ABSTRACT
Objectives:
The aim of this study was to assess growth and nutritional biomarkers of preterm infants fed human milk (HM) supplemented with a new powdered HM fortifier (nHMF) or a control HM fortifier (cHMF). The nHMF provides similar energy content, 16% more protein (partially hydrolyzed whey), and higher micronutrient levels than the cHMF, along with medium-chain triglycerides and docosahexaenoic acid.
Methods:
In this controlled, multicenter, double-blind study, a sample of preterm infants ≤32 weeks or ≤1500 g were randomized to receive nHMF (n = 77) or cHMF (n = 76) for a minimum of 21 days. Weight gain was evaluated for noninferiority (margin = –1 g/day) and superiority (margin = 0 g/day). Nutritional status and gut inflammation were assessed by blood, urine, and fecal biochemistries. Adverse events were monitored.
Results:
Adjusted mean weight gain (analysis of covariance) was 2.3 g/day greater in nHMF versus cHMF; the lower limit of the 95% CI (0.4 g/day) exceeded both noninferiority (P < 0.001) and superiority margins (P = 0.01). Weight gain rate (unadjusted) was 18.3 (nHMF) and 16.8 g · kg−1 · day−1 (cHMF) between study days 1 and 21 (D1–D21). Length and head circumference (HC) gains between D1 and D21 were not different. Adjusted weight-for-age z score at D21 and HC-for-age z score at week 40 corrected age were greater in nHMF versus cHMF (P = 0.013, P = 0.003 respectively). nHMF had higher serum blood urea nitrogen, pre-albumin, alkaline phosphatase, and calcium (all within normal ranges; all P ≤ 0.019) at D21 versus cHMF. Both HMFs were well tolerated with similar incidence of gastrointestinal adverse events.
Conclusions:
nHMF providing more protein and fat compared to a control fortifier is safe, well-tolerated, and improves the weight gain of preterm infants.
Keywords: growth, human milk, low birth weight
What Is Known
Due in part to variability in human milk composition, incidence of postnatal growth restriction is more frequently reported in very-low-birth-weight infants fed fortified human milk compared to those fed preterm formulas.
The optimal composition of human milk fortifier and nutritional recommendations for preterm infants fed fortified human milk are still debated.
What Is New
A new human milk fortifier containing partially hydrolyzed protein, fat, and carbohydrate provides a higher protein:energy ratio while achieving lower osmolality versus a current fortifier.
In preterm infants, the new fortifier improves weight gain and reduces postnatal growth restriction compared to the current fortifier.
Feeding of human milk (HM) rather than preterm formulas provides many benefits to preterm infants (eg, accelerated gut maturation (1); protection against infections (2), sepsis (3), necrotizing enterocolitis (2), and retinopathy of prematurity (4); possible protective effect on neurodevelopment (5)) that are mediated by protective biomolecules and trophic factors in HM. HM, however, provides inadequate protein and micronutrients to support the rapid growth and bone mineralization of preterm infants. These deficits are particularly acute in the smallest infants (birthweight <1500 g) who have the highest protein and mineral needs (6). Fortification of mother's own milk or banked HM is therefore recommended for all preterm infants with birthweight <1800 g to improve nutrient accretion and in-hospital growth (7,8).
Feeding fortified HM helps support adequate growth and bone mineralization (9), and is associated with favorable neurodevelopmental outcomes (10), although evidence for improved outcomes other than in-hospital growth is limited (11). The nutritional content, however, of some currently available fortifiers may be inadequate for many preterm infants. Incidence of postnatal growth restriction is more frequently reported in very-low-birth-weight infants fed fortified HM compared to those fed preterm formulas (12,13). In addition, the nutritional profile of HM from mothers of premature infants varies greatly (14) and may differ from published reference compositional data, which may lead to less-than-recommended intakes of protein and energy (15,16). These nutritional inadequacies may worsen with use of donor HM, which is often from mothers of term infants >1-month postpartum (17).
A new powdered HM fortifier has been developed with a higher protein:energy ratio (protein provided as partially hydrolyzed whey), non-protein energy from lipids and carbohydrate, and higher electrolyte and vitamin levels (enriching HM in line with ESPGHAN (18) and expert group (19) recommendations) versus a control fortifier. When mixed with HM containing 1.5 g protein/100 mL (2–4 week milk) (20–22), it provides 3.6 g protein/100 kcal (within the ESPGHAN-recommended ranges (18) for protein and energy intakes for a minimal intake volume of 140 mL/kg/day in very-low-birth-weight infants up to 1.8 kg body weight), with osmolality below the recommended threshold of 450 mOsm/kg (23,24).
This study evaluated growth and nutritional biomarkers during a 21-day interval in clinically stable preterm infants receiving the new HM fortifier (nHMF) compared to infants fed a control fortifier (cHMF). The primary objective was to assess weight gain velocity (grams per day); evaluations of other growth parameters (including weight gain velocity in gram per kilograms per day) and intervals (eg, to 40 weeks corrected age [W40CA]), feeding tolerance, adverse events, time to full fortification/full enteral feeding, and markers of protein-energy, electrolytes, bone metabolic status, gut inflammation, and maturity of gastrointestinal (GI) function were also conducted as secondary outcomes. We hypothesized that weight gain of infants fed nHMF would be both noninferior (lower limit of 95% confidence interval [CI] of mean difference >–1 g/day) and superior (lower limit of 95% CI of mean difference >0 g/day) to that of infants fed cHMF.
METHODS
Study design and participants
This was a controlled, double-blind, randomized, parallel-group study conducted in neonatal intensive care units (NICUs) at 11 metropolitan hospitals in France, Belgium, Germany, Switzerland, and Italy. NICU size ranged from 25 to 45 beds. Clinically stable male and female preterm infants with gestational age ≤32 weeks or birthweight ≤1500 g and born to mothers who had agreed to provide expressed or donor breastmilk for the entire 21-day study duration were enrolled in the study from April 2011 to March 2014. Infants were excluded if they had a history of or current systemic, metabolic, or chromosomic disease, any congenital anomalies of the GI tract, were small for gestational age (defined in this study as bodyweight ≤5th percentile (25)), or were receiving steroids or preterm formula during the study period. For multiple births, the first sibling was randomized and other siblings were allocated to the same group. The study was reviewed and approved by an institutional review board/independent Ethics Committee at each study site. Each subject's parent/legal representative provided written informed consent before participating in the study.
Infants tolerating ≥100 mL · kg−1 · day−1 of HM for >24 hours were randomized to receive either nHMF or cHMF for a minimum of 21 days; infants continued to receive their allocated study fortifier (or were transitioned to a routine/standard fortifier) until NICU discharge or medical decision to stop fortification, and fortification was stopped after discharge. The fortifiers were both cow's milk-based and provided similar energy supplementation (17 kcal/100 mL of HM). For every 100 mL of HM, nHMF provided 1.4 g partially hydrolyzed whey protein, 0.7 g lipids (primarily medium chain triglycerides and docosahexaenoic acid), 1.3 g carbohydrate (maltodextrin), with a blend of micronutrients. cHMF (FM85 Human Milk Fortifier, Nestlé, Switzerland) provided 1.0 g extensively hydrolyzed whey protein, no lipids, 3.3 g carbohydrate (lactose and maltodextrin), with a blend of micronutrients. nHMF contained higher concentrations of some vitamins and electrolytes compared to cHMF, but both contained similar levels of minerals, including calcium (as calcium glycerophosphate and calcium phosphate) and phosphorus. Table 1 presents the estimated composition and osmolality of preterm HM (22) fortified with each fortifier. Fortifiers were fed beginning at half-strength (Fortification Strength Increase day 1; FSI1), then advanced per hospital practice, with full-strength fortification occurring once infants could maintain intakes of 150 to 180 mL · kg−1 · day−1 (ie, full enteral feeds; study day 1 [D1]). A study plan schematic is presented in Figure 1.
TABLE 1.
Calculated∗ nutrient composition of fortified preterm human milk
| Preterm HM + nHMF | Preterm HM + cHMF | ||||||
| 4 g fortifier alone | 4 g fortifier per 100 kcal milk | 4 g fortifier per 100 mL milk | 5 g fortifier alone | 5 g fortifier per 100 kcal milk | 5 g fortifier per 100 mL milk | Recommended intake range (per 100 kcal)† | |
| Nutrient | |||||||
| Energy, kcal | 17.4 | 100 | 84.6 | 17.4 | 100 | 84.5 | |
| Protein, g | 1.42 | 3.6 | 3.04 | 1.0 | 3.10 | 2.62 | 3.2–4.1 |
| Protein source | Partially hydrolyzed whey | Extensively hydrolyzed whey | |||||
| Fat, g | 0.72 | 5.00 | 4.23 | 0.02 | 4.16 | 3.52 | 4.4–6 |
| MCT, g | 0.50 | 0.59 | 0.50 | 0 | 0 | 0 | |
| DHA, mg | 6.3 | 19.3 | 16.3 | 0 | 11.8 | 10.0 | (16.4–) 50–55 |
| Carbohydrate, g | 1.30 | 10.17 | 8.60 | 3.30 | 12.53 | 10.60 | 10.5–12 |
| Carbohydrate source | Maltodextrin | Lactose and maltodextrin | |||||
| Calcium, mg | 76 | 119 | 101 | 75 | 118 | 100 | 109–182 |
| Phosphorus, mg | 44 | 69 | 58 | 45 | 70 | 59 | 55–127 |
| Magnesium, mg | 4.0 | 8.6 | 7.3 | 2.4 | 6.7 | 5.7 | 7.3–13.6 |
| Sodium, mg | 36.7 | 76.5 | 64.7 | 20.0 | 56.8 | 48.0 | 63–105 |
| Potassium, mg | 48.4 | 116.4 | 98.4 | 42.0 | 108.8 | 92.0 | 71–177 |
| Chloride, mg | 32.1 | 106.6 | 90.1 | 17.0 | 88.7 | 75.0 | 95–161 |
| Iron, mg | 1.80 | 2.23 | 1.89 | 1.30 | 1.64 | 1.39 | 1.8–2.7 |
| Zinc, mg | 0.94 | 1.55 | 1.31 | 0.80 | 1.38 | 1.17 | 1.3–2.3 |
| Manganese, μg | 8.08 | 9.98 | 8.44 | 5.00 | 6.34 | 5.36 | 0.9–13.6 |
| Copper, mg | 0.05 | 0.11 | 0.09 | 0.04 | 0.09 | 0.08 | 0.09–0.21 |
| Iodine, μg | 16.9 | 36.6 | 30.9 | 15.0 | 34.3 | 29.0 | 9–50 |
| Selenium, μg | 3.7 | 7.2 | 6.1 | 1.5 | 4.6 | 3.9 | 4.5–9 |
| Vitamin A, IU | 1183 | 1754 | 1483 | 500 | 946 | 800 | 1217–3333 |
| Vitamin D, IU | 150 | 187 | 158 | 100 | 128 | 108 | 100–350 |
| Vitamin E, IU | 4.4 | 5.6 | 4.7 | 2.2 | 3.0 | 2.5 | 2.2–11.1 |
| Vitamin K, μg | 8.0 | 9.8 | 8.3 | 4.0 | 5.1 | 4.3 | 4–25 |
| Thiamin, mg | 0.15 | 0.19 | 0.16 | 0.05 | 0.07 | 0.06 | 0.13–0.27 |
| Riboflavin, mg | 0.20 | 0.27 | 0.23 | 0.10 | 0.15 | 0.13 | 0.18–0.36 |
| Vitamin B6, mg | 0.13 | 0.16 | 0.14 | 0.05 | 0.07 | 0.06 | 0.05–0.27 |
| Vitamin B12, μg | 0.20 | 0.26 | 0.22 | 0.10 | 0.14 | 0.12 | 0.09–0.73 |
| Niacin, mg | 1.50 | 2.02 | 1.71 | 0.80 | 1.19 | 1.01 | 0.9–5 |
| Folic acid, μg | 40.0 | 51.0 | 43.1 | 40.0 | 51.0 | 43.1 | 32–91 |
| Pantothenic acid, mg | 0.70 | 1.10 | 0.93 | 0.40 | 0.74 | 0.63 | 0.45–1.9 |
| Biotin, μg | 3.50 | 4.78 | 4.04 | 3.00 | 4.19 | 3.54 | 1.5–15 |
| Vitamin C, mg | 20.0 | 28.9 | 24.4 | 10.0 | 17.0 | 14.4 | 18–50 |
| Osmolality‡, mOsm/kg | 390 | 441 | |||||
cHMF = control human milk fortifier; DHA = docosahexaenoic acid; HM = human milk; nHMF = new human milk fortifier; MCT = medium chain triglycerides.
*Calculated based on preterm human milk composition from Tsang et al, 2005 (22).
†Recommended nutrient intakes for fully enterally fed preterm very low birth weight infants (19).
‡Measured immediately after fortification at room temperature (25°C).
FIGURE 1.
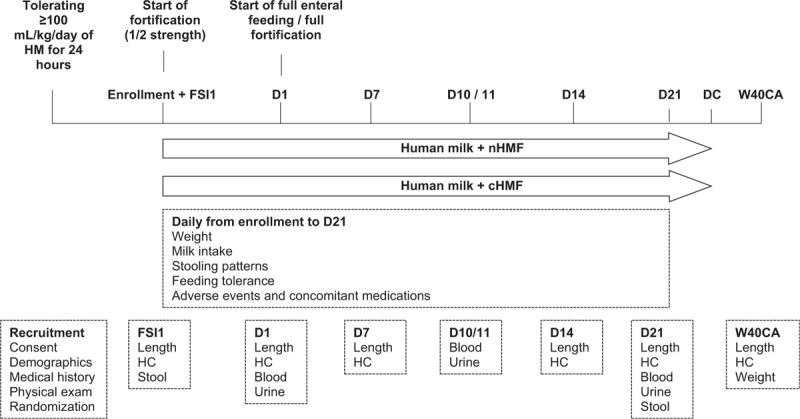
Study design. cHMF = control human milk fortifier; D1 = study day 1; D7 = study day 7; D10/11 = study day 10/11; D14 = study day 14; D21 = study day 21; DC = discharge (note that infants continued to receive their allocated study fortifier [or were transitioned to a routine/standard fortifier] until neonatal unit discharge or medical decision to stop fortification if length of stay was >21 days, and fortification was stopped after discharge) ; FSI1 = fortification strength increase day 1; HC = head circumference; HM = human milk; nHMF = new human milk fortifier; W40CA = week 40 corrected age.
Study Procedures
Growth
Infant nude weight (to the nearest 1 g) was measured daily by trained nursery personnel using a calibrated electronic scale (Baby Scale 717, Seca, Semur-en-Auxois, France). Recumbent length and head circumference (HC; both to the nearest 0.1 cm) were measured at FSI1, D1, and weekly thereafter. At least 2 trained examiners measured recumbent length using a length board (Mobile Measuring Board 417, Seca, Semur-en-Auxois, France) while maintaining proper body alignment and full body extension with feet flexed. HC was measured using a nonelastic measuring tape (Measuring Tape 212 or 218, Seca, Semur-en-Auxois, France) placed over the largest circumference of the skull (above the supraorbital ridges while covering the most prominent part of the frontal bulge anteriorly). The same calibrated equipment was used for anthropometric measures for each infant at all sites. Weight-for-age, length-for-age, and HC-for-age z scores were calculated using Fenton (25). Weight gain velocity (grams per kilograms per day) was calculated using the average of the start and end weights as the denominator.
Markers of Protein-energy, Electrolyte, and Bone Metabolic Status
Blood and urine samples were collected at D1, D10/11, and D21 and analyzed for serum creatinine and prealbumin, blood urea nitrogen (BUN), urinary urea, hemoglobin, hematocrit, electrolyte status, and bone metabolic status. All blood and urine parameters were analyzed as part of routine clinical assessments at each NICU. Since 24-hour urine collections were not performed in this study owing to logistical infeasibility, urinary markers were corrected for 24-hour creatinine excretion (26) assuming a standard urinary excretion in preterm infants of 10 mg · kg−1 · day−1(27).
Feeding Tolerance and Adverse Events
Feeding tolerance was evaluated by trained nursery staff who recorded daily milk intake (milliliters), stool pattern (defecation frequency and stool consistency [5 = hard, 4 = formed, 3 = soft, 2 = liquid, or 1 = watery]), presence of abdominal distention, and incidence of spitting-up (defined as return of a small amount of swallowed food, usually a mouthful, and usually occurring during or shortly after feeding) and vomiting (defined as return of a larger amount of food with more complete emptying of the stomach, and usually occurring sometime after feeding). In addition, frequency, type, and attribution to fortifier intake of adverse events (AEs; including clinical and laboratory) were evaluated using physician-reported information recorded using standardized forms from enrollment to W40CA. AEs were categorized by the reporting investigator as “serious” in accordance with International Conference on Harmonization criteria (28) and as “related to the intervention” based on detailed, standardized criteria provided in the protocol.
Statistical Analysis
Sample size was based on a previous study (29), which investigated growth and zinc status in preterm infants fed fortified HM. In the present trial, a group-sequential design was chosen (Wang and Tsiatis) (30) with 1 interim analysis. To detect a noninferior weight gain in infants fed with nHMF versus cHMF from D1 to D21 (noninferiority margin –1 g/day, expected weight gain difference 2 g/day, standard deviation 4.73 g/day, type I error 5%, power 80%) (29), 192 subjects (males and females combined) were needed. A computer-generated list of random numbers was used to allocate group assignments. Minimization algorithm with allocation ratio 1:1 and second best probability of 15% was used. Stratification factors were center, sex, and birthweight (100g intervals). Group coding was used with 2 nonspeaking codes per group; fortifier packaging was coded accordingly but otherwise identical in appearance. Infants were enrolled and assigned to their intervention by the study investigators or trained delegates. All study personnel (both site- and sponsor-based) and participants (infants’ families) were blind to group assignment. Noninferiority was demonstrated if the lower limit of the 2-sided 95% CI of the difference in weight gain from D1 to D21 was larger than the noninferiority margin. Superiority was evaluated if noninferiority was demonstrated. Weight gain was analyzed in the intent-to-treat (ITT) and per-protocol populations by analysis of covariance (ANCOVA) adjusting for D1 postmenstrual age and weight, sex, and center (random effect). Sensitivity analyses were conducted using ANCOVA models that adjusted for covariates that were determined post hoc to be significantly different between groups and which may have confounded the primary results (eg, mother smoking status). Secondary endpoints were analyzed in the ITT population only. For noninferiority and superiority tests, 1-sided P values are provided and should be compared to a reference value of 0.025. For other tests, 2-sided P values are provided and should be compared to a reference value of 0.05. 95% CIs provide estimates for feeding effects on all endpoints. Based on prespecified guidelines in the independent Data Monitoring Committee's (DMC) charter, a single interim analysis was conducted when 134 subjects had completed their D21 visit. The interim analysis was planned to occur when the first 100 infants completed at least 21 days of full fortification; however, the analysis was conducted using data from 134 infants owing to unforeseen delays in conducting the analysis (eg, performing statistical programming, data cleaning, and query resolution) while recruitment continued. The type 1 error rate was adjusted to account for the analysis being conducted at ∼70% enrollment rather than the planned 52%. The DMC consisted of independent experts (2 clinicians, 1 biostatistician) who reviewed growth, formula intake, and key biochemical data as well as AEs. The purpose of the interim analysis was to examine unblinded growth velocity results and determine whether the trial could be stopped early for success or futility, or whether the targeted sample size required adjustment (the interim statistical analysis plan was finalized before unblinding, and the analysis was unblinded only to the DMC to facilitate ethical decision-making) (31). On April 2, 2014, the DMC recommended to stop the trial, as noninferiority and superiority in regard to the primary outcome had been demonstrated. The sponsor was notified of this decision on April 3, 2014, and the final study population included infants enrolled through March 31, 2014.
RESULTS
A total of 274 infants were screened, with 153 enrolled and randomized to either nHMF (n = 77) or cHMF (n = 76) (Fig. 2). Demographic and baseline anthropometry data are summarized in Table 2. There was no evidence of imbalance between the 2 groups with respect to infant characteristics. A significantly lower percentage of mothers and fathers of infants in the nHMF group, however, smoked during pregnancy. Number of twins was similar in each group.
FIGURE 2.
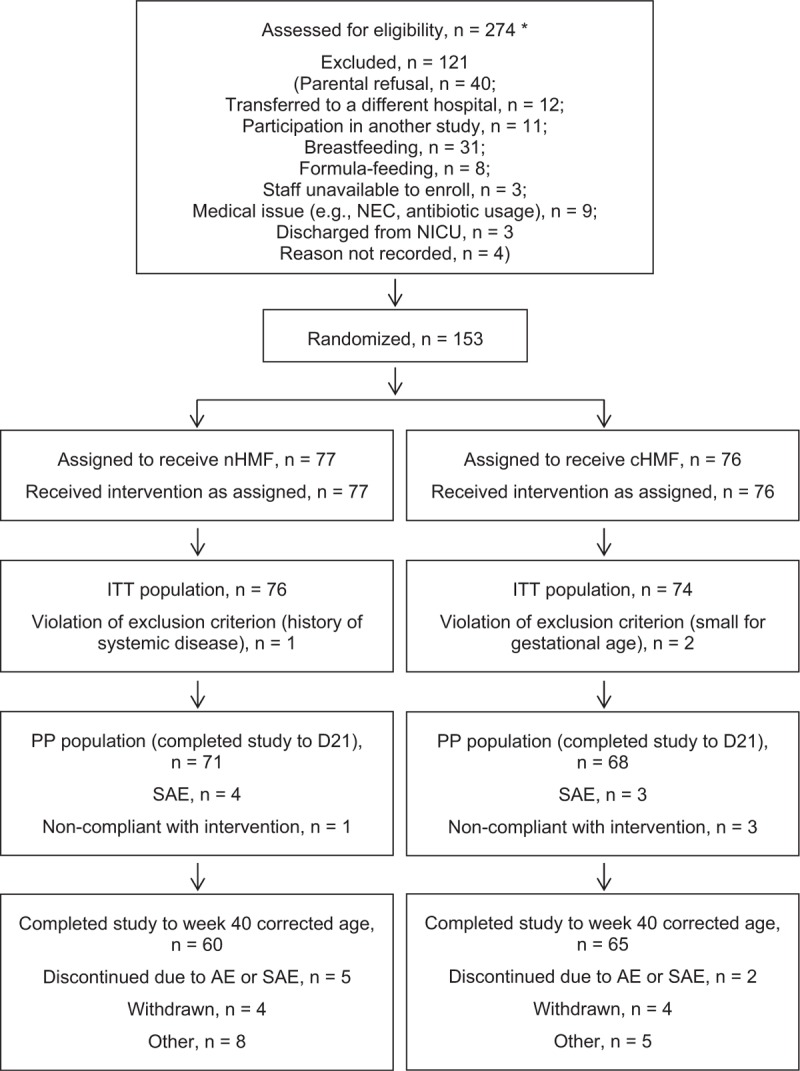
Flow of study participants. AE = adverse event; cHMF = control human milk fortifier; D21 = study day 21; ITT = intent-to-treat; NEC = necrotizing enterocolitis; nHMF = new human milk fortifier; NICU = neonatal intensive care unit; PP = per-protocol; SAE = serious adverse event. ∗Although screening procedures were standardized across sites, some variability in prescreening procedures did occur. Based on the typical clinical characteristics of infants who were admitted to each NICU during the study interval, the total number of infants who would have been theoretically considered eligible for the study was higher than the number shown here.
TABLE 2.
Demographic and baseline characteristics of infants and parents
| nHMF (n = 76) | cHMF (n = 74) | |
| Infant characteristics | ||
| Sex | ||
| Boys | 38 (50) | 35 (47) |
| Delivery type | ||
| Vaginal | 24 (32) | 20 (27) |
| Twin | 18 (24) | 16 (22) |
| Birth weight, g | 1147 ± 258 | 1156 ± 289 |
| Birth weight by birth weight category | ||
| <1000 g | ||
| n (%) | 24 (32) | 26 (35) |
| Birth weight, g | 850.5 ± 118.9 | 847.3 ± 105.1 |
| ≥1000 g | ||
| Birth weight, g | 1283.6 ± 175.4 | 1323.9 ± 206.2 |
| Birth length, cm | 37.1 ± 2.7 | 37.1 ± 3.1 |
| Birth head circumference, cm | 26.5 ± 2.7 | 26.7 ± 2.5 |
| Gestational age at birth, weeks | 28.8 ± 2.1 | 28.7 ± 1.8 |
| Postnatal age at study time points, days* | ||
| FSI1 | 13 (11, 18) | 14 (10, 20) |
| Day 1 | 16 (13, 20) | 17 (13, 23) |
| Day 21 | 36 (33, 40) | 37 (33, 43) |
| Week 40 corrected age | 76 (66, 91) | 76 (67, 83) |
| Apgar score | ||
| 1 min | 5.8 ± 2.5 | 5.8 ± 2.3 |
| 5 min | 8.0 ± 1.8 | 7.7 ± 1.9 |
| Parent characteristics | ||
| Smoking status | ||
| Mother smoker during pregnancy | 6 (9) | 18 (29) |
| Father smoker | 3 (5) | 12 (21) |
| Mother drank alcohol during pregnancy | 0 (0) | 4 (6) |
| Mother's age, y | 31.1 ± 5.1 | 30.8 ± 5.5 |
| Mother's BMI before pregnancy, kg/m2* | 23.2 (20.6, 27.2) | 21.3 (19.7, 26.1) |
| Mother's weight gain during pregnancy, kg | 11.2 ± 6.8 | 9.2 ± 5.2 |
BMI = body mass index; cHMF = control human milk fortifier; FSI1 = fortification strength increase day 1; nHMF = new human milk fortifier . Data are presented as n (%) for categorical variables and mean ± SD for continuous variables except where noted.
*Data are presented as median (Q1, Q3).
The majority (84% and 87% by volume in nHMF and cHMF, respectively) of milk provided to infants was pasteurized. Donor milk was always pasteurized and accounted for 49% and 51% of the fortified HM volume provided in the nHMF and cHMF groups, respectively. There was no significant difference in mean volume of fortified milk intake between groups (152.7 ± 13.0 and 152.6 ± 17.2 mL · kg−1 · day−1 in nHMF and cHMF, respectively). Protein intake estimated using standard values for preterm HM composition per 100 mL (22) was significantly greater in nHMF compared to cHMF (4.48 ± 0.38 vs 3.81 ± 0.43 g · kg−1 · day−1, respectively; P < 0.001) because of higher protein content of the nHMF. Estimated energy intake was not significantly different between groups (125 kcal · kg−1 · day−1 in both groups). There was no significant difference in number of days between FSI1 and D1, but adjusted time between birth and D1 was significantly shorter in nHMF (16.8 ± 5.4 vs 18.7 ± 8.8 days; −8.5% [95% CI: −15.0%, −1.0%]).
Growth
In the ITT population, adjusted weight gain from D1 to D21 was 2.3 g/day higher in nHMF, with the 95% CI ranging from 0.4 to 4.2 g/day, demonstrating noninferiority (P < 0.001) and superiority (P = 0.01) of nHMF. Per-protocol results were similar. Weight gain from D1 to D21 remained significantly higher in nHMF when expressed in grams per kilogram per day (Table 3). Weight-for-age z scores (Fig. 3) remained stable from FSI1 to D21 in nHMF, but continued to decrease in cHMF (P = 0.007 vs D1). At D21, weight-for-age z score was significantly higher in nHMF compared to cHMF (0.12 [95% CI: 0.03, 0.22]). Length and HC gains during the D1 to D21 period were not significantly different between groups (Table 3), with comparable results observed from analyses of unadjusted means (Table 4). Length-for-age z scores at D21 (Fig. 3) were significantly lower than D1 values in cHMF (P = 0.041). Additionally, at W40CA, adjusted HC-for-age z scores were significantly higher in nHMF compared to cHMF (0.41 [95% CI: 0.14, 0.68]). Mean weight, length, and HC at D1, D21, and W40CA are summarized in Table 5.
TABLE 3.
Anthropometric gains from D1 to D21
| Treatment group | |||||
| n | nHMF | n | cHMF | P* | |
| Weight gain, g · kg−1 · day−1 | 64 | 18.3 ± 3.7 | 67 | 16.8 ± 3.7 | 0.013† |
| Length gain, cm/wk | 55 | 1.23 ± 0.62 | 65 | 1.18 ± 0.49 | 0.842 |
| HC gain, cm/wk | 57 | 1.04 ± 0.32 | 65 | 0.96 ± 0.26 | 0.125 |
cHMF = control human milk fortifier; D1 = study day 1 (first day of full-strength fortification); D21 = study day 21; HC = head circumference; nHMF = new human milk fortifier. Data are presented as unadjusted mean ± SD.
*One-sided superiority P value based on analysis of covariance model adjusted for postmenstrual age and relevant anthropometric measure at D1, sex, and center.
†Adjusted difference in weight gain (nHMF–cHMF): mean difference = 1.18 g · kg−1 · day−1; 95% CI = 0.14, 2.21.
FIGURE 3.
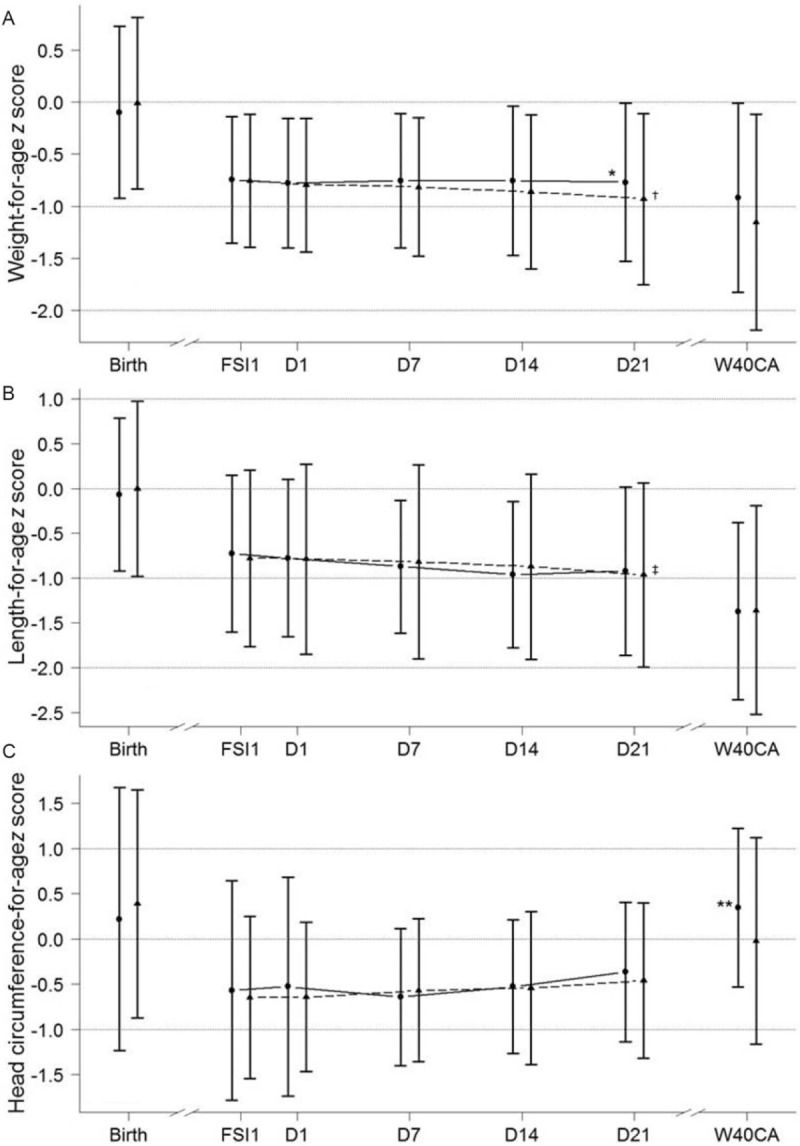
Mean ± SD weight-for-age (panel A), length-for-age (panel B), and head circumference-for-age (panel C) z scores for the overall ITT population. Circle symbols/solid line represents nHMF. Triangle symbols/dashed line represents cHMF. FSI1 = fortification strength increase day 1; ITT = intent-to-treat; SD = standard deviation; W40CA = week 40 corrected age; z scores calculated using Fenton preterm growth chart (25). ∗P = 0.013 vs cHMF (by analysis of covariance, adjusting for value at D1, sex, and center); †P = 0.007 vs day 1 (by t test); ‡P = 0.041 vs day 1 (by t test); ∗∗P = 0.003 vs cHMF (by analysis of covariance, adjusting for value at D1, sex, and center).
TABLE 4.
Body length and head circumference gains between study days 1 and 21, by infant sex and by birth weight category
| Unadjusted length gain, cm/wk* | Unadjusted head circumference gain, cm/wk* | |||||||||
| nHMF | cHMF | nHMF | cHMF | |||||||
| n | Mean ± SD | n | Mean ± SD | P† | n | Mean ± SD | n | Mean ± SD | P† | |
| Overall | 55 | 1.23 ± 0.62 | 65 | 1.18 ± 0.49 | 0.842 | 57 | 1.04 ± 0.32 | 65 | 0.96 ± 0.26 | 0.126 |
| Boys | 27 | 1.40 ± 0.65 | 28 | 1.18 ± 0.49 | 0.364 | 28 | 1.12 ± 0.28 | 28 | 0.99 ± 0.22 | 0.062 |
| Girls | 28 | 1.08 ± 0.56 | 37 | 1.17 ± 0.50 | 0.510 | 29 | 0.97 ± 0.35 | 37 | 0.93 ± 0.29 | 0.598 |
| <1000 g | 19 | 1.07 ± 0.52 | 21 | 1.27 ± 0.52 | 0.563 | 19 | 1.04 ± 0.34 | 21 | 0.94 ± 0.28 | 0.223 |
| ≥1000 g | 36 | 1.32 ± 0.66 | 44 | 1.13 ± 0.48 | 0.499 | 38 | 1.05 ± 0.32 | 44 | 0.96 ± 0.26 | 0.270 |
cHMF = control human milk fortifier; nHMF = new human milk fortifier.
*Data are presented as unadjusted mean ± SD.
†Superiority P value for gain differences adjusted for postmenstrual age and the relevant anthropometric measure at D1, sex, and center by analysis of covariance.
TABLE 5.
Weight, length, and head circumference at selected study time points
| nHMF | cHMF | |||||
| Variable | n | Mean | SD | n | Mean | SD |
| Weight, g | ||||||
| D1 | 72 | 1346 | 271 | 74 | 1347 | 270 |
| D21 | 64 | 1884 | 336 | 67 | 1863 | 328 |
| W40CA | 60 | 3076 | 519 | 63 | 2897 | 416 |
| Length, cm | ||||||
| D1 | 67 | 38.7 | 2.5 | 74 | 38.7 | 2.8 |
| D21 | 58 | 41.8 | 2.4 | 65 | 42.0 | 2.7 |
| W40CA | 60 | 47.6 | 2.6 | 62 | 47.3 | 2.5 |
| Head circumference, cm | ||||||
| D1 | 68 | 27.7 | 2.5 | 73 | 27.6 | 1.9 |
| D21 | 59 | 30.2 | 2.2 | 66 | 30.3 | 2.0 |
| W40CA | 59 | 35.3 | 1.4 | 64 | 34.6 | 1.5 |
cHMF = control human milk fortifier; D1 = study day 1; D21 = study day 21; nHMF = new human milk fortifier; SD = standard deviation; W40CA = week 40 corrected age.
Protein-Energy Status
BUN decreased progressively in cHMF (P = 0.004 for D21 vs D1), whereas it increased in nHMF (P < 0.001 for D10/11 vs D1 [data not shown]) and remained stable up to D21 (Table 6). Prealbumin levels were similar at D1 and increased in both groups during the study (Table 6). The increase from D1 to D21, however, was only significant in nHMF (P = 0.004). At D21, adjusted mean prealbumin in nHMF was significantly higher (+11.8% [95%CI: +2.3%, +22.2%]) than in cHMF. Urinary urea excretion (corrected for creatinine excretion) at D1 was similar in the 2 groups (Table 6). Urea excretion remained steady in cHMF but increased sharply in nHMF (P < 0.001 for D10/11 vs D1 [data not shown]), after which it remained stable (to D21). At D21, urea excretion was significantly higher in nHMF versus cHMF (+108.7% [95% CI: +66.0%, +162.5%]).
TABLE 6.
Markers of protein-energy status, electrolytes, and bone metabolic status at study days 1 and 21
| nHMF | cHMF | ||||||||
| Variable | n | Median | IQR | Geometric mean | n | Median | IQR | Geometric mean | P* |
| Serum creatinine, μmol/L | |||||||||
| D1 | 69 | 44.0 | 36.2–48.0 | 41.5 | 70 | 44.1 | 38.0–51.8 | 43.5 | 0.303 |
| D21 | 63 | 28.0 | 23.5–32.0 | 26.7 | 65 | 30.0 | 25.0–35.0 | 29.5 | 0.001 |
| BUN, mmol/L | |||||||||
| D1 | 70 | 3.10 | 1.70–4.56 | 2.89 | 71 | 2.50 | 1.65–4.67 | 2.73 | 0.585 |
| D21 | 63 | 3.90 | 3.05–4.65 | 3.89 | 64 | 2.15 | 1.50–2.63 | 2.15 | <0.001 |
| Serum prealbumin, mg/L | |||||||||
| D1 | 51 | 100 | 80–120 | 96.8 | 46 | 90 | 80–100 | 87.8 | 0.073 |
| D21 | 46 | 116 | 91.3–140 | 113.8 | 41 | 100 | 90–120 | 98.1 | 0.015 |
| Urinary urea†, mmol/10 mg creatinine | |||||||||
| D1 | 47 | 2.7 | 2.0–4.7 | 2.8 | 53 | 2.5 | 1.9–3.3 | 2.5 | 0.302 |
| D21 | 42 | 5.8 | 4.6–6.8 | 5.1 | 40 | 2.8 | 2.0–3.3 | 2.7 | <0.001 |
| Serum calcium, mmol/L | |||||||||
| D1 | 50 | 2.44 | 2.31–2.53 | 2.41 | 54 | 2.47 | 2.38–2.56 | 2.44 | 0.445 |
| D21 | 50 | 2.47 | 2.40–2.54 | 2.46 | 48 | 2.43 | 2.34–2.53 | 2.43 | 0.019 |
| Serum phosphorus, mmol/L | |||||||||
| D1 | 68 | 1.99 | 1.85–2.22 | 1.96 | 71 | 1.94 | 1.76–2.25 | 1.94 | 0.816 |
| D21 | 62 | 2.10 | 1.93–2.23 | 2.05 | 64 | 2.12 | 1.93–2.26 | 2.08 | 0.681 |
| Alkaline phosphatase, U/L | |||||||||
| D1 | 67 | 353.0 | 298.5–459.5 | 377.9 | 63 | 333.0 | 250.0–438.5 | 343.8 | 0.208 |
| D21 | 62 | 320.5 | 273.3–405.5 | 337.5 | 62 | 270.5 | 233.0–354.3 | 297.5 | 0.010 |
| Urinary calcium †, mmol/10 mg creatinine | |||||||||
| D1 | 60 | 0.11 | 0.07–0.19 | 0.12 | 69 | 0.14 | 0.09–0.20 | 0.12 | 0.985 |
| D21 | 55 | 0.14 | 0.09–0.23 | 0.15 | 54 | 0.21 | 0.13–0.32 | 0.19 | 0.011 |
| Urinary phosphorus†, mmol/10 mg creatinine | |||||||||
| D1 | 59 | 0.41 | 0.12–0.66 | 0.22 | 65 | 0.34 | 0.14–0.65 | 0.23 | 0.867 |
| D21 | 52 | 0.68 | 0.44–1.10 | 0.53 | 52 | 0.71 | 0.40–0.92 | 0.58 | 0.896 |
| Urinary calcium:phosphorus molar ratio | |||||||||
| D1 | 59 | 0.39 | 0.15–0.90 | 0.50 | 64 | 0.41 | 0.16–1.34 | 0.47 | 0.824 |
| D21 | 53 | 0.22 | 0.12–0.48 | 0.28 | 53 | 0.31 | 0.19–0.60 | 0.34 | 0.054 |
| Serum sodium, mmol/L | |||||||||
| D1 | 71 | 138.0 | 137.0–140.0 | 138.6 | 72 | 138.6 | 136.6–140.0 | 138.5 | 0.891 |
| D21 | 65 | 138.0 | 136.4–140.0 | 138.0 | 64 | 138.0 | 137.0–139.9 | 138.3 | 0.449 |
| Serum potassium, mmol/L | |||||||||
| D1 | 71 | 4.73 | 4.30–5.32 | 4.83 | 72 | 4.77 | 4.40–5.10 | 4.78 | 0.685 |
| D21 | 64 | 4.74 | 4.29–5.10 | 4.72 | 64 | 4.51 | 4.14–4.88 | 4.54 | 0.091 |
| Serum chloride, mmol/L | |||||||||
| D1 | 71 | 106.0 | 104.0–109.0 | 106.1 | 72 | 105.0 | 102.8–108.0 | 105.2 | 0.148 |
| D21 | 63 | 105.0 | 103.0–107.0 | 104.6 | 62 | 105.0 | 104.0–107.0 | 105.3 | 0.111 |
BUN = blood urea nitrogen; cHMF = control human milk fortifier; D1 = study day 1; D21 = study day 21; IQR = interquartile range; nHMF = new human milk fortifier.
*D1 geometric mean values were log-transformed and analyzed using t test; D21 geometric mean values were log-transformed and analyzed using analysis of covariance (adjusting for the relevant biochemical parameter at D1, sex, and center).
†Corrected for urinary creatinine excretion of 10 mg/kg body weight/day.
Bone Metabolic Status
Serum calcium concentrations were generally stable during the study (Table 6), with mean values for both groups within the normal range (32). Nevertheless, adjusted mean serum calcium concentration in nHMF was minimally but significantly higher than in cHMF at D21 (+1.9% [95% CI: +0.3%, +3.5%]). Serum phosphorus increased slightly in the 2 groups (Table 6). At D1, relative hypophosphatemia (<1.55 mmol/L) was observed in 13 infants in both groups; this was corrected in 11 infants by D10/11 and 12 infants by D21. At D1, serum alkaline phosphatase was not significantly different in nHMF versus cHMF (P = 0.208). Thereafter, serum alkaline phosphatase decreased significantly in both groups (D21 vs D1: P = 0.005 for nHMF, P < 0.001 for cHMF), with mean values significantly higher in nHMF versus cHMF at D10/11 (+8.6% [95% CI: +1.0%, +16.8%]; data not shown) and D21 (+12.1% [95% CI: +2.8%, +22.3%]) (Table 6). Declines from baseline were significantly greater in cHMF versus nHMF at D10/11 (P < 0.001; data not shown) and D21 (P = 0.035). At D1, spot urinary excretions of calcium and phosphorus corrected for urinary creatinine excretion were similar in the 2 groups (Table 6). Calcium excretion tended to increase slowly during the study in both groups, with mean concentration significantly lower in nHMF compared to cHMF at D21 (P = 0.011). Phosphorus excretion increased in both groups, resulting in a decreased median urinary calcium:phosphorus molar ratio in both groups (Table 6).
Electrolytes
Serum electrolyte concentrations were stable during the study and similar in both groups (Table 6). Urinary sodium and potassium concentrations were significantly higher (sodium: +31.1% [95% CI: +1.7%, +68.9%], potassium: +22.5% [95% CI: +1.0%, +48.6%]) in nHMF compared to cHMF at D21 (Table 7).
TABLE 7.
Markers of kidney function, blood count, and urinary electrolyte status at study days 1 and 21
| nHMF | cHMF | ||||||||
| Variable | n | Median | IQR | Geometric mean | n | Median | IQR | Geometric mean | P* |
| Urinary creatinine, μmol/L | |||||||||
| D1 | 63 | 1300.0 | 785.5–1685.5 | 1224.7 | 69 | 1105.0 | 900.0–1500.0 | 1182.3 | |
| D21 | 57 | 1030.0 | 660.0–1609.0 | 1000.3 | 55 | 854.0 | 618.0–1273.0 | 900.8 | 0.447 |
| Serum hemoglobin, mmol/L | |||||||||
| D1 | 68 | 2.08 | 1.84–2.29 | 2.14 | 72 | 2.02 | 1.84–2.26 | 2.18 | |
| D21 | 63 | 1.71 | 1.56–1.91 | 1.83 | 66 | 1.69 | 1.50–1.98 | 1.76 | 0.936 |
| Serum hematocrit, % | |||||||||
| D1 | 68 | 0.40 | 0.35–0.43 | 0.39 | 72 | 0.39 | 0.35–0.43 | 0.38 | |
| D21 | 63 | 0.32 | 0.29–0.38 | 0.33 | 66 | 0.33 | 0.28–0.38 | 0.33 | 0.805 |
| Urinary sodium, mmol/L | |||||||||
| D1 | 66 | 37.0 | 23.3–57.3 | 37.5 | 69 | 32.0 | 19.4–54.0 | 31.2 | |
| D21 | 59 | 34.0 | 21.1–48.0 | 33.3 | 56 | 23.0 | 14.3–36.4 | 24.0 | 0.037 |
| Urinary potassium, mmol/L | |||||||||
| D1 | 66 | 25.9 | 13.6–37.0 | 23.6 | 69 | 21.8 | 15.0–32.2 | 20.0 | |
| D21 | 59 | 30.0 | 16.9–45.0 | 27.6 | 57 | 22.9 | 16.9–30.4 | 22.8 | 0.040 |
| Urinary chloride, mmol/L | |||||||||
| D1 | 60 | 37.0 | 26.3–60.0 | 40.2 | 67 | 33.0 | 20.5–55.0 | 34.2 | |
| D21 | 54 | 31.0 | 17.8–43.8 | 30.7 | 55 | 26.0 | 18.0–39.5 | 27.8 | 0.558 |
cHMF = control human milk fortifier; D1 = study day 1; D21 = study day 21; IQR = interquartile range; nHMF = new human milk fortifier .
*D21 geometric mean values were log-transformed and analyzed using analysis of covariance (adjusting for the relevant biochemical measure at D1, sex, and center).
Stool Characteristics and Feeding Tolerance
Stool frequency from D1 to D21 was not significantly different in nHMF and cHMF (3.9 ± 1.05 vs 3.6 ± 0.93 stools/day; 0.29 [95% CI: −0.05, 0.63]). Stool consistency was slightly more “formed” in nHMF compared to cHMF during this interval (3.1 ± 0.26 vs 3.0 ± 0.27; 0.12 [95% CI: 0.02, 0.21]). Most infants (>90%) had stool consistency scores of “soft.” There were no significant differences between groups in frequencies of spitting-up, vomiting, or abdominal distention. There also were no group differences in incidence of AEs indicative of feeding intolerance (all P ≥ 0.25).
Adverse Events
The overall incidence of AEs was significantly larger in nHMF (103 events in 56 infants, including 26 events categorized as GI disorders, 18 as infections or infestations, and 5 as metabolism and nutrition disorders) compared to cHMF (78 events in 41 infants, including 21 events categorized as GI disorders, 18 as infections or infestations, and 1 as metabolism and nutrition disorder; odds ratio: 2.26 [95% CI: 1.10, 4.47]). Other AEs that occurred more frequently in nHMF included several that were classified by study investigators as unlikely to be related to consumption of milk fortifiers (eg, cardiac disorders [16 events in nHMF vs 5 in cHMF], eye disorders [10 events in nHMF vs 3 in cHMF]). The number of AEs considered related to study product intake as determined by physician report was low (3 events in nHMF [2 events of hyponatremia, 1 of vomiting] and 0 events in cHMF). No significant difference was demonstrated in overall incidence of serious AEs between the 2 groups (7 events in 7 infants [including 2 events of necrotizing enterocolitis, 0 events of bronchopulmonary dysplasia, 0 events of sepsis, 0 events of retinopathy] in nHMF and 12 events in 11 subjects [including 4 events of necrotizing enterocolitis, 1 event of bronchopulmonary dysplasia, 0 events of sepsis, 0 events of retinopathy] in cHMF; odds ratio: 0.54 [95% CI: 0.17, 1.58]).
DISCUSSION
This study demonstrated that weight gain from D1 of full fortification until D21 in preterm infants fed HM fortified with a new fortifier designed to add 1.4 g partially hydrolyzed protein and 0.7 g fat to 100 mL of HM was significantly greater than weight gain in infants fed HM fortified with an isocaloric control fortifier designed to add 1.0 g extensively hydrolyzed protein and no fat. The mean difference was 2.3 g/day or 1.2 g · kg−1 · day−1, consistent with our hypothesized difference of 2 g/day, and which indicates the superiority of the new fortifier compared to the control with regard to weight gain. In addition, the weight gain benefit tended to persist until discharge, with a significantly higher adjusted weight gain difference in the nHMF group compared to cHMF from FSI1 to W40CA (2.01 g/day; P = 0.009). In the nHMF group, weight-for-age z scores were stable from FSI1 to D21 and average weight gain exceeded 18 g · kg−1 · day−1, matching recommended rates of postnatal weight gain to mimic intrauterine growth (33,34). Consistent with the increased protein content of the new fortifier, the nHMF group had significantly higher serum prealbumin concentrations, suggesting an increase in nitrogen retention compared to cHMF. The lack of difference, however, in length gain during the study may be in part the result of the relatively limited period of protein supplementation (only 21 days) or because mean length gains in both groups were already quite high (ie, ≥1.1 cm/week), whereas the significantly higher HC-for-age z score at W40CA in the nHMF group may be because of the increased protein and lipid content of the new fortifier. In contrast, the absence of a significant difference at earlier timepoints could be attributable to the relatively high variability of HC gain (31% and 27% for nHMF and cHMF, respectively, from D1 to D21) induced by the natural dolichocephalic evolution of the skull that occurs in preterm infants (35). Feeding tolerance and stool patterns were similar in each group, and AEs related to feeding were low and not significantly different between groups, consistent with fortified HM osmolality values slightly lower in nHMF versus cHMF and below the recommended cutoff (23,24) in both groups.
Although there was no evidence of imbalance between the 2 fortifier groups with respect to infant baseline characteristics, significant differences in maternal weight gain, smoking, and alcohol usage during pregnancy were observed. As these may be confounding factors in the analysis of weight gain, post hoc ANCOVAs including these parameters were performed. The post hoc results were essentially the same as the main results, indicating that differences in maternal baseline characteristics did not confound the results. Additionally, to determine the possible impact of including clustered data from twins in the analyses, a sensitivity analysis on weight gain (grams per day) from D1 to D21 accounting for the correlated multiple-birth data was performed. Again, these results were similar to those of the main analysis (weight gain 3.2 g/day higher in nHMF [95% CI: 0.5, 5.9 g/day]).
Our results are consistent with those of previous studies (36–42). A recent meta-analysis of 5 studies (comprising 352 infants with birthweight ≤1750 g and gestational age ≤34 weeks) compared growth of infants fed HM fortified with either lower-protein or higher-protein fortifier (43). Infants receiving higher-protein fortifier had significantly greater weight (mean difference 1.77 g/kg/day), length (0.21 cm/week), and HC gains (0.19 cm/week) compared to those receiving lower-protein fortifier (43). Miller et al (39) used a higher-protein fortifier similar in protein content to the one used in the present study, and reported a higher bodyweight at study end among infants in the higher-protein HMF group (mean difference 220 g), but no significant differences in length or HC. In contrast, Moya et al (40) observed a significantly higher achieved weight, length, and HC in the experimental group compared to controls, but their fortifier had a slightly higher protein content (3.2 g/100 mL) versus the one used in the present study (3.04 g/100 mL), plus the intervention lasted 28 rather than 21 days.
Energy and protein content of HM samples were not analyzed in this study but estimated according to Tsang et al (22). Variability of protein, fat, and energy content of HM fed to preterm infants in the NICU is high (15,21). In addition, fat content may be reduced during processing of HM from expression to administration (44), which could be exacerbated with the use of continuous tube feeding (45). In our study, percentage of intake from mother's own milk, donor milk, and pasteurized HM was assessed. Pasteurized donor milk accounted for 51% of the fortified HM provided during the study, whereas 56% of mother's own milk was also pasteurized. Considering that protein content of donor HM is lower than that of mother's own milk (46) and that all the required processing steps (eg, collection, transfer, refrigeration, pasteurization, tube feeding) may significantly decrease fat and energy content (47), the characteristics of the HM used in the present study suggests that protein and energy content could be overestimated when based on a theoretical composition of preterm HM.
In the present study, the mean increase in protein supplementation provided by nHMF compared to cHMF was 0.65 g · kg−1 · day−1 or 7.4 mmol · kg−1 · day−1 of nitrogen, from which approximately 6.14 mmol · kg−1 · day−1 of nitrogen (83%) is absorbed (based on data from balance studies) (48). During the study, urea production increased significantly in the nHMF group leading to an increase in BUN of 1.7 mmol/L at D21 and in urea excretion of 2.3 mmol · kg−1 · day−1 (2.3 mmol/10 mg creatinine). These data suggest that the nitrogen balance was improved to ∼3.8 mmol nitrogen (52% of nitrogen intake) in preterm infants fed nHMF compared to control. This relatively limited protein utilization could result from reduced energy bioavailability of HM, and an increase in energy supply could improve protein utilization in preterm infants fed fortified HM. These data also suggest that specific nutritional recommendations should be formulated for infants fed fortified HM. Nevertheless, the increase in nitrogen retention (∼3.8 mmol · kg−1 · day−1) appears to be higher than the nitrogen content of the higher weight gain observed with the nHMF (12% of the 1.5 g · kg−1 · day−1 corresponding to 2 mmol · kg−1 · day−1 of nitrogen), suggesting an increase in lean body mass accretion and a moderate reduction in fat mass gain as previously demonstrated in preterm infants fed protein-fortified HM (49).
Indices of bone metabolism were satisfactory in both groups, with a significant decrease in serum alkaline phosphatase observed in both groups and 98% of the infants having normal serum phosphorus concentrations at D21. Adequate postnatal bone mineralization is difficult to obtain in preterm infants owing to the interruption of mineral transplacental transfer (50). Although elevated alkaline phosphatase activity may be associated with reduced bone mineralization when mineral intake is deficient (51), the decrease in enzyme levels observed in the presence of normal serum phosphorus values, as well as the low urinary calcium and moderate urinary phosphorus excretion observed in both groups in this study, suggest that intakes were adequate to promote bone mineralization and limit postnatal osteopenia. Mean serum creatinine concentration decreased significantly in both groups suggesting a similar maturation of renal function during this period. Urinary electrolyte concentrations were higher in nHMF versus cHMF at D21, likely in parallel with the higher electrolyte content of nHMF.
A lack of HM composition data (allowing estimation of nutritional balance) is a limitation of our study, although standardized accurate techniques are still not available in the NICU. Additionally, the composition of the faster weight gain can only be estimated as lean body mass and/or bone mineralization were not determined. As a result, nutrient absorption and metabolism can only be estimated from serum and urinary metabolite concentrations. Lastly, the results need to be confirmed in a broader population of preterm infants commonly admitted to the NICU including SGA infants and partially breast-fed infants, as these infants were excluded by design. Strengths of this study include the size and multiple sites (11 pediatric hospitals in 4 European countries), which enhances external validity.
In conclusion, these results indicate that the new HM fortifier, made with partially hydrolyzed whey protein and a higher protein:energy ratio is safe, well-tolerated, and improves weight gain of preterm infants compared to control fortifier. Providing some energy as fat and replacing extensively hydrolyzed with partially hydrolyzed protein in the new HM fortifier allows a reduction in osmolality <400 mOsm/kg immediately after fortification. Protein intakes from HM supplemented with the new fortifier are within the range of the most recent nutritional recommendations for preterm infants.
Acknowledgments
The authors thank the families of the infants who participated in the study, as well as the research staff at each participating institution. The authors also thank Christelle Perdrieu and Samir Dahbane from the Clinical Development Unit at the Nestlé Research Center for assistance with trial management and Philippe Steenhout, Medical Director at Nestlé Nutrition, for input on study design and assistance with trial supervision.
Footnotes
This study was sponsored by Nestlé Nutrition. J.J., L.A., and N.P.H. are employees of Nestlé SA. J.R., J.M.H., C.B., J.C.P., F.M., A.R., E.S., M.R., U.S., B.G., and J.S. received research funding from Nestlé Nutrition. J.R., J.C.P., and C.B. are consultants for Nestlé Nutrition. U.S. has been a speaker, consultant, and expert panel participant for Nestlé, Danone, and Bledina over the past 3 years. V.d.H. has no conflicts of interest to declare.
This study was sponsored by Nestlé Nutrition.
Portions of these data were presented in abstract form at the 1st Congress of joint European Neonatal Societies, Budapest, Hungary, 15–20 September 2015.
REFERENCES
- 1.Garcia C, Duan RD, Brevaut-Malaty V, et al. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: an overview. Cell Mol Biol (Noisy-le-grand) 2013; 59:108–131. [PubMed] [Google Scholar]
- 2.Corpeleijn WE, Kouwenhoven SM, Paap MC, et al. Intake of own mother's milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012; 102:276–281. [DOI] [PubMed] [Google Scholar]
- 3.Patel AL, Johnson TJ, Engstrom JL, et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol 2013; 33:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manzoni P, Stolfi I, Pedicino R, et al. Human milk feeding prevents retinopathy of prematurity (ROP) in preterm VLBW neonates. Early Hum Dev 2013; 89 suppl 1:S64–S68. [DOI] [PubMed] [Google Scholar]
- 5.Koo W, Tank S, Martin S, et al. Human milk and neurodevelopment in children with very low birth weight: a systematic review. Nutr J 2014; 13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson S, Wojcik B, Barker A, et al. Guidelines for the use of human milk fortifier in the neonatal intensive care unit. University of Iowa Neonatology Handbook. 2011. Available at: http://www.uichildrens.org/iowa-neonatology-handbook/feeding/human-milk Accessed on January 22, 2017. [Google Scholar]
- 7.Adamkin DH, Radmacher PG. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin Perinatol 2014; 41:405–421. [DOI] [PubMed] [Google Scholar]
- 8.Moro GE, Arslanoglu S, Bertino E, et al. XII. Human milk in feeding premature infants: consensus statement. J Pediatr Gastroenterol Nutr 2015; 61 suppl 1:S16–S19. [DOI] [PubMed] [Google Scholar]
- 9.Einloft PR, Garcia PC, Piva JP, et al. Supplemented vs. unsupplemented human milk on bone mineralization in very low birth weight preterm infants: a randomized clinical trial. Osteoporos Int 2015; 26:2265–2271. [DOI] [PubMed] [Google Scholar]
- 10.Gibertoni D, Corvaglia L, Vandini S, et al. Positive effect of human milk feeding during NICU hospitalization on 24 month neurodevelopment of very low birth weight infants: an Italian cohort study. PLoS ONE 2015; 10:e0116552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JV, Embleton ND, Harding JE, et al. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev 2016; 5:CD000343. [DOI] [PubMed] [Google Scholar]
- 12.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999; 103 (6 pt 1):1150–1157. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor DL, Jacobs J, Hall R, et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J Pediatr Gastroenterol Nutr 2003; 37:437–446. [DOI] [PubMed] [Google Scholar]
- 14.Weber A, Loui A, Jochum F, et al. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatr 2001; 90:772–775. [PubMed] [Google Scholar]
- 15.Corvaglia L, Aceti A, Paoletti V, et al. Standard fortification of preterm human milk fails to meet recommended protein intake: bedside evaluation by near-infrared-reflectance-analysis. Early Hum Dev 2010; 86:237–240. [DOI] [PubMed] [Google Scholar]
- 16.Arslanoglu S, Moro GE, Ziegler EE. Preterm infants fed fortified human milk receive less protein than they need. J Perinatol 2009; 29:489–492. [DOI] [PubMed] [Google Scholar]
- 17.Arslanoglu S, Corpeleijn W, Moro G, et al. Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr 2013; 57:535–542. [DOI] [PubMed] [Google Scholar]
- 18.Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010; 50:85–91. [DOI] [PubMed] [Google Scholar]
- 19.Koletzko B, Poindexter B, Uauy R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev Nutr Diet 2014; 110:297–299. [DOI] [PubMed] [Google Scholar]
- 20.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 2014; 14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Halleux V, Rigo J. Variability in human milk composition: benefit of individualized fortification in very-low-birth-weight infants. Am J Clin Nutr 2013; 98 suppl:529S–535S. [DOI] [PubMed] [Google Scholar]
- 22.Tsang RC, Uauy R, Koletzko B, et al. Nutrition of the Preterm Infant, Scientific Basis and Practical Guidelines. Cincinnati: Digital Educational Publishing, Inc; 2005. [Google Scholar]
- 23.Kreissl A, Zwiauer V, Repa A, et al. Effect of fortifiers and additional protein on the osmolarity of human milk: is it still safe for the premature infant? J Pediatr Gastroenterol Nutr 2013; 57:432–437. [DOI] [PubMed] [Google Scholar]
- 24.Billeaud C, Senterre J, Rigo J. Osmolality of the gastric and duodenal contents in low birth weight infants fed human milk or various formulae. Acta Paediatr Scand 1982; 71:799–803. [DOI] [PubMed] [Google Scholar]
- 25.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta 2000; 294:139–155. [DOI] [PubMed] [Google Scholar]
- 27.Al-Dahhan J, Stimmler L, Chantler C, et al. Urinary creatinine excretion in the newborn. Arch Dis Child 1988; 63:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ICH Expert Working Group. Guideline for good clinical practice E6(R1). 1996. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf Accessed on January 22, 2017. [Google Scholar]
- 29.Spalinger JH, Schmidt M, Berger TM, et al. Comparison of two human milk fortifiers: effects on growth and zinc status in premature infants. J Pediatr Gastroenterol Nutr 2004; 39 suppl 1:1126. [Google Scholar]
- 30.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics 1987; 43:193–199. [PubMed] [Google Scholar]
- 31.Knottnerus JA, Spigt MG. When should an interim analysis be unblinded to the data monitoring committee? J Clin Epidemiol 2010; 63:350–352. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson JF, Pesce MA. Nelson WE, Behrman RE, Kliegman R, Arvin AM. Laboratory Testing and Reference Values (Table 670-2) in Infants and Children. Nelson Textbook of Pediatrics. Philadelphia: W.B. Saunders; 1996. 2031–2084. [Google Scholar]
- 33.Fenton TR, Nasser R, Eliasziw M, et al. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr 2013; 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117:1253–1261. [DOI] [PubMed] [Google Scholar]
- 35.McCarty DB, Peat JR, Malcolm WF, et al. Dolichocephaly in preterm infants: prevalence, risk factors, and early motor outcomes. Am J Perinatol 2016; 34:372–378. [DOI] [PubMed] [Google Scholar]
- 36.Porcelli P, Schanler R, Greer F, et al. Growth in human milk-fed very low birth weight infants receiving a new human milk fortifier. Ann Nutr Metab 2000; 44:2–10. [DOI] [PubMed] [Google Scholar]
- 37.Reis BB, Hall RT, Schanler RJ, et al. Enhanced growth of preterm infants fed a new powdered human milk fortifier: a randomized, controlled trial. Pediatrics 2000; 106:581–588. [DOI] [PubMed] [Google Scholar]
- 38.Berseth CL, Van Aerde JE, Gross S, et al. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004; 114:e699–e706. [DOI] [PubMed] [Google Scholar]
- 39.Miller J, Makrides M, Gibson RA, et al. Effect of increasing protein content of human milk fortifier on growth in preterm infants born at <31 wk gestation: a randomized controlled trial. Am J Clin Nutr 2012; 95:648–655. [DOI] [PubMed] [Google Scholar]
- 40.Moya F, Sisk PM, Walsh KR, et al. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics 2012; 130:e928–e935. [DOI] [PubMed] [Google Scholar]
- 41.Alan S, Atasay B, Cakir U, et al. An intention to achieve better postnatal in-hospital-growth for preterm infants: adjustable protein fortification of human milk. Early Hum Dev 2013; 89:1017–1023. [DOI] [PubMed] [Google Scholar]
- 42.Thoene M, Hanson C, Lyden E, et al. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014; 6:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu TT, Dang D, Lv XM, et al. Human milk fortifier with high versus standard protein content for promoting growth of preterm infants: A meta-analysis. J Int Med Res 2015; 43:279–289. [DOI] [PubMed] [Google Scholar]
- 44.Vieira AA, Soares FV, Pimenta HP, et al. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk's macronutrient concentrations. Early Hum Dev 2011; 87:577–580. [DOI] [PubMed] [Google Scholar]
- 45.Igawa M, Murase M, Mizuno K, et al. Is fat content of human milk decreased by infusion? Pediatr Int 2014; 56:230–233. [DOI] [PubMed] [Google Scholar]
- 46.Wojcik KY, Rechtman DJ, Lee ML, et al. Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc 2009; 109:137–140. [DOI] [PubMed] [Google Scholar]
- 47.de Halleux V, Peiltain C, Santerre T, et al. Use of donor milk in the neonatal intensive care unit. Semin Fetal Neonatal Med 2017; 22:23–29. [DOI] [PubMed] [Google Scholar]
- 48.Picaud JC, Putet G, Rigo J, et al. Metabolic and energy balance in small- and appropriate-for-gestational-age, very low-birth-weight infants. Acta Paediatr Suppl 1994; 405:54–59. [DOI] [PubMed] [Google Scholar]
- 49.Putet G, Rigo J, Salle B, et al. Supplementation of pooled human milk with casein hydrolysate: energy and nitrogen balance and weight gain composition in very low birth weight infants. Pediatr Res 1987; 21:458–461. [DOI] [PubMed] [Google Scholar]
- 50.Pieltain C, de Halleux V, Senterre T, et al. Prematurity and bone health. World Rev Nutr Diet 2013; 106:181–188. [DOI] [PubMed] [Google Scholar]
- 51.Rusk C. Rickets screening in the preterm infant. Neonatal Netw 1998; 17:55–57. [PubMed] [Google Scholar]


