Significance
Color vision variation is prevalent among neotropical monkeys. Captive studies indicate that trichromacy should confer a fruit feeding advantage. This hypothesis, however, has yet to be supported by field studies. We collected behavioral and genetic data from 72 capuchins and analyzed ca. 20,000 fruit intake events across 27 plant species. Controlling for plant species and phenological condition, we find that trichromats eat reddish, conspicuous fruits more quickly than do dichromatic (red-green colorblind) groupmates. We demonstrate an advantage of trichromacy for consuming fruit among wild monkeys. Previous research has revealed dichromatic advantage for cryptic tasks; our results suggest fruit foraging plays an important role in the maintenance of primate opsin polymorphism via balancing selection.
Keywords: sensory ecology, opsin genes, color vision, frugivory, platyrrhine
Abstract
Intraspecific color vision variation is prevalent among nearly all diurnal monkeys in the neotropics and is seemingly a textbook case of balancing selection acting to maintain genetic polymorphism. Clear foraging advantages to monkeys with trichromatic vision over those with dichromatic “red-green colorblind” vision have been observed in captive studies; however, evidence of trichromatic advantage during close-range foraging has been surprisingly scarce in field studies, perhaps as a result of small sample sizes and strong impacts of environmental or individual variation on foraging performance. To robustly test the effects of color vision type on foraging efficiency in the wild, we conducted an extensive study of dichromatic and trichromatic white-faced capuchin monkeys (Cebus capucinus imitator), controlling for plant-level and monkey-level variables that may affect fruit intake rates. Over the course of 14 months, we collected behavioral data from 72 monkeys in Sector Santa Rosa, Costa Rica. We analyzed 19,043 fruit feeding events within 1,602 foraging bouts across 27 plant species. We find that plant species, color conspicuity category, and monkey age class significantly impact intake rates, while sex does not. When plant species and age are controlled for, we observe that trichromats have higher intake rates than dichromats for plant species with conspicuously colored fruits. This study provides clear evidence of trichromatic advantage in close-range fruit feeding in wild monkeys. Taken together with previous reports of dichromatic advantage for finding cryptic foods, our results illuminate an important aspect of balancing selection maintaining primate opsin polymorphism.
Intraspecific color vision variation is nearly ubiquitous among neotropical monkeys as a result of allelic variation of the X-linked M/L opsin gene (OPN1LW) (1). The enduring persistence of this polymorphism, its widespread occurrence across diverse taxa, and the extent of opsin gene diversity suggest that balancing selection maintains the allelic variation (2). The mechanism underlying balancing selection, however, is unresolved and remains a subject of extensive debate (3–7). Central to all current hypotheses is the prediction that trichromats will have an advantage over dichromatic group members, whether this advantage is global (heterozygote superiority hypothesis) or contextual (niche divergence, mutual benefit of association, or frequency-dependent hypotheses). It has long been suggested that trichromacy should assist the search for ripe fruit amid green leaves, given the stability of the color signal, even in the dappled lighting of forests (8, 9). For a majority of New World monkeys, fruit is an important dietary component, and many fruits have hues (yellowish-to-reddish) that are distinct from those of leaves (greenish) in the red-green range of the chromatic spectrum, thus making them more conspicuous to trichromatic animals (10–12). Because effective fruit detection and selection could improve foraging efficiency, natural selection favoring an enhanced ability to perceive different fruit hues could be an evolutionarily significant force shaping primate sensory adaptation (11, 13).
To date, direct evidence of feeding advantages for trichromatic primates is absent in the wild. Some captive experiments have demonstrated clear differences between the intake rates of dichromats and trichromats (14, 15). Surprisingly, however, wild studies report similar intake rates among trichromats and dichromats (16–18), despite theoretical models predicting an increased ability for trichromats to find and select their dietary species (19, 20). Studies of the feeding rates of wild animals suffer a disadvantage relative to captive experiments, in that many stochastic variables influence the measures of interest. These variables include consumer-level factors, such as previous experience, skill, social dominance rank, sex, and body size, all of which may affect foraging ability and access to resources (21–23). For example, juvenile wedge-capped capuchins spend significantly more time foraging than adults (24), but are less efficient (22, 23, 25). In addition to consumer-level variables, food patch-level variables including plant species (fruit and seed size) and phenology (fruit abundance and ripeness) affect foraging rates of wild frugivorous birds and primates (26, 27). Nutritional reward also varies with plant species, making some fruits less profitable than others (28). Previous field studies evaluating the potential for trichromatic advantage in the wild have called attention to the relatively small number of monkeys in their studies and/or the limited number of plant species included (16–18, 29, 30), factors that have limited their power to detect small effect sizes or generalize their results. Therefore, increased sample sizes and improvements in the ability to account for plant-level characteristics may reveal previously undetected effects of color vision phenotype on fruit intake rates of primates.
To address this possibility, we study a relatively large number of free-ranging white-faced capuchins (Cebus capucinus imitator; n = 72; Dataset S1) and conduct foraging observations on males and females of varying age class and color vision phenotype. Male capuchins, as in other polymorphic platyrrhines, are dichromatic (red-green colorblind), whereas females can be either dichromatic (homozygous for the M/L opsin gene) or trichromatic (heterozygous; Fig. 1). We account for the conspicuity of ripe fruit color against a leaf background to different color vision types, the plant species, and the identities of individual food patches during foraging observations to control for patch-level variables (tree size, phenological state). Our overarching aim is to determine whether color vision phenotype affects fruit intake rates of wild primates once variation resulting from sex, age, or food patch characteristic is controlled for. We hypothesize that if discriminating among long wavelength hues is important for fruit detection and selection, then monkeys with different color vision types will eat fruits at the same rates. We predict that trichromatic advantage will be evident through increased numbers of conspicuous fruits eaten per duration of foraging time (intake rate), and that this will result in increased nutritional uptake.
Fig. 1.
Juvenile white-faced capuchins forage for the fruit of Allophylus occidentalis (A and B) and a katydid insect (C and D). Images simulate capuchin trichromacy (A and C; λmax 532 and 561) and dichromacy (C and D; λmax 532), using customized software (31), demonstrating the increased difficulty of visually discerning the ripe fruits, but not the leaf-camouflaged insect.
Results
We collected behavioral data over the course of 14 mo from 72 monkeys in Sector Santa Rosa, Costa Rica. For 27 plant species, we had sufficient data to compare across color vision type and age class. In total, we analyzed 19,043 fruit intake events within 1,602 foraging bouts (Dataset S2). Twenty-one plant species, 77.78% of species in our study, produced ripe fruit we modeled to be more visually conspicuous to monkeys with trichromatic vision than dichromatic monkeys; three plant species produced relatively inconspicuously (cryptically) colored fruits that were similar in chroma and luminance to background leaves (Dataset S3). The final three species produced dark fruits that should be highly conspicuous to all vision phenotypes (31). Ripe fruits ranged in mean diameter from 0.08 to 5.16 cm, and differed in nutritional profile across species (Dataset S2). Considerable variation in nutrition was evident within each fruit color category (Dataset S2). However, fruits in different color categories did not differ significantly from each other in size of ripe fruit (Kruskal-Wallis test, χ2 = 1.2804; df = 2; P = 0.5272), energy content per dry mass (χ2 = 1.6436; df = 2; P = 0.4396), or energy intake rates (χ2 = 0.4783; df = 2; P = 0.7873; Dataset S2).
To test for effects of sex on fruit intake rate, we ran a linear mixed model including only dichromats (Table 1, “Sex model”). We found that sex did not have a significant effect on fruit intake rates [χ2 = 0.002; df = 1; P = 0.965; dichromatic females: least-squares mean ± SE (LSmean) = −2.281 ± 0.230; dichromatic males: LSmean ± SE = −2.279 ± 0.229]. Therefore, we treat data from male and female dichromats as belonging to a single color vision category for our linear mixed model and exclude sex from further analysis, as it is confounded with color vision type (i.e., it is not possible to have male trichromats).
Table 1.
Results from linear mixed-effects models predicting fruit feeding rates of wild white-faced capuchin monkeys (Cebus capucinus imitator) in a tropical dry forest
| Model Name (dataset) | Variables and Interactions | χ2 | df | P value |
| Sex model (only dichromats, n = 47) | ||||
| Age class | 7.284 | 2 | 0.026* | |
| Sex | 0.002 | 1 | 0.965 | |
| Conspicuity | 0.117 | 2 | 0.943 | |
| Ripe fruit index | 6.455 | 3 | 0.091 | |
| Age class × sex | 0.178 | 1 | 0.673 | |
| Main model (all, n = 72) | ||||
| Age class | 6.280 | 2 | 0.043* | |
| Color vision type | 13.083 | 1 | <0.001* | |
| Conspicuity | 0.743 | 2 | 0.690 | |
| Ripe fruit index | 7.877 | 3 | 0.049* | |
| Age class × Color vision type | 2.805 | 2 | 0.246 | |
| Color vision type × Conspicuity | 10.169 | 2 | 0.006* | |
| Ripe fruit index × Color vision type | 3.098 | 3 | 0.377 | |
| Dominance rank model (mature monkeys >5 y, n = 41) | ||||
| Color vision type | 3.446 | 1 | 0.063 | |
| Conspicuity | 0.184 | 2 | 0.912 | |
| Rank class | 0.643 | 2 | 0.808 | |
| Ripe fruit index | 3.080 | 3 | 0.379 | |
| Color vision type × conspicuity | 7.182 | 2 | 0.028* | |
| Color vision type × rank class | 3.441 | 2 | 0.179 | |
| Ripe fruit index × color vision type | 2.939 | 3 | 0.401 |
indicates statistical significance (P < 0.05).
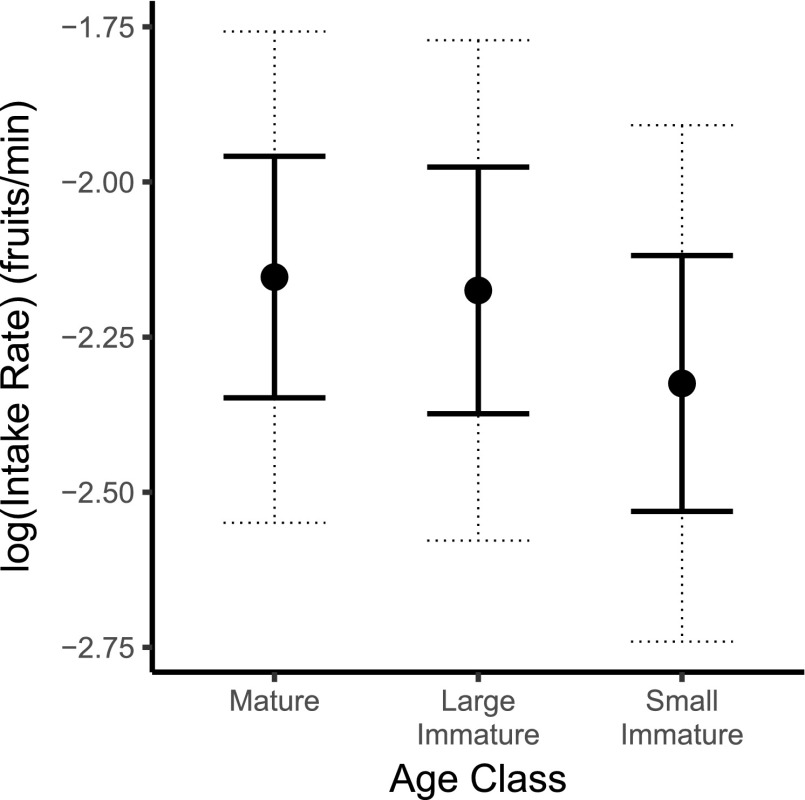
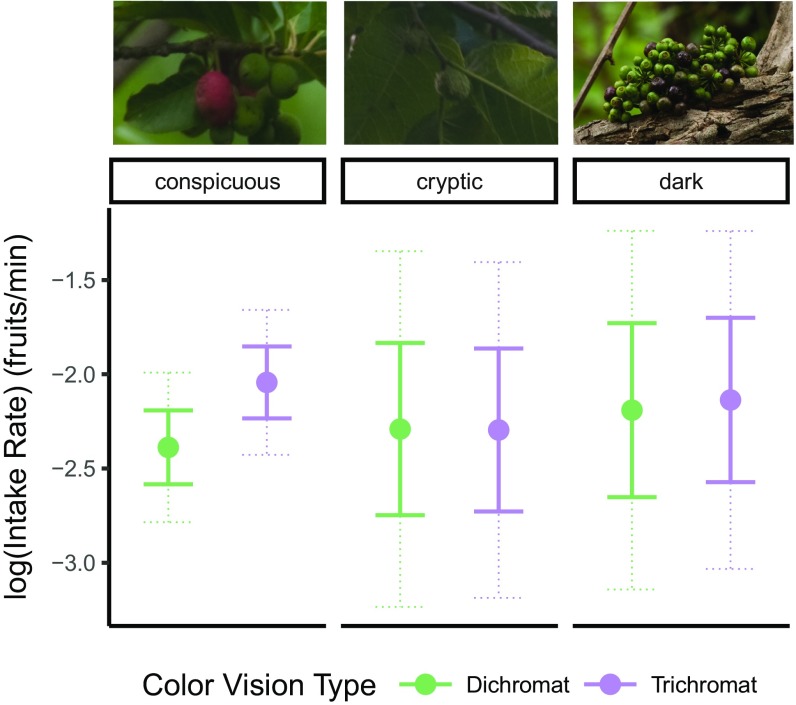
Age class and color vision type had a significant effect on fruit intake rate (Table 1, “Main model”). Mature monkeys (LSmean = −2.153 ± 0.195) and large immatures (LSmean = −2.175 ± 0.199) fed at faster rates than small immatures (LSmean = −2.325 ± 0.206; Fig. S1). In addition to a significant main effect of color vision type on fruit intake rate (χ2 = 13.083; df = 1; P < 0.001; Table 1), we found a significant interaction between color vision and fruit conspicuity (χ2 = 10.169; df = 2; P = 0.006, Table 1). When the intake rates of dichromats and trichromats are examined as least-squares means, controlling for the other variables in the model (i.e., plant species, fruit patch visit ID, monkey ID and age class), we see that trichromats (LSmean = −2.043 ± 0.191) consumed conspicuous fruits at a higher rate than did dichromats (LSmean = −2.388 ± 0.196; Fig. 2; t = 4.06; df = 199.1; P < 0.001), but that there was no significant difference attributable to color vision type when cryptic (greenish) or dark fruits were compared (Fig. 2; cryptic: t = −0.03; df = 197.5; P > 0.999; and dark: t = 0.33; df = 270.2; P > 0.999). Similar effects of color vision are seen if analyses are restricted to only juvenile monkeys (n = 31; main effect of color vision, χ2 = 6.984; df = 1; P = 0.008), only mature monkeys (n = 41; main effect of color vision, χ2 = 3.178; df = 1; P = 0.075), or only females (n = 40; main effect of color vision, χ2 = 2.683; df = 1; P = 0.100). In all cases, trichromats had faster feeding rates for conspicuously colored fruits, although in the latter two subsets of data, the effect of color vision was not significant.
Fig. S1.
Fruit intake rates [mean ±SE (bold lines) and 95% confidence intervals (dashed lines)] of different age classes are plotted as least-squares (LS) means for mature (≥6 y), large immature (≥3 y), and small immature (<3 y) monkeys. Negative values reflect log-transformation of data before analysis.
Fig. 2.
Fruit intake rates [mean, ±SE (bold lines) and 95% confidence intervals (dashed lines)] are plotted as least-squares (LS) means for dichromatic and trichromatic monkeys.
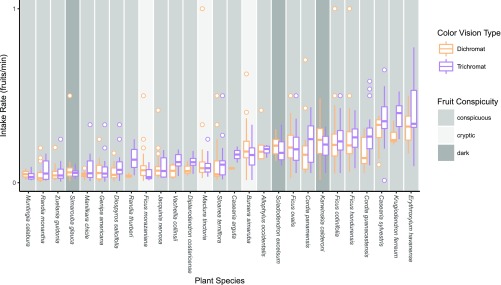
To demonstrate the effect of fruit conspicuity and plant species on intake rates, we plotted the values for dichromatic and trichromatic monkeys by plant species, with background shading indicating conspicuity category (Fig. 3). Species with larger fruits were among those consumed more slowly, including Genipa americana, Randia monantha, and Zuelania guidonia. The faster intake rate of trichromats is most evident for the reddish (conspicuous) fruits of Krugiodendron ferreum, and yellowish fruits of Diospyros salicifolia and Vachellia collinsii. In contrast, dichromats had similar intake rates to trichromats for cryptic fruits, including the evergreen fruit of Maclura tinctoria, as well as for dark-fruiting species (e.g., Sciadodendron excelsum).
Fig. 3.
Fruit intake rates (fruits eaten/second) of dichromatic and trichromatic wild capuchin monkeys across different plant species and fruit conspicuity categories of fruits consumed in Sector Santa Rosa, Costa Rica.
To test for the possible influence of social dominance on foraging efficiency, we ran a model including dominance rank class along with color vision type, using the foraging data for mature monkeys that were old enough to have a dominance rank assigned (i.e., 6 y of age by the end of the study). We found that the main effect of color vision was not significant (χ2 = 3.446; df = 1; P = 0.063), but there was a significant interaction between color vision type and fruit conspicuity (χ2 = 7.182; df = 2; P = 0.028; Table 1, “Dominance rank model”), with trichromats having faster intake rates than dichromats for conspicuous fruits (trichromats: LSmean = −2.178 ± 0.242; dichromats: LSmean = −2.226 ± 0.249). Dominance rank class did not have a significant main effect on intake rate (χ2 = 0.428; df = 2; P = 0.808). If we restrict the model to include only females (dichromats and trichromats) or only males (dichromats only), dominance still did not have a significant effect on intake rate (females: χ2 = 0.720; df = 2; P = 0.698; and males: χ2 = 1.142; df = 2; P = 0.565).
Discussion
We demonstrate a significant difference in fruit intake rates of wild monkeys that is attributable to color vision phenotype. Overall, trichromats ate fruits at a faster rate than dichromats. However, differences between dichromats and trichromats were not present for all fruit color categories. This is likely because the chromatically cryptic fruits produced by some plants were relatively inconspicuous to both trichromats and dichromats, and the dark fruits were similarly visible to all visual systems, collapsing the performance gap between trichromatic and dichromatic monkeys for fruits in the cryptic and dark categories (17). For yellowish-to-reddish (conspicuous) fruits, trichromats had a higher intake rate. This effect held when we analyzed foraging data of juvenile monkeys, and for our analysis of mature monkeys, but only in the model in which social dominance rank was included. Color vision did not have a significant effect in analyses that included only females (all ages). The juvenile result suggests effects of color vision type may be particularly strong during juvenescence, perhaps as a result of increased demands for resource acquisition in this critical window of development (23–25). In addition, as they age, capuchins may learn to effectively use nonvisual senses during foraging (18), lessening the importance of trichromacy among older monkeys. The lack of a significant effect of color vision in the female-only analysis may reflect decreased statistical power due to sample size, or possibly an undetected effect of sex, although we found the intake rates of dichromatic males and dichromatic females were not significantly different. Overall, by controlling for phenological variation and tree species, as well as monkey age, an effect of color vision type on fruit intake is evident, mirroring results found in controlled captive experiments more than a decade ago (14).
We found no significant main effect of social dominance rank on intake rate in our study, although including dominance rank in the model improved our ability to detect an effect of color vision for mature monkeys. Dominance has previously been shown to affect aspects of foraging efficiency in capuchins and other primates, including priority of access to productive feeding trees, monopolization of central feeding locations, intake rates, and patch residence times (32–35). It is possible that we do not see a main effect of dominance in the present analyses because our method was to assess fruit intake efficiency once the monkey was already engaged in food searching behavior, and to control for food patch identity. By removing time spent in nonforaging behaviors (e.g., fleeing, waiting, other submissive behaviors) and controlling for plant-level variables, we removed many of the contexts in which dominant individuals have an advantage. Still, our dominance results should be treated with caution because of the limitations of our study. First, dominance hierarchies were assigned separately for females and for males. Thus, we could not create a linear numerical hierarchy including all mature monkeys, but used a within-sex calculation of dominance position followed by categorization as “high,” “mid,” and “low” rank. When we analyzed effects of dominance separately for males and females, sample size may have been an issue. We also did not consider the effect of tree size or quality on agonism rates, which can affect how dominance impacts intake rates (35). Finally, dominance hierarchies were constructed using social data collected in the months before, between, and after our three periods of foraging data collection. Although dominance hierarchies appeared to be stable across each of our 4-mo periods of observation, dominance assessment should ideally coincide with the timing of foraging data collection. Additional studies should be conducted to further explore the relationship between dominance and vision type. However, trichromatic and dichromatic monkeys in our study were relatively evenly distributed across each dominance class (Dataset S1), so dominance rank is unlikely to have confounded our conclusions based on color vision phenotype.
The mean energy content per gram dry weight and net energy gain per minute feeding for conspicuously colored fruits was similar to the energy content of and gain from cryptic and dark fruits. Importantly, plant species with conspicuously colored fruits accounted for more than 75% of the species included in this study. Because of the high representation of conspicuous fruits in the capuchin diet and the higher intake rates by trichromatic monkeys, trichromacy likely confers an overall nutritional benefit in the context of fruit foraging. Other studies of capuchins and primates with polymorphic color vision similarly highlight the large proportion of chromatically conspicuous fruits in their natural diets (7, 16, 19, 30), suggesting trichromatic advantage in the context of frugivory could be widespread. However, while fruits comprise 50–80% of the annual diet for our study population (28, 36), alternative sources of nutrition are essential for capuchins and other primates. In future studies, the relative nutritional contributions of invertebrates and other dietary components, along with the intake of macro- and micronutrients by monkeys with differing color vision phenotypes, should be considered (37). Examination of the ratio of nonprotein energy to protein consumed would also be useful. This ratio is increasingly realized to be an important indicator of health outcomes among primates (e.g., ref. 38).
Our study adds insight to the mechanism of balancing selection that has preserved polymorphic color vision in New World monkeys for >20 My (39). This polymorphism is often described as an early (9) or intermediate stage (40) during the evolution of primate trichromatic vision. These terms risk teleological interpretation and frame the search for a unifying visual target, such as ripe fruit, to account for the different expressions of trichromatic vision across primate lineages. In consequence, polymorphic color vision is often cast as a suboptimal, transitional trait on the path to uniform trichromatic vision. Our results affirm the value of trichromatic vision for discriminating natural fruits, but prior research has found that dichromats have a different foraging advantage: efficient gleaning of invertebrate prey attributed to an improved ability to break camouflage. This ability is hypothesized to contribute to the maintenance of polymorphic color vision (41, 42), and indeed, dichromatic and trichromatic females have similar levels of reproductive success, as measured by birth rates and infant survival rates (43).
This combination of findings is important because it indicates that the enduring preservation of polymorphic color vision cannot be attributed solely to the foraging advantages of trichromatic vision (heterosis), but rather to discrete foraging advantages associated with each visual phenotype. The polymorphic color vision of primates is perhaps best viewed as an alternative stable state that evolved under different ecological pressures than those that favored the evolution of uniform trichromatic vision in howling monkeys, Old World monkeys, and apes (44–48).
Future studies examining niche divergence within species with differing color vision types may reveal that differential foraging ability leads to divergent diets and decreased intragroup competition. A previous study examining the potential for niche divergence did not find large-scale differences in the diets of dichromats and trichromats (29), but a more refined level of examination with a larger sample size may generate new insight. The potential for mutual benefit of association among individuals with different phenotypes is also worthy of investigation (3, 4, 7). If trichromats are better able to locate profitable areas of a fruiting tree, dichromatic monkeys might benefit from foraging near them. Finally, detailed study of allele frequencies, variation within and between species, and social and ecological correlates may generate new insight into the mechanism of natural selection maintaining this intriguing color vision system.
Materials and Methods
Study Site and Subjects.
Data were collected in Sector Santa Rosa in the Área de Conservación, Guanacaste, Costa Rica. Sector Santa Rosa is a seasonal tropical dry forest (49). Annual rainfall is typically between 800 and 2,600 mm, with an average of 1,473 mm, and maximum temperatures vary between 21.6 °C and 34.4 °C (20, 50). Long-term field studies of white-faced capuchins in the park began in 1983 and have been ongoing (51).
White-faced capuchins are medium-sized omnivorous primates. They preferentially consume ripe fruits and have a highly diverse vegetative diet (52). In addition, they forage on invertebrates throughout the year, and seasonal variation in diet is common (20, 36, 53). Capuchins engage in extractive foraging and other complex behaviors, have relatively large brains, and are long-lived with extended periods of juvenile development (54, 55). We observed 72 white-faced capuchins (Cebus capucinus imitator) across four social groups: GN, CP, LV, and EX (Dataset S1). We classified our study subjects into three age classes: mature (≥6 y), large-juvenile (3–5 y), and small-juvenile (1–2 y), and dominance status, categorized as high-, mid, or low-ranking, was determined for individuals who reached 6 y of age by the end of the study (SI Materials and Methods). The color vision genotypes of all monkeys in the study have previously been reported (20). At the time of study, A.D.M. was aware that males would be dichromatic and knew the genotypes of five out of 24 adult females from a previous study (41); however, color vision types were intentionally not revealed to five assistants collecting behavioral data.
Data Collection.
Research was conducted January–May 2007, September 2007–January 2008, and May–September 2008. When a monkey fed on fruit, we recorded a “fruit patch visit” and noted the plant species, diameter at breast height, and phenology, specifically the percentage of canopy coverage and the maturity of each phenophase (leaves, fruits, and flowers), using a five-point scale: 0%, 1–24%, 25–49%, 50–74%, 75–100% (see ref. 52 for detailed methods). Each fruit patch visit was given a unique ID. If a monkey returned on a subsequent day, we assigned a new fruit patch ID, as phenological conditions may have changed. We calculated a ripe fruit index for each fruit patch by multiplying the fruit canopy coverage by the proportion of ripe fruit. Patches without ripe fruit received a score of 0, patches with <25% ripe fruit coverage a score of 1, patches with 25–49% ripe fruit coverage a score of 2, and patches with ≥50% ripe fruit coverage a score of 3. For 23 of the 27 fruit species, we were able to collect sufficient quantities of ripe fruit (>16 g dry mass) for nutritional analyses at Dairy One Forage Lab. Using these data, combined with mean intake rates for each species, we calculated mean energy intake per minute as a measure of nutritive value for each fruit species (Dataset S2). Using spectral reflectance data and color models, we additionally classified fruits into one of three color categories: more conspicuous to trichromats than dichromats (“conspicuous”), similar in color to background leaves for both trichromats and dichromats (“cryptic”), or relative visible to both dichromats and trichromats due to strong luminance contrast (“dark”) (SI Materials and Methods and Dataset S3).
To record behavioral data, we conducted modified focal animal samples (56) ranging in duration from 1 to 5 min, depending on subject visibility, for as many individuals in the fruit patch as possible. We define a foraging bout as the time from when an individual began foraging in the fruit patch until the end of the last foraging state behavior. We recorded all fruit ingest events within this period. We ended the focal if the monkey ceased foraging for more than 30 s, began feeding on a different species, or exited the tree. Focal individuals were selected based on visibility, and we followed a rotation through age-sex classes.
Statistical Analyses.
After exploring several error distributions, we found that our data best complied with the assumptions of a linear mixed model (LMM). We used LMMs to analyze the effect of plant-level and primate-level variables on intake rate (fruits consumed per minute of foraging behavior). Because a lognormal distribution best characterized our intake rate data, we performed a log-transformation before analysis. The models were fit in R version 3.3.2 (57), using the lmer function from the package lme4 (58). Significance was estimated using a Wald χ2 test conducted using the Anova function from the package car (59). Because of the X-linked nature of color vision variation, it was not possible to include both sex and color vision phenotype simultaneously in the model. We therefore initially tested for an effect of sex by including only dichromatic females and males in the analysis (“Sex model,” Table 1). We set as fixed effects categorical variables representing the sex, the age class, and the fruit color conspicuity category, an ordinal variable representing the ripe fruit index, and an interaction between sex and age class. We allowed intercepts in the sex model to vary across random effects of categorical variables representing animal identity, tree species, and a categorical variable representing the fruit patch visit identity of the food tree (i.e., a snapshot of phenological condition of a given tree during a visit by one or more monkeys). All reported P values are based on two-tailed tests.
Sex did not have a significant main effect on fruit intake rate, and was not involved in a significant interaction with age (Table 1); therefore, our “Main model” included color vision type as a fixed effect, and we included data from males and females in the analysis. The main model also included age class, fruit conspicuity, and ripe fruit index as fixed effects, along with relevant interaction terms (Table 1). For the main model, we allowed the slope of the main effect of color vision phenotype to vary across the same random effects as described for the “Sex model,” along with all intercepts. To test for an effect of dominance rank, we ran a LMM of intake rates for all mature individuals, including rank class as a fixed effect along with color vision type, fruit conspicuity, ripe fruit index, and relevant interaction terms (Table 1). Intercepts and the slope of color vision phenotype were again set to vary across random effects of animal identity, tree species, and fruit patch visit identity. Because male and female ranks were assessed relative to other members of the same sex only, we also ran a LMM to test for a dominance effect for males and females separately. We allowed for rank changes across years as necessary for some individuals, reflecting changes in group membership or dominance status (Dataset S1).
To test whether fruits of colors we modeled to be more conspicuous to trichromatic monkeys (“conspicuous fruits,” Dataset S2) were eaten more quickly by trichromats, we conducted planned comparisons of the least-squares means by color vision type for conspicuous fruits, cryptic fruits, and dark fruits. We used the Holm–Bonferroni method to adjust P values for multiple comparisons. All code for LMM models written for this project is available on GitHub (https://github.com/kchiou/capuchin_color_vision).
SI Materials and Methods
Age Classification.
Age categorizations followed ref. 29, and we analyze small juveniles and large juveniles separately, as foraging efficiency changes over the juvenile period (60). White-faced capuchins are typically weaned by 1 y of age, and we excluded infants (<1 y), as they do not forage independently.
Dominance Hierarchies.
Dominance hierarchies were determined for mature individuals, 6 y of age and older, as part of the long-term social data collected collaboratively for capuchin monkeys in Sector Santa Rosa. We used all recorded instances of agonistic behavior, which includes dominance followed by submission, or formalized submission only, collected during 10-min focal animal samples in conjunction with data collected ad libitum to construct dominance hierarchies using the I&SI method in MatMan 1.1 (61). Males and females are ranked in separate hierarchies based on the outcomes of dyadic interactions that include contact and noncontact aggression, supplants, cowers, avoid, grimace, and flee behaviors (see ref. 62 for detailed methods). Dominance hierarchies were based on a dataset that was collected in the months before, between, and after, but not during, the periods of fieldwork in which the foraging data analyzed here were collected. Dominance relationships were studied separately for males and females, and thus we could not create a sex-integrated hierarchy. For groups with six or more individuals in the hierarchy, that is, females in GN and CP groups), we could assess linearity of hierarchies and found hierarchies to be significantly linear using Landau’s linearity index (h′). For groups EX and LV, there were fewer than six females included in the analysis, a sample size too low to determine significance using this test. However, we are confident in our rank categorizations because of the very high level of directional consistency in dominance interactions between females. As in other studies of wild capuchins (55, 63, 64), we were unable to determine whether male dominance was significantly linear below the alpha level because of low numbers of males and the rarity of agonistic interactions between male dyads.
A dominance rank of “3” in a small social group is very different from the same linear rank in a large group, and because capuchin hierarchies are somewhat relaxed versus strictly despotic, we assigned individuals to high-, mid-, and low-ranking dominance classes within each social group, and this variable was included in the linear mixed model. For males, the alpha male alone was listed as high ranking, as sharp definitions between alpha and subalpha occur among males in this species (64). Midranking males were the one to two males ranking below the alpha (depending on group size), and low-ranking males included the one to three lowest ranking males of the group. Among females, individuals were divided into high-, mid-, and low-ranking groups so that, so far as possible, each dominance class had a similar sample size within each social group. In borderline cases, females were placed in same rank class as other members of their matriline, as kinship plays a role in capuchin dominance (62).
Fruit Nutritional Analyses.
Fruit nutritional data (Dataset S2) for capuchin foods were measured in Sector Santa Rosa between 2009 and 2011 (53). Tens to hundreds of fruits were collected from multiple trees during the period in which each species was eaten (typically 1 mo). The parts of fruits consumed by the monkeys were separated from nonconsumed portions and dried at 30 °C in a commercial food dehydrator (Model FD-1020; Nesco American Harvest Gardenmaster Pro). A minimum of 16 g dried fruit matter was required for each species, and samples were shipped to Dairy One Forage Laboratory, New York, where the macronutrient composition (e.g., crude protein, crude fat, water-soluble carbohydrates, neutral detergent fiber, total ash, and organic matter) of each species was measured. For the fruits of plant species included in our behavioral analysis, we report the estimated energy (kJ) per gram dry mass, calculated as follows: Energy (kL) = [16.74 × (CP + WSC)] + (37.66 × CF), where CP is the proportion of dry mass as crude protein, WSC is the proportion of dry mass as water-soluble carbohydrates, and CF is the proportion of dry mass as crude fat. Neutral detergent fiber was not included in calculated energy uptake, as it is unlikely capuchins are able to extract significant amounts of energy from difficult-to-digest plant materials such as fiber, given their fast gut passage times (65). To calculate the mean energy intake rate, based on estimated dry matter consumed per minute, we used the values published in ref. 53, as this study recorded individual bites of fruits, whereas the present study recorded eat/reject dichotomously, with an “eat” being scored if the whole fruit was consumed or partially consumed (>2 bites). The former method therefore resulted in a more accurate estimate for large fruits, which are typically not consumed in entirety by capuchins. However, for the three species of Ficus trees producing small red figs (F. cotinifolia, F. ovalis, and F. hondurensis), as well as Allophylus occidentalis, Jacquinia nervosa, and Randia thurberi, we used feeding rates calculated in the present study, as ref. 53 did not differentiate feeding rates among the Ficus species nor have feeding rate data for the other three plants. For smaller fruits, the feeding rates between the studies were very similar (e.g., mean Ficus rate from ref. 53: 19.17 fruits/min; our study: F. cotinifolia, 16.76; F. ovalis, 21.97; and F. hondurensis, 28.05 fruits/min), and we do not anticipate that using our values for these species introduced bias.
Color Vision Models and Assigning Color Categories to Fruits.
Spectral reflectance data from ripe fruits were collected on-site in Sector Santa Rosa, using a portable spectrophotometer (USB4000; Ocean Optics Inc.) and LS-1 light source. The spectrometer was calibrated before data collection, using a white reflectance standard (WS-1-SL). We took five measurements per surface per item to calculate a mean reflectance value for each plant part for each species. Irradiance data used in these models were recorded under forest shade and blue sky in Sector Santa Rosa, using the portable spectrometer and an irradiance probe fitted with a cosine corrector (Ocean Optics Inc.). For fruit species consumed in the late dry season (March–early May), we modeled conspicuity under a blue sky illuminant, as the dry forest lacks a canopy of green leaves at this time. For species consumed in the wet season and early dry season, we modeled conspicuity under a forest shade illuminant.
To predict whether ripe fruits were more conspicuous from background leaves to trichromats than to dichromats, we conducted a “just-noticeable-difference” (JND) analysis, following the bright daylight version of this model (19). The JND calculation models chromatic differences in the color space of different phenotypes, accounting for sources of neural noise. We interpret a difference of 1 JND or greater between any two phenotypes to indicate a significant perceptual difference that could translate into a sensory advantage. The value of 1 JND is based on data from humans in laboratory conditions (66), and an assumption of our study is that this value is also meaningful for other primates with highly acute color vision (44). All analyses were carried out in MATLAB R2015. We performed separate JND calculations for the six possible capuchin phenotypes: three trichromatic and three dichromatic. To be conservative, we considered the ripe fruit of a plant species to be more “conspicuous” to trichromats if at least one trichromatic phenotype had a modeled advantage over a dichromatic phenotype (Dataset S3). The fruits of three species, Karwinskia calderoni, Sciadodendron excelsum, and Simarouba glauca, turn deep purple/nearly black when they are ripe. These fruits were categorized as “dark,” and dark fruits of these species have previously been shown to be highly visible to both dichromatic and trichromatic phenotypes (31). We considered fruits that were similar in color to background leaves, and for which JND values calculated for trichromats and dichromats were less than 1 JND apart, to be chromatically “cryptic” to both trichromats and dichromats (Dataset S3).
Supplementary Material
Acknowledgments
We thank R. Blanco Segura and M. M. Chavarria and staff from the Área de Conservación Guanacaste and Ministerio de Ambiente y Energía. Warmest thanks also to A. Guadamuz, A. Blauel, J. Hogan, B. Klug, M. Lemmon, M. Myers, N. Parr, and L. Weckman. We thank V. Schoof for data on male dominance hierarchies. Funding was provided by the Wenner-Gren Foundation, the Leakey Foundation, and the National Sciences and Engineering Council of Canada (A.D.M.), a Sigma Xi grant-in-aid of research (to E.R.W.), the Japan Society for the Promotion of Science 15H02421 (S.K.), and the Canada Research Chairs Program and an National Sciences and Engineering Council of Canada Discovery grant (to A.D.M. and L.M.F.). This research adhered to the laws of Costa Rica, the United States, and Canada and complied with protocols approved by the Área de Conservación Guanacaste and by the Canada Research Council for Animal Care through the University of Calgary’s Life and Environmental Care Committee.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.J.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705957114/-/DCSupplemental.
References
- 1.Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364:2957–2967. doi: 10.1098/rstb.2009.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiwatashi T, et al. An explicit signature of balancing selection for color-vision variation in new world monkeys. Mol Biol Evol. 2010;27:453–464. doi: 10.1093/molbev/msp262. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura S, et al. Polymorphic color vision in primates: Evolutionary considerations. In: Hirai H, Imai H, Go Y, editors. Post-Genome Biology of Primates. Springer Japan; Tokyo: 2012. pp. 93–120. [Google Scholar]
- 4.Mollon JD, Bowmaker JK, Jacobs GH. Variations of colour vision in a new world primate can be explained by polymorphism of retinal photopigments. Proc R Soc Lond B Biol Sci. 1984;222:373–399. doi: 10.1098/rspb.1984.0071. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs RL, Bradley BJ. Considering the influence of nonadaptive evolution on primate color vision. PLoS One. 2016;11:e0149664. doi: 10.1371/journal.pone.0149664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surridge AK, Osorio D, Mundy NI. Evolution and selection of trichromatic vision in primates. Trends Ecol Evol. 2003;18:198–205. [Google Scholar]
- 7.Veilleux CC, et al. Group benefit associated with polymorphic trichromacy in a Malagasy primate (Propithecus verreauxi) Sci Rep. 2016;6:38418. doi: 10.1038/srep38418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen G. The Colour-Sense: Its Origin and Development: An Essay in Comparative Psychology. Trübner; London: 1879. [Google Scholar]
- 9.Mollon JD. “Tho’ she kneel’d in that place where they grew...” The uses and origins of primate colour vision. J Exp Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Osorio D, Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proc Biol Sci. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- 11.Regan BC, et al. Frugivory and colour vision in Alouatta seniculus, a trichromatic platyrrhine monkey. Vision Res. 1998;38:3321–3327. doi: 10.1016/s0042-6989(97)00462-8. [DOI] [PubMed] [Google Scholar]
- 12.Sumner P, Mollon JD. Catarrhine photopigments are optimized for detecting targets against a foliage background. J Exp Biol. 2000;203:1963–1986. doi: 10.1242/jeb.203.13.1963. [DOI] [PubMed] [Google Scholar]
- 13.Sussman RW, Tab Rasmussen D, Raven PH. Rethinking primate origins again. Am J Primatol. 2013;75:95–106. doi: 10.1002/ajp.22096. [DOI] [PubMed] [Google Scholar]
- 14.Caine NG, Mundy NI. Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proc Biol Sci. 2000;267:439–444. doi: 10.1098/rspb.2000.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AC, Buchanan-Smith HM, Surridge AK, Osorio D, Mundy NI. The effect of colour vision status on the detection and selection of fruits by tamarins (Saguinus spp.) J Exp Biol. 2003;206:3159–3165. doi: 10.1242/jeb.00536. [DOI] [PubMed] [Google Scholar]
- 16.Vogel ER, Neitz M, Dominy NJ. Effect of color vision phenotype on the foraging of wild white-faced capuchins, Cebus capucinus. Behav Ecol. 2007;18:292–297. [Google Scholar]
- 17.Hiramatsu C, et al. Importance of achromatic contrast in short-range fruit foraging of primates. PLoS One. 2008;3:e3356. doi: 10.1371/journal.pone.0003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melin AD, et al. Fig foraging by dichromatic and trichromatic Cebus capucinus in a tropical dry forest. Int J Primatol. 2009;30:753–775. [Google Scholar]
- 19.Osorio D, Smith AC, Vorobyev M, Buchanan‐Smith HM. Detection of fruit and the selection of primate visual pigments for color vision. Am Nat. 2004;164:696–708. doi: 10.1086/425332. [DOI] [PubMed] [Google Scholar]
- 20.Melin AD, Young HC, Mosdossy KN, Fedigan LM. Seasonality, extractive foraging and the evolution of primate sensorimotor intelligence. J Hum Evol. 2014;71:77–86. doi: 10.1016/j.jhevol.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Rose LM. Sex differences in diet and foraging behavior in white-faced capuchins (Cebus capucinus) Int J Primatol. 1994;15:95–114. [Google Scholar]
- 22.Fragaszy DM. Time budgets and foraging behavior in wedge-capped capuchins (Cebus olivaceus): Age and sex differences. In: Taub DM, King FA, editors. Current Perspectives in Primate Social Dynamics. Van Nostrand Reinhold; New York: 1986. pp. 159–174. [Google Scholar]
- 23.Fragaszy DM, Boinski S. Patterns of individual diet choice and efficiency of foraging in wedge-capped capuchin monkeys (Cebus olivaceus) J Comp Psychol. 1995;109:339–348. doi: 10.1037/0735-7036.109.4.339. [DOI] [PubMed] [Google Scholar]
- 24.Fragaszy DM. Sex and age differences in the organization of behavior in wedge-capped capuchins, Cebus olivaceus. Behav Ecol. 1990;1:81–94. [Google Scholar]
- 25.Agostini I, Visalberghi E. Social influences on the acquisition of sex-typical foraging patterns by juveniles in a group of wild tufted capuchin monkeys (Cebus nigritus) Am J Primatol. 2005;65:335–351. doi: 10.1002/ajp.20120. [DOI] [PubMed] [Google Scholar]
- 26.Levey DJ. Seed size and fruit-handling techniques of avian frugivores. Am Nat. 1987;129:471–485. [Google Scholar]
- 27.Vogel ER. Rank differences in energy intake rates in white-faced capuchin monkeys, Cebus capucinus: The effects of contest competition. Behav Ecol Sociobiol. 2005;58:333–344. [Google Scholar]
- 28.Bergstrom ML, Emery Thompson M, Melin AD, Fedigan LM. Using urinary parameters to estimate seasonal variation in the physical condition of female white-faced capuchin monkeys (Cebus capucinus imitator) Am J Phys Anthropol. 2017;163:707–715. doi: 10.1002/ajpa.23239. [DOI] [PubMed] [Google Scholar]
- 29.Melin AD, Fedigan LM, Hiramatsu C, Kawamura S. Polymorphic color vision in white-faced capuchins (Cebus capucinus): Is there foraging niche divergence among phenotypes? Behav Ecol Sociobiol. 2008;62:659–670. [Google Scholar]
- 30.Bunce JA, Isbell LA, Grote MN, Jacobs GH. Color vision variation and foraging behavior in wild neotropical titi monkeys (Callicebus brunneus): Possible mediating roles for spatial memory and reproductive status. Int J Primatol. 2011;32:1058–1075. [Google Scholar]
- 31.Melin AD, Kline DW, Hickey CM, Fedigan LM. Food search through the eyes of a monkey: A functional substitution approach for assessing the ecology of primate color vision. Vision Res. 2013;86:87–96. doi: 10.1016/j.visres.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Vogel ER, Janson CH. Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. Am J Primatol. 2007;69:533–550. doi: 10.1002/ajp.20368. [DOI] [PubMed] [Google Scholar]
- 33.Hall CL, Fedigan LM. Spatial benefits afforded by high rank in white-faced capuchins. Anim Behav. 1997;53:1069–1082. [Google Scholar]
- 34.Chancellor RL, Isbell LA. Food site residence time and female competitive relationships in wild gray-cheeked mangabeys (Lophocebus albigena) Behav Ecol Sociobiol. 2009;63:1447–1458. doi: 10.1007/s00265-009-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janson C. Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella) Behav Ecol Sociobiol. 1985;18:125–138. [Google Scholar]
- 36.Mosdossy KN, Melin AD, Fedigan LM. Quantifying seasonal fallback on invertebrates, pith, and bromeliad leaves by white-faced capuchin monkeys (Cebus capucinus) in a tropical dry forest. Am J Phys Anthropol. 2015;158:67–77. doi: 10.1002/ajpa.22767. [DOI] [PubMed] [Google Scholar]
- 37.Raubenheimer D, Rothman JM. Nutritional ecology of entomophagy in humans and other primates. Annu Rev Entomol. 2013;58:141–160. doi: 10.1146/annurev-ento-120710-100713. [DOI] [PubMed] [Google Scholar]
- 38.Rothman JM, Raubenheimer D, Chapman CA. Nutritional geometry: Gorillas prioritize non-protein energy while consuming surplus protein. Biol Lett. 2011;7:847–849. doi: 10.1098/rsbl.2011.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boissinot S, et al. Origins and antiquity of X-linked triallelic color vision systems in new world monkeys. Proc Natl Acad Sci USA. 1998;95:13749–13754. doi: 10.1073/pnas.95.23.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin RD, Ross CF. The Primate Visual System. Wiley; New York: 2005. The evolutionary and ecological context of primate vision; pp. 1–36. [Google Scholar]
- 41.Melin AD, Fedigan LM, Hiramatsu C, Sendall CL, Kawamura S. Effects of colour vision phenotype on insect capture by a free-ranging population of white-faced capuchins, Cebus capucinus. Anim Behav. 2007;73:205–214. [Google Scholar]
- 42.Melin AD, Fedigan LM, Young HC, Kawamura S. Can color vision variation explain sex differences in invertebrate foraging by capuchin monkeys? Curr Zool. 2010;56:300–312. [Google Scholar]
- 43.Fedigan LM, Melin AD, Addicott JF, Kawamura S. The heterozygote superiority hypothesis for polymorphic color vision is not supported by long-term fitness data from wild neotropical monkeys. PLoS One. 2014;9:e84872. doi: 10.1371/journal.pone.0084872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melin AD, et al. Howler monkey foraging ecology suggests convergent evolution of routine trichromacy as an adaptation for folivory. Ecol Evol. 2017;7:1421–1434. doi: 10.1002/ece3.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- 46.Lucas PW, et al. Evolution and function of routine trichromatic vision in primates. Evolution. 2003;57:2636–2643. doi: 10.1111/j.0014-3820.2003.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 47.Dominy NJ, Svenning J-C, Li W-H. Historical contingency in the evolution of primate color vision. J Hum Evol. 2003;44:25–45. doi: 10.1016/s0047-2484(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 48.Hernández Salazar LT, Dominy NJ, Laska M. The sensory systems of Alouatta: Evolution with an eye to ecology. In: Kowalewski MM, Garber PA, Cortés-Ortiz L, Urbani B, Youlatos D, editors. Howler Monkeys, Developments in Primatology: Progress and Prospects. Springer; New York: 2015. pp. 317–336. [Google Scholar]
- 49.Janzen DH. Costa Rican Natural History. University of Chicago Press; Chicago: 1983. [Google Scholar]
- 50.Fedigan LM, Jack K. Neotropical primates in a regenerating Costa Rican dry forest: A comparison of howler and capuchin population patterns. Int J Primatol. 2001;22:689–713. [Google Scholar]
- 51.Fedigan LM, Jack KM. Tracking neotropical monkeys in Santa Rosa: Lessons from a Costa Rican dry forest. In: Kappeler PM, Watts DP, editors. Long-Term Field Studies of Primates. Springer; Berlin: 2012. pp. 165–184. [Google Scholar]
- 52.Melin AD, et al. The behavioral ecology of color vision: Considering fruit conspicuity, detection distance and dietary importance. Int J Primatol. 2014;35:258–287. [Google Scholar]
- 53.Bergstrom ML. 2015. Seasonal effects on the nutrition and energetic condition of female white-faced capuchin monkeys. PhD dissertation (Univ of Calgary, Calgary, Alberta, Canada)
- 54.Rilling JK, Insel TR. Evolution of the cerebellum in primates: Differences in relative volume among monkeys, apes and humans. Brain Behav Evol. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- 55.Fragaszy DM, Visalberghi E, Fedigan LM. The Complete Capuchin: The Biology of the Genus Cebus. Cambridge Univ Press; Cambridge, UK: 2004. [Google Scholar]
- 56.Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 57.R Core Team 2017 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org. Accessed August 7, 2017.
- 58.Bates D, et al. 2016 lme4: Linear Mixed-Effects Models Using “Eigen” and S4. Available at https://cran.r-project.org/web/packages/lme4/index.html. Accessed July 1, 2016.
- 59.Fox J, et al. 2015 car: Companion to Applied Regression. Available at https://cran.r-project.org/web/packages/car/index.html. Accessed July 1, 2016.
- 60.Eadie EC. Ontogeny of foraging competence in capuchin monkeys (Cebus capucinus) for easy versus difficult to acquire fruits: A test of the needing to learn hypothesis. PLoS One. 2015;10:e0138001. doi: 10.1371/journal.pone.0138001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Vries H. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim Behav. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- 62.Bergstrom ML, Fedigan LM. Dominance style of female white-faced capuchins. Am J Phys Anthropol. 2013;150:591–601. doi: 10.1002/ajpa.22231. [DOI] [PubMed] [Google Scholar]
- 63.Perry S. Male-male social relationships in wild white-faced capuchins, Cebus capucinus. Behaviour. 1998;135:139–172. [Google Scholar]
- 64.Schoof VAM, Jack KM. The association of intergroup encounters, dominance status, and fecal androgen and glucocorticoid profiles in wild male white-faced capuchins (Cebus capucinus) Am J Primatol. 2013;75:107–115. doi: 10.1002/ajp.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valenta K, Fedigan LM. How much is a lot? Seed dispersal by white-faced capuchins and implications for disperser-based studies of seed dispersal systems. Primates. 2008;49:169–175. doi: 10.1007/s10329-008-0087-0. [DOI] [PubMed] [Google Scholar]
- 66.Sperling HG, Harwerth RS. Red-green cone interactions in the increment-threshold spectral sensitivity of primates. Science. 1971;172:180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.