Abstract
Background:
Vascular and lung function develop and decline over the life course; both predict cardiovascular events and mortality but little is known of how they develop over time. We analysed their relationship in a multiethnic cohort study to test whether lung function from early adolescence to young adulthood affected vascular indices.
Methods:
‘DASH’ (http://dash.sphsu.mrc.ac.uk) included 6643 children aged 11–13 years in 2003; a representative 10% sample (n = 665) participated in a pilot follow-up in 2013. Psychosocial, anthropometric, blood pressure (BP), and lung function measures were collected in both surveys; aortic pulse wave velocity (PWV) and augmentation index (AIx) were measured at aged 21–23 years. Relationships between forced expiratory volume Z-scores in 1 s (zFEV1), after global initiative-ethnic adjustments and BP, PWV, and AIx were tested in linear regression and general estimating statistical models.
Results:
In total, 488 people with complete data were included. At 11–13 years, SBP was positively associated with zFEV1 (coefficient = 1.90, 95% confidence interval 1.11–2.68, P < 0.001); but not at 21–23 years. The 10-year increase in zFEV1 was associated with rise in SBP (1.38, 0.25–1.51, P < 0.05) in mixed effect models adjusted for age, sex, ethnicity, waist to height ratio, employment, reported racism, smoking, and alcohol use but DBP change was unrelated. In fully adjusted models, neither PWV nor central AIx were associated with zFEV1 at 11–13 years or 21–23 years (P > 0.05).
Conclusion:
Forced expiratory volume change is positively and independently associated with SBP change from adolescence to young adulthood, suggesting earlier lung function plays important roles in SBP development. Vascular indices were unrelated to lung function or its change.
Keywords: augmentation index, blood pressure, forced expiratory volume, longitudinal studies, pulse wave velocity
INTRODUCTION
In adulthood, impaired lung function is inversely related to the incidence of stroke [1], myocardial infarction, and cardiovascular mortality [2], independently of (after adjustment for) traditional cardiovascular risk factors in middle-age populations. Given lung function's significance for adult vascular health, surprisingly few studies have examined the interrelationships of their developmental trajectories from childhood. Lung development starts in utero and continues through adolescence and early adulthood [3,4], which raises the question whether suboptimal lung growth and development could affect later vascular function and blood pressure (BP).
Whether associations between low lung function and incidence of vascular events could be related to or mediated by its link with BP and vessel dysfunction remain unclear. The inverse association between lung function and BP has been reported in several adult studies [5–7]. In middle aged (45–59 years) and elderly (>60 years) population, lung function parameters measured at age 40 were stronger predictors of arterial stiffness [aortic pulse wave velocity (PWV) and pressure or flow wave reflection measured as augmentation index (AIx)] than lung function at age 60 [8–10]. Furthermore, lung function and vascular function may track each other through convergent inflammatory or metabolic pathways throughout life, or genetic susceptibility. Such low-grade systemic inflammation is indicated by higher levels of C-reactive protein, fibrinogen, and other systemic inflammatory markers [11].
There is increasing evidence of ethnic differences in the development of both lung function and BP. Lung function is lower among South Asians and Black African origin children compared with White European peers even after adjustment for age, sex, and height, findings which led to the recent development of ethnic specific equations [12].
A faster rise of BP has been observed among African origin children in the United States [13] and among all ethnic minority groups in the United Kingdom [14,15] than their White counterparts. These studies signal that ethnic differences in growth velocities in early life and postnatally are important determinants of cardiorespiratory development.
To date, information is lacking on the determinants of cardiorespiratory development from an ethnically diverse population in early adulthood, a time when physical health is at its peak and when early signs of disease begin to appear. Studies that tracked growth from childhood and collected biomarkers and vascular measures are mainly of White Europeans with few in Europe varying in ethnic make-up [16,17]. The Determinants of Adolescent Social Well-Being and Health (DASH) study, established in 2002, has followed health and social exposures of over 6000 young Londoners (United Kingdom), including 80% ethnic minorities, over the last 12 years. A pilot follow-up study of the cohort, now in their early 1920s, was recently completed. We used the DASH study to examine potential associations between lung function and BP, PWV, and AIx from early adolescence to early 1920s, and whether any observed association is influenced by sex, health behaviours, and socioeconomic circumstances (SEC).
METHOD
Details of the Determinants of Adolescent Social Well-Being and Health study can be found elsewhere
In 2002–2003, a total of 6643 students, aged 11–13 years, from 51 secondary schools in 10 London boroughs, were recruited [18]. In 2005–2006, 4785 (88% of children in 49 schools, 72% of the initial cohort), participated in the first follow-up aged 14–16 year. A 10% subsample (N = 665, 97% of those contacted) participated in the pilot follow-up, completed in March 2014. The subsample consisted of 107 White UK, 102 Black Caribbean, 132 Black African, 99 Indian, 111 Bangladeshi or Pakistani, and 115 other (mainly mixed) ethnicities. We first located the sample and 81% (5414 of 6643) of the cohort was traced through friendship networks, social media, and community campaigns. We then randomly selected 100 (50 per sex) per ethnic group, and pragmatically attempted, by repeated random selection, to ensure representation across the London boroughs, schools, and SEC at 11–13 years.
The study was approved by the Multicentre Research Ethics Committee and additionally at age 21–23 years from National Health Service Local Research Ethics Committees. Written informed consent was obtained from all participants. Ethnicity was self-reported, checked against reported parental ethnicity, and grandparents’ country of birth. Bangladeshis and Pakistanis were combined because of small sample sizes.
Spirometry data management and exclusion criteria
Spirometric assessments were undertaken using a portable Micro-plus spirometer (Micro Medical Ltd., Kent, UK) at 11–13 years and 21–23 years in accordance with published guidelines [19]. Details of data quality and acceptability from 11–13 years survey have been published [20]. Data collected at 21–23 years were overread by a senior respiratory physiologist (University College London Great Ormond Street Institute of Child Health) to ensure appropriate quality control.
As the cohort is a healthy community sample of young people, the forced expired volume in 1 s (FEV1) provides the optimal survey measure of airway caliber, with lower values or rapid decline in FEV1 being associated with higher morbidity or unfavourable prognosis [21]. Missing data, implausible values or extreme outliers (−3< or >3 Z-scores) for height and FEV1, (e.g.: when FEV1 between 11–13 years and 21–23 years differed by <100 ml), or those with current asthma were excluded (n = 177; Figure S1).
After adjusting for sex, age, and standing height, FEV1 was expressed as Z-scores using the Global Lung Function Initiative (GLI-2012) ethnic-specific equations [12]. As reliable spirometric equations for South Asians are lacking, the GLI-Black equation, which provided a better approximation for interpreting South Asian data was used instead [22]. Within a healthy population, the mean (SD) FEV1 Z-scores would approximate 0(1). A Z-score difference in FEV1 is equivalent to 12% of predicted FEV1 at these ages.
Anthropometry, blood pressure, arterial stiffness, and wave reflection measurements
Field staff was trained for 1 week prior to the start of fieldwork, and were recertified at 6-month intervals. Equipments were calibrated regularly by the field supervisors. During adolescence, assessments were conducted in schools and at 21–23 years, in community locations (e.g. local general practitioner's surgeries, local community pharmacies, survey clinic rooms at Kings College London).
Height was measured using portable stadiometers (Leicester, UK), to the nearest 0.1 cm and weight using Salter electronic scales, to the nearest 0.1 kg. Waist circumference (cm) was measured midway between the 10th rib and the top of the iliac crest, and 0.5 cm subtracted to correct for measurement over T-shirt or vest. The mean of two duplicate measures was derived for the waist to height ratio (WHtR). SBP, and DBP were measured at both time points using validated OMRON M5-I semiautomatic devices (Kyoto, Japan), and appropriately sized cuffs, after participant had sat quietly for a timed 5 min, with more than 1 min between three readings. The mean of second and third readings was analysed, as previously reported [14]. At 21–23 years, central SBP (cSBP), pulse, PWV, central AIx (AIxao), and brachial BP were also measured using the Arteriograph 24-h device (TensioMed, Budapest, Hungary), previously calibrated and standardized [23].
Social exposures
A self-administered questionnaire measured smoking, alcohol consumption, racism, and SEC at 11–13 years and 21–23 years. Reported racism was assessed using validated questions on unfair treatment on grounds of race, skin colour, country of birth, or religion in various locations (school, neighbourhoods, and work) [24]. In adolescence, SEC was assessed using parental employment and the Family Affluence Scale [25] based on number of cars, computers, and holidays. In adulthood, SEC was measured using own education and employment.
Statistical analyses
Continuous variables (SBP, DBP, WHtR, FEV) did not require transformation as the Shapiro–Wilk test indicated normal distributions. For BP, we first examined the cross-sectional associations between FEV1 Z-score and BP at 11–13 years and at 21–23 years, using linear regression models. We then used linear mixed models to examine the association between the change in FEV1 and the change in BP, between 11–13 years and 21–23 years. In these latter analyses zFEV1, sex, and ethnicity at 11–13 years were added as fixed covariates, and change in zFEV1 and remaining social exposures were added as time varying covariates. All variables were added stepwise and two models (model with age, sex, ethnicity, WHtR, and the model with further adjustment for all social exposures) were presented. For outcomes that were measured only at 21–23 years (cSBP, PWV, Aix, and FEV1), linear regression models were used to examine associations between these outcomes and forced expiratory volume Z-scores in 1 s (zFEV1) at 11–13 years and at 21–23 years. Two sets of models were run. The first set was with zFEV1 at 11–13 years to examine, whether at this critical period of adolescence, there was an association with these outcomes at 21–23 years. The second repeated the analyses with zFEV1 at 21–23. All the analyses were performed STATA 13 (Stat Corp., College Station, Texas, USA). P < 0.05 were considered to be statistically significant.
RESULTS
Study population
After exclusions, 488 subjects (53.1% males) were included in the analyses. At age 11–13 years, the longitudinal sample's profile for most anthropometric parameters, BP and FEV1 did not vary significantly by gender (Table 1). At 21–23 years, men were taller and had higher BP, brachial SBP, cSBP, and PWV but a lower heart rate and AIxao. Mean zFEV1 for all ethnic groups and at both ages were within ± 0.5 Z-scores; Table S1). The 10-year increase in height, SBP, and DBP was greater for Whites than for other ethnic minority groups (Table S2). At 21–23 years, Whites also had a higher PWV and lower AIxao (Table S1).
TABLE 1.
Sample characteristics by sex
| Variables | 11–13 years | 21–23 years | ||
| Male | Female | Male | Female | |
| Age (years) | 12.6 (12.5,12.7) | 12.5 (12.4,12.6) | 22.9 (22.8,23.0) | 22.7 (22.6,22.8) |
| Height (cm) | 155.0 (153.7,156.3) | 155.7 (154.7,156.7) | 176.1 (175.2,176.8) | 163.7 (162.8,164.5) |
| Waist to height ratio | 0.40 (0.41,0.44) | 0.43 (0.42,0.44) | 0.48 (0.47,0.49) | 0.49 (0.08,0.50) |
| SBP (mmHg) | 109.2 (107.94,110.50) | 107.2 (106.0,108.3) | 120.2 (118.9,121.4) | 107.1 (105.8,108.4) |
| DBP (mmHg) | 66.5 (65.5,67.5) | 66.7 (65.8,67.6) | 73.5 (72.5,74.5) | 71.4 (70.4,72.4) |
| FEV1 (l) | 2.4 (2.3,2.5) | 2.3 (2.3,2.4) | 3.9 (3.7,4.1) | 2.9 (2.8,2.9) |
| zFEV1 | −0.22 (1.08) | −0.32 (1.09) | −0.43 (0.98) | −0.43 (1.01) |
| Pulse (beats/min) | 66.4 (65.0,67.7) | 71.3 (69.8,72.7) | ||
| zFEV1 (%) | ||||
| −1 to +1 Z score | 62.1 (56.3,68.1) | 61.6 (55.2,68.1) | 62.5 (56.6,68.3) | 65.8 (59.4,72.1) |
| Less than 1 Z score | 24.5 (19.4,29.7) | 28.8 (22.7,34.8) | 28.6 (23.2,34.1) | 26.5 (20.6,32.4) |
| >1 Z score | 13.4 (9.3,17.5) | 9.6 (5.7,13.5) | 8.9 (5.5,12.3) | 7.8 (4.2,11.3) |
| Brachial SBP (mmHg) | 121.8 (120.3,123.3) | 112.7 (111.0,114.4) | ||
| acSBP (mmHg) | 109.6 (108.2,111.0) | 103.6 (102.2,105.0) | ||
| PWV (m/s)b | 7.4 (7.19,7.6) | 6.8 (6.6,6.9) | ||
| AIxao (%) | 12.1 (11.0,13.3) | 15.6 (14.1,17.0) | ||
| Current smoker (%) | 0.01 (−0.001,0.2) | 0.02 (0.001,0.03) | 32.7 (27.1,38.3) | 37.4 (31.0,43.9) |
| Current alcohol user (%) | 25.7 (20.4,30.9) | 24.7 (18.9,30.4) | 53.2 (47.2,59.2) | 61.2 (54.7,67.7) |
| Reported racism (%) | 20.4 (15.6,25.3) | 20.1 (14.8,25.4) | 43.9 (37.9,49.8) | 43.4 (36.8,50.0) |
| Parental/own employment (%) | 75.8 (70.7,81.0) | 77.6 (72.1,83.2) | 48.3 (42.3,54.3) | 52.5 (45.9,59.2) |
| Family Affluence Scalec | ||||
| ≥3 | 53.5 (47.5,59.5) | 53.0 (46.3,59.6) | ||
| 1–2 | 27.5 (22.4,32.9) | 29.7 (23.6,35.8) | ||
| Completed a university degree (%) | 40.9 (35.0,46.8) | 57.5 (51.0,64.1) | ||
The Determinants of Adolescent Social Well-Being and Health study.
Values are mean (95% CI) or percentage (95% CI). Z score of FEV1 are mean (SD).
AIxao, central augmentation index; CI: confidence interval; cSBP, central SBP; PWV, pulse wave velocity; zFEV1, forced expiratory volume Z-scores in 1 s.
acSBP, central SBP.
bPWV, pulse wave velocity.
cFamily affluence scale comprises of number of holidays last year, computers, cars, or vans.
Forced expiratory volume Z-scores in 1 s and blood pressure
Analyses of the cross-sectional data at 11–13 years showed that zFEV1 was positively and independently associated with SBP at 11–13 year (Table 2). Unadjusted, an increase in 1 zFEV1 score was associated with +2.1 [95% confidence interval (CI) 1.3–2.9] mmHg rise in SBP. Adjusting for age, sex, ethnicity, and WHtR reduced this effect a little [+1.90 (1.11–2.68) mmHg]. There was no significant change on further adjustment of racism, parental employment, current smoking, and alcohol use. zFEV1 was not associated with DBP at 11–13 years. At 21–23 years, zFEV1 was positively but not significantly associated with SBP (Table 2). Figure 1 shows the predicted means by ethnicity, derived from the core model, adjusted for age, sex, and WHtR. A similar pattern was observed across the ethnic groups, with a stronger association between zFEV1 and SBP at 11–13 years than 21–23 years. zFEV1 was not associated with DBP (Table S3).
TABLE 2.
Cross-sectional and longitudinal associations between forced expiratory volume in 1 s and SBP from early childhood to young adults
| Variables | SBP at 11–13 yearsa | SBP at 21–23 yearsa | Δ SBP |
| zFEV1 | 2.1 (1.3,2.9)b | 0.5 (−0.3,1.4) | 0.02 (−1.2,1.1) |
| ΔzFEV1 | 1.4 (0.3,2.5)c | ||
| Ethnicity (White UK: ref) | |||
| Black Caribbean | −0.3 (−3.2,2.5) | −1.7 (−4.8,1.4) | −0.8 (−3.3,1.6) |
| Black African | −0.1 (−2.9,2.7) | −2.0 (−5.1,1.1) | −0.8 (−3.2,1.6) |
| India | −1.4 (−4.4,1.6) | −4.0 (−7.2,−0.8)c | −2.5 (−5.0,0.0) |
| Pakisitani/Bangladeshi | −5.4 (−8.5,−2.5)b | −6.8 (−10.2, −3.4)b | −6.4 (−9.0, −3.8)b |
| Others | −1.0 (−3.7,1.7) | −3.8 (−6.8, −0.8)c | −2.4 (−4.8, −0.1)c |
| Female | −1.6 (−3.2,0.1) | −13.9 (−15.6, −12.1)b | −7.7 (−9.1, −6.3) |
| Age | 2.9 (1.2,4.3)b | 0.3 (−0.9,1.5) | 0.4 (0.3,0.6)b |
| Waist to height ratio | 26.9 (12.4,41.4)b | 31.9 (20.0,43.9) | 26.4 (16.4,36.3)b |
| Reported racism (no: ref) | |||
| Yes | −1.3 (−3.4,0.8) | 0.2 (−2.0,1.6) | −0.9 (−4.6,2.8) |
| Parental/own employment (yes: ref) | |||
| No | −0.6 (−2.9,1.7) | −1.0 (−3.1,1.1) | −0.2 (−1.8,1.3) |
| Current smoking (no: ref) | |||
| Yes | −6.3 (−13.3,0.6) | 0.2 (−2.1,2.5) | 2.5 (0.4,4.6)c |
| Alcohol use (No: ref) | |||
| Yes | −1.3 (−3.4,0.9) | 0.3 (−1.9,2.5) | 0.8 (−2.4,0.8) |
The Determinants of Adolescent Social Well-Being and Health study.
zFEV1, lifestyle factors (alcohol and smoking), lifestyle factors, employment, reported racism.
aLinear models adjusted for age, sex, ethnicity and waist to height ratio and reported racism, employment, lifestyle factors (smoking and alcohol use; 11–13 years R2 = 0.17, 21–23 years R2 = 0.36).
bData were statistically significant at P < 0.001.
cData were statistically significant at P < 0.05.
FIGURE 1.
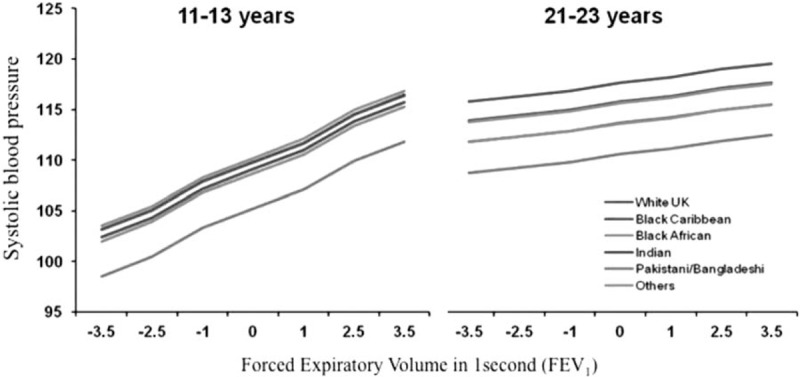
Predicted mean systolic blood pressure by zFEV1 (superscript for footnote) at 11–13 years and at 21–23 years, derived from linear regression models adjusted for age, sex, ethnicity, and waist to height ratio. DASH, The Determinants of Adolescent Social Well-being and Health; zFEV1, forced expiratory volume Z-scores in 1 s.
In analysis of the longitudinal data, changes in zFEV1 between 11–13 years and 21–23 years were associated with SBP between 11–13 years and 21–23 years (Table 2). Adjusting for age, gender, ethnicity, changes in WHtR were positively associated with SBP, the pooled average being +1.38 (0.3–1.6) mmHg, unaltered by lifestyle and social exposures. Changes in zFEV1 were not associated with DBP between 11–13 years and 21–23 years (Table S4).
The associations between forced expiratory volume Z-scores in 1 s and central SBP, pulse wave velocity, and central augmentation index
zFEV1 at 11–13 years or 21–23 years was not significantly associated with cSBP, PWV, or AIx at 21–23 years (Table 3). PWV was significantly associated with sex (women lower), age, WHtR, and heart rate, the latter two also reducing AIx, which was unaffected by age or social exposures.
TABLE 3.
The influence of forced expiratory volume in 1 s from early childhood to young adults on central blood pressure, pulse wave velocity, and augmentation index at 21–23 year
| Variables | cSBP | PWV | AIXao |
| zFEV1 at 21–23 years | 0.4 (−2.1,3.0) | 0.03 (−0.2,0.1) | 0.2 (−1.1,1.5) |
| zFEV1 at 11–13 years | −0.7 (−3.3,1.8) | 0.005 (−0.2,0.2) | −0.5 (−1.7,0.7) |
| Age | −2.6 (−6.6,1.5) | 0.7 (0.2,0.7)a | −1.6 (−3.5,0.4) |
| Female | −5.5 (−9.5,−1.5)a | −0.7 (−1.0,−0.4)a | 2.3 (−0.3,4.9) |
| Ethnicity (White UK: ref) | |||
| Black Caribbean | −2.4 (−8.5,3.6) | −0.1 (−-0.6,0.3) | 0.1 (−3.1,3.3) |
| Black African | 1.4 (−5.2,8.0) | −0.3 (−0.7,0.2) | 3.0 (−0.3,6.3) |
| India | −5.0 (−11.5,1.5) | −0.2 (−0.7,0.2) | 1.5 (−2.0,4.9) |
| Pakisitani/Bangladeshi | −3.4 (−11.4,4.6) | −0.5 (−1.0,0.1) | 3.3 (−0.5,7.1) |
| Others | −1.2 (−7.1,4.8) | −0.3 (−0.8,0.1) | 0.9 (−2.1,4.0) |
| Waist to height ratio | 65.8 (27.6,104.0)a | 2.9 (0.4,5.4)b | −0.3 (−0.5, −0.2a |
| Pulse | −0.03 (−0.2,0.1) | 0.04 (0.03,0.05)a | −0.3 (−0.4, −0.2)a |
| brSBP | – | 0.01 (−0.00,0.02) | 0.1 (0.04,0.2)c |
The Determinants of Adolescent Social Well-Being and Health study.
Linear models adjusted for age, sex, ethnicity and waist to height ratio, pulse and (brachial blood pressure for PWV and AIXao) at 21–23 years and reported racism, employment, education, smoking and alcohol using at 21–23 years and adolescent adjustment (R2 = 0.17, 0.29, and 0.23).
AIxao, central augmentation index; PWV, pulse wave velocity.
aData were statistically significant at P < 0.001.
bData were statistically significant at P < 0.05.
cData were statistically significant at P < 0.01.
DISCUSSION
To the best of our knowledge, this is the first longitudinal study with a diverse ethnic composition, as yet from a pilot follow-up, to report an association between lung function and SBP between adolescence and early adulthood. The longitudinal changes in lung function are positively associated with SBP during this part of the life course when considerable growth occurs. The mechanisms underlying these associations are not well understood but could illustrate how greater lung growth marginally, but likely reversibly, impairs venous return to the heart over this growth period, with a reflex rise in SBP.
Normal developmental trajectory for lung function shows that it increases in childhood, peaks in young adulthood and then gradually decreases with age [12,26,27]. A US-based longitudinal study (N = 508) showed that a decline in lung function from average peak age (29.4 years) to 35 years significantly predicted incidence of hypertension between mean ages 35–45 years. In correspondence with DASH findings, the cross-sectional Wheezing Illnesses Study Leidsche Rijn (WHISTLER) study of 5-year-olds (N = 382) in the Netherlands showed a positive relationship between FEV1 and SBP (+4.8 mmHg/l, 95% CI: −0.3 to 10.0). WHISTLER also showed a positive relationship with DBP (+4.6 mmHg/l, 95% CI: −0.2 to 9.4) adjusted for age, sex, weight, and height [28]. The results of WHISTLER and DASH, though discrepant in relation to DBP, suggest that the positive relationship between BP and lung function is normal physiological development in childhood and in early adolescence. As the WHISTLER result suggests a litre change in FEV1 was associated with ∼5 mmHg in SBP change in a 5-year-old child, when mean (SD) FEV1 would be only 1.3(0.2) l, a change of 200 ml (1 SD) in FEV1 would be associated with a 1 mmHg increase in SBP. Similar results were observed here in DASH.
As with lung function, the association between PWV and lung function has not been studied before in healthy children. A cross-sectional study of asthmatic children (aged 6.1–15.3 years) in Switzerland showed that carotid-femoral PWV was inversely associated (r2=0.20, P = 0.004) with FEV1[29]. It is noteworthy that the effect size of about +2 mmHg increase in SBP these studies with 1 Z-score of FEV1 is comparable across these studies of 5-year-old and of 11–13 years in DASH. Perhaps in contrast to the DASH findings, a cross-sectional community-based study of 249 8-year-old children in Australia showed FEV1 was inversely associated with carotid (not brachial, as here) AIx75 (coefficient = −0.17, P = 0.03) and forced vital capacity (coefficient = −0.29, P < 0.001) [30].
Our results showed that lung function is associated with SBP at 11–13 years, and the change in lung function from 11 to 23 years is associated with the change in SBP. At 21–23 years lung function was not associated with SBP or PWV. There are very few studies that have examined cardiorespiratory health in healthy young adults. Garcia-Larsen et al.[31] showed no association between lung function and hypertension in 25-year-olds. We are not aware of any studies that have examined the association of lung function and PWV in young adults. One possible explanation for the lack of association between lung function and PWV is that PWV was measured only at 21–23 years (not at 11–13 years). At 11–23 years, lung and vascular function are expected to be within a normal range. Thus, these findings cannot be extrapolated to older age when other risk factors can affect both parameters. In addition, pollutants can deeply influence BP [32] as well as lung function [33], even in adolescence. The impact of pollution is the focus of separate analyses.
Strengths and weaknesses of study
Social exposures, for example, deprivation, influence growth [34], which in turn influences cardiorespiratory development. The studies cited above did not examine potential moderating influences from social exposures. DASH contains information on socioeconomic disadvantage and behaviours, which enabled an examination of their association on the zFEV1-cardiovascular disease measure. DASH contains a diverse sample, has high retention rates and low item-nonresponse, mainly because of enormous community support. Lung function was not measured at the second follow-up of the cohort at 14–16 years, which could have provided valuable insight into the pattern of changing associations with BP in adolescence. The lack of data before age 11 years and the relatively small sample sizes for the ethnic groups in the pilot follow-up are limitations. Prior to DASH a large-scale study with an explicit focus on ethnicity had not been attempted. The use of the GLI-2012 ethnic-specific equations rather than absolute FEV also strengthened the analysis. These robust equations have been shown to be appropriate for London school children [35] and the small offset (≤0.5 Z-scores) in mean FEV1 in all ethnic groups observed in this study suggest a sample size issue. Quanjer et al. suggested that a dataset of at least 300 (150 men and 150 women) would be required to validate reference values to avoid spurious differences because of sampling error [36]. Despite relatively small numbers, these findings in a tight age range provide strong justification for future studies on the degree and how childhood and adolescent lung function influence vascular development to improve understanding of the developmental interaction of cardio-respiratory systems.
ACKNOWLEDGEMENTS
We acknowledge the invaluable support of participants and their parents, the Participant Advisory Group, schools, civic leaders, local GP surgeries and community pharmacies, the Clinical Research Centre at Queen Mary University of London, the Clinical Research Facility at University College Hospital, the survey assistants and nurses involved with data collection, Rachel Bonner, senior respiratory physiologist (UCL GOS ICH) for spirometry training and over-reading of data for quality control, the Primary Care Research Network, and Professor Sanders and J.K.C at the Diabetes and Nutritional Sciences Division at Kings College London for hosting the feasibility study. H.S. is the Principal Investigator of DASH. All authors contributed to study design, analyses, and writing of the article. The study was funded by the MRC (MC_U130015185/MC_ UU_12017/13), Chief Scientist Office (SPHSU13), North Central London Research Consortium, and the Primary Care Research Network.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 2
The present longitudinal study aims to demonstrate a relationship between vascular and lung function considering that the alterations of both systems can predict cardiovascular events. The most interesting and intriguing finding is that forced expiratory volume modifications are associated with systolic blood pressure values from adolescence (11-13 years) to young adulthood (10 years after). Obviously, the study is descriptive and it is not possible to establish a clear cause-effect relationship, also considering that lung function is not related to aortic pulse way velocity, one of the main determinants of systolic blood pressure. However, the study demonstrates an important relationship between pulmonary and vascular function, which might be of great relevance in the pathogenesis of cardiovascular disease.
Footnotes
Abbreviations: AIx, augmentation index; BP, blood pressure; FEV1, forced expired volume in 1s; GLI-2012, Global Lung Function Initiative; PWV, pulse wave velocity; SEC, socioeconomic circumstances; WHtR, waist to height ratio; zFEV1, forced expiratory volume Z-scores
REFERENCES
- 1.Gulsvik AK, Gulsvik A, Skovlund E, Thelle DS, Mowe M, Humerfelt S, Wyller TB. The association between lung function and fatal stroke in a community followed for 4 decades. J Epidemiol Community Health 2012; 66:1030–1036. [DOI] [PubMed] [Google Scholar]
- 2.Tarnoki DL, Tarnoki AD, Lazar Z, Medda E, Littvay L, Cotichini R, et al. Genetic and environmental factors on the relation of lung function and arterial stiffness. Respir Med 2013; 107:927–935. [DOI] [PubMed] [Google Scholar]
- 3.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med 2013; 1:728–742. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008; 371:340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WL, Wang CC, Wu LW, Kao TW, Chan JY, Chen YJ, et al. Relationship between lung function and metabolic syndrome. PLoS One 2014; 9:e108989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engstrom G, Wollmer P, Valind S, Hedblad B, Janzon L. Blood pressure increase between 55 and 68 years of age is inversely related to lung function: Longitudinal results from the cohort study ’men born in 1914’. J Hypertens 2001; 19:1203–1208. [DOI] [PubMed] [Google Scholar]
- 7.Paek YJ, Jung KS, Hwang YI, Lee KS, Lee DR, Lee JU. Association between low pulmonary function and metabolic risk factors in korean adults: the korean national health and nutrition survey. Metabolism 2010; 59:1300–1306. [DOI] [PubMed] [Google Scholar]
- 8.Bolton CE, Cockcroft JR, Sabit R, Munnery M, McEniery CM, Wilkinson IB, et al. Lung function in mid-life compared with later life is a stronger predictor of arterial stiffness in men: the caerphilly prospective study. Int J Epidemiol 2009; 38:867–876. [DOI] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Aortic stiffness for cardiovascular risk prediction: just measure it, just do it!. J Am Coll Cardiol 2014; 63:647–649. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rooyen Y, Schutte AE, Huisman HW, Eloff FC, Du Plessis JL, Kruger A, van Rooyen JM. Inflammation as possible mediator for the relationship between lung and arterial function. Lung 2016; 194:107–115. [DOI] [PubMed] [Google Scholar]
- 12.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multiethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Resp J 2012; 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Blood pressure differences by ethnic group among united states children and adolescents. Hypertension 2009; 54:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding S, Whitrow M, Lenguerrand E, Maynard M, Teyhan A, Cruickshank JK, Der G. Emergence of ethnic differences in blood pressure in adolescence: The determinants of adolescent social well being and health study. Hypertension 2010; 55:1063–1069. [DOI] [PubMed] [Google Scholar]
- 15.Cruickshank JK, Silva MJ, Molaodi OR, Enayat ZE, Cassidy A, Karamanos A, et al. Ethnic differences in and childhood influences on early adult pulse wave velocity: The determinants of adolescent, now young adult, social wellbeing, and health longitudinal study. Hypertension 2016; 67:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson W, Li L, Kuh D, Hardy R. How has the age-related process of overweight or obesity development changed over time? Co-ordinated analyses of individual participant data from five united kingdom birth cohorts. PLoS Med 2015; 12:e1001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gishti O, Jaddoe VWV, Hofman A, Wong TY, Ikram MK, Gaillard R. Body fat distribution, metabolic and inflammatory markers and retinal microvasculature in school-age children. The generation r study. Int J Obes 2015. [DOI] [PubMed] [Google Scholar]
- 18.Harding S, Whitrow M, Maynard MJ, Teyhan A. Cohort profile: The dash (determinants of adolescent social well being and health) study, an ethnically diverse cohort. Int J Epidemiol 2007; 36:512–517. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005; 26:319–338. [DOI] [PubMed] [Google Scholar]
- 20.Whitrow MJ, Harding S. Ethnic differences in adolescent lung function: Anthropometric, socioeconomic, and psychosocial factors. Am J Respir Crit Care Med 2008; 177:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968. [DOI] [PubMed] [Google Scholar]
- 22.Lum S, Bountziouka V, Quanjer P, Sonnappa S, Wade A, Beardsmore C, et al. Challenges in collating spirometry reference data for south-asian children: an observational study. PLoS One 2016; 11:e0154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faconti L, Silva MJ, Molaodi OR, Enayat ZE, Cassidy A, Karamanos A, et al. Can arterial wave augmentation in young adults help account for variability of cardiovascular risk in different british ethnic groups? J Hypertens 2016; 34:2220–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger N, Sidney S. Racial discrimination and blood pressure: the cardia study of young black and white adults. Am J Public Health 1996; 86:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currie C, Molcho M, Boyce W, Holstein B, Torsheim T, Richter M. Researching health inequalities in adolescents: the development of the health behaviour in school-aged children (hbsc) family affluence scale. Soc Sci Med 2008; 66:1429–1436. [DOI] [PubMed] [Google Scholar]
- 26.Strachan DP, Sheikh A. A life course approach to respiratory and allergic diseases. 2nd ed.2004; Oxford: Oxford University Press, 240–259. [Google Scholar]
- 27.Quanjer PH, Brazzale DJ, Boros PW, Pretto JJ. Implications of adopting the global lungs initiative 2012 all-age reference equations for spirometry. Eur Respir J 2013; 42:1046–1054. [DOI] [PubMed] [Google Scholar]
- 28.Eising JB, van der Ent CK, van der Gugten AC, Grobbee DE, Evelein AM, Numans ME, Uiterwaal CS. Life-course of cardio-respiratory associations. Eur J Prev Cardiol 2015; 22:140–149. [DOI] [PubMed] [Google Scholar]
- 29.Steinmann M, Abbas C, Singer F, Casaulta C, Regamey N, Haffner D, et al. Arterial stiffness is increased in asthmatic children. Eur J Pediatr 2015; 174:519–523. [DOI] [PubMed] [Google Scholar]
- 30.Ayer JG, Belousova EG, Harmer JA, Toelle B, Celermajer DS, Marks GB. Lung function is associated with arterial stiffness in children. PLoS One 2011; 6:e26303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Larsen V, Bustos P, Amigo H, Potts J, Rona RJ. Ventilatory function and cardiovascular disease risk factors: a cross-sectional study in young adults. BMC Pulm Med 2014; 14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, et al. Particulate matter air pollution and ambient temperature: opposing effects on blood pressure in high-risk cardiac patients. J Hypertens 2015; 33:2032–2038. [DOI] [PubMed] [Google Scholar]
- 33.Astell-Burt T, Maynard MJ, Lenguerrand E, Whitrow MJ, Molaodi OR, Harding S. Effect of air pollution and racism on ethnic differences in respiratory health among adolescents living in an urban environment. Health Place 2013; 23:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwok MK, Schooling CM, Subramanian SV, Leung GM, Kawachi I. Pathways from parental educational attainment to adolescent blood pressure. J Hypertens 2016; 34:1787–1795. [DOI] [PubMed] [Google Scholar]
- 35.Lum S, Bountziouka V, Sonnappa S, Wade A, Cole TJ, Harding S, et al. Lung function in children in relation to ethnicity, physique and socioeconomic factors. Eur Respir J 2015; 46:1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quanjer PH, Stocks J, Cole TJ, Hall GL, Stanojevic S, Global Lungs I. Influence of secular trends and sample size on reference equations for lung function tests. Eur Respir J 2011; 37:658–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


