Abstract
Atypical hemolytic uremic syndrome (aHUS) is a devastating disease characterized by thrombus formation in the microvasculature and is associated with complement dysregulation. The recommended treatment is eculizumab, an humanized monoclonal antibody, which binds complement protein C5 and thereby preventing the assembly of the terminal complement complex (TCC, soluble C5b-9) and the generation of C5a, an anaphylatoxin.
The objective of the study was to identify a reliable biomarker, which estimates the degree of complement inactivation under normal and pathophysiological conditions, such as an infection. Here we report on serial measurements of the soluble form of C5b-9, measured in plasma and the TCC capacity (soluble C5b9 after ex-vivo activation, measured in serum) in seven patients with aHUS who were treated with eculizumab. By measuring the latter we were able to assess the maximum possible soluble C5b-9 production under a potent complement trigger. Patients were followed up over a median duration of 3.8 years either on biweekly (q2w) or a three-weekly (q3w) maintenance therapy interval.
As expected, eculizumab treatment resulted in a profound decrease of TCC capacity (median 55, IQR 44–88 AU/ml) compared to baseline (1249, 529–1806 AU/ml, p < 0.0001) and there was no significant difference in TCC capacity measurements on q2w vs. q3w maintenance dosing schedule. However, during q3w maintenance significantly more single measurements of TCC capacity above the lower cut-off level were detected (50% on q3w vs. 5% on q2w maintenance, p < 0.045). Higher TCC capacity levels were associated with lower eculizumab levels. The TCC capacity may represent a novel and easy to perform parameter to determine level of complement inhibition in patients treated with eculizumab, especially because it identifies the residual capacity of inhibition at pathophysiological stages.
Keywords: Atypical hemolytic uremic syndrome, Thrombotic microangiopathies, Complement activation, Terminal complement complex, Eculizumab
1. Introduction
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA), leading to thrombus formation in small vessels, hemolytic anemia, thrombocytopenia and renal impairment due to systemic complement activation (Riedl et al., 2014). In 60% of aHUS patients, the disease can be attributed to specific mutations in components or regulators of the alternative complement pathway, such as factor H (Richards et al., 2001), MCP (Fremeaux-Bacchi et al., 2006), factor I (Bienaime et al., 2010), factor B (Goicoechea de Jorge et al., 2007), C3 (Roumenina et al., 2012) and thrombomodulin (Delvaeye et al., 2009). Additionally, autoantibodies against factor H, predominantly in the setting of a CFHR1/3 deletion, may also cause aHUS (Hofer et al., 2013). Specifically, these alterations result in complement activation with ongoing cleavage of C5 and subsequent excessive levels of the anaphylatoxin C5a and the potentially lytic C5b-9 or terminal complement complex (TCC) (Noris and Remuzzi, 2013). In this scenario, uncontrolled complement activation causes endothelial cell damage, inflammation and a prothrombotic state that manifests clinically as TMA (Markiewski et al., 2007; Noris and Remuzzi, 2009; Noris and Remuzzi, 2013). Recently a mutation in the DGKε gene, encoding diacylglycerol kinase ε, a protein involved in cell metabolism has also been linked to aHUS in infants (Lemaire et al., 2013; Bruneau et al., 2015).
Long-term prognosis is devastating with up to 77% of patients with aHUS developing end stage renal disease or death within the first 3 years after diagnosis (Noris and Remuzzi, 2009). Prognosis in aHUS is markedly improved by blocking the terminal complement pathway with eculizumab, a monoclonal anti-C5 antibody (Rother et al., 2007; Legendre et al., 2013; Licht et al., 2015; Loirat et al., 2016). Two prospective trials and one retrospective analysis of a pediatric cohort have confirmed efficacy and safety of eculizumab in patients with aHUS (Simonetti et al., 2012; Legendre et al., 2013; Licht et al., 2015). However, despite the positive outcome with eculizumab treatment, a standard procedure for monitoring for treatment response and the efficacy of complement inhibition has not been established.
Routinely, C3 levels have been measured to assess complement activation. However, low C3 levels are only reported in about 50% of aHUS patients during active disease and are unable to assess the efficacy of eculizumab treatment (Loirat and Fremeaux-Bacchi, 2011; Cugno et al., 2014; Noris et al., 2014). Previous work by our group and others has shown that SC5b-9 levels are significantly elevated in aHUS patients during a TMA event compared to patients in clinical remission or healthy subjects (Prufer et al., 2006; Cataland et al., 2014).
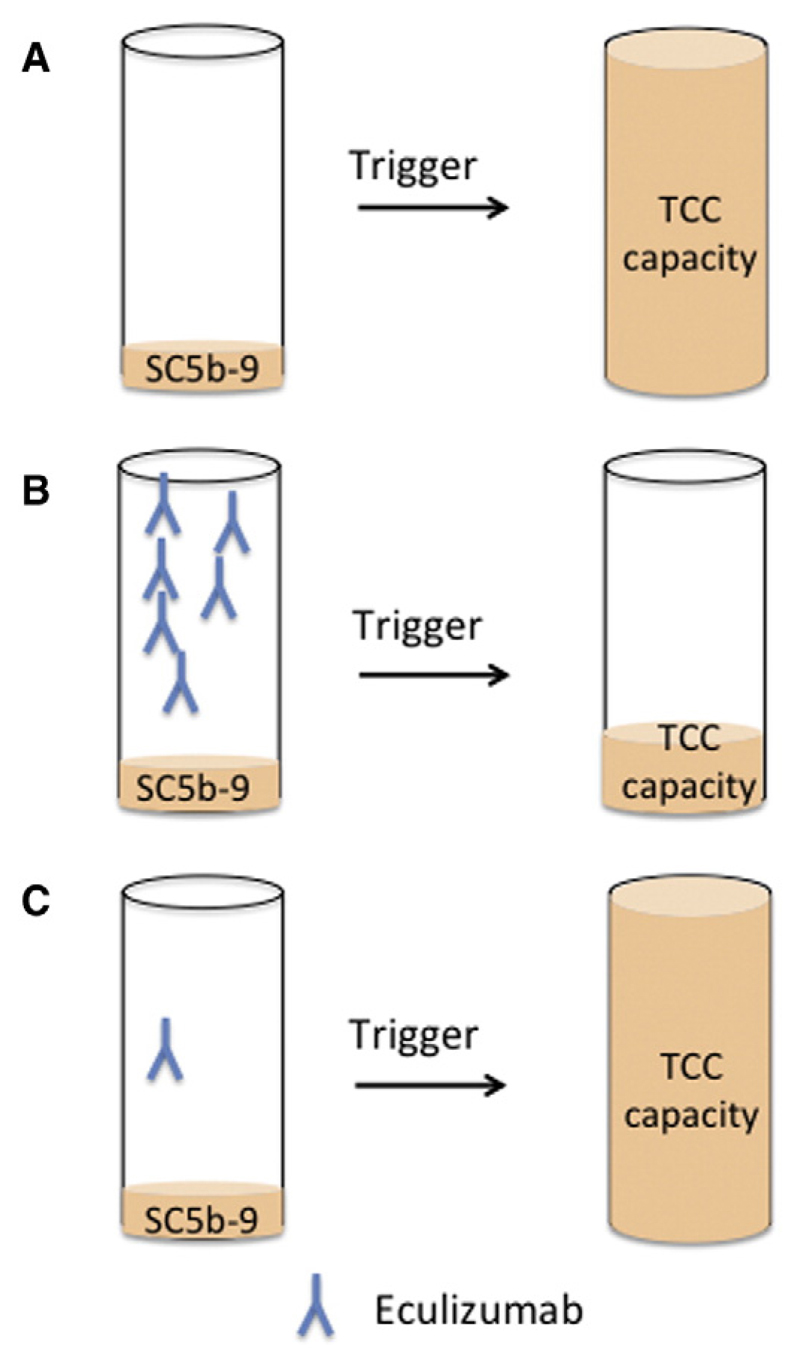
In this study we measured SC5b-9 levels, the TCC capacity and eculizumab levels in aHUS patients treated with eculizumab over time. SC5b-9 reflects the non-lytic soluble C5b-9 bound to S-protein/vitronectin in the fluid phase (Hadders et al., 2012). The TCC capacity represents the maximal available SC5b-9 after ex-vivo stimulation (Prufer et al., 2006). Zymosan, a potent activator of the alternative pathway of complement (Unsworth et al., 1993), was added to patient's serum for triggering complement activation (Fig. 1).
Fig. 1.
Principles of TCC capacity measurement with and without eculizumab therapy. Zymosan activates the alternative complement pathway and uses all available C5 (and other complement proteins) to form SC5b-9. Eculizumab binds to C5 and therefore prevents the formation of SC5b-9. Only free C5 can be activated and will contribute to SC5b-9 levels. (A) High TCC capacity values are expected when patient is not treated with eculizumab. (B) Very low levels of TCC capacity are expected when eculizumab blockade is sufficient. (C) When the eculizumab concentration is at its lower level, the strong in vitro trigger will lead to an increase of TCC capacity, i.e. the patient is at potential risk in case of an infection, despite a controlled low SC5b-9 concentrations in the native sample.
The aim of this study was to assess whether the TCC capacity, measured by an easy to perform ELISA, is a good biomarker to predict sufficient complement blockade during eculizumab treatment even under pathophysiological conditions. Therefore we studied aHUS patients treated permanently with eculizumab with two regimens: i) on q2w (every-other-week) maintenance and ii) on q3w (every-third-week) maintenance. TCC capacity levels were compared with circulating eculizumab and eculizumab-C5 complex levels in plasma.
2. Material and methods
2.1. Study design
Since 2002 180 patients with atypical HUS, defined by the triad of thrombocytopenia, hemolytic anemia and reduced renal function have been enrolled in the international aHUS registry (www.hus-online.at). Before enrollment the patient or the guardian gave informed consent. The study was approved by the local hospital ethic commission and is according to the Helsinki Declaration of 1975.
2.2. Patient cohort
From November 2008 to April 2013 serial complement assays were performed in 7 patients with aHUS whilst they were being treated with eculizumab. Table 1 gives a detailed overview of these seven aHUS patients (unpublished, Zimmerhackl et al., 2010; Tschumi et al., 2011). Diagnostic workup included screening for CFH, CFI, MCP, C3, CFB mutations and factor H antibodies.
Table 1.
Overview of patient cohort.
| Age at disease onset | Age at eculizumab therapy onset | Genetics | PE before eculizumab | Dosing of eculizumab | |
|---|---|---|---|---|---|
| #1 | 4 years (Zimmerhackl et al., 2010) | 10 years (Nov 08) | CFH mutation (SCR20) | 10 days | 900 mg once 600 mg q2w/q3w |
| #2 | 1.2 years | 4 years (June 09) | CFH mutation (SCR20) | Chronic PE treatment | 600 mg once 300 mg q2w/qw3w |
| #3 | 25 years | 25 years (June 09) | CFH mutation (SCR20) | Intensive PE treatment (for 1 month) | 900 mg induction 1200 mg q2w |
| #4 | 7.5 years (Tschumi et al., 2011) | 8.5 years (June 09) | CFH mutation (SCR10) | Chronic PE treatment (3 ×/week) | 600 mg q2w/q3w |
| #5 | 1.5 years | 2 years (May 10) | Polymorphism CFH | Chronic PE treatment (12 in total) | 600 mg for induction 300 mg q2w/q3w |
| #6 | 32 years | 33 years (April 11) | Several polymorphisms in C3, CFB, CFH and CFI | Daily PE treatment | 900 mg induction 1200 mg q2w/q3w |
| #7 | 22 years | 31 years (Jan 12) | C3 mutation | No | 900 mg induction 1200 mg q2w/q3w |
CFB, complement factor B; CFH, complement factor H; CFI, complement factor I; q2w, maintenance dosing biweekly; q3w, maintenance dosing every three weeks; PE, plasma exchange; SCR, short consensus repeats.
Six out of seven patients received plasma exchange prior to eculizumab commencement, which was stopped in all before receiving the first eculizumab infusion. The treating physician at each institution determined the dosage and treatment interval.
Meningococcal vaccination was performed in each patient and additional oral antibiotic prophylaxis (penicillin in 3 and cefuroxime in 1 patient) was given to 4 patients. No meningococcal infection was reported. eGFR was calculated with MDRD formula in adults and height-independent Pottel equation in children (Pottel et al., 2012).
2.3. Samples
SC5b-9 levels were measured in EDTA plasma, the TCC capacity in serum. Blood samples were drawn from the patients either before the first eculizumab infusion was administered (prior, baseline) or right before the next eculizumab infusion was given - to determine trough levels, either on q2w or q3w dosing interval. Serum and plasma samples were centrifuged within 1 h, sent on dry ice and stored at −20 °C (<6 months) or at −80 °C (>6 months). The number of samples collected from each patient varies from 1–58 per time period (prior, q2w, q3w). Ninety-eight healthy adult blood donors (without an apparent infection) were used as controls. The median age of the controls was 37 years (IQR: 28–50 years), 46% were male. Data is given as median and interquartile range (IQR), unless indicated otherwise.
2.4. SC5b-9 ELISA and TCC capacity
To determine TCC capacity (maximal possible formation of SC5b-9) 10% baker's yeast (zymosan) solution (Unsworth et al., 1993) was added to serum and incubated over night in a 37 °C warm waterbath (Fig. 1). SC5b-9 and TCC capacity were determined by using a sandwich ELISA based on a neoepitope-specific anti C9 antibody (WU13-15, Hycult Biotech Inc., The Netherlands), in detail described elsewhere (Prufer et al., 2006). In short, ELISA plates were coated overnight with WU13-15, samples were added the next day. A biotinylated C6 antibody was used as a secondary antibody, followed by Streptavidin alkaline phosphatase (Sigma-Aldrich, Vienna, Austria) and p-Nitrophenyl phosphate (Sigma-Aldrich) as a substrate. Absorbance was measured at 405 nm (reference wave length 490 nm). Pooled zymosan activated serum was used as standard curve. AU/ml was calculated using a linear regression curve and log/lin axis scales. A 1/100 dilution of the standard curve was set as 10 AU/ml. Plasma samples were diluted 1:4 and TCC capacity samples 1:100 (trough level)–1:400 (baseline and controls). The concentration of patient samples was calculated using Microsoft Excel.
2.5. Eculizumab levels and eculizumab-C5 complexes
The eculizumab concentration in the serum samples was measured by an ELISA specifically developed for this purpose. To capture eculizumab, the 96-well plates were coated with purified 0.5 μg/well of purified C5 (Calbiochem®, San Diego, CA, USA) diluted in carbonate buffer (pH 9.6) overnight at 4 °C. Serum samples have been diluted in phosphate buffered saline, supplemented with 1% bovine serum albumin (PBS/BSA). Eculizumab detection has been performed using horse radish peroxidase-conjugated goat-antihuman IgG (JacksonImmunoResearch Laboratories, Inc., West Grove, PA, USA). A standard was produced by adding eculizumab (Alexion Pharmaceuticals, Cheshire, CT, USA) to PBS/BSA in two-fold dilution steps to create a range of 0.8–50 ng/ml. Eculizumab-C5 complexes were detected as described elsewhere (Hallstensen et al., 2015).
2.6. Statistics
Statistical analysis was performed using Mann–Whitney-U test and Kruskal-Wallis as indicated. The values used for q2w and q3w maintenance were taken 1 month after. Graphpad Prism 6 was used for data analysis and for preparation of figures. A p-value < 0.05 was considered statistically significant.
2.7. Determination of cut-off level for TCC capacity
The reference interval for TCC capacity was calculated using IBM SPSS software version 21 as follows (Lumsden and Mullen, 1978; Zurakowski et al., 1999; Katayev et al., 2010): The IQR of TCC capacity in controls was calculated and the upper (75th percentile + (1.5 × IQR)) and lower (25th percentile −(1.5 × IQR)) limits were determined. All values above or below these percentiles (n = 3) were considered as outliers and removed. As TCC capacity did not show a normal distribution (Kolmogorov-Smirnov p < 0.0001), a natural log-transformation was applied before calculating the 2.5th percentile to determine the lower cut-off level for TCC capacity in controls. Values were converted back to the original units by calculating the antilog of the logarithmically transformed values. The lower cut-off level for TCC capacity was calculated as 150 AU/ml.
3. Results
3.1. No difference of SC5b-9 levels under eculizumab therapy
Prior to receiving eculizumab patients showed an elevated SC5b-9 concentration compared to controls (2.5, IQR 1.9–3.9 AU/ml vs. 1.2, IQR 0.8–1.8 AU/ml; p < 0.02). At this time point, 6/7 patients received plasma exchange. SC5b-9 levels remained unchanged upon transition from plasma exchange to eculizumab q2w and q3w maintenance therapy (2.3, IQR 1.6–3.8 vs. 2.3, IQR 1–16.3, n.s.).
3.2. Terminal complement complex (TCC) capacity decreases under eculizumab therapy
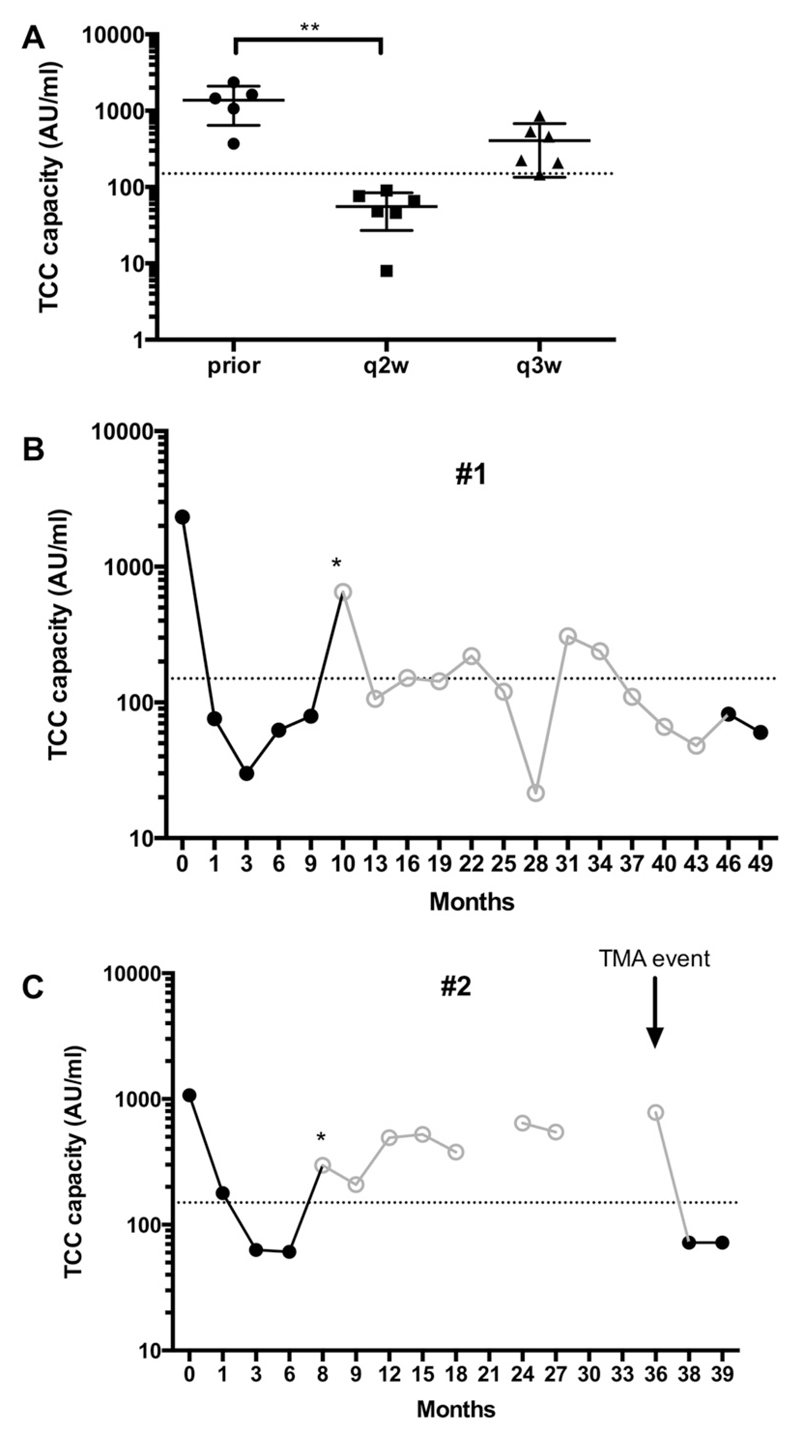
At baseline, prior to the first eculizumab infusion, the TCC capacity showed a median level of 1249 AU/ml (IQR 529–1806 AU/ml) compared to healthy controls with a median of 505 AU/ml (IQR 290–856 AU/ml; p = 0.09). A significant decline (p < 0.0001) was observed under eculizumab treatment, with a median of 55 AU/ml (44–88 AU/ml) during biweekly administration, indicating inhibition of terminal complement activation (Fig. 2A). This was observed as early as after the first infusion of eculizumab.
Fig. 2.
TCC capacity levels decreases under eculizumab therapy. (A) TCC capacity levels (logarithmic scale) are displayed for patients at different time points: prior eculizumab, on q2w maintenance and q3w maintenance therapy. A significant decrease compared to TCC levels prior eculizumab therapy was observed for q2w maintenance levels. **p < 0.05 (Kruskal-Wallis). (B) TCC capacity levels over time in patient #1. (C) TCC capacity levels over time in patient #2. Open dots/line in grey indicate q3w maintenance interval. Dotted line indicates lower cut-off level of 150 AU/ml. Asterisk indicates switch to q3w maintenance. q2w, maintenance dosing biweekly; q3w, maintenance dosing every three weeks.
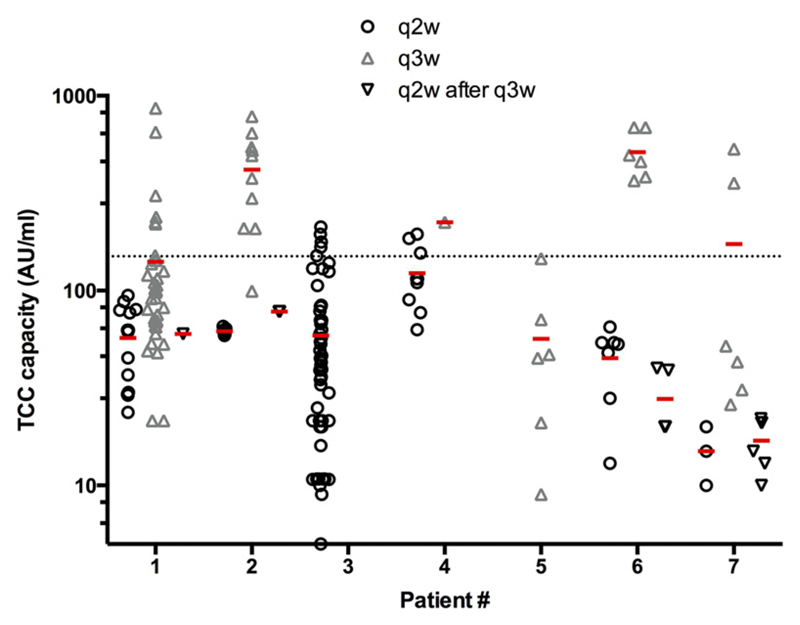
After treatment extension to a three-week maintenance schedule (q3w) an overall trend to an increase of the TCC capacity was observed (209 AU/ml, IQR 106–510 AU/ml, p = 0.06) (Fig. 2A). Furthermore, a significant higher number of single measurements above the cut-off level (150 AU/ml, 2.5th percentile of healthy controls) was detected in patients with the q3w dosing regime (50%, IQR 26–77% on q3w vs. 5%, IQR 0–12% on q2w; p = 0.02, Mann–Whitney-U-test) (Fig. 3). This was seen in patient #1 and #2 (detailed in Fig. 2B and C), but also #4 and #6 already after the first three-week interval and in patient #7 three months later with an intercurrent illness (not detailed).
Fig. 3.
Significant more single measurements above the cut-off level when on q3w maintenance therapy. For each patient (x-axis) single measurements under q2w maintenance (black circle), q3w maintenance (grey triangle) and after re-intensification (q2w after q3w maintenance, black triangle) are displayed on the y-axis (logarithmic scale). Significant more values above the cut-off level of 150 AU/ml (dotted line) were observed under q3w maintenance. The red line indicates the mean. q2w, maintenance dosing biweekly; q3w, maintenance dosing every three weeks.
3.3. Eculizumab levels decrease over time on q3w dosing
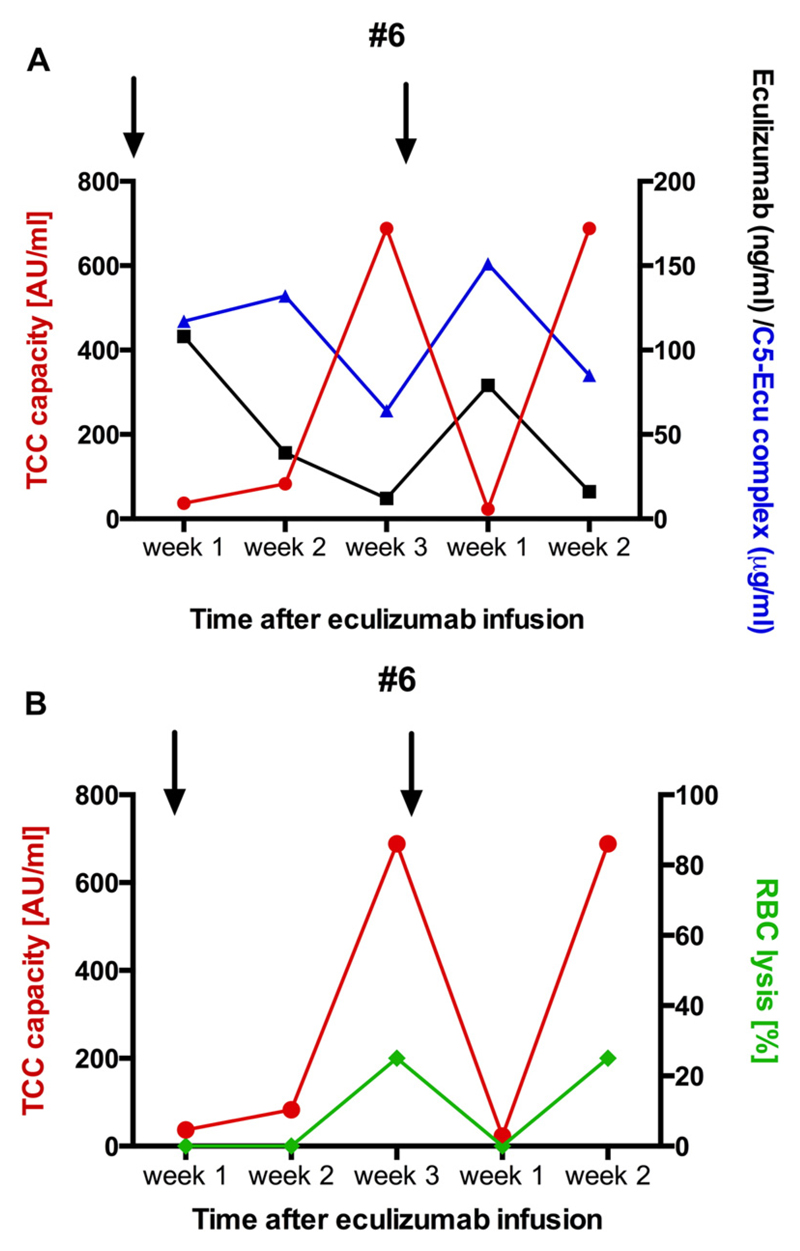
In 3 patients we measured eculizumab levels and eculizumab-C5 complexes in addition to the TCC capacity. Eculizumab levels and eculizumab-C5 complexes decreased over time when patients were on q3w dosing (Fig. 4, Table 2). In patient #6 we had samples available before the 1st and 2nd q3w dosage. Increased TCC capacity was seen at 3 weeks, prior to the 1st dosage and then again 2 weeks later. Higher TCC capacity values were associated with lower eculizumab levels and eculizumab-C5 complexes (Fig. 4A). In one patient with high TCC capacity and low eculizumab levels (the same patient #6) we were able to detect lysis of rabbit erythrocytes, indicating that the non-inhibited C5b-9 can be inserted in cell membranes and induce cell damage (Fig. 4B).
Fig. 4.
TCC capacity, eculizumab and E-C5 complexes in a patient after transition to q3w. (A) TCC capacity (red, left x-axis), eculizumab (black, right x-axis) and eculizumab-C5 complexes (blue, right x-axis) were measured in patient #6 for the first 5 week after treatment extension to q3w. TCC capacity levels are high 3 weeks and 2 weeks after eculizumab infusion, respectively. Concomitantly eculizumab levels and E-C5 complexes decreased. Arrows indicated eculizumab infusion. (B) High TCC capacity levels are associated with lysis of rabbit RBCs, indicating that serum samples can form C5b-9 complexes to lyse RBCs.
Table 2.
TCC capacity, eculizumab levels and E-C5 complexes in two patients.
| TCC capacity (AU/ml) | E level (ng/ml) | E-C5 complex (μg/ml) | Interval | Time | |
|---|---|---|---|---|---|
| #1 | 30 | 547 | 166 | q2w | 3.5 months after start |
| 651 | 622 | 168 | q3w | Prior 2nd dose on q3w | |
| 137 | 116.5 | 119 | q3w | 17 months on q3w | |
| 307 | 68 | 90 | q3w | 30 months on q3w | |
| #2 | 65.5 | 161 | 143 | q2w | 4 weeks after start |
| 297 | 39 | 100 | q3w | Prior 1st dose on q3w | |
| 524 | 19.5 | 85 | q3w | 6 months on q3w, infection | |
| 78 | 89 | 93 | q2w | 2 months on q2w, 2 months after TMA event |
q2w, maintenance dosing biweekly; q3w, maintenance dosing every three weeks; E, eculizumab;
3.4. One aHUS patients develops a TMA episode under q3w dosing
Seven months after commencing eculizumab treatment patient #2 was switched to a three weeks interval, which resulted in an immediate increase of TCC capacity (from median 63 AU/ml at q2w to 297 AU/ml, maximum 642 AU/ml), indicating less complement inhibition at day 21, prior to next eculizumab infusion (Fig. 2B). Twenty-eight months later the girl suffered from a TMA event, triggered by an upper respiratory tract infection. Platelets decreased to 108 × 109/l, LDH was elevated and eGFR decreased from 52 to 33 ml/min/1.73 m2. A re-induction and a q2w administration of eculizumab as maintenance therapy (TCC capacity 78 AU/ml) were able to normalize platelet counts (218 × 109/l) and increase eGFR to 56 ml/min/1.73 m2.
4. Discussion
Over the last years, eculizumab became the recommended treatment of choice for most patients with aHUS (Loirat et al., 2016). There is an increasing need to quantify the level of complement inhibition in patients treated with eculizumab. This is the first analysis studying the TCC capacity – in contrast to SC5b-9 - to determine level of treatment induced C5 inhibition.
As eculizumab blocks the generation of the terminal pathway, detecting SC5b-9 seemed to be an ideal marker for disease monitoring. However, in this study we were not able to see a difference in plasma levels of SC5b-9 during treatment with eculizumab. This is in line with other reports, which were not able to show that treatment with eculizumab alters the SC5b-9 levels (Mache et al., 2009; Davin et al., 2010; Weitz et al., 2011; Radhakrishnan et al., 2012; Noris et al., 2014). Complement inhibition resulted in a profound decrease of the TCC capacity in all patients during eculizumab treatment (q2w maintenance). We thus analyzed complement activity by determining the TCC capacity in patients receiving eculizumab via a q2w or q3w dosing interval.
A q2w maintenance dosing schedule was described as sufficient for blocking terminal complement activation (Legendre et al., 2013), and delayed next dosing was associated with hemolysis and deterioration of renal function in one patient (Chatelet et al., 2010). Earlier reports already indicated that termination of treatment can lead to TMA recurrence (Nurnberger et al., 2009; Legendre et al., 2013). This is not unexpected as the elimination half-life of eculizumab was reported as 11 ± 3 days and in a pediatric cohort as 14.5 days. (Rother et al., 2007; Greenbaum et al., 2016). In paroxysmal nocturnal hemoglobinuria (PNH), another complement disorder, it was recommended that eculizumab dosing interval should not be expanded beyond 17 days (Nakayama et al., 2016). TCC capacity levels of patients on q3w maintenance therapy were more frequently above the lower cut-off level of healthy controls than patients on q2w maintenance. Some levels were as high as levels detected in healthy controls, indicating lack of full terminal complement inhibition over the course of q3w dosing. The elevated TCC capacity was associated with clinical TMA or possible ongoing renal damage. One patient demonstrating higher TCC capacity levels had a TMA recurrence. Three additional patients showed a decline or persistently impaired eGFR even after initial eGFR improvement when maintenance therapy was switched from q2w to q3w, the latter also being associated with elevated TCC capacity levels. Furthermore, we were able to show that high TCC capacity levels are associated with low circulating eculizumab concentration and reduced eculizumab-C5 complexes.
Other complement assays to determine complement activation have been studied in patients with aHUS on eculizumab treatment. Using more global functional assays, such as APH50 and CH50 or the Complement system Screen WIESLAB, others reported an absence of total complement activity of classical and alternative pathway after eculizumab administration (Zuber et al., 2012; Cugno et al., 2014; Volokhina et al., 2015). Volokhina and co-workers reported a complete complement blockade for up to 4 weeks after the last eculizumab dosage. In their study eculizumab-C5 complexes decreased only when dosage interval extended 5 weeks (Volokhina et al., 2015). Compared to our study they followed their patients up to dose 16 (<12 months) and discontinued then and some of the eculizumab-C5 complexes were measured in serum. We however, have a follow-up of patients on chronic q3w dosing for up to 40 months. Noris and colleagues found CH50 not helpful for eculizumab monitoring and employed C5b-9 deposition on endothelial cells as monitoring tool (Noris et al., 2014). However, the latter test needs confocal microscopy and is not an easy assay to perform.
The assay we propose here, TCC capacity, however is easy to perform, as it is ELISA based. Additionally it reflects TCC levels after maximal stimulation, mimicking a patient on eculizumab experiencing a complement triggering condition, like an infection. Infections are known to trigger aHUS, possibly via induction of complement activation (Loirat and Fremeaux-Bacchi, 2011; Fremeaux-Bacchi et al., 2013). One patient in our cohort with incomplete complement inhibition suffered from a TMA episode following an infection. During q2w maintenance despite being challenged with intercurrent illness and infections, no TMA episode/decline in renal function was observed in our cohort or reported in literature (except DGKε – associated HUS) (Lemaire et al., 2013; Loirat et al., 2016).
Eculizumab therapy has clearly improved long-term outcome in patients with aHUS. Up to now, no reliable and comprehensively studied tool to monitor complement blockade in this patient cohort is available. Although only measured in a small cohort, the TCC capacity might be a good parameter to determine grade of complement inhibition in these patients, especially if patients experience a complement triggering event such as an infection. Further studies are needed to address the question to which extent the terminal pathway has to be blocked to prevent ongoing terminal pathway activation and subsequent TMA and to compare the available tools.
In conclusion, therapeutic complement inhibition can be determined by measuring the novel biomarker TCC capacity by an easy to perform ELISA, which can indicate successful complement inhibition even in pathophysiological conditions. The cost of eculizumab has led to several patients receiving the drug in off label extended intervals. Our study indicates that in some patients with sufficient complement inhibition even under triggering conditions treatment intervals can be delayed and money saved. Other patients, however, could suffer from these too optimistically extended intervals and are then at risk for ongoing organ damage, a sudden TMA episode or progressive decline in renal function. This state of being “at risk”, however, can be assessed, as in this case complement inhibition would not be sufficient when tested for the TCC capacity.
Acknowledgements
M. Riedl, J. Hofer, T. Giner and A. Rosales performed the TCC ELISA. L.B. Zimmerhackl, M. Riedl, R. Würzner and T. Jungraithmayr were responsible for concept and design of study, the latter three also performed data analysis and prepared the manuscript. K. Häffner, G. Simonetti, U. Walden, D. Heininger and G. Mayer performed testing of clinical laboratory data, wrote clinical course of their patient and together with Günter Weiss critically revised the document. All authors gave final approval of the document.
Parts of this work have been presented at the ASN 2012, the IPNA 2013 and the HUS 2015.
We are grateful for the patients and parents for taking part in this study. We thank Dr. Christoph Licht and Dr. Damien Noone for proof reading the manuscript. This work was supported by the Nationalbank Jubiläumsfond Austria (projects 12711 and 13655) received by Lothar Bernd Zimmerhackl and Therese Jungraithmayr, the Innsbruck Medical University, Austria and by the FWF-funded doctoral program HOROS (FWF-W1253-B24 received by Reinhard Würzner).
Glossary
- aHUS
atypical hemolytic uremic syndrome
- APH50
Alternative complement activity
- CFB
Complement factor B
- CFH
Complement factor H
- CFI
Complement factor I
- CH50
Total complement activity
- CKD
Chronic kidney disease
- DGKε
Diacylglycerol kinase ε
- eGFR
Estimated Glomerular Filtration Rate
- LDH
Lactate dehydrogenase
- q2w
Dosing every 2 weeks
- q3w
Dosing every 3 weeks
- SC5b-9
C5b-9 bound to S protein
- SCR
Short consensus repeat
- TCC
Terminal complement complex
- TMA
Thrombotic microangiopathy
Footnotes
Disclosures
Magdalena Riedl and Therese Jungraithmayr have been on scientific advisory boards for Alexion. Reinhard Würzner has received unrestricted educational grants from Alexion and Eurodiagnostica and has attended meetings organised and financed by these companies (including royalties).
References
- Bienaime F, Dragon-Durey MA, Regnier CH, Nilsson SC, Kwan WH, Blouin J, Jablonski M, Renault N, Rameix-Welti MA, Loirat C, Sautes-Fridman C, et al. Mutations in components of complement influence the outcome of factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010;77:339–349. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- Bruneau S, Neel M, Roumenina LT, Frimat M, Laurent L, Fremeaux-Bacchi V, Fakhouri F. Loss of DGK epsilon induces endothelial cell activation and death independently of complement activation. Blood. 2015;125:1038–1046. doi: 10.1182/blood-2014-06-579953. [DOI] [PubMed] [Google Scholar]
- Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–3738. doi: 10.1182/blood-2013-12-547067. [DOI] [PubMed] [Google Scholar]
- Chatelet V, Lobbedez T, Fremeaux-Bacchi V, Ficheux M, Ryckelynck JP, Hurault de Ligny B. Eculizumab: safety and efficacy after 17 months of treatment in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome: case report. Transplant Proc. 2010;42:4353–4355. doi: 10.1016/j.transproceed.2010.09.125. [DOI] [PubMed] [Google Scholar]
- Cugno M, Gualtierotti R, Possenti I, Testa S, Tel F, Griffini S, Grovetti E, Tedeschi S, Salardi S, Cresseri D, Messa P, et al. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12:1440–1448. doi: 10.1111/jth.12615. [DOI] [PubMed] [Google Scholar]
- Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis. 2010;55:708–711. doi: 10.1053/j.ajkd.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaime F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V, Moulton EA, Kavanagh D, Dragon-Durey MA, Blouin J, Caudy A, Arzouk N, Cleper R, Francois M, Guest G, Pourrat J, et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17:2017–2025. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, Lopez-Trascasa M, Sanchez-Corral P, Morgan BP, Rodriguez de Cordoba S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Hadders MA, Bubeck D, Roversi P, Hakobyan S, Forneris F, Morgan BP, Pangburn MK, Llorca O, Lea SM, Gros P. Assembly and regulation of the membrane attack complex based on structures of C5b6 and sC5b9. Cell Rep. 2012;1:200–207. doi: 10.1016/j.celrep.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstensen RF, Bergseth G, Foss S, Jaeger S, Gedde-Dahl T, Holt J, Christiansen D, Lau C, Brekke OL, Armstrong E, Stefanovic V, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2015;220:452–459. doi: 10.1016/j.imbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Hofer J, Janecke AR, Zimmerhackl LB, Riedl M, Rosales A, Giner T, Cortina G, Haindl CJ, Petzelberger B, Pawlik M, Jeller V, et al. Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2013;8:407–415. doi: 10.2215/CJN.01260212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180–186. doi: 10.1309/AJCPN5BMTSF1CDYP. [DOI] [PubMed] [Google Scholar]
- Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian CBC, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Fremeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, Ji W, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45:531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA, Goodship T, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;5:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden JH, Mullen K. On establishing reference values. Can J Comp Med. 1978;42:293–301. [PMC free article] [PubMed] [Google Scholar]
- Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V, Kirschfink M, Zipfel PF, Roedl S, Vester U, Ring E. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Usuki K, Echizen H, Ogawa R, Orii T. Eculizumab dosing intervals longer than 17 days may be associated with greater risk of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria. Biol Pharm Bull. 2016;39:285–288. doi: 10.1248/bpb.b15-00703. [DOI] [PubMed] [Google Scholar]
- Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, Tripodo C, Bettoni S, Donadelli R, Valoti E, Tedesco F, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger J, Philipp T, Witzke O, Opazo Saez A, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M. Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med. 2009;360:542–544. doi: 10.1056/NEJMc0808527. [Erratum appears in N Engl J Med. 2009 Jun 4; 360(23):2487 Note: Philipp, Thomas [added]] [DOI] [PubMed] [Google Scholar]
- Pottel H, Hoste L, Martens F. A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr Nephrol. 2012;27:973–979. doi: 10.1007/s00467-011-2081-9. [DOI] [PubMed] [Google Scholar]
- Prufer F, Scheiring J, Sautter S, Jensen DB, Treichl R, Wurzner R, Zimmerhackl LB. Terminal complement complex (C5b-9) in children with recurrent hemolytic uremic syndrome. Semin Thromb Hemost. 2006;32:121–127. doi: 10.1055/s-2006-939768. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, Pluthero F, Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366:1165–1166. doi: 10.1056/NEJMc1106619. [DOI] [PubMed] [Google Scholar]
- Richards A, Buddles MR, Donne RL, Kaplan BS, Kirk E, Venning MC, Tielemans CL, Goodship JA, Goodship TH. Factor H mutations in hemolytic uremic syndrome cluster in exons 18–20, a domain important for host cell recognition. Am J Hum Genet. 2001;68:485–490. doi: 10.1086/318203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl M, Fakhouri F, Le Quintrec M, Noone DG, Jungraithmayr TC, Fremeaux-Bacchi V, Licht C. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- Roumenina LT, Frimat M, Miller EC, Provot F, Dragon-Durey MA, Bordereau P, Bigot S, Hue C, Satchell SC, Mathieson PW, Mousson C, et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012;119:4182–4191. doi: 10.1182/blood-2011-10-383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti G, Vilalta R, Lapeyraque AL, Gruppo R, Sherwinter J, Smith J, Thornburg C, Jungraithmayr T, Wuehl E, Al-Akash S, Davin JC, et al. Efficacy and safety of eculizumab treatment for atypical haemolytic uremic syndrome (aHUS) in paediatric patients: subgroup analysis of a retrospective study. Nephrol Dial Transplant. 2012;27:ii11–ii12. [Google Scholar]
- Tschumi S, Gugger M, Bucher BS, Riedl M, Simonetti GD. Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol. 2011;26:2085–2088. doi: 10.1007/s00467-011-1989-4. [DOI] [PubMed] [Google Scholar]
- Unsworth DJ, Wurzner R, Brown DL, Lachmann PJ. Extracts of wheat gluten activate complement via the alternative pathway. Clin Exp Immunol. 1993;94:539–543. [PMC free article] [PubMed] [Google Scholar]
- Volokhina EB, van de Kar NC, Bergseth G, van der Velden TJ, Westra D, Wetzels JF, van den Heuvel LP, Mollnes TE. Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome. Clin Immunol. 2015;160:237–243. doi: 10.1016/j.clim.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Weitz M, Amon O, Bassler D, Koenigsrainer A, Nadalin S. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatr Nephrol. 2011;26:1325–1329. doi: 10.1007/s00467-011-1879-9. [DOI] [PubMed] [Google Scholar]
- Zimmerhackl LB, Hofer J, Cortina G, Mark W, Wurzner R, Jungraithmayr TC, Khursigara G, Kliche KO, Radauer W. Prophylactic eculizumab after renal transplantation in atypical hemolytic-uremic syndrome. N Engl J Med. 2010;362:1746–1748. doi: 10.1056/NEJMc1001060. [DOI] [PubMed] [Google Scholar]
- Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V, French Study Group for a, H.C.G Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- Zurakowski D, Di Canzio J, Majzoub JA. Pediatric reference intervals for serum thyroxine, triiodothyronine, thyrotropin, and free thyroxine. Clin Chem. 1999;45:1087–1091. [PubMed] [Google Scholar]