Significance
Past and current treatments for type 1 diabetes (T1D) have all suffered from adverse effects due to severe immune suppression or a lack of efficacy. A better understanding of the mechanisms and limitations of these therapies may help to maximize patient responses to treatment or elicit new ideas for a cure. Here, we discover a role for intestinal type 1 regulatory T (Tr1) cells in preventing autoimmune diabetes and provide mechanistic insight to explain the better efficacy of combination therapy in disease treatment. Our results also highlight the influence of dysbiotic gut microbiota on promotion of intestinal Tr1 cells and suggest that strategies targeting mucosal tissue to induce Tr1 in vivo might be used as a therapeutic approach for T1D.
Keywords: gut microbiota, IL-10-producing Tr1 cells, cell migration, diabetes suppression
Abstract
Growing insight into the pathogenesis of autoimmune diseases and numerous studies in preclinical models highlights the potential of regulatory T cells to restore tolerance. By using non-obese diabetic (NOD) BDC2.5 TCR-transgenic (Tg), and IL-10 and Foxp3 double-reporter mice, we demonstrate that alteration of gut microbiota during cohousing experiments or treatment with anti-CD3 mAb significantly increase intestinal IL-10–producing type 1 regulatory T (Tr1) cells and decrease diabetes incidence. These intestinal antigen-specific Tr1 cells have the ability to migrate to the periphery via a variety of chemokine receptors such as CCR4, CCR5, and CCR7 and to suppress proliferation of Th1 cells in the pancreas. The ability of Tr1 cells to cure diabetes in NOD mice required IL-10 signaling, as Tr1 cells could not suppress CD4+ T cells with a dominant-negative IL-10R. Taken together, our data show a key role of intestinal Tr1 cells in the control of effector T cells and development of diabetes. Therefore, modulating gut-associated lymphoid tissue to boost Tr1 cells may be important in type 1 diabetes management.
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by the destruction of insulin-producing β-cells in the islets of Langerhans in the pancreas (1). Past and current attempts to address this immune-mediated disease include systemic immunosuppressive drugs, monoclonal antibodies for targeting T cells or antigen presenting cells, autoantigen immunization, and regulatory T cell (Treg)-based immunotherapy. However, most of these trials have suffered from adverse effects due to severe immune suppression or a lack of efficacy (2–6).
Although anti-CD3 monoclonal antibody (mAb) treatment showed only modest success in previous clinical studies, it remains a potentially powerful approach in a subgroup of T1D patients through its function of preserving β-cells and decreasing the requirement for insulin (7–9). As such, further investigations of anti-CD3 mAb in newly diagnosed patients and the “at-risk” population are currently underway (AbATE extension study and teplizumab prevention trial at TrialNet). In addition, there is also a growing consensus that strategic combinations of therapeutic agents may provide additional benefits. Several recent preclinical experiments have identified the synergistic effect of a combination treatment of anti-CD3, autoantigen, and interleukin-10 (IL-10). Combining therapeutic approaches further improved the efficacy of anti-CD3 mAb alone (10–12). In studies on the potential mechanism of action of this combined approach, we observed that anti-CD3 mAb, autoantigen, and treatment with IL-10 are all capable of boosting IL-10–secreting T cells in vivo. For example, in the presence of exogenous IL-10, IL-10–producing T cells can be generated (13) and functionally sustained (14). Oral administration of autoantigen at low concentrations results in induction of regulatory T cells that secrete IL-10 and/or TGF-β (15, 16). After injection of OKT-3 or humanized monoclonal hOKT3γ1 (Ala-Ala), serum IL-10 levels were significantly increased in 63% of patients, and IL-10 expression was induced in ∼10% of peripheral CD4 T cells on day 12 of drug treatment (17). All these observations suggest that IL-10–secreting Treg cells may play an important role in controlling diabetes.
Previously, we found that anti-CD3 mAb treatment leads to the accumulation of IL-10+ type 1 regulatory T (Tr1) cells in the small intestine, where they control pathogenic Th17 cells in colitis and experimental autoimmune encephalomyelitis (18–20). However, whether these regulatory T cells migrate from the periphery into the gastrointestinal tract in response to anti-CD3 or are generated in situ is unknown. Moreover, given that diabetes is likely driven by Th1 cells and the pancreas is the target organ, the mechanism whereby these intestinal resident IL-10+ T cells block the effector function of Th1 cells merits thorough study.
The intestinal environment has long been recognized to play a larger role in body function and health in addition to its role in processing and absorbing food. In fact, the connection between the intestinal microbe and the development of T1D has been demonstrated in both animal models (21–23) and human studies (24, 25). However, the underlying mechanisms by which gut microbes could trigger diabetes or protect from this disease are not fully understood. In comparison with other animal housing conditions, our non-obese diabetic (NOD) mouse colony has a much lower diabetes incidence. As our mouse room also houses large numbers of mice lacking inflammasome components or effectors, such as Asc−/−, Nlrp3−/−, and Il18−/−, and these inflammasome-deficient mice harbor an altered intestinal microbiota that affect the disease susceptibility (26), we hypothesized that exposure to some of these dysbiotic microbiota may have an effect on the immune system and prevent diabetes development. Furthermore, microbiota have been shown to be capable of modulating the development of Th17 (27) and Treg cells (28); whether this beneficial outcome seen in our animal facility is due to the induction of intestinal Tr1 cells remains to be explored.
Here, we show that after alteration of gut microbiota through cohousing or administration of anti-CD3, the number of intestinal Tr1 cells was significantly increased. Up-regulation of IL-27 and TGF-β in the small intestine may account for the increased Tr1 cell differentiation. These regulatory T cells are mobile and have the ability to migrate back to the periphery and sites of inflammation via different chemokine receptors, such as CCR4, CCR5, and CCR7. We also demonstrated that Tr1 cells could directly suppress diabetogenic T cells via IL-10 signaling and significantly delay disease development. Therefore, strategies targeting mucosal tissue to induce Tr1 cells in vivo might be used as a therapeutic approach to prevent diabetes or treat people with newly diagnosed T1D.
Results
Colonization with Dysbiotic Gut Microbiota Promotes Intestinal IL-10–Producing CD4+ T Cells and Protects NOD Mice from T1D.
To investigate whether the lower diabetes incidence in our animal room where NOD mice were housed somehow reflects colonization of gut flora from dysbiotic inflammasome-deficient mice, we cohoused female NOD mice purchased from the Jackson Laboratory at 3 wk of age with dysbiotic mice from our facility and monitored disease development. There was a clear difference in the cumulative disease incidence between the mice held under the two housing conditions. While 78% of noncohoused NOD mice developed diabetes by 25 wk of age, only 37% of cohoused NOD mice developed the disease (Fig. 1A). Concomitantly, intestinal IL-10–producing CD4+ T cells were also significantly induced in cohoused Foxp3RFP and IL-10eGFP double-reporter NOD mice (Fig. 1 B and C). Although the percentage of intestinal CD4+Foxp3+ T cells decreased, the total number was not significantly changed (Fig. 1 B and C). These results suggested that the protective function of dysbiotic gut microbiota might be attributed to the elevation of IL-10–producing CD4+ T cells.
Fig. 1.
Diabetes incidence and the changes of intestinal Tregs in cohoused and noncohoused NOD mice. (A) Cumulative incidence curves for female NOD mice in two housing conditions. Mice were cohoused with dysbiotic inflammasome-deficient mice after weaning. Diabetes development was monitored twice per week starting at 10 wk of age. The difference between the two groups was analyzed by log-rank test (P = 0.06). Percentages (B) and total numbers (C) of IL-10–producing and Foxp3+ T Cells in the intestine of cohoused Foxp3RFP IL-10eGFP double-reporter mice were plotted. Cells were gated on CD4+TCRβ+ cells. Data are pool of two independent experiments and indicated as the mean ± SEM. Open circles, noncohoused reporter mice; black circles, cohoused reporter mice.
Tr1 Cells Are Generated in the Small Intestine in Response to Anti-CD3.
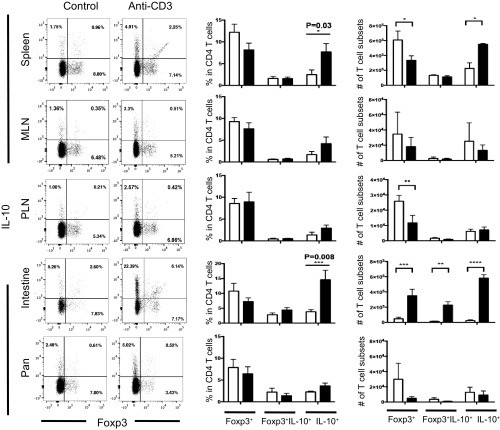
To determine whether intestinal IL-10+ CD4+ T cells are generated in situ or migrate from the periphery, we used the anti-CD3–induced immune tolerance mouse model. From our past experience, administration of anti-CD3 leads to a strong induction of IL-10–producing cells in the gut. In accordance with previous findings (19), the frequency of Tr1 cells (CD4+Foxp3−IL-10+) was elevated in all examined organs, with the highest level seen in the intestine. By contrast, the frequencies of CD4+Foxp3+IL-10− and CD4+Foxp3+IL-10+ T cells were not significantly changed by this treatment (Fig. S1). More importantly, the total numbers of Tr1 cells were increased (Fig. 2A and Fig. S1), supporting the hypothesis that the IL-10 producers are generated de novo. Since activation of CD4+ T cells in the presence of IL-27 or TGF-β plus IL-27 results in the differentiation of IL-10–producing Tr1 cells (29, 30), we isolated total intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) from anti-CD3–treated and untreated mice to measure the mRNA expression level of these cytokines. Tgfb1, 2, 3 and Il27 mRNA significantly increased in the proximal part of the small intestine (duodenum and jejunum) following anti-CD3 treatment (Fig. 2B), implying that Tr1 cells may be generated in response to these inducers in both locations.
Fig. S1.
Analysis of different T cell subsets after anti-CD3 treatment. Cells were isolated from different organs as indicated. Foxp3 RFP and IL-10 eGFP expression were measured in freshly isolated cells. Cells were gated on CD4+TCRβ+ events (Left). Percentages and numbers of CD4+Foxp3−IL-10+, CD4+Foxp3+IL-10+, and CD4+Foxp3+IL-10− cells were compared between control mice (white bar) and mice treated with anti-CD3 (black bar). Data represent the mean ± SEM (n = 6 per group) and were analyzed by two-way ANOVA, multiple comparisons test. Results are representative of three independent experiments. MLN, mesenteric lymph node; Pan, pancreas; PLN, pancreatic lymph node.
Fig. 2.
Tr1 cells are generated within small intestine after anti-CD3 treatment. (A) Total numbers of CD4+Foxp3−IL-10+, CD4+Foxp3+IL-10+, and CD4+Foxp3+IL-10− cells were pooled from different mouse organs and compared between control mice (white bar) and mice treated with anti-CD3 (black bar). Foxp3 RFP and IL-10 eGFP expression was measured in freshly isolated CD4+TCRβ+ cells. Data represent the mean ± SEM (n = 6 per group) and were analyzed by two-way ANOVA, multiple comparisons test. Results are representative of three independent experiments. (B) TGFβ1,2,3 and IL-27 mRNA levels (mean ± SEM, n = 3) of IEL and LPL isolated from a different part of the small intestine in control mice (white bar) and mice treated with anti-CD3 (black bar). Data were normalized to mouse HPRT. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Intestinal Tr1 Cells Migrate into the Periphery via Chemokine Receptors to Suppress Diabetes Development in Vivo.
To test whether intestinal Tr1 cells could suppress diabetes development, we sorted these cells from anti-CD3–treated, BDC2.5 double-reporter mice and cotransferred with BDC2.5 CD4+CD25− effector T cells (Teff) into NOD-severe combined immunodeficiency (scid) mice. As expected, mice injected with Teff alone all became diabetic within 11–16 d. By contrast, cotransferring effectors with intestinal Tr1 at a 1:1 ratio significantly delayed diabetes for an average of 29 d (P = 0.001) (Fig. 3A). To analyze the mechanism whereby Tr1 cells act upon pancreas-infiltrating leukocytes, we repeated the experiment with a reduced number of Tr1 cells and cotransferred with Teff at a 1:5 ratio. Although the reduced number of Tr1 cells did not delay the onset of diabetes, the frequency of IFN-γ–producing CD4+ T cells in the pancreata was significantly reduced compared with that in the mice transferred with Teff cells alone (Fig. 3B).
Fig. 3.
Intestinal Tr1 cells have ability to migrate and suppress diabetes development in vivo. (A) BDC2.5 TCR-Tg CD4+CD25− cells (1 × 105) were injected into NOD-scid mice either alone or coinjected with sorted intestinal Tr1 cells (1 × 105) from BDC2.5 TCR-Tg double-reporter NOD mice. Diabetes incidence was measured for 60 d by urine glucose readings, and positive readings were confirmed by blood glucose analysis. Data were analyzed by log-rank (Mantel–Cox) test. (B) BDC2.5 TCR-Tg CD4+CD25− cells (1 × 105) were injected into NOD-scid mice either alone (open circles) or coinjected with sorted intestinal Tr1 cells (0.2 × 105) from BDC2.5 TCR-Tg double-reporter NOD mice (black circles). Percentages of IFN-γ+ cells were measured in various organs. Each circle represents one mouse. Horizontal bars indicate the mean ± SEM. One experiment of two is shown. Data were analyzed by unpaired Student’s t test. (C) Representative experiment with chemokine receptor staining of Tr1 (red line) and non-Tr1 (blue line) cells isolated from intestine, spleen, and mesenteric lymph node (MLNs). T cells were stained with anti-CCR4, CCR5, CCR7, and CCR9 mAbs. Profile of expression was assessed by flow cytometry. Histogram outlines indicate cytokine-specific staining, and shaded histograms indicate isotype control staining. Results are representative of at least two independent experiments. (D) Representative FACS plots of chemokine receptor expression on Tr1 and non-Tr1 cells isolated from small intestine after anti-CD3 mAb treatment. A portion of Tr1 cells coexpresses CCR4, CCR5, CCR7, and CCR9. (E) NOD-scid mice were intrarectally administered with 0.5 × 106 in vitro-differentiated Tr1 cells. (Top) Schematic of the experiment. Cells were isolated from spleen, LN, MLN, small instestine, colon, and pancreas 2 wk after cell administration. The absolute number of CD4 T cells was determined by flow cytometry. Data were pooled from two independent experiments and are indicated as the mean ± SEM of six mice in total. LN, lymph node; SI, small intestine.
To perform a regulatory function in the diabetes setting, intestinal Tr1 cells have to migrate to the periphery or be recruited to the site of inflammation. Chemokine receptors determine whether T cells remain in the tissue or return to the circulation. For example, CCR7 is a critical signal that determines T cell exit from peripheral tissue (31, 32), CCR5 is specific for homing to the sites of inflammation (33), and CCR4 and CCR9 chemokine receptors are expressed on skin- (34), islet- (35), and gut-homing (36) T lymphocytes, respectively. We therefore carried out experiments to measure the expression level of CCR4, CCR5, CCR7, and CCR9 in intestinal Tr1 cells and also compared their expression levels with those in non-Tr1 (IL-10− CD4+) cells. Interestingly, Tr1 cells isolated from intestine express all of the above chemokine receptors, and their expression levels were much higher than those in non-Tr1 cells, suggesting that these IL-10–producing cells might readily be mobilized to migrate into the periphery and to other tissues. In addition, this characteristic was specific to the intestine, as we did not see the same phenotype in the Tr1 cells isolated from spleen or mesenteric lymph nodes, with the exception of CCR9 (Fig. 3 C and D).
To further confirm the migration ability of Tr1 cells, we rectally administered 0.5 × 106 sorted GFP+ IL-10+ CD4 T cells into NOD-scid mice and analyzed the egress of these cells 2 wk after injection. The majority of CD4+ T cells were found in the spleen and lymph node (LN), with lesser amounts in the small intestine, colon, and pancreas tissue (Fig. 3E). Therefore, Tr1 cells not only can actively migrate from intestine into the circulation and further to lymphoid and nonlymphoid tissue, but also are able to cross an epithelial barrier from the luminal side.
IL-10 Signaling in CD4+ T Cells Is Critical in Controlling Diabetes Development.
To study the cellular target of Tr1 cells, we generated transgenic mice on the NOD background, in which IL-10 signaling is specifically blocked in T cells by the overexpression of a dominant-negative IL-10R under the CD4 promoter (CD4–DN–IL-10R). Of note, these transgenic mice do not differ from NOD mice in their incidence of spontaneous diabetes. We injected 10 μg of anti-CD3 antibody for 5 consecutive days into recent-onset diabetic CD4–DN–IL-10R transgenic or nontransgenic NOD mice to compare the diabetes reversal rate for each group. In response to anti-CD3, diabetes remission was generally maintained within 6 wk after the onset of diabetes and treatment in NOD mice, although some of the treated mice experienced disease relapse when they were monitored for a longer period (Fig. 4A). This phenomenon resembled what was seen in clinical trials, namely that anti-CD3 can mediate the extension of the honeymoon phase in newly diabetic patients (37, 38). Although the difference in the rate of diabetes reversion did not reach statistical significance in the 12-wk window between NOD mice and CD4–DN–IL-10R transgenic mice treated with anti-CD3 (47% in NOD vs. 25% in CD4–DN–IL-10R NOD, P = 0.1), the reversal rate is significantly higher in NOD mice within a time window of 6 wk (P = 0.03) (Fig. 4B). Therefore, direct suppression of CD4 T cells via the IL-10–signaling pathway at least partially contributes to the effect of anti-CD3 treatment in vivo.
Fig. 4.
IL-10 signaling in CD4+ T cells is critical in controlling diabetes development. (A) Individual glycemia values of anti-CD3–treated recent-onset diabetic NOD mice (Left) and CD4–DN–IL-10R NOD mice (Right). Blood glucose concentrations were monitored until 12 wk posttreatment initiation. (B) Percentages of nondiabetic mice in NOD (n = 15) and CD4–DN–IL-10R NOD (n = 12) mice after anti-CD3 treatment. *P = 0.03. Statistical significance between groups was calculated using a log-rank (Mantel–Cox) test. (C) IL-10R expression was measured by flow cytometry. Cells were gated on CD4+CD25− T cells, and Th1 (CD4+IFNγ+) cells were isolated from the spleen of a prediabetic and diabetic mouse. Results are representative of at least two independent experiments.
We found previously that Tr1 cells can control Th17 cells directly through the IL-10 receptor, which is expressed in the latter cells (19). To investigate if diabetogenic CD4 T cells also express IL-10R, we isolated CD4+CD25− T cells from spleens of BDC2.5 NOD mice and found that these T cells expressed a high level of IL-10R. When mice became diabetic, about 30% of CD4 T cells expressed INF-γ, and these Th1 cells also expressed a significant amount of this cytokine receptor (Fig. 4C). Therefore, Tr1 cells in principle are able to control diabetogenic T cells directly in both a prediabetic environment and fully differentiated Th1 cells at the late stage of disease.
Tr1 Cells Generated in Vitro from Memory or Total CD4 T Cells Have a Different Capacity to Suppress Diabetes Development.
To explore the therapeutic possibility of the use of Tr1 cells to treat autoimmune diabetes, we differentiated and expanded antigen-specific Tr1 cells in vitro and tested their stability and function in an adoptive transfer model. Total or memory CD4+ T cells were isolated from BDC2.5 TCR-transgenic NOD mice. Upon culture with IL-27 and TGF-β (30), 29.8 ± 4.55% IL-10–producing Tr1 cells were generated from memory CD4+ T cells, while only 8.84 ± 1.44% Tr1 cells were generated from total CD4+ T cells (Fig. 5A). This result is in line with previous findings (39) and suggests that memory T cells are the major source of this regulatory cell type. With regards to their suppressive function, Tr1 cells that were differentiated from total CD4+ T cells did not prevent diabetes (Fig. 5B), while Tr1 cells differentiated from memory CD4+ T cells significantly delayed disease development (Fig. 5C). Moreover, although BDC2.5 Teff cells isolated from CD4–DN–IL-10R transgenic NOD mice caused a similar diabetes incidence and secreted a comparable amount of IFN-γ as the normal BDC2.5 Teff cells (Fig. 5 C and D), Tr1 cells generated from the memory pool were no longer able to suppress these diabetogenic T cells, further confirming that Tr1 regulatory cells control the effector T cells via the IL-10–signaling pathway.
Fig. 5.
In vitro-generated Tr1 cells have a different capacity in suppressing diabetes development. (A) Total CD4 and memory CD4 T cells were cultured for 5 d with anti-CD3 and anti-CD28 in the presence of mouse recombinant TGF-β and IL-27. Percentages of Tr1 cells were compared after in vitro differentiation. Statistical significance was determined by using a paired Student t test. Data are means ± SEM of four independent experiments. (B) BDC2.5 TCR-Tg CD4+CD25− cells (1 × 105) were injected into NOD-scid mice either alone or coinjected with in vitro-differentiated Tr1 cells from total CD4 T cells. (C) BDC2.5 TCR-Tg CD4+CD25− Teff cells (1 × 105) from wild-type or CD4–DN–IL-10R NOD mice were injected into NOD-scid either alone or coinjected with in vitro-differentiated Tr1 cells from memory CD4 T cells. Statistical significance was determined by log-rank (Mantel–Cox) test. (D) Cytokine production by CD4 T cells was analyzed intracellularly with flow cytometry and compared between wild-type and CD4–DN–IL-10R NOD mice. Percentages of the IFN-γ–producing T cells are indicated. Data are representative of three independent experiments.
Due to the difference in suppressive function of Tr1 cells generated from two different sources of CD4 T cells, we further studied if cells differentiated from memory CD4 T cells are more stable in vivo. We sorted Tr1 cells differentiated from total or memory CD4+ T cells and then injected these cells into NOD-scid mice. Two weeks after injection, Tr1 cells were able to migrate into various tissues. Although the majority of the cells lost IL-10 expression, 5 to ∼25% of IL-10 producer cells remained, independent of the original cellular source (Fig. S2A). This raised the issue of whether Tr1 cells might become pathogenic after loss of IL-10 expression. By intracellular staining, we observed that these exTr1 cells secrete IFN-γ and minimal amounts of IL-17 (Fig. S2B). Nevertheless, the loss of the ability to secrete IL-10 did not render those cells diabetogenic in NOD-scid mice in a 75-d observation window.
Fig. S2.
Cytokine expression of Tr1 cells measured after 2 wk adoptive transfer into lymphopenic mice. Tr1 cells were differentiated from either total or memory CD4 T cells and then injected into NOD-scid mice. (A) Two weeks later, IL-10 eGFP expression was measured in freshly isolated cells from NOD-scid mice. Cells were gated on CD4+TCRβ+ events. (B) Cells from the spleen were also restimulated with PMA, and Ionomycin and intracellular cytokine staining for IL-17A and IFN-γ was performed. Plots were gated on CD4+TCRβ+ events. Data are representative of at least three independent experiments.
Discussion
In this paper, we highlight the importance of Tr1 cells and their suppressive mechanism in blocking diabetes development. Given that combinations of anti-CD3 mAb, autoantigen, and IL-10 treatment can revert autoimmune diabetes in NOD mice to a greater extent than monotherapy or any of the two-way combinations (11), it is possible that the synergy obtained through combined therapy may be due to increased induction of Tr1 cells. Although the mechanisms of anti-CD3 treatment for autoimmune diabetes are also thought to involve Foxp3+ T cells, the evidence has been conflicting (10, 40–42). In some studies, Foxp3+ Tregs have been expanded to various degrees after a given treatment while, in others, Treg numbers were decreased. In our study, we also observed systematically reduced levels of Foxp3+ T cells. Instead, the numbers of Tr1 cells were significantly increased, implying that this cell type may contribute significantly to the efficacy of the treatment.
It has been well recognized that, even with all these therapies, not all patients will respond. Data from clinical studies suggest that there are “responders” and “nonresponders” (43). Therefore, identification of the genetic, metabolic, and immunological features that differentiate responders and nonresponders may help to tailor therapies for subjects to improve efficacy and safety and to guide how combinations might be constructed. When monitoring children with newly diagnosed T1D for 3 mo, Sanda et al. (44) found that higher FoxP3 expression in T cells at diagnosis predicted worse future glycemic control, while higher mean numbers of IL-10+ T cells were associated with better future glucose control as measured by HbA1C. Therefore, quantifying IL-10+ T cell numbers might be a better way to distinguish the populations who might be at high risk for developing disease, identify trends, and serve as an immunological biomarker that could predict the treatment outcome.
Compared with treatment, preventing healthy or at risk people from developing disease is even more important. Because we showed that dysbiotic gut microbiota can induce intestinal Tr1 cells and that these cells also have the ability to migrate into the periphery and other organs, Tr1 cells may serve to patrol in the steady state to regulate pathogenic immune responses by suppressing, for example, autoreactive T cells that have escaped thymic deletion. Once immune tolerance is overcome, autoimmune cells will react quickly and start to attack human tissues. Therefore, a deficit of Tr1 cells might be an underlying first trigger to initiate human autoimmune diseases. In fact, in newly diagnosed T1D patients and first-degree relatives, fewer antigen-specific IL-10–secreting cells were found compared with healthy controls (45, 46). Therefore, modulation of gut-associated lymphoid tissue (GALT) to boost Tr1 cells could represent a means to affect the natural history of autoimmune diabetes. Multiple strategies targeting mucosal tissue to modulate local and systemic immune responses have demonstrated success, such as oral administration of probiotic bacteria (47). Oral administration of a mixture of different strains of viable lyophilized probiotic bacteria in early diabetic NOD mice, including Bifidobacteria, Lactobacilli, and Streptococcus salivarius, induces IL-10–producing cells in GALT and prevents islet destruction and the onset of clinical signs of diabetes. Interestingly, Bifidobacterium and Lactobacillus species are widely used in the food industry for production of yogurt and cheese, which are thought to be beneficial in reducing the risk of diabetes. By contrast, early exposure to a particular diet, such as cows’ milk (48, 49), gluten, and other cereal components (50, 51), may trigger or promote autoimmune reactivity. From this point of view, diet, which includes various antigens and also has an impact on gut microbiota, is critically important in T1D management. Manipulating diet to boost protective immune responses might be a good way to modify the disease incidence.
Several studies have explored the potential of Tr1 cells as therapeutic agents in a number of settings (52–54). To explore the possibility of in vitro-expanded Tr1 cells as an adoptive cell therapy, we differentiated IL-10–producing cells from either total or memory CD4 T cells. Surprisingly, we found that only Tr1 cells generated from memory T cells could suppress diabetogenic T cells. However, no matter from which cell pool the regulatory cells were generated, both Tr1 populations showed lineage plasticity, similar to what has been previously reported for in vitro-expanded Foxp3+ Tregs (55, 56). Cells that previously expressed IL-10, called exTr1 cells, acquired effector-like properties by producing cytokines like IFN-γ or IL-17. Although these cells did not elicit autoimmunity, at least in a 75-d observation window, further investigation of the stability, function, and phenotypic and genotypic characteristics of the different source of Tr1 cells will be essential to further secure the ongoing clinical trials.
Materials and Methods
Mice.
NOD mice and BDC2.5 transgenic NOD mice were purchased from The Jackson Laboratories. Foxp3RFP (57) IL-10eGFP double-reporter mice (20) and dominant-negative IL-10R mice (CD4–DN–IL-10R) (58) were backcrossed to a NOD background for 10 generations. Age- and sex-matched littermates between 8 and 16 wk old were used.
Inflammasome-deficient mice (Asc−/− and Nlrp3−/−) with dysbiotic gut microbiota were used for the cohousing experiments. Female diabetes-prone NOD mice or Foxp3RFP IL-10eGFP double-reporter mice were cohoused with dysbiotic mice at a 1:1 ratio for 3–6 mo. All animal procedures were approved by the Institutional Animal Care and Use Committee of Yale University.
Intestinal Lymphocyte Isolation.
Mice were injected with anti-CD3 (10–15 μg per mouse, 2C11) or PBS intraperitoneally two times every other day. After removal of the Peyer’s Patches, IEL and LPL were isolated via incubation with 5 mM EDTA at 37 °C for 30 min (for IEL), followed by further digestion with collagenase from Clostridium histolyticum (#2139; Sigma) and DNase at 37 °C for 1 h (for LPL). Cells were then further separated with a Percoll gradient.
RT-PCR.
Intestinal lymphocytes were isolated from two independent experiments, each using three mice injected with or without anti-CD3 mAb. Total RNA was extracted from cells using TRIzol reagent and reverse-transcribed into cDNA. Samples were run in duplicate or triplicate, and mRNA expression levels were calculated as relative to the expression of Hprt.
Flow Cytometry.
Cell suspensions were prepared from spleen, lymph nodes, pancreas, and intestine of control mice or mice treated with anti-CD3. Samples were stained with fluorochrome-labeled mAbs against cell-surface antigens and analyzed on a LSRII flow cytometer (BD Biosciences). The following mAbs were used: anti-CD4 (RM4-4), TCR-β (H57-597), IL-10R (1B1.3a), CCR4 (2G12), CCR5 (HM-CCR5), CCR7 (4B12), CCR9 (CW-1.2), Armenian Hamster IgG Isotype Ctrl (HTK888), Biotin Rat IgG2a, κ isotype Ctrl (RTK2758), mouse IgG2a, and κ isotype Ctrl (MOPC-173). All mAbs were from BioLegend, and data were analyzed using FlowJo.
For intracellular cytokine staining, the cells were restimulated for 4 h at 37 °C with phorbol 12-myristate 13-acetate (PMA) (50 ng/mL; Sigma) and ionomycin (1 μg/mL; Sigma) in the presence of Golgistop (BD Bioscience). Cells were then fixed and permeabilized with BD Cytofix/Cytoperm buffer and stained at 4 °C with anti-IL-17A (catalog no. 560184; BD Bioscience) and anti-IFNγ (catalog no. 554412; BD Bioscience) antibodies for 30 min. Lymphocytes were resuspended in PBS and 0.5% FBS.
Adoptive Transfer.
CD4+CD25− Teff cells and CD4+Foxp3−IL-10+ (Tr1) cells were FACS-sorted from spleen and intestine of BDC2.5 double-reporter NOD mice, respectively. A total of 100,000 Teff cells were i.v. injected into 6-wk-old NOD-scid recipients with or without the same number or five times fewer of Tr1 cells. Disease development was monitored by testing the urine glucose.
In Vitro Tr1 Cell Differentiation.
We FACS-sorted total or memory CD4+ T cells (CD44hi CD62Llo) and activated them with plate-bound monoclonal antibodies to CD3 (2 μg/mL, 145–2C11) in the presence of mouse recombinant TGF-β (2 ng/mL), IL-27 (25 ng/mL), and antibodies to CD28 (2 μg/mL, PV-1). All cytokines were purchased from R&D. Click’s (Irvine Scientific) or RPMI (Sigma) media were supplemented with 10% FBS, l-glutamine (2 mM), penicillin (100 U/mL), and β-mercaptoethanol (40 nM). After 4–5 d of culture, the cells were acquired by FACS.
Intrarectal Administration of Tr1 Cells.
IL-10 eGFP+ Tr1 cells were sorted after in vitro differentiation. NOD-scid recipient mice were fasted overnight before cell administration. The next day, mice were anesthetized with an intrperitoneal injection of ketamine/xylazine and then intrarectally injected with 0.5 × 106 cells with a gavage needle. Mice were monitored until full recovery and maintained in fasting for another night. Two weeks later, mice were euthanized to assess the T cell distribution.
mAb Treatment and Blood Glucose Monitoring.
Anti-mouse CD3 mAb 10 μg/d (145-2C11) was administered i.p. in newly onset diabetic mice for 5 consecutive days. A diagnosis of diabetes was made when mice had blood glucose levels of at least 250 mg/dL on two consecutive occasions. Blood glucose was measured in the morning twice a week using a Turebalance Glucose Meter (NIPRO Diagnostics). Diabetes remission was defined as the absence of glycosuria, and glycemia values >250 mg/dL never occurred in any of the treated mice.
Statistical Analysis.
Differences in diabetes incidence were assessed using the Mantel–Cox log-rank test. Statistical significance of other comparisons was tested using paired or unpaired two-tailed Student’s t test or two-way ANOVA (multiple comparisons test) as indicated. Graphs were plotted and statistics calculated with GraphPad Prism v. 4.00 for Macintosh (GraphPad Software).
Acknowledgments
We thank C. Lieber, E. Hughes-Picard, and J. Alderman for expert administrative assistance and B. Hu and H. Xu for technical help and scientific discussion. This work was supported by NIH Grant R01 DK 51665 (to R.A.F.); Yale Diabetes Research Center Grant P30 DK045735; the American Diabetes Association Research Foundation Postdoctoral Fellowship (to H.Y.); and the Dr. Keith Landesman Memorial Fellowship of the Cancer Research Institute (to N.G.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705599114/-/DCSupplemental.
References
- 1.Mathis D, Vence L, Benoist C. β-Cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein J, et al. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319:599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 3.Keymeulen B, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 4.Keller RJ, Eisenbarth GS, Jackson RA. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet. 1993;341:927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Trial–Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 6.Marek-Trzonkowska N, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherry N, et al. Protégé Trial Investigators Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold KC, et al. AbATE Study Team Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagopian W, et al. Protégé Trial Investigators Teplizumab preserves C-peptide in recent-onset type 1 diabetes: Two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresson D, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takiishi T, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–1725. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert S, et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes. 2014;63:2876–2887. doi: 10.2337/db13-1236. [DOI] [PubMed] [Google Scholar]
- 13.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 14.Brockmann L, et al. IL-10 receptor signaling is essential for TR1 cell function in vivo. J Immunol. 2017;198:1130–1141. doi: 10.4049/jimmunol.1601045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Lee WH, Yun P, Snow P, Liu CP. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. J Immunol. 2003;171:733–744. doi: 10.4049/jimmunol.171.2.733. [DOI] [PubMed] [Google Scholar]
- 16.Hancock WW, Polanski M, Zhang J, Blogg N, Weiner HL. Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration is associated with selective expression of interleukin-4 and -10, transforming growth factor-beta, and prostaglandin-E. Am J Pathol. 1995;147:1193–1199. [PMC free article] [PubMed] [Google Scholar]
- 17.Herold KC, et al. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esplugues E, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurkovetskiy L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriegel MA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostic AD, et al. DIABIMMUNE Study Group The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Goffau MC, et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569–1577. doi: 10.1007/s00125-014-3274-0. [DOI] [PubMed] [Google Scholar]
- 26.de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6:a016287. doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 30.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 32.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baeke F, et al. The vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation. J Immunol. 2011;186:132–142. doi: 10.4049/jimmunol.1000695. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JJ, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 35.Montane J, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121:3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zabel BA, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatenoud L, Bluestone JA. CD3-specific antibodies: A portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 38.Herold KC, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito T, et al. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belghith M, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 41.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med. 2010;207:1879–1889. doi: 10.1084/jem.20100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–2022. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herold KC, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanda S, Roep BO, von Herrath M. Islet antigen specific IL-10+ immune responses but not CD4+CD25+FoxP3+ cells at diagnosis predict glycemic control in type 1 diabetes. Clin Immunol. 2008;127:138–143. doi: 10.1016/j.clim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Petrich de Marquesini LG, et al. IFN-gamma and IL-10 islet-antigen-specific T cell responses in autoantibody-negative first-degree relatives of patients with type 1 diabetes. Diabetologia. 2010;53:1451–1460. doi: 10.1007/s00125-010-1739-3. [DOI] [PubMed] [Google Scholar]
- 46.Chujo D, et al. Adult-onset type 1 diabetes patients display decreased IGRP-specific Tr1 cells in blood. Clin Immunol. 2015;161:270–277. doi: 10.1016/j.clim.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Calcinaro F, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 48.Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17:13–19. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 49.Knip M, et al. Finnish TRIGR Study Group Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 51.Norris JM, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 52.Mfarrej B, et al. Generation of donor-specific Tr1 cells to be used after kidney transplantation and definition of the timing of their in vivo infusion in the presence of immunosuppression. J Transl Med. 2017;15:40. doi: 10.1186/s12967-017-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geissler EK. The ONE Study compares cell therapy products in organ transplantation: Introduction to a review series on suppressive monocyte-derived cells. Transplant Res. 2012;1:11. doi: 10.1186/2047-1440-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andolfi G, et al. Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4+ T cells. Mol Ther. 2012;20:1778–1790. doi: 10.1038/mt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey-Bucktrout SL, Bluestone JA. Regulatory T cells: Stability revisited. Trends Immunol. 2011;32:301–306. doi: 10.1016/j.it.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamanaka M, et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]