trials presented with a variety of foods selected mixed diets in which protein provided 11–21% of metabolizable energy (ME). Compared with experimentally manipulated diets (higher and lower in protein), the protein levels selected by bears were shown to maximize growth rates under ad libitum feeding (25, 26). Spawning salmon are suboptimally high in protein (83.7% of ME from protein), but elderberry fruit have protein levels similar to those selected by captive bears (12.8% of ME from protein). We believe macronutrient optimization is the most parsimonious explanation for the food switching we observed, but further research is needed to directly test this hypothesis. Although bears in other ecosystems often mix protein-rich salmon with low-protein, carbohydrate-rich berries to optimize macronutrient intake (26), elderberries have more protein than most berries available to brown bears (27), and they occur in dense clusters that should reduce the constraint of handling time (27). These differences likely allow bears eating red elderberries to rapidly gain weight without mixing foods. Although there is increasing recognition that energetic costs can drive large-scale patterns of space use in animals (28), costs associated with assimilation (i.e., due to the macronutrient composition of prey) are rarely considered in such analyses and are often assumed to be constant across prey taxa (28).
Our phenology analysis suggests the temporal overlap of elderberry and salmon is increasing. Salmon spawning phenology showed no significant trend over the time-series (t = −1.60; P = 0.12), but red elderberry phenology became 2.5 d earlier per decade (t = −3.11; P = 0.003). If these trends continue, by 2070, the average onset of berry availability would occur during the average peak of salmon availability, such that the anomalous phenological overlap we observed in 2015 would be typical. How would this affect bears? When two resources become synchronized in time, their combined duration of availability decreases, which can decrease consumer fitness (29). However, synchronizing two resources also creates redundancy in the resources available at a specific time of year, providing insurance in case one resource fails (30). In our study system, the outcome of these two effects will likely depend on how critical early-season foraging opportunities are for bears and whether there are alternative late-season resources to replace elderberries. Regardless, coastal brown bear populations will continue to depend on healthy salmon runs to meet much of their energy needs.
There is a strong need for research on phenological change to move beyond two species interactions (i.e., between a predator and a single prey taxa) and begin to address the complex multispecies interactions that are pervasive in real food webs (31, 32). It is widely recognized that indirect effects can strongly mediate ecological dynamics such that species interactions cannot be understood without accounting for the effects of shared competitors, prey, and predators (33–35). In this study, salmon and elderberry interacted indirectly via a shared relationship with brown bears. Phenological shifts in elderberry likely triggered prey switching away from salmon, attenuating a trophic linkage with disproportionate ecological significance (15–19). We suggest that similarly strong and underappreciated responses are occurring elsewhere as climate change reschedules species interactions across ecosystems.
Methods
Study Area.
We conducted this work in the Karluk watershed (638,000 km2) of Kodiak Island, Alaska (Fig. 1A), which has a dense population of brown bears (∼250 individuals/1,000 km2) (36). Although four species of salmon spawn in Karluk waters, sockeye salmon (Fig. 1C) are the most abundant; the average sockeye salmon run size during the last 56 y (after commercial harvest) was 549,507 ± 34,029 (SEM). Pink salmon are also abundant (average run size = 491,375 ± 96,849 SEM), but spawn primarily in the Karluk River, where they are less vulnerable to bears. Sockeye salmon in our focal system spawn in rivers, along lakeshores, and in lake tributaries. Sockeye salmon are most vulnerable to bear predation in tributary streams (21). In addition, sockeye salmon in smaller tributaries experience greater overall predation rates and allow selective predation of prespawn fish that are higher in energy content (22, 23). The tributary streams in Karluk are 3–16 m wide, representing the size of stream in which bears can prey on prespawn fish and kill a large fraction of the run [∼25–75% (21)].
In addition to salmon, Kodiak brown bears routinely consume several species of berries, including red elderberry (Fig. 1B), salmonberry (Rubus spectabilis Pursh), crowberry (Empetrum nigrum), and blueberry (Vaccinium spp.) (37). Red elderberry is found primarily at midelevation slopes, with greater concentrations on northern aspects (Fig. 1A). Prior studies found that red elderberry was the dominant fruit item found in bear scats on Kodiak during late summer and fall (38).
Elderberry Phenology.
To estimate elderberry phenology, we reviewed images from a time-lapse camera placed near a salmon spawning stream in 2010 and in 2013–2016 (Fig. S3). There were numerous red elderberry shrubs within the image viewshed, which had an area of ∼10,000 m2 (Fig. S3). For each year, we noted the first date when ripe red elderberries were visible on at least half of the shrubs. We defined this as the “ripening date” for each year. Although we have observed spatial variation in elderberry phenology across elevations and aspects, our time-lapse method still provides a consistent index of ripening date to calibrate an elderberry development model. Due to bird consumption, decomposition, and other factors, red elderberries decline in abundance after initial ripening.
Fig. S3.
Example of image used to estimate bear occupancy of salmon spawning habitat and red elderberry phenology. Picture from a time-lapse camera at Canyon Creek. A Kodiak brown bear is in the stream, and the fruits of red elderberry are visible in the foreground at right and to the right of the stream.
To place observed red elderberry phenology into historical context, we compiled a 1949–2016 record of air temperature. We acquired daily minimum and maximum temperatures for our field site (Karluk Lake) and the Kodiak Benny Benson State Airport in Kodiak, Alaska, from the National Climatic Data Center. Data for Karluk Lake were limited to spring–fall of 1965–1969, while daily records starting in 1949 were available for the airport. There were strong positive correlations between Karluk Lake and airport minimum (Pearson’s r = 0.82; P < 0.0001) and maximum (Pearson’s r = 0.81, P < 0.0001) air temperatures. To benefit from the longer airport time series, we fit least squares linear regression models of Karluk maximum and minimum temperatures as functions of airport temperatures and then used these models to predict 1949–2016 Karluk Lake maximum/minimum temperatures (Fig. S1A).
To understand how red elderberry phenology varies with climate, we modeled ripening date as a function of growing degree days (39) (GDDs), a method frequently used in research and application to predict the phenology of temperate vegetation and insects (40–47). GDD models predict phenology from daily temperature data on the basis of two parameters: B, the minimum (base) temperature required for development, and Y, the cumulative growing degrees required to reach a specific stage of development (e.g., fruiting) (39). We found no published values for B or Y in red elderberries, so we estimated them using the 5 y of observed red elderberry fruiting phenology (from 2010 and 2013–2016) and standard methods (39, 48).
To select B, we calculated the GDDs for each of the five observed ripening dates with a B ranging from 0 to 10 °C. We calculated growing degrees (GD) for each day using the equation: {GD = [(max temp − min temp)/2] − B; if GD < 0, GD = 0} (39). GDDs is the cumulative sum of GDs starting from January 1 (39). We selected the value of B that minimized the SD of GDDs required to produce ripe berries in the 5 observed years [2 °C; minimum SD = 16.9 GDDs (42, 48)]. Y was the mean of the five GDD values calculated using B (941 GDDs). Finally, using our estimates of B and Y, we hindcast the red elderberry ripening date for each year in the 1949–2016 record of air temperature. We calculated 95% confidence intervals for each ripening date, using the 95% confidence intervals on the mean of Y (Fig. S1C).
We validated our model using leave-one-out cross-validation, in which we removed one of the five observed elderberry ripening dates, reestimated the B and Y parameters, and then predicted the ripening date for the excluded year. Leave-one-out cross-validation predictions were closely correlated with observed dates of elderberry ripening (Pearson’s r = 0.99; Fig. S1D) and exhibited a mean absolute error of 1.4 d.
Bear Habitat Use.
To assess interannual patterns of bear space use, we analyzed data from three complementary sources: spatially extensive aerial surveys that provided annual snapshots of the entire bear population’s use of salmon predation sites, time-lapse cameras that provided a temporally continuous index of bear activity on salmon streams throughout the salmon spawning season, and GPS telemetry that provided location data for a subset of female individuals with fine spatial and temporal resolution.
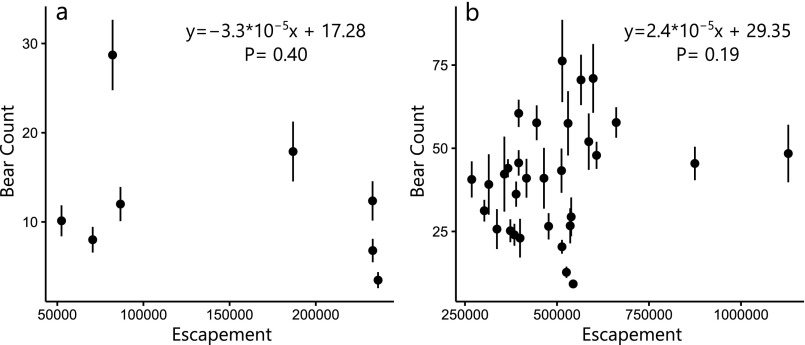
To quantify interannual variation in the degree to which bears were foraging on salmon, we conducted aerial counts (from a small airplane) of the number of bears (excluding cubs) seen along 13 streams and rivers, and used the mean annual bear count as an index of bear foraging on salmon. Survey frequency varied (range = 4–22/y; median = 9/y), but generally occurred in the early morning or late evening two to three times per week from early July through late August. We compared annual mean bear counts (±1 SEM) to indices of phenological overlap between elderberry and salmon. We compared bear counts from the Karluk watershed (133 observations across seven streams from 2008 to 2015) to the estimated temporal overlap between salmon and elderberries (Methods). We used least squares linear regression to test the hypothesis that increasing salmon and elderberry overlap was associated with decreased bear activity at salmon predation sites. To test this relationship across a larger temporal and spatial extent (1982–2015, ∼900 km2), we also analyzed bear counts from similar aerial surveys conducted beyond the Karluk watershed (317 observations across six streams). Because we lacked basin-specific information on salmon spawning phenology, we used estimated elderberry ripening date as a proxy for resource overlap and tested the effect of elderberry phenology on bear counts at salmon predation sites, using least squares linear regression. Elderberry ripening date should act as a reliable proxy for resource overlap because salmon phenology is relatively consistent through time, and thus, resource overlap is primarily driven by variation in the date of ripening (Fig. 2B). We also used least squares regression to test for effects of interannual variation in sockeye salmon abundance on bear counts and found no statistically significant relationships (Fig. S4).
Fig. S4.
Aerial bear counts vs. sockeye salmon escapement (abundance remaining after fishery harvest). (A) Mean bear counts across seven salmon spawning streams in the Karluk watershed vs. abundance of early sockeye salmon run (before July 15), which spawns primarily in streams. Data from 113 aerial surveys spanning 2008–2015. Points show annual mean bear count (n = 8), error bars = 1 SEM, and gray line shows least squares regression. (B) Mean bear counts vs. sockeye salmon escapement into Ayakulik and Dog Salmon watersheds. Data from 317 aerial surveys (1982–2015, n = 31 due to missing years) of six streams and rivers outside of the Karluk watershed. Error bars = 1 SEM, and gray line shows least squares regression.
To determine whether salmon predation varied intra-annually in relation to elderberry availability, we monitored bear occurrence at four tributary streams where salmon are both abundant and vulnerable, using time-lapse photography (streams indicated by yellow stars in Fig. 1A). Although bears occasionally use streams for purposes other than foraging on salmon (e.g., travel corridor or water source), a prior study found that bears were detected by time-lapse cameras 61 times more frequently when salmon were present compared with when salmon were absent (15). Each year, three cameras were placed along each of the four streams (a total of 12 cameras). Cameras were placed in the same location with the same viewshed to ensure count effort was consistent across years. Cameras were deployed in late May or early June before salmon runs began, and were removed in late August in 2013/2014 and late September in 2015, after spawning concluded. Cameras were programmed to take a photo every 5 min during daylight hours. We counted the number of bears (excluding cubs) in each photo and then summed counts by day. These counts do not census the bears that used these streams but, rather, index overall bear use and have been corroborated by more direct monitoring techniques, such as GPS telemetry (15).
To test whether bears switched to using elderberry habitat when ripe elderberries were available, we analyzed the space use of individually collared female brown bears. More than 101,000 GPS locations were recorded from 36 individuals over the course of 47 bear-years (some bears carried collars for more than 1 y). In 2010, 2014, and 2015, 20, 12, and 15 bears carried collars, respectively. We captured bears in the southwestern region of Kodiak Island, Alaska, by firing immobilization darts from a helicopter (49). We fitted each bear with a GPS radio collar programmed to record a location every hour from mid-May through mid-November. We screened GPS locations for accuracy, removing locations with a positional dilution of precision greater than 10 (n = 6,297) (50). All capture procedures were approved by the U.S. Fish and Wildlife Service Institutional Animal Care and Use Committee (IACUC permit #2012008, 2015-001).
To quantify temporal patterns of bear habitat use, we divided the location data by week. For each week, we calculated the proportion of all observations located in salmon habitat and the proportion located in red elderberry habitat (these were independent; i.e., a bear could be in both habitats at once). We defined salmon habitat as areas within 50 m of a stream, river, or lake beach known to be used by spawning salmon (20) (Fig. 1A shows stream and river sites). To evaluate use of elderberry, we used a 30 × 30 m raster of percentage red elderberry canopy cover taken from the Kodiak Land Cover Classification (51) (Fig. 2). We defined elderberry habitat as any area containing at least 10% elderberry cover (this accounted for 10.7% of the landscape). We assumed bears located in elderberry-classified habitats were indeed foraging on elderberry because past diet studies found that elderberry was the dominant nonsalmon diet item (38, 52) (Fig. S2). We assumed that bears detected in salmon spawning habitats (whether by GPS collar, aerial survey, or camera) were foraging on salmon because bears rarely use these habitats except for when salmon are present (21, 53). In our focal system, bear detections at salmon spawning sites increase 61-fold when salmon are spawning (20).
Bear Fecal Analysis.
To directly test the assumption that bears were eating red elderberry when they overlapped temporally with salmon, we conducted a fecal survey on August 21, 2015, during which both salmon and elderberries were available. We conducted the survey by walking along the four study streams monitored by time-lapse cameras. We classified each encountered scat (n = 161) by its dominant prey item: salmon, elderberries, or other. Elderberry scats are easily recognized by the presence of elderberry stems and seeds, whereas salmon scats have a distinct color and odor, and often contain salmon bones. This survey occurred adjacent to habitat used by bears eating salmon, so although it is spatially biased, it is likely to overestimate use of salmon, rather than red elderberry.
Estimating Salmon and Elderberry Macronutrient Composition.
We compared the protein content of salmon and elderberries with those of optimal diets determined by ad libitum feeding experiments on captive brown bears (25). We quantified protein content as the percentage of metabolizable energy in each food source (26, 54, 55), using published macronutrient values for salmon (23) and carbon-nitrogen analysis (N X 6.25) for elderberries (56). We concluded that bears do not digest elderberry seeds because the crude protein content in seeds extracted from feces was not lower than that of seeds in fresh fruit (11.5% digested vs. 10.2% fresh; n = 3) (56). Due to the difficulty of retaining the nonseed portion of the berry after isolating it from the seed, we estimated the crude protein content of the nonseed portion of elderberries by difference (i.e., nonseed protein = total protein − seed protein). The average crude protein content of whole dried elderberry was 12.7%. The undigested seeds accounted for 36 ± 6% of the dried berry’s mass and accounted for 29% of its total crude protein. Thus, the remaining 64% of the berry was composed of 14% crude protein. Carbohydrate content in the nonseed portion of the berry was estimated as the difference between the digestible dry matter and the protein in the nonseed dry matter, assuming no lipids (25). The metabolizable energy provided by carbohydrates, protein, and fat was estimated for salmon and elderberries using Atwater specific factors (25), and then the percentage of metabolizable energy provided by protein was calculated by dividing protein metabolizable energy by total metabolizable energy.
Salmon Abundance and Phenology.
We estimated salmon abundance in four tributaries that are known to be heavily used by bears and are disproportionately important foraging habitats due to their large salmon runs and small size, which renders salmon highly vulnerable to bears (21, 57, 58) (Fig. 1A). We used a time-lapse photography double-sampling method paired with stream specific estimates of mortality rates (59). These camera systems have been shown to estimate salmon abundance accurately (<10% error), do not obstruct salmon passage, and minimize bear disruption due to human presence.
To estimate historical patterns of spawning phenology, we first calculated the average lag time between the median date of sockeye salmon passage at the Karluk river weir [date at which half of the early run (before July 15th) sockeye salmon had moved through the weir] and the date of peak sockeye salmon abundance in the four tributary streams we monitored in 2013–2015. We estimated past peak spawning dates by adding the resulting lag value (46 d) to the median weir passage dates from 1961 to 2016.
Acknowledgments
We thank Lisa Eby, Chris Servheen, Bonnie Ellis, and Taal Levi for helpful discussion and comments. We thank the staff at the Kodiak National Wildlife Refuge and Flathead Lake Biological Station for logistical and administrative support. Three anonymous reviewers provided helpful comments that improved the manuscript. This work was funded by the US Fish and Wildlife Service Refuge Program and US Fish and Wildlife Service Inventory and Monitoring Program. W.W.D. and J.A.S. were supported by the Jessie M. Bierman Professorship during data collection and analysis. W.W.D. was supported by the Oregon State University Department of Fisheries and Wildlife during writing and manuscript preparation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10309.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705248114/-/DCSupplemental.
References
- 1.Armstrong JB, Takimoto G, Schindler DE, Hayes MM, Kauffman MJ. Resource waves: Phenological diversity enhances foraging opportunities for mobile consumers. Ecology. 2016;97:1099–1112. doi: 10.1890/15-0554.1. [DOI] [PubMed] [Google Scholar]
- 2.Thackeray SJ, et al. Phenological sensitivity to climate across taxa and trophic levels. Nature. 2016;535:241–245. doi: 10.1038/nature18608. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Thackeray SJ, et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol. 2010;16:3304–3313. [Google Scholar]
- 5.Winder M, Schindler D. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85:2100–2106. [Google Scholar]
- 6.Both C, Bouwhuis S, Lessells CM, Visser ME. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. [DOI] [PubMed] [Google Scholar]
- 7.Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proc Biol Sci. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philos Trans R Soc Lond B Biol Sci. 2010;365:3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang LH, Rudolf VHW. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett. 2010;13:1–10. doi: 10.1111/j.1461-0248.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly A, Caffarra A, O’Neill BF. A review of climate-driven mismatches between interdependent phenophases in terrestrial and aquatic ecosystems. Int J Biometeorol. 2011;55:805–817. doi: 10.1007/s00484-011-0426-5. [DOI] [PubMed] [Google Scholar]
- 11.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant-pollinator interactions. Ecol Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch WW. Switching in general predators : Experiments on predator specificity and stability of prey populations. Ecol Monogr. 1969;39:335–354. [Google Scholar]
- 13.Estes JA. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 14.Hilderbrand GV, Jenkins SG, Schwartz CC, Hanley TA, Robbins CT. Effect of seasonal differences in dietary meat intake on changes in body mass and composition in wild and captive brown bears. Can J Zool. 1999;1630:1623–1630. [Google Scholar]
- 15.Hilderbrand GV, et al. The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can J Zool. 1999;77:132–138. [Google Scholar]
- 16.Carlson SM, Hilborn R, Hendry AP, Quinn TP. Predation by bears drives senescence in natural populations of salmon. PLoS One. 2007;2:e1286. doi: 10.1371/journal.pone.0001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisi PJ, Schindler DE. Spatial variation in timing of marine subsidies influences riparian phenology through a plant-pollinator mutualism. Ecosphere. 2011;2:1–14. [Google Scholar]
- 18.Meehan E, Seminet-Reneau E, Quinn T. Bear predation on Pacific salmon facilitates colonization of carcasses by fly maggots. Am Midl Nat. 2005;153:142–151. [Google Scholar]
- 19.Helfield JM, Naiman RJ. Keystone interactions: Salmon and bear in riparian forests of Alaska. Ecosystems (N Y) 2006;9:167–180. [Google Scholar]
- 20.Deacy W, Leacock W, Armstrong JB, Stanford JA. Kodiak brown bears surf the salmon red wave: Direct evidence from GPS collared individuals. Ecology. 2016;97:1091–1098. doi: 10.1890/15-1060.1. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TP, Cunningham CJ, Wirsing AJ. Diverse foraging opportunities drive the functional response of local and landscape-scale bear predation on Pacific salmon. Oecologia. 2016;183:415–429. doi: 10.1007/s00442-016-3782-3. [DOI] [PubMed] [Google Scholar]
- 22.Gende S, Quinn T, Hilborn R. Brown bears selectively kill salmon with higher energy content but only in habitats that facilitate choice. Oikos. 2004;104:518–528. [Google Scholar]
- 23.Hendry AP, Berg OK. Secondary sexual characters, energy use, senescence, and the cost of reproduction in sockeye salmon. Can J Zool. 1999;77:1663–1675. [Google Scholar]
- 24.Gende SM, Quinn TP, Willson MF. Consumption choice by bears feeding on salmon. Oecologia. 2001;127:372–382. doi: 10.1007/s004420000590. [DOI] [PubMed] [Google Scholar]
- 25.Robbins CT, et al. Optimizing protein intake as a foraging strategy to maximize mass gain in an omnivore. Oikos. 2007;116:1675–1682. [Google Scholar]
- 26.Erlenbach JA, Rode KD, Raubenheimer D, Robbins CT. Macronutrient optimization and energy maximization determine diets of brown bears. J Mammal. 2014;95:160–168. [Google Scholar]
- 27.Welch C, Keay J, Kendall K, Robbins C. Constraints on frugivory by bears. Ecology. 1997;78:1105–1119. [Google Scholar]
- 28.Brandt SB, Mason DM, Patrick EV. Spatially-explicit models of fish growth rate. Fisheries. 1992;17:23–35. [Google Scholar]
- 29.Pettorelli N, Mysterud A, Yoccoz NG, Langvatn R, Stenseth NC. Importance of climatological downscaling and plant phenology for red deer in heterogeneous landscapes. Proc Biol Sci. 2005;272:2357–2364. doi: 10.1098/rspb.2005.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naeem S, Li S. Biodiversity enhances ecosystem reliability. Nature. 1997;390:507–509. [Google Scholar]
- 31.Visser ME. Phenology: Interactions of climate change and species. Nature. 2016;535:236–237. doi: 10.1038/nature18905. [DOI] [PubMed] [Google Scholar]
- 32.Thackeray SJ. Casting your network wide: A plea to scale-up phenological research. Biol Lett. 2016;12:20160181. doi: 10.1098/rsbl.2016.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wootton JT. The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 34.Menge BA. Indirect effects in marine rocky intertidal interaction webs: Patterns and importance. Ecol Monogr. 1995;65:21–74. [Google Scholar]
- 35.Roemer GW, Coonan TJ, Garcelon DK, Bascompte J, Laughrin L. Feral pigs facilitate hyperpredation by golden eagles and indirectly cause the decline of the island fox. Anim Conserv. 2001;4:307–318. [Google Scholar]
- 36.Barnes VG, Smith RB, Van Daele LJ. 1988. Density estimates and estimated population of brown bears on Kodiak and adjacent islands, 1987 (Alaska Department of Fish and Game, Juneau, Alaska)
- 37.Van Daele M, et al. Salmon consumption by Kodiak brown bears (Ursus arctos middendorffi) with ecosystem management implications. Can J Zool. 2013;91:164–174. [Google Scholar]
- 38.Sorum M. 2013. Behavior-specific resource selection by Kodiak brown bears. PhD dissertation (University of Idaho, Moscow, ID)
- 39.McMaster GS, Wilhelm WW. Growing degree-days: One equation, two interpretations. Agric Meteorol. 1997;87:291–300. [Google Scholar]
- 40.Parry ML, Carter TR. The effect of climatic variations on agricultural risk. Clim Change. 1985;7:95–110. [Google Scholar]
- 41.Wielgolaski F-E. Starting dates and basic temperatures in phenological observations of plants. Int J Biometeorol. 1999;42:158–168. [Google Scholar]
- 42.Bonhomme R. Bases and limits to using “degree.day” units. Eur J Agron. 2000;13:1–10. [Google Scholar]
- 43.Zavalloni C, Andresen JA, Flore JA. Phenological models of flower bud stages and fruit growth of ‘Montmorency’ sour cherry based on growing degree-day accumulation. J Am Soc Hortic Sci. 2006;131:601–607. [Google Scholar]
- 44.Nufio CR, McGuire CR, Bowers MD, Guralnick RP. Grasshopper community response to climatic change: Variation along an elevational gradient. PLoS One. 2010;5:e12977. doi: 10.1371/journal.pone.0012977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgson JA, et al. Predicting insect phenology across space and time. Glob Change Biol. 2011;17:1289–1300. [Google Scholar]
- 46.Diamond SE, et al. Unexpected phenological responses of butterflies to the interaction of urbanization and geographic temperature. Ecology. 2014;95:2613–2621. [Google Scholar]
- 47.Cayton HL, Haddad NM, Gross K, Diamond SE, Ries L. Do growing degree days predict phenology across butterfly species? Ecology. 2015;96:1473–1479. [Google Scholar]
- 48.Yang S, Logan J, Coffey DL. Mathematical formulae for calculating the base temperature for growing degree days. Agric Meteorol. 1995;74:61–74. [Google Scholar]
- 49.Taylor W, Reynolds H, Ballard W. Immobilization of grizzly bears with tiletamine hydrochloride and zolazepam hydrochloride. J Wildl Manage. 1989;53:978–981. [Google Scholar]
- 50.Lewis JS, Rachlow JL, Garton EO, Vierling LA. Effects of habitat on GPS collar performance: Using data screening to reduce location error. J Appl Ecol. 2007;44:663–671. [Google Scholar]
- 51.Fleming MD, Spencer P. 2007 Kodiak Archipelago land cover classification users guide. Available at akevt.gina.alaska.edu/data/Kodiak_UsersGuide_v1.1.pdf. Accessed December 28, 2015.
- 52.Clark WK. Seasonal food habits of the Kodiak bear. Trans North Am Wildl Nat Resour Conf. 1957;22:145–151. [Google Scholar]
- 53.Gill I, Helfield J. Alternative foraging strategies among bears fishing for salmon: A test of the dominance hypothesis. Can J Zool. 2012;775:766–775. [Google Scholar]
- 54.Raubenheimer D. Towards a quantitative nutritional ecology: The right-angled mixture triangle. Ecol Monogr. 2011;81:407–427. [Google Scholar]
- 55.Coogan SCP, Raubenheimer D. Might macronutrient requirements influence grizzly bear-human conflict? Insights from nutritional geometry. Ecosphere. 2016;7:1–15. [Google Scholar]
- 56.Jones DB. 1941. Factors for converting percentages of nitrogen in foods and feeds into percentages of proteins. (US Department of Agriculture, Washington, DC) Circular no. 183, pp 1–22.
- 57.Gende S, Quinn T. The relative importance of prey density and social dominance in determining energy intake by bears feeding on Pacific salmon. Can J Zool. 2004;85:75–85. [Google Scholar]
- 58.Quinn TP, Wetzel L, Bishop S, Overberg K, Rogers DE. Influence of breeding habitat on bear predation and age at maturity and sexual dimorphism of sockeye salmon populations. Can J Zool. 2001;79:1782–1793. [Google Scholar]
- 59.Deacy WW, Leacock WB, Eby LA, Stanford JA. A time-lapse photography method for monitoring salmon (Oncorhynchus spp.) passage and abundance in streams. PeerJ. 2016;4:e2120. doi: 10.7717/peerj.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hewitt DG, Robbins CT. Estimating grizzly bear food habits from fecal analysis. Wildl Soc Bull. 1996;24:547–550. [Google Scholar]
- 61.Holmes EE, Ward EJ, Wills K. MARSS: Multivariate autoregressive state-space models for analyzing time-series data. R J. 2012;4:11–19. [Google Scholar]
- 62.Zeileis A. 2016 dynlm: Dynamic linear regression. R package version 0.3-5. Available at cran.r-project.org/package=dynlm. Accessed March 15, 2017.