Abstract
A large body of research data suggests that traditional dietary habits and lifestyle unique to the Mediterranean region (Mediterranean diet, MD) lower the incidence of chronic diseases and improve longevity. These data contrast with troubling statistics in the United States and other high income countries pointing to an increase in the incidence of chronic diseases and the projected explosion in cost of medical care associated with an aging population. In 2013, the MD was inscribed by UNESCO in the “Representative List of the Intangible Cultural Heritage of Humanity.” The 2015–2020 Dietary Guidelines for Americans included the MD as a healthy dietary pattern. Therefore, specific objectives of this article are to provide an overview of the nutritional basis of this healthful diet, its metabolic benefits, and its role in multiple aspects of disease prevention and healthy aging. Whereas recommendations about the MD often focus on specific foods or bioactive compounds, we suggest that the eating pattern as a whole likely contributes to the health promoting effects of the MD.
Mortality attributable to chronic diseases is projected to increase as the US population ages. At the same time, calorie, carbohydrate, and portion size intake have risen along with greater amounts of food and calories per meal, while the population has adopted lifestyles that are more sedentary. Together, these factors have contributed to the increase in noncommunicable diseases. The 48 million individuals (~15% of households) in the United States who are unable to acquire adequate food to meet their needs must also be considered because poverty can exacerbate the risk of some chronic diseases.1 During the last 3 decades, various dietary strategies and visual representations (ie, pyramids, plates) have been developed by US and health organizations elsewhere in the world to promote the rebalancing of sources of calories with more physical activity to ensure dietary adequacy and reduce the burden of chronic diseases. The Scientific Report of the 2015–2020 Dietary Guidelines of the US Department of Health and Human Services and US Department of Agriculture (USDA)2 recognized that dietary patterns of the American public are suboptimal and causally related to poor individual and population health, as well as higher chronic disease rates.
The Dietary Guidelines for Americans1 suggested the adoption of healthy eating patterns characterized by higher consumption of fruits, vegetables, and whole grains and lower intake of calories, saturated fat, sodium, refined grains, and added sugars. Moreover, such a pattern would help address underconsumption of vitamin D, calcium, potassium, and fiber, nutrients that have been identified as of public concern for most of the US population. One such healthy eating pattern contributing to overall health is the Mediterranean diet (MD). This article describes how many different foods and beverages act together in this pattern to contribute to overall health outcomes. The MD is a dietary pattern or model that integrates a number of variations on a basic theme adapted to an individual country's heritage and cultures. Compared with western dietary patterns, the MD favors local and seasonal food production to a greater degree. During the last 2 decades, many studies have reported on the association between consumption of such an MD and reduced risk of chronic diseases, leading to the development of an MD pyramid.3 Studies of the MD and reduced risk of cardiovascular diseases (CVDs) contributed the highest number of scientific citations, but clearly, there are also publications suggesting reductions in other chronic diseases including cancer, diabetes, and neurodegenerative diseases (Figure 1).4 This article highlights some opportunities and challenges for the adoption of an MD eating pattern to reduce chronic disease risk and promote healthy aging.
FIGURE 1.
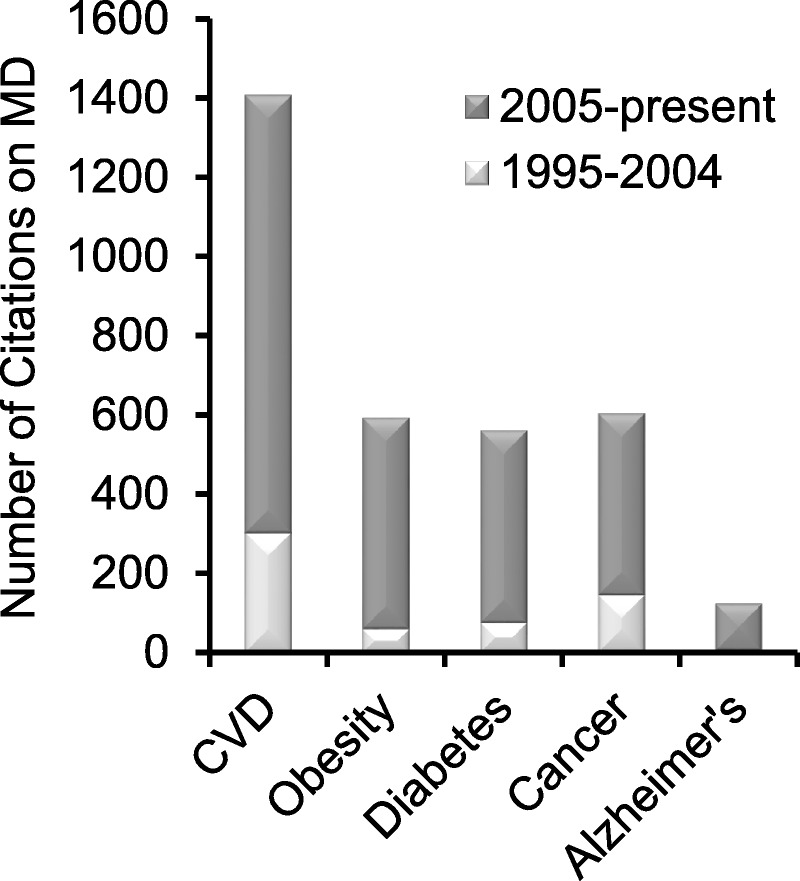
Studies on MD and health during the last 20 years. Bars represent number of citations in PubMed for two time periods (1995–2004 and 2005–present) for combinations of MD studies and specific chronic diseases.4
PREVENTION OF CHRONIC DISEASES
CVD and Metabolic Syndrome
Fact: Cardiovascular diseases include coronary heart disease (CHD) (coronary artery disease, ischemic heart disease), stroke, high blood pressure (hypertension), and rheumatic heart disease. In 2010, approximately 84 million men and women 20 years and older in the United States (35% of the population)1 were affected by some form of CVD. Reports from the National Health and Nutritional Examination Survey indicate that the prevalence of metabolic syndrome (MetS) is approximately 28% among US adults, with a persistent increase during the last 3 decades.5
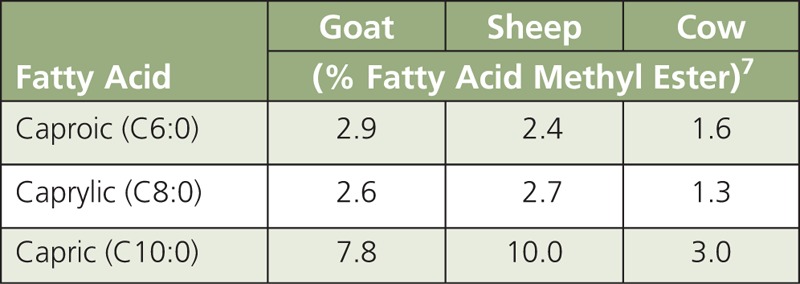
Fats, oils, and metabolites. In Ancel Keys'6 Seven Countries Study on the MD, the principal disease outcome analyzed was CVD and, particularly, CHD. Although the overall fat intake in Crete was similar to that of populations living in East Finland, approximately 36% and 39% of total energy intake (TEI), respectively, the Crete population had an approximately 30-fold lower rate of CHD. In East Finland, consumption of saturated fatty acids (SFAs) contributed approximately 24% of TEI compared with only approximately 8% in Crete. It is now recognized that monounsaturated fatty acids (MUFAs) found in extra virgin olive oil (EVOO) and n-3 polyunsaturated fatty acids (PUFA) mainly from fish contributed to the beneficial effects of the MD observed in the Crete’s population. A traditional MD would provide ~36–40% of TEI from fat, of which 7–10% is SFA; 19–25% MUFA; and 3–6% PUFA. Although the MD and US diet provide comparable levels of TEI from SFA (~7–11%), in the MD eating pattern the higher consumption of olive oil is mainly responsible for the higher energy intake (19–25% vs ~12.5%) from MUFA (Table 1). In the traditional MD pattern, the intake of dairy products and meats is lower than in the Western diet,8 and animal fats are mainly from goats and sheep, which provide a higher content in medium-chain fatty acids (MCFA ≤ 12 C) that are less atherogenic than long-chain fatty acids (LCFA ≥ 13 C). A notable difference in the SFA composition of milk from goats and sheep versus cow’s milk is the far higher levels of MCFA, namely caproic (C6:0), caprylic (C8:0), and capric (C10:0) fatty acids (Table 2). For example, goat’s milk typically contains 15-18% MCFA compared to only 5-9% in cow’s milk.9,10 In Mediterranean countries, milk from various ruminant species (cow, goat, and sheep) is processed to a large extent into cheese or yogurt, rather than being consumed raw or converted into butter. Lipolysis during cheese aging increases free MCFA several fold.11 Ruminant species that spend more time grazing also tend to produce in n-3 α-linolenic acid (ALA), i.e., ~2.5% of total fatty acids in lamb meat compared to 1.3% in beef.12 These observations suggest that both quantity and type of fat should be taken into consideration when assessing nutritional benefits of the MD eating pattern. For example, the most commonly used vegetable fat in the United States is soybean oil rich in n-6 linoleic acid (LA).1 Conversely, Mediterranean populations tend to consume a larger percentage of PUFA of marine origin. In Spain, consumption of fish approximates ~55 g/d13 compared to only ~16 g/d in the United States. Data for the years 2005–2006 estimated that in the United States, LA and n −3 PUFA contributed ~7 and ~0.05% % of TEI, respectively.14 In contrast, the baseline consumption of n-3 PUFA in the 2003–2004 Prevención con Dieta Mediterránea (PREDIMED) cohort was 0.32% of TEI, almost ten times higher than US consumption.15 Thus, there are important differences between the Mediterranean16 and US diet not only in the quantity and the source of MUFA, but also in the quantity of n-6 and n-3 PUFA. Results of the PREDIMED trial pointed out that a MD with relatively high fat intake (35–40%), mostly from EVOO, was associated with primary prevention of CVD.15 Higher intakes of nuts and EVOO were also associated with a lower incidence of type-2 diabetes.17 These observations raise the concern about recommending a low-fat diet, which coupled with the widespread availability of zero- or low-fat foods (e.g., milk, yogurt), may actually favor higher consumption of refined starch-and sugar-rich foods, thus contributing to excessive energy intake, overweight, obesity, and related complications (i.e., CVD). In support of this concern, there is some evidence that compared to carbohydrates, both MUFA and PUFA in the MD eating pattern tend to reduce low-level density lipoprotein cholesterol (LDL-C) and triglycerides (TG) while increasing high-density lipoprotein (HDL-C).18 These metabolic benefits of the MD may be even greater in individuals with underlying insulin resistance.
TABLE 1.
Dietary Estimates of Fat Intake (as % Total Energy Intake) in Mediterranean Countries and the United States Compared to a Model MD7
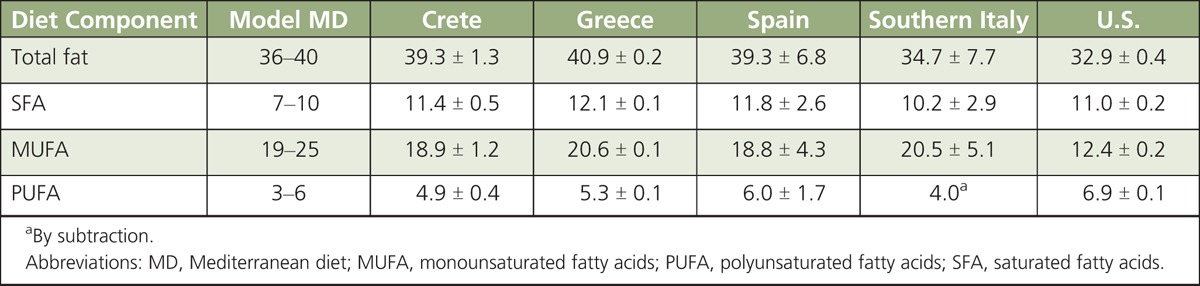
TABLE 2.
Main Medium-Chain Fatty Acids in Milk From Ruminant Species
Studies on the MD conducted nearly 40 years ago concluded that a fat intake contributing ~35–40% of TEI, with MUFA plus PUFA providing ~20–30% TEI and SFA providing 9%, was associated with a lower risk of CVD.19 Some nutritionists contend that dietary recommendations to further reduce SFA (i.e., below ~10% of TEI) may favor higher consumption of protein and carbohydrates at the expense of healthy fatty acids from fish, olive oil, and dairy products (i.e., conjugated linoleic acid and MCFA). Clearly, a major tenet of the MD eating pattern is that fats present in olive oil (18:1) and fish (eicosapentaenoic acid, EPA; docosahexaenoic acid, DHA) should not be equated to those present in vegetable oils rich in n-6 LA for which obesogenic and diabetogenic effects have been observed in some preclinical studies.20
In general, results from clinical, cross-sectional, and prospective studies support the health benefits of a MD eating pattern against MetS. The clinical diagnosis of MetS is having at least three of the following conditions: a waist circumference > 102 cm for men and > 88 cm for women; high TG levels (≥150 mg/dL); low HDL-C (<40 mg/dL for men and <50 mg/dL for women); high blood pressure (BP ≥130/85 mmHg) on at least two separate measurements; and high fasting glucose (≥100 mg/dL or ≥5.6 mmol/L).21 Metabolomic studies have tentatively identified deacetoxy oleuropein as a putative metabolite biomarker of a healthy diet associated with olive oil consumption. Oleuropein is a secoiridoid glycoside with antioxidant, cardioprotective, and antiatherogenic properties. Oleuropein aglycone (and its derivatives) and quercetin were identified as the main compounds in olive oil exerting antiproliferative effects in the gut.22 Metabolomics-based studies have also correlated the health benefits of a MD-like diet (i.e., fish consumption) to increased urinary excretion of 1-methylhistidine p-hydroxyphenylacetate, and reduced production of trimethylamine-N-oxide, a risk factor for CVD. Moreover, metabolomics measurements from double-blind, randomized, placebo-controlled dietary interventions identified increased production of phenylacetylglutamin as another metabolite biomarker of high-vegetable intake characteristic of the MD. Thus, metabolomic approaches hold great promise for advancing our understanding of the effects of a MD eating pattern on the host-microbiota interactions, and further the prevention of chronic diseases including CVD and MetS.
Wine. Epidemiological data and various clinical trials support a correlation between moderate red wine consumption and lower risk of CVD. For example, results of the MONICA (Monitoring System for Cardiovascular Disease) project that followed seven million men and women between 35 and 64 years of age in 37 countries for a period of 10 years concluded that populations residing in France had a ~3-fold lower annual mortality due to CVD when compared to populations residing in other industrialized nations. This “French paradox” was attributed in part to regular consumption of wine in spite of the fact the French group had risk factors for CVD similar to those of other countries, and a ~3-fold higher intake of SFA. On the other hand, it is well established that drinking too much (higher than ~2 drinks/d) increases mortality due to all causes and CVD.23 Current MD pattern and lifestyle recommendations include regular, albeit moderate, consumption of red wine (1 drink/d for women, 2 drinks/d for men), mainly with meals.1,24 Nevertheless, clinical judgment is needed to determine if alcohol consumption is appropriate based on individual medical history and the person’s predisposition to binge drinking and alcohol dependency.25
Aging
Fact: By 2050, adults 65 years of age and older are expected to comprise almost one-fifth of the population in the United States. Along with an increase in life expectancy (from 71 to 79 years since 1970), hypertension, arthritis, heart disease, diabetes, and cancer account today for a disproportionate fraction of health care expenditure.26
Observational studies with older adults illustrate the positive association of a MD eating pattern on health through reductions in BP, blood cholesterol, diabetes, inflammatory markers, coronary syndromes, CVD, body mass index (BMI), and obesity. Compared to a typical high SFA and carbohydrate diet, a MD showed improvements in lipid profiles and inflammatory markers.27,28 In studies of more than 1,500 healthy adults, participants (62 years of age and older) adhering to a MD had significantly less body fat compared to their non-adherent controls. Older individuals at high risk of CVD and consuming a MD with EVOO had a 40 % reduction in risk of developing diabetes, and those subjects consuming the MD with mixed nuts had an 18 % reduction in relative risk of diabetes compared to those consuming a low-fat control diet.26 Because the MD eating pattern relies heavily on EVOO, fruits, vegetables, whole grains, and lean meats and fish, it may provide a pattern that consists of foods with high nutrient density while helping to alleviate the progression of chronic health conditions (i.e., type-2 diabetes, cancer) during aging.
Neurodegenerative Diseases
Fact: During the period 2000–2013, US death rates for dementia among persons 75–84 years of age increased 21% for men and 31% for women. Among individuals 85 years of age or older, dementia-associated death rates for women and men were ~4,000 and 3,200 per 100,000 population, respectively.29
Age-related diseases affecting the brain and neuromuscular system comprise Alzheimer’s (AD), Parkinson’s (PD), and different forms of dementia ranging from mild cognitive decline to vascular dementia. AD accounts for ~70 % of all cases of dementia in elderly people. Higher adherence to a MD eating pattern has been associated with reduced incidence of AD and of cognitive decline. Compared to a Western diet rich in n-6 PUFA, SFA and meats, the abundance of fish, grains, fruits and vegetables in the MD may also be protective against PD and frailty.29 Fish oil and moderate wine consumption have been linked to slower cognitive decline and reduced risk of AD in observational studies. Another study documented a possible preventive role of phenols in EVOO against cognitive decline in individuals (age 55–80) at high risk of CVD.30 Lastly, prospective investigations conducted in Greece on subjects 65 years of age or older supported an inverse association between adherence to a MD and cognitive decline in older subjects.31 In a rodent model, dietary supplementation with oleuropein aglycone, a secoiridoid abundant in EVOO, has been shown to improve learning and synaptic functions by increasing brain levels of acetylated histone-4 at lysine-5 (AcH4K5), a marker of epigenetic activation, matched by a decreased expression of the epigenetic repressor histone deacetylase-2. Perturbations in gene expression are emerging factors in the pathogenesis of neurologic diseases, and a MD eating pattern rich in oleuropein aglycone may contribute to the prevention of AD through epigenetic mechanisms.32
Breast Cancer
Fact: Breast cancer is the third leading cause of cancer death in the United States. In 2012, an estimated 3 million women had a history of breast cancer.1 However, only a small number of breast tumors are hereditary, whereas the vast majority of breast cancer cases are sporadic.
Estimates from epidemiological studies suggest that ~25% of breast cancer cases could be prevented through adoption of a MD eating pattern over the typical Western diet.33 For example, in a case–control study with 2,396 Asian American women 25 to 74 years of age, a MD pattern was associated with a 35% lower risk for breast cancer.34 The Four-Corners study also reported a reduced risk for breast cancer in Hispanic as well as non-Hispanic white women adopting MD patterns.35 A study from Cyprus of 935 breast cancer cases and 817 women controls between 40 and 70 years of age was suggestive of a protective association for the MD eating pattern characterized by higher fruit/vegetables/fish intake.36 The protective effects of the MD against breast cancer have been related to reduced circulating estrogens, and increased intake of carotenoids that are known to lower oxidative stress.37 On the other hand, a study from France with 1,359 women that included 437 cases of breast cancer reported no significant association between adherence to the MD and breast cancer risk. Biases inherent to the adoption of case–control approaches versus other experimental models may explain why the latter study concluded that the MD eating pattern had no effects on breast cancer risk.38 In support of a protective association (~15% risk reduction) between adherence to a MD eating pattern and breast cancer development are large prospective studies of 65,374 French39 and 91,779 US40 women. Also, secondary analyses from the EPIC (European Prospective Investigation into Cancer and Nutrition) cohort showed that a higher MD score controlling for alcohol consumption was associated with a 6% lower risk for breast cancer.41 This protective association was greatest in women diagnosed with estrogen receptor (ER)- and progesterone receptor (PR)-negative breast disease. Similarly, large studies in the United Kingdom,42 Sweden,43 United States (Nurses’ Health Study),44 and Greece (EPIC)45 did not report a reduction in overall risk, but found protective associations for ER-negative breast tumors44 and in post-menopausal women.42,45
Wine. Flavonoids and phytoalexins are components widely distributed in vegetables and fruits including grapes. Some flavonoids have been shown to exert inhibitory activity against aromatase, the enzyme involved in estrogen production, which is a risk factor for breast cancer. For example, flavan-3ols (proanthocyanidins) and flavones (e.g. apigenin, luteolin) may reduce breast cancer risk by 7 and 10%, respectively. One study conducted in Northern Italy between 1983 and 2004 on over 20,000 cases of several major cancers and 18,000 controls suggested that intake of the phytoalexin resveratrol from fruits and vegetables, rather than wine consumption, was associated with a ~40% reduction in risk.46 In fact, alcohol consumption is associated with higher breast density and risk for breast cancer. Overall the estimated association is ~30-50% increase in breast cancer risk from 15–30 g/d of alcohol consumption.47
Fats and phenols. Epidemiological studies have reported that intake of olive oil reduced breast cancer risk.48 However, findings have not always been consistent. Oleic acid was shown to antagonize the expression and action of the human epidermal growth factor-2 (HER-2) oncogene in breast cancer cells. Phenolic compounds in olive oil (i.e., squalene, hydroxytyrosol) may protect against oxidative stress. Secondary analyses within the PREDIMED study reported lower breast cancer risk rates (per 1,000 person-years) for the MD with EVOO (Relative Risk, RR = 1.1) compared to the MD with nuts (RR = 1.8) and low-fat (RR = 2.9) control group.49 These protective effects of a MD rich in EVOO contrast with those of n-6 fatty acids (i.e. LA), which have been identified as risk factors due to elevation in prostaglandin E2 (PGE2) and cytokines known to promote inflammation and carcinogenesis.50 Preclinical studies support an anticancer role for n-3 fatty acids through inhibition of inflammation, proliferation, and angiogenesis, and induction of apoptosis. However, population studies have been inconclusive and advised that a threshold of intake of DHA and EPA may be necessary to elicit some protection against breast cancer.51
Inflammatory Bowel Disease
Fact: Approximately 1.6 million Americans have inflammatory bowel disease (IBD), an increase of 200,000 since 2011. As many as 70,000 new cases of IBD are diagnosed in the US each year. There may be as many as 80,000 children in the United States with IBD.52
Historically, the prevalence of IBD in the Mediterranean region has been lower compared to that of industrialized countries such as the United Kingdom, United States, Canada, and New Zealand.53
Olive oil. In vitro studies attribute the anti-inflammatory properties of olive oil to repression of cyclooxygenase-2 (COX-2) expression and PGE2 synthesis by phenolic compounds such as hydroxytyrosol, tyrosol, and β-sitosterol rather than to oleic acid alone. In animal studies, EVOO-enriched diet (5 %) improved ulcerative colitis (UC) and reduced inflammation. Importantly, the supplementation with olive oil polyphenols (i.e., hydroxytyrosol, 40 mg/kg of diet) improves results over EVOO alone.54 In Spanish CHD patients (average 68 years of age), daily doses of 50 ml of unprocessed EVOO over two 3-week periods reduced inflammatory markers (interleukin-6, IL-6; c-reactive protein, CRP), compared to control subjects given refined olive oil.55 Intervention groups consuming 25 ml/d of olive oil for 12 weeks had increased anti-inflammatory activity of HDL-C, which was reflected in decreased expression of intracellular adhesion molecule-1,56 also a marker of increased risk of IBD. These observations suggest that the phenolic compounds found in EVOO, and possibly other dietary oils (e.g., rice bran oil, argan oil), and not just MUFA, may prevent IBD and other chronic diseases.57
n-3 fatty acid, grain fiber, and wine polyphenols. Mixtures of fish oils (i.e., ~50% EPA and DHA) were shown to downregulate proinflammatory COX-2 in human colon cancer cells,58 and in animal models of IBD, fish n-3 PUFA attenuated experimental UC.59 Clinical trials found that n-3 fatty acid supplementation decreased blood levels of cytokines (IL-1β, IL-6) and markers of systemic inflammation (i.e. tumor necrosis factor-α, TNFα), while increasing blood levels of adiponectin60 and lowering risk of UC.61 In rodent models of Crohn’s disease, administration of a fiber-supplemented diet (5% psyllium seed by weight) decreased inflammatory markers (nitric oxide and TNFα) in colonic and cecal tissue. In cancer-free subjects of the Italian cohort of the EPIC study, dietary grain fiber intake was associated with reduced inflammatory markers in blood.62 Similarly, grain fiber intake (20–30 g of germinated barley) decreased endoscopic and clinical activity parameters in a clinical trial with UC patients.63 In rodent models of experimental IBD, supplemental resveratrol reduced inflammatory markers in cecal and colonic tissues. Oral consumption of 500 mg/d of resveratrol was associated with a decrease in plasma TNFα and CRP, and lower nuclear factor kappa-B (NF-κB) activity in peripheral blood mononuclear cells (PBMC) of UC patients. A meta-analysis of 17 randomized controlled trials, in which the study period ranged from 12 weeks to 48 months and the participants were ≥19 years old, concluded that adherence to a MD eating pattern decreased CRP and markers of inflammation (i.e., IL-6).64 Finally, crossover trials concluded that subjects adhering to a Mediterranean eating pattern had decreased postprandial expression of the p65 subunit of NF-κB and TNFα compared to a SFA-rich and a low-fat/high-carbohydrate (LFHC) diet.65 Taken together, results of preclinical and clinical studies indicate that foods and bioactive compounds commonly present in the MD tend to lower the risk of systemic inflammation and IBD.
Colorectal Cancer
Fact: In the United States, colorectal cancer (CRC) is the second leading cause of cancer death and ~1.2 million adult men and women have a history of CRC.1
Secondary analyses of the EPIC study in an Italian cohort of men and women (42,275 participants) with no cancer history concluded that adherence to the MD was inversely related to risk of CRC irrespective of gender. The protective effects were mainly for distal colon and rectal cancer and not for proximal colon cancer. In a cohort of men and women aged 25 to 70 years from various European countries, adherence to a MD eating pattern was associated with an 8–11% decrease in CRC risk.66
Olive oil and n-3 fatty acids. Phenolic compounds present in olive oil have been shown to induce apoptosis while reducing markers of inflammation (NF-kB, COX-2) and proliferation of colon cancer cells. Preclinical studies in models of familial adenomatous polyposis (FAP) documented that EVOO as well as phenolic extracts containing oleuropein and hydroxytyrosol induced expression of cannabinoid receptor-1 (CNR1), which is known to repress growth of colonic adenomas with inactivated adenomatous polyposis coli (APC) gene.67 Loss of APC functions predisposes to uncontrolled cell proliferation. In FAP patients less than 18 years of age, 6-month dietary intervention with EPA decreased the number of polyps by ~13%. Studies to better assess the impact of age, gender, timing and dose of exposure to a MD rich in n-3 PUFA on development of CRC are needed.
Fiber. In the MD eating pattern, fiber is derived mostly from cereals, fruits, and vegetables, which positively influence colonic fermentation through higher production of acetate and butyrate. In a recent analysis from the EPIC study, the risk of CRC was inversely associated with intakes of total fruit and vegetables, and total fiber.68 In accord with these findings, earlier analyses in men and women (25 to 70 years of age) enrolled in the EPIC study indicated that doubling fiber intake from ~ 15–20 g/d to ~40 g/d reduced CRC risk by ~40%.69 Higher fiber intake (~30 g/d) has been associated with reduced risk (~27%) of developing adenomas and insulin resistance. The inverse association in the EPIC study between cereal fiber intake and CRC risk (~11% per 10 g/d) was based on a large number (4,517) of patient cases,70 and has been confirmed in a meta-analysis of prospective studies.71
Red meat. Nitroso compounds (NOC) are formed when organic substrates (e.g., amines) present in foods interact with nitrogen additives (nitrites, nitrates, nitrogen oxide) in the milieu of the low pH of the stomach. Consumption of meats containing nitrogen additives supports the intestinal production of genotoxic NOC, which induce mutations in oncogenes (i.e., G > A in K-ras) by causing deamination of DNA bases leading to DNA mismatch and the formation of abasic sites. The formation of NOC-specific DNA adducts [i.e., O-(6)-carboxymethyl guanine)] is generally higher in subjects on a high red meat diet compared with subjects on a vegetarian or high red meat plus fiber (~30 g/d) diet.72 A dietary analysis of men and women 29 to 69 years of age within the Spanish cohort of the EPIC study showed that intake of potential NOC-generating compounds was lower in the cohort adopting a MD eating pattern.73
Microbiome. Moderate red wine consumption in healthy men has been reported to increase the number of gut health-promoting Enterococcus, Bacteroidetes, Bifidobacterium, and Prevotella species while decreasing the number of deleterious Clostridium species. Bacteria from the Clostridium genera possess 7α-dehydroxylase enzyme activity, which is responsible for the synthesis of potentially carcinogenic secondary bile acids.74 Foods commonly present in the MD (i.e., fruits) tend to support beneficial Bifidobacteria and Lactobacilli, which downregulate the expression of inflammatory factors and CRP.75
Epigenetics
Fact: Epigenetic mechanisms influence gene expression and modify disease risk.76
Epigenetics relates to non-mutational mechanisms that influence gene expression. DNA methylation is a one-carbon nutrient related epigenetic modification. High intake of vegetables and fruits is associated with a lower prevalence of global DNA hypomethylation, which is linked to increased risk of cancer.76 Conversely, increased DNA methylation of tumor suppressor genes is a risk factor in tumorigenesis. For example, our group reported that in-utero exposure to the grape phytoalexin resveratrol may prevent in rodent female offspring DNA hypermethylation and transcriptional repression of the breast cancer susceptibility gene, BRCA-1 by compounds that activate the aromatic hydrocarbon receptor (AhR).77 Ligands of the AhR include xenobiotics (i.e., dioxins, polycyclic aromatic hydrocarbons found in grilled foods, UV light) and metabolites (i.e., arachidonic acid) of n-6 LA. These epigenetic data support the idea that maternal nutrition with bioactive food components commonly present in the MD eating pattern reduce the risk of breast tumorigenesis in offspring. This notion extends to fish oils rich in EPA and DHA, whose supplementation to overweight women during pregnancy has been shown to protect fetal development and growth of infants. These protective effects of fish oils were associated with increased DNA methylation of IGF2,78 which controls intrauterine and postnatal growth. Therefore, epigenetic mechanisms might be key targets for food components of the MD eating pattern and affect fetal programming as well as adult susceptibility to chronic diseases.
In preclinical studies, treatment of colon cancer cells with EVOO (100 ppm), olive oil phenolic extract (50 μM), or the olive oil polyphenol hydroxytyrosol (50 μM) lowered methylation of the tumor suppressor CNR166 while decreasing cell proliferation. Interestingly, EVOO extracts devoid of phenols did not alter CNR1 methylation and mRNA levels, suggesting that the phenolic fraction of EVOO, and not just MUFA, may be likely responsible for epigenetic protection against CRC. Complex polyphenols in EVOO (i.e., secoiridoids) have been shown to induce cellular levels of AcH3K18 while inhibiting growth of colon cancer cells. In a cohort of women at higher risk for developing cervical intraepithelial neoplasia grade 2+, PBMC from subjects consuming MD-like foods were more likely to have higher DNA methylation in markers of cervical cancer compared to women consuming high sugar beverages, starchy foods, margarine, butter, refined grains, desserts and sweets, fatty meat, sausages, and bacon.79 Overall, a MD eating pattern may exert anticancer effects through maintenance of DNA methylation in gene regions that are expected be hypermethylated (i.e., silenced) under healthy conditions, while promoting epigenetic activation of tumor suppressor genes. This hypothesis deserves to be tested.
Cognitive diseases and circadian rhythm. Disturbances in genes that control circadian rhythm pathways have been linked to higher risk of being overweight and development of MetS. For example, studies examined the relationships between intake of MUFA and PUFA and DNA methylation of clock circadian regulator gene (CLOCK) in normal-weight, overweight, and obese women who followed weight reduction programs. In Mediterranean cohorts with high intake of olive oil and MUFA, CpG hypomethylation of CLOCK was linked to higher CLOCK expression. Conversely, PUFA were shown to increase DNA methylation of CLOCK, thus reducing its expression. Data also indicated that patients who snacked frequently had higher DNA methylation levels in CLOCK.80 Interestingly, the supplementation with olive oil was found to increase DNA methylation of fatty acid desaturase (FADS-2) in healthy subjects and renal patients. Therefore, olive oil may reduce biosynthesis of PUFA via epigenetic regulation of FADS2.81 Studies are clearly needed to assess the role of epigenetic modifications induced by a MD (and its components) on endpoints of cognitive disease and circadian rhythm.
Physical Activity
Fact: In the United States, only ~20% of all adults meet physical activity guidelines.1
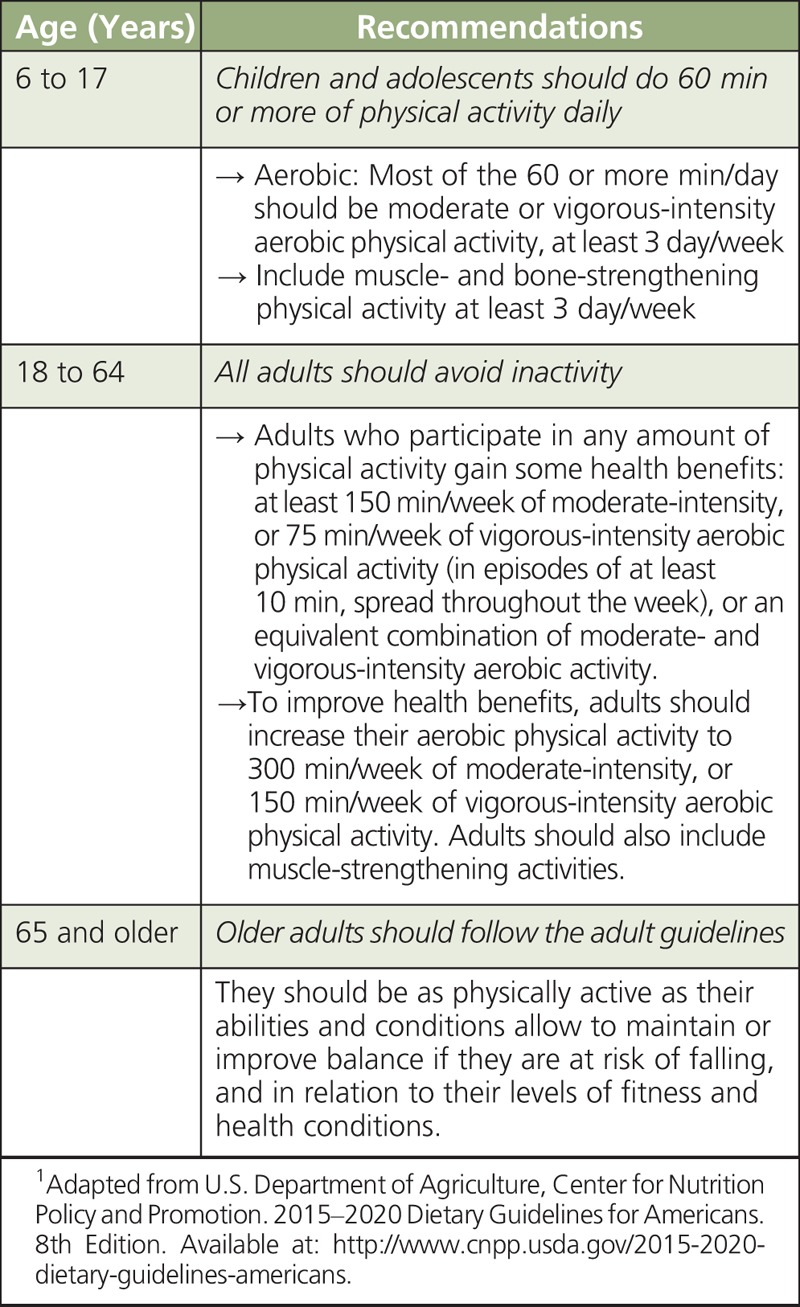
In traditional Mediterranean populations, daily physical tasks (i.e. worker effect) rather than planned exercise contributed to energy expenditure and the prevention of overweight and obesity. Therefore, the adoption of a MD eating pattern by more sedentary populations residing in industrialized countries may not exert the same benefits unless it is coupled to regular physical activity. Improved insulin sensitivity in response to increasing physical activity underscores the importance of exercise in addition to diet for the prevention of type-2 diabetes and associated complications. Daily moderate physical activity equivalent to brisk walking for 30–60 minutes is a safe and effective recommendation for minimizing the risk of CVD.82 Table 3 summarizes key USDA recommendations for physical activity based on age. These include suggestions that children and adolescents (6 to 17 years) should do 1 hour or more of physical activity daily; all adults (18 to 64 years) should avoid inactivity; and older adults (65 years and older) should follow adult guidelines.
TABLE 3.
Physical Activity for Americans: USDA Recommendations1
Building an MD Plate
Changes in food consumption during the last few decades
Fact: The National Health and Nutritional Examination Survey estimated that between 1971 and 2000, the average total energy consumption among US adults increased by ~22 % in women, from 1542 to 1886 kcal/d (+344 kcal/d), and by ~10 % in men, from 2450 to 2693 kcal/d (+243 kcal/d; Figure 2).83
FIGURE 2.
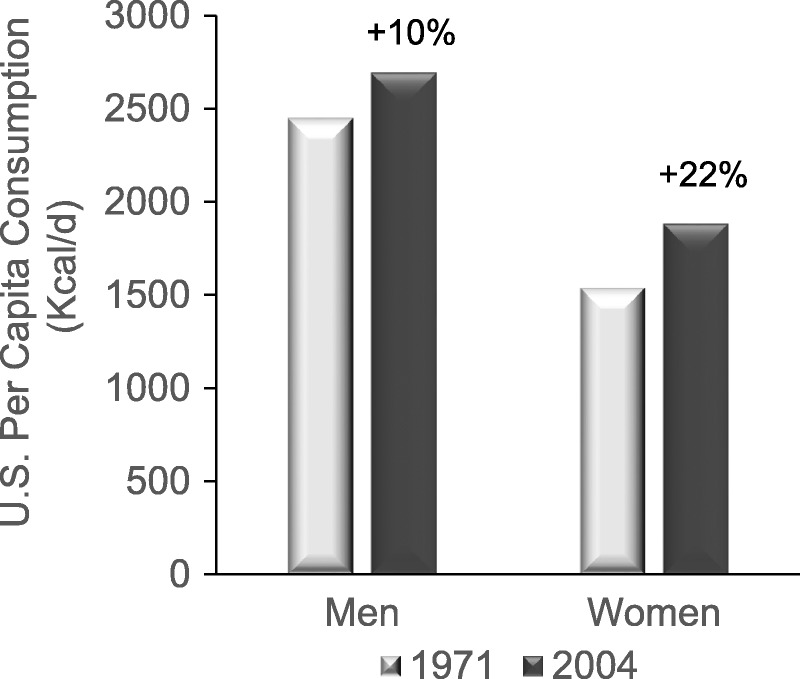
Change in total energy consumption among US adults during the period 1971–2000.83
Analysis of U.S. Census Bureau data for major food commodities and commercially available fruits and vegetable84 from 1980 to 2009 (Figure 3) revealed that per capita consumption of eggs (Figure 3A) and total red meat (beef, veal, lamb and mutton, and pork, Figure 3B) decreased by ~9% and 16%, respectively. Conversely, during the same period there was an increase in the per capita consumption of flour and cereal products (~35 %), and total dairy products (~12 %). Per capita consumption of total vegetables and fish and shellfish (Figure 3C) increased by ~15 % and 27 %, respectively, whereas there were no major changes in consumption of total fruits and butter. Interestingly, there were major increases in the per capita consumption of poultry (~70%); total cheese (~87%) with mozzarella cheese consumption increasing from 3 to ~11 lbs per capita; salad and cooking oils (~145%); high-fructose corn syrup (~165%); and non-frozen yogurt (~413%, Figure 3D). Whereas these food estimates reflect overall availability rather than direct intake by population and geographical subgroups, they are nevertheless informative of food consumption changes that occurred in the United States during the last few decades. In support of this interpretation, one study14 confirmed large increases in the percentage calorie contribution for soybean oil, poultry, seed oils, and sugars, and a reduction in calorie contribution for beef, eggs, dairy, vegetables, grains and legumes, during the second part of the 20th century. Notable has been the increase in the estimated per capita consumption of soybean oil, which in 1999 was estimated at ~7.4% of TEI. Overall, these consumption data suggested that during the last few decades the average per capita consumption for certain foods, e.g., fruits, vegetables, and fish was relatively stable, whereas there have been substantial increases in the average consumption of foods rich in n-6 LA and sugars. Perhaps not surprisingly, a recent study provided evidence that overall US dietary quality were poor, in particular for subgroups of lower socioeconomic status, and called for an increase in whole fruit consumption (3 serving/d) and cutting back on consumption of sugar-sweetened beverages.85
FIGURE 3.
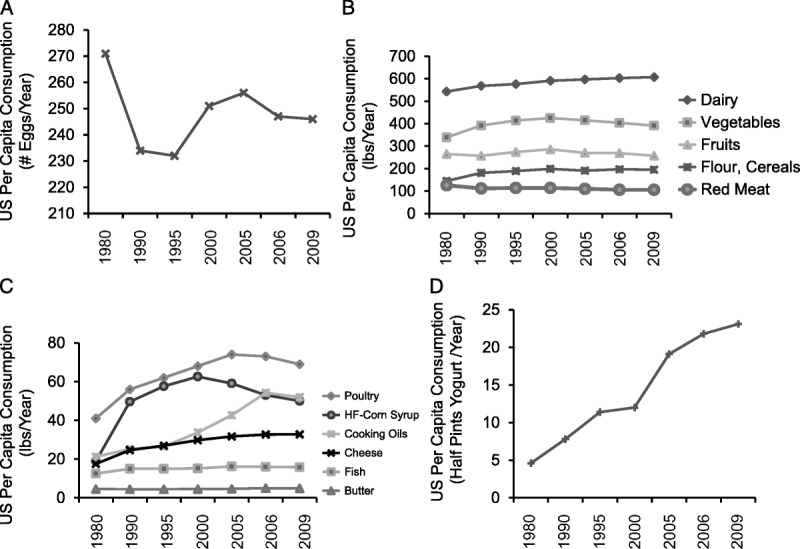
US changes in per capita consumption of major food commodities and commercially available fruits and vegetables from 1980 to 2009. A) Number (#) of eggs consumed/per capita/year); B) Foods consumed at > 100 lbs/per capita/year; C) Foods consumed at < 100 lbs/capita/year; and D) Non-frozen yogurt (consumed lbs/per capita/year).84
Shifting to Healthier Eating Patterns
Fact: Healthy eating patterns, including the MD, decrease the risk of chronic diseases.86
Two notable differences exist between the MD and average Western eating pattern. The former is higher in MUFA, fish, fruits and vegetables, and lower in meat consumption. Suggested actions for reducing the incidence of chronic diseases are:
increase intake of foods high in MUFA such as EVOO, avocados, avocado oil rich in 18:1, and nuts (almonds, hazelnuts, macadamia nuts, pistachios, pecans).86 Although walnuts contain more n-6 LA (~38%), (~9%) and n-3 α-linolenic acid (~9%)87;
increase consumption of fruits and vegetables including legumes (e.g., beans and peas), and limit consumption of calories from refined grains and sugars1;
BOX 1 and BOX 2 provide three examples of Mediterranean recipes and related nutritional parameters. For recipes that contribute to the base of the MD (i.e., pasta, rice, cereals, couscous, bread dishes), ~45 to 65% of TEI is likely derived from carbohydrates. Conversely, for fish and meat-based recipes with olive oil, a higher percentage of TEI is expected to come from fat and protein (~40-60%). For most MD dishes, MUFA, mainly from olive oil, would contribute the highest TEI from fat except for dairy-based dishes for which SFA may provide ~40-50% of TEI. Vegetables-based recipes are expected to provide the highest fiber/serving (e.g., braised lentils; crudites, and couscous with vegetables). Dishes that contribute the highest total cholesterol/serving (~80 mg) would include shrimp and dairy-based desserts.88
BOX 1.
Mediterranean Recipes87
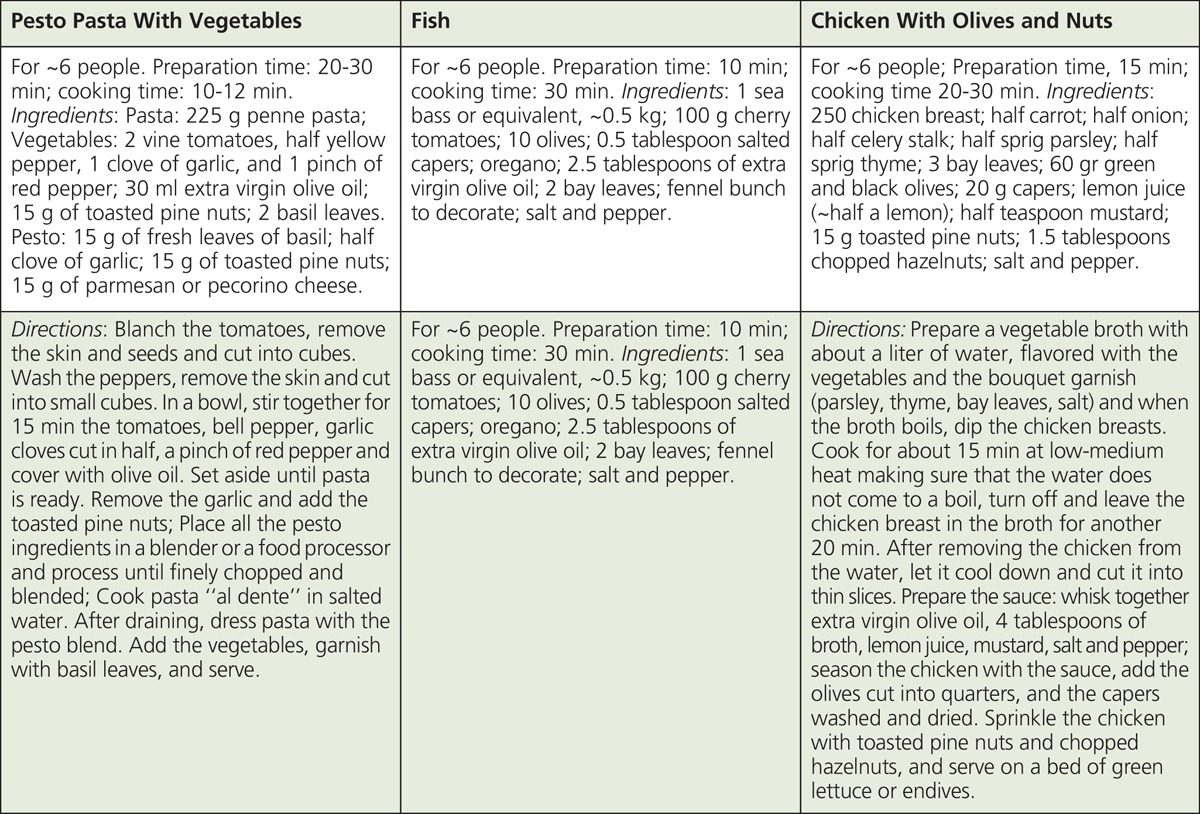
BOX 2.
Estimated Nutritional Composition of Mediterranean Recipes87
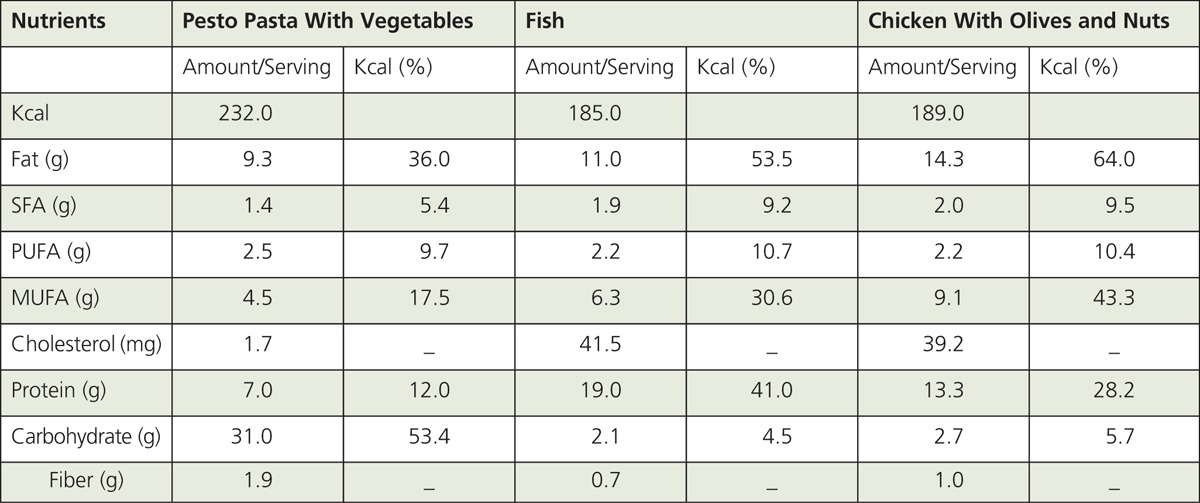
With respect to commercially available olive oils, tree variety, age of olives, and type of processing are expected to influence phenolic and fatty acid content. For example, concentrations of phenolic compounds in olive oil may vary greatly, ranging from ~250 mg/kg for the Arbequina variety to ~550–600 mg/kg for Cornicabra and Picual varieties. Based on the information that phenolic compounds in olive oil antagonize biological processes (e.g., inflammation, proliferation) linked to chronic diseases, implementation of labeling requirements for phenolic fractions may better inform consumers about the potential health benefits associated with olive oil consumption. The quality of olive oil may be influenced by the MUFA fraction. A good quality EVOO should have at least ~73% oleic acid and less than ~10% LA producing an oleic/LA ratio greater than 7.89 However, chemical and sensory testing indicated that some EVOO commercially available in the United States did not meet extra virgin standards for reasons that included fatty acid oxidation by exposure to elevated temperatures, light, and/or aging; adulteration with cheaper refined olive oils; processing flaws; and improper oil storage. Therefore, regulatory food agencies (e.g., USDA) should consider moving towards the implementation of olive oil grade standards to make them consistent with the International Olive Council (IOC) recommendations.
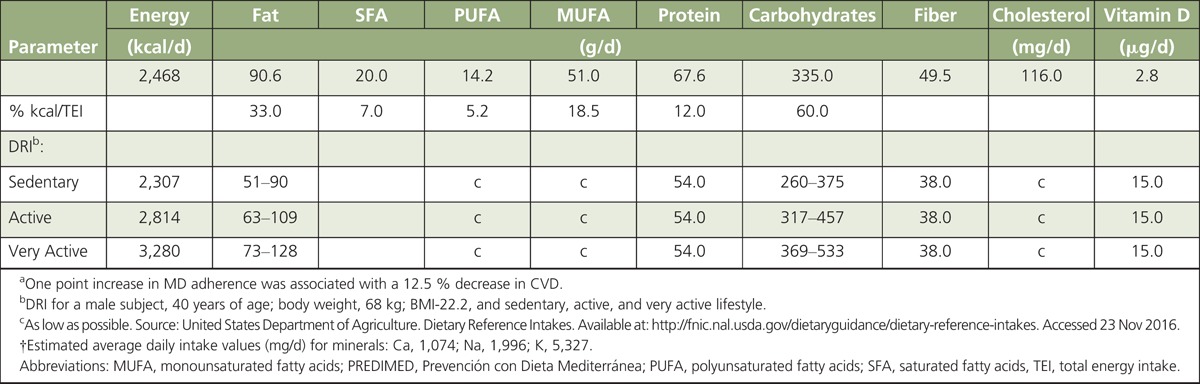
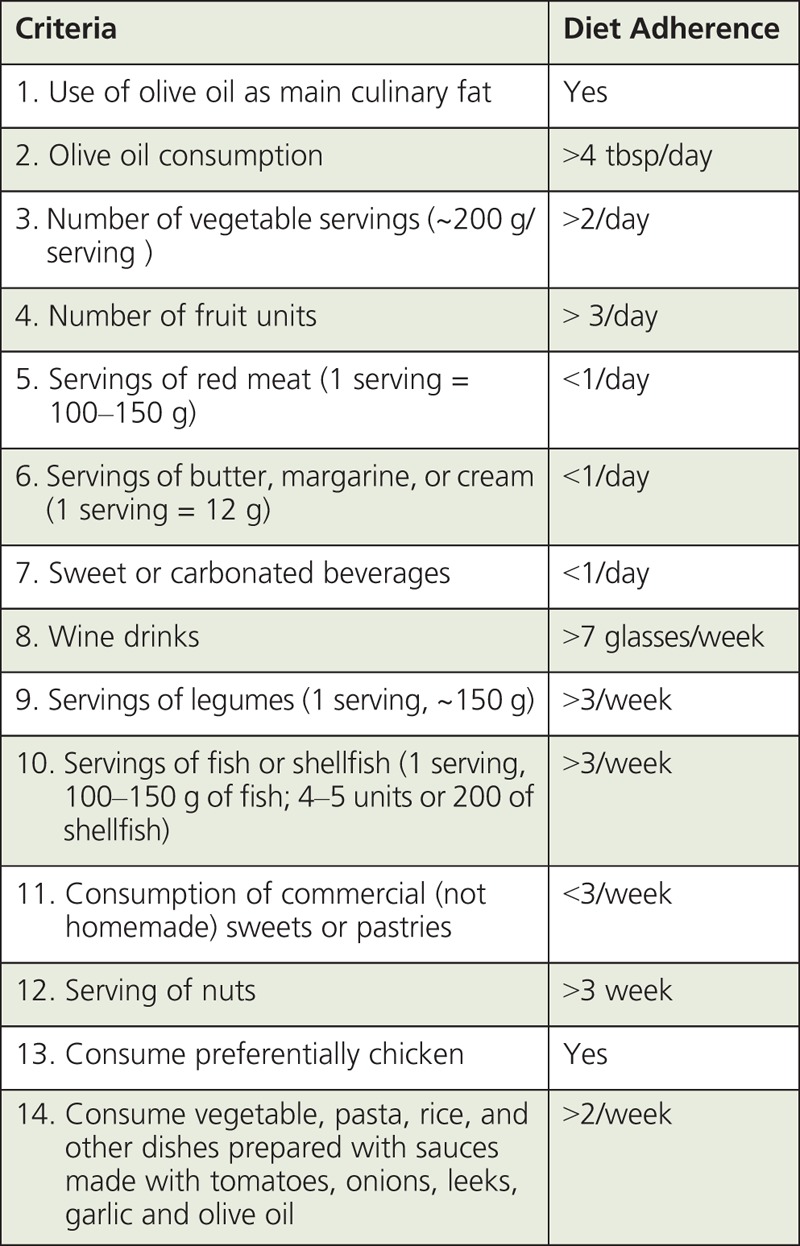
Table 4 presents average nutrition parameters of a weekly food plan (BOX 3). We developed this plan adopting a 14-item food questionnaire criteria used in the PREDIMED trial (BOX 4), in which a 1-point increase in diet adherence decreased the risk of CVD by 12.5%.90 When we compared the MD nutrition parameters with the USDA dietary reference intake (DRI) goals for a male individual (40 years of age; body weight 68 kg; height 175 cm; BMI = 22.2) with different physical activities (sedentary, active, and very active), the average MD pyramid values for macronutrients (daily energy, fat, carbohydrates, and fiber) were in good agreement with the USDA-DRI requirement targets. MUFA plus PUFA in the MD weekly plan contributed on average ~24% of TEI. Intake levels of MUFA plus PUFA approaching 20-30% of TEI have been linked to reduced risk of CVD. Average daily protein intake of the MD plan (~68 g/d) was above the USDA-DRI reference (54 g/d), but below the maximum recommended target (~15%) of TEI. The MD plan provided also a good fit overall with USDA recommendations for calcium, sodium, and potassium although recipes used to develop the weekly MD food pyramid did not include table salt (target <2.3 g/d). In contrast, estimated vitamin D intake levels (average ~2.8 μg/d) from foods listed in BOX 3 were mostly below the USDA-DRI requirements (15 μg/d) (Table 4). Only on day 1 of the MD food plan, did intake levels of vitamin D increase to ~10 μg primarily from fish. These estimates of vitamin D intake reinforced the concern that even a MD-like eating pattern rich in fish and fish oils may not provide on average sufficient daily levels of vitamin D.1 Surveys in Europe and the United States (NHANES) indicated that the average vitamin D intake ranged from ~150 to 300 IU/d (~3.8–7.5 μg/d). These estimates were well below the recommended DRI levels.91 Finally, we estimated that moderate wine consumption based on the PREDIMED standards (~1 glass/d) contributed ~130 to 200 kcal/d, in line with the recommendation that alcohol should contribute <10 % of TEI.92
TABLE 4.
Average Nutrition Parametersa of a Mediterranean Weekly Plan88 Developed Using a 14-Item Food PREDIMED Questionnaire,90†
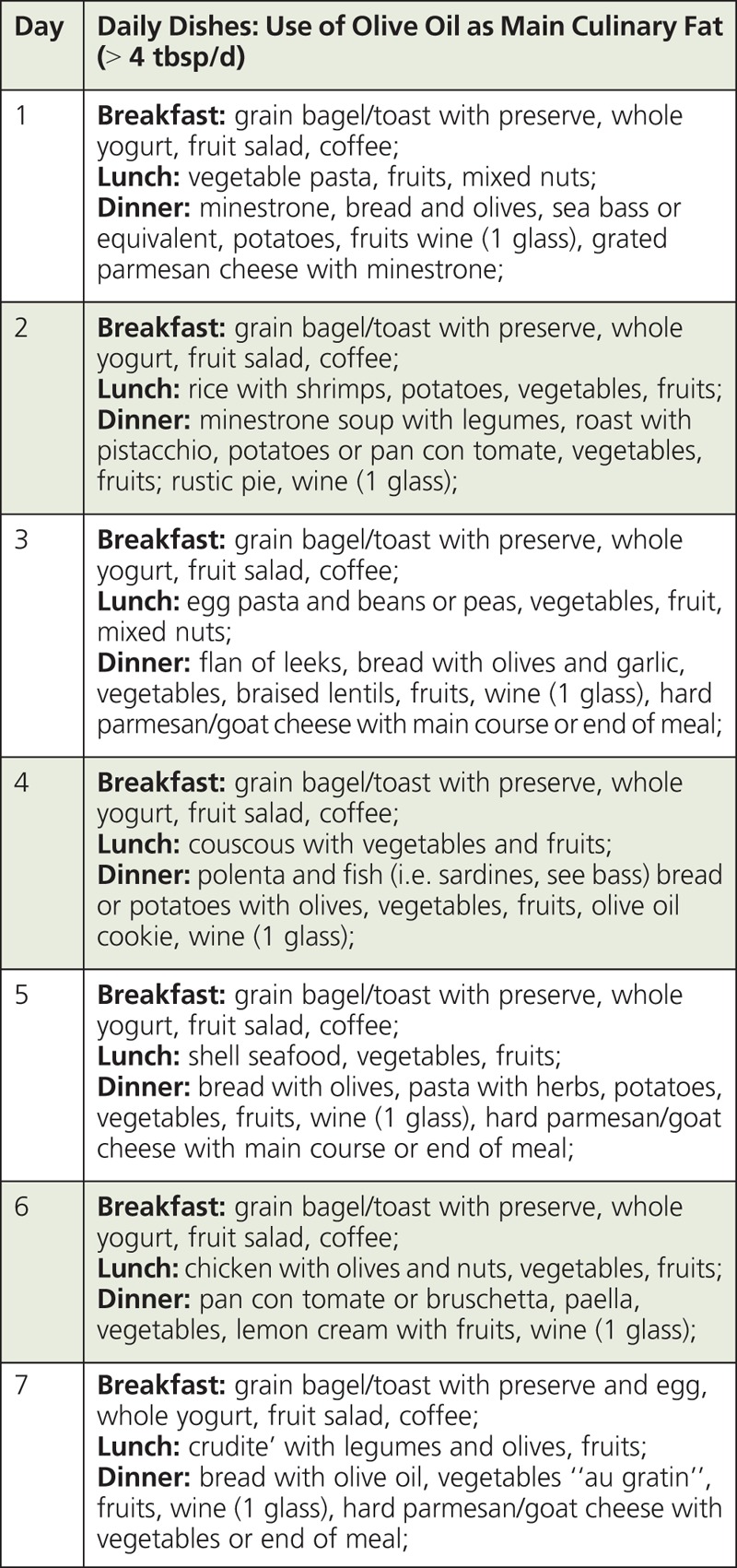
BOX 3.
Example of a Weekly MD plan87
BOX 4.
14-Item Questionnaire of Mediterranean Diet Adherence89
SUMMARY AND CONCLUSIONS
Many elements of the MD eating pattern associate with improved health and longevity. Mediterranean recipes and foods can be organized into a dietary pattern that helps individuals meet target USDA-DRI for energy, macronutrients (fat, carbohydrate, protein, and fiber), and selected minerals (calcium, sodium, and potassium). However, certain factors may reduce the potential health benefits of a MD eating pattern. For example, although oleic acid from EVOO and nuts is a major contributor to TEI in the MD eating pattern, the important role of phenolic compounds, not just MUFA, should be emphasized as large variations in phenolic profile and content may reduce grade of olive oil commercially available and health benefits of olive oil consumption.93 Another major caveat is that requirements for vitamin D may not be easily met through adoption of a MD. Therefore, in addition to fish-based recipes (>3 times/week), milk products (e.g. liquid milk, cheese, etc), and adequate sun exposure, supplemental vitamin D may be needed to alleviate inadequacies in vitamin D intake.1 Finally, because some of the healthier foods (i.e., fish, nuts, EVOO, berries) are expensive and therefore not largely accessible, or are not produced in the United States, a major question is what it would actually take to shift current US dietary patterns across ethnic and socioeconomic strata. We suggest that new initiatives led by food industry and public health organizations (i.e. USDA, NIH, etc.) should realign agricultural and food production with public health programs so that more people can transition to a healthier eating pattern. Major economic incentives to produce and market foods to protect the health of the US public should be developed not only because of increased risk of chronic diseases, but also in response to ballooning medical costs, which for obesity and diabetes were estimated at ~ $150 billion in 2008, and ~ $250 billion in 2012, respectively.1 Clearly, savings for medical expenditures related to chronic diseases could be redirected to improve the quality of life for people residing in the United States and society at large.

No caption available
No caption available
Acknowledgment
The authors wish to thank Dr. Michele A. Zacks, The University of Arizona Cancer Center for help with editorial review of the manuscript.
Footnotes
This study was supported by funding from NIFA (2015-67017-23144, US Department of Agriculture Conference Grant), US Department of Defense Breast Cancer Research Program (W81XWH-14-1-0470), and Cancer Center Support Grant (P30 CA023074).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition December 2015. Available at http://health.gov/dietaryguidelines.
- 2.U.S. Department of Agriculture. Scientific report of the 2015 Dietary Guidelines Advisory Committee to the Secretary of Agriculture and the Secretary of Health and Human Services. http://health.gov/dietaryguidelines/2015-scientificreport. Accessed 15 Aug 2015.
- 3.Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274–2284. [DOI] [PubMed] [Google Scholar]
- 4.Romagnolo DF, Selmin OI. Mediterranean Diet and Lifestyle in a Modern World Context. In: Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:15–26. [Google Scholar]
- 5.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care. 2011;34:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keys A, Menotti A, Karvonen MI. The diet and 15-year death rate in the Seven Countries Study. Am J Epidemiol. 1986;124:903–915. [DOI] [PubMed] [Google Scholar]
- 7.Gerber M, Hoffman R. Mediterranean oils and fats, and disease risk. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:71–88. [Google Scholar]
- 8.Marangoni F, Martiello A, Galli C. Dietary fat intake of European countries in the Mediterranean area: An update. World Rev Nutr Diet. 2007;97:67–84. [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Juarez M, Ramos M, Haenlein GF. Physico-chemical characteristics of goat and sheep milk. Small Rumin Res. 2007;68:88–113. [Google Scholar]
- 10.German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46:57–92. [DOI] [PubMed] [Google Scholar]
- 11.Collins YF, McSweeney PL, Wilkinson MG. Lipolysis and free fatty acid catabolism in cheese: a review of current knowledge. Int Dairy J. 2003;13:841–866. [Google Scholar]
- 12.McCance RA, Widdowson EM, Paul AA, Southgate DA. McCance and Widdowson’s. The composition of foods. 4th revised and extended ed./by A.A. Paul and D.A.T. Southgate/[for the] Ministry of Agriculture, Fisheries and Food [and the] Medical Research Council. edn. H.M.S.O. Amsterdam; Oxford: Elsevier/North-Holland Biomedical Press, London; 1978. [Google Scholar]
- 13.Engeset D, Alsaker E, Lund E, et al. Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006;119:175–182. [DOI] [PubMed] [Google Scholar]
- 14.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 16.Linseisen J, Welch AA, Ocke M, et al. Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: results from the 24-h dietary recalls. Eur J Clin Nutr 2009;63(Suppl 4):S61–S80. [DOI] [PubMed] [Google Scholar]
- 17.Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC. The Mediterranean diet: science and practice. Public Health Nutr. 2006;9:105–110. [DOI] [PubMed] [Google Scholar]
- 19.Dayton S, Pearce ML, Hashmoto S, Dixon WJ, Tomiyasu U. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation. 1969;39–40(Suppl 2):1–63. [Google Scholar]
- 20.Deol P, Evans JR, Dhahbi J, et al. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS One 2015;10:e0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Arroyo S, Gomez-Martinez A, Rocamora-Reverte L, et al. Application of nanoLC-ESI-TOF-MS for the metabolomic analysis of phenolic compounds from extra-virgin olive oil in treated colon-cancer cells. J Pharm Biomed Anal 2012;63:128–134. [DOI] [PubMed] [Google Scholar]
- 23.Gronbaek M, Becker U, Johansen D, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med 2000;133:411–419. [DOI] [PubMed] [Google Scholar]
- 24.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. [DOI] [PubMed] [Google Scholar]
- 25.Sumpio BJ, Cordova AC, Sumpio BE. Wine, Polyphenols, and Cardioprotection. In: Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:97–108. [Google Scholar]
- 26.Porter Starr KN, Bales CW. The Value of the Mediterranean Diet for Older Adults: Emphasis on Obesity Interventions. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:141–152. [Google Scholar]
- 27.Fito M, Guxens M, Corella D, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–1203. [DOI] [PubMed] [Google Scholar]
- 28.Sofi F, Fabbri A, Casini A. Inflammation and Cardiovascular Disease and Protection by the Mediterranean Diet. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:89–96. [Google Scholar]
- 29.Selmin OI, Romagnolo AP, Romagnolo DF. Mediterranean diet and neurodegenerative diseases. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:153–164. [Google Scholar]
- 30.Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis 2012;29:773–782. [DOI] [PubMed] [Google Scholar]
- 31.Trichopoulou A, Kyrozis A, Rossi M, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr 2015;54:1311–1321. [DOI] [PubMed] [Google Scholar]
- 32.Luccarini I, Grossi C, Rigacci S, et al. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-β toxicity: biochemical, epigenetic and functional correlates. Neurobiol Aging. 2015;36(2):648–663. doi: 10.1016/j.neurobiolaging.2014.08.029. pii: S0197-4580(14)00591-0. [DOI] [PubMed] [Google Scholar]
- 33.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 34.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009;89:1145–1154. [DOI] [PubMed] [Google Scholar]
- 35.Murtaugh MA, Sweeney C, Giuliano AR, et al. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr 2008;87:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demetriou CA, Hadjisavvas A, Loizidou MA, et al. The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 2012;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson CA, Stendell-Hollis NR, Rock CL, Cussler EC, Flatt SW, Pierce JP. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bessaoud F, Daurès JP, Gerber M. Dietary factors and breast cancer risk: a case control study among a population in Southern France. Nutr Cancer. 2008;60(2):177–187. [DOI] [PubMed] [Google Scholar]
- 39.Cottet V, Touvier M, Fournier A, et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol 2009;170:1257–1267. [DOI] [PubMed] [Google Scholar]
- 40.Link LB, Canchola AJ, Bernstein L, et al. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr 2013;98:1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckland G, Travier N, Cottet V, et al. Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer 2013;132:2918–2927. [DOI] [PubMed] [Google Scholar]
- 42.Cade JE, Taylor EF, Burley VJ, Greenwood DC. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr. 2011;65:920–928. [DOI] [PubMed] [Google Scholar]
- 43.Couto E, Sandin S, Löf M, Ursin G, Adami HO, Weiderpass E. Mediterranean dietary pattern and risk of breast cancer. PLoS One. 2013;8:e55374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136:466–472. [DOI] [PubMed] [Google Scholar]
- 45.Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr. 2010;92:620–625. [DOI] [PubMed] [Google Scholar]
- 46.La Vecchia C, Bosetti C. Diet and cancer risk in Mediterranean countries: open issues. Public Health Nutr. 2006;9:1077–1082. [DOI] [PubMed] [Google Scholar]
- 47.McDonald JA, Goyal A, Terry MB. Alcohol Intake and Breast Cancer Risk: Weighing the Overall Evidence. Curr Breast Cancer Rep. 2013; 5(3). doi:10.1007/s12609-013-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trichopoulou A, Katsouyanni K, Stuver S, et al. Consumption of olive oil and specific food groups in relation to breast cancer risk in Greece. J Natl Cancer Inst 1995;87:110–116. [DOI] [PubMed] [Google Scholar]
- 49.Toledo E, Salas-Salvadó J, Donat-Vargas C, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 2015;175:1752–1760. [DOI] [PubMed] [Google Scholar]
- 50.Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008;49:133–143. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Lim SY, Shin A, et al. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case control study. BMC Cancer 2009;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chron’s & Colitis Foundation of America. The facts about inflammatory bowel disease. 2014:1–24. ( http://www.ccfa.org/resources/facts-about-inflammatory.html. accessed on 11/22/2016).
- 53.Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol 2011;106(Suppl 1):S2–S25. [DOI] [PubMed] [Google Scholar]
- 54.Sánchez-Fidalgo S, Sánchez de Ibargüen L, Cárdeno A, Alarcón de la Lastra C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur J Nutr. 2012;51(4):497–506. [DOI] [PubMed] [Google Scholar]
- 55.Fitó M, Cladellas M, de la Torre R, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr 2008;62:570–574. [DOI] [PubMed] [Google Scholar]
- 56.Loued S, Berrougui H, Componova P, Ikhlef S, Helal O, Khalil A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br J Nutr. 2013;110:1272–1284. [DOI] [PubMed] [Google Scholar]
- 57.Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2:75–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Llor X, Pons E, Roca A, et al. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin Nutr 2003;22:71–79. [DOI] [PubMed] [Google Scholar]
- 59.Huang CH, Hou YC, Yeh CL, Yeh SL. A soybean and fish oil mixture with different n-6/n-3 PUFA ratios modulates the inflammatory reaction in mice with dextran sulfate sodium-induced acute colitis. Clin Nutr. 2015;34:1018–1024. [DOI] [PubMed] [Google Scholar]
- 60.Paoli A, Moro T, Bosco G, et al. Effects of n-3 polyunsaturated fatty acids(ω-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar Drugs 2015;13:996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22(5):602–606. [DOI] [PubMed] [Google Scholar]
- 62.Chuang SC, Vermeulen R, Sharabiani MT, et al. The intake of grain fibers modulates cytokine levels in blood. Biomarkers 2011;16:504–510. [DOI] [PubMed] [Google Scholar]
- 63.Kanauchi O, Suga T, Tochihara M, et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol 2002;37(Suppl 14):67–72. [DOI] [PubMed] [Google Scholar]
- 64.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer. 2014;135:1884–1897. [DOI] [PubMed] [Google Scholar]
- 65.Camargo A, Delgado-Lista J, Garcia-Rios A, et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br J Nutr 2012;108:500–508. [DOI] [PubMed] [Google Scholar]
- 66.Bamia C, Lagiou P, Buckland G, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol 2013;28:317–328. [DOI] [PubMed] [Google Scholar]
- 67.Di Francesco A, Falconi A, Di Germanio C, et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J Nutr Biochem 2015;26:250–258. [DOI] [PubMed] [Google Scholar]
- 68.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100(Suppl 1):394S–398S. [DOI] [PubMed] [Google Scholar]
- 69.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003;361:1496–1501. [DOI] [PubMed] [Google Scholar]
- 70.Murphy N, Norat T, Ferrari P, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One 2012;7:e39361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose–response meta-analysis of prospective studies. BMJ 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewin MH, Bailey N, Bandaletova T, et al. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: implications for colorectal cancer risk. Cancer Res 2006;66:1859–1865. [DOI] [PubMed] [Google Scholar]
- 73.Jakszyn P, Agudo A, Berenguer A, et al. Intake and food sources of nitrites and N-nitrosodimethylamine in Spain. Public Health Nutr 2006;9:785–791. [DOI] [PubMed] [Google Scholar]
- 74.Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet, inflammatory bowel diseases, and colon cancer. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:181–201. [Google Scholar]
- 75.Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632–15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross SA. Epigenetics of Mediterranean diet: altering disease risk. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:203–216. [Google Scholar]
- 77.Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol Carcinog. 2015;54:261–269. [DOI] [PubMed] [Google Scholar]
- 78.Lee HS, Barraza-Villarreal A, Biessy C, et al. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiol Genomics 2014;46:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piyathilake CJ, Badiga S, Kabagambe EK, et al. A dietary pattern associated with LINE-1 methylation alters the risk of developing cervical intraepithelial neoplasia. Cancer Prev Res (Phila). 2012;5:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milagro FI, Gómez-Abellán P, Campión J, Martínez JA, Ordovás JM, Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int. 2012;29:1180–1194. [DOI] [PubMed] [Google Scholar]
- 81.Hoile SP, Clarke-Harris R, Huang RC, et al. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS One. 2014;9(10):e109896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hingle MD, Laddu DR, Going SB. Physical activity and the Mediterranean diet. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:219–228. [Google Scholar]
- 83.National Health and Nutrition Examination Survey (NHANES). United States, 1971–2000. Trends in Intake of Energy and Macronutrients -United States, 1971–2000. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5304a3.htm.
- 84.US Census Bureau, Statistical Abstract of the United States: 2012. Health and Nutrition. Available at http://www.census.gov. Accessed 23 Nov 2016.
- 85.Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Briggs MA, Fleming JA, Kris-Etherton PM. Food-Based approaches for achieving nutritional adequacy with the Mediterranean, DASH, and USDA food patterns. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:239–259. [Google Scholar]
- 87.Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romagnolo DF, Jackson KA, Sparks PL, Selmin OI. Building the Mediterranean pyramid: Part B – Balancing the plate. In: Romagnolo DF, Selmin OI, eds. Mediterranean Diet. Dietary Guidelines and Impact on Health and Disease. AG Switzerland: Springer International Publishing; 2016:275–288. [Google Scholar]
- 89.International Olive Council. Available at http://www.internationaloliveoil.org/. Visited on 23 Nov 2016.
- 90.Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varela-Moreiras G, Ruiz E, Valero T, Avila JM, del Pozo S. The Spanish diet: an update. Nutr Hosp. 2013;28(suppl 5):13–20. [DOI] [PubMed] [Google Scholar]
- 93.Frankel EN, Mailer RJ, Shoemaker CF, Wang SC, Flynn JD. Tests indicate that imported “extra virgin” olive oil often fails international and USDA standards. UC Davis Olive Center, July 2010. Robert Mondavi Institute for Wine and Food Science, University of California, Davis.


