As a foundation for the next guidelines revision, we confirmed with additional precision the risk estimates previously reported for combinations of human papillomavirus and cytology screening.
Key Words: management guidelines, cervical cancer, screening
Abstract
Objectives
The next round of the American Society for Colposcopy and Cervical Pathology (ASCCP)-sponsored cervical cancer screening and management guidelines will recommend clinical actions based on risk, rather than test-based algorithms. This article gives preliminary risk estimates for the screening setting, showing combinations of the 2 most important predictors, human papillomavirus (HPV) status and cytology result.
Materials and Methods
Among 1,262,713 women aged 25 to 77 years co-tested with HC2 (Qiagen) and cytology at Kaiser Permanente Northern California, we estimated 0–5-year cumulative risk of cervical intraepithelial neoplasia (CIN) 2+, CIN 3+, and cancer for combinations of cytology (negative for intraepithelial lesion or malignancy [NILM], atypical squamous cells of undetermined significance [ASC-US], low-grade squamous intraepithelial lesion [LSIL], atypical squamous cells cannot exclude HSIL [ASC-H], high-grade squamous intraepithelial lesion [HSIL], atypical glandular cells [AGC]) and HPV status.
Results
Ninety percent of screened women had HPV-negative NILM and an extremely low risk of subsequent cancer. Five-year risks of CIN 3+ were lower after HPV negativity (0.12%) than after NILM (0.25%). Among HPV-negative women, 5-year risks for CIN 3+ were 0.10% for NILM, 0.44% for ASC-US, 1.8% for LSIL, 3.0% for ASC-H, 1.2% for AGC, and 29% for HSIL+ cytology (which was very rare). Among HPV-positive women, 5-year risks were 4.0% for NILM, 6.8% for ASC-US, 6.1% for LSIL, 28% for ASC-H, 30% for AGC, and 50% for HSIL+ cytology.
Conclusions
As a foundation for the next guidelines revision, we confirmed with additional precision the risk estimates previously reported for combinations of HPV and cytology. Future analyses will estimate risks for women being followed in colposcopy clinic and posttreatment and will consider the role of risk modifiers such as age, HPV vaccine status, HPV type, and screening and treatment history.
Five years have passed since the last American Society for Colposcopy and Cervical Pathology (ASCCP)-sponsored consensus conference on cervical cancer screening and management, held in 2012.1 The current guidelines succeeded in introducing the important principle of “equal management of equal risk” and in harmonizing management strategies by applying this principle to different combinations of test results. However, current recommendations remain undeniably complicated and, in some instances, controversial.
Formal preparation for the next round of ASCCP-sponsored guidelines has now begun, with the goal of making the recommendations both simpler and more precise.2 Algorithm diagrams for each test result will be replaced with fully risk-based, tailored recommendations for each woman. Based on level of risk of precancer/cancer, the guidelines will suggest a recommended action. For example, in response to risk assessment, the guidelines might recommend that the clinician “consider treatment” (if risk is extremely high), “perform colposcopy and biopsy” (if risk is high), “retest in 1 year” (or some other interval chosen by the guidelines group, for intermediate risk), “rescreen routinely” at an agreed interval (for low risk), or “exit screening” (if risk of subsequent cancer is virtually zero or risk of testing outweighs disease risk).
The estimated risks of cervical intraepithelial neoplasia (CIN) 2 or worse (CIN 2+), CIN 3 or worse (CIN 3+), or cancer (for that day or in the subsequent 1 to 5 years) will form a comprehensive, underlying risk database now under construction. A woman's risk estimate will depend on whatever is known about present human papillomavirus (HPV) and cytology test results, history of test results and treatments, and important modifying factors such as age and HPV vaccination status.
At present, we anticipate that recommendations will be provided for the following 3 situations: the screening clinic, the colposcopy clinic, and follow-up posttreatment. A simple electronic presentation of risk estimates and related recommendations via a smartphone application and other formats will be created.
Importantly, recommendations will be based on the latest evidence and will be easily modifiable, by release to users of a downloadable software update. Once “risk-action thresholds” are decided by consensus, subsequent guideline updates will create minimal disruption and we can hope for some stability in the form and logic of recommendations. The inevitable continued introduction of new test methods and strategies mandating revisions to the risk estimates can be incorporated into the algorithm, and the output to clinicians of the recommendations themselves will stay the same and familiar: e.g., treatment, colposcopy, retest in 1 year or some other intensified interval, routine screening, or exit.
An extensive data analysis effort is needed to produce risk estimates for all of the many combinations of tests and other predictive factors that might be presented by a woman, including the strong possibility of missing information for some factors such as history. The National Cancer Institute (NCI) epidemiologists and statisticians, in collaboration with the ASCCP, have taken on the responsibility of identifying important data sets, of negotiating access with the data providers when possible and of performing and openly releasing for use by anyone (not exclusively the ASCCP) because many risk calculations are needed to cover the kinds of populations, settings, and test results likely to be faced. Data sources for the risk calculations will be varied and will include both clinical trials and observational “big data” from routine clinical practices.
Deciding the correct management for various levels of risk will be the responsibility of clinical organizations, with a major multiorganizational effort organized by the ASCCP. A separate ASCCP research effort is underway to determine how best to reach these difficult decisions, which involve many factors related to risk perception and acceptable levels of safety.
As we embark on construction of the master risk database that will underlie the guidelines, we plan a series of publications addressing all the component topics, to permit public feedback and to foster acceptance of the finished product. For each of the articles in this series, the important issues will be the following:
What risk strata can we establish that are reliable?
How many women from the whole population are in these strata? Some (like HPV-negative atypical glandular cells [AGC]) are very small while others (HPV-positive negative for intraepithelial lesion or malignancy [NILM]) influence many women.
What are the risks for women whose test results and other characteristics place them in the strata?
Given the risks and numbers in the strata, what preliminary conclusions can be drawn about optimal clinical actions? The ultimate decisions will be made through the guidelines process.
Although no single database can provide all the information we need and many publications will be forthcoming, this article is the first in a series of Journal of Lower Genital Tract Disease presentations of risk estimates making use of the invaluable experience with HPV and cytology co-testing from Kaiser Permanente Northern California (KPNC). This first article gives preliminary risk estimates for the combinations of HPV testing and cytology in the screening setting. Future articles will refine risk estimates by consideration of HPV typing, various biomarkers, past test and treatment history, age, vaccination status, and other predictive factors and will also address risk estimates in the colposcopy clinic and in the posttreatment setting.
For each of the articles in the series involving KPNC data, the methods will be virtually uniform and will be kept brief by referencing rather than repeating details. The results section will tabulate the risk estimates produced by NCI analyses. The discussion section describing the implications of the data will be written under the direction of an expert clinician (in this case, R.S.G.) to promote a clear and relevant connection to the readers, who are intended to be users of the guidelines.
It is important to re-emphasize that the risk estimates presented in each of the Journal of Lower Genital Tract Disease papers will be preliminary and subject to updates by subsequent analyses leading up the release of the official data set. That data set will bring together data from all available trials and observational studies near the time of the next guidelines conference (tentatively conceived as taking place sometime late in 2019).
The guidelines research effort is approved annually by the KPNC Institutional Review Board and was judged exempt from review at National Institutes of Health. It is important to note that all potentially identifying information is maintained at KPNC and not included in analytic datasets analyzed by NCI.
MATERIALS AND METHODS
The KPNC/NCI guidelines cohort has been previously described.3 Starting in 2003, women 30 to 65 years old were routinely co-tested with Hybrid Capture 2 (HC2) and cytology. The current analysis includes histopathologic end point data for women aged 25 to 77 years through 2015, comprising more than a million women's outcomes. The estimates for women aged 25 to 29 years are not based on a random sample and are presented mainly because of the large number of women tested for triage of atypical squamous cells of undetermined significance (ASC-US).
Cytology was performed at KPNC regional and local laboratories. Human papillomavirus status was based on HC2 (Qiagen, Germantown, MD) testing performed on a second cervical specimen (collected at the same time as the cytology specimen) at the regional laboratory. Hybrid Capture 2 was used as per manufacturer's instructions to identify infection with high-risk HPV types. Cytology results were reported based on the 2001 Bethesda System. Between 2003 and 2009, conventional cytology slides were first processed using the BD FocalPoint Slide Profiler (BD Diagnostics, Burlington, NC) primary screening and directed quality control system and then manually reviewed. In 2009, KPNC switched to liquid-based cytology tested with BD SurePath. Clinical outcomes were obtained by matching to KPNC computerized cytology and histopathology records.
We restricted the analytic sample to 1,262,713 women with HPV and cytology results, excluding women with missing HPV results or with cytology reports of missing, uncertain, or not cervical. Cytology results were categorized as the following: NILM, ASC-US, low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells cannot exclude HSIL (ASC-H), AGC, high-grade squamous intraepithelial lesion or worse (HSIL+), and inadequate. We reported HPV status as negative versus positive for infection with any of the 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), recognizing that HC2 also detects through cross-reaction a percentage of closely related HPV types (e.g., 53, 66, 67, 70, 82, and 82v).4
For this analysis, we used Logistic-Weibull models to estimate cumulative risks of different histologic outcomes (CIN 2+, CIN 3+, and cancer) at different time points (0–5 years after co-testing), stratified by cytology and HPV results.5 Follow-up time started at the initial co-testing results. In the text, we discuss mainly 5-year risks, because the patterns are virtually the same regardless of the period chosen. The tables present other periods for use as desired, and the eventual guidelines will provide even more detail; it is still unsettled if and when it matters to use risk estimates for a particular time point to guide management. The most obvious issue is estimating the need for immediate colposcopy presented by screening combinations that do not currently lead to immediate colposcopy at KPNC (especially HPV-positive NILM and HPV-negative ASC-US). Should colposcopy referral depend strictly on the risk of actually finding CIN 2+ or CIN 3+ if referred that day or also incorporate notions of how many would regress and which women are at risk of precancer in the near future?
RESULTS
The average follow-up of the 1,262,713 women with cytology-HPV co-testing in the KPNC population was 3.5 years (median = 3, minimum = 0, maximum = 13, interquartile range = 0–6 years).
The cytology and HPV co-test results at study enrollment are tabulated in Table 1. Unsatisfactory cytology results were rare (0.28%). Of the total cohort, 90% had enrollment results of HPV-negative NILM. The 10% of women with nonnormal cytology and/or HPV positivity included 3.8% with HPV-positive NILM, 2.5% with HPV-negative ASC-US+ (mainly HPV-negative ASC-US), and 3.7% with HPV-positive ASC-US+. As expected, HPV positivity generally increased with severity of cytologic abnormality: ASC-US (48%), LSIL (85%), AGC (28%), ASC-H (78%), and HSIL+ (95%).
TABLE 1.
Distribution of Cytologya and HPV Results in the Study Populationa
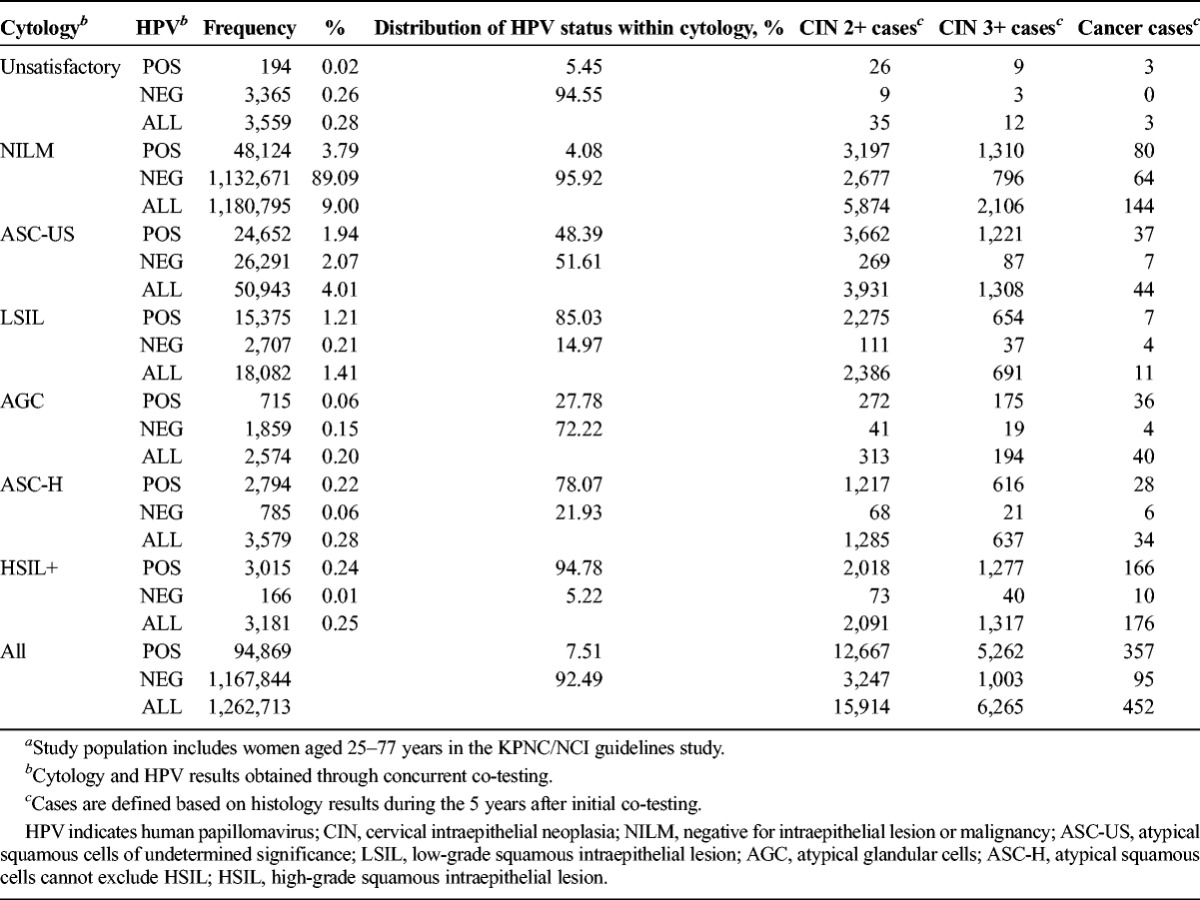
Total numbers of CIN 2+, CIN 3+, and cancers found in women with each enrollment co-test combination are also shown in Table 1 and presented individually in Tables 2 to 4 with risk estimates at different follow-up time points. Overall, CIN 2 was as common as CIN 3 and invasive cancers were rare. CIN 3 was slightly more commonly diagnosed at enrollment, because the first KPNC co-test detected a larger number of prevalent precancers than that during follow-up. In contrast, CIN 2 was more common in follow-up (4,320 at enrollment vs 5,330 in follow-up).
TABLE 2.
Cumulative Risk of CIN 3+ at Years 0 Through 5, by Cytology and HPV Results (n = 6,265)a
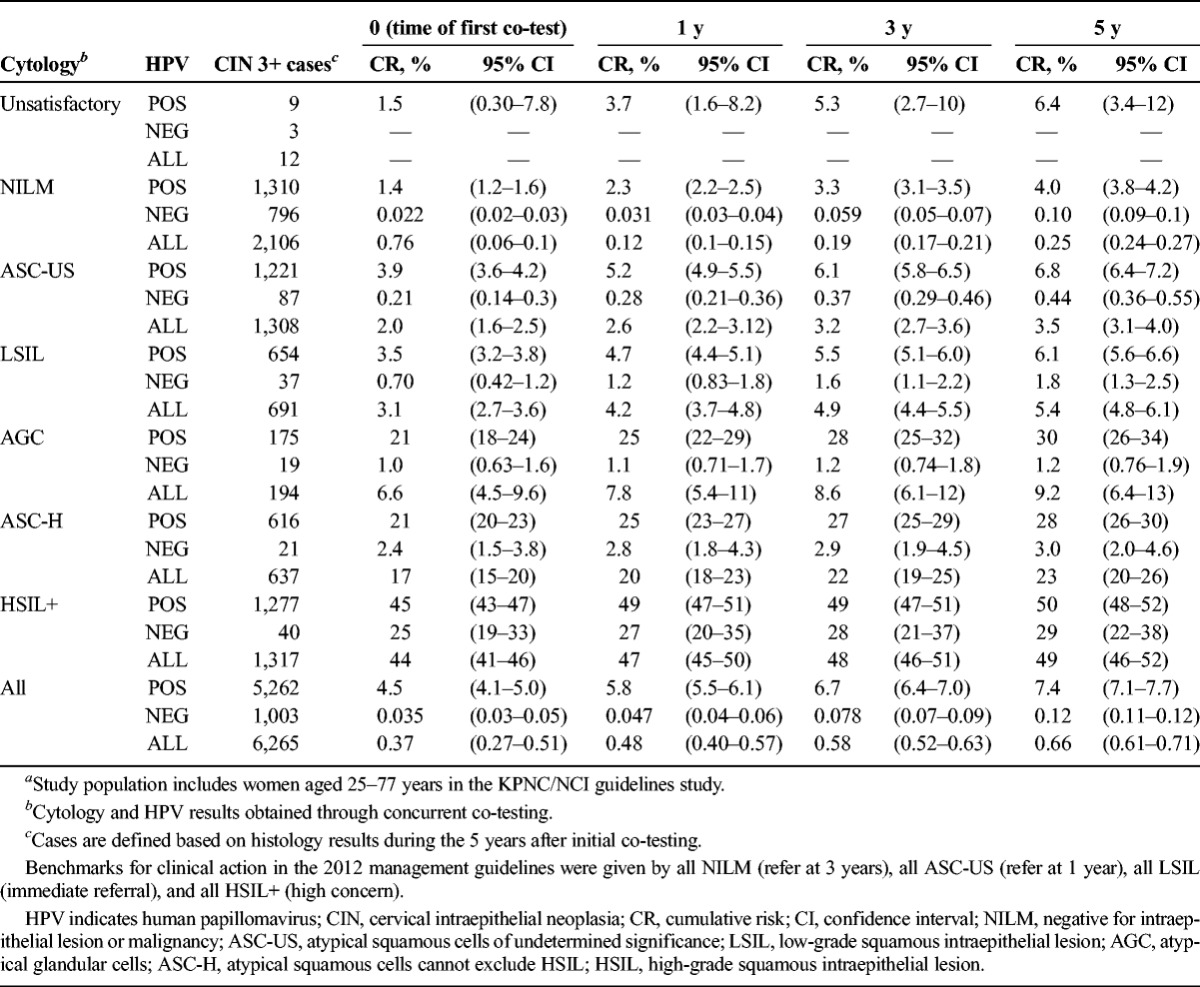
TABLE 4.
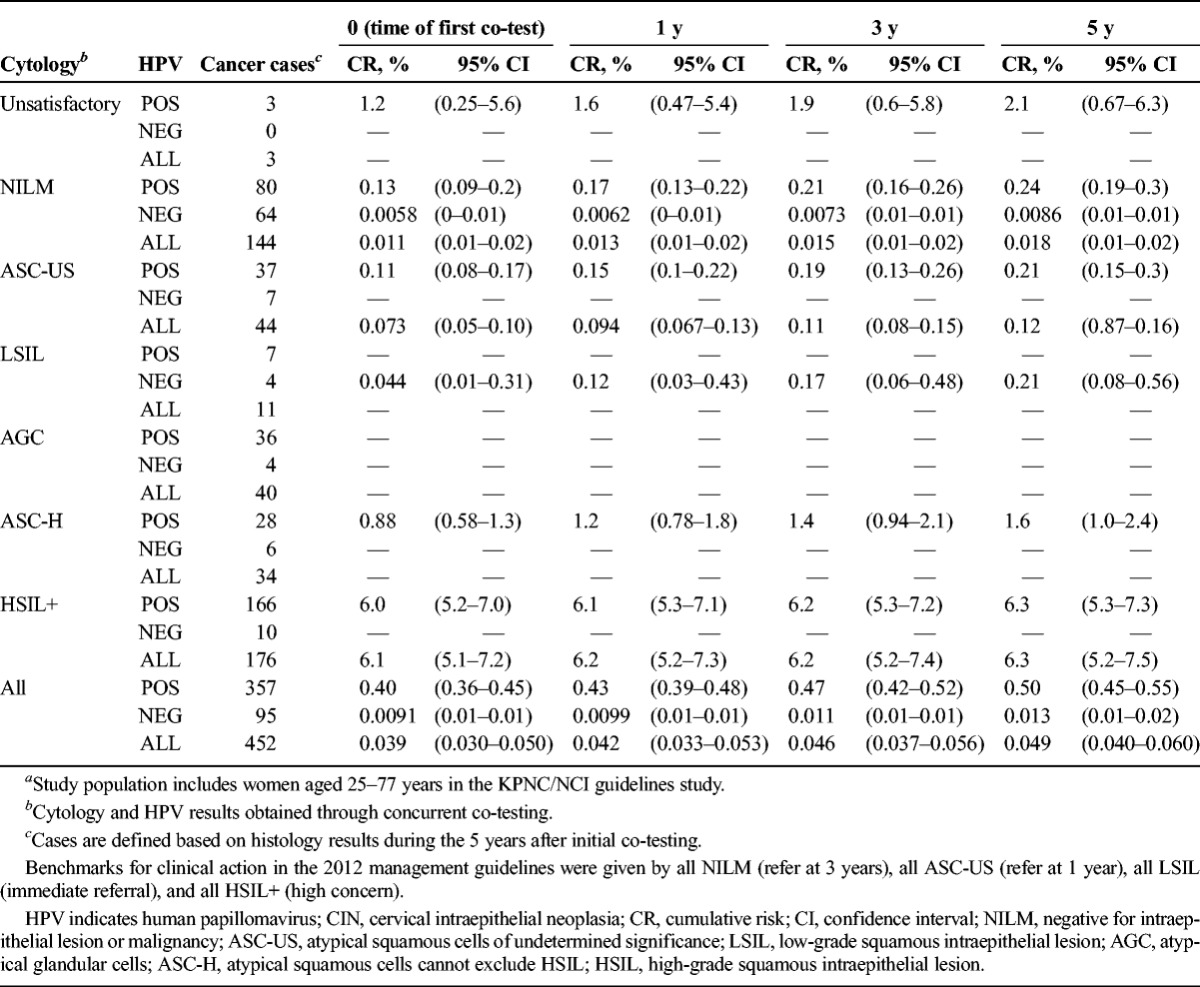
Cumulative Risk of Cancer at Years 0 Through 5, by Cytology and HPV Results (n = 452)a
Generally, risk estimates predicted by different combinations of cytology and HPV status followed similar patterns for CIN 2+ and CIN 3+ (see Tables 2, 3).
TABLE 3.
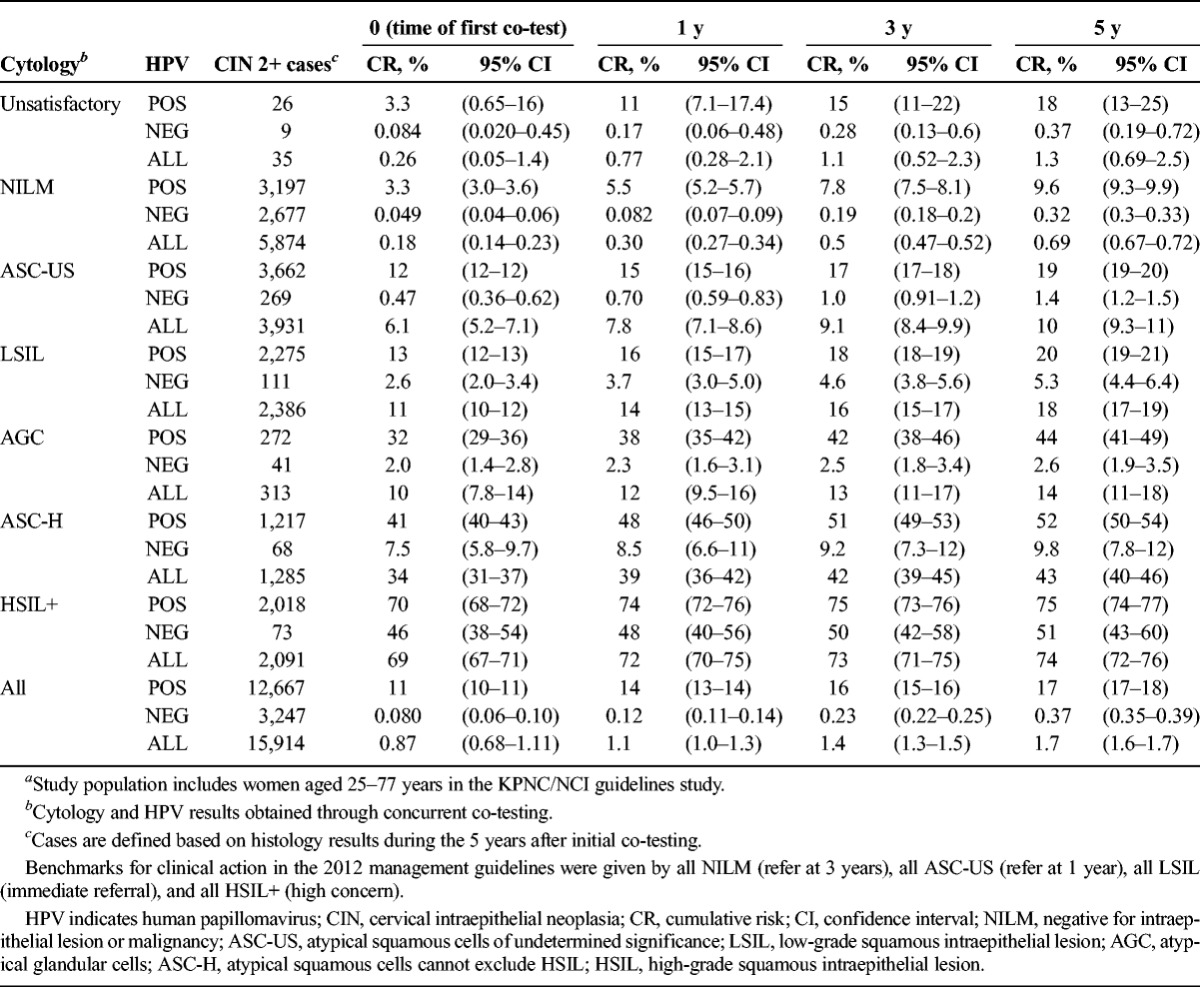
Cumulative Risk of CIN 2+ at Years 0 Through 5, by Cytology and HPV Results (n = 15,914)a
We focus the detailed description of patterns for risk of CIN 3+ (see Table 2) and highlight a few differences specific to CIN 2+ (see Table 3). We discuss the rare cancer outcomes (see Table 4) separately.
Table 2 shows risk of CIN 3+ (6,265 cases or 0.66% overall 5-year risk). Slightly more than half (3,243) of cases were found at enrollment, the time of first co-test. Five-year cumulative risks ranged from 0.25% (NILM) to 49% (HSIL+) when stratified by cytology and from 0.12% (HPV-negative) to 7.4% (HPV-positive) when stratified by HPV status. Thus, 5-year risk for HPV-negative women (0.12%) was substantially less than for NILM cytology (0.25%) and was quite close to the risk after negative co-testing (0.10%).
When HPV results were negative, risks of CIN 3+ tended to be low regardless of cytologic result, except when the cytologic result was HSIL+ (29% risk). However, HPV-negative HSIL+ represented only 0.01% of test results (and roughly the same percentage of negative HPV test results, given that 90% of results were HPV negative).
The HPV-positive women overall (ignoring cytologic result) had a higher 5-year risk of CIN 3+ (7.4%) than those with classical cytologic signs of HPV infection, i.e., LSIL ignoring HPV status (5.4%). Among HPV-positive women, risk varied little when the cytologic result was NILM (4.0%), ASC-US (6.8%), or LSIL (6.1%); however, risk of CIN 3+ was greatly increased for HPV-positive women with more worrisome cytology: ASC-H (28%), AGC (30%), or HSIL+ (50%).
It is noteworthy that HPV-positive ASC-US and overall LSIL (mainly HPV-positive) posed such similar results that they seemed to represent the same underlying state. Both represent HPV induced cytologic abnormality, without evidence of precancer. Conversely, HPV-negative ASC-US predicted risks that were just slightly more than for NILM on an absolute scale.
In contrast to ASC-US (which HPV testing can stratify well in terms of risk), ASC-H proved not to be cleanly divisible by HC2 test result. The 5-year risk of CIN 3+ for HPV-positive ASC-H (28%) was not as high as that for HSIL+ (50%). HPV-negative ASC-H predicted a 5-year risk of CIN 3+ (3.0%) that was still substantially greater than that of NILM (0.25%).
Atypical glandular cells were shown to be especially dependent on HPV status when considering its risk. HPV-positive AGC had a high 5-year risk (44% for CIN 2+, 30% for CIN 3+), but HPV-negative AGC had a low 5-year risk (2.6% for CIN 2+, 1.2% for CIN 3+).
In Table 3, the histopathologic outcome was switched to CIN 2+, with very few changes in patterns. The overall risk of CIN 2+ was 1.7%, based on 15,914 women with that histologic outcome. We observed that average time-to-diagnosis of incident (not at enrollment) CIN 2+ (3.5 years) was no shorter than that of incident CIN 3+ (3.3 years).
Greater numbers of CIN 2+ than those of CIN 3+ made it possible to calculate risks for inadequate cytology categories. Among women with inadequate cytology, risk of CIN 2+ (1.3%) was very similar to the risk for the whole population, overall or stratified by HPV status. Thus, inadequate cytology did not elevate risk; rather, it seemed to simply reflect missing cytology information.
As shown in Table 4, risk of cancer was 0.05% with only 452 cases. Most cancer cases (65%) were prevalent at the time of the first KPNC co-test (see Table 4). We did not have enough cancer cases to estimate risks for all combinations of HPV and cytology. Enrollment cytology was negative for 144 cancer cases, HPV testing was negative for 95, and co-testing was negative for 64. Viewed instead in terms of risk, only 0.0086% of women with enrollment HPV-negative NILM developed cancer within 5 years and the comparable figure for HPV- negative women (ignoring cytologic result, as in primary HPV screening) was 0.013%. The most commonly found enrollment co-test combination preceding diagnosis of cancer was HPV-positive HSIL+, but that only accounted for 27% of the total.
When we ordered the 5-year risk of CIN 3+ for all possible cytology and HPV categories, we observed that with the exception of HSIL+ results, risk clearly divided between HPV negative and positive women, regardless of cytology. As described earlier, HSIL+ risks remained high independently of HPV status. For all other cytology categories, HPV-negative results predicted risks less than 3.5%, equivalent to the risk of an ASC-US cytologic result ignoring HPV results. On the other hand, HPV positivity predicted risk of 4.0% (HPV-positive NILM) to 50% (HPV-positive HSIL+).
Previous guidelines compared co-test results to the risk “benchmarks” associated with cytology. However, the interpretation of cytology can change over time. In particular, KPNC shifted to cytologic interpretations informed by HPV status. This tended to increase the percentage of ASC-US results that were HPV positive, decreased HPV positivity very slightly in NILM, and very slightly increased the risk after ASC-US cytology.
DISCUSSION
We extended the KPNC analysis that was conducted at the time of the last ASCCP-sponsored cervical screening consensus conference.6–10 Simply put, this new, much larger analysis confirmed previous findings with increased precision. Human papillomavirus status was the most important test result, and cytologic results were important to know when HPV was positive.
What specifics and general lessons can a clinician take away from the complex tables found in this first article on screening? If the results from KPNC can be generalized, the most definite conclusions come from the extremes of risk. A woman with HPV-positive HSIL+ is at very high risk for CIN 3+ (44%) even at her initial visit. If risk is judged high enough, there might be a place for Loop Electrosurgical Excision Procedure (LEEP) without biopsy because colposcopically directed biopsies can miss small lesions.11,12
At the other extreme, 90% of screened women had HPV-negative NILM, predicting an extremely low risk for CIN 3+ at 3 and 5 years, 0.06% and 0.10%, respectively. There is debate if the risk level of 0.1% for CIN 3+ (with a vanishingly low risk of new cancer diagnoses when prevalent cases found at enrollment are excluded) justifies a 5-year interval based on the large number of women that have a result of HPV-negative NILM.13 Serious consideration of delayed screening is warranted for this group. Moreover, the 5-year risk for HPV-negative women overall (i.e., primary HPV testing) was 0.12%, only the slightest bit more than 0.10% for co-testing. The guidelines group will need to consider whether tiny differences in safety justify co-testing over primary HPV screening.14
We have known for some time15 that HPV-positive ASC-US and LSIL have very similar risks, which is again confirmed by this very large cohort. They are functionally synonymous, as HPV infections with nonnormal cytology.
Human papillomavirus–positive ASC-H is not quite the same as HSIL+ (and HPV-negative ASC-H not quite like normal). High variability in the classification of ASC-H16 means that the management of HPV-positive ASC-H, although intensive, might not require the same attention as HSIL in some settings.
The cytologic result of AGC warrants special comment. Given that HPV-positive AGC and HPV-negative AGC carry such different risks for CIN 3+ (30% vs 1.2%), one would assume that HPV could be used as a good triage tool for this cytologic abnormality. Women with AGC must be assessed for not only cervical glandular abnormalities but also endometrial abnormalities. Because AGC comprises only 0.2% of all cytologic abnormalities, the guidelines group might continue to refer all women with AGC to colposcopy.
We are in the process of separating the very rare cytologic results of AIS and cancer from the HSIL+ group, to clarify what differences in predicted risk these indicate.
The results for CIN 2+ were the same as for CIN 3+, yet we know that CIN 3 is a better surrogate end point for cancer risk.17 Cervical intraepithelial neoplasia 2 is a poorly reproduced diagnosis,18 many cases are caused by HPV types not found to cause cervical cancer, and the regression rate is very high. Nonetheless, in the KPNC data set, the same conclusions would be drawn about pooled HPV testing and cytology using either end point. When the topic of HPV typing is addressed, we expect greater differences.19
The risk prediction performed from the KPNC database is extremely precise because of huge numbers, but there are limitations. The use of logistic-Weibull models smooths the curves plotting risk by time and speeds computation, based on a few reasonable simplifying bounding assumptions on when a case diagnosed at time X actually developed, in a setting where disease can only be diagnosed when women come in for testing. The logistic-Weibull model cannot entirely overcome the fact that women with HPV-positive NILM or HPV-negative ASC-US are not immediately referred to colposcopy at KPNC. We need other sources of data to observe directly how many cases of CIN 2+, CIN 3+, and cancer would occur if all were referred immediately rather than asked to return (at 1 year for HPV-positive NILM). More generally, we need to repeat similar calculations in multiple cohorts and trial data sets, to consider how variable risks are depending on setting. The one comparison performed to date was reassuring in that the HPV-cytology combinations generated risks similar to those seen at KPNC.20
In summary, this update confirmed the core risk estimates for combinations of HPV testing and cytology results that informed the current guidelines. Future articles will address 2 additional situations (colposcopy population,21 posttreatment22). Risk estimates and conclusions will be expanded to include HPV typing, biomarkers, clinical risk factors such as history that are important to consider like age, and a history of HPV vaccination.
Footnotes
The National Cancer Institute has received cervical cancer screening assays in-kind or at reduced cost from BD, Cepheid, Hologic, and Roche. P.E.C. has received human papillomavirus tests and testing for research at a reduced or no cost from Qiagen, Roche, MTM, and Norchip. The rest of the authors have declared they have no conflicts of interest.
P.E.C. and M.S. contributed equally in the study.
The Kaiser Permanente Northern California Institutional Review Board approved use of the data, and National Institutes of Health Office of Human Subjects Research and Albert Einstein College of Medicine Institutional Review Board deemed this study exempt from review.
W.K.K. was still affiliated with Kaiser Permanente Northern California before retirement in March 2017.
REFERENCES
- 1.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Wentzensen N, Khan MJ, et al. Preparing for the next round of asccp-sponsored cervical screening and management guidelines. J Low Genit Tract Dis 2017;21:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castle PE, Kinney WK, Cheung LC, et al. Why does cervical cancer occur in a state-of-the-art screening program? Gynecol Oncol 2017;pii: S0090-8258:30898–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castle PE, Solomon D, Wheeler CM, et al. Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol 2008;46:2595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung LC, Pan Q, Hyun N, et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. [published online ahead of print June 28, 2017]. Stat Med 2017. doi: 10.1002/sim.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis 2013;17(5 Suppl 1):S56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. J Low Genit Tract Dis 2013;17(5 Suppl 1):S50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 2+ and CIN 3+ among women with HPV-positive and HPV-negative LSIL Pap results. J Low Genit Tract Dis 2013;17(5 Suppl 1):S43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV testing of ASC-US Pap results. J Low Genit Tract Dis 2013;17(5 Suppl 1):S36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis 2013;17(5 Suppl 1):S28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol 2015;33:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentzensen N, Schiffman M, Silver MI, et al. ASCCP Colposcopy Standards: risk–based colposcopy practice. J Low Genit Tract Dis 2017;21:230–4. [DOI] [PubMed] [Google Scholar]
- 13.Kinney W, Wright TC, Dinkelspiel HE, et al. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol 2015;125:311–5. [DOI] [PubMed] [Google Scholar]
- 14.Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst 2014;106:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbyn M, Buntinx F, Van Ranst M, et al. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst 2004;96:280–93. [DOI] [PubMed] [Google Scholar]
- 16.Sherman ME, Castle PE, Solomon D. Cervical cytology of atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H): characteristics and histologic outcomes. Cancer 2006;108:298–305. [DOI] [PubMed] [Google Scholar]
- 17.Carreon JD, Sherman ME, Guillén D, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol 2007;26:441–6. [DOI] [PubMed] [Google Scholar]
- 18.Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001;285:1500–5. [DOI] [PubMed] [Google Scholar]
- 19.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 20.Gage JC, Hunt WC, Schiffman M, et al. Similar risk patterns after cervical screening in two large U.S. populations: implications for clinical guidelines. Obstet Gynecol 2016;128:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katki HA, Gage JC, Schiffman M, et al. Follow-up testing after colposcopy: five-year risk of CIN 2+ after a colposcopic diagnosis of CIN 1 or less. J Low Genit Tract Dis 2013;17(5 suppl 1):S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katki HA, Schiffman M, Castle PE, et al. Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV and Pap cotesting in posttreatment management. J Low Genit Tract Dis 2013;17(5 Suppl 1):S78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


